Effect of Iron Availability on the Growth and Microcystin Content of Natural Populations of Microcystis spp. from Reservoirs in Central Argentina: A Microcosm Experiment Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Microcystis spp. Collection, Sample Characterization and Laboratory Acclimation
2.2. Experimental Design
2.3. Physicochemical and Biological Determinations of Microcosm Samples
2.4. Data and Statistical Analysis
3. Results and Discussion
3.1. Characterization of Phytoplankton Community during Microcystis spp. Blooms
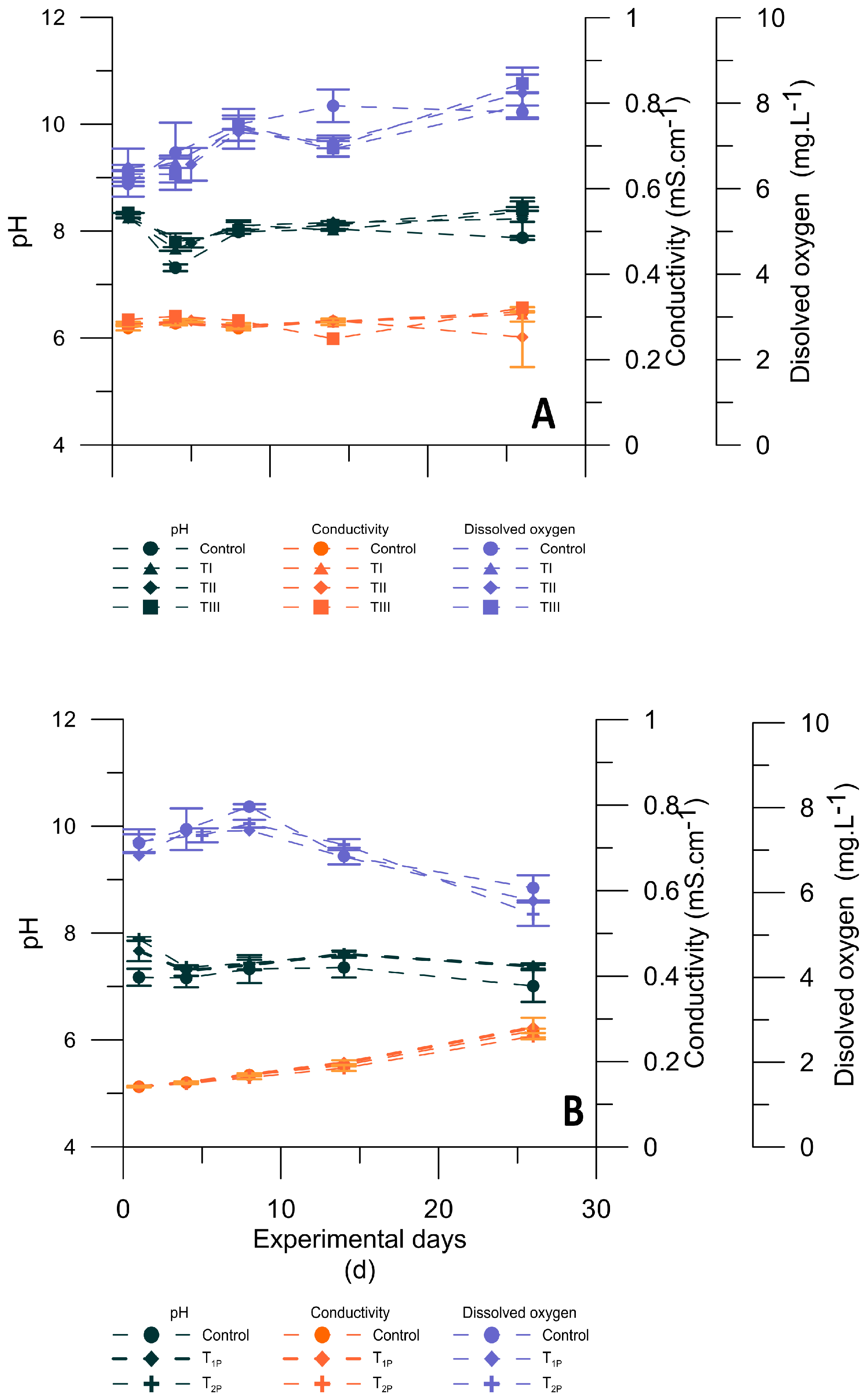
3.2. Physicochemical Conditions during Microcosm Experiments
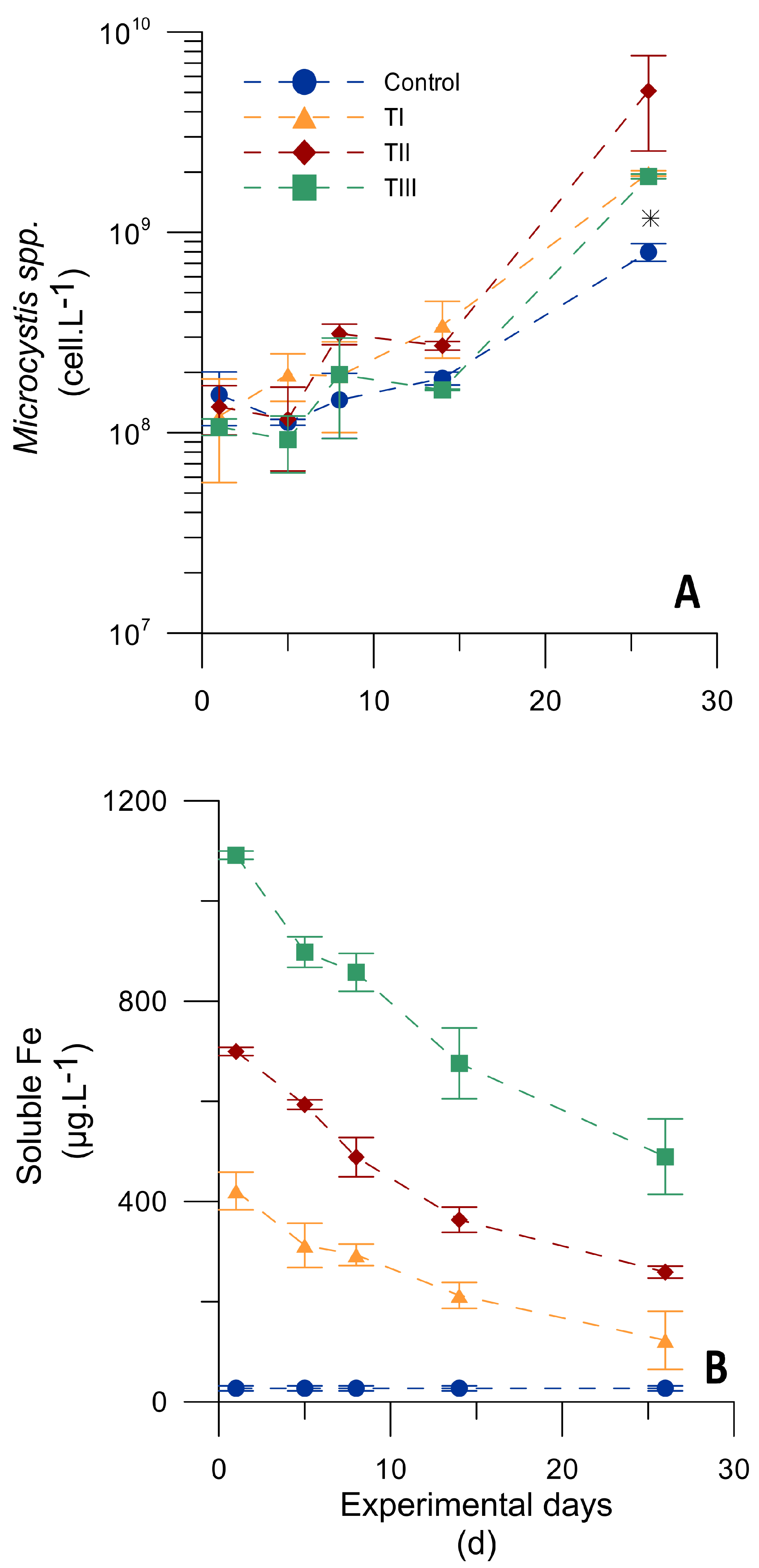
3.3. Effect of Iron Availability on Microcystis spp. Growth
3.3.1. Microcosm Experiment Testing Different Iron Concentrations
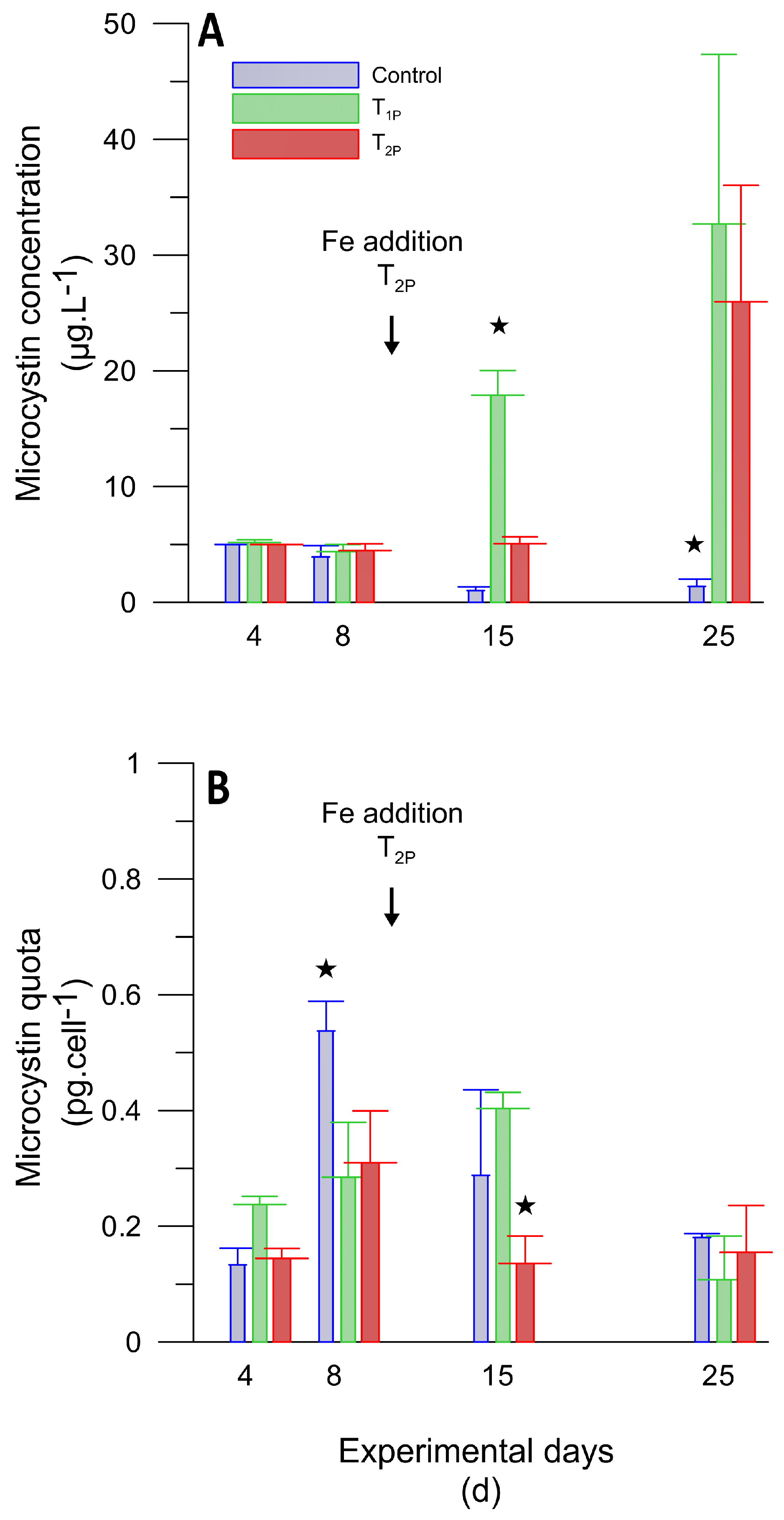
3.3.2. Microcosm Experiment Testing Different Iron Pulses
3.4. Total MC Concentration and MC Quota under Different Iron Addition Treatments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Le Moal, M.; Gascuel-Odoux, C.; Ménesguen, A.; Souchon, Y.; Étrillard, C.; Levain, A.; Moatar, F.; Pannard, A.; Souchu, P.; Lefebvre, A.; et al. Eutrophication: A new wine in an old bottle? Sci. Total Environ. 2019, 651, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Smith, V.H.; Joye, S.B.; Howarth, R.W. Eutrophication of freshwater and marine ecosystems. Limnol. Oceanogr. 2006, 51, 351–355. [Google Scholar] [CrossRef]
- Wurtsbaugh, W.A.; Paerl, H.W.; Dodds, W.K. Nutrients, eutrophication and harmful algal blooms along the freshwater to marine continuum. WIREs Water 2019, 6, e1373. [Google Scholar] [CrossRef]
- Oliveira, M.; Machado, A.V. The role of phosphorus on eutrophication: A historical review and future perspectives. Environ. Technol. Rev. 2013, 2, 117–127. [Google Scholar] [CrossRef]
- Rast, W.; Thornton, J.A. Trends in eutrophication research and control. Hydrol. Process. 1996, 10, 295–313. [Google Scholar] [CrossRef]
- Schindler, D.W.; Hecky, R.E.; Findlay, D.L.; Stainton, M.P.; Parker, B.R.; Paterson, M.J.; Beaty, K.G.; Lyng, M.; Kasian, S.E.M. Eutrophication of lakes cannot be controlled by reducing nitrogen input: Results of a 37-year whole-ecosystem experiment. Proc. Natl. Acad. Sci. USA 2008, 105, 11254–11258. [Google Scholar] [CrossRef]
- Dokulil, M.T.; Teubner, K. Cyanobacterial dominance in lakes. Hydrobiologia 2000, 438, 1–12. [Google Scholar] [CrossRef]
- Moss, B.; Kosten, S.; Meerhoff, M.; Battarbee, R.W.; Jeppesen, E.; Mazzeo, N.; Havens, K.; Lacerot, G.; Liu, Z.; De Meester, L.; et al. Allied attack: Climate change and eutrophication. Inland Waters 2011, 1, 101–105. [Google Scholar] [CrossRef]
- Paerl, H.W.; Paul, V.J. Climate change: Links to global expansion of harmful cyanobacteria. Water Res. 2012, 46, 1349–1363. [Google Scholar] [CrossRef]
- Chen, L.; Giesy, J.P.; Adamovsky, O.; Svirčev, Z.; Meriluoto, J.; Codd, G.A.; Mijovic, B.; Shi, T.; Tuo, X.; Li, S.C.; et al. Challenges of using blooms of Microcystis spp. in animal feeds: A comprehensive review of nutritional, toxicological and microbial health evaluation. Sci. Total Environ. 2021, 764, 142319. [Google Scholar] [CrossRef]
- Chorus, I.; Welker, M. (Eds.) Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management, 2nd ed.; Taylor & Francis: London, UK, 2021. [Google Scholar]
- Harke, M.J.; Steffen, M.M.; Gobler, C.J.; Otten, T.G.; Wilhelm, S.W.; Wood, S.A.; Paerl, H.W. A review of the global ecology, genomics, and biogeography of the toxic cyanobacterium, Microcystis spp. Harmful Algae 2016, 54, 4–20. [Google Scholar] [CrossRef]
- Müller, M.N.; Mardones, J.I.; Dorantes-Aranda, J.J. Editorial: Harmful Algal Blooms (HABs) in Latin America. Front. Mar. Sci. 2020, 7, 34. [Google Scholar] [CrossRef]
- Aguilera, A.; Haakonsson, S.; Martin, M.V.; Salerno, G.L.; Echenique, R.O. Bloom-forming cyanobacteria and cyanotoxins in Argentina: A growing health and environmental concern. Limnologica 2018, 69, 103–114. [Google Scholar] [CrossRef]
- Ruibal-Conti, A.L.; Guerrero, J.M.; Regueira, J.M. Levels of microcystins in two Argentinean reservoirs used for water supply and recreation: Differences in the implementation of safe levels. Environ. Toxicol. Int. J. 2005, 20, 263–269. [Google Scholar] [CrossRef]
- Ruiz, M.; Galanti, L.; Ruibal, A.L.; Rodriguez, M.I.; Wunderlin, D.A.; Amé, M.V. First report of microcystins and anatoxin-a co-occurrence in San Roque reservoir (Córdoba, Argentina). Water Air Soil Pollut. 2013, 224, 1593. [Google Scholar] [CrossRef]
- Oberholster, P.J.; Botha, A.M.; Grobbelaar, J.U. Microcystis aeruginosa: Source of toxic microcystins in drinking water. Afr. J. Biotechnol. 2004, 3, 159–168. [Google Scholar] [CrossRef]
- Pavlova, V.; Furnadzhieva, S.; Rose, J.; Andreeva, R.; Bratanova, Z.; Nayak, A. Effect of temperature and light intensity on the growth, chlorophyll a concentration and microcystin production by Microcystis aeruginosa. Gen. Appl. Plant Physiol. 2010, 36, 148–158. [Google Scholar]
- Utkilen, H.; Gjølme, N. Iron-stimulated toxin production in Microcystis aeruginosa. Appl. Environ. Microbiol. 1995, 61, 797–800. [Google Scholar] [CrossRef]
- Benayache, N.-Y.; Nguyen-Quang, T.; Hushchyna, K.; McLellan, K.; Afri-Mehennaoui, F.-Z.; Bouaïcha, N. An overview of cyanobacteria harmful algal bloom (CyanoHBA) issues in freshwater ecosystems. In Limnology- Some New Aspects of Inland Water Ecology; IntechOpen: London, UK, 2019; pp. 13–37. [Google Scholar]
- Davis, T.W.; Berry, D.L.; Boyer, G.L.; Gobler, C.J. The effects of temperature and nutrients on the growth and dynamics of toxic and non-toxic strains of Microcystis during cyanobacteria blooms. Harmful Algae 2009, 8, 715–725. [Google Scholar] [CrossRef]
- Downing, J.A.; Watson, S.B.; McCauley, E. Predicting cyanobacteria dominance in lakes. Can. J. Fish. Aquat. Sci. 2001, 58, 1905–1908. [Google Scholar] [CrossRef]
- Gobler, C.J.; Burkholder, J.M.; Davis, T.W.; Harke, M.J.; Johengen, T.; Stow, C.A.; Van de Waal, D.B. The dual role of nitrogen supply in controlling the growth and toxicity of cyanobacterial blooms. Harmful Algae 2016, 54, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Harris, T.D.; Wilhelm, F.M.; Graham, J.L.; Loftin, K.A. Experimental manipulation of TN: TP ratios suppress cyanobacterial biovolume and microcystin concentration in large-scale in situ mesocosms. Lake Reserv. Manag. 2014, 30, 72–83. [Google Scholar] [CrossRef]
- Amé, M.V.; Wunderlin, D.A. Effects of iron, ammonium and temperature on microcystin content by a natural concentrated Microcystis aeruginosa population. Water Air Soil Pollut. 2005, 168, 235–248. [Google Scholar] [CrossRef]
- Dai, R.; Wang, P.; Jia, P.; Zhang, Y.; Chu, X.; Wang, Y. A review on factors affecting microcystins production by algae in aquatic environments. World J. Microbiol. Biotechnol. 2016, 32, 51. [Google Scholar] [CrossRef]
- Facey, J.A.; Apte, S.C.; Mitrovic, S.M. A review of the effect of trace metals on freshwater cyanobacterial growth and toxin production. Toxins 2019, 11, 643. [Google Scholar] [CrossRef]
- Lukač, M.; Aegerter, R. Influence of trace metals on growth and toxin production of Microcystis aeruginosa. Toxicon 1993, 31, 293–305. [Google Scholar] [CrossRef]
- Jiang, Y.; Ji, B.; Wong, R.N.S.; Wong, M.H. Statistical study on the effects of environmental factors on the growth and microcystin production of bloom-forming cyanobacterium-Microcystis aeruginosa. Harmful Algae 2008, 7, 127–136. [Google Scholar] [CrossRef]
- Steffen, M.M.; Belisle, B.S.; Watson, S.B.; Boyer, G.L.; Wilhelm, S.W. Status, causes and controls of cyanobacterial blooms in Lake Erie. J. Great Lakes Res. 2014, 40, 215–225. [Google Scholar] [CrossRef]
- Berman-Frank, I.; Quigg, A.; Finkel, Z.V.; Irwin, A.J.; Haramaty, L. Nitrogen-fixation strategies and Fe requirements in cyanobacteria. Limnol. Oceanogr. 2007, 52, 2260–2269. [Google Scholar] [CrossRef]
- Glass, J.B.; Wolfe-Simon, F.; Anbar, A.D. Coevolution of metal availability and nitrogen assimilation in cyanobacteria and algae. Geobiology 2009, 7, 100–123. [Google Scholar] [CrossRef]
- Molot, L.A.; Watson, S.B.; Creed, I.F.; Trick, C.G.; Mccabe, S.K.; Verschoor, M.J.; Sorichetti, R.J.; Powe, C.; Venkiteswaran, J.J.; Schiff, S.L. A novel model for cyanobacteria bloom formation: The critical role of anoxia and ferrous iron. Freshw. Biol. 2014, 59, 1323–1340. [Google Scholar] [CrossRef]
- Ceballos-Laita, L.; Marcuello, C.; Lostao, A.; Calvo-Begueria, L.; Velazquez-Campoy, A.; Bes, M.T.; Fillat, M.F.; Peleato, M.L. Microcystin-LR binds iron, and iron promotes self-assembly. Environ. Sci. Technol. 2017, 51, 4841–4850. [Google Scholar] [CrossRef]
- Martin-Luna, B.; Sevilla, E.; Hernandez, J.A.; Bes, M.T.; Fillat, M.F.; Peleato, M.L. Fur from Microcystis aeruginosa binds in vitro promoter regions of the microcystin biosynthesis gene cluster. Phytochemistry 2006, 67, 876–881. [Google Scholar] [CrossRef]
- Alexova, R.; Fujii, M.; Birch, D.; Cheng, J.; Waite, T.D.; Ferrari, B.C.; Neilan, B.A. Iron uptake and toxin synthesis in the bloom-forming Microcystis aeruginosa under iron limitation. Environ. Microbiol. 2011, 13, 1064–1077. [Google Scholar] [CrossRef]
- Lyck, S.; Gjølme, N.; Utkilen, H. Iron starvation increases toxicity of Microcystis aeruginosa CYA 228/1 (Chroococcales, Cyanophyceae). Phycologia 1996, 35, 120–124. [Google Scholar] [CrossRef]
- Sevilla, E.; Martin-Luna, B.; Vela, L.; Bes, M.T.; Fillat, M.F.; Peleato, M.L. Iron availability affects mcyD expression and microcystin-LR synthesis in Microcystis aeruginosa PCC7806. Environ. Microbiol. 2008, 10, 2476–2483. [Google Scholar] [CrossRef]
- Buratti, F.M.; Manganelli, M.; Vichi, S.; Stefanelli, M.; Scardala, S.; Testai, E.; Funari, E. Cyanotoxins: Producing organisms, occurrence, toxicity, mechanism of action and human health toxicological risk evaluation. Arch. Toxicol. 2017, 91, 1049–1130. [Google Scholar] [CrossRef]
- Geng, L.; Qin, B.; Yang, Z. Unicellular Microcystis aeruginosa cannot revert back to colonial form after short-term exposure to natural conditions. Biochem. Syst. Ecol. 2013, 51, 104–108. [Google Scholar] [CrossRef]
- Wu, Z.X.; Song, L.R. Physiological comparison between colonial and unicellular forms of Microcystis aeruginosa Kütz.(Cyanobacteria). Phycologia 2008, 47, 98–104. [Google Scholar] [CrossRef]
- Xiao, M.; Li, M.; Reynolds, C.S. Colony formation in the cyanobacterium Microcystis. Biol. Rev. 2018, 93, 1399–1420. [Google Scholar] [CrossRef]
- Quirós, R.; Drago, E. The environmental state of Argentinean lakes: An overview. Lakes Reserv. Res. Manag. 1999, 4, 55–64. [Google Scholar] [CrossRef]
- Rodríguez, M.I.; Ruiz, M. Limnology of the San Roque Reservoir. In The Suquía River Basin (Córdoba, Argentina). The Handbook of Environmental Chemistry; Wunderlin, D., Ed.; Springer: Cham, Switzerland, 2016; pp. 37–59. [Google Scholar] [CrossRef]
- Halac, S.; Mengo, L.; Guerra, L.; Lami, A.; Musazzi, S.; Loizeau, J.L.; Ariztegui, D.; Piovano, E.L. Paleolimnological reconstruction of the centennial eutrophication processes in a sub-tropical South American reservoir. J. South Am. Earth Sci. 2020, 103, 102707. [Google Scholar] [CrossRef]
- Cao, Z.; Li, P.; Li, Z.H. A latest review on the application of microcosm model in environmental research. Environ. Sci. Pollut. Res. 2021, 28, 60438–60447. [Google Scholar] [CrossRef] [PubMed]
- Belzile, N.; Chen, Y.; Gunn, J.M.; Tong, J.; Alarie, Y.; Delonchamp, T.; Lang, C. The effect of selenium on mercury assimilation by freshwater organisms. Can. J. Fish. Aquat. Sci. 2006, 63, 1–10. [Google Scholar] [CrossRef]
- INA-SCIRSA. Characterization of Water Quality and Meteorological Variables Related to Cyanobacterial Extreme Bloom Events in the San Roque Reservoir. 2018. Available online: https://www.ina.gov.ar/archivos/publicaciones/2018_Pussetto%20et%20al_Caracterizaci%C3%B3n%20Calidad%20Agua%20Eventos%20Extremos.pdf (accessed on 28 October 2022).
- Amaral, V.; Bonilla, S.; Aubriot, L. Growth optimization of the invasive cyanobacterium Cylindrospermopsis raciborskii in response to phosphate fluctuations. Eur. J. Phycol. 2014, 49, 134–141. [Google Scholar] [CrossRef]
- Vogel, A.; Jeffery, G.H. Fundamental theoretical principles of reactions in solutions. In Vogel’s Textbook of Quantitative Chemical Analysis; Wiley and Sons: New York, NY, USA, 1989; pp. 15–70. [Google Scholar]
- APHA. Standard Methods: For the Examination of Water and Waste Water, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar] [CrossRef]
- Hillebrand, H.; Dürselen, C.-D.; Kirschtel, D.; Pollingher, U.; Zohary, T. Biovolume calculation for pelagic and benthic microalgae. J. Phycol. 1999, 35, 403–424. [Google Scholar] [CrossRef]
- Zar, J.H. Statistical significance of mutation frequencies, and the power of statistical testing, using the poisson distribution. Biom. J. 1984, 26, 83–88. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 28 October 2022).
- Puddick, J.; Thomson-Laing, G.; Wood, S.A. Microcystins in New Zealand: A review of occurrence, congener diversity and cell quotas. New Zealand. J. Bot. 2019, 57, 93–111. [Google Scholar] [CrossRef]
- Almanza, V.; Pedreros, P.; Laughinghouse, H.D.; Félez, J.; Parra, O.; Azócar, M.; Urrutia, R. Association between trophic state, watershed use, and blooms of cyanobacteria in south-central Chile. Limnologica 2019, 75, 30–41. [Google Scholar] [CrossRef]
- Halac, S.; Bazán, R.; Larrosa, N.; Nadal, A.F.; Ruibal Conti, A.L.; Rodríguez, M.I.; Ruiz, M.; Lopéz, A.G. First report on negative association between cyanobacteria and fecal indicator bacteria at San Roque reservoir (Argentina): Impact of environmental factors. J. Freshw. Ecol. 2019, 34, 273–291. [Google Scholar] [CrossRef]
- Whitton, B.A.; Potts, M. Introduction to the Cyanobacteria. In The Ecology of Cyanobacteria: Their Diversity in Time and Space; Whitton, B.A., Potts, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar] [CrossRef]
- Fujii, M.; Rose, A.L.; David Waite, T. Iron uptake by toxic and nontoxic strains of Microcystis aeruginosa. Appl. Environ. Microbiol. 2011, 77, 7068–7071. [Google Scholar] [CrossRef]
- Xing, W.; Huang, W.; Li, D.; Liu, Y. Effects of iron on growth, pigment content, photosystem II efficiency, and siderophores production of Microcystis aeruginosa and Microcystis wesenbergii. Curr. Microbiol. 2007, 55, 94–98. [Google Scholar] [CrossRef]
- Walsby, A.E.; McAllister, G.K. Buoyancy regulation by Microcystis in Lake Okaro. N. Z. J. Mar. Freshw. Res. 1987, 21, 521–524. [Google Scholar] [CrossRef]
- Ibelings, B.W.; Mur, L.R.; Walsby, A.E. Diurnal changes in buoyancy and vertical distribution in populations of Microcystis in two shallow lakes. J. Plankton Res. 1991, 13, 419–436. [Google Scholar] [CrossRef]
- Paerl, H.W.; Otten, T.G. Harmful Cyanobacterial Blooms: Causes, Consequences, and Controls. Microb. Ecol. 2013, 65, 995–1010. [Google Scholar] [CrossRef]
- Tammeorg, O.; Nürnberg, G.; Niemistö, J.; Haldna, M.; Horppila, J. Internal phosphorus loading due to sediment anoxia in shallow areas: Implications for lake aeration treatments. Aquat. Sci. 2020, 82, 1–10. [Google Scholar] [CrossRef]
- Visser, P.M.; Ibelings, B.W.; Mur, L.R.; Walsby, A.E. The ecophysiology of the harmful cyanobacterium Microcystis. In Harmful Cyanobacteria; Huisman, J., Matthijs, H.C.P., Visser, P.M., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 109–142. [Google Scholar] [CrossRef]
- Bormans, M.; Sherman, S.; Webster, I.T. Is buoyancy regulation in cyanobacteria an adaptation to exploit separation of light and nutrients? Mar. Freshw. Res. 1999, 50, 897–906. [Google Scholar] [CrossRef]
- Li, H.; Murphy, T.; Guo, J.; Parr, T.; Nalewajko, C. Iron-stimulated growth and microcystin production of Microcystis novacekii UAM 250. Limnologica 2009, 39, 255–259. [Google Scholar] [CrossRef]
- Wood, S.A.; Dietrich, D.R.; Craig Cary, S.; Hamilton, D.P. Increasing Microcystis cell density enhances microcystin synthesis: A mesocosm studies. Inland Waters 2012, 2, 17–22. [Google Scholar] [CrossRef]
- Sabart, M.; Pobel, D.; Briand, E.; Combourieu, B.; Salençon, M.J.; Humbert, J.F.; Latour, D. Spatiotemporal variations in microcystin concentrations and in the proportions of microcystin-producing cells in several Microcystis aeruginosa populations. Appl. Environ. Microbiol. 2010, 76, 4750–4759. [Google Scholar] [CrossRef]

| Physiochemical Variables in Sp, Sm and A | Subsurface Layer (0.5 m below Surface) | Deepest Layer (1 m above the Bottom) | |||||
|---|---|---|---|---|---|---|---|
| Median | Min | Max | Median | Min | Max | ||
| Water temperature (°C) | Sp | 21.3 | 13.1 | 27.6 | 18.7 | 12.9 | 21.1 |
| Su | 24.6 | 21.1 | 28.7 | 22.8 | 16.7 | 25.9 | |
| A | 18.9 | 12.0 | 24.5 | 18.4 | 11.5 | 23.4 | |
| Conductivity (µS·cm−1) | Sp | 272 | 194 | 492 | 267 | 103 | 494 |
| Su | 213 | 128 | 335 | 180 | 76.6 | 329 | |
| A | 178 | 100 | 338 | 178 | 108 | 336 | |
| pH | Sp | 8.2 | 6.5 | 9.8 | 7.6 | 6.1 | 9.2 |
| Su | 8.4 | 6.8 | 9.7 | 7.2 | 6.1 | 8.5 | |
| A | 7.6 | 6.0 | 9.2 | 7.6 | 6.1 | 8.5 | |
| Dissolved oxygen (mg·L−1) | Sp | 9.3 | 2.8 | 15,3 | 4.7 | 0.0 | 11.3 |
| Su | 8.4 | 4.4 | 16.7 | 1.0 | 0.0 | 9.6 | |
| A | 8.0 | 3.7 | 22.3 | 6.7 | 0.0 | 11.1 | |
| Total phosphorous (µg·L−1) | Sp | 66 | 14 | 392 | 61 | 10 | 322 |
| Su | 94 | 10 | 1117 | 127 | 30 | 420 | |
| A | 74 | 35 | 240 | 76 | 27 | 756 | |
| Dissolved inorganic nitrogen (µg·L−1) | Sp | 435 | 143 | 849 | 532 | 146 | 1248 |
| Su | 147 | 56 | 570 | 345 | 85 | 975 | |
| A | 346 | 138 | 655 | 376 | 146 | 713 | |
| Total iron (µg·L−1) | Sp | 120 | ≤50 | 830 | 160 | ≤50 | 1300 |
| Su | 120 | ≤50 | 950 | 290 | ≤50 | 2600 | |
| A | 140 | ≤50 | 310 | 180 | ≤50 | 1570 | |
| Chlorophyll a (µg·L−1) | Sp | 25.0 | ≤2.0 | 876 | 3.3 | ≤2.0 | 114 |
| Su | 78.2 | ≤2.0 | 1068 | 4.1 | ≤2.0 | 88 | |
| A | 27.1 | ≤2.0 | 482 | 6.0 | ≤2.0 | 186 | |
| Phytoplankton Composition at the Reservoir Center | Cell Abundance (Cell·L−1) | Contribution to Total Abundance (%) | |
|---|---|---|---|
| sample-2017 | |||
| Cyanobacteria | Microcystis spp. | 3.0 × 107 | 95.2 |
| Dynophyceae | Ceratium furcoides | 1.2 × 106 | 3.81 |
| Diatomeae | Aulacoseira granulata | 1.0 × 105 | 0.32 |
| Cyclotella sp. | 1.8 × 105 | 0.57 | |
| Chlorophyceae | Monorraphydium sp. | 3.3 × 104 | 0.10 |
| Cryptista | Cryptomonas sp. | 2.0 × 103 | 0.006 |
| sample-2018 | |||
| Cyanobacteria | Microcystis spp. | 7.3 × 107 | 97.3 |
| Aphanocapsa sp. | 1.8 × 106 | 2.40 | |
| Dolichospermum spp. | 3.7 × 105 | 0.49 | |
| Diatomeae | Cyclotella sp. | 8.5 × 103 | 0.01 |
| Nitzschia sp. | 1.4 × 103 | 0.002 | |
| Chlorophyceae | Pediastrum sp. | 3.4 × 104 | 0.04 |
| Zygnematophyceae | Staurastrum sp. | 4.2 × 103 | 0.005 |
| Cryptista | Chroomonas sp. | 2.5 × 104 | 0.05 |
| Cryptomonas sp. | 1.4 × 103 | 0.002 | |
| Iron Treatments | Specific Growth Rate (µ) during the Last 12 Days (d−1) | SFe Decreasing Rate throughout the Experiment [(µg·L−1)·d−1] | SRP Decreasing Rate throughout the Experiment [(µg·L−1)·d−1] |
|---|---|---|---|
| Control | 0.121 ± 0.002 (a) | n.d. | −3.02 ± 0.94 (a) r2 = 0.68 |
| (no iron addition) | |||
| TI | 0.147 ± 0.017 (a) | −17.95 ± 0.64 (a) | −3.19 ± 0.71 (a) |
| (400 µ Fe·L−1) | r2 = 0.87 | r2 = 0.68 | |
| TII | 0.239 ± 0.028 (b) | −21.77 ± 0.46 (a) | −4.12 ± 0.61 (a) |
| (700 µ Fe·L−1) | r2 = 0.95 | r2 = 0.88 | |
| TIII | 0.204 ± 0.002 (b) | −28.37 ± 6.89 (a) | −4.96 ± 0.22 (a) |
| (1100 µ Fe·L−1) | r2 = 0.98 | r2 = 0.95 |
| Iron Treatments | Specific Growth Rate (µ) during the Last 11 Days (d−1) | SFe Decreasing Rate throughout the Experiment [(µg·L−1)·d−1] | SRP Decreasing Rate throughout the Experiment [(µg·L−1)·d−1] |
|---|---|---|---|
| Control | 0.129 ± 0.017 (a) | n.d. | −0.80 ± 0.18 (a) |
| (no iron addition) | r2 = 0.93 | ||
| −28.64 ± 1.83 * r2 = 0.82 −13.87 ± 4.72 ** (a) r2 = 0.92 | |||
| T1P | 0.444 ± 0.122 (b) | −1.29 ± 0.37 (a) | |
| (700 µ Fe·L−1) | r2 = 0.78 | ||
| T2P | |||
| (350 µ Fe·L−1 + | 0.580 ± 0.139 (b) | −17.60 ± 1.97 ** (a) | −1.26 ± 0.58 (a) |
| 350 µ Fe·L−1) | r2 = 0.84 | r2 = 0.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Halac, S.R.; Ruibal-Conti, A.L.; Mengo, L.d.V.; Ullmer, F.; Cativa, A.; Bazan, R.; Rodriguez, M.I. Effect of Iron Availability on the Growth and Microcystin Content of Natural Populations of Microcystis spp. from Reservoirs in Central Argentina: A Microcosm Experiment Approach. Phycology 2023, 3, 168-185. https://doi.org/10.3390/phycology3010011
Halac SR, Ruibal-Conti AL, Mengo LdV, Ullmer F, Cativa A, Bazan R, Rodriguez MI. Effect of Iron Availability on the Growth and Microcystin Content of Natural Populations of Microcystis spp. from Reservoirs in Central Argentina: A Microcosm Experiment Approach. Phycology. 2023; 3(1):168-185. https://doi.org/10.3390/phycology3010011
Chicago/Turabian StyleHalac, Silvana Raquel, Ana Laura Ruibal-Conti, Luciana del Valle Mengo, Florencia Ullmer, Aldana Cativa, Raquel Bazan, and Maria Ines Rodriguez. 2023. "Effect of Iron Availability on the Growth and Microcystin Content of Natural Populations of Microcystis spp. from Reservoirs in Central Argentina: A Microcosm Experiment Approach" Phycology 3, no. 1: 168-185. https://doi.org/10.3390/phycology3010011
APA StyleHalac, S. R., Ruibal-Conti, A. L., Mengo, L. d. V., Ullmer, F., Cativa, A., Bazan, R., & Rodriguez, M. I. (2023). Effect of Iron Availability on the Growth and Microcystin Content of Natural Populations of Microcystis spp. from Reservoirs in Central Argentina: A Microcosm Experiment Approach. Phycology, 3(1), 168-185. https://doi.org/10.3390/phycology3010011





