Abstract
In a paradigm shift in plastic wastes due to the COVID-19 pandemic, wetlands such as mangroves are threatened by a new form of pollution, plastics, on top of the eutrophication of estuarine waters due to nitrogen and phosphorus wastes/effluents that lead to cyanobacterial proliferation. Both plastic and nutrient pollution lead to prosperity of cyanotoxin-producing cyanobacteria that flourish in both and disperse leading to the detriment of fauna and flora in the mangrove ecosystem due to resulting toxicities. Although cyanotoxins are still a relatively poorly studied phenomenon in mangroves, their presence does create a focus of attention due to biofilm formation and the resultant flotation and sinking properties that are linked to cyanobacterial mats on plastic debris. Sri Lanka, being the first country in the world to conserve all its mangrove wetlands, does have a responsibility to prevent the invasion of plastics to this protected ecosystem, and binding with the Ramsar Convention, precluding plastic waste and their concomitant footprint, is a task at hand to the relative authorities. The path ahead mandates that we study the properties of plastics for cyanobacterial proliferation, biofilm formation, the fates of such plastics (flotation, dispersal and sinking), the cyanotoxin production changes that are attributed—or linked—to plastic pollution and the resultant impacts on mangrove ecosystems. Cyanotoxins are long-lived, and it is paramount that we find the necessary mechanisms to eliminate or curtail their production in mangrove ecosystems while establishing surveillance and monitoring of both the producers and the harmful agents. Cyanobacteria although vehicles for nitrogen fixation and replenishing of nutrients to an N-depleted ecosystem such as the mangroves, could lead to enhancements in cyanotoxins production. However, this phenomenon remains ambiguous and poorly studied in applied phycology in relation to mangroves. “New normal” plastics are lodged mostly on the surfaces of bark, prop roots, and pneumatophores, which are the localities where the highest level of new nitrogen is fixed, and this may lead to the proliferation of N-fixing, cyanotoxin-producing cyanobacteria, which may have repercussions on both flora and fauna of mangroves. Therefore, it is crucial that we monitor plastic pollution and find mechanisms for sanitizing plastics-imprinted mangroves to lessen the harmful footprint resulting from plastic overload.
1. Cyanobacteria in Mangrove Ecosystems
Sri Lanka is the first country to protect all its mangrove forests in alignment with the Ramsar Convention. Mangroves can be identified as secluded but adjoined marginal and intertidal ecosystems that border the marine macrocosm, are associated with lagoons/estuaries and have high productivity but are known to host a limited biodiversity of specialized plant types [1]. Mangroves are implicated in the protection and stabilization of coastlines. Mangroves face inhospitable conditions stemming from high salinity, low oxygen, strong light intensity and powerful winds and, consequently, the vegetation associated with mangroves are known for their adaptive features, such as thick cuticles, salt-secreting glands, aerating roots (Figure 1) and propagates through vivipary [2]. Mangroves are found in 123 countries in the world, mostly in the tropics and sometimes in temperate regions [3].
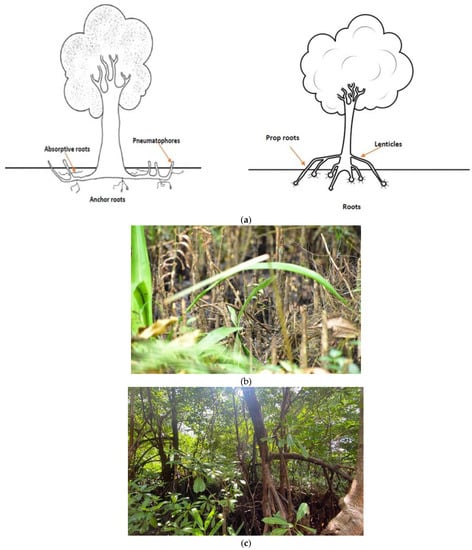
Figure 1.
(a) Illustrations of root adaptations of mangrove tree species (reproduced with permission from [4]). Below are images from a mangrove plot in Ittapana, Sri Lanka, where the pneumatophores (b) and prop roots (c) are shown.
The reported estimates of net primary productivity (NPP) for mangroves range from 2 to 50 Mg C ha−1 year−1, which is comparable to, if not overtakes, rival tropical forest productivity [5]. Although mangrove ecosystems are rich in carbon, they are poor in nutrients, primarily nitrogen. In that landscape, it is proposed that the high primary productivity of mangroves in nutrient-limiting conditions is achieved via nutrient cycling and nutrient conservation [5]. The primary nutrient inputs come in the form of tidal flushing, nitrogen fixation, microbial activity, leaf litter, and abundant macrofauna, while nutrient conservation is performed in mangrove forests using evergreen canopies, high root/shoot ratios, high photosynthetic nitrogen-use efficiency, and sclerophylly [5].
Mangroves provide habitats to many types of macro and microorganisms, namely bacteria and cyanobacteria, crustaceans, mollusks, birds, reptiles and fish [6]. One such microorganism is cyanobacteria that can be found as a key component of mangrove ecosystems, especially on submerged parts, aerial roots and trunks (Figure 1b,c). Cyanobacteria in mangrove ecosystems is an emerging field of study. The number and diversity of cyanobacteria in mangrove ecosystems have been underestimated and few studies have attempted to resolve the breadth of cyanobacteria in the soil, sediment and phytoplanktonic compartment that is a hallmark of such ecosystems. Most studies on cyanobacteria have focused traditionally on the soil and sediments [7], however, there is a vast biodiversity of mangrove cyanobacteria in the water sources that has been relatively neglected thus far. Cyanobacteria in mangrove ecosystems are known for their recycling/biomineralization of organic stocks through photosynthesis, nitrogen and carbon fixation, and phosphorus acquisition [8]. Cyanobacteria within enclosed water bodies that are classified as mangroves are capable of containing cyanobacterial taxa that are synonymous with many kinds of toxins (such as cyanotoxins). Cyanotoxins are known for their high toxicity and harm to humans and other lifeforms [9].
Cyanobacterial mats in mangroves come in three major forms: metaphyton (floating mats), epiphyton (periphytic algae) and epipelon (bottom mats) [10]. Metaphyton is produced by the sloughing of the epiphyton and epipelon substrata that float on water. The characteristics of cyanobacteria are suited for formation of mats due to their intrinsic ability for matrix binding using EPS/mucilage formation, while forging biochemical links that come in the form of nitrogen-fixing cyanobacteria that benefit the surrounding ecosystem by N-supply.
There is a complexity of lifeforms attached to the periphyton of mangrove ecosystems, which consists of submerged surfaces that are laden with cyanobacteria, microalgae, bacteria and many other microscopic and macroscopic lifeforms [10]. Periphyton can be called a scaffolding aggregate, which is made of an amalgamation of organisms, on the surface of plants, rocks, sediments and many other types of surfaces [10]. Cyanobacteria, too, provide niche advantages to the mangrove ecosystem, namely xenobiotic biosorption, bioremediation and secretion of plant growth-promoting compounds [11]. Cyanobacteria are also suggested as biostimulatory and bioremediatory, which means it is possible to recover marginalized, fragmented and eroding mangrove ecosystems using cyanobacteria [11]. Cyanobacteria are identified as key organisms for nitrogen fixation in the mangrove ecosystem and are more important than heterotrophic bacteria for fixed nitrogen stocks [12]. In such environments, the presence of organic matter and polysaccharides improves the overall nitrogen fixation and the nitrogen stocks in the ecosystem. However, with their widespread assistance in gifting a plethora of benefits, there is one evil in the form of cyanotoxins, especially in the face of bloom formation due to eutrophication stemming from high phosphorus effluents [13] as well as nitrogenous compounds [5].
Traditionally, cyanobacteria in mangrove ecosystems have been underestimated, and this factor is made worse by the high number of unclassified cyanobacteria and cyanobacteria misannotated due to the use of morphological methods only, which have less discriminatory power and resolution. An example of a case of misidentification is the genus Moorea, which was classified as Lyngbya from morphological means. Therefore, systematic polyphasic studies are required for the elucidation of the biodiversity of cyanobacteria in mangrove ecosystems. The molecular loci that have been traditionally used to characterize cyanobacteria are 16S rDNA, phycocyanin, ITS regions, rpoB (encoding the β-subunit of RNA polymerase) gene, PEP carboxylase gene [14] and, for cyanotoxin producers, toxin synthesizing-related loci that form the central cog for the accurate discrimination, topped up with morphological and biochemical methods.
The aims of this review are as follows:
- To detail the footprint of cyanotoxin production in mangrove ecosystems.
- To question the ambiguity in cyanotoxin production in relation to N:P ratios and biological nitrogen fixation.
- To elucidate the landscape of plastic-opulent estuarine mangroves with the emerging role of the plastisphere on cyanobacterial biofilm formation, buoyancy, sinking and dispersal.
- To examine the articles of the Ramsar Convention that are impacted by the emergence of diverse types of plastic pollution in wetlands and their downstream reverberations.
- To showcase the nexus between N-fixation and plastic pollution that exacerbates the putative cyanotoxin footprint in mangroves.
- Navigating the path ahead: what science should ideally do in the face of plastic pollution in mangroves and associated wetlands.
2. Mangrove Cyanotoxins and Their Legacy
Cyanotoxins can be classified as secondary metabolites that can have a toxic footprint on eukaryotic organisms and are catalogued according to the target of toxicity and mode of action: hepatotoxic (microcystins and nodularins), neurotoxic (anatoxin-a and analogues, saxitoxin and analogues), dermatotoxic (lipopolysaccharides) and cytotoxic (cylindrospermopsins) [15]. Cyanotoxin research, with the exception of Australia, has traditionally been conducted in countries of few or no mangroves, which has resulted in a dearth of review articles on cyanobacteria in a mangrove ecosystem [11].
The presence of cyanotoxins is reliant on the composition of cyanobacterial communities due to the species specificity of cyanotoxin production. Microcystins are released primarily by Microcystis, Planktothrix or Anabaena species [16]. Cylindrospermopsins are produced primarily by Cylindrospermopsis and Aphanizomenon species [16]. Meanwhile, for genera such as Limnothrix, there are yet no records of biogenic cyanotoxins [16]. It is also known that there are marriages of toxigenic and non-toxigenic cyanobacteria in water columns, and the relative contributions of taxa appear to be a determining factor for cyanotoxin chemo-diversity [16]. Variation prone environmental parameters such as light, temperature and nutrients are also critical determinants of toxin cocktails.
A study in Guadalupe identified three species of water bloom-forming cyanobacteria in the periphyton of a marine mangrove: they were identified using 16S rDNA as belonging to the genera Oscillatoria and Planktothricoides [17]. A separate study identified 34 species in 15 genera and 5 families in the mangroves of the Red Sea in Saudi Arabia, where the highest enrichment of species (25-31) was found in benthic mats, while pneumatophore mats recorded a lower diversity (10-12) [18]. Species biodiversity and species evenness were high in the benthic mats compared to pneumatophore mats [18]. In the same study, nine species of cyanobacteria were cultured, and, out of those cultivable species, four showed cyanotoxin production, namely microcystins (one species) and saixotoxins (three species) [18]. It was concluded that aquatic food webs in the mangroves are susceptible to the bioaccumulation and biomagnification of cyanotoxins that may relay harmful consequences to the impacted lifeforms [18]. Furthermore, there was higher species similarity between different sampled sites from where cyanobacteria originated from; however, there was high species diversity between benthic and pneumatophore mats from the same sampling site [18].
Another key study that focused on the Brazilian mangrove system showed how cyanobacteria were isolated from soil, water and periphytic environments of Cardoso Island and Bertioga mangroves had strong cyanobacterial diversity with five orders, seven families and eight genera [19]. The genera were Synechococcus, Cyanobium, Cyanobacterium, Chlorogloea, Leptolyngbya, Phormidium, Nostoc and Microchaete [19]. The sequenced 16S rDNA from the above genera matched database-available cyanobacterial species (Genbank) within an identity range of 92.5% to 99.7% [19]. The conclusions emphasized the broad diversity of molecular genetics between the isolated individual species, which was not apparent from morphology. Some clades were composed fully of newly-identified cyanobacteria that were not significant matches to any hit in Genbank [19]. It was also shared in the same study that some morphotypes of Leptolyngbia and Nostoc could possess new generic identities [19]. What was alarming though was that in the same study, the scientists identified peptide synthetase, polyketide synthase, microcystin and saxitoxin genes in 20.5%, 100%, 37.5% and 33.3% of the isolated samples, respectively [19]. In a gamut of 44 cyanobacteria collected for the study, a rather ominous landscape capable of cyanotoxin production was shown, mainly via the polyketide synthases, which are encoded as large gene clusters and are involved in the production of both cylindrospermopsin and microcystin [19]. Genes such as cyrA, cyrB, cyrC, cyrJ, mycE, sxtA, sxtB and sxtL have been used recently to identify cyanotoxin-producing cyanobacteria [15]. The above genes include ones for the synthesis of cylindrospermopsin (cyrA, cyrB and cyrC), microcystin producers (mycE) and saxitoxin-producing cyanobacteria (sxtA, sxtB and sxtL).
In a study performed in Iran, 120 cyanobacterial cultures were obtained from a mangrove ecosystem (Khoor-e-Khooran mangrove forest), which included Phormidium, Oscillatoria, Spirulina and Nostoc genera that were present at 25%, 20%, 10% and 10%, respectively [20]. The above genera were identified by molecular methods focused on the sequenced 16S rDNA loci. In the same study, the microcystin gene was detected in selective cyanobacteria, namely Microcystis sp. strain KH 3, Microcystis sp. strain KH 4 and Microcystis sp. strain KH 11, but was negative for nodularin and cylindrospermopsin genes, showing their capacity to produce microcystins [20].
The importance of identifying and classifying toxic bloom-forming cyanobacteria from non-bloom-forming counterparts is key, since recovery of mangrove ecosystems could be helped by non-toxin-producing cyanobacteria. Bioremediation by indigenous and introduced cyanobacteria, transforming debris, xenobiotics and biodegradable plastics into innocuous byproducts and fates is both low-cost and low-risk, which makes them a key component of remediation efforts.
The breadth of cyanobacteria in mangrove ecosystems is provided in Table 1. This is incorporating the microorganisms stated in the review of [11] and studies since then. There is a species richness of cyanobacteria in mangrove ecosystems. We propose that cyanobacteria from mangrove sediments, vegetation and fauna need to be classified in culture-dependent and culture-independent methods such as metagenomics to scale the whole breadth of the blue-green microorganisms and their genetic footprints for cyanotoxin production.
There is always the danger of undetected, undocumented and unknown cyanotoxins that are yet to be characterized using contemporary assays or tools of molecular biology [21]. This has been demonstrated by assays using cyanobacterial extracts, where the detected/documented bioactivity cannot be explained by the toxins known to the scientific community and to the specific cyanobacterium [21]. There is also the impact of known toxic effects that may have been erroneously attributed to the causative cyanobacterium, or, in other words, mistaken etiology that may contaminate the knowledge pool acquired by molecular and biochemical methods. Strong cytotoxicity that is higher than normal levels, the presence of tissue-specific toxicity events that are not detected with known cyanotoxins and toxicities that cannot be explained by the knowledge base of biochemical pathways are all indicative of the complexity and richness of cyanotoxins and their effects [21]. There may also be combined toxic effects from mixed cyanotoxin pools that may be difficult to separate into individual factors and that may contribute towards the complexity of cyanotoxin-related phenomena and their biochemical etiology. There is also the notion of chemo-specificity that may arise from clonal specificity within a single population, which has been shown to be true for Microcystis, Planktothrix, Dolichospermum (Anabaena) and Lyngbya species, and the resulting blooms will contain a cocktail of related compounds with different bioactivities, which could result in higher damage to the ecosystem and species in harm’s way [21].
The mangrove ecosystem, being one of strong fluxes with fickle conditions including spatiotemporal changes in community structures of the cyanobacteria, can have an effect on phenomena such as dominance, species richness, chemo-opulence of individual toxins that may lead to events of morbidities and mortalities of a broad spectrum of unrelated species. The species richness of such an ecosystem may need interventions from omics techniques that can unveil the spectrum of cyanobacteria as a consequence of the variation in the ecosystem. The fact that mangroves are a buffer zone may also contribute towards the richness of cyanobacterial species including documented and undocumented species that may live in “ecotones”, where there may be a magnified “edge effect” or a higher species diversity than the marine and freshwater ecosystems individually.
Cyanotoxins are known to travel up food chains [22] and can be biomagnified in top levels of the food web. Mangroves are known for high productivity, a high species diversity, complex trophic levels and a few economically-important species such as fish and shrimp that are dependent on consumption of available food sources [23]. The roots of mangrove plants have established trophic chains that may be in harm’s way due to the presence of cyanotoxins in near vicinities. It is said that shrimps and crabs in mangrove ecosystems connect the primary producers with the higher trophic levels and, consequently, are classified as keystone species [23]. Cyanotoxins are known to impact aquatic vertebrates and invertebrates, provisioning acute effects, chronic repercussions such as depletion of fecundity and changes in niche behavior. For example, higher levels of BMAA, a potent toxin associated with neurodegenerative diseases, has been reported in pink shrimp and blue crabs, and, although biomagnification was not observed for BMAA, it cannot be eliminated conclusively as a potential threat when traveling up the food chain [24]. Even Azolla microphylla that can adapt to brackish water of up to 6 ppm salinity is known to produce BMAA via the partner symbiotic cyanobacteria, examples of which include Nostoc spp. and Fischerella spp. [25].

Table 1.
A subset of cyanobacterial species reported in mangroves from different countries known for their cyanotoxin production.
Table 1.
A subset of cyanobacterial species reported in mangroves from different countries known for their cyanotoxin production.
| Species/Genera | Habitat | Country | Reference |
|---|---|---|---|
| Gloeothece sp. | Sediment, water | Brazil, Egypt, India | [11] |
| Hydrocoleum sp. | Sediment | Egypt, India, Mexico, Saudi Arabia | [11] |
| Bostrychia sp. | Plant, mud and rock in the mangrove | Southern Africa, Europe | [11,26] |
| Chamaecalyx sp. | Epiphytic | Mozambique, Mexico | [11] |
| Acaryochloris sp. | Epiphytic biofilms on a red alga (Gelidium caulacantheum) colonizing the pneumatophores of a temperate mangrove (Avicennia marina) | Australia | [11] |
| Nostoc sp. | Sediment, water | Brazil, India, Tanzania, Cardoso Island, Bertioga | [11,27] |
| Anabaena sp. | Avicennia pneumatophores, rhizosphere, sediment, water | India, Mexico, Saudi Arabia, Tanzania | [11] |
| Arthrospira sp. | Epiphytic, sediment | Mozambique, Saudi Arabia, Tanzania | [11] |
| Calothrix sp. | Epiphytic, Avicennia marina pneumatophores, Brugiera gymnorrhiza knee roots, rhizosphere, rock, sediment, water, algae- and seagrass-associated | Brazil, Egypt, India, Mexico, Mozambique, Saudi Arabia, South Africa, Tanzania | [11] |
| Chroococcus sp. | Epiphytic, Avicennia pneumatophores, Brugiera gymnorrhiza knee roots, epiphytic, rhizosphere, sediment, water, associated with Bostrychia and Rhizoclonium algae | Brazil, Egypt, India, Mexico, Mozambique, Saudi Arabia, South Africa, Tanzania | [11] |
| Coleofasciculus sp. | Avicennia pneumatophores, Brugiera gymnorrhiza knee roots, Rhizophora mucronata prop roots, ephiphytic, rock surfaces, sediment, algae-associated, among Microcoleus tenerrimus | Brazil, Egypt, India, Mozambique, Saudi Arabia, South Africa, Tanzania | [11,28] |
| Dermocarpa sp. | Epiphytic, Avicennia marina pneumatophores, Brugiera gymnorrhiza knee roots, rhizosphere, sediment, associated with Bostrychia, Caloglossa, Enteromorpha and Rhizoclonium algae | India, Saudi Arabia, South Africa | [11] |
| Dichothrix sp. | Avicennia pneumatophores, Rhizophora prop roots, rhizosphere, sediment | India, Saudi Arabia | [11,29] |
| Gloeocapsa sp. | Epiphytic, Avicennia marina pneumatophores, rhizosphere, sediment, water | Brazil, Egypt, India, Saudi Arabia, Tanzania | [11] |
| Hydrococcus sp. | Epiphytic, associated with Bostrychia, Caloglossa, Enteromorpha and Rhizoclonium algae | Mozambique, South Africa | [11] |
| Lyngbya sp. | Epiphytic, Avicennia pneumatophores, Brugiera gymnorrhiza knee roots, Rhizophora roots/trunks, rhizosphere, rock, sediment, algae- and seagrass-associated, among Coleofasciculus (Microcoleus) chtonoplastes and Porphyrosiphon martensianus | Brazil, Egypt, India, Mexico, Mozambique, Saudi Arabia, South Africa, Tanzania | [11,18] |
| Merismopedia sp. | Rhizosphere, sediment, water, among Oscillatoria | Brazil, India, Saudi Arabia, Tanzania | [11,30] |
| Microcystis sp. | Epiphytic, rhizosphere, sediment, water | India | [11,20] |
| Nodularia sp. | Epiphytic, sediment, water | India, Mozambique, Tanzania | [11,31] |
| Oscillatoria sp. | Epiphytic, Aegiceras corniculatum aerial roots, Avicennia pneumatophores, Brugiera gymnorrhiza knee roots, Suaeda maritima aerial roots, rhizosphere, rock, sediment, water, algae- and seagrass-associated, among Coleofasciculus (Microcoleus) chtonoplastes | Brazil, Egypt, India, Mexico, Mozambique, Saudi Arabia, South Africa, Tanzania | [11,32] |
| Phormidium sp. | Epiphytic, Avicennia pneumatophores, Rhizophora roots/trunks, rhizosphere, rock, sediment, water, algae-associated, among Phormidium simplicissimum or Microcoleus tenerrimus | Brazil, Egypt, India, Mexico, South Africa, Tanzania | [11,33] |
| Pseudanabaena sp. | Epiphytic, sediment, water | India, Mexico, Saudi Arabia | [11,34] |
| Raphidiopsis sp. | Avicennia marina pneumatophores, sediment | Egypt, India | [11] |
| Rivularia sp. | Avicennia pneumatophores, Ceriops tagal bark, Rhizophora prop roots, sediment | Egypt, Saudi Arabia, South Africa, Tanzania | [11] |
| Schizothrix sp. | Aegiceras corniculatum aerial roots, Avicennia marina pneumatophores, Brugiera gymnorrhiza knee roots, Rhizophora mucronata prop roots, sediment, among Scytonema insulare | Brazil, Egypt, India, Mexico, Saudi Arabia, South Africa, Tanzania | [11] |
| Scytonema sp. | Avicennia pneumatophores, Brugiera knee roots, Ceriops tagal bark, Rhizophora roots/ trunks, rhizosphere, rock, sediment, water | Brazil, Egypt, India, Saudi Arabia, South Africa, Tanzania | [11] |
| Synechococcus sp. | Rhizosphere, sediment, water, associated to Bostrychia algae | Brazil, India, Saudi Arabia, Tanzania | [11] |
| Trichodesmium sp. | Rhizosphere, sediment, water | India, Tanzania | [11,35] |
| Xenococcus sp. | Epiphytic, Avicennia schaueriana pneumatophores, Rhizophora roots/trunks, sediment, algae-associated, among Coleofasciculus (Microcoleus) chthonoplastes | Brazil, India, Mexico, Mozambique, Saudi Arabia, South Africa | [11] |
3. Nitrogen Fixation: Can the New Nitrogen Induce Cyanotoxin Production in N-Poor Ecosystems?
A positive relationship has been reported between N-levels and cyanobacterial biovolume, although this is not a global consensus. Aphanizomenon gracile and the largely invasive species Cylindrospermopsis raciborskii are known to have strong biovolumes when the N:P ratio reaches higher levels, both being diazotrophs [16]. The above factors should be taken with caution, as it is suggested that cyanobacteria should not be taken as a single entity—and so too N-fixing Nostoc spp.—when fickle factors that result in nutrient loading leave a legacy of cyanotoxins [16]. Nitrogen forms, too, have a strong role to play in cyanotoxin production, which consequently determines the downstream effects of toxins and the resulting toxicity [36]. It has been reported that total nitrogen, ammonium and dissolved organic nitrogen all have roles to play in the proliferation of microcystin producers and the holistic toxicity of the blue-green community [36].
Surplus nitrogen has been reported to provoke biological invasions, modify the competitive ability among species and change the footprint in dominance patterns, with subsequent losses in biodiversity [37]. Cyanobacteria that are capable of producing cyanotoxins can present as biological invasions due to nutrient enrichment, can change the competition and dominance patterns from non-toxigenic species to toxigenic species and can form blooms due to eutrophication with the concomitant erosion of biodiversity. Anthropogenic N-enrichment of mangroves can also augment nitrous oxide emissions, giving rise to the abetment of climate change. Biological nitrogen fixation is estimated to be between 2 and 10 mgN.m−2 d−1 within mangrove forests [37].
Research carried out on traits that aid cyanobacterial prosperity and cyanotoxin production has shown atmospheric nitrogen fixation to be a positive determinant when the ratio of N:P is austere, such as in mangrove ecosystems. The individual rates of nitrogen fixation vary a great deal between genera: for example, Aphanizomenon flos-aquae strains can fix atmospheric nitrogen at a stronger rate compared to several Anabaena strains, while Cylindrospermopsis raciborskii is considered to be a relatively poor nitrogen fixer [38]. However, Nostocales cyanobacteria are not always able to deliver the nitrogen fixation stocks, and it has been documented that, due to N-fixation being a high energy taxing system, light abundance and non-turbidity are helpful features for filament growth and fixing nitrogen via nitrogenase enzyme function [16]. The levels of molybdenum are a crucial weighing factor for the success of mangrove plant species and have been documented to be non-limiting for some mangrove plants and even toxic in higher concentrations for plants such as Xylocarpus moluccensis. Molybdenum, too, is an essential cofactor for a subset of nitrogen-fixing microorganisms, including diazotrophic bacteria and cyanobacteria, and, consequently, may be freely available for endophytic colonizers of mangrove plants due to its unlimiting nature [39]. The role of alternate nitrogenases—namely vanadium and iron-dependent—in mangrove ecosystems is an emerging frontier as of the contemporary. Some of the microorganisms that are capable of nitrogen fixation, isolated from mangrove roots or sediments, can be listed as Marinobacterium mangrovicola, Listonella anguillarum, Vibrio campbelli, Azotobacter, Azospirillum and Microcoleus sp. [12].
Mangrove ecosystems are known to be limited or depleted in nitrogen stocks, and the enclosed wood webs have developed methods to conserve the nitrogen currencies. They are (1) efficient transfer of soil nitrogen to trees and microbes, (2) strong N-use efficiency (3), low losses of intact nitrogen through export of soluble nitrogen and nitrous oxide production, (4) production of humic and fulvic acids, which, in turn, are capable of increasing nitrogen fixation of microbes, (5) nitrogen fixation in prop roots, wood, pneumatophores and soil and sediment surfaces, and (6) a large population of dead root parts below the surface layers of soil. In fact, tidal and planktonic microbial populations play a large role in the production and conservation of nitrogen stocks [40].
In relation to rates of nitrogen fixation (mg N m−2 d −1) at several striata, mainly soil cover of mangroves, cyanobacteria mats, roots above ground (pneumatophores and prop roots), belowground roots and rhizomes, foliar litter on the floor of mangrove forests, senescent leaves and microbial crusts found on tree stems, three factors appear to be the primary reasons for nitrogen budgets, namely bark (on average 100.95 mg N m−2 d −1), prop roots and pneumatophores (on average 31.78 mg N m−2 d −1) and cyanobacterial mats (on average 9.69 mg N m−2 d −1) [40].
Although field and laboratory studies have illuminated the linkage between nitrogen in water bodies to the presence of toxic cyanobacteria and cocktails of cyanotoxins, the role of nitrogen fixation and the contribution of “new nitrogen” to the production of cyanotoxins has not been comprehensively studied in mangroves, and we take into consideration two studies from lakes to state the current understanding on cyanotoxin production due to nitrogen fixation. However, it is now known that mangroves are receptors of enhanced reactive nitrogen creation due to large scale utilization of urea and other nitrogen (N) fertilizers, legume crop rotations and cultivation and N emissions from fossil fuels [37].
A study that was performed in Wisconsin, USA, centered on a lake named Mendota, demonstrated that an early summer elevation of nitrogen fixation took place concomitant with the depletion of nitrogen stocks in the lake, driving Microcystis proliferation [41]. On a week-by-week basis, when the microcystin-producing cyanobacteria were monitored by PCR amplification of the phycocyanin intergenic spacer sequence, it was shown that there was an upheaval of Microcystis proliferation in early summer, coinciding with the detection of large rises in nitrogen fixation [41]. The maximum recorded microcystin concentrations were synonymous with Microcystis spp. dominance of the lake. Similarly, the late summer drop in nitrogen stocks too increased nitrogen fixation, with a concomitant rise of Aphanizomenon spp. in the lake waters [41]. Therefore, the conclusions formed from this study were that new nitrogen synthesized by nitrogen fixation is capable of driving the proliferation of cyanotoxin-producing cyanobacteria in early and later summer, and it is likely that the same phenomenon may be present in other ecosystems where nitrogen fixation may induce temporal and spatial variation, including mangroves.
In a study performed in 102 lakes in North Germany, the hypothesis, that, potentially, N2-fixing Nostocales taxa would be favored for low N:P ratios, was disproven [16]. In this study, N-fixing A. gracile and C. raciborskii were able to prosper in stronger biovolumes when N:P ratios were high and not when N:P ratios were low, the latter favoring nitrogen fixation [16]. It was concluded that cyanotoxin-producing cyanobacteria display miscellaneous responses to the rudimentary level of nitrogen in the ambience [16]. That said, strong microcystin concentrations have been documented in nutrient-depleted dimictic lakes dominated by Planktothrix species, such as Planktothrix rubescens, which is identified as a potent microcystin producer [16].
Therefore, it is timely that similar studies are performed on mangrove ecosystems to unearth the contribution of fixed “new nitrogen” to cyanotoxin production, especially due to the N-inadequacy in mangrove ecosystems. The four main types of nitrogen in tidal waters of mangroves—dissolved organic nitrogen (DON), ammonium (NH4+), nitrate (NO3 −) and nitrite (NO2−)—are, in fact, in the micromolar (µM) range with respective values of 0.1 to 60 µM, 0 to 120 µM, 0 to 5 µM and 0 to 37 µM [40]. Furthermore, on average, the total nitrogen concentrations in mangrove forests are on par with those from tropical terrestrial forests for leaves and wood, but distinctly lower compared to levels from forest soils and roots. Therefore, the gross nitrogen fixation—an underestimated and patchy mechanism in mangroves—should be calculated for bark, prop roots and pneumatophores to elucidate their contributions to otherwise low nitrogen levels and then aligned with the emergence of toxigenic communities and the cyanotoxin footprint to understand the contribution of nitrogen fixation to toxin production, especially in mats and the water columns of mangroves.
4. Cyanotoxins and the Role of the “Plastisphere”
It is an alarming statistic that, in the history of our planet, 7 billion metric tons of plastic waste has been manufactured until the contemporary, of which only 9% has been recycled and 79% has been discarded to landfills or to waterbodies and other environments [42]. This has been exacerbated by the advent of COVID-19 with its footprint of plastic pollution. The emergence of the COVID-19 pandemic has had serious repercussions on wetlands, especially estuarine ecosystems that overlap with mangroves. Now, the footprint of plastic pollution has resulted in a new sphere to be accommodated and that is the plastisphere. Since the 2010s, there have been studies that have centered on the role of primary producers and the role that the plastisphere plays in the prosperity and the proliferation of such producers on surfaces that are designated as “plastispheric” [43]. The eighth continent, which is what the plastisphere is duly called, has repercussions that are biotic, abiotic, genetic, chemical and macroscopical, such as the following phenomena: evolution of bacteria/cyanobacteria, distribution of antibiotic resistance genes, higher horizontal gene transfer, floating islands on oceans and “Sea Snow”, aggregation of persistent organic pollutants on plastic surfaces and creation of vectors for the dispersal of alien invasive species [44]. Microbes in the plastisphere have been divided into divergent and non-divergent from other non-plastic-based communities and also involve members which are able to decompose the plastic substrate as well as types that are capable of being pathogenic and carrying/transporting antibiotic-resistance genes [42].
A major contribution of plastics enters the estuarine localities and is fragmented to plastic shrapnel, called microplastic, which is characteristically <5 mm [45]. Plastics come in a myriad of forms and are divided by shape and other parameters (Figure 2). Most plastics that do not enter reuse or recycling are candidates to enter wetlands, in particular, estuarine mangrove ecosystems [46]. The most popular plastics are polypropylene and polystyrene in relation to marine microplastics, which are made from both engineered plastic spheres as well as fragments resulting from the breakdown of plastics [47]. Although newer plastics are shown to be susceptible to biodegradation, there is an augmenting argument on the lethality/toxicity of the resulting chemo-molecules, as well as aiding to release harmful chemicals such as polymer additives.
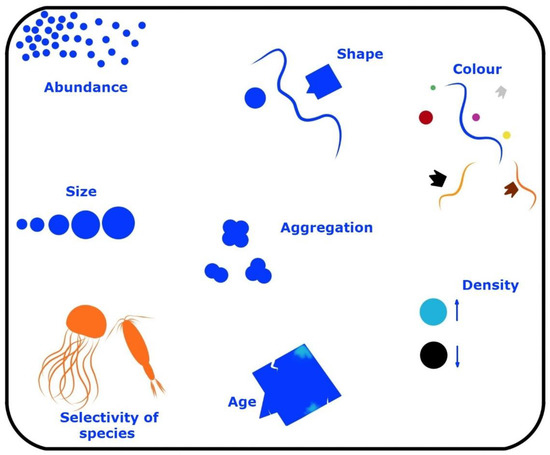
Figure 2.
The many factors that differ in plastic forms as well as their species selectivity. This image was adapted with permission from an illustration that was presented in [48].
During the time frame of 2011–2015, there were many reports of biofilm-forming heterotrophic bacteria that were shown to form an adhesive layer called a biofilm, found also in a secondary group of microorganisms, cyanobacteria [43]. Cyanobacteria can have repercussions on plastic fragments by two methods [49] [Figure 3]: (1) the adhesion of biofilm-forming cyanobacteria onto plastics that are of the flotation nature, which may be given extra buoyancy by the gas vesicles that are found in cyanobacteria, even increasing the longevity and travelled distance of the resulting composite, and (2) the ultimate sinking of floating plastics due to the load/density of cyanobacterial mats, which may influence the sedimentation potential and properties and relegate to be sunken material/sediments.
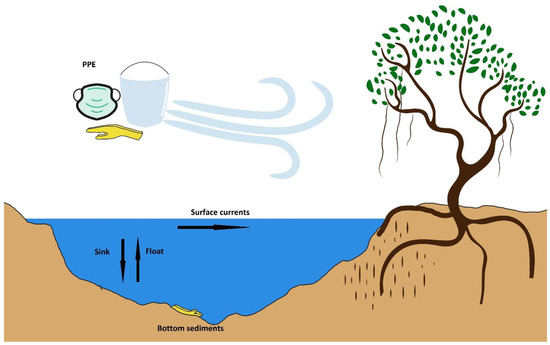
Figure 3.
Plastic products accumulating in the mangroves, adapted with permission from an illustration in [50].
The genus Dolichospermum [51], which is a revised genus comprising planktonic cyanobacteria formerly known by the genus name Anabaena, forms conspicuous cyanobacterial blooms containing endotoxins and is known to bind to plastics. In a recent review, it was stated that the genus Microcystis is a candidate for interactions with nanoplastics, while laboratory ware is known to bind microcystins [52]. It, too, was reported in the same review that amino-modified polystyrene nanoplastics are capable of increasing microcystin production in an immunoassay, as well as providing a source for the enhanced extracellular release of the resulting toxins [52]. In another study, it was reported that microcystins can adsorb and accumulate on microplastics, posing a threat to ambient biota via bioaccumulation [53]. Cyanobacteria are too contenders for the transmission of persistent organic pollutants via adhesion to microplastics [53]. Cyanobacteria of the genus Phormidium are also known for their colonization of the plastisphere, especially in marine environments [54]. The following genera, too, have been reported to be capable of colonizing plastics, namely Chroococcales (genus Microcystis), Oscillatoriales (genus Rivularia), Nostocales (genera Calothrix and Scytonema), Pleurocapsales (genus Pleurocapsa) and Synechococcales (genera Synechococcus, Prochlorothrix and Leptolyngbya) [54]. It is reported that the physiological plasticity of cyanobacteria is a crucial determinant of plastic colonization.
Mangroves as a buffer between marine and freshwater ecosystems are known for their bioaccumulation of macro-, micro- and nano-plastics, especially in estuarine systems. The distribution of plastics in mangroves is distinct from other ecosystems due to the stagnant waters, high density of vegetation and strong biomass. The anthropogenic stressor of plastics can be a vulnerability to the mangrove ecosystem, due to their entrapment in stagnant water, aerial roots, sediments and other susceptible areas of physico-environment as well as their bioaccumulation inside macrofauna. Approximately 4.8 to 12.7 million tons of plastic waste annually, due to the absence of waste collection, become sources of plastics to mangroves [55]. Plastic encroachment on knee roots and pneumatophore-bearing species can result in hypoxic/anoxic devastation of the mangrove flora by smothering. In particular, mangrove species such as Avicennia, Laguncularia spp. and Sonneratia spp. that bear aerial roots are known to be suffocated more strongly than other genera [55].
In a study performed in South Africa using four mangrove-dominated estuarine regions, it was shown that microfibers were the predominant microplastic type in estuarine water (69%) and mangrove sediments (51%) [56]. In the same study, it was shown that, overall, the predominant polymers were polyethylene (43%) and polypropylene (23%); meanwhile, there were seven types of concomitant synthetic polymers in total in the collection sites, namely polyethylene (PE), polypropylene (PP), polystyrene (PS), polyamide (PA 6/nylon) and polyurethane (PUR) [56].
5. Mangroves in the New Normal: The Ramifications on Ramsar Convention
There are implications on the Ramsar Convention due to the proliferation of hygienic and safety products that enter the wetlands as waste. The Ramsar Convention’s strategic action plan works on the three pillars: 1. wise use of the wetlands, 2. establishment and management of protected Ramsar sites and 3. international cooperation for the wise use and conservation of the wetlands. Therefore, the implications of plastic pollution should be relevant to the three pillars, or, in simple terms, how to sustainably “use, manage and protect” the wealth of mangroves.
The point from the Ramsar Convention that need to be focused on in the new normal stemming from the COVID-19 pandemic is Article 3.2, which draws the importance of conservation and remedial and curative protection of the wetlands ecosystem, as stated in, “Each Contracting Party shall arrange to be informed at the earliest possible time if the ecological character of any wetland in its territory and included in the List has changed, is changing or is likely to change as the result of technological developments, pollution or other human interference. Information on such changes shall be passed without delay to the organization or government responsible for the continuing bureau duties specified in Article 8” (https://www.ramsar.org/sites-countries/change-in-ecological-character [accessed on 3 February 2023].
To be informed means to have data on the type of pollution, pollution load, source of pollution, the short-term and long-term impacts on the wetland ecosystem, the impacts on biodiversity—both flora and fauna, the impacts on the functioning of the wetlands and, in the case of mangroves, as a buffer stabilizing the interface between saltwater and freshwater ecosystems. The technological developments associated with COVID-19 primarily involve the mass scale production of mega-, micro-, and nano-plastics that can have repercussions on the biology of the wetlands. Personal protective equipment comes in the form of a plethora of plastics, polyurethane (PU), polypropylene (PP), polycarbonate (PC), low-density polyethylene (LDPE), polyvinyl chloride (PVC), high-density polyethylene (HDPE), polystyrene (PS), polyethylene terephthalate (PET), etc., [57]. Out of the above, PS and LDPE are non-prone to be recycled, while PET and HDPE are recycle-friendly and PVC and PP, on most occasions, are unrecycled back to reuse [57]. Masks, face shields and takeout containers are made from PP, PC and PS, while hand sanitizer bottles, goggles and gloves are made from PVC [57].
Although no substantial data were available for the direct impact of different types of plastics on mangrove vegetation, the effects of plastic types on the aquatic macrophyte Nelumbo nucifera (lotus) were available. Although not directly relevant to mangrove vegetation, the data do reveal the extent to which distinct types of plastics can inhibit plant growth and seed germination in aquatic plants (Table 2).

Table 2.
The effects of types of plastics on Nelumbo nucifera (lotus) biology [58].
As a measure of dealing with the impending crisis of the pandemic, the World Health Organization has made projections of the sheer numbers and loads of plastic utilities that are manufactured on a monthly basis: a staggering 89 million face masks, 76 million gloves, 30 million gowns, 1.6 million goggles and 2.9 million hand sanitizers, just for the purpose of protecting front line health workers from COVID-19. In India, the land of water and rivers, 25 lakhs of personal protective equipment are required for a single day to battle the COVID-19 pandemic [57].
The question is how much of the above litters or trickles to the stagnant waters of wetlands, and, for the case of this review, to estuarine mangroves. The pandemic itself will have a lasting footprint on mangroves or “the ecological character of any wetland in its territory and included in the List has changed, is changing or is likely to change as the result of technological developments, pollution or other human interference”. [59] Leakage and mismanagement of plastic could have a lasting legacy on mangroves and other wetlands.
The global plastic packaging market size was anticipated to grow from USD 909.2 billion in 2019 to 1012.6 billion by 2021 due to the Coronavirus pandemic [https://www.businesswire.com/– Accessed 03 Feb 2023]. The global facemask market is projected to be valued at 10.2 billion USD by 2026. Plastic constitutes 83.02 % of litter that amasses on the mangrove forest floor and 93.4 % of litter in the canopy of the mangrove ecosystem [60]. The extent of microplastics in mangrove ecosystems is reported to be 1.22–6390 microplastics per kilogram of mangrove sediments [61]. Collectively, plastics compose 70% of the total marine debris in relation to mangrove forests, which draws the focus on the magnitude of the problem.
In a study that assessed root development, stress responses of exposed plants and the conservation of trees in the face of plastic cover percentages (0%, 50% and 100%) for a six-week period on the island of Java, Indonesia, it was revealed that anoxic conditions were more prevalent at 100% plastic cover, and that, at 50% cover, there was adaptability and resilience on the part of the mangrove flora, suggesting that mangrove species are capable of retaining their canopies and growing new pneumatophores in the face of plastic pollution that can interfere with cellular respiration [62]. However, 100% suffocated mangrove plants eventually perished with the plastic cover, while, for the 50% suffocated trees, although showing extension of roots and foliage renewal (investments in growth) during the course of the experiment, their responses to partial suffocation remain unclear in the contemporary [62]. Another study performed in Saudi Arabia along the red sea mangroves exhibited that microplastics below 0.5 mm predominated in mangrove sediments and demonstrated why they were found comparatively in lesser magnitudes in surface waters [63]. Therefore, the threat is imminently valid, but it may not be all gloom and doom on the part of mangroves.
Heterocystous cyanobacteria, due to nitrogen fixation in anoxic environments, will be able to build up their protein stocks in the absence of oxygen, although there is a high energy demand of 12-16 ATPs that is needed to break apart the 3 covalent bonds of elemental dinitrogen gas. The ability of roots to rise above ground in mangroves (Figure 1) aids in the escape of the plant species to better handle plastic cover of soil sediments and plant parts, and, consequently, they are adaptable to some extent to the rising threat of plastics. Systems overload can happen again, as it has since COVID-19 began. There needs to be a taskforce locally to quantify and treat the damage done by plastics of many nature on mangrove ecosystems. The impact on the production of cyanotoxins stemming from ambient hygienic product pollution overlaps with more traditional plastics, and research on biodegradable plastics as well as plastic morphometrics and shapes (Figure 2) that can preclude the lodgment of plastics in mangrove sediments and make them more accessible to biodegradation are the needs of the future. Research on the adhesion properties of cyanotoxins to plastic surfaces are also needed for each polymer that is used for production purposes. The study of upward relay of cyanotoxins in food chains, through internalized plastics, also needs to be performed to ascertain their harmful nature at different trophic levels in the face of biomagnification. Scientists also need to research whether plastics are “adjuvants” to other pollutants and if the synergy of such harmful pollutants can be a threat bigger than its individual components.
6. The Path Ahead: Surveillance, Mitigation, Challenges and Newer Technologies
Surveillance of cyanotoxin producers that can harm local species that are dependent on the water is a prophylactic exercise. Of particular importance is the monitoring and management of mangrove water bodies. Cyanotoxins as well as cyanotoxin-producing cyanobacteria can be detected using conventional methods, namely ELISAs for cyanotoxins and PCRs for the identification of cyanotoxin-producing genes harbored inside cyanotoxin producers, while cell counts of cyanobacteria that are elaborated as cells per mL or biovolume are the initial indicator [15]. WHO has addressed the issue of cyanotoxins by using biovolume values of above 0.2 mm3/L for further scrutiny of highly hazardous water bodies [15]. Mass spectrometry is too a valid technological tool for the detection and quantification of cyanotoxins [15], as well determining breakdown products of cyanotoxins, performed by coupling HPLC to mass spectrometry [64]. Chlorophyll A bequeaths a cheaper measurement for cyanotoxins, and there have been documented correlations between microcystins and chlorophyll A screening [65]. Whole animal bioassays are avoided due to the availability of the above screening methods [52].
Plastics render a whole new addition to the cyanotoxin problem that humans are faced with worldwide. Floating or lodged plastics are known to carry harmful chemicals such as persistent organic pollutants as well as antibiotics and are implicated in carrying toxin-producing cyanobacteria. Cyanobacteria are known to adhere to plastic surfaces and to produce polysaccharides that induce the drowning of cyanobacteria-carrying plastic fragments to marine or mangrove sediments. In a recent study, it was shown that, irrespective of thickness of biofilms and biovolume, single polythenes stayed afloat (buoyant), while the sinking velocities of two other plastics, single polystyrene and polyethylene terephthalate, remained uninfluenced by the above parameters [66]. However, stemming from the amalgamation of anoxic, iron-enriched water from the hypolimnion mixing with high water striata, there was aggregation and subsequent sinking of the single polythene, along with biomatter that included cyanobacterial colonies [66]. Therefore, cyanobacteria can abet the floating and sinking properties of microplastics under specific environmental conditions. In a freshwater ecosystem, summer induced the highest sinking of polypropylene plastic fragments (or loss of buoyancy) compared to other seasons, and biofilm development was fastest on smaller plastic fragments compared to their larger counterparts [67]. It was determined in the above publication that sinking of plastics were different according to size of plastic, temperature/season and water chemistry, while biofilm formation was a critical determining factor for buoyancy and sinking [67].
The role of cyanotoxin production on the plastisphere is a new frontier for scientists to tackle, along with other dangers that the plastisphere presents, such as antibiotics and antibiotic resistance gene transfers, persistence of organic chemicals, transmission of harmful pathogens and transfer of harmful products and byproducts along food chains, all of which could produce adverse effects to mankind and megafauna. In fact, simple laboratory experiments based on the interaction of microplastics with cyanobacteria in a beaker filled with water from a specific ecosystem (for example, mangroves) can be performed to determine their adsorption, biovolumes, identities of cyanobacteria through isolation and 16S rDNA sequencing, time course of the biofilms based on water source, flotation and sinking properties and toxin production by biofilm-forming cyanobacteria. Sampling from polluted mangroves should be a concurrent exercise to further the knowledge base on what type of cyanobacteria are capable of forming biofilms on mega- and micro-plastics, their ability to produce cyanotoxins being of key value. Some cyanotoxins are known to have strong half-lives—microcystins and cylindrospermopsins that have been traced back 200 and 4700 years, respectively –, which makes the quest to unravel the biochemical and biotic relationships of great importance [68]. Such long-lived cyanotoxins may too adhere to plastics and be carried by water currents to newer localities.
In fact, even cultured cyanotoxin-producing bacteria can be used to monitor biofilm formation and to measure their potential for cyanotoxin production while being bound to plastic surfaces. Metagenomics is too a sound weapon for the determination of the spectrum of cyanobacteria inhabiting the mangrove ecosystem and plastisphere. The advantage of metagenomics is the ability to sample microorganisms recalcitrant to culturing in the laboratory and the capacity to study a broad range of plastic samples from multiple sites for the presence of cyanobacteria.
There are setbacks to monitoring and surveillance of cyanobacteria in the plastisphere.
- The inability to decipher harmful and non-harmful bacteria by microscopical means.
- The temporal fluctuation of harmful cyanobacteria between seasons.
- The recalcitrance of harmful toxigenic cyanobacteria to cultivation.
- The false positives in PCR presented by dead cyanobacterial cells and environmental DNA that may not be indicative of cyanobacterial colonization.
- The differences in plastic fragment sizes, shapes, edges, etc., that specify the cyanobacterial communities.
We propose here the need to find suitable genetic loci such as the gas vesicle protein genes (example: GvpC, etc.) to decipher the cyanobacteria that are capable of buoyancy and adhesion to variable plastic surfaces. GvpC genes (coding for outer gas vesicle proteins) are particularly divergent in gene locus size and sequence and can be used as a specific indicator of biofilm-forming species on plastispheres. The GvpC proteins in cyanobacteria have multiple repeats of 4–6 domains, which aid in the zooming in on the preliminary identity without sequencing the loci (Figure 4), which makes this an inexpensive tool suitable for developing countries. Basically, the same principle as micro- or mini-satellites is employed here (variable sequence size), basing the approximate genus-level identification on a PCR band size, which is variable between genera. For example, the 5N of the GvpC gene (5 repeats of 31–33 residues), which includes the diazotrophic genera Aphanizomenon, Dolichospermum (planktonic) and Anabaena, forming the ADA clade that is made of cyanobacteria that are able to form large cyanobacterial blooms and produce cyanotoxins such as microcystin, anatoxin-a and saxitoxin in a subgroup-specific manner [69]. Four repeats in GvpC proteins in Microcystis aeruginosa PCC 7806 and Calothrix sp. strain PCC 7601 and three in Anabaena/Nostoc sp. strain PCC 7120 resemble the diversity in lengths of GvpC proteins [70]. Therefore, preliminary understanding of buoyant toxigenic cyanobacteria can be attained by means of a PCR band size of amplified GvpC loci, which can be further sequenced, if necessary, for a higher level of precise identification [70].

Figure 4.
Sequence alignment of six GvpC proteins from 4(N) [QIR36639.1-Tolypothrix sp. PCC 7910; WP_096646036.1-Calothrix brevissima]; 5(N) [WP_096668701.1-Dolichospermum compactum; WP_027402301.1-Aphanizomenon flos-aquae]; 6(N) [WP_085729044.1-Cylindrospermopsis raciborskii; WP_063873376.1-Nodularia spumigena]. Sequence conservation at both N and C termini will be conducive for primer design. PCR will multiply sequences of different lengths in relation to 4N, 5N and 6N sequence repeats.
On the topic of plastic pollution, cyanobacteria (e.g., Anabaena sp. PCC 7120) have been shown to interact with the plastic particles [71]. Induced intracellular Reactive Oxygen Species (ROS) are a factor in the interaction between the cyanobacteria and plastic, hinting that the cyanobacteria are stressed by its interaction with the plastic substrate [71]. However, there are some mechanisms that are helpful for the cyanobacteria to achieve degradation of the plastic surfaces, namely bio-deterioration, bio-fragmentation, assimilation, mineralization and degradation (Figure 5). Bio-deterioration is facilitated by the development of a biofilm on the superficially-degraded plastic particles stemming from abiotic abrasion forces, and colonized microbial communities produce a further spectrum of polymeric compounds that enter the pores of the plastic and cause cracks [71,72]. The release of acid compounds, such as nitrous acid, can be used to further weaken the integrity of the plastic. Bio-fragmentation, on the other hand, is microbial and enzyme-effectuated, namely using oxygenases (mono- and di-oxygenases), hydrolases (lipases and proteases) and lyases [72]. Assimilation is carrier/transporter-based internalization of the plastic residue that is further catabolized inside the cells, while mineralization is the complete conversion of organic polymers into carbon dioxide. Degradation of microplastics by cyanobacteria can be a boon or a bane, and we need to study the process further before addressing its benefits or harms.
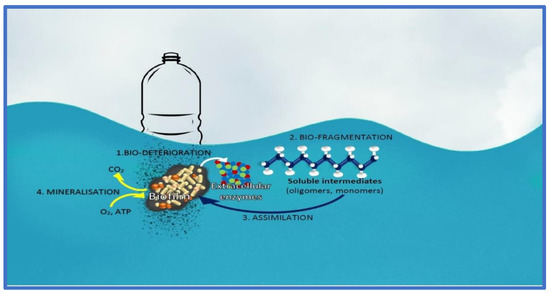
Figure 5.
The biodegradation mechanisms of plastics in mangrove waters illustrated as a drawing. This was illustrated using the diagram in [72] to which permission was provided by the author team.
Surface roughness of microplastics requires proper characterization and classification during the storyline from surface pollution and extent of microbial deterioration/fragmentation to subsequent phenomena. As the plastic surface properties are key factors to weigh in, the numerical description of SEM pictures and quantitative parameters appear to be the way forward, while AFM (Atomic Force Microscopy) can be useful for precise 3D analyses of topography/shape [44].
Cyanobacteria impact plastic fragments by two methods: enhanced flotation properties or buoyancy and the ultimate sinking of floating plastics due to the load/density of cyanobacterial mats. Both such fates should be studied in detail. In particular, the gas vesicle proteins of cyanobacteria require a strong focus to understand if they assist in the flotation properties. Gas vesicles have been reported in five phyla of bacteria including cyanobacteria. Microcystis aeruginosa has an 8.7-kb gene cluster with eleven or twelve genes that are capable of synthesis of gas vesicle proteins [70]. Gas vesicles in cyanobacteria are formed entirely from proteins, comprising a dozen or so in number [70]. Gas vesicle synthesis, gas vesicle collapse and carbohydrate ballast formation are thought to be key for cyanobacterial buoyancy, which makes the case of gas vesicle proteins an important area of study. The authors acknowledge here that air bubbles produced during processes such as photosynthesis may also assist the flotation properties.
The role of plastic surface features to the type of toxin-producing cyanobacteria should be a valuable factor for future ecosystem well-being, and knowing the toxin spectrum with plastic surface type can be useful from both prophylactic and curative perspectives. Finding ways to detach/weaken the surface-binding properties of cyanobacteria to microplastics can be a useful direction of future research. Simple indicator tests using the glycobiology/S-layer of cyanobacterial cell walls can be explored as one way forward to expedite the identification process. The peptidoglycan layer of cyanobacteria is found in a central zone between that of Gram-negative (2–6 nm) and Gram-positive (20–40 nm) bacteria, although designated coarsely as Gram-negative [73]. Cyanobacterial availability and counts can also be useful to demarcate the purity or polluted status of the mangrove ecosystem, just as the decline in lichens are indicators of sulfur dioxide pollution in cities, or coliform bacteria are indicators of river water contamination.
Furthermore, many a crusade can be embarked on using synthetic biology and nanotechnologies, and emerging biotreatment technologies can foster social targets, such as the removal or degradation of plastics from the ambient mangrove environment [71] and, in doing so, can stop the further dispersal of cyanotoxin-producing cyanobacteria. Plastic pollution can be tackled by applied science endeavors that have the potential to unveil newer microorganisms such as Idionella sakiensis that degrades Polyethylene Terephthalate plastics [74], to be used in global bioremediation. In fact, plastic-infested mangroves may be a treasure chest for such microorganisms.
Scalable biology-based solutions are also sought to reduce the footprint of toxins emanating from toxic bloom-forming cyanobacteria, including north-south linkages that aim to develop cyanolytic mechanisms to be integrated to water purification techniques to tackle endemic problems such as the Chronic Kidney Disease of an Unknown etiology (CKDu) in Sri Lanka (a collaboration between Robert Gordon University and the University of Sri Jayewardenepura). Mangrove protection is just one step forward in the conservation of a valuable buffer biome, keeping it free from cyanotoxins will be a crusade in the right direction to protect not just the ecosystem but also the ecosystem consumers from primary producers to humans. Biology-based techniques such as cyanolytic microbes [75] that are synthesized in a capsule or powdered form, or recombinant enzymes that can degrade cyanotoxins manufactured in a convenient form, are two methods of tackling the impending problem at hand.
7. Conclusions
A total of 100% of mangrove forests in Sri Lanka are protected, and many countries are bound to follow suit in this quest to keep the mangrove ecosystem pristine and away from anthropogenic phenomena. Still, the new normal and the escalating proliferation of hygiene and safety accompaniments has led to the displacement of “protective wear” from urban households to coastal wetlands. In mangroves, such protective equipment serves as transportation vehicles for dispersal, flotation and sedimentation of biofilm-forming cyanobacteria, leaving behind a legacy of harmful cyanotoxins in newer localities that were prior to that impoverished in harmful bloom-forming aquatic lifeforms.
Mangroves are known for their N-poverty and, thus, the legacy of the “new nitrogen” produced by diazotrophs in plant compartments and the plant–ambience interface are bound to play a role in cyanotoxin production, which, again, is a causative agent supplementary to the increase in plastic consumption in the contemporary “new normal”. Plastics are most prevalent lodged on barks, prop roots and pneumatophores, which are the main localities of microbial nitrogen fixation, which, again, brings into perspective the potential harm humans can cause mangrove forests. Plastics lodged on mangrove plant matter may lead to unprecedented levels of cyanotoxin producers on plastic surfaces, and, consequently may provoke stronger cyanotoxin producing communities which could have a negative footprint on mangrove forests.
Author Contributions
Conceptualization, D.G. and P.M.; methodology, D.G.; writing—original draft preparation, D.G. and S.A.; writing—review and editing, D.G., S.A. and P.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Any data pertaining to the publication will be made available upon request.
Acknowledgments
We thank Priyan Perera for the photographs in Figure 1.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kuenzer, C.; Bluemel, A.; Gebhardt, S.; Quoc, T.V.; Dech, S. Remote sensing of mangrove ecosystems: A review. Remote Sens. 2011, 3, 878–928. [Google Scholar] [CrossRef]
- Barbier, E.B. The protective service of mangrove ecosystems: A review of valuation methods. Mar. Pollut. Bull. 2016, 109, 676–681. [Google Scholar] [CrossRef]
- Faridah-Hanum, I.; Latiff, A.; Hakeem, K.R.; Ozturk, M. Mangrove Ecosystems of Asia: Status, Challenges and Management Strategies; Springer Science & Business Media: New York, NY, USA, 2013. [Google Scholar]
- Janssen-Stelder, B.; Augustinus, P.; Van Santen, W. Sedimentation in a Coastal Mangrove System, Red River Delta, Vietnam. Proceedings in Marine Science; Elsevier: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Reef, R.; Feller, I.C.; Lovelock, C.E. Nutrition of mangroves. Tree Physiol. 2010, 30, 1148–1160. [Google Scholar] [CrossRef] [PubMed]
- Mearns, A.J.; Reish, D.J.; Oshida, P.S.; Morrison, A.M.; Rempel-Hester, M.A.; Arthur, C.; Rutherford, N.; Pryor, R. Effects of pollution on marine organisms. Water Environ. Res. 2016, 88, 1693–1807. [Google Scholar] [CrossRef] [PubMed]
- Kollimalai, S.; Kandasamy, K. Cyanobacterial diversity from mangrove sediment of south east coast of India. Asian J. Biodivers. 2013, 4. [Google Scholar]
- Lee, R.Y.; Joye, S.B. Seasonal patterns of nitrogen fixation and denitrification in oceanic mangrove habitats. Mar. Ecol. Prog. Ser. 2006, 307, 127–141. [Google Scholar] [CrossRef]
- Bownik, A. Harmful algae: Effects of alkaloid cyanotoxins on animal and human health. Toxin Rev. 2010, 29, 99–114. [Google Scholar] [CrossRef]
- Rejmánková, E.; Komárková, J. A function of cyanobacterial mats in phosphorus-limited tropical wetlands. Hydrobiologia 2000, 431, 135–153. [Google Scholar] [CrossRef]
- Alvarenga, D.O.; Rigonato, J.; Branco, L.H.Z.; Fiore, M.F. Cyanobacteria in mangrove ecosystems. Biodivers. Conserv. 2015, 24, 799–817. [Google Scholar] [CrossRef]
- Alfaro-Espinoza, G.; Ullrich, M.S. Bacterial N2-fixation in mangrove ecosystems: Insights from a diazotroph-mangrove interaction. Front. Microbiol. 2015, 6, 445. [Google Scholar] [CrossRef]
- Barcellos, D.; Queiroz, H.M.; Nóbrega, G.N.; de Oliveira Filho, R.L.; Santaella, S.T.; Otero, X.L.; Ferreira, T.O. Phosphorus enriched effluents increase eutrophication risks for mangrove systems in northeastern Brazil. Mar. Pollut. Bull. 2019, 142, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.A.; Coomes, M.W.; Smith, T.E. Isolation and sequence of the phosphoenolpyruvate carboxylase gene of the marine cyanobacterium Synechococcus PCC 7002. J. Biol. Sci. 2008, 8, 1261–1270. [Google Scholar] [CrossRef]
- Ribeiro, M.S.F.; Tucci, A.; Matarazzo, M.P.; Viana-Niero, C.; Nordi, C.S.F. Detection of Cyanotoxin-Producing Genes in a Eutrophic Reservoir (Billings Reservoir, São Paulo, Brazil). Water 2020, 12, 903. [Google Scholar] [CrossRef]
- Dolman, A.M.; Rücker, J.; Pick, F.R.; Fastner, J.; Rohrlack, T.; Mischke, U.; Wiedner, C. Cyanobacteria and cyanotoxins: The influence of nitrogen versus phosphorus. PLoS ONE 2012, 7, e38757. [Google Scholar] [CrossRef] [PubMed]
- Guidi-Rontani, C.; Jean, M.R.N.; Gonzalez-Rizzo, S.; Bolte-Kluge, S.; Gros, O. Description of new filamentous toxic Cyanobacteria (Oscillatoriales) colonizing the sulfidic periphyton mat in marine mangroves. FEMS Microbiol. Lett. 2014, 359, 173–181. [Google Scholar] [CrossRef]
- Mohamed, Z.A.; Al-Shehri, A.M. Biodiversity and toxin production of cyanobacteria in mangrove swamps in the Red Sea off the southern coast of Saudi Arabia. Bot. Mar. 2015, 58, 23–34. [Google Scholar] [CrossRef]
- Silva, C.S.; Genuário, D.B.; Vaz, M.G.; Fiore, M.F. Phylogeny of culturable cyanobacteria from Brazilian mangroves. Syst. Appl. Microbiol. 2014, 37, 100–112. [Google Scholar] [CrossRef]
- Zaheri, A.; Bahador, N.; Yousefzadi, M.; Arman, M. Molecular identification and toxicity effects of cyanobacteria species isolated from the Khoor-e-Khooran mangrove forest, Persian Gulf. Iran. J. Fish. Sci. 2021, 20, 572–589. [Google Scholar]
- Chorus, I.; Welker, M. Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management; CRC Press: Boca Raton, FL, USA; on behalf of the World Health Organization: Geneva, Switzerland, 2021; p. 858. [Google Scholar]
- Wijewickrama, M.M.; Manage, P.M. Accumulation of Microcystin-LR in grains of two rice varieties (Oryza sativa L.) and a leafy vegetable, Ipomoea aquatica. Toxins 2019, 11, 432. [Google Scholar] [CrossRef]
- Muro-Torres, V.M.; Amezcua, F.; Soto-Jiménez, M.; Balart, E.F.; Serviere-Zaragoza, E.; Green, L.; Rajnohova, J. Primary sources and food web structure of a tropical wetland with high density of mangrove forest. Water 2020, 12, 3105. [Google Scholar] [CrossRef]
- Ferrão-Filho, A.D.S.; Kozlowski-Suzuki, B. Cyanotoxins: Bioaccumulation and effects on aquatic animals. Mar. Drugs 2011, 9, 2729–2772. [Google Scholar] [CrossRef]
- Pushpakumara, B.L.D.U.; Gunawardana, D. Preliminary data on the presence of an alternate vanadium nitrogenase in a culturable cyanobiont of Azolla pinnata R. Brown: Implications on Chronic Kidney Disease of an unknown etiology (CKDu). Data Brief 2018, 21, 2590–2597. [Google Scholar] [CrossRef] [PubMed]
- Nedumaran, T. Seaweed: A fertilizer for sustainable agriculture. In Sustainable Agriculture towards Food Security; Springer: Singapore, 2017; pp. 159–174. [Google Scholar]
- Rigonato, J.; Alvarenga, D.O.; Fiore, M.F. Tropical cyanobacteria and their biotechnological applications. In Diversity and Benefits of Microorganisms from the Tropics; Springer: Cham, Switzerland, 2017; pp. 139–167. [Google Scholar]
- Pramanik, P.J.; Mukherjee, J. Euryhalinema mangrovii gen. nov., sp. nov. and Leptoelongatus litoralis gen. nov., sp. nov.(Leptolyngbyaceae) isolated from an Indian mangrove forest. Phytotaxa 2019, 422, 058–074. [Google Scholar]
- Liu, L.; Wu, Y.; Hongxia, J.; Riding, R. Calcified rivulariaceans from the Ordovician of the Tarim Basin, Northwest China, Phanerozoic lagoonal examples, and possible controlling factors. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2016, 448, 371–381. [Google Scholar] [CrossRef]
- Salma, U.; Bengen, D.G.; Kurniawan, F. Impact of mangrove and seagrass ecosystem on marine productivity of Pramuka Island, Seribu Islands, Indonesia. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2022; Volume 1109, p. 012103. [Google Scholar]
- Genuario, D.B.; Vaz, M.G.M.V.; Hentschke, G.S.; Sant’Anna, C.L.; Fiore, M.F. Halotia gen. nov., a phylogenetically and physiologically coherent cyanobacterial genus isolated from marine coastal environments. Int. J. Syst. Evol. Microbiol. 2015, 65, 663–675. [Google Scholar] [CrossRef]
- Ram, A.T.; Shamina, M. Cyanobacterial diversity from seven mangrove environments of Kerala, India. World News Nat. Sci. 2017, 9, 91–97. [Google Scholar]
- Gueye, M.; Ba, N.; Ngom, A.; Mbaye, M.S.; Noba, K. Cyanophytes of the Joal-Fadiouth Lagoon (Senegal). GSC Biol. Pharm. Sci. 2020, 12, 156–161. [Google Scholar] [CrossRef]
- Singh, T.; Bhadury, P. Description of a new marine planktonic cyanobacterial species Synechococcus moorigangaii (Order Chroococcales) from Sundarbans mangrove ecosystem. Phytotaxa 2019, 393, 263–277. [Google Scholar] [CrossRef]
- Briand, M.J.; Bonnet, X.; Goiran, C.; Guillou, G.; Letourneur, Y. Major sources of organic matter in a complex coral reef lagoon: Identification from isotopic signatures (δ13C and δ15N). PLoS ONE 2015, 10, e0131555. [Google Scholar] [CrossRef] [PubMed]
- Monchamp, M.E.; Pick, F.R.; Beisner, B.E.; Maranger, R. Nitrogen forms influence microcystin concentration and composition via changes in cyanobacterial community structure. PLoS ONE 2014, 9, e85573. [Google Scholar] [CrossRef]
- Reis, C.R.G.; Nardoto, G.B.; Oliveira, R.S. Global overview on nitrogen dynamics in mangroves and consequences of increasing nitrogen availability for these systems. Plant and soil. 2017, 2017. 410, 1–19. [Google Scholar] [CrossRef]
- Willis, A.; Chuang, A.W.; Burford, M.A. Nitrogen fixation by the diazotroph Cylindrospermopsis raciborskii (Cyanophyceae). J. Phycol. 2016, 52, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, J.A.; George, S.J.; Rubio, L.M. Molybdenum trafficking for nitrogen fixation. Biochemistry 2009, 48, 9711–9721. [Google Scholar] [CrossRef] [PubMed]
- Alongi, D.M. Nitrogen Cycling and Mass Balance in the World’s Mangrove Forests. Nitrogen 2020, 1, 167–189. [Google Scholar] [CrossRef]
- Beversdorf, L.J.; Miller, T.R.; McMahon, K.D. The role of nitrogen fixation in cyanobacterial bloom toxicity in a temperate, eutrophic lake. PLoS ONE 2013, 8, e56103. [Google Scholar] [CrossRef] [PubMed]
- Wright, R.J.; Langille, M.G.I.; Walker, T.R. Food or just a free ride? A meta-analysis reveals the global diversity of the Plastisphere. ISME J. 2021, 15, 789–806. [Google Scholar] [CrossRef]
- Amaral-Zettler, L.A.; Zettler, E.R.; Mincer, T.J. Ecology of the plastisphere. Nat. Rev. Microbiol. 2020, 18, 139–151. [Google Scholar] [CrossRef]
- Dąbrowska, A. Microbial Degradation of Microplastics. In Recent Advances in Microbial Degradation; Springer: Singapore, 2021; pp. 373–387. [Google Scholar]
- Andrady, A.L. The plastic in microplastics: A review. Mar. Pollut. Bull. 2017, 119, 12–22. [Google Scholar] [CrossRef]
- Razeghi, N.; Hamidian, A.H.; Wu, C.; Zhang, Y.; Yang, M. Scientific studies on microplastics pollution in Iran: An in-depth review of the published articles. Mar. Pollut. Bull. 2021, 162, 111901. [Google Scholar] [CrossRef]
- Astner, A.F.; Hayes, D.G.; O’Neill, H.; Evans, B.R.; Pingali, S.V.; Urban, V.S.; Young, T.M. Mechanical formation of micro-and nano-plastic materials for environmental studies in agricultural ecosystems. Sci. Total Environ. 2019, 685, 1097–1106. [Google Scholar] [CrossRef]
- Botterell, Z.L.; Beaumont, N.; Dorrington, T.; Steinke, M.; Thompson, R.C.; Lindeque, P.K. Bioavailability and effects of microplastics on marine zooplankton: A review. Environ. Pollut. 2019, 245, 98–110. [Google Scholar] [CrossRef]
- Clerico, E.M.; Ditty, J.L.; Golden, S.S. Specialized techniques for site-directed mutagenesis in cyanobacteria. In Circadian rhythms; Humana Press: Totowa, NJ, USA, 2007; pp. 155–171. [Google Scholar]
- De-la-Torre, G.E.; Aragaw, T.A. What we need to know about PPE associated with the COVID-19 pandemic in the marine environment. Marine pollution bulletin 2021, 163, 111879. [Google Scholar] [CrossRef]
- Xu, B.; Liu, F.; Cryder, Z.; Huang, D.; Lu, Z.; He, Y.; Wang, H.; Lu, Z.; Brookes, P.C.; Tang, C.; et al. Microplastics in the soil environment: Occurrence, risks, interactions and fate–a review. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2175–2222. [Google Scholar] [CrossRef]
- Metcalf, J.S.; Codd, G.A. Co-occurrence of cyanobacteria and cyanotoxins with other environmental health hazards: Impacts and implications. Toxins 2020, 12, 629. [Google Scholar] [CrossRef]
- Karakolis, E.G.; Nguyen, B.; You, J.B.; Graham, P.J.; Rochman, C.M.; Sinton, D. Digestible fluorescent coatings for cumulative quantification of microplastic ingestion. Environ. Sci. Technol. Lett. 2018, 5, 62–67. [Google Scholar] [CrossRef]
- Nava, V.; Leoni, B. A critical review of interactions between microplastics, microalgae and aquatic ecosystem function. Water Res. 2021, 188, 116476. [Google Scholar] [CrossRef]
- van Bijsterveldt, C.E.; van Wesenbeeck, B.K.; Ramadhani, S.; Raven, O.V.; van Gool, F.E.; Pribadi, R.; Bouma, T.J. Does plastic waste kill mangroves? A field experiment to assess the impact of macro plastics on mangrove growth, stress response and survival. Sci. Total Environ. 2021, 756, 143826. [Google Scholar] [CrossRef] [PubMed]
- Govender, J.; Naidoo, T.; Rajkaran, A.; Cebekhulu, S.; Bhugeloo, A. Towards characterising microplastic abundance, typology and retention in mangrove-dominated estuaries. Water 2020, 12, 2802. [Google Scholar] [CrossRef]
- Parashar, N.; Hait, S. Plastics in the time of COVID-19 pandemic: Protector or polluter? Sci. Total Environ. 2021, 759, 144274. [Google Scholar] [CrossRef]
- Esterhuizen, M.; Kim, Y.J. Effects of polypropylene, polyvinyl chloride, polyethylene terephthalate, polyurethane, high-density polyethylene, and polystyrene microplastic on Nelumbo nucifera (Lotus) in water and sediment. Environ. Sci. Pollut. Res. 2022, 29, 17580–17590. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, D. The ‘ecological character’ of wetlands: A foundational concept in the Ramsar Convention, yet still cause for debate 50 years later. Marine Freshw. Res. 2021, 73, 1127–1133. [Google Scholar] [CrossRef]
- Garcés-Ordóñez, O.; Mejía-Esquivia, K.A.; Sierra-Labastidas, T.; Patiño, A.; Blandón, L.M.; Díaz, L.F.E. Prevalence of microplastic contamination in the digestive tract of fishes from mangrove ecosystem in Cispata, Colombian Caribbean. Marine pollution bulletin. 2020, 154, 111085. [Google Scholar] [CrossRef]
- Maghsodian, Z.; Sanati, A.M.; Tahmasebi, S.; Shahriari, M.H.; Ramavandi, B. Study of microplastics pollution in sediments and organisms in mangrove forests: A review. Environ. Res. 2022, 208, 112725. [Google Scholar] [CrossRef]
- van Bijsterveldt, C.E.; van Wesenbeeck, B.K.; van der Wal, D.; Afiati, N.; Pribadi, R.; Brown, B.; Bouma, T.J. How to restore mangroves for greenbelt creation along eroding coasts with abandoned aquaculture ponds. Estuarine, coastal and shelf science. 2020, 235, 106576. [Google Scholar] [CrossRef]
- Martin, C.; Baalkhuyur, F.; Valluzzi, L.; Saderne, V.; Cusack, M.; Almahasheer, H.; Krishnakumar, P.K.; Rabaoui, L.; Qurban, M.A.; Arias-Ortiz, A.; et al. Exponential increase of plastic burial in mangrove sediments as a major plastic sink. Sci. Adv. 2020, 6, eaaz5593. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Guo, J.; Huang, F.; Massey, I.Y.; Huang, R.; Li, Y.; Wen, C.; Ding, P.; Zeng, W.; Liang, G. Removal of Microcystin-LR by a Novel Native Effective Bacterial Community Designated as YFMCD4 Isolated from Lake Taihu. Toxins 2018, 10, 363. [Google Scholar] [CrossRef]
- Howard, M.D.; Nagoda, C.; Kudela, R.M.; Hayashi, K.; Tatters, A.; Caron, D.A.; Busse, L.; Brown, J.; Sutula, M.; Stein, E.D. Microcystin prevalence throughout lentic waterbodies in coastal southern California. Toxins 2017, 9, 231. [Google Scholar] [CrossRef] [PubMed]
- Leiser, R.; Wu, G.; Neu, T.R.; Potthoff, K.W. Biofouling, metal sorption and aggregation are related to sinking of microplastics in a stratified reservoir. Water Res. 2020, 176, 115748. [Google Scholar] [CrossRef]
- Chen, X.; Xiong, X.; Jiang, X.; Shi, H.; Wu, C. Sinking of floating plastic debris caused by biofilm development in a freshwater lake. Chemosphere 2019, 222, 856–864. [Google Scholar] [CrossRef]
- Henao, E.; Rzymski, P.; Waters, M.N. A Review on the Study of Cyanotoxins in Paleolimnological Research: Current Knowledge and Future Needs. Toxins 2019, 12, 6. [Google Scholar] [CrossRef]
- Österholm, J.; Rafael, P.; David, F.; Kaarina, S. Phylogenomic Analysis of Secondary Metabolism in the Toxic Cyanobacterial Genera Anabaena, Dolichospermum and Aphanizomenon. Toxins 2020, 12, 248. [Google Scholar] [CrossRef]
- Mlouka, A.K.; Comte, A.M.; Castets, C.; Bouchier, N. Tandeau de Marsac. The gas vesicle gene cluster from Microcystis aeruginosa and DNA rearrangements that lead to loss of cell buoyancy. J. Bacteriol. 2014, 186, 2355–2365. [Google Scholar] [CrossRef] [PubMed]
- Barone, G.D.; Ferizović, D.; Biundo, A.; Lindblad, P. Hints at the Applicability of Microalgae and Cyanobacteria for the Biodegradation of Plastics. Sustainability 2020, 12, 10449. [Google Scholar] [CrossRef]
- Jacquin, J.; Cheng, J.; Odobel, C.; Conan, P.; Pujo-pay, M.; Meistertzheim, A.; Jean-francois, G. Microbial ecotoxicology of marine plastic debris: A review on colonization and biodegradation by the ‘plastisphere’. Front. Microbiol. 2018, 10, 865. [Google Scholar] [CrossRef]
- Hoiczyk, E.; Hansel, A. Cyanobacterial cell walls: News from an unusual prokaryotic envelope. J. Bacteriol. 2000, 182, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Palm, G.J.; Reisky, L.; Böttcher, D.; Müller, H.; Michels, E.A.; Walczak, M.C.; Berndt, L.; Weiss, M.S.; Bornscheuer, U.T.; Weber, G. Structure of the plastic-degrading Ideonella sakaiensis MHETase bound to a substrate. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Idroos, E.S.; Manage, P.M. Bioremediation of Microcystins by Two Native Bacteria; Bacillus Cereus and Rahnella Aquatilis. Asian J. Microbiol. Biotechnol. Environ. Sci. 2018, 20, 24–32. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).