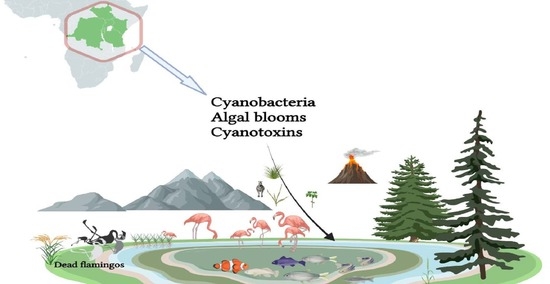
Lacustrine Cyanobacteria, Algal Blooms and Cyanotoxins in East Africa: Implications for Human and Ecological Health Protection
Abstract
1. Introduction
2. Materials and Methods
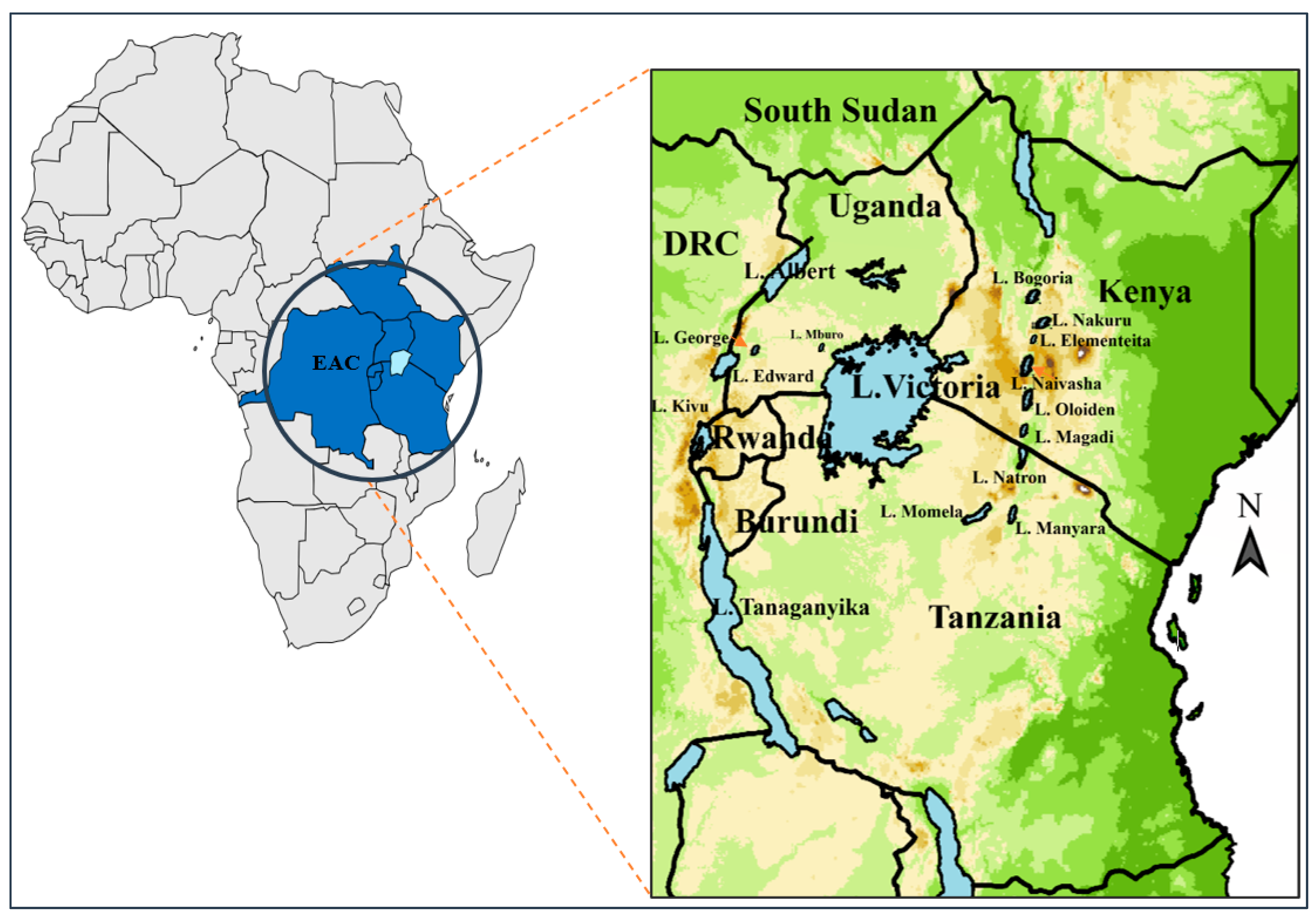
3. Occurrence of Cyanobacteria, Algal Blooms and Phycotoxins in EAC Lakes
3.1. DRC
3.2. Kenya
3.3. Tanzania
3.4. Uganda
3.5. Rwanda
4. Toxicity, Human and Ecological Health Implications of Cyanotoxins in EAC Lakes
4.1. MCs
4.2. Anatoxin-a
4.3. Homoanatoxin-a
4.4. Cylindrospermopsin
4.5. Nodularins
5. Conclusions and Recommendations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Opoku, E.E.O.; Yan, I.K.-M. Industrialization as driver of sustainable economic growth in Africa. J. Int. Trade Econ. Dev. 2019, 28, 30–56. [Google Scholar] [CrossRef]
- Naudé, W. Industrialization under Medieval Conditions? Global Development after COVID-19. Discussion Working paper, IZA DP No. 13829. 2020. Available online: https://docs.iza.org/dp13829.pdf (accessed on 26 November 2022).
- Elfaki, K.E.; Handoyo, R.D.; Ibrahim, K.H. The Impact of Industrialization, Trade Openness, Financial Development, and Energy Consumption on Economic Growth in Indonesia. Economies 2021, 9, 174. [Google Scholar] [CrossRef]
- Bongomin, O.; Ocen, G.G.; Nganyi, E.O.; Musinguzi, A.; Omara, T. Exponential Disruptive Technologies and the Required Skills of Industry 4.0. J. Eng. 2020, 2020, 4280156. [Google Scholar] [CrossRef]
- United Nations Industrial Development Organization. Industrialization as the Driver of Sustained Prosperity; United Nations Industrial Development Organization: Vienna, Austria, 2020; Available online: https://www.unido.org/sites/default/files/files/2020-04/UNIDO_Industrialization_Book_web4.pdf (accessed on 26 November 2022).
- Band-Schmidt, C.; Durán-Riveroll, L.; Bustillos-Guzmán, J.; Leyva-Valencia, I.; López-Cortés, D.; Núñez-Vázquez, E.J.; Hernández-Sandoval, F.; Ramírez-Rodríguez, D. Paralytic Toxin Producing Dinoflagellates in Latin America: Ecology and Physiology. Front. Mar. Sci. 2019, 6, 42. [Google Scholar] [CrossRef]
- Ndlela, L.L.; Oberholster, P.J.; Van Wyk, J.H.; Cheng, P.H. An overview of cyanobacterial bloom occurrences and research in Africa over the last decade. Harmful Algae 2016, 60, 11–26. [Google Scholar] [CrossRef]
- Kat, M. Special meeting on causes, dynamics and effects of exceptional marine blooms and related events. Int. Counc. Explor. Sea C 1984, 3. [Google Scholar]
- Padmakumar, K.B.; Menon, N.R.; Sanjeevan, V.N. Is Occurrence of Harmful Algal Blooms in the Exclusive Economic Zone of India on the Rise? Int. J. Oceanogr. 2012, 2012, 263946. [Google Scholar] [CrossRef]
- Klijnstra, M.D.; Faassen, E.J.; Gerssen, A. A Generic LC-HRMS Screening Method for Marine and Freshwater Phycotoxins in Fish, Shellfish, Water, and Supplements. Toxins 2021, 13, 823. [Google Scholar] [CrossRef]
- Jacquet, S.; Briand, J.-F.; Leboulanger, C.; Avois-Jacquet, C.; Oberhaus, L.; Tassin, B.; Vinçon-Leite, B.; Paolini, G.; Druart, J.-C.; Anneville, O.; et al. The proliferation of the toxic cyanobacterium Planktothrix rubescens following restoration of the largest natural French lake (Lac du Bourget). Harmful Algae 2005, 4, 651–672. [Google Scholar] [CrossRef]
- Reinl, K.L.; Brookes, J.D.; Carey, C.C.; Harris, T.D.; Ibelings, B.W.; Morales-Williams, A.M.; Yokota, K.; Zhan, Q. Cyanobacterial blooms in oligotrophic lakes: Shifting the high-nutrient paradigm. Freshw. Biol. 2021, 66, 1846–1859. [Google Scholar] [CrossRef]
- Lihepanyama, D.L.; Ndakidemi, P.A.; Treydte, A.C. Spatio–TemporalWater Quality Determines Algal Bloom Occurrence and Possibly Lesser Flamingo (Phoeniconaias minor) Presence in Momella Lakes, Tanzania. Water 2022, 14, 3532. [Google Scholar] [CrossRef]
- Olokotum, M.; Mitroi, V.; Troussellier, M.; Semyaloa, R.; Bernarde, C.; Montuelle, B.; Okello, W.; Quiblier, C.; Humbert, J.-F. A review of the socioecological causes and consequences of cyanobacterial blooms in Lake Victoria. Harmful Algae 2020, 96, 101829. [Google Scholar] [CrossRef] [PubMed]
- Saulnier-Talbot, É.; Gregory-Eaves, I.; Simpson, K.; Efitre, J.; Nowlan, T.; Taranu, Z.; Chapman, L. Small Changes in Climate Can Profoundly Alter the Dynamics and Ecosystem Services of Tropical Crater Lakes. PLoS ONE 2014, 9, e86561. [Google Scholar] [CrossRef] [PubMed]
- Baguma, G.; Musasizi, A.; Twinomuhwezi, H.; Gonzaga, A.; Nakiguli, C.K.; Onen, P.; Angiro, C.; Okwir, A.; Opio, B.; Otema, T.; et al. Heavy Metal Contamination of Sediments from an Exoreic African Great Lakes’ Shores (Port Bell, Lake Victoria), Uganda. Pollutants 2022, 2, 407–421. [Google Scholar] [CrossRef]
- Kimambo, O.N.; Gumbo, J.R.; Chikoore, H. The occurrence of cyanobacteria blooms in freshwater ecosystems and their link with hydro-meteorological and environmental variations in Tanzania. Heliyon 2019, 5, e01312. [Google Scholar] [CrossRef] [PubMed]
- Ayugi, B.; Dike, V.; Ngoma, H.; Babaousmail, H.; Mumo, R.; Ongoma, V. Future Changes in Precipitation Extremes over East Africa Based on CMIP6 Models. Water 2021, 13, 2358. [Google Scholar] [CrossRef]
- Kaggwa, M.; Straubinger-Gansberger, N.; Schagerl, M. Cyanotoxins in small artificial dams in Kenya utilised for cage fish farming—A threat to local people? Afr. J. Aquat. Sci. 2018, 43, 123–129. [Google Scholar] [CrossRef]
- Mbabazi, D.; Orach-Meza, F.L.; Makanga, B.; Hecky, R.E.; Balirwa, J.; Ogutu-Ohwayo, R.; Verburg, P.; Namuleno, G.; Muhumuza, E.; Luyiga, J. Trophic structure and energy flow in fish communities of two lakes of the Lake Victoria basin. Uganda J. Agric. Sci. 2004, 9, 348–359. [Google Scholar]
- Jones, M.R.; Pinto, E.; Torres, M.A.; Dörr, F.; Mazur-Marzec, H.; Szubert, K.; Tartaglione, L.; Dell’Aversano, C.; Miles, C.O.; Beach, D.G. CyanoMetDB, a comprehensive public database of secondary metabolites from cyanobacteria. Water Res. 2021, 196, 117017. [Google Scholar] [CrossRef]
- Gerssen, A.; Gago-Martinez, A. Emerging marine biotoxins. Toxins 2019, 11, 314. [Google Scholar] [CrossRef]
- Simiyu, B.; Amukhuma, H.; Sitoki, L.; Okello, W.; Kurmayer, R. Interannual variability of water quality conditions in the Nyanza Gulf of Lake Victoria, Kenya. J. Great Lakes Res. 2022, 48, 97–109. [Google Scholar] [CrossRef]
- Kimambo, O.N.; Gumbo, J.R.; Chikoore, H.; Msagati, T. Harmful Algal Blooms in Aquaculture Systems in Ngerengere Catchment, Morogoro, Tanzania: Stakeholder’s Experiences and Perception. Int. J. Environ. Res. Public Health 2021, 18, 4928. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, I.; Silsbe, G.; Verreth, J.; van Donk, E.; Nagelkerke, L. Dynamics and limitations of phytoplankton biomass along a gradient in Mwanza Gulf, southern Lake Victoria (Tanzania). Freshw. Biol. 2014, 59, 127–141. [Google Scholar] [CrossRef]
- Kondowe, B.; Masese, F.; Raburu, P.; Singini, W.; Walumona, R. Water quality and ecology of Lake Kanyaboli, Kenya: Current status and historical changes. Lakes Reserv. Res. Manag. 2022, 27, e12401. [Google Scholar] [CrossRef]
- Hecky, R.E.; Bugenyi, F.; Ochumba, P.; Talling, J.; Mugidde, R.; Gophen, M.; Kaufman, L. Deoxygenation of the deep water of Lake Victoria, East Africa. Limnol. Oceanogr. 1994, 39, 1476–1481. [Google Scholar] [CrossRef]
- Cocquyt, C.; Plisnier, P.-D.; Mulimbwa, N.; Nshombo, M. Unusual massive phytoplankton bloom in the oligotrophic Lake Tanganyika. Plant Ecol. Evol. 2021, 154, 351–361. [Google Scholar] [CrossRef]
- Ehrenfels, B.; Bartosiewicz, M.; Mbonde, A.; Baumann, K.; Dinkel, C.; Junker, J.; Kamulali, T.; Kimirei, I.; Niederdorfer, R.; Odermatt, D.; et al. Diazotrophic Cyanobacteria are Associated With a Low Nitrate Resupply to Surface Waters in Lake Tanganyika. Front. Environ. Sci 2021, 9, 716765. [Google Scholar] [CrossRef]
- Sarmento, H.; Darchambeau, F.; Descy, J. Phytoplankton of Lake Kivu; Descy, J.P., Darchambeau, F., Schmid, M., Eds.; Lake Kivu. Aquatic Ecology Series; Springer: Dordrecht, The Netherlands, 2012; Volume 5, pp. 67–83. [Google Scholar]
- Rugema, E.; Darchambeau, F.; Sarmento, H.; Stoyneva-Gärtner, M.; Leitao, M.; Thiery, W.; Latli, A.; Descy, J.P. Long-term change of phytoplankton in Lake Kivu: The rise of the greens. Freshw. Biol. 2019, 64, 1940–1955. [Google Scholar] [CrossRef]
- Hecky, R.; Kling, H. Phytoplankton ecology of the great lakes in the rift valleys of Central Africa. Arch. Hydrobiol. Beih. Ergeb. Limnol. 1987, 25, 197–228. [Google Scholar]
- Sarmento, H.; Leitao, M.; Stoyneva, M.; Couté, A.; Compère, P.; Isumbisho, M.; Descy, J. Diversity of pelagic algae of Lake Kivu (East Africa). Cryptogam. Algol. 2007, 28, 245–269. [Google Scholar]
- Sarmento, H.; Unrein, F.; Isumbisho, M.; Stenuite, S.; Gasol, J.; Descy, J. Abundance and distribution of picoplankton in tropical, oligotrophic Lake Kivu, eastern Africa. Freshw. Biol. 2008, 53, 756–771. [Google Scholar] [CrossRef]
- Sarmento, H.; Isumbisho, M.; Descy, J.-P. Phytoplankton ecology of Lake Kivu (eastern Africa). J. Plankton Res. 2006, 28, 815–829. [Google Scholar] [CrossRef]
- Ballot, A.; Krienitz, L.; Kotut, K.; Wiegand, C.; Metcalf, J.S.; Codd, G.A.; Pflugmacher, S. Cyanobacteria and cyanobacterial toxins in three alkaline Rift Valley lakes of Kenya—Lakes Bogoria, Nakuru and Elmenteita. J. Plankton Res. 2004, 26, 925–935. [Google Scholar] [CrossRef]
- Ballot, A.; Krienitz, L.; Kotut, K.; Wiegand, C.; Pflugmacher, S. Cyanobacteria and cyanobacterial toxins in the crater lakes Sonachi and Simbi, Kenya. Harmful Algae 2005, 4, 139–150. [Google Scholar] [CrossRef]
- Kotut, K.; Ballot, A.; Krienitz, L. Toxic cyanobacteria and their toxins in standing waters of Kenya: Implications for water resource use. J. Water Health 2006, 4, 3–18. [Google Scholar] [CrossRef]
- Tuite, C.H. Standing crop densities and distribution of Spirulina and benthic diatoms in East African alkaline saline lakes. Freshw. Biol. 1981, 11, 345–360. [Google Scholar] [CrossRef]
- Melack, J.M. Photosynthesis and growth of Spirulina platensis (Cyanophyta) in an equatorial lake (Lake Simbi, Kenya). Limnol. Oceanogr. 1979, 24, 753–760. [Google Scholar] [CrossRef]
- Finlay, B.J.; Curds, C.R.; Bamforth, S.S.; Bafort, J.M. Ciliated protozoa and other microorganisms from two African soda lakes (Lake Nakuru and Lake Simbi, Kenya). Arch. Protistenkd. 1987, 133, 81–91. [Google Scholar] [CrossRef]
- Dadheech, P.K.; Glöckner, G.; Casper, P.; Kotut, K.; Mazzoni, C.J.; Mbedi, S.; Krienitz, L. Cyanobacterial diversity in the hot spring, pelagic and benthic habitats of a tropical soda lake. FEMS Microbiol. Ecol. 2013, 85, 389–401. [Google Scholar] [CrossRef]
- Kibichii, S.; Shivoga, W.A.; Muchiri, M.; Enanga, E.; Miller, S.N. Seasonality in water quality and its influence on the abundance and distribution of phytoplankton and chironomid larvae in Lake Nakuru, Kenya. Int. Ver. Für Theor. Und Angew. Limnol. Verh. 2008, 30, 333–338. [Google Scholar] [CrossRef]
- Krienitz, L.; Kotut, K. Fluctuating algal food populations and the occurrence of lesser flamingos (Phoeniconaias minor) in three Kenyan rift valley lakes. J. Phycol. 2010, 46, 1088–1096. [Google Scholar] [CrossRef]
- Vareschi, E. The ecology of Lake Nakuru (Kenya). Oecologia 1978, 32, 11–35. [Google Scholar] [CrossRef]
- Sitoki, L.; Kurmayer, R.; Rott, E. Spatial variation of phytoplankton composition, biovolume, and resulting microcystin concentrations in the Nyanza Gulf (L. Victoria, Kenya). Hydrobiologia 2012, 691, 109–122. [Google Scholar] [CrossRef]
- Githukia, C.; Onyango, D.; Lusweti, D.; Ramkat, R.; Kowenje, C.; Miruka, J.; Lung’ayia, H.; Orina, P. An Analysis of Knowledge, Attitudes and Practices of Communities in Lake Victoria, Kenya on Microcystin Toxicity. Open J. Ecol. 2022, 12, 198–210. [Google Scholar] [CrossRef]
- Roegner, A.; Sitoki, L.; Weirich, C.; Corman, J.; Owage, D.; Umami, M.; Odada, E.; Miruka, J.; Ogari, Z.; Smith, W.; et al. Harmful Algal Blooms Threaten the Health of Peri-Urban Fisher Communities: A Case Study in Kisumu Bay, Lake Victoria, Kenya. Expo. Health 2020, 12, 835–848. [Google Scholar] [CrossRef]
- Simiyu, B.; Oduor, S.; Rohrlack, T.; Sitoki, L.; Kurmayer, R. Microcystin content in phytoplankton and in small fish from Eutrophic Nyanza Gulf, L. Victoria, Kenya. Toxins 2018, 10, 275. [Google Scholar] [CrossRef] [PubMed]
- Hamisi, M.; Lugomela, C.; Lyimo, T.; Bergman, B.; Díez, B. Plankton composition, biomass, phylogeny and toxin genes in Lake Big Momela, Tanzania. Afr. J. Aquat. Sci. 2017, 42, 109–121. [Google Scholar] [CrossRef]
- Lugomela, C.; Pratap, H.B.; Mgaya, Y.D. Cyanobacteria Blooms: A Possible Cause of Mass Mortality of Lesser Flamingos in Lake Manyara and Lake Big Momela, Tanzania. Harmful Algae 2006, 5, 534–541. [Google Scholar] [CrossRef]
- Nonga, H.E.; Mdegela, R.H.; Sandvik, M.; Lie, E.; Miles, C.O.; Skaare, J.U. Cyanobacteria and cyanobacterial toxins in the alkaline-saline Lakes Natron and Momela, Tanzania. Tanzan. Vet. Assoc. Proc. 2017, 32, 108–116. [Google Scholar]
- Miles, C.O.; Sandvik, M.; Nonga, H.E.; Rundberget, T.; Wilkins, A.L.; Rise, F.; Ballot, A. Identification of microcystins in a Lake Victoria cyanobacterial bloom using LC-MS with thiol derivatization. Toxicon 2013, 70, 21–31. [Google Scholar] [CrossRef]
- Mchau, G.; Machunda, R.; Kimanya, M.; Makule, E.; Gong, Y.; Mpolya, E.; Meneely, J.; Elliott, C.; Greer, B. First Report of the Co-occurrence of Cylindrospermopsin, Nodularin and Microcystins in the Freshwaters of Lake Victoria, Tanzania. Expo. Health 2021, 13, 185–194. [Google Scholar] [CrossRef]
- Busobozi, E. Eutrophication in Ugandan Crater Lakes. A Case Study of Six Crater Lakes Located in Kabarole District Western Uganda. Master’s Thesis, University of Canterbury Christchurch, Christchurch, New Zealand, 2017. [Google Scholar]
- Nankabirwa, A.; De Crop, W.; Van der Meeren, T.; Cocquyt, C.; Plisnier, P.-D.; Balirwa, J.; Verschuren, D. Phytoplankton communities in the crater lakes of western Uganda, and their indicator species in relation to lake trophic status. Ecol. Indic. 2019, 107, 105563. [Google Scholar] [CrossRef]
- Stoyneva-Gärtner, M.P.; Morana, C.; Borges, A.V.; Okello, W.; Bouillon, S.; Deirmendjian, L.; Lambert, T.; Roland, F.; Nankabirwa, A.; Nabafu, E.; et al. Diversity and ecology of phytoplankton in Lake Edward (East Africa): Present status and long-term changes. J. Great Lakes Res. 2020, 46, 741–751. [Google Scholar] [CrossRef]
- Ganf, G.G. Phytoplankton Biomass and Distribution in a Shallow Eutrophic Lake (Lake George, Uganda). Oecologia 1974, 16, 9–29. [Google Scholar] [CrossRef]
- Okello, W.; Ostermaier, V.; Portmann, C.; Gademann, K.; Kurmayer, R. Spatial isolation favours the divergence in microcystin net production by Microcystis in Ugandan freshwater lakes. Water Res. 2010, 44, 2803–2814. [Google Scholar] [CrossRef]
- Kamanyi, J.R.; Ogwang, O.; Twongo, E. Plankton identified in stomach contents of Oreochromis niloticus (Pisces, Cichlidae) and the water system of Lakes Edward, George, and Kazinga channel—Uganda. Afr. J. Trop. Hydrobiol. Fish 1996, 7, 49–54. [Google Scholar] [CrossRef]
- Nyakoojo, C.; Byarujali, S.M. An ecological study of two shallow, equatorial lakes: Lake Mburo and Lake Kachera, Uganda. Afr. J. Ecol. 2010, 48, 860–864. [Google Scholar] [CrossRef]
- Kayiira, D. Algal Community of Lake Mburo and Murchison Bay, Lake Victoria. Master’s Thesis, Makerere University, Kampala, Uganda, 2007. [Google Scholar]
- Byarujali, S.M. Phytoplankton production in L. Mburo—Western Uganda. In Proceedings of the First Conference on Ecology and Sustainable Natural Resource Management for Development, Mweya, Queen Elizabeth National Park, Uganda, 27 February–3 March 1995; pp. 284–290. [Google Scholar]
- Poste, A.E. Microcystin in Ugandan Lakes: Production Dynamics, Accumulation in Fish, and Risk Evaluation. Ph.D. Thesis, University of Waterloo, Waterloo, ON, Canada, 2010. [Google Scholar]
- Nyakairu, G.; Nagawa, C.; Mbabazi, J. Assessment of cyanobacteria toxins in freshwater fish: A case study of Murchison Bay (Lake Victoria) and Lake Mburo, Uganda. Toxicon 2010, 55, 939–946. [Google Scholar] [CrossRef]
- Semyalo, R.; Rohrlack, T.; Naggawa, C.; Nyakairu, G.W. Microcystin concentrations in Nile Tilapia (Oreochromis niloticus) caught from Murchison Bay, L. Victoria and Lake Mburo: Uganda. Hydrobiologia 2009, 638, 235–244. [Google Scholar] [CrossRef]
- Olokotum, M.; Humbert, J.-F.; Quiblier, C.; Okello, W.; Semyalo, R.; Troussellier, M.; Marie, B.; Baumann, K.; Kurmayer, R.; Bernard, C. Characterization of Potential Threats from Cyanobacterial Toxins in Lake Victoria Embayments and during Water Treatment. Toxins 2022, 14, 664. [Google Scholar] [CrossRef]
- Mukankomeje, R.; Plisnier, P.-D.; Descy, J.-P.; Massaut, L. Lake Muzahi, Rwanda: Limnological features and phytoplankton production. Hydrobiologia 1993, 257, 107–120. [Google Scholar] [CrossRef]
- Mukankomeje, R.; Laviolette, F.; Descy, J.-P. Régime alimentaire de Tilapia, Oreochromis niloticus, du Lac Muhazi (Rwanda). Ann. De Limnol. 1994, 30, 297–312. [Google Scholar] [CrossRef]
- Fazi, S.; Butturini, A.; Tassi, F.; Amalfitano, S.; Venturi, S.; Vazquez, E.; Clokie, M.; Wanjala, S.W.; Pacini, N.; Harper, D.M. Biogeochemistry and biodiversity in a network of saline–alkaline lakes: Implications of ecohydrological connectivity in the Kenyan Rift Valley. Ecohydrol. Hydrobiol. 2018, 18, 96–106. [Google Scholar] [CrossRef]
- Okello, W.; Portmann, C.; Erhard, M.; Gademann, K.; Kurmayer, R. Occurrence of microcystin-producing cyanobacteria in Ugandan freshwater habitats. Environ. Toxicol. 2010, 25, 367–438. [Google Scholar] [CrossRef]
- Ochumba, P.B.O.; Kibbara, D.I. Observations on blue-green algal blooms in the open waters of Lake Victoria, Kenya. Afr. J. Ecol. 1989, 27, 23–34. [Google Scholar] [CrossRef]
- Muruga, B.N.; Wagacha, J.M.; Kabaru, J.M.; Amugune, N.; Duboise, S.M. Effect of physicochemical conditions on growth rates of cyanobacteria species isolated from Lake Magadi, a soda lake in Kenya. WebPub J. Sci. Res. 2014, 2, 41–50. [Google Scholar]
- Okello, W.; Kurmayer, R. Seasonal development of cyanobacteria and microcystin production in Ugandan freshwater lakes. Lakes Reserv. Res. Manag. 2011, 16, 123–135. [Google Scholar] [CrossRef]
- Rastogi, R.; Madamwar, D.; Incharoensakdi, A. Bloom Dynamics of Cyanobacteria and Their Toxins: Environmental Health Impacts and Mitigation Strategies. Front. Microbiol 2015, 6, 1254. [Google Scholar] [CrossRef]
- Diez-Quijada, L.; Benítez-González, M.; Puerto, M.; Jos, A.; Cameán, A.M. Immunotoxic Effects Induced by Microcystins and Cylindrospermopsin: A Review. Toxins 2021, 13, 711. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). Microcystin-LR. IARC Monographs on the Identification of Carcinogenic Hazards to Humans. Available online: https://monographs.iarc.who.int/list-of-classifications (accessed on 2 December 2022).
- Otero, P.; Silva, M. The role of toxins: Impact on human health and aquatic environments. In the Pharmacological Potential of Cyanobacteria; Academic Press: Cambridge, MA, USA, 2022; pp. 173–199. [Google Scholar]
- WHO. Cyanobacterial Toxins: Microcystins. Background Document for Development of WHO Guidelines for Drinking-Water Quality and Guidelines for Safe Recreational Water Environments; World Health Organization: Geneva, Switzerland, 2020; WHO/HEP/ECH/WSH/2020.6; Available online: https://apps.who.int/iris/bitstream/handle/10665/338066/WHO-HEP-ECH-WSH-2020.6-eng.pdf (accessed on 2 December 2022).
- Codd, G.A.; Metcalf, J.S.; Morrison, L.F.; Krienitz, L.; Ballot, A.; Pflugmacher, S.; Wiegand, C.; Kotut, K. Susceptibility of flamingos to cyanobacterial toxins via feeding. Vet. Rec. 2003, 152, 722–723. [Google Scholar]
- Nowicka-Krawczyk, P.; Mühlsteinová, R.; Hauer, T. Detailed characterization of the Arthrospira type species separating commercially grown taxa into the new genus Limnospira (Cyanobacteria). Sci. Rep. 2019, 9, 36831. [Google Scholar] [CrossRef] [PubMed]
- Fox, D.L.; McBeth, J.W. Some dietary carotenoids and blood-carotenoid levels in flamingos. Comp. Biochem. Physiol. 1970, 34, 707–713. [Google Scholar] [CrossRef]
- Fox, D.; Smith, V.E.; Wolfson, A.A. Carotenoid selectivity in blood and feathers of lesser (African), Chilean and greater (European) flamingos. Comp. Biochem. Physiol. 1967, 23, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.; Metallinos-Katsaras, E.; Grivetti, L. Coturnism: Human Poisoning By European Migratory Quail. J. Cult. Geogr. 1987, 7, 51–65. [Google Scholar] [CrossRef]
- Koenig, R. The pink death: Die-offs of the lesser flamingo raise concern. Science 2006, 313, 1724–1725. [Google Scholar] [CrossRef] [PubMed]
- Ballot, A.; Pflugmacher, S.; Wiegand, C.; Kotut, K.; Krause, E.; Metcalf, J.S.; Morrison, L.F.; Codd, G.A.; Krienitz, L. Cyanobacterial toxins, a further contributory cause of mass deaths of flamingos at Kenyan Rift Valley lakes. In Abstracts of the Xth International Conference on Harmful Algae; Tradewinds Conference Center: St. Pete Beach, FL, USA, 2002; p. 20. [Google Scholar]
- Nonga, H.; Sandvik, M.; Miles, C.; Lie, E.; Mdegela, R.; Mwamengele, G.; Semuguruka, W.; Skaare, J. Possible involvement of microcystins in the unexplained mass mortalities of Lesser Flamingo (Phoeniconaias minor Geoffroy) at Lake Manyara in Tanzania. Hydrobiologia 2011, 678, 167–178. [Google Scholar] [CrossRef]
- Fyumagwa, R.D.; Bugwesa, Z.; Mwita, M.; Kihwele, E.S.; Nyaki, A.; Mdegela, R.H.; Mpanduji, D.G. Cyanobacterial toxins and bacterial infections are the possible causes of mass mortality of lesser flamingos in Soda lakes in northern Tanzania. Res. Opin. Anim. Vet. Sci. 2013, 3, 1–6. [Google Scholar]
- Krienitz, L.; Ballot, A.; Kotut, K.; Wiegand, C.; Pütz, S.; Metcalf, J.S.; Codd, G.A.; Pflugmacher, S. Contribution of hot spring cyanobacteria to the mysterious deaths of Lesser Flamingos at Lake Bogoria, Kenya. FEMS Microbiol. Ecol. 2003, 43, 141–148. [Google Scholar] [CrossRef]
- Metcalf, J.S.; Morrison, L.F.; Krienitz, L.; Ballot, A.; Krause, E.; Kotut, K.; Pütz, S.; Wiegand, C.; Pflugmacher, S.; Codd, G.A. Analysis of the cyanotoxins anatoxin-a and microcystins in Lesser Flamingo feathers. Toxicol. Environ. Chem. 2006, 88, 159–167. [Google Scholar] [CrossRef]
- Metcalf, J.S.; Banack, S.A.; Kotut, K.; Krienitz, L.; Codd, G.A. Amino acid neurotoxins in feathers of the Lesser Flamingo, Phoeniconaias minor. Chemosphere 2013, 90, 835–839. [Google Scholar] [CrossRef] [PubMed]
- Riley, B. “Lake That Turns Animals to Stone” Not so Deadly as Photos Suggest. 2013. Available online: https://www.nationalgeographic.com/science/article/lake-that-turns-animals-to-stone-not-so-deadly-as-photos-suggest (accessed on 4 December 2022).
- Straubinger-Gansberger, N.; Gruber, M.; Kaggwa, M.N.; Lawton, L.; Oduor, S.O.; Schagerl, M. Sudden flamingo deaths in Kenyan Rift Valley lakes. Wildl. Biol. 2014, 20, 185–189. [Google Scholar] [CrossRef]
- Krienitz, L.; Ballot, A.; Casper, P.; Codd, G.A.; Kotut, K.; Metcalf, J.S.; Morrison, L.F.; Pflugmacher, S. Contribution of toxic cyanobacteria to massive deaths of lesser flamingos at saline-alkaline lakes of Kenya. Int. Ver. Limnol. 2005, 29, 783–786. [Google Scholar] [CrossRef]
- Ndetei, R.; Muhandiki, V.S. Mortalities of Lesser Flamingos in Kenyan Rift Valley saline lakes and the implications for sustainable management of the lakes. Lakes Reserv. Res. Manag. 2005, 10, 51–58. [Google Scholar] [CrossRef]
- Kock, N.D.; Kock, R.A.; Wambua, J.; Kamau, G.J.; Mohan, K. Mycobacterium avium related epizootic in free ranging Lesser Flamingos. Kenya J. Wildl. Dis. 1999, 35, 297–300. [Google Scholar] [CrossRef]
- Kihwele, E. Seasonal Variations in the Abundance of Lesser Flamingos (Phoeniconaiais minor) in Relation to Some Limnological Parameters in Lake Manyara, Tanzania. Master’s Thesis, University of Dar es Salaam, Dar es Salaam, Tanzania, 2010. [Google Scholar]
- Foss, A.J.; Miles, C.O.; Samdal, I.A.; Løvberg, K.E.; Wilkins, A.L.; Rise, F.; Jaabæk, J.A.H.; McGowan, P.C.; Aubel, M.T. Analysis of free and metabolized microcystins in samples following a bird mortality event. Harmful Algae 2018, 80, 117–129. [Google Scholar] [CrossRef]
- Carmichael, W.W.; Li, R. Cyanobacteria toxins in the Salton Sea. Saline Syst. 2006, 2, 5. [Google Scholar] [CrossRef]
- Alonso-Andicoberry, C.; García-Villada, L.; Lopez-Rodas, V.; Costas, E. Catastrophic mortality of flamingos in a Spanish national park caused by cyanobacteria. Vet. Rec. 2002, 151, 706–707. [Google Scholar]
- Matsunaga, H.; Harada, K.I.; Senma, M.; Ito, Y.; Yasuda, N.; Ushida, S.; Kimura, Y. Possible cause of unnatural mass death of wild birds in a pond in Nishinomiya, Japan: Sudden appearance of toxic cyanobacteria. Nat. Toxins 1999, 7, 81–84. [Google Scholar] [CrossRef]
- Henriksen, P.; Carmichael, W.W.; An, J.; Moestrup, Ø. Detection of an anatoxin-a (s)-like anticholinesterase in natural blooms and cultures of cyanobacteria/blue-green algae from Danish lakes and in the stomach contents of poisoned birds. Toxicon 1997, 35, 901–913. [Google Scholar] [CrossRef]
- Sileo, L.; Grootenhuise, J.G.; Tuite, G.H.; Hopcraft, H.D. Microbacteriosis in the Lesser Flamingo of Lake Nakuru, Kenya. J. Wildl. Dis. 1979, 15, 387–390. [Google Scholar] [CrossRef]
- Kairu, J.K. Heavy metals residue in birds of Lake Nakuru, Kenya. Afr. J. Ecol. 1996, 34, 397–400. [Google Scholar] [CrossRef]
- Saltwork Consultants Pty Ltd. Lake Nakuru’s Flamingo Connection—Cycles of Feast and Famine in Schizohaline Waters. Available online: https://www.saltworkconsultants.com/lake-nakuru-flamingo-connection-cycles-of-feast-and-famine-in-schizohaline-waters/ (accessed on 1 December 2022).
- Nelson, Y.M.; Thampy, R.J.; Motellin, G.K.; Raini, J.A.; Disante, C.J.; Lion, L.W. Model for trace metal exposure in filter feeding flamingo at an alkaline rift valley lake, Kenya. Environ. Toxicol. Chem. 1998, 17, 2302–2309. [Google Scholar] [CrossRef]
- Miller, E.; Brunner, E.; Driscoll, C.; McGowan, P. Botulism.Or Is It? Wildl. Rehabil. Bull. 2013, 31, 1–12. [Google Scholar] [CrossRef]
- WWF–LNCDP. Annual Report. World Wide Fund for Nature–Lake Nakuru Conservation and Development Project (WWF–LNCDP); WWF–LNCDP: Nakuru, Kenya, 1994. [Google Scholar]
- IUCN. Flamingo. Bulletin of the IUCN-SSC/Wetlands International. 2015. Available online: https://www.wetlands.org/wp-content/uploads/2015/11/Flamingo-Newsletter-14-2006.pdf (accessed on 1 December 2022).
- Papadimitriou, T.; Katsiapi, M.; Vlachopoulos, K.; Christopoulos, A.; Laspidou, C.; Moustaka-Gouni, M.; Kormas, K. Cyanotoxins as the “common suspects” for the Dalmatian pelican (Pelecanus crispus) deaths in a Mediterranean reconstructed reservoir. Envirn. Pollut. 2018, 234, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, C.; McGowan, P.; Miller, E.; Carmichael, W. Case Report: Great blue heron (Ardea herodias) morbidity and mortality investigation in Maryland’s Chesapeake Bay. In Proceedings of the Southeast Fish and Wildlife Conference, Baltimore, MD, USA, 24 October 2002. [Google Scholar]
- Kumssa, T.; Bekele, A. Current Population Status and Activity Pattern of Lesser Flamingos (Phoeniconaias minor) andGreater Flamingo (Phoenicopterus roseus) in Abijata-Shalla Lakes National Park (ASLNP), Ethiopia. Int. J. Biodivers. 2014, 2014, 295362. [Google Scholar] [CrossRef]
- Kihwele, E.; Lugomela, C.; Howell, K. Temporal Changes in the Lesser Flamingos Population (Phoenicopterus minor) in Relation to Phytoplankton Abundance in Lake Manyara, Tanzania. Open J. Ecol. 2014, 4, 145–161. [Google Scholar] [CrossRef]
- Childress, B.; Hughes, B.; Harper, D.; van den Bossche, W. East African flyway and key site network of the Lesser Flamingo (Phoenicopterus minor) documented through satellite tracking. J. Afr. Ornithol. 2009, 78, 463–468. [Google Scholar] [CrossRef]
- Childress, B.; Nagy, S.; Hughes, B. International Single Species Action Plan for the Conservation of the Lesser Flamingo (Phoeniconaias minor); CMS Technical Series No. 18, AEWA Technical Series No. 34; AEWA: Bonn, Germany, 2008. [Google Scholar]
- Fiorucci, L.; Grande, F.; Macrelli, R.; Schnitzer, P.; Crosta, L. Hand-Rearing of Three Lesser Flamingo Chicks (Phoeniconaias minor). Animals 2020, 10, 1251. [Google Scholar] [CrossRef]
- Botana, L.; James, K.; Crowley, J.; Duphard, J.; Lehane, M.; Furey, A. Anatoxin-a and Analogues: Discovery, Distribution, and Toxicology. In Phycotoxins: Chemistry and Biochemistry; Blackwell Publishing: Hoboken, NJ, USA, 2007; pp. 141–158. [Google Scholar]
- Aráoz, R.; Molgó, J.; Tandeau de Marsac, N. Neurotoxic cyanobacterial toxins. Toxicon 2010, 56, 813–828. [Google Scholar] [CrossRef]
- Osswald, J.; Rellán, S.; Gago, A.; Vasconcelos, V. Toxicology and detection methods of the alkaloid neurotoxin produced by cyanobacteria, anatoxin-a. Environ. Int. 2007, 33, 1070–1089. [Google Scholar] [CrossRef]
- Soliakov, L.; Gallagher, T.; Wonnacott, S. Anatoxin-a-evoked [3H]dopamine release from rat striatal synaptosomes. Neuropharmacology 1995, 34, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Aas, P.; Eriksen, S.; Kolderup, J.; Lundy, P.; Haugen, J.E.; Skulberg, O.M.; Fonnum, F. Enhancement of acetylcholine release by homoanatoxin-a from Oscillatoria formosa. Environ. Toxicol. Pharmacol. 1996, 2, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.A.; Selwood, A.I.; Rueckert, A.; Holland, P.T.; Milne, J.R.; Smith, K.F.; Smits, B.; Watts, L.F.; Cary, C.S. First report of homoanatoxin-a and associated dog neurotoxicosis in New Zealand. Toxicon 2007, 50, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, D.J.; Saker, M.L. The Palm Island mystery disease 20 years on: A review of research on the cyanotoxin cylindrospermopsin. Environ. Toxicol. Chem. 2003, 18, 78–93. [Google Scholar] [CrossRef]
- Chichova, M.; Tasinov, O.; Shkodrova, M.; Mishonova, M.; Sazdova, I.; Ilieva, B.; Doncheva-Stoimenova, D.; Kiselova-Kaneva, Y.; Raikova, N.; Uzunov, B.; et al. New Data on Cylindrospermopsin Toxicity. Toxins 2021, 13, 41. [Google Scholar] [CrossRef]
- Froscio, S.; Humpage, A.; Wickramasinghe, W.; Shaw, G.; Falconer, I. Interaction of the cyanobacterial toxin cylindrospermopsin with the eukaryotic protein synthesis system. Toxicon 2008, 51, 191–198. [Google Scholar] [CrossRef]
- Humpage, A.R.; Fenech, M.; Thomas, P.; Falconer, I.R. Micronucleus induction and chromosome loss in transformed human white cells indicate clastogenic and aneugenic action of the cyanobacterial toxin, cylindrospermopsin. Mutat. Res. 2000, 472, 155–161. [Google Scholar] [CrossRef]
- Thomas, A.D.; Saker, M.L.; Norton, J.H.; Olsen, R.D. Cyanobacterium Cylindrospermopsis raciborskii as a probable cause of death in cattle in northern Queensland. Aust. Vet. J 1998, 76, 592–594. [Google Scholar] [CrossRef]
- Đorđević, N.; Simić, S.; Ćirić, A. First identification of the cylindrospermopsin (cyn)-producing cyanobacterium Cylindrospermopsis raciborskii (Woloszyńska) Seenayya & Subba Raju in Serbia. Fresenius Env. Bull. 2015, 24, 3736–3742. [Google Scholar]
- Beasley, V.R. Harmful Algal Blooms (Phycotoxins). In Reference Module in Earth Systems and Environmental Sciences; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Chen, Y.; Shen, D.; Fang, D. Nodularins in poisoning. Clin. Chim. Acta 2013, 425, 18–29. [Google Scholar] [CrossRef]
- Zegura, B.; Straser, A.; Filipič, M. Genotoxicity and potential carcinogenicity of cyanobacterial toxins—A review. Mutat. Res. 2011, 727, 16–41. [Google Scholar] [CrossRef]
- Flores, M.; Goodrich, D.W. Retinoblastoma Protein Paralogs and Tumor Suppression. Front. Genet. 2022, 13, 818719. [Google Scholar] [CrossRef] [PubMed]
- Štern, A.; Rotter, A.; Novak, M.; Filipič, M.; Žegura, B. Genotoxic effects of the cyanobacterial pentapeptide nodularin in HepG2 cells. Food Chem. Toxicol. 2019, 124, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Sivonen, K. Cyanobacterial toxins and toxin production. Phycologia 1996, 190, 267–275. [Google Scholar] [CrossRef]
- Harding, W.R.; Rowe, N.; Wessels, J.C.; Beattie, K.A.; Codd, G.A. Death of a dog attributed to the cyanobacterial (blue-green algal) hepatotoxin nodularin in South Africa. J. S. Afr. Vet. Assoc. 1995, 66, 256–259. [Google Scholar] [PubMed]
- Main, D.C.; Berry, P.H.; Peet, R.L.; Robertson, J.P. Sheep mortalities associated with the blue green alga Nodularia spumigena. Aust. Vet. J. 1977, 53, 578–581. [Google Scholar] [CrossRef]



| Lake | Fish Species | MCs Content (Average/Range, μg kg−1) 1 | References |
|---|---|---|---|
| L. Victoria (Murchison Bay) | Clarias gariepinus | 23.9 | [64] |
| Haplochromis spc (filleted and whole) | 35.6 and 19.9 | ||
| Lates niloticus | 13.5 | ||
| Oreochromis leucostictus | 30.3 | ||
| Oreochromis niloticus | 13.7 | ||
| Protopterus aethiopicus | 4.1 | ||
| Rastrineobola argentea (whole/dry from market) | 36.2–41.2 | ||
| Synodontis afrofischeri | 28.8 | ||
| Synodontis victoriae | 16.7 | ||
| Tilapia zilli | 15.5 | ||
| Oreochromis niloticus (gut, liver and muscle) | 1479.24, 48.07 and 9.65 | [65] | |
| Lates niloticus (gut, liver and muscle) | 27.78, 3.74 and 1.86 | ||
| L. Victoria (Napoleon Gulf) | Astatoreochromis alluaudi | 6.2 | [64] |
| Bagrus docmac | 15.1 | ||
| Brycinus sadleri | 24.6 | ||
| Haplochromis spc (filleted and whole) | 13.0–17.1 and 15.3 | ||
| Lates niloticus (filleted and gutted/beheaded) | 7.3 and 12.9 | ||
| Mormyrus kannume | 21.1 | ||
| Oreochromis leucostictus | 3.2–4.3 | ||
| Oreochromis niloticus (filleted and gutted/beheaded) | 9.8 and 6.1 | ||
| Oreochromis variabilis | 30.1 | ||
| Protopterus aethiopicus | 2.8 | ||
| Rastrineobola argentea (whole/dry from market) | 83.7 | ||
| Synodontis afrofischeri | 31.0 | ||
| Synodontis victoriae | 16.7 | ||
| Tilapia zilli (filleted and gutted) | 8.4 and 3.4 | ||
| L. Victoria (open lake at Rusinga channel and Nyanza Gulf) | Rastrineobola argentea | 14 and 25–109 | [49] |
| Lake Mburo | Bagrus docmac | 13.4 | [64] |
| Clarias gariepinus | 20.6 | ||
| Haplochromis spc (filleted) | 2.5–5.6 | ||
| Haplochromis spc (gutted/beheaded) | 5.4–11.8 | ||
| Haplochromis spc (whole) | 12.1 | ||
| Oreochromis esculentus | 17.9 | ||
| Oreochromis leucostictus | 8.4 | ||
| Oreochromis leucostictus (gutted/head removed) | 7.4 | ||
| Protopterus aethiopicus | 2.5 | ||
| Oreochromis niloticus (gut, liver and muscle) | 1312.08, 73.10 and 208.65 | [65] | |
| Lake Nkuruba | Poecelia reticulata | 4.5 to 73.3 | [64] |
| Tilapia zilli (filleted and whole) | 11.7 and 42.5 | ||
| Oreochromis leucostictus (filleted and gutted/beheaded) | 8.3 and 17.2 | ||
| Lake George | Bagrus docmac | 9.1 | |
| Clarias gariepinus | 6.1 | ||
| Oreochromis leucostictus | 21.2 | ||
| Oreochromis niloticus | 10.2 | ||
| Protopterus aethiopicus | 2.4 | ||
| Oreochromis esculentus | 6.3 | ||
| Haplochromis squamipinnis (filleted and gutted) | 6.7 and 11.8 | ||
| Lake Saka | Astatoreochromis alluaudi (filleted and gutted/beheaded) | 71.3 and 10.5 | [64] |
| Astatoreochromis alluaudi (whole) | 32.5 | ||
| Barbus neumayerii (gutted/beheaded) | 9.5 | ||
| Haplochromis spc (filleted) | 52.1 | ||
| Haplochromis spc (gutted/beheaded and whole) | 23.2–1189.3 and 21.3–215.2 | ||
| Lates niloticus | 16.4 | ||
| Oreochromis niloticus | 17.0 | ||
| Tilapia zilli (filleted and whole) | 4.9 and 898.7 | ||
| Lake Edward | Bagrus docmac | 6.2 | |
| Barbus bynni | 5.3 | ||
| Clarias gariepinus | 8.6 | ||
| Haplochromis spc | 10.0 | ||
| Haplochromis squamipinnis | 8.6 | ||
| Oreochromis leucostictus | 21.9 | ||
| Oreochromis niloticus | 8.0 | ||
| Protopterus aethiopicus | 5.3 | ||
| Lake Albert | Lates niloticus | 3.9–11.6 | |
| Tilapia zilli | 2.7–6.2 |
| Waterbody (Country) | Report (s) | Year | Reference(s) |
|---|---|---|---|
| East Africa | |||
| Lake Nakuru (Kenya) | 0.00003 to 0.0004 µg MC-LR eq kg−1 and 0.00004–0.0058 µg kg−1 in liver, stomach/intestine | 2001–2003 | [94] |
| 35,000 birds died | 2006 | [44] | |
| Lake Bogoria and Lake Nakuru (Kenya) | 40,000 birds died | 1991 | [93] |
| More than 30,000 birds died; 0.00021 and 0.00093 µg MC-LR eq kg−1 fresh weight, ATX ranged between 0.00106 and 0.00582 µg kg−1 fresh weight | 1993 | [85,86,95,96] 1 | |
| 50,000 birds died | 1995/1996 | [94] | |
| Lake Bogoria | 30,000 birds died | 1999/2000 | [89,93] |
| 0.00003–0.0009 µg MC-LR eq kg−1 and 0.00004–0.0002 µg kg−1 in liver, stomach/intestine | 2001–2003 | [94] | |
| 30,000 birds died | 2008 | [44,93] | |
| 2000 birds died | 2009 | [93] | |
| Lake Natron and Empakai crater (Tanzania) | 43,800 birds died. Total MCs (MC-RR, -YR, -LR and -RY) were 0.1–4.5 μg mL−1 | July–August 2004 | [51,85,88] 2 |
| Lake Big Momela (Tanzania) | 15 and 50 individuals per day for 2004 case; elevated levels (up to 150 million filaments L−1 of A. fusiformis were quantified in sampled scum; no MCs detected | ||
| Lake Manyara (Tanzania) | 521 deaths per month; bird livers contained 0.0003–0.0541 µg kg−1 wet weight of MCs. Corynebacteria species, Pasteurella multocida and Proteus species were found in visceral organs of all carcasses tested | 2004, August–October 2008 | [87,88,97] 3* |
| Other regions | |||
| The Salton Sea (USA) | Over 20,000 deaths of Eared grebe (Podiceps nigricollis). Water contained up to 0.001 µg kg−1 DW and UDT to 0.00011 µg kg−1 DW in grebe liver tissues | 1990–2006 | [98,99] |
| Doñana National Park (Spain) | 579 Greater flamingos (Phoenicopterus roseus) died; MCs at concentrations of 0.44 μg kg−1 of liver wet weight and 0.625 μg kg−1 in crop contents | 2001 | [100] |
| Pond in Nishinomiya (Japan) | 20 spot-billed ducks died; MCs were detected in water (0.512 μg kg−1 cyanobacterial cell powder) | 1995 | [101] |
| Lake Knudsø (Denmark) | 3 ducks, 16 ducklings, 1 coot, coot chicks | 23rd and 26th June 1981 | [102] ** |
| 2 ducks and crows (unknown number) | 12th and 17th June 1988 | ||
| 2 grebes; 14–19 birds (black-necked and crested grebes, seagulls and a duck) and 2 grebes, respectively. ATX was recorded at 2.30 μg kg−1 | 10th June, 1st and 4th July 1993 | ||
| Birds (unreported number), and 1 coot, 1 duck. ATX was recorded at 3.30 μg kg−1 while MCs occurred at 0.0001 to 0.0009 μg kg−1 | 28th June and 6th July 1994 | ||
| 1 duck | 9th July 1995 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omara, T.; Nagawa, C.B.; Kyarimpa, C.; Böhmdorfer, S.; Rosenau, T.; Lugasi, S.O.; Matovu, H.; Odongo, S.; Ssebugere, P. Lacustrine Cyanobacteria, Algal Blooms and Cyanotoxins in East Africa: Implications for Human and Ecological Health Protection. Phycology 2023, 3, 147-167. https://doi.org/10.3390/phycology3010010
Omara T, Nagawa CB, Kyarimpa C, Böhmdorfer S, Rosenau T, Lugasi SO, Matovu H, Odongo S, Ssebugere P. Lacustrine Cyanobacteria, Algal Blooms and Cyanotoxins in East Africa: Implications for Human and Ecological Health Protection. Phycology. 2023; 3(1):147-167. https://doi.org/10.3390/phycology3010010
Chicago/Turabian StyleOmara, Timothy, Christine Betty Nagawa, Christine Kyarimpa, Stefan Böhmdorfer, Thomas Rosenau, Solomon Omwoma Lugasi, Henry Matovu, Silver Odongo, and Patrick Ssebugere. 2023. "Lacustrine Cyanobacteria, Algal Blooms and Cyanotoxins in East Africa: Implications for Human and Ecological Health Protection" Phycology 3, no. 1: 147-167. https://doi.org/10.3390/phycology3010010
APA StyleOmara, T., Nagawa, C. B., Kyarimpa, C., Böhmdorfer, S., Rosenau, T., Lugasi, S. O., Matovu, H., Odongo, S., & Ssebugere, P. (2023). Lacustrine Cyanobacteria, Algal Blooms and Cyanotoxins in East Africa: Implications for Human and Ecological Health Protection. Phycology, 3(1), 147-167. https://doi.org/10.3390/phycology3010010