Monthly Variation in Mycosporine-like Amino Acids from Red Alga Dulse (Devaleraea inkyuleei, Formerly Palmaria palmata in Japan)
Abstract
1. Introduction
2. Materials and Methods
2.1. Algal Sample Preparation
2.2. Extraction of Crude MAAs from Dulse
2.3. HPLC Analysis of MAAs
2.4. Protein, Phycoerythrin (PE), and Sugar Content
2.5. Abiotic Data in Hakodate
2.6. Compositional Comparison of Usujiri Dulse from 2019 to 2021
2.7. Ethanol Extraction of MAAs
2.8. Statistical Analysis
3. Results and Discussion
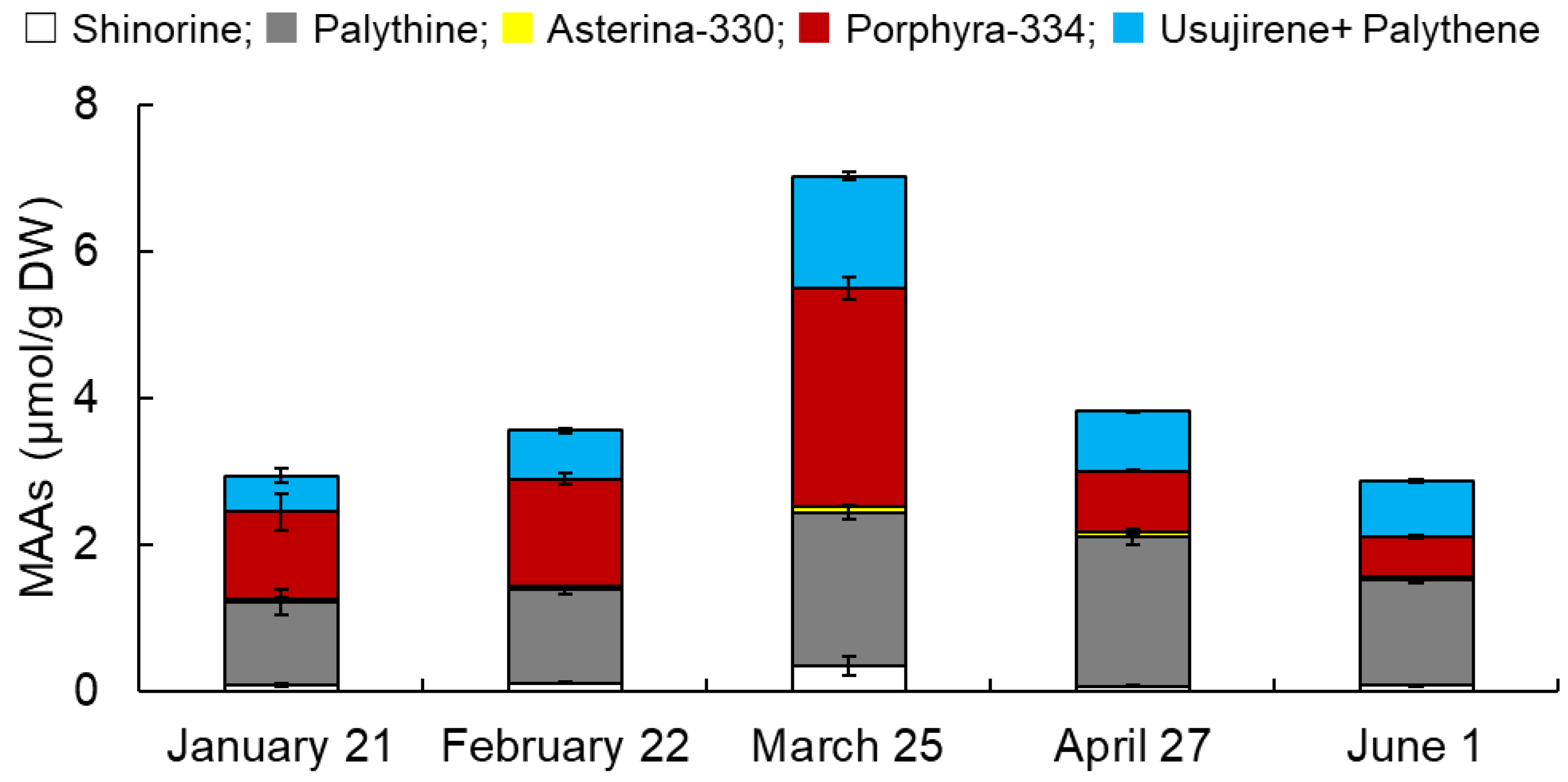
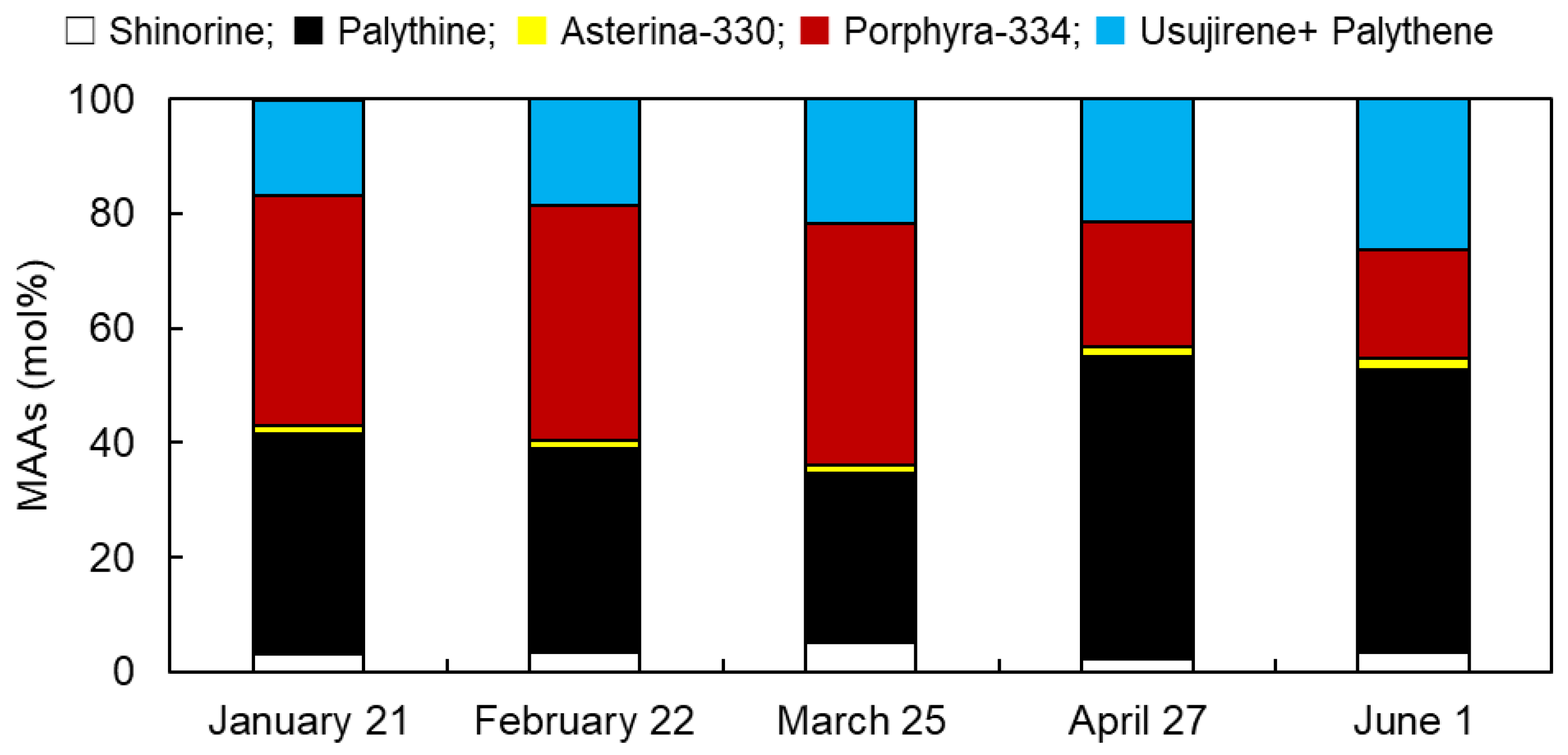
3.1. Monthly Variations in MAAs from Usujiri Dulse in 2021
3.2. Monthly Variations in MAA Content
3.3. Changes in MAAs, Proteins, Saccharides, and Erythemal UV Intensity
3.4. Evaluation of Ethanol Extraction of MAAs from Dulse
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suh, S.-S.; Hwang, J.; Park, M.; Seo, H.H.; Kim, H.-S.; Lee, J.H.; Moh, S.H.; Lee, T.-K. Anti-Inflammation Activities of Mycosporine-Like Amino Acids (MAAs) in Response to UV Radiation Suggest Potential Anti-Skin Aging Activity. Mar. Drugs 2014, 12, 5174–5187. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Cho, W.K.; Kim, H.-I.; Paek, S.H.; Jang, S.J.; Jo, Y.; Choi, H.; Lee, J.H.; Moh, S.H. Transcriptome Profiling of Human Follicle Dermal Papilla Cells in response to Porphyra-334 Treatment by RNA-Seq. Evid. Based Complement. Alternat. Med. 2021, 2021, 6637513. [Google Scholar] [CrossRef] [PubMed]
- Vega, J.; Schneider, G.; Moreira, B.R.; Herrera, C.; Bonomi-Barufi, J.; Figueroa, F.L. Mycosporine-Like Amino Acids from Red Macroalgae: UV-Photoprotectors with Potential Cosmeceutical Applications. Appl. Sci. 2021, 11, 5112. [Google Scholar] [CrossRef]
- Rosic, N.N. Recent advances in the discovery of novel marine natural products and mycosporine-like amino acid UV-absorbing compounds. Appl. Microbiol. Biotechnol. 2021, 105, 7053–7067. [Google Scholar] [CrossRef]
- Ryu, J.; Park, S.-J.; Kim, I.-H.; Choi Hee, Y.; Nam, T.-J. Protective effect of porphyra-334 on UVA-induced photoaging in human skin fibroblasts. Int. J. Mol. Med. 2014, 34, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Orfanoudaki, M.; Hartmann, A.; Alilou, M.; Gelbrich, T.; Planchenault, P.; Derbré, S.; Schinkovitz, A.; Richomme, P.; Hensel, A.; Ganzera, M. Absolute Configuration of Mycosporine-Like Amino Acids, Their Wound Healing Properties and In Vitro Anti-Aging Effects. Mar. Drugs 2020, 18, 35. [Google Scholar] [CrossRef]
- Lawrence, K.P.; Gacesa, R.; Long, P.F.; Young, A.R. Molecular photoprotection of human keratinocytes in vitro by the naturally occurring mycosporine-like amino acid palythine. Br. J. Dermatol. 2018, 178, 1353–1363. [Google Scholar] [CrossRef]
- Danovaro, R.; Bongiorni, L.; Corinaldesi, C.; Giovannelli, D.; Damiani, E.; Astolfi, P.; Greci, L.; Pusceddu, A. Sunscreens Cause Coral Bleaching by Promoting Viral Infections. Environ. Health Perspect. 2008, 116, 441–447. [Google Scholar] [CrossRef]
- Pangestuti, R.; Siahaan, E.A.; Kim, S.-K. Photoprotective Substances Derived from Marine Algae. Mar. Drugs 2018, 16, 399. [Google Scholar] [CrossRef]
- Barnes, P.W.; Williamson, C.E.; Lucas, R.M.; Robinson, S.A.; Madronich, S.; Paul, N.D.; Bornman, J.F.; Bais, A.F.; Sulzberger, B.; Wilson, S.R.; et al. Ozone depletion, ultraviolet radiation, climate change and prospects for a sustainable future. Nat. Sustain. 2019, 2, 569–579. [Google Scholar] [CrossRef]
- Núñez-Pons, L.; Avila, C.; Romano, G.; Verde, C.; Giordano, D. UV-Protective Compounds in Marine Organisms from the Southern Ocean. Mar. Drugs 2018, 16, 336. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Gómez, F.; Korbee, N.; Casas-Arrojo, V.; Abdala-Díaz, R.T.; Figueroa, F.L. UV Photoprotection, Cytotoxicity and Immunology Capacity of Red Algae Extracts. Molecules 2019, 24, 341. [Google Scholar] [CrossRef] [PubMed]
- Bonomi-Barufi, J.; Figueroa, F.L.; Korbee, N.; Momoli, M.M.; Martins, A.P.; Colepicolo, P.; Van Sluys, M.-A.; Oliveira, M.C. How macroalgae can deal with radiation variability and photoacclimation capacity: The example of Gracilaria tenuistipitata (Rhodophyta) in laboratory. Algal Res. 2020, 50, 102007. [Google Scholar] [CrossRef]
- Karentz, D.; McEuen, F.S.; Land, M.C.; Dunlap, W.C. Survey of mycosporine-like amino acid compounds in Antarctic marine organisms: Potential protection from ultraviolet exposure. Mar. Biol. 1991, 108, 157–166. [Google Scholar] [CrossRef]
- Jain, S.; Prajapat, G.; Abrar, M.; Ledwani, L.; Singh, A.; Agrawal, A. Cyanobacteria as efficient producers of mycosporine-like amino acids. J. Basic Microbiol. 2017, 57, 715–727. [Google Scholar] [CrossRef]
- Sinha, R.P.; Singh, S.P.; Häder, D.-P. Database on mycosporines and mycosporine-like amino acids (MAAs) in fungi, cyanobacteria, macroalgae, phytoplankton and animals. J. Photochem. Photobiol. B Biol. 2007, 89, 29–35. [Google Scholar] [CrossRef]
- Geraldes, V.; Pinto, E. Mycosporine-Like Amino Acids (MAAs): Biology, Chemistry and Identification Features. Pharmaceuticals 2021, 14, 63. [Google Scholar] [CrossRef]
- Karsten, U.; Sawall, T.; Wiencke, C. A survey of the distribution of UV-absorbing substances in tropical macroalgae. Phycol. Res. 1998, 46, 271–279. [Google Scholar] [CrossRef]
- Chuang, L.F.; Chou, H.N.; Sung, P.J. Porphyra-334 isolated from the marine algae Bangia atropurpurea: Conformational performance for energy conversion. Mar. Drugs 2014, 12, 4732–4740. [Google Scholar] [CrossRef]
- Conde, F.R.; Carignan, M.O.; Churio, M.S.; Carreto, J.I. In Vitro cis–trans photoisomerization of palythene and usujirene. Implications on the in vivo transformation of mycosporine-like amino acids. Photochem. Photobiol. 2003, 77, 146–150. [Google Scholar] [CrossRef]
- Conde, F.R.; Churio, M.S.; Previtali, C.M. The photoprotector mechanism of mycosporine-like amino acids. Excited-state properties and photostability of porphyra-334 in aqueous solution. J. Photochem. Photobiol. B Biol. 2000, 56, 139–144. [Google Scholar] [CrossRef]
- Torres, P.; Santos, J.; Chow, F.; Ferreira, M.J.P.; Santos, D. Comparative analysis of in vitro antioxidant capacities of mycosporine like amino acids (MAAs). Algal Res. 2018, 34, 57–67. [Google Scholar] [CrossRef]
- Pallela, R.; Na-Young, Y.; Kim, S. Anti-photoaging and photoprotective compounds derived from marine organisms. Mar. Drugs 2010, 8, 1189–1202. [Google Scholar] [CrossRef]
- Guillerme, J.-B.; Couteau, C.; Coiffard, L. Applications for marine resources in cosmetics. Cosmetics 2017, 4, 35. [Google Scholar] [CrossRef]
- Korbee, N.; Huovinen, P.; Figueroa, F.L.; Aguilera, J.; Karsten, U. Availability of ammonium influences photosynthesis and the accumulation of mycosporine-like amino acids in two Porphyra species (Bangiales, Rhodophyta). Mar. Biol. 2005, 146, 645–654. [Google Scholar] [CrossRef]
- Jiang, H.; Gao, K.; Helbling, E.W. UV-absorbing compounds in Porphyra haitanensis (Rhodophyta) with special reference to effects of desiccation. Environ. Boil. Fishes 2007, 20, 387–395. [Google Scholar] [CrossRef]
- Carreto, J.I.; Carignan, M.O. Mycosporine-like amino acids: Relevant secondary metabolites. Chemical and ecological aspects. Mar. Drugs 2011, 9, 387–446. [Google Scholar] [CrossRef] [PubMed]
- Briani, B.; Sissini, M.N.; Lucena, L.A.; Batista, M.B.; Costa, I.O.; Nunes, J.M.C.; Schmitz, C.; Ramlov, F.; Maraschin, M.; Korbee, N.; et al. The influence of environmental features in the content of mycosporine-like amino acids in red marine algae along the Brazilian coast. J. Phycol. 2018, 54, 380–390. [Google Scholar] [CrossRef]
- Pliego-Cortés, H.; Bedoux, G.; Boulho, R.; Taupin, L.; Freile-Pelegrín, Y.; Bourgougnon, N.; Robledo, D. Stress tolerance and photoadaptation to solar radiation in Rhodymenia pseudopalmata (Rhodophyta) through mycosporine-like amino acids, phenolic compounds, and pigments in an Integrated Multi-Trophic Aquaculture system. Algal Res. 2019, 41, 101542. [Google Scholar] [CrossRef]
- Diehl, N.; Michalik, D.; Zuccarello, G.C.; Karsten, U. Stress metabolite pattern in the eulittoral red alga Pyropia plicata (Bangiales) in New Zealand—Mycosporine-like amino acids and heterosides. J. Exp. Mar. Bio. Ecol. 2019, 510, 23–30. [Google Scholar] [CrossRef]
- Véliz, K.; Chandía, N.; Karsten, U.; Lara, C.; Thiel, M. Geographic variation in biochemical and physiological traits of the red seaweeds Chondracanthus chamissoi and Gelidium lingulatum from the south east Pacific coast. J. Appl. Phycol. 2019, 31, 665–682. [Google Scholar] [CrossRef]
- Klisch, M.; Sinha, R.P.; Richter, P.R.; Häder, D.-P. Mycosporine-like amino acids (MAAs) protect against UV-B-induced damage in Gyrodinium dorsum Kofoid. J. Plant Physiol. 2001, 158, 1449–1454. [Google Scholar] [CrossRef]
- Oren, A.; Gunde-Cimerman, N. Mycosporines and mycosporine-like amino acids: UV protectants or multipurpose secondary metabolites? FEMS Microbiol. Lett. 2007, 269, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rosic, N.N.; Dove, S. Mycosporine-Like Amino Acids from Coral Dinoflagellates. Appl. Environ. Microbiol. 2011, 77, 8478–8486. [Google Scholar] [CrossRef] [PubMed]
- Rødde, R.S.H.; Vårum, K.M.; Larsen, B.A.; Myklestad, S.M. Seasonal and geographical variation in the chemical composition of the red alga Palmaria palmata (L.) Kuntze. Bot. Mar. 2004, 47, 125–133. [Google Scholar] [CrossRef]
- Orfanoudaki, M.; Hartmann, A.; Kamiya, M.; West, J.; Ganzera, M. Chemotaxonomic Study of Bostrychia spp. (Ceramiales, Rhodophyta) Based on Their Mycosporine-Like Amino Acid Content. Molecules 2020, 25, 3273. [Google Scholar] [CrossRef]
- Skriptsova, A.V.; Suzuki, M.; Semenchenko, A.A. Morphological variation in Northwest Pacific Devaleraea mollis and description of D. inkyuleei sp. nov. (Palmariaceae, Rhodophyta). Phycologia 2022, 61, 606–615. [Google Scholar] [CrossRef]
- Nishida, Y.; Saburi, W.; Miyabe, Y.; Kishimura, H.; Kumagai, Y. Characterization of Antioxidant Activity of Heated Mycosporine-like Amino Acids from Red Alga Dulse Palmaria palmata in Japan. Mar. Drugs 2022, 20, 184. [Google Scholar] [CrossRef]
- Sato, N.; Furuta, T.; Takeda, T.; Miyabe, Y.; Ura, K.; Takagi, Y.; Yasui, H.; Kumagai, Y.; Kishimura, H. Antioxidant activity of proteins extracted from red alga dulse harvested in Japan. J. Food Biochem. 2019, 43, e12709. [Google Scholar] [CrossRef]
- Furuta, T.; Miyabe, Y.; Yasui, H.; Kinoshita, Y.; Kishimura, H. Angiotensin I converting enzyme inhibitory peptides derived from phycobiliproteins of dulse Palmaria palmata. Mar. Drugs 2016, 14, 32. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Kishimura, H.; Kinoshita, Y.; Saburi, W.; Kumagai, Y.; Yasui, H.; Ojima, T. Enzymatic production of xylooligosaccharides from red alga dulse (Palmaria sp.) wasted in Japan. Process Biochem. 2019, 82, 117–122. [Google Scholar] [CrossRef]
- Kobayashi, M.; Kumagai, Y.; Yamamoto, Y.; Yasui, H.; Kishimura, H. Identification of a Key Enzyme for the Hydrolysis of β-(1-3)-Xylosyl Linkage in Red Alga Dulse Xylooligosaccharide from Bifidobacterium adolescentis. Mar. Drugs 2020, 18, 174. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, Y.; Miyabe, Y.; Takeda, T.; Adachi, K.; Yasui, H.; Kishimura, H. In Silico Analysis of Relationship between Proteins from Plastid Genome of Red Alga Palmaria sp. (Japan) and Angiotensin I Converting Enzyme Inhibitory Peptides. Mar. Drugs 2019, 17, 190. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, Y.; Tsubouchi, R.; Miyabe, Y.; Takeda, T.; Adachi, K.; Yasui, H.; Kishimura, H. Complete sequence of mitochondrial DNA of red alga dulse Palmaria palmata (Linnaeus) Weber & Mohr in Japan. Mitochondrial DNA Part B 2019, 4, 3177–3178. [Google Scholar] [CrossRef] [PubMed]
- Miyabe, Y.; Furuta, T.; Takeda, T.; Kanno, G.; Shimizu, T.; Tanaka, Y.; Gai, Z.; Yasui, H.; Kishimura, H. Structural Properties of Phycoerythrin from Dulse Palmaria palmata. J. Food Biochem. 2017, 4, 12301. [Google Scholar] [CrossRef]
- Nishida, Y.; Kumagai, Y.; Michiba, S.; Yasui, H.; Kishimura, H. Efficient Extraction and Antioxidant Capacity of Mycosporine-Like Amino Acids from Red Alga Dulse Palmaria palmata in Japan. Mar. Drugs 2020, 18, 502. [Google Scholar] [CrossRef] [PubMed]
- Nishida, Y.; Miyabe, Y.; Kishimura, H.; Kumagai, Y. Monthly Variation and Ultraviolet Stability of Mycosporine-like Amino Acids from Red Alga Dulse Palmaria palmata in Japan. Phycology 2021, 1, 119–128. [Google Scholar] [CrossRef]
- Beer, S.; Eshel, A. Determining phycoerythrin and phycocyanin concentrations in aqueous crude extracts of red algae. Mar. Freshw. Res. 1985, 36, 785–792. [Google Scholar] [CrossRef]
- Ekman, P.; Yu, S.; Pedersen, M. Effects of altered salinity, darkness and algal nutrient status on floridoside and starch content, α-galactosidase activity and agar yield of cultivated Gracilaria sordida. Br. Phycol. J. 1991, 26, 123–131. [Google Scholar] [CrossRef]
- Shick, J.M.; Romaine-Lioud, S.; Ferrier-Pages, C.; Gattuso, J.P. Ultraviolet-B radiation stimulates shikimate pathway-dependent accumulation of mycosporine-like amino acids in the coral Stylophora pistillata despite decreases in its population of symbiotic dinoflagellates. Limnol. Oceanogr. 1999, 44, 1667–1682. [Google Scholar] [CrossRef]
- Bhatia, S.; Sharma, K.; Namdeo, A.G.; Chaugule, B.B.; Kavale, M.; Nanda, S. Broad-spectrum sun-protective action of Porphyra-334 derived from Porphyra vietnamensis. Pharmacogn. Res. 2010, 2, 45–49. [Google Scholar] [CrossRef]
- Kudo, I.; Matsunaga, K. Environmental Factors Affecting the Occurrence and Production of the Spring Phytoplankton Bloom in Funka Bay, Japan. J. Oceanogr. 1999, 55, 505–513. [Google Scholar] [CrossRef]
- Isada, T.; Hirawake, T.; Nakada, S.; Kobayashi, T.; Sasaki, K.; Tanaka, Y.; Watanabe, S.; Suzuki, K.; Saitoh, S.-I. Influence of hydrography on the spatiotemporal variability of phytoplankton assemblages and primary productivity in Funka Bay and the Tsugaru Strait. Estuar. Coast. Shelf Sci. 2017, 188, 199–211. [Google Scholar] [CrossRef]
- Yuan, Y.V.; Westcott, N.D.; Hu, C.; Kitts, D.D. Mycosporine-like amino acid composition of the edible red alga, Palmaria palmata (dulse) harvested from the west and east coasts of Grand Manan Island, New Brunswick. Food Chem. 2009, 112, 321–328. [Google Scholar] [CrossRef]
- Lalegerie, F.; Stiger-Pouvreau, V.; Connan, S. Temporal variation in pigment and mycosporine-like amino acid composition of the red macroalga Palmaria palmata from Brittany (France): Hypothesis on the MAA biosynthesis pathway under high irradiance. J. Appl. Phycol. 2020, 32, 2641–2656. [Google Scholar] [CrossRef]
- Karsten, U.; Wiencke, C. Factors Controlling the Formation of UV-absorbing Mycosporine-like Amino Acids in the Marine Red Alga Palmaria palmata from Spitsbergen (Norway). J. Plant Physiol. 1999, 155, 407–415. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamamoto, R.; Mune Mune, M.A.; Miyabe, Y.; Kishimura, H.; Kumagai, Y. Monthly Variation in Mycosporine-like Amino Acids from Red Alga Dulse (Devaleraea inkyuleei, Formerly Palmaria palmata in Japan). Phycology 2023, 3, 127-137. https://doi.org/10.3390/phycology3010008
Yamamoto R, Mune Mune MA, Miyabe Y, Kishimura H, Kumagai Y. Monthly Variation in Mycosporine-like Amino Acids from Red Alga Dulse (Devaleraea inkyuleei, Formerly Palmaria palmata in Japan). Phycology. 2023; 3(1):127-137. https://doi.org/10.3390/phycology3010008
Chicago/Turabian StyleYamamoto, Ryuya, Martin Alain Mune Mune, Yoshikatsu Miyabe, Hideki Kishimura, and Yuya Kumagai. 2023. "Monthly Variation in Mycosporine-like Amino Acids from Red Alga Dulse (Devaleraea inkyuleei, Formerly Palmaria palmata in Japan)" Phycology 3, no. 1: 127-137. https://doi.org/10.3390/phycology3010008
APA StyleYamamoto, R., Mune Mune, M. A., Miyabe, Y., Kishimura, H., & Kumagai, Y. (2023). Monthly Variation in Mycosporine-like Amino Acids from Red Alga Dulse (Devaleraea inkyuleei, Formerly Palmaria palmata in Japan). Phycology, 3(1), 127-137. https://doi.org/10.3390/phycology3010008







