Abstract
Many marine algae are strong accumulators of halogens. Commercial iodine production started by burning seaweeds in the 19th century. The high iodine content of certain seaweeds has potential pharmaceutical and nutritional applications. While the metabolism of iodine in brown algae is linked to oxidative metabolism, with iodide serving the function of an inorganic antioxidant protecting the cell and thallus surface against reactive oxygen species with implications for atmospheric and marine chemistry, rather little is known about the regulation and homoeostasis of other halogens in seaweeds in general and the ecological and biological role of marine algal halogenated metabolites (except for organohalogen secondary metabolites). The present review covers these areas, including the significance of seaweed-derived halogens and of halogens in general in the context of human diet and physiology. Furthermore, the understanding of interactions between halogenated compound production by algae and the environment, including anthropogenic impacts, effects on the ozone layer and global climate change, is reviewed together with the production of halogenated natural products by seaweeds and the potential of seaweeds as bioindicators for halogen radionuclides.
1. General Introduction to Algae
Algae are a heterogenous group of photosynthetic, oxygen-producing, mostly aquatic organisms, that lack the complexity of a typical plant structure (leaves, stem, roots and complex reproductive structures). These simple organisms are critically important; it is estimated that 50–80% of the oxygen produced on Earth originates from algae and about half of the global total annual productivity is carried out by algae in the oceans [1,2]. Furthermore, algae influence climate by controlling processes such as biogenic calcification, oceanic sequestration of CO2 and release of dimethylsulfide [3], along with the production of a wide array of natural compounds that play a primary role in ecosystem functioning and influence the environment and surrounding organisms [4], thus fulfilling very important ecosystem services. Algae vary in cellular structure, photosynthetic pigments and biochemical characters. This variation is used to organise them into different phyla and classes. There are at least 350,000 known species of algae [5,6] ranging in size from single celled picoplankton of 1–3 μm to giant kelps over 60 m long. The number of algal phyla has been repeatedly revised over the years. According to van den Hoek et al. [7] there are 16 algal phyla, yet this has more recently been revised upwards because of molecular phylogenies and Tree-of-Life projects [8,9,10].
The marine macroscopic forms or seaweeds live mainly in the littoral zone and are ecologically significant, not only important as primary producers and in sequestering carbon and combating climate change [11,12], but also as nurseries [13,14] and habitats for other marine organisms [15,16]. Seaweeds are taxonomically distributed in three major groups: Phaeophyceae, or brown algae, due to their content of the golden brown pigment fucoxanthin; Chlorophyta, which are green in color due to the dominance of chlorophyll a and b; and Rhodophyta, or red algae, that take their color from their red phycoerythrin content [17]. Brown algae comprise around 2000 described species and although many are filamentous, others have evolved complex multicellularity [18,19], with larger forms usually consisting of stem-like stipe, leaf-like blades, and being mostly attached to a solid substrate by a root-like holdfast. Large, multicellular Laminariales and Fucales were found to be the strongest iodine accumulators and to exhibit the most elaborate halogen metabolism among the brown algae [20,21,22,23,24,25]. Green algae are one of the most diverse groups of organisms on Earth, with an estimated 600 genera and 10,000 species. The macrophytes among this group range in morphology from filaments and multinucleated species to taxa with parenchymatous tissue [26]; these could be attached by a holdfast or free floating. The Rhodophyta are also a large group, containing over 7000 currently recognised species [6]. The majority of species (6793) are found in the class Florideophyceae, and mostly consist of multicellular marine algae, in the form of filaments, sheets or calcareous algae, including many notable seaweeds [6,27].
2. Halogens
2.1. Definition, Discovery and History
The halogens are a family of nonmetal elements in group 17 (VIIA) of the periodic table, consisting of six elements: fluorine, chlorine, bromine, iodine, astatine, and tennessine. The name ‘halogen’ was proposed by Jons Jakob Berzelius in 1842 based upon the Greek words “hal” (salts) and “gen” (“to produce”) [28]. Halogens are found in the environment mostly in the form of ions or compounds; because of their great reactivity their elemental forms tend to be short-lived. In their elemental forms, the halogens are very reactive diatomic molecules, which exist at room temperature and atmospheric pressure in different physical states: fluorine and chlorine are gases, bromine is a liquid, while iodine is solid. Of particular interest in the context of the present review, certain seaweeds—in particular, members of the Fucales and Laminariales—release molecular iodine. There are no stable (nonradioactive) isotopes of astatine and tennessine. The halogen atoms have seven electrons in their outer shells, so that addition of only one electron gives the corresponding anions (halide ions) with a stable (octet) electron configuration and an oxidation number of −1. On Earth, the halogens mostly occur as salts (halides: F−, Cl−, Br−, and I−; halite: IO3−) either dissolved in water or in solid deposits. Among these, chloride is the major anion in seawater [29] which contains 536 mM chloride, 800 μM bromide and 0.5 μM iodide [21,30,31]. Iodide also occurs in high concentrations of natural gas deposits, e.g., in the Kanto Province in Japan, where it is commercially extracted in parallel to natural gas production [24]. The main solid forms of iodine are iodargyrite (AgI) [32,33,34,35], calcium iodate Ca(IO3)2 and caliche (occurring mostly in Chile) which is CaCO3 containing nitrates and iodates [24]. The solar system contains 0.4 ppm F, 4.8 ppm Cl, 23 ppb Br, and 3 ppb I, making the halogens the 25th (F), 18th (Cl), 34th (Br), and 50th (I) most abundant elements in the solar system [36].
2.1.1. Fluorine
F, atomic number 9 with an atomic mass of 18.998404 Da, is derived from Latin “fluere” 9 (=“to flow”, [28]). In the early 1800s, Andre-Marie Ampère in France and Humphry Davy in England, corresponded about a potentially novel element. In 1813, Davy announced its discovery and named it fluorine following a suggestion by Ampère [37,38]. In 1886, the isolation of elemental fluorine was reported by Henri Moissan after nearly 74 years of effort by other chemists (some of whom may have died from poisoning in this effort), for which he was awarded the 1906 Nobel Prize [38,39]. It makes up about 0.06–0.08% of the Earth’s crust but the average crustal abundance is low with an average of 300 μg/g [40]. Fluorine is widely distributed in many types of rocks and soils, air, plants, animals and has a low concentration in seawater [41]. However, it is the most electronegative and reactive of all elements, and rarely occurs naturally in the elemental state [42].
2.1.2. Chlorine
Cl, atomic number 17 with an atomic mass of 35.5 Da. The name is derived from the Greek word χλωρός (khlôros), meaning “greenish yellow” [28]. Chloride was first discovered by Carl Wilhelm Scheele, a Swedish scientist, in 1774 [43]. Since chlorine is very reactive, it is found in nature only in combination with other elements [39]. In nature, the major mineral source of chloride is rock salts, besides being found in dissolved salts in seawater and from the prehistoric evaporation of salt lakes. It makes up about 0.031% of the earth’s crust [28].
2.1.3. Bromine
Br, atomic number 35 with an atomic mass of 79.904 Da. The name is derived from the Greek word βρώμος (bromos), meaning “bad smell” or “stench” [28]. The element bromine (as Br2) was first prepared by Balard in 1826. Bromine is concentrated in the upper mantle crust of the Earth and seawater, principally in the form of inorganic bromides. It has been estimated that the total bromine content in the crust of the earth is 0.00016% [44].
2.1.4. Iodine
I, atomic number 53 with an atomic mass of 126.9 Da. The discovery of the element is credited to Courtois in the context of the Napoleonic Wars in 1811 while manufacturing saltpetre (used to make gunpowder) from ashes of seaweed [24,37,45,46]. After the initial discovery, Joseph Gay-Lussac, another French chemist, gave the new element its name iodine which is derived from the Greek word ιώδης (iodes) due to its purple colour. Iodine is present mostly as iodate (IO3−) and iodide (I−) in natural waters, with total iodine levels ranging from ~60 µg/L in seawater [47] to about 5 µg/L in estuaries to less than 0.2 µg/L in rivers (in some Triassic mountain regions of northern Italy). Correspondingly, freshwater trout contain 20 µg/kg of iodine compared to saltwater fishes (herring) with about 500–800 µg/kg [48].
2.1.5. Astatine
At, atomic number 85 with an atomic mass of 209.9871 Da, has been reported in 1940 by Dale R. Coson, Kenneth Ross Mackenzie and Emilio Segrè. The name comes from the Greek άστατος (astatos), meaning unstable [28]. Astatine is a highly unstable radioactive chemical element of the halogen group. Astatine appears in the Earth’s crust when the radioactive elements uranium and thorium decay. The total amount of astatine present in the Earth’s crust does not exceed 50 mg, making it one of the rarest elements [39].
2.1.6. Tennessine
Ts, atomic number 117, was first synthesised in 2010 by a Russian-American team [49]. It was named after the American state which houses the Oak Ridge Laboratory, which contributed to the discovery; the ending –ine (rather than –ium) underlines its position in the halogen group, although whether this is also reflected in its chemical properties remains to be established.
2.2. The Global Cycles of Halogens
The oceans are the largest reservoirs of bioavailable chlorine, bromine and iodine on the planet [50] and, from there, the elements are transferred to the atmosphere. In the atmosphere, iodine reaches concentrations of 5–20 ng m−3 in gaseous forms and 1–5 ng m−3 as particulate iodine [51]. Iodine atoms, formed by photolysis of their precursors, for example HOI, I2 and organoiodines [52], are rapidly oxidised via ozone into higher iodine oxides (I2O2, I2O3, and I2O4) [53,54] and iodic acid (HIO3) [55], which may nucleate under certain conditions and form new aerosol particles [55,56]. Particles that grow to larger sizes influence climate directly, by scattering light, and indirectly by producing cloud condensation nuclei (CCN) [55].
Iodine in the form of gas and aerosol is carried by the wind and rain to land areas [24,30,57,58]. In rainwater, the iodine appears at concentrations of around 2 μg L−1 [59]. Once found on land, iodine is distributed in different ways: it is again mobilised by volatilisation into the atmosphere by abiotic and biotic processes, fixed in soil and biomass, or carried to the ocean through water streams [57,58,60,61].
The biogeochemical cycle of bromine is dominated by major sources of bromine to the atmosphere, such as sea spray, saline lakes, volcanoes, and marshes, but also anthropogenic sources [62]. Bromine is lost from the atmosphere mainly through photochemical oxidation of gaseous emissions to soluble forms which are ultimately deposited, and deposition of sea spray aerosol. The bromine cycle is linked to those of chlorine and iodine and contributes to ozone destruction in the stratosphere and troposphere [62,63].
The chlorine cycle shares features with the bromine cycle—in particular, the large-scale transfer of chlorine into the troposphere from sea spray is similar to that of bromine [64,65]. The troposphere receives inputs of both inorganic and organic chlorine from the ocean. Due to the much higher stability of organic chlorine compounds compared to brominated and iodinated compounds, the former tends to be much more long-lived, which results in significant amounts of chlorinated compounds reaching the stratosphere. The major atmospheric sinks of chlorine are similar to those of bromine, although Cl atoms are also capable of oxidation of alkanes, providing additional sinks.
More recent reviews are available about the environmental role of fluorine [66], chlorine [67] and bromine [62].
2.3. Inorganic Biochemistry of the Halogens
2.3.1. Halogen Oxidation States
Having introduced the halogens and their biogeochemical cycles briefly in Section 2.1 and Section 2.2, we will in this section link the chemical properties of the halogens to their roles in biology, including other forms of life than the seaweeds, which are the main topic of this review, and will be treated in detail in the remaining sections. The discussion of the biological roles of the halogens will focus on fluorine, chlorine, bromine, and iodine. The only relation of the recently synthesised tennessine with life is that it is completely anthropogenic [49]; the 211At isotope is promising for targeted α-therapy, provided that its easy removal from biomolecules by biological dehalogenation is bypassed [68]. The redox properties of the four most important halogens are compared to each other and that of oxygen (being the most abundant medium and most readily available oxidant in the environment in, respectively, its reduced and elemental forms) in water at pH 7 and 25 °C in Figure 1 in an oxidation state (or Frost) diagram. In such a diagram, strong oxidants are at the top right, and strong reductants at the bottom left; the slope of the line connecting the oxidant and the reductant of a redox couple is a measure of the reduction potential, i.e., the oxidising power, of the oxidant. The elemental forms are in the origin, and the minimum corresponds to the most stable form of an element (which for the halogens are the aforementioned halide ions). When an intermediate species takes a position above the line connecting the surrounding species, it indicates a thermodynamic tendency to disproportionate into those surrounding species; a position below the line implies a tendency for the surrounding species to comproportionate.
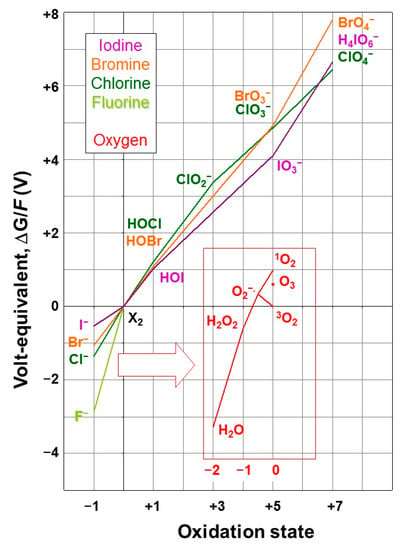
Figure 1.
Oxidation state diagram of the halogens and (inset) oxygen (Adapted from [69,70,71,72]).
In its triplet ground state, molecular oxygen (3O2) is a relatively inert oxidant; its spin isomer (singlet oxygen, 1O2), its allotrope (ozone, O3), and the products of its reduction by one electron (superoxide, O2−), two electrons (hydrogen peroxide, H2O2), and three electrons (OH radical, not included in Figure 1), are kinetically and thermodynamically stronger oxidants and are, therefore, collectively referred to as reactive oxygen species (ROS). It can be seen from the diagram that, along with H2O2, elemental fluorine (F2) is the strongest oxidant in the system, and it is not possible to oxidise it beyond the elemental level. For the other halogens, oxidised forms exist up to oxidation states of +5 and +7 (Figure 1, top right); of these, only iodate (I5+) is stable in water. Of the elemental forms, only bromine and iodine are stable in aqueous solution, in strong association with bromide and iodide, if available, yielding Br3− and I3−, respectively. In the presence of molecular oxygen, the predominant form of I is iodate. As will be discussed in more detail in Section 4, the oxidation of iodide to iodate, and the incorporation of the latter into calcium carbonate precipitates, has allowed the I/Ca ratio in such minerals to be used as an accurate indicator of the degree of oxidation in the atmosphere at the time of the mineral deposition [73,74,75]. Because of the lack of reactivity of the stable halide anions, some oxidative activation is required for halogens to undergo reactions such as incorporation into organic compounds.
The diagram (Figure 1) also shows that H2O2 can oxidise the halides X− (except fluoride) to the corresponding hypohalous acids HOX, which are not dissociated at neutral pH, and are, in turn, relatively strong oxidants, but also that, since they are located above the line connecting the surrounding oxidation states, they have a thermodynamic propensity to disproportionate into the halide and halite, which is stronger at high pH. The partly oxidised (elemental and hypohalous) halogen species can evaporate from water, thereby escaping possible dis- and com-proportionations, and be further oxidised in the gas phase by dioxygen and ozone, thus forming the more highly oxidised halogen species without the limitation of stability in aqueous solution. The various hypohalous acids are close together in the diagram in Figure 1.
2.3.2. Enzymatic Incorporation of Halide into Halocarbons
Because of the lack of reactivity of the stable halide anions, some oxidative activation is required for halogens to undergo reactions such as incorporation into organic compounds; as discussed above, this is not possible for fluorine, which will be discussed separately in Section 2.3.5. An overview of enzymes involved in biological halogenation strategies, collectively known as halogenases [76,77], is given in Figure 2. Enzymes that activate halide X− with H2O2 as the oxidant are called haloperoxidases. The resulting hypohalous acid HOX can be conceived as a combination of OH− with a halogen with a formal charge of +1, which readily reacts as an electrophile, in particular with activated moieties, such as the hydroxyl-substituted phenyl ring in the side chain of the amino acid tyrosine. The less electronegative a halide is, the more readily it is oxidised to the hypohalous acid; haloperoxidases are therefore named after the most electronegative element that they can activate. The first haloperoxidase that was discovered [78], the chloroperoxidase from the fungus Caldariomyces fumago, contains a heme cofactor [79], like the mammalian haloperoxidases [80], which will be discussed in more detail in Section 6. The haloperoxidases from many other organisms, including seaweeds, depend on vanadate [81,82] and will be discussed in more detail in Section 5. The chemical reaction of hypohalous acid with hydrogen peroxide gives singlet oxygen [83], the more reactive spin isomer of triplet oxygen (Figure 1), and its formation is also a feature of enzymic catalysis by heme- [84] and vanadate-dependent haloperoxidases [85]; its significance does not appear to have been further explored. It should be noted that, in addition to the heme- and vanadate-dependent peroxidases, cofactor-free haloperoxidases exist (not included in Figure 2) which only occur in bacteria; the first was discovered in Pseudomonas pyrrocinia, that incorporates chlorine at the 7-position in tryptophan [86]. When the structure of a related bromoperoxidase from Streptomyces aureofaciens was elucidated, it was found to contain a catalytic triad with a nucleophilic serine residue, like the hydrolases to which it is related [87]. For haloperoxidation, a carboxylic acid is needed, that is proposed to react with the serine to form an ester, which is attacked by H2O2 to form a peracid, so that the enzyme could also be called a perhydrolase [88]; the peracid reacts with the halide to produce the halogenating intermediate, which is not known with certainty, but must be electrophilic. The perhydrolase activity is considered by some to be a side activity of hydrolases, such as lipases and esterases [89]. While the important effects of haloperoxidases in human physiology will be discussed in Section 6, some examples from other forms of life are mentioned here. Singly brominated tyrosines are at the core of metal-halogen biominerals [90] which make the tips of crab claws resistant to fracture [91]. Multiply halogenated amino acids with iodine and bromine are proposed to play a role in protein cross-linking in the jaws of the marine worm Nereis [92]. Another amino acid that is susceptible to electrophilic bromination is tryptophan; the degradation products of 6-Br-tryptophan (Figure 3) are responsible for the fluorescence in, for example, the swell shark Cephaloscyllium ventriosum [93]. Other examples of biological halide oxidation, that apparently do not involve haloperoxidase, are the microbial oxidations of iodide by α-proteobacteria to elemental iodine [94,95,96], by Pseudomonas iodooxidans to iodate [97], and by the manganese-oxidising bacterium Roseobacter via extracellular superoxide [98].
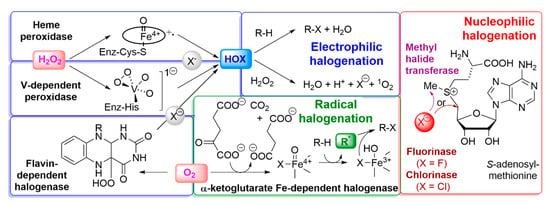
Figure 2.
Enzymatic incorporation of halogens in biomolecules (adapted from [76]). The sections and relevant intermediates for resp. electrophilic, radical, and nucleophilic halogenation are highlighted in blue, green, and red, respectively; in the nucleophilic section, the fluorinase/chlorinase (brown) and methyl halide transfer (violet) are distinguished. Oxidants (O2, H2O2) are highlighted in pink.
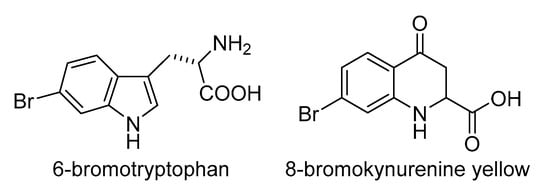
Figure 3.
Brominated tryptophan and a kynurenine degradation product.
Another group of halogenases does not depend on hydrogen peroxide, but on molecular oxygen as the oxidant; oxygen-dependent halogenationproceeds either with reduced flavin as a coreductant in the flavin-dependent halogenases (Figure 2, bottom left), or with α-ketoglutarate as a coreactant and non-heme iron in the radical halogenases (Figure 2, bottom middle) [76,77]. The first non-metal halogenase was discovered in the bacterium Streptomyces aureofaciens, where it is involved in the biosynthesis of 6-demethylchlortetracycline [99]; it was then established by work on a related enzyme that this group of halogenases has reduced flavin as a cofactor [100]. The electrophilic HOX intermediate, already familiar from the haloperoxidases, is produced via a flavin hydroperoxide intermediate (Figure 2, bottom left) [101]. In the structure of the halogenase CmlS, which is responsible for chlorine incorporation in the precursor of chloramphenicol in the bacterium Streptomyces venezuelae, the substrate binding site is approx. 10 Å away from the flavin, and the HOCl is proposed to react with a lysine residue near this binding site to produce an even more reactive chloramine intermediate [102]. This fixation of the reactive halogen near the binding site could be the explanation for the high selectivity [103] of this class of halogenases compared to the haloperoxidases. Recently, a flavin-dependent halogenase with iodine specificity has been discovered in a virus, specifically, a marine cyanophage [104].
The first halogenase dependent on non-heme iron, α-ketoglutarate, and molecular oxygen was discovered in the bacterium Pseudomonas syringae, where it is involved in the biosynthesis of the chlorine-containing phytotoxin syringomycin E [105]. Structural [106] and spectroscopic studies [107,108] reveal that O2 and the α-ketoglutarate coreactant reacts with the iron to give a non-heme iron-oxo intermediate, which abstracts an H atom from the organic substrate in a non-activated position to give an organic radical (Figure 2, bottom middle). The advantage of non-heme over heme iron in this reaction is that it cannot only coordinate O2 and the coreactant in adjacent positions, but also the halide, which reacts with the organic radical to give the halogenated product. A related enzyme from the cyanobacterium Hapalosiphon welwitschii plays a role in the production of welwitindolinone and related indole monoterpenoids [109,110,111].
2.3.3. Halogen Oxyanions as Electron Acceptors
Although much of the highly oxidised halogens, the (per)halates and organohalogen compounds, currently in the environment, is anthropogenic, there has been enough material of geo- or bio-chemical origin around for a sufficient time for life forms to evolve to take advantage of their presence in various ways. In spite of their thermodynamic instability, the chlorine species with the highest oxidation states play a role in biology [112], because, like molecular oxygen, they combine a high thermodynamic reduction potential with kinetic inertness. Some organisms can reduce both the potentially explosive perchlorate (by perchlorate reductases [113] containing molybdenum in the active site [114]) and chlorate, others only chlorate (by chlorite reductase), both to the level of chlorite [67]. Some of the organisms that reduce perchlorate can also reduce bromate, nitrate, and iodate. A feature that distinguishes the biological reduction of the (per)chlorates from other oxyanion electron acceptors, which proceed with the formation of water, is that the decomposition of the chlorite intermediate by chlorite dismutase is one of the few (along with superoxide dismutase, catalase, and the oxygen-evolving centre of photosynthesis) biological processes to produce molecular oxygen, as would be expected based on their higher reduction potential. Other examples of biological processes involving highly oxidised halogen species, albeit without oxygen evolution, include iodate reduction by Pseudomonas [115] and the specific reduction of bromate (a carcinogen) by the bacterium Rhodococcus [116].
2.3.4. Dissimilatory and Assimilatory Organohalide Degradation
Another class of metabolism, where halogen-containing molecules act as the final electron acceptor, is that of so-called organohalide (or organohalogen) respiration, which produces halide and the reduced organic compound [117,118,119] in a bacterium, tentatively named Dehalococcoides ethenogenes, belonging to the emerging group of organohalide-respiring bacteria. In PceA, a tetrachloroethene reductive dehalogenase from the bacterium Sulfurospirillum multivorans organohalogen reduction involves a corrinoid cofactor in connection with a 4Fe-4S cluster [120]. Because of its inverse relation with the strength of the carbon-halogen bond, the reduction potential of the organohalogens by H2 increases going from those containing F (for which no respiration is observed) via Cl and Br to I [121]. The free energy of the reduction of perchloroethylene (PCE) to trichloroethylene (TCE) is comparable to that of nitrate [122]; this places organohalogens between chlorate and iodate in Figure 1, and makes them much better electron acceptors than sulphate. Organisms that feature this kind of metabolism of organohalogens not only occur in environments where they can be expected to be of anthropogenic origin, but also in so-called pristine environments where they are of (bio)geochemical origin [123]. An example of biological reductive dehalogenation that does not involve a corrinoid cofactor is the bacterial tetrabromopyrrole debrominase, which has two cysteine residues in the active site [124].
The reductive type of dehalogenation, which is the distinguishing feature of organohalide respiration and is dissimilatory, i.e., has an energetic benefit for the microorganism, should be distinguished [125] from the oxidative dehalogenation in other organisms, which is assimilatory, i.e., the benefit to the organism is in the carbon utilization [126,127]. In addition to the aforementioned reductive pathways, humans have a variety of deiodination pathways [128], including oxidative ones involving cytochrome P450 [129,130], either alone, or in combination with nucleophilic deiodination with glutathione [131,132].
2.3.5. Halide Binding to Proteins and Nucleophilic Halogenation
In contrast to the most common trace elements, the transition metals, halides do not bind to proteins by coordination to amino acid side chains; they are themselves likely to be ligands for transition metals. It is perhaps surprising that charge complementarity hardly plays a role in halide-protein interactions; for example, the main role of the positively charged amino acids in the active site of vanadium-dependent chloroperoxidase (see Section 5; [133]) is to compensate for the negative charge of the vanadate cofactor. Their interaction with proteins depends very much on the possibility to be an acceptor for hydrogen bonds. For example, the voltage-gated CLC channel has a binding side for the chloride ion that consists of a glutamic acid and two backbone amides [134]. A survey of halide binding sites in protein structures deposited in the Protein Data Bank reveals that the ions are only partly dehydrated to varying degrees [135]; chloride and bromide are typically surrounded by three, iodide by two water molecules. The technique of infrared multiple-photon dissociation (IRMPD) spectroscopy, by which infrared spectra can be taken of mass spectrometric fragments with a free-electron laser, allows H-bonds of halide ions with peptides to be studied in the gas phase [136]. The dehydration of fluoride ion plays an important role in the catalysis by fluorinase (5′-fluoro-5′-deoxy adenosine synthetase (FDAS) from the bacterium Streptomyces cattleya [137] which acts by nucleophilic displacement of the methionine leaving group in S-adenosyl-methionine (SAM) by the fluoride ion (Figure 2, brown arrow). The reaction proceeds with the inversion of the configuration that is characteristic of a bimolecular (SN2) substitution mechanism [138]. Although computational studies do not agree on the necessity to desolvate the fluoride ion for it to be a good nucleophile [139,140], crystallographic studies show that it loses all but two water molecules upon binding to fluorinase, and that the remaining ones are displaced upon binding of the coreactant SAM in its proximity [141,142]. Because fluoride cannot be activated by oxidation (Figure 1), the nucleophilic substitution featured in the fluorinase reaction is the only biochemical pathway for fluorocarbon formation. In Streptomyces cattleya, SAM fluorination leads ultimately to the production of fluorothreonine and fluoroacetate; the latter is also known as a toxin from some tropical plants, such as the gifblaar Dichapetalum cymosum. Thesedo not contain a fluorinase, however, and the mechanism of fluorine introduction by plants is as yet unknown [143]. An analogous enzyme, chlorinase, has been discovered in a marine bacterium Salinispora tropica [144], is active on chloride, bromide, and iodide, but not fluoride, and plays a role in the biosynthesis of the chlorine-containing proteasome inhibitor salinosporamide A [145]. In another group of SAM-dependent enzymes for halogenation, the methyl halide transferases, which have been found in various species, including a red alga [146] and a microalga [147], it is the homocysteinyl-adenosyl, not the methionine, that is the leaving group, leading to a methylhalide product (Figure 2, purple arrow). Only the crystal structure of the SAM-bound form, not including the halide, has been elucidated [148]. While the haloperoxidases, to be discussed in more detail in Section 5, are responsible for the production of polyhalomethanes in algae, the methyl transferases are responsible for methyl halide formation [149,150].
2.4. Significance of Halogens
2.4.1. Environment
Natural halogenated compounds affect the atmosphere, the marine and terrestrial environment. The geo-, atmospheric and marine chemistry of iodine has been reviewed by [24], also with an emphasis on microbial metabolism [24,151] and is summarised in Figure 4.
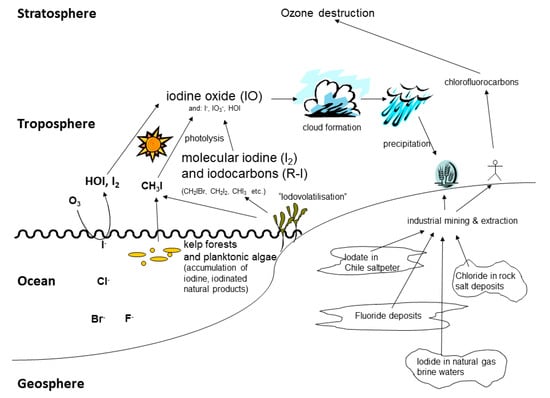
Figure 4.
Schematic overview of the biogeochemical cycle of the halogens.
Halogens have a significant impact on global stratospheric ozone [152,153]. Since the late 1970s, stratospheric ozone levels above the Antarctic were observed to be declining due to anthropogenic emissions of halocarbons (in particular, chlorofluorocarbons/CFCs) which has been labelled the “ozone hole” [154,155]. The ozone hole was first detected in the Antarctic in 1984 [155,156] and, a few years later, also in the Arctic [157]. Concern for the ozone layer, which shields the world from dangerous UV irradiation, arose in the early 1970s, chiefly because of the studies by Paul Crutzen highlighting the danger to the ozone layer by nitrogen oxides stemming from supersonic stratospheric aviation [158] and by F. Sherwood Rowland and Mario Molina who predicted the ozone-damaging potential of CFCs [159], for which they shared the 1995 Nobel Prize in chemistry [160,161]. Ralph Cicerone, Richard Stolarski and co-workers proposed in the 1970s that one atom of chlorine would be able to destroy 100,000 molecules of ozone [154]. Bromine is up to 40–100 times more efficient in destroying the ozone molecules than chlorine in the stratosphere [162]. The role of chlorine and bromine radicals in polar stratospheric ozone loss was demonstrated in airborne measurements [163]. The combination of theoretical calculations since the 1970s [159], supported by the discovery of the Antarctic ozone hole in the early 1980s [155], led world leaders to sign the Montreal Protocol (Protocol on Ozone Depleting Substances (ODS)) mandating the phase-out of CFCs in 1987 [156], which is now considered one of the most successful international environmental treaties of all time [164,165]. Indeed, as a result of this treaty and its consequent amendments, the atmospheric concentrations of very long-lived CFCs and other synthetic ozone-depleting substances (ODSs) have been declining since the late 1990s (later for CFC replacement gases), and, consequently, both the Antarctic and the Arctic ozone holes have now started recovering [166,167]. Most recently, iodine radicals, which are efficient at destroying ozone in the gas and particulate phases, have been found in significant amounts in the lower stratosphere, resulting from transport of natural oceanic emissions [168].
In the past 20 years, atmospheric chemists have come to realise that halogens exert a powerful influence on the chemical composition of the troposphere and, through that influence, can affect the fate of pollutants and influence climate. Of particular note for the climate is that halogen cycles affect methane, ozone, and particles, all of which are powerful climate-forcing agents through direct and indirect radiative effects. This influence comes partly from the high reactivity of atomic halogen radicals (with reactivity in this series Cl > Br > I) and halogen oxides (e.g., ClO, BrO, IO, and higher oxides), known as reactive halogen species [63,169,170,171,172,173] which are generated photochemically [174]. Of particular interest is the impact of reactive halogen species on tropospheric ozone, a key greenhouse gas and air pollutant [175]. Together, reactive iodine and bromine are responsible for up to 50% of ozone destruction in the marine boundary layer (MBL) [173,176]. Bromine and iodine compounds can also impact climate through modification of nitrogen oxide (NOx) and hydrogen oxide radical (HOx) cycles with resulting effects on the lifetimes of other climatically important trace gases [177,178], and through oxidation of dimethyl sulfide (DMS) [176]. Iodine has particularly significant impacts on tropospheric photochemistry, ultimately impacting climate by reducing the radiative forcing of ozone (O3) and air quality by reducing extreme O3 concentrations in polluted regions [170,171,172].
The rapid reaction of sea-surface iodide with O3 is currently believed to be the largest single source of gaseous iodine to the atmosphere [179,180]. Due to increased anthropogenic O3, this release of iodine is believed to have increased dramatically over the twentieth century, by as much as a factor of three [181,182]. However, marine biological production of very short-lived organic halogenated compounds (VSLH) can represent a strong local to regional source of iodine and an important source of iodine to the stratosphere [168], and is a globally important source of atmospheric bromine [176].
In the geosphere, the abundance of halogens in the mantle may provide information about the origin and evolution of the Earth and other planets. The halogen cycle on Mars has received a lot of interest, in terms of the concentration of halogens being significantly enriched in the crust when compared to the Earth [183]. The surface of Mars is relatively rich in perchlorate, which present a possible hazard to astronauts because of its inhibition of iodide uptake by the thyroid (see Section 2.4.2; [184]), but could also be considered a favourable factor for human settlement because, taking advantage of its (bio)chemistry discussed in Section 2.3.3, it may be used as a possible source of molecular oxygen [185]. Detailed knowledge about the abundance of halogens in hydrothermal fluids and minerals can help to reconstruct paleo-environments (e.g., composition of ancient seawaters [186]), to deduce the sources and evolution of hydrothermal fluids, and to provide constraints on the petrogenesis of magmatic, metamorphic, and sedimentary processes. Analysis of minerals (chert) considered representative of Precambrian sediments has revealed that the Br/Cl and I/Cl ratios in the Archaean ocean were higher than the current ones [187]. Moreover, planktonic iodine-to-calcium ratios (I/Ca) are a useful biogeochemical proxy for oxygen depletion in seawater [75], e.g., as applied recently for reconstructing conditions in the glacial Southern Ocean [188], but also for much more distant eras of the Earth’s history, such as the latest Ordovician glacial maximum [189]. Tandem proxy reconstructions provide evidence of a downward expansion of oxygen depletion in the eastern Pacific during the last glacial, with no indication of greater oxygenation in the upper reaches of the water column. Extrapolation of quantitative deep-water oxygen reconstructions showed that the respired carbon reservoir of the glacial Pacific was substantially increased, establishing it as an important component of the coupled mechanism that led to low levels of atmospheric carbon dioxide during the glacial [190]. Analysis of an extensive compilation of iodine-to-calcium ratios (I/Ca) in marine carbonates supports a major rise in the partial pressure of oxygen in the atmosphere at ~400 million years (Ma) ago and reveals a step-change in the oxygenation of the upper ocean to relatively sustainable near-modern conditions at ~200 Ma ago. An Earth system model demonstrates that a shift in organic matter remineralization to greater depths, which may have been due to increasing size and biomineralization of eukaryotic plankton, likely drove the I/Ca signals at ~200 Ma ago [74].
2.4.2. Human Physiology and Medicine
Iodine plays a central role in thyroid physiology, being the essential element in thyroid hormones (THS) and the major constituent regulator of thyroid gland function [191]. Chatin was the first to publish the hypothesis, in 1851, that iodine deficiency was the cause of goitre [192,193]. In 1896, Baumann [194] and Roos [195] discovered iodine in the thyroid [193]. Iodine from the diet is rapidly and efficiently absorbed (>90%) throughout the gastrointestinal tract and duodenum [196]. Dietary iodine in organic form is converted into the iodide ion before it is absorbed. Under normal circumstances, the plasma iodide is cleared from circulation, mostly by the thyroid and kidney. Normally about 120 micrograms of iodide are taken daily up by the thyroid gland for the synthesis of the thyroid hormones triiodothyronine (Figure 5; molecular formula C15H11I3NO4) and thyroxine (molecular formula C15H11I4NO4) [197]. Through these hormones, iodine is involved in controlling metabolism, cell growth and maturation, and the development and growth of tissues [198]. Nonhormonal iodine is found in a variety of body tissues, including mammary, physiological glands, eye, gastric mucosa, cervix and salivary glands [199]. The typical daily requirement for iodine during adult life is 150 µg, but this requirement is increased during pregnancy and lactation to around 200 µg; during the neonatal period it is 40 µg. Severe iodine deficiency results in hypothyroidism, endemic goitre, cretinism, brain disorders, decreased fertility, retarded psychomotor development, speech and hearing impairments, increased prenatal death, and increased overall mortality [200]. High iodine intake may also cause disturbances in thyroid function [201], but two or three milligrams of sodium iodide per week will prevent goitre and ten milligrams has been shown to cause no harm. In Chinese adults, those consuming non-iodised salt had a significantly (25–36%) increased risk of thyroid nodules compared with those consuming iodised salt [202]. Solomon et al. [203] found a high incidence of autoimmune hyperthyroidism, Graves’ disease, and of multinodular toxic goitre in an area with low iodine intake in a cross-sectional study.
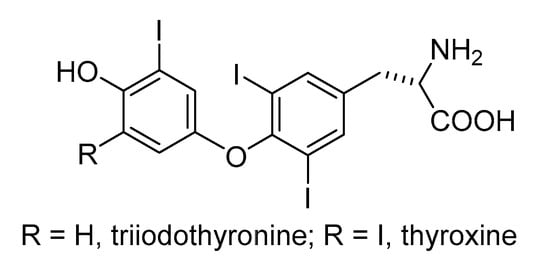
Figure 5.
The thyroid hormones.
Several studies have shown iodide to be a potent antioxidant [204,205]. It has been hypothesised that the ancestral function of iodine accumulation in the thyroid in the evolution of vertebrates was to provide a simple antioxidant system, before the evolution of the thyroid hormone system [205]. Micromolar amounts of I−, which acts as a primitive electron-donor through peroxidase enzymes, can scavenge free oxygen radicals and increase the total antioxidant status in human blood cells [22]. The importance of antioxidants as protective substances against chronic and degenerative diseases, such as cancer and cardiovascular diseases, has been studied for many years. Iodine has also recently been mentioned as a therapy for the maintenance of healthy breast tissue in both animal [206] and clinical studies [207], yet the mechanisms responsible remain unclear. Indeed, Japanese women have been shown to have a lower incidence of breast cancer than that found in most other populations [208], and Japan has one of the highest iodine intakes in the world. It has, however, not been documented that iodine intake is the causative factor, but inorganic iodine has experimentally been shown to suppress breast cancer in Sprague–Dawley rats [209].
The mammalian peroxidases, such as the myeloperoxidase from leukocytes [210], lactoperoxidase from milk [211], and thyroid peroxidase from the thyroid [212] contain (modified) hemes. The XOH/X− couples are strong oxidants, as reflected in the fact that household bleach is a solution of sodium hypochlorite, but also occur in blood plasma in the oxidative burst as a result of the oxidation of chloride and bromide with H2O2 by myeloperoxidase, leading to the hypohalous acids which are the active species in the antimicrobial action. The higher chloride concentration in blood makes HOCl formation more likely than HOBr formation, but some systems appear to have a preference for the formation of HOBr. One example is the sulfilimine link formation between methionine and lysine in collagen IV fibres by peroxidasin, which is only effective with HOBr, not HOCl, providing a rare example of an essential role for bromine in man [213]. The chemical factor that determines the difference in reactivity between the halogen species in these cases is the polarizability or “softness” of the electron cloud, which increases with its size, going from fluorine via chlorine and bromine to iodine. The sulfilimine link formation proceeds via a sulfonium halide which, when formed with the relatively soft bromide, stimulates attack of the lysine nitrogen, producing the sulfilimine link, whereas the harder chloride promotes non-productive attack of water, producing the (non-linking) sulfoxide. Type IV collagen is an important component of the glomerular and tubular basement membrane in the kidney (among others, the concentration of 6-bromotryptophan in blood is inversely correlated with the progression of chronic kidney disease [214], simply because it is a marker for the presence of bromide ion). Another example of preferential bromide activation is the formation of bromotyrosine in eosinophils [215,216], which is proposed as a potential biomarker for monitoring eosinophil-related inflammatory conditions, such as asthma [217].
In humans, MIT and DIT by-products released after proteolysis are deiodinated by the flavin-dependent iodotyrosine deiodinase (IYD), so the excess iodine and the tyrosine moieties can be salvaged [218]; specific selenocysteine-dependent (sec-dependent) iodothyronine deiodinases (DIOs) activate/deactivate the THs by deiodination of the inner or the outer ring of the iodothyronine molecule [219]. In spite of being surrounded by electron lone pairs, halogens in molecules have Lewis acid (electron-acceptor) properties, due to the presence of a σ-hole in the electrostatic surface potential. This gives rise to a specific type of noncovalent interaction called the halogen bond by analogy to the well-known hydrogen bond [220]. An important example is the aforementioned association of the diatomic bromine and iodine molecules with their respective halide ions, which act as the Lewis base. Such interactions can also play a role in the interaction of organohalogens with other biomolecules, for example, the interaction of the thyroid hormone (see Section 2.4.2) with proteins, which involves C–I···O=C [221] and C–I···Se–C (selenocysteine) halogen bonds, respectively [222].
2.4.3. Dietary Iodine and Other Halogens in Seaweeds
In the previously mentioned health context, seaweeds have a special significance as dietary sources. The human body requires iodine intake from external sources, chiefly to sustain healthy function of the thyroid. Iodine is an essential element that is naturally present in many foods, added to some food products, and available as a dietary supplement. The recommended daily intake (RDI) of iodine is 150 micrograms (μg) per day for adults. For women who are pregnant or nursing, the requirements are higher [223]. In fact, one-third of the population is at risk of deficiency, particularly those who live in areas that have only a small amount of iodine in the soil and which are far from coastal areas which tend to have iodine-rich air, including central and eastern European countries. The thyroid relies on iodine to make hormones to help control growth, energy production, reproduction and the repair of damaged cells. Iodine deficiency can lead to swelling of the thyroid gland, known as goitre, and hypothyroidism, which can cause fatigue, muscle weakness and weight gain [224]. In contrast, high consumption of seaweed in Japan and Korea resulting in an excessive supply of iodine to lactating mothers has been linked to neonatal iodine toxicity and consequent hypothroidism [225]. Iodine oversupply has been reported to negatively affect individuals with underlying thyroid disorders [226,227]. Among marine-derived foodstuffs, seaweeds constitute the major supplier of iodine [228]. The use of seaweeds in Asian cooking has been long established and is enjoying increasing popularity in the Western diet [229]. Some Asian seaweed dishes may exceed the tolerable upper iodine intake level of 1100 μg/day [230]. By combining information from dietary records, food surveys, urinary iodine analysis and seaweed iodine content, it is estimated that the Japanese iodine intake, largely from seaweeds, averages 1000–3000 μg/day (1–3 mg/day). However, seaweed preparation, such as washing, drying, and cooking, can readily reduce iodine content [231,232]. Iodine in seaweed foodstuffs is water-soluble in cooking and may vaporise in humid storage conditions, making average iodine content of prepared foods difficult to estimate. Twenty samples of supermarket soups with kelp or kelp broth were analyzed by Nishiyama et al. to determine iodine content, revealing a minimum concentration of 660 μg/L (0.66 mg/L) and a maximum concentration of 31,000 μg/L (31 mg/L) [227]. A study that surveyed seaweed samples from various Asian countries for their iodine content included the three most popular seaweed products in Japan: nori (Porphyra sp.), wakame (Undaria) and kombu (Laminaria japonica). Dried iodine contents range from 16 μg/g in nori to over 8000 μg/g in kelp flakes, while Japanese kombu and wakame contain an estimated 2353 μg/g and 42 μg/g iodine, respectively [230,233]. Al-Adilah et al. [234] investigated the most common seaweeds in the Arabian Gulf for their iodine content. Interestingly, iodine was detectable in all seaweed samples collected from Kuwait, with concentrations ranging from 49.59 μg g−1 DW in Codium papillatum, 129.04 μg g−1 DW in Chondria sp. and 925.10 μg g−1 DW in Sargassum asperifolium. Certain species of seaweed can concentrate bromide [235]. If seaweeds with elevated levels of bromine and low levels of iodine are consumed when the body is in an iodine deficient state, inhibition of thyroid hormone synthesis due to bromine’s attachment to tyrosine residues on thyroglobulin in place of iodine is plausible [236]. Long-term intervention studies (particularly well-powered, appropriately designed randomised-controlled trials) are necessary to assess whether dietary seaweed/seaweed fibre impacts positively on human health.
The strong focus on iodine in studies involving edible seaweeds is likely due to the importance of iodine for thyroid function, but also because especially kelps and fucoids are very potent iodine accumulators. Bromine has been comparatively neglected in such studies and has rarely been investigated in this context, e.g., for its bioavailability alongside iodine in edible seaweeds [237].
Fluoride, the ionic form of fluorine, plays an important role in dental health, but has also adverse effects on other aspects of human health. Fluoride acts as an antibacterial agent in the mouth giving greater protection against acid attack on teeth, but also stabilises hydroxyapatite by OH to F substitution [238,239,240]. Subsequent studies and reviews revealed that fluoride also affects the human skeletal structure, as high fluoride intakes increase the accretion, resorption and calcium turnover rates of bone tissue affecting the homeostasis of bone mineral metabolism [241]. Fluoride is cytotoxic because of its resemblance to the hydroxy anion. Therefore, fluoride exporters exist for eukaryotic cells [242]. Deletion of the genes of such transporters in yeast yields a mutant that can be contained with fluoride as an inorganic antibiotic [243].
In recent years, a novel potential application of bromoform-rich red seaweeds, especially of the genus Asparagopsis, has emerged: Bromoform is a potent inhibitor of methanogenesis which is attributed to its binding to B12 required in the methyl transferase reaction responsible for the formation of the precursor methyl malonyl-CoA [244]. Asparagopsis has been found to be a major emitter of volatile bromoform [245] and to produce large amounts of this compound for its antimicrobial defence [245,246] with the haloform reaction likely involved in its biosynthesis [247]. With ruminants—chiefly cows and sheep—being major emitters of methane [248], and methane having a global warming potential of at least 25 times that of carbon dioxide [249], there is significant interest to quell this important source of a potent greenhouse gas. As a feed supplement, Asparagopsis effectively suppresses methane production from lactating dairy cows [250], beef steers [251] and sheep [252]. Given the knowledge of the action mechanism of bromoform on methanogenesis [244], it is not surprising that adding Asparagopsis to cattle feed has a near-immediate effect on methane production, before ultimately changing the diversity of the rumen microbiome [253]. Significantly, the effects of bromoform contained in Asparagopsis and, to a lesser extent, also other seaweeds on suppressing methane production could be reproduced in vitro in rumen-like microbial communities cultured in the laboratory [254,255,256,257]. The subject, especially bromoform biosynthesis in Asparagopsis, methanogenesis in ruminants, the status of commercial Asparagopsis cultivation, and the mechanism of inhibition of methane formation in ruminants, has recently been reviewed [258].
3. Algae and Iodine Speciation in the Ocean
Iodide and iodate are the only forms of iodine that are taken up by brown macroalgae [259,260]. Iodate is reduced to iodide at the surface of Ectocarpus cells by cell surface reductases and then transported into these cells as iodide without reentering the bulk solution [261]. This is further supported by samples collected in the Macrocystis kelp forest showing reduced levels of total iodine, iodide and iodate, with iodate levels being by far the most affected [262].
A recent study [261] indicates that growing microalgae do not appear to reduce iodate to iodide. Nevertheless, there are indications that iodate reduction can be caused by the release of cellular reductants accompanying cell senescence towards the end of phytoplankton bloom. Furthermore, support is given to the notion that macroalgae, such as giant kelp (Macrocystis pyrifera), can take up both iodide and iodate from seawater (even though more slowly). The study proposes a mechanism whereby iodate is reduced to iodide at the cell surface by cell surface reductases and is taken up directly as such without reentering the surrounding seawater.
The antioxidant function of iodide (below) is characterised by an efflux of the latter into the surrounding seawater [22,259,263]. Conducting aquarium experiments, Truesdale et al. [260] observed that both Laminaria digitata and Fucus serratus increase iodide levels and that L. digitata also decreases iodate levels in seawater. This is also reflected in the iodide/iodate speciation of the seawater surrounding the forests of the giant kelp Macrocystis pyrifera [264]. When diver-operated syringe sampling on the surface of Macrocystis thalli, instead of boat-based sampling with Niskin bottles is applied, the effects observed are even stronger [261]. Most recently, again using diving-based sampling using syringes, it was shown that this also applies to the Laminaria forests [265] which are typical especially of the rocky infralittoral of the cold-temperate and Arctic North Atlantic [266].
4. Algal Halogen Accumulation, Metabolism, Biochemistry
Studies on the distribution of certain halogens in seaweeds show the equivalent concentrations of halogens in seaweeds are in the order I > F > Br > Cl, whereas in seawater the order of concentrations is the exact opposite, i.e., Cl > Br > F > I [31].
The biochemistry and physiology of iodine in seaweeds has been extensively reviewed [20,23,24,25,267]. The brown alga Laminaria digitata is the strongest accumulator of iodine among all living systems [22,261,268]. It accumulates iodine to more than 30,000 times the concentration found in seawater, representing an average content of 1% of dry weight [269], however >99% of this is in an inorganic state (iodide; [22]).
Even though iodine had been discovered in seaweed ashes, not until the late 19th century did algal iodine metabolism receive any research interest. During this period Eschle [270] investigated the iodine content of Fucus vesiculosus and Laminaria digitata. As early as 1894, Golenkin reported the release of free iodine (detected by a blue stain of starch on paper) by the red alga Bonnemaisonia asparagoides [268]. Several decades later this was confirmed by the studies of Sauvageau [271] on red algae and of Kylin [272] and Dangeard [273] in the 1920s. The latter scientists were the first to report the emission of molecular iodine (I2) from kelp (L. digitata) surfaces, termed “iodovolatilisation”. The rise of nuclear physics and the availability of radioisotopes enabled studies of the uptake mechanism of iodine in brown algae, notably those of Tong and Chaikoff on the Pacific kelp Nereocystis luetkeana [274], of Bailey and Kelly on Ascophyllum nodosum [275], and of Shaw on Laminaria [276,277].
There is a strong geographic bias in studies of iodine metabolism in seaweeds, with the large majority stemming from the North Atlantic and, to a smaller extent, from the Pacific coast of North America [276,278,279] and the Far East. Among the latter region, especially the study by Saenko et al. [235], surveys of a range of red, brown and green algae from the cold-temperate Sea of Japan and Sea of Okhotsk have been performed. In the early 1960s and in one of the first such studies for warm-temperate and tropical seaweeds, Indian scientists reported iodine levels in seaweeds from the coast of Gujarat [280]. Our group recently contributed the first report of iodine and fluorine concentrations in seaweeds of the Arabian Gulf [234], with the iodine levels observed in this study being in a range comparable to those reported from the same genera elsewhere [237,261,268]. There is a conspicuous lack of studies from the Southern Hemisphere.
It was discovered that kelps of the genus Laminaria (Figure 6) accumulate iodide as a unique inorganic antioxidant in its apoplast in order to protect its surface against several aqueous and gaseous oxidants [22]. Upon reaction with ozone, volatile molecular iodine is released—providing the biochemical explanation for “iodovolatilisation”—resulting in aerosol formation and impacting atmospheric processes [22,281]. More recently, molecular iodine emissions were also quantified in Fucalean brown algae [278]. Consistent with the function of an antioxidant protecting the thallus surface [22], iodine accumulation in Laminaria is mostly in the outer, cortical cell layers [279] yet uncertainty remains at present whether the accumulation is intracellular or apoplastic (i.e., in the cell wall). More recently, laser desorption ionization (LDI) and desorption electrospray-ionization techniques (DESI), coupled with mass spectrometry, confirmed the predominance of inorganic I species on the surface of fresh algae, and a peripheral iodine localization when applied on micro-sections. While iodide is the most suitable halide against most reactive oxygen species (ROS, see Section 2.3.1), bromide complements iodide for detoxifying superoxide [282]. The iodide antioxidant system appears to be more widespread among brown and red algae. Key features were detected in the brown algal genome model Ectocarpus [150] and the world’s largest seaweed, Macrocystis pyrifera (giant kelp; [262]). For several of these studies, X-ray absorption spectroscopy (XAS) has become an ideal non-invasive tool to probe the chemical speciation and redox state of bromine and iodine in situ [21,22,153,249,283]. Besides the exploration of the halide antioxidant system [22,282], the technique enabled the detection of different modes of bromine storage in brown and red algae as well as diatoms and dinoflagellates, and the presence of an important intermediate of the haloform reaction [284,285], an indication that the latter is operative in the biosynthesis of bromoform in the red alga Asparagopsis armata [247]. It has also enabled elucidation of the speciation of organobromine and organochlorine compounds in edible seaweeds [286], but also the bonding of chlorine in particulate organic matter in the ocean, showing that it exists primarily in concentrated aliphatic forms consistent with lipid chlorination [283]. When applied in X-ray spectromicroscopic studies, it was shown that the distributions of Clorg and inorganic Cl−(Clinorg) in oak leaf material vary dramatically with decay stage [287] and XAS enabled investigation of enzymatic formation of organochlorines in decaying plant material [288]. In situ X-ray spectroscopy and spectromicroscopy, furthermore, showed that Brorg is ubiquitous throughout diverse sedimentary environments, occurring in correlation with Corg and metals such as Fe, Ca, and Zn [289]. Analysis of sinking particulate carbon from the seawater column links the Brorg observed in sediments to biologically produced Brorg compounds that persist through humification of natural organic matter (NOM) [289].

Figure 6.
Kelps of the genus Laminaria include the strongest iodine accumulators among all living systems. They accumulate iodide as an inorganic antioxidant, impacting atmospheric processes [22] but also iodine speciation in coastal seawater [265]. (Photograph taken by FCK near Sandhaven, Aberdeenshire, Scotland, 25 July 2021, edited by Susan De Goër, Roscoff).
Seaweed polysaccharides and derived oligosaccharides stimulate defence responses and protection against pathogens in seaweeds [290,291,292,293,294,295,296,297,298,299,300]. In the kelp Laminaria digitata, oligoguluronates are a breakdown product of the cell wall following a bacterial attack; H2O2 is produced during the oxidative burst following the elicitation by oligoguluronates [290,301], but also by bacteria-derived lipopolysaccharides [302], prostaglandin A2 [303] as well as polyunsaturated free fatty acids and methyl jasmonate [304]. Palmer et al. reported increased halocarbons and I2 emissions by L. digitata [281]. When subjected to oligoguluronates, the emission of iodinated compounds, i.e., CH2I2 and CH2ClI, seemed stronger than when subjected to H2O2. However, when the seaweed was exposed to H2O2, the emissions of the brominated compounds CHBr3 and CHBr2Cl seemed stronger than the emission of iodinated compounds. Similar trends were also reported by [305] in the red seaweed Meristiella gelidium [306]. Increased amounts of halogenated compounds of up to eight-fold were also produced through the oxidative burst response to agar oligosaccharides in Gracilaria sp. [307]. In the filamentous brown algal model Ectocarpus, infection by the basal oomycete Eurychasma dicksonii [308,309] resulted in an upregulation of vanadium haloperoxidase activity [310], providing supporting evidence for the notion proposed by Wever et al. [311] that HOBr production by this enzyme has a defence function in marine algae.
Tissue-specific differences in the rates of uptake of iodine correlated with haloperoxidase activity, but not with the iodine contents [269]. The state of iodine in the stipe, the meristematic area and the distal blade of the brown macroalga Laminaria digitata (Phaeophyceae) has been investigated by several workers [274,312,313]. The total number of moles of I2 emitted by stipes was approximately 10 times higher than those emitted from other thallus parts.
While we know much less about the halogen metabolism in Macrocystis as compared to Laminaria, Manley [314,315,316,317] reported uptake kinetics of iodide in the range of 8 × 10−3 f mol cm−2 min−1, tissue concentrations of iodide of 0.48 mg g−1 and methyl iodide emissions of around 1–3 ng CH3I g−1 FW h−1. Our own results [262] are also entirely in accord with the hypothesis that the halogen metabolism/biochemistry of Macrocystis is indeed similar to that of Laminaria. Thus, we show that (a) iodide is taken up by Macrocystis in a concentration dependent manner consistent with a facilitated diffusion mechanism; (b) Macrocystis produces an oxidative burst of H2O2 upon exposure to elicitors such as oligoguluronates; (c) it also releases a burst of iodide upon oxidative stress; and (d) iodide and bromide are primarily localised in the apoplast. Finally, since VPO catalysed reactions are thought to be important in halogen emissions, it is significant that a recent transcriptomic analysis of Macrocystis across depth and season showed that VPOs were present and that transcript abundance was 5–10 times higher in the surface exposed tissues vs. those at depth, consistent with its role in an oxidative stress response [318].
Using laboratory incubation studies, Goodwin et al. have determined emission rates of bromoform and dibromomethane from Macrocystis and concluded that this organism would be an important contributor to the global bromine budget [319]. Another in situ bag incubation study at Zuma Beach in Malibu (CA, USA) found release of 20–150 pptv of I2, 20 ppb iodoform, 0.8 ppb methyl iodide and 80 ppb bromoform by Macrocystis blades [320]. It is notable that these emissions occurred even in the absence of ozone in the air. Finally, field studies at Zuma Beach [320] and at Scripps Pier in San Diego (CA, USA) [321], using API-MS and/or LP-DOAS (long path differential optical absorption spectroscopy), found 1–4 pptv I2 in the atmosphere at these locations, which showed no tidal influence but a strong diurnal effect with concentrations very low during mid-day and much higher at night. Both authors conclude that the low steady state concentrations of I2 were consistent with the fast photolysis of I2 and the nocturnal offshore winds that would lead to low I2 even in the presence of large emissions that likely derived from nearby kelp beds. The observation of several ppt of iodine monoxide (IO) and even more iodine dioxide (OIO) were considered proof that atmospheric iodine chemistry is occurring at a significant scale on the southern Californian coast, likely the result of emissions from Macrocystis. Emissions from Macrocystis are likely of global significance. Crude estimates of global emissions of molecular iodine alone by Macrocystis, based on published emission rates from the related kelp Laminaria [22,284,322] and worldwide Macrocystis canopy biomass [323], suggest values in the range of 10–100 Gg(I)/yr. Given the high reactivity of molecular iodine with ozone (far greater than the iodocarbons), such emissions are expected to be of both regional and global atmospheric significance.
Little is known about the biological significance of fluorine in algae. Fluoride possibly increases the growth and metabolic activities of brown seaweeds [324]. On the other hand, it was found that aluminofluoride and beryllofluoride, which are structural analogues of vanadate and phosphate, inhibited apo-bromoperoxidase (a key enzyme in halogen metabolism of brown and red algae; [25]) from the North Atlantic brown alga Ascophyllum nodosum [325]. Haloperoxidases cannot catalyse fluorination reactions because hydrogen peroxide lacks the thermodynamic potential to oxidise fluoride (Figure 1); thus, enzymes catalysing fluorination reactions are not peroxidases, but instead incorporate fluorine by nucleophilic substitution (Section 2.3.5) [322]. At present, it is not clear whether algae of any phylum contain fluorinating enzymes; in fact, the only fluorinase so far known to science is from the bacterium Streptomyces cattleya [137,143]. It should be noted that among all marine organisms investigated so far, anywhere in the world, only one marine-derived Streptomyces xinghaiensis strain has been reported to produce a fluorinated metabolite, the structurally simple fluoroacetate [326,327]. No fluorinated compounds have been reported from the entire breadth of algal diversity, including seaweeds. They are mainly producers of chlorinated, brominated and iodinated compounds due to the presence of vanadium haloperoxidases (e.g., [322,328]). The lack of reports on organofluorine compounds in algae may also be due to traditionally much less investigator effort focusing on algae in the natural products community compared to bacteria, fungi, sponges, etc. Considering that algae also harbor abundant and diverse bacterial communities, especially on their surfaces as biofilms, it would be surprising that there are no fluorinated compounds. Young and Langille [329] found that fluoride content ranges from 3.02 to 18.86, 4.78 to 17.82 and 4.35 to 20.04 mg kg−1 dry weight in green, brown and red algae, respectively. Even though variations in fluoride content are observed in each class of algae, red algae, in general, tend to contain more fluoride compared to brown and green algae. Recently, there has been increasing interest in the potential of dried seaweed biomass for biosorption of fluoride from fluoride-contaminated water. In this context, it was found that dried Padina sp. (Phaeophyta) from the Red Sea [330] and Gracilaria sp. (Rhodophyta) from the Bay of Bengal [331] could be applied as eco-friendly biosorbents for fluoride. Our recent study has surveyed seaweeds of the Kuwaiti coast (Arabian/Persian Gulf) for iodine and fluorine levels [234].
5. Algal Halogenated Natural Products
Besides simple halogenated alkanes and iodinated tyrosines, marine macroalgae produce halogenated fatty acids, oxylipins and a variety of halogenated secondary metabolites, including halogenated terpenoids, indoles, acetogenins, naphthalene derivatives and phenols. The halogenated fatty acids and oxylipins are though rare in macroalgae. Halogenated fatty acids (3-bromo-2-heptanoic acids and 3-bromo-2-nonanoic acids) have been found in red alga of genus Bonnemaisonia [332], while halogenated oxylipins have only been reported in brown algae. The chlorinated oxylipins egregiachlorides A, B and C were found in Egregia menziesii [333], and eiseniachlorides A, B and C, and iodinated oxylipins eiseniaiodides A, B, in Eisenia bicyclis [334].
Most of the halogenated secondary metabolites (mentioned above) are found in red and brown algae, and fewer in green algae 411. In a recent review focussing on the distribution, natural occurrence, and biological properties of halogenated aromatic secondary compounds from macroalgae, 89% of the metabolites were reported from red, 9% from brown and 2% from green macroalgae [335]. Most of these metabolites were brominated followed by chlorinated and iodinated. Surprisingly, less than 1% of the secondary metabolites from brown algae contain bromine or chlorine compared with 7% of green algal compounds and 90% of those reported for red algae [336]. In fact, iodination is more frequent in brown algae than in red and green algal metabolites [337]. The selective incorporation of bromine or iodine during the biosynthesis of halogenated metabolites and halogenation degree in different red and brown algae offers an interesting area of further investigation.
The distribution of these halogenated secondary metabolites is not even among red seaweed families, with most of the metabolites being reported from the Rhodomelaceae family, especially from the genera Laurencia, Rhodomela, Symphyocladia, Polysiphonia and Odonthalia [335,338]. The great chemical diversity of halogenated metabolites in especially Laurencia spp. demonstrates the presence of many versatile V-haloperoxidase-mediated biosynthetic pathways [335,338]. Macroalgal halogenated secondary metabolites have been extensively studied and reviewed and providing a detailed account would be far beyond the scope of this paper. For this, the reader is referred to the reviews by Gribble [339,340,341,342,343,344], Cardozo et al. [345], Blunt et al. [346], Cabrita et al. [4], Wang et al. [338] and Jesus et al. [335] for isolation, characterization of halogenated metabolites and their biological activities. Few comprehensive reviews are available for the biosynthetic routes of these organohalogens. However, the review of Paul and Pohnert [149] provides an excellent overview of biosynthesis of volatile halogenated compounds in algae and their significance in algal physiology and ecology. The review by Wang et al. [338] provides a comprehensive description of chemical diversity of 697 halogenated secondary metabolites (mainly including halogenated diterpenes, triterpenes, sesquiterpenes, C15-acetogenins, indoles and phenols) from the Rhodomelaceae family, along with approaches towards their synthesis and biosynthesis, as well as their chemotaxonomic significance, biological activities and potential functions.
The broad suite of halogenated compounds found in, and released from algae, are thought to act as a defence mechanism. They help protect macroalgae from grazing, control bacterial, fungal and microalgal epiphytes, and limit fungal and bacterial infection [149,307]. For example, bromoform, dibromoacetic acid and halogenated methanes are potential antibacterial, antifouling, ichthyotoxic agents in Asparagopsis armata, Ulvella lens and Lithophyllum species [246,347]. Brominated furanones released from the red alga Delisea pulchra interfere with bacterial communication by affecting bacterial mobility and attachment to the algal surface and are also have effective anti-fouling and anti-feedant potential [348,349,350]. Bromophycolides and callophycoic acids, one of the largest group of algal antifungal compounds found in the red alga Callophycus serratus [351] are involved in surface-mediated defence against pathogenic microbes in this macroalga [352]. It has been found that halogenated metabolites, such as brominated terpenoids in Laurencia spp., brominated furanones in D. pulchra, and halogenated heptan-2-ones in Bonnemaisonia hamifera, are localised in specialised gland cells that release these metabolites onto the algal surface [149,353,354,355]. Such gland cells have also been found in A. armata where stalk-like structures connect the gland cells with the outer wall of the pericentral cells that might provide a mechanism to transport halogenated metabolites onto the algal surface [356].
In fact, most of the halogenated secondary metabolites identified from macroalgae are biologically active and exhibit antibacterial, antifungal, anti-viral, anti-inflammatory, antiproliferative, antifouling, antifeedant, cytotoxic, ichthyotoxic and insecticidal properties [4,335,338,343,344]. It is beyond our scope to discuss in detail here the ecological, physiological and biological activities of all the halogenated secondary metabolites isolated from macroalgae to date and thus the above-mentioned reviews are referred to for this context. However, despite the known bioactive potential of these compounds, their biological evaluations have been limited to a few bioassay models. Not much is known about their structure–activity relationships and mode of action. Research in these directions might help in developing a better understanding of their mechanism of action at cellular and molecular levels leading to the discovery of novel analogues or biosynthetic routes that can be utilised in drug discovery [338]. Moreover, further studies on how macroalgal microbionts affect the production of these organohalogen compounds could reveal interesting ecological interactions.
6. Vanadium Haloperoxidases
Although the previously mentioned (Section 2.3.2 and Section 2.4.2) heme peroxidases also occur in microalgae [357], the vanadium-dependent haloperoxidases (vHPOs) are key enzymes in algal halogen metabolism. The subject has been reviewed extensively [24,25,82,358,359,360,361,362,363,364,365,366]. They play a role in iodine uptake [259,269], biosynthesis of halogenated natural products [328,367,368] and can be upregulated upon pathogen attack [310]. As mentioned, haloperoxidases are therefore named after the most electronegative element that they can activate; the chloroperoxidase from the fungus Curvularia inaequalis, which was the first vanadate haloperoxidase to have its structure elucidated [369], will react with chloride, bromide, and iodide, whereas the brown alga Laminaria digitata contains a bromoperoxidase, active on bromide and iodide, as well as an iodoperoxidase, specific for iodide [370]. vBPO was the first vHPO isolated and characterised from the brown alga Ascophyllum nodosum [371,372,373] and, interestingly, it was found to brominate its own tyrosine residues [374], in line with the electrophilic nature of the halogenating intermediate, HOBr. Not only activated aromatic rings, such as those of tyrosine, or the pyrrole ring of indole in tryptophan, are susceptible to electrophilic attack, but also the C=C bond in biomolecules, such as terpenes, which gives a bromonium ion that can give rise to a variety of brominated cyclisation products [328,367,368], and the enol tautomer of a ketone, which can undergo multiple halogenations and produces polyhalomethanes upon hydrolysis of the halogenated ketone [247,284]. In the last four decades, vHPOs have been characterised from various cyanobacteria, as well as red and brown algae [81,370,375,376,377,378,379,380,381,382,383,384]. vHPOs have been reported from green algae [385] but this is not confirmed. These enzymes have the same conservative metal ion binding sites; the histidine residues, the region for vanadium ions, and amino acid series have a high degree of homology in terms of gene structure [386]. A vanadium-containing iodoperoxidase has been identified in the marine microorganism Zobellia galactanivorans [387]. Recent bioinformatics and phylogenetic analyses of genomic and transcriptomic sequencing data of algal and fungal vHPOs have revealed that they are likely monophyletic and of bacterial origin, yet with two distinct clades, one comprising cyanobacterial, brown and red algal enzymes, and another one comprising bacterial enzymes. vHPOs are phylogenetically derived from bacterial acid phosphatases [82,387].
7. Algal Halogen Sources to the Atmosphere
Over the past 50 years, it has become clear that many organisms have the ability to produce natural organohalogen compounds, and the reported diversity of this class of compounds has grown from a dozen in 1954 to more than 5000 compounds by 2014 [388]. These naturally halogenated compounds are formed by living organisms, such as marine algae [4,389,390,391,392,393], fungi [394,395], bacteria, sponges, lichens, higher plants, insects, and mammals, which has been reviewed [341,342,396].
Seaweeds have been identified as major emitters of organic and inorganic iodine and of organic bromine to the coastal atmosphere in different parts of the world—in particular, the cold-temperate North Atlantic. Nevertheless, a strong sampling bias and major uncertainties remain, both with regard to the regions, and the species studied. In particular, a major knowledge gap exists along the coasts of the world that are dominated by giant kelp (Macrocystis pyrifera) forests (Pacific North America from Baja California to SE Alaska, Peru, Chile, Argentina, and Sub-Antarctic Islands) which have a far greater geographic extent and seaweed standing stock than the coasts dominated by the much-studied Laminaria digitata. Furthermore, unlike Laminaria, a large portion of giant kelp biomass occurs floating at the sea surface, thereby directly exposing its blades to the atmosphere and thus potentially increasing the air-sea flux of kelp-derived products.
Production of halocarbons by seaweeds was first discovered by Lovelock [397]. A plethora of studies have since detected halocarbon production in both microalgae [398] and seaweeds 249. The haloperoxidase-mediated production of hypobromous acid (HOBr) by seaweeds has been considered a defence reaction which may impact the biosphere [311]. Many macroalgae produce halocarbons as defence mechanisms, chiefly the oxidative burst, which are activated against mechanical and chemical stress [248,284,399,400]. Oligoagars trigger an oxidative burst and rapid increases in respiration and halogenating activity when agar, agarose, or the agarose degradation products, neoagarotetraose and neoagarohexaose, were added to the growth medium [294,307], while similarly, in the brown alga Laminaria, oligoalginates enhance the production of iodinated and brominated halocarbons [281].
Marine algae (phytoplankton and seaweeds) are important intermediaries in the chemical transformation of seawater-dissolved halides to volatile halocarbons. Different levels of CHBr3, CH2Br2, CHBr2Cl, and CH3I have been observed in seaweed beds. More than 1140 metabolites have been reported in the Phaeophyceae in 2007 [401]. The red algal genus Laurencia (Rhodomelaceae, Ceramiales), the most prolific synthesiser of secondary halogenated metabolites, has been reported to produce numerous, diverse, unique compounds [402]. The uptake of radio-iodide (131I) has previously been demonstrated in several species of seaweed, including Laminaria digitata (mentioned as L. flexicaulis, [403]), Ascophyllum nodosum [404,405], Nereocystis luetkeana [274] and Fucus ceranoides [313].
The natural environment—such as light intensity, desiccation, tissue, wounding, or grazing—can influence the emission of halogenated compounds by seaweeds [406], but also the uptake and release of iodine from seawater (recently reviewed by Küpper and Carrano, 2019).
Rates of halocarbon production may vary considerably based on species—for example, the diatom Amphora sp. had a higher range of CH3I emission rates than the cyanobacterium Synechococcus sp. and the chlorophyte Parachlorella sp. [407]—but also depending on geographical location. Although tropospheric halogenated compounds are well studied in coastal environments, the presence of halogens above the open ocean environment has not been widely reported. Marine macroalgae are thought to be the dominant source of CH3I in the coastal ocean [247,401,408,409,410]. In contrast, in the open ocean, there is proof of several production pathways: production through photochemical degradation of organic matter [399,411], production by marine biota [151,400,412], or substitution when dust contacts seawater containing iodide or when marine water vapour condenses on dust containing iodide [413].
It is evident that most studies investigating halocarbon emission by seaweeds were conducted on temperate and some polar seaweed species with just a few on tropical species. In the polar regions, there have been reports of high concentrations of bromoform in sea ice and snowpack which originate from biogenic production by ice algae [414]. Brown seaweeds from the temperate region, including Laminariales and Fucales, release large amounts of iodinated compounds [245]. It has been reported that brown seaweeds, namely Sargassum binderi, Padina australis, and Turbinaria conoides, which were dominant in a tropical coral reef, emitted various volatile halogenated [415].
The way irradiation by sunlight affects the emission of halocarbons by seaweeds could be caused by changes in photosynthetic activity [385,391,406,408,416,417]. Several studies found increased halocarbon emissions by seaweeds during higher irradiances [245,385]. Correlations were observed between emission of bromo-and iodinated compounds with photosynthetic activity [415]. In several incubation experiments, production of polyhalogenated compounds of the green seaweed Ulva lactuca was found to be three times higher in the light compared to the dark [385]. Additionally, Keng et al. [415] found that increase in irradiance resulted in significant changes in the emission rates of CHBr3, CH2Br2, CH2BrI and CH2I2 by T. conoides. It has been suggested [409,410,418,419] that the algal emissions of VHC into the Arctic atmosphere may be related to the high irradiance during spring and early summer.
However, some other investigations do not suggest that high irradiance levels cause higher halocarbon emissions by algae. Porphyridium purpureum cultures (a unicellular Rhodophyte) were used as a model to investigate the light stress in the release of volatile halogenated organic compounds, such as CH3I. The results from Porphyridium purpureum culture samples show no increase in the CH3I release rate in the high light-exposed cultures relative to the lower light controls [420]. In agreement with this study, Hughes et al. [421] concluded that light-induced stress did not induce iodocarbon release in any of the three species of marine phytoplankton from different algal classes: the prymnesiophyte Emiliania huxleyi, the prasinophyte Tetraselmis sp., and the diatom Thalassiosira pseudonana. In a study with two cold-water marine diatoms (Thalassiosira antarctica and Porosira glacialis), a series of laboratory experiments was performed to investigate the link between the availability of photosynthetically active radiation (PAR) and brominating activity, as measured by the bromination of phenol red [422]. Consistent with this, no link was found in the halocarbon emission rate and photosynthesis by the four tropical seaweeds, Kappaphycus alvarezii (Doty) Doty ex P.C. Silva, Sargassum binderi Sonder ex J. Agardh (syn. S. aquifolium (Turner) C. Agardh), Sargassum siliquosum J. Agardh and Turbinaria conoides (J. Agardh) Kützing [423]. However, there was a correlation between percentage decrease in the maximum quantum yield of photosynthesis (Fv/Fm) and halocarbon emission rates, which was significant only for CH2BrCl emission by Padina australis Hauck.
The effect of temperature on halocarbon emissions by seaweeds was also observed over the years. Abrahamsson et al. [424] concluded that the production of certain halocarbons may increase with temperature in certain species of brackish-water algae. For Arctic macroalgae, halogenating activity has been found to increase with temperature for the brown macroalga Laminaria saccharina (L.) [425]. Various species of Antarctic macroalgae showed approximately 10 to 50-fold lower emission of methyl halides compared to temperate macroalgae [426]. A hotspot of iodine emissions from sea ice in the Weddell Sea is ascribed to diatoms [427].
The effect of pH on halocarbon emission by seaweeds was shown to be species-specific. The highest percentage changes in emissions for the halocarbons of interest were observed at the lower pH levels of 7.2 and 7.4, especially in Padina australis and Sargassum spp., compared to Kappaphycus alvarezii and Turbinaria conoides [423]. Ethyl bromide production from the red alga Endocladia muricata was maximal in the pH range of 7.5–7.6. At pH values higher than 7–6 the activity decreased rapidly and no methyl bromide production could be detected at pH 9.2 [416].
The effect of pH on halocarbon emission by seaweeds was also shown to be compound specific. Keng et al. [415] indicate that the emission of specific halocarbons is possibly enhanced under lowered pH conditions. For instance, there was a significant negative correlation of CH3I with decrease in seawater pH for three of the species studied; K. alvarezii, P. australis and S. siliquosum.
Natural and anthropogenic emissions of these volatile halocarbons create a pool of atmospheric halogen radicals, providing precursors for cloud condensation nuclei [428].
Uncertainties in global oceanic emissions of halogenated compounds are high, both on the process level and for the global oceanic emission flux. “Top-down” estimates, i.e., calculated from atmospheric abundances, suggest global bromoform emissions of between 5.4–7 Gmol Br yr−1 and for dibromomethane between 0.7–1.4 Gmol Br yr−1 using different atmospheric transport models (e.g., [429,430,431,432]). Global bottom-up emissions estimated by extrapolating oceanic and atmospheric in situ measurements are between 2.8–10.3 Gmol Br yr−1 for CHBr3 and between 0.8–3.5 Gmol Br yr−1 for CH2Br2 (e.g., [433,434,435,436]). CH3I is a ubiquitous compound which is mainly emitted from the ocean and is the dominant gaseous organic iodine species in the troposphere [437,438], although more reactive iodine compounds (in an atmospheric sense, since they form more stable radicals because the methyl iodide is a strong alkylating (methylating) reagent, whereas the second halogen diminishes the susceptibility of the first in a nucleophilic substitution reaction) such as CH2ICl and CH2I2 are important sources of iodine to the Marine Boundary Layer (MBL) [52]. A top-down estimate of annual ocean CH3I emissions [439] is 191 Gg (I) year −1, falling within an earlier estimate of 114–320 Gg (I)/year. However, field data covering seven cruises across the Atlantic, Pacific, and Southern Oceans suggest a total oceanic source more than a factor of two higher at ∼550 Gg (I)/year [433].
A major drawback of both top-down and bottom-up approaches is that they do not capture coastal emissions well. In the top-down case this is due to the coarse resolution of such models. In contrast, the bottom-up approaches rely on subdividing the ocean into either the main basins or into near shore, shelf and open ocean regions, and then apply a coarse data extrapolation. Data show at least a 100-fold difference in surface seawater concentrations (and sea-air fluxes) of CHBr3 between very shallow kelp-rich coastlines and the open ocean. This is highly significant considering that coastal waters with a depth up to 180 m represent 7.5% of the world ocean area [440]. Differences between and uncertainties in global model emission estimates are thus highly dependent upon the approach used to capture coastal emissions, although this remains largely unrecognised.
It is well established that coastal regions contain elevated concentrations of VSLH in both air and water, especially those close to macroalgal forests and around islands [245,435,436]. In the case of organobromine species, a number of incubation and direct field measurements suggest that algae are most likely the largest source of CHBr3 and CH2Br2, comprising between 25–80% [433,434,435,441] of the global inventory. In coastal regions macroalgae (seaweeds) are the major contributors, while phytoplankton are a possible source in open ocean regimes. The situation vis-à-vis iodine and iodinated hydrocarbons is less clear. It was originally thought that biologically produced CH3I and CH2I2 would be the most important atmospherically active volatile iodine containing species. However, it has been subsequently shown that, in fact, inorganic iodine emissions dominate over the organoiodine species in coastal regions, where I2 is believed to be the major species, and open ocean regions, where HOI dominates [281,442,443]. In addition, it has been shown that both abiotic as well as biotic sources may be important. Abiotic emission of I2 and HOI occurs at both the air-sea interface and possibly in sea-spray aerosols via reaction of dissolved iodide with ozone [179,180]. These reactive iodine species are also emitted via biological processes, either by (a) reaction of I− effluxed by stressed algae with ozone or other reactive oxygen species (ROS) and/or (b) via vanadium-dependent haloperoxidase (VPO) catalysed reactions of I− with H2O2. There is overwhelming evidence that in coastal environments the biological reactions mediated by macroalgae are the dominant source of these emissions while the abiotic and/or biological reactions involving phytoplankton are most important in the open ocean.
8. Seaweeds as Bioindicators for Radioactive Iodine
Since marine algae can selectively concentrate many inorganic elements, including iodine, they are being used as bioindicators to monitor the level of environmental pollution and for decontamination of some toxic elements or radionuclides in wastewaters [444,445,446]. Californian giant kelp (M. pyrifera) has been used as a natural dosimeter for detecting radioactive contamination from the Fukushima accident in Japan [447]. More recently, secondary ion mass spectrometry (SIMS) nanoprobe analyses have detected both 127I and 129I in L. digitata collected near the nuclear reprocessing plant at Le Hague, France [448].
9. General Conclusions
Halogens interact with the Earth’s geo-, bio-, hydro- and atmosphere through their biogeochemical cycle, in which marine algae have a key role at the interface between the Ocean, the atmosphere and land. Halogens have important roles in atmospheric processes and how the latter are impacted by human activities, but also in human physiology and medicine. Certain algae accumulate high levels of iodine and bromine, rendering them suitable as biomonitors of halogen radionuclides; they possess vanadium haloperoxidases as key enzymes in halogen metabolism and produce a plethora of halogenated natural products.
Author Contributions
The Abstract was written by H.A.-A. and F.C.K.; Section 1 was written by D.A.-B.; Section 2.1 was written by M.C.F., H.A.-A. and F.C.K.; Section 2.2 was written by H.A.-A., F.C.K., L.J.C. and M.C.F.; Section 2.3.1 was written by M.C.F., F.C.K., L.J.C. and H.A.-A.; Section 2.3.2, Section 2.3.3, Section 2.3.4 and Section 2.3.5 were written by M.C.F.; Section 2.4.1 was written by H.A.-A., M.C.F. and F.C.K.; Section 2.4.2 was written by H.A.-A. and F.C.K.; Section 3 was written by H.A.-A., C.J.C. and F.C.K.; Section 4 was written by P.K., C.J.C. and F.C.K.; Section 5 was written by P.K., F.C.K. and M.C.F.; Section 6 was written by M.C.F., P.K. and F.C.K.; Section 7 was written by L.J.C., C.J.C., F.C.K. and H.A.-A.; Section 8 was written by H.A.-A. and F.C.K.; F.C.K. and D.A.-B. provided general mentoring of H.A.-A.’s PhD and reviewed this text. All authors have read and agreed to the published version of the manuscript.
Funding
We are grateful to the Kuwait Institute for Scientific Research (KISR) for PhD funding for Hanan Al-Adilah and to the European Commission for a Marie Curie International Incoming Fellowship (Horizon 2020 Research and Innovation Programme of the European Union under the Marie Skłodowska-Curie grant agreement No 839151) to Puja Kumari. We would equally like to thank the UK Natural Environment Research Council for their support to FCK (program Oceans 2025—WP 4.5 and grants NE/D521522/1 and NE/J023094/1) and LJC (grant NE/N009983/1). This work also received support from the Marine Alliance for Science and Technology for Scotland pooling initiative. MASTS is funded by the Scottish Funding Council (grant reference HR09011) and contributing institutions. MCF, FCK and HAA thank the Lorentz Center (funded by the Netherlands Organization for Scientific Research, NWO, and the University of Leiden) for the organization of the workshop ‘IODINE: Biogeochemical Cycle of Iodine and Human Health’ (4–6 October 2017) which partly inspired this review. LJC acknowledges funding from the European Research Council (ERC) under the European Union’s Horizon 2020 programme (project O3-SML; grant agreement no. 833290).
Acknowledgments
We are grateful to the Kuwait Institute for Scientific Research (KISR) for Ph.D. funding for H.A.-A. and to the European Commission for a Marie Curie International Incoming Fellowship (Horizon 2020 Research and Innovation Programme of the European Union under the Marie Skłodowska-Curie grant agreement No 839151) to P.K. We would equally like to thank the UK Natural Environment Research Council for their support to F.C.K. (program Oceans 2025—WP 4.5 and grants NE/D521522/1 and NE/J023094/1) and L.J.C. (grant NE/N009983/1). This work also received support from the Marine Alliance for Science and Technology for Scotland pooling initiative. MASTS is funded by the Scottish Funding Council (grant reference HR09011) and contributing institutions. M.C.F., F.C.K. and H.A.-A. thank the Lorentz Center (funded by The Netherlands Organization for Scientific Research, NWO, and the University of Leiden) for the organization of the workshop ‘IODINE: Biogeochemical Cycle of Iodine and Human Health’ (4–6 October 2017) which partly inspired this review. L.J.C. acknowledges funding from the European Research Council (ERC) under the European Union’s Horizon 2020 programme (project O3-SML; grant agreement no. 833290).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Carr, M.E.; Friedrichs, M.A.M.; Schmeltz, M.; Aita, M.N.; Antoine, D.; Arrigo, K.R.; Asanuma, I.; Aumont, O.; Barber, R.; Behrenfeld, M.; et al. A comparison of global estimates of marine primary production from ocean color. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2006, 53, 741–770. [Google Scholar] [CrossRef]
- Huang, Y.; Nicholson, D.; Huang, B.; Cassar, N. Global Estimates of Marine Gross Primary Production Based on Machine Learning Upscaling of Field Observations. Glob. Biogeochem. Cycles 2021, 35, e2020GB006718. [Google Scholar] [CrossRef]
- Keller, M.D.; Bellows, W.K.; Guillard, R.R.L. Dimethyl sulfide production in marine phytoplankton. ACS Symp. Ser. 1989, 393, 167–182. [Google Scholar]
- Cabrita, M.T.; Vale, C.; Rauter, A.P. Halogenated compounds from marine algae. Mar. Drugs 2010, 8, 2301–2317. [Google Scholar] [CrossRef]
- Brodie, J.; Zuccarello, G.C. Systematics of the species rich algae: Red algal classification, phylogeny and speciation. In Reconstructing the Tree of Life: Taxonomy and Systematics of Species Rich Taxa; Hodkinson, T.R., Parnell, J.A.N., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 324–334. [Google Scholar]
- Guiry, M.D.; Guiry, G.M. AlgaeBase; World-Wide Electronic Publication; National University of Ireland: Galway, Ireland, 2021; Available online: http://www.algaebase.org (accessed on 1 December 2021).
- Van den Hoek, C.; Mann, D.G.; Jahns, H.M. Algae; Cambridge University Press: Cambridge, MA, USA, 1997. [Google Scholar]
- Moon-van der Staay, S.Y.; De Wachter, R.; Vaulot, D. Oceanic 18S rDNA sequences from picoplankton reveal unsuspected eukaryotic diversity. Nature 2001, 409, 607–610. [Google Scholar] [CrossRef]
- Baldauf, S.L. The deep roots of eukaryotes. Science 2003, 300, 1703–1706. [Google Scholar] [CrossRef]
- Baldauf, S.L. An overview of the phylogeny and diversity of eukaryotes. J. Syst. Evol. 2008, 46, 263–273. [Google Scholar]
- Krause-Jensen, D.; Lavery, P.; Serrano, O.; Marba, N.; Masque, P.; Duarte, C.M. Sequestration of macroalgal carbon: The elephant in the Blue Carbon room. Biol. Lett. 2018, 14, 20180236. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O.; Northrop, E.; Lubchenco, J. The ocean is key to achieving climate and societal goals. Science 2019, 365, 1372. [Google Scholar] [CrossRef]
- Holbrook, S.J.; Carr, M.H.; Schmitt, R.J.; Coyer, J.A. Effect of giant kelp on local abundance of reef fishes—The importance of ontogenic resource requirements. Bull. Mar. Sci. 1990, 47, 104–114. [Google Scholar]
- Kitada, S.; Nakajima, K.; Hamasaki, K.; Shishidou, H.; Waples, R.S.; Kishino, H. Rigorous monitoring of a large-scale marine stock enhancement program demonstrates the need for comprehensive management of fisheries and nursery habitat. Sci. Rep. 2019, 9, 5290. [Google Scholar] [CrossRef]
- Teagle, H.; Hawkins, S.J.; Moore, P.J.; Smale, D.A. The role of kelp species as biogenic habitat formers in coastal marine ecosystems. J. Exp. Mar. Biol. Ecol. 2017, 492, 81–98. [Google Scholar] [CrossRef]
- Hind, K.R.; Starko, S.; Burt, J.M.; Lemay, M.A.; Salomon, A.K.; Martone, P.T. Trophic control of cryptic coralline algal diversity. Proc. Natl. Acad. Sci. USA 2019, 116, 15080–15085. [Google Scholar] [CrossRef]
- Hurd, C.; Harrison, P.; Bischof, K.; Lobban, C. Seaweed Ecology and Physiology, 2nd ed.; Cambridge University Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Knoll, A.H. The Multiple Origins of Complex Multicellularity. Annu. Rev. Earth Planet. Sci. 2011, 39, 217–239. [Google Scholar] [CrossRef]
- Cock, J.M.; Godfroy, O.; Macaisne, N.; Peters, A.F.; Coelho, S.M. Evolution and regulation of complex life cycles: A brown algal perspective. Curr. Opin Plant Biol. 2014, 17, 1–6. [Google Scholar] [CrossRef]
- Küpper, F.C. Iodine in Seaweed: Two Centuries of Research; Springer: Berlin, Germany, 2015; pp. 591–596. [Google Scholar]
- Küpper, F.C.; Feiters, M.C.; Olofsson, B.; Kaiho, T.; Yanagida, S.; Zimmermann, M.B.; Carpenter, L.J.; Lu, Z.; Jonsson, M.; Kloo, L. Purple fumes: The importance of iodine. Sci. Sch. 2013, 27, 45–53. [Google Scholar]
- Küpper, F.C.; Carpenter, L.J.; McFiggans, G.B.; Palmer, C.J.; Waite, T.J.; Boneberg, E.M.; Woitsch, S.; Weiller, M.; Abela, R.; Grolimund, D.; et al. Iodide accumulation provides kelp with an inorganic antioxidant impacting atmospheric chemistry. Proc. Natl. Acad. Sci. USA 2008, 105, 6954–6958. [Google Scholar] [CrossRef]
- Küpper, F.C.; Carrano, C.J. Key aspects of the iodine metabolism in brown algae: A brief critical review. Metallomics 2019, 11, 756–764. [Google Scholar] [CrossRef]
- Küpper, F.C.; Feiters, M.C.; Olofsson, B.; Kaiho, T.; Yanagida, S.; Zimmermann, M.B.; Carpenter, L.J.; Luther, G.W., III; Lu, Z.; Jonsson, M.; et al. Commemorating two centuries of iodine research: An interdisciplinary overview of current research. Angew. Chem.-Int. Ed. 2011, 50, 11598–11620. [Google Scholar] [CrossRef]
- Küpper, F.C.; Kroneck, P.M.H. Iodine Bioinorganic Chemistry: Physiology, Structures, and Mechanisms. In Iodine Chemistry and Applications; Kaiho, T., Ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2015; pp. 557–589. [Google Scholar]
- Pröschold, T.; Leliaert, F. Systematics of the Green Algae: Conflict of Classic and Modern Approaches. Syst. Assoc. Spec. Vol. 2007, 75, 123. [Google Scholar]
- Thomas, D.N. Seaweeds; The Natural History Museum: London, UK, 2002. [Google Scholar]
- Downs, A.J.; Adams, C.J. The Chemistry of Chlorine, Bromine, Iodine and Astatine: Pergamon Texts in Inorganic Chemistry; Elsevier: Amsterdam, The Netherlands, 2017; Volume 7. [Google Scholar]
- Rao, C.K.; Singbal, S. Seasonal Variations in Halides in Marine Brown Algae from PORBANDAR and Okha Coasts (NW Coast of India); CSIR–NIScPR: Delhi, India, 1995; Volume 24, pp. 137–141. [Google Scholar]
- Truesdale, V.W.; Luther, G.W.; Canosamas, C. Molecular-Iodine Reduction In Seawater—An improved rate-equation considering organic compounds. Mar. Chem. 1995, 48, 143–150. [Google Scholar] [CrossRef]
- Rao, C.K.; Indusekhar, V. Distribution of Certain Cations and Anions in Seaweeds and Seawater of Saurashtra Coast and Their Geochemical Significance; CSIR–NIScPR: Delhi, India, 1989. [Google Scholar]
- Pekov, I.V.; Lykova, I.S.; Bryzgalov, I.A.; Ksenofontov, D.A.; Zyryanova, L.A.; Litvinov, N.D. Uniquely high-grade iodide mineralization in the oxidation zone of the Rubtsovskoe base-metal deposit, Northwest Altai, Russia. Geol. Ore Depos. 2011, 53, 683–698. [Google Scholar] [CrossRef]
- Gołębiowska, B.; Pieczka, A.; Rzepa, G.; Matyszkiewicz, J.; Krajewski, M. Iodargyrite from Zalas (Cracow area, Poland) as an indicator of Oligocene–Miocene aridity in Central Europe. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2010, 296, 130–137. [Google Scholar] [CrossRef]
- Reich, M.; Palacios, C.; Alvear, M.; Cameron, E.; Leybourne, M.; Deditius, A. Iodine-rich waters involved in supergene enrichment of the Mantos de la Luna argentiferous copper deposit, Atacama Desert, Chile. Miner. Depos. 2009, 44, 719–722. [Google Scholar] [CrossRef]
- Millsteed, P.W. Marshite-miersite solid solution and iodargyrite from Broken Hill, New South Wales, Australia. Mineral. Mag. 1998, 62, 471–475. [Google Scholar] [CrossRef]
- Lodders, K. Solar system abundances and condensation temperatures of the elements. Astrophys. J. 2003, 591, 1220. [Google Scholar] [CrossRef]
- Davy, H. XXXI. Some experiments and observations on the substances produced in different chemical processes on fluor spar. Phil. Trans. R. Soc. 1814, 104, 74–93. [Google Scholar]
- Wisniak, J. The history of fluorine—from discovery to commodity. Indian J. Chem. Technol. 2002, 9, 363–372. [Google Scholar]
- Banks, R. Isolation of flourine by Moissan: Setting the scene. J. Fluor. Chem. 1986, 33, 3–26. [Google Scholar] [CrossRef]
- TH, Y.T. Relationship between Natural Water Quality and Health; United Nations Educational, Scientific and Cultural Organization: Paris, Frence, 1983. [Google Scholar]
- Kendrick, M.A. Halogens in seawater, marine sediments and the altered oceanic lithosphere. In The Role of Halogens in Terrestrial and Extraterrestrial Geochemical Processes; Springer: Cham, Switzerland, 2018; pp. 591–648. [Google Scholar]
- Weast, R.C.; Lide, D.; Astle, M.; Beyer, W. Handbook of Chemistry and Physics. –1989–1990; CRC Press: Boca Raton, FL, USA, 1989. [Google Scholar]
- Coley, N. Animal chemists and urinary stone. Ambix 1971, 18, 69–93. [Google Scholar] [CrossRef]
- Wisniak, J. The History of Bromine from Discovery to Commodity; NISCAIR-CSIR: New Delhi, India, 2002; pp. 263–271. [Google Scholar]
- Gay-Lussac, J.-L. Sur la combinaison de l’iode avec l’oxygène. Ann. Chim. 1813, 88, 319–321. [Google Scholar]
- Gay-Lussac, L.-J. Sur un nouvel acide formé avec la substance découverte par M. Courtois. Ann. Chim. 1813, 88, 311–318. [Google Scholar]
- Chance, R.J.; Tinel, L.; Sherwen, T.; Baker, A.R.; Bell, T.; Brindle, J.; Campos, M.L.A.M.; Croot, P.; Ducklow, H.; Peng, H.; et al. Global sea-surface iodide observations, 1967–2018. Sci. Data 2019, 6, 286. [Google Scholar] [CrossRef]
- Venturi, S.; Donati, F.M.; Venturi, A.; Venturi, M. Environmental iodine deficiency: A challenge to the evolution of terrestrial life? Thyroid 2000, 10, 727–729. [Google Scholar] [CrossRef] [PubMed]
- Oganessian, Y.T.; Abdullin, F.S.; Bailey, P.D.; Benker, D.E.; Bennett, M.E.; Dmitriev, S.N.; Ezold, J.; Hamilton, J.H.; Henderson, R.A.; Itkis, M.G.; et al. Synthesis of a New Element with Atomic NumberZ=117. Phys. Rev. Lett. 2010, 104, 142502. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.T. The marine geochemistry of iodine. Rev. Aquat. Sci. 1991, 4, 45–73. [Google Scholar]
- Moyers, J.L.; Duce, R.A. Gaseous and particulate iodine in the marine atmosphere. J. Geophys. Res. Earth Surf. 1972, 77, 5229–5238. [Google Scholar] [CrossRef]
- Jones, C.E.; Hornsby, K.E.; Sommariva, R.; Dunk, R.M.; Mc Figgans, G.; Von Glasow, R.; Carpenter, L.J. Quantifying the contribution of marine organic gases to atmospheric iodine. Geophys. Res. Lett. 2010, 37, L18804. [Google Scholar] [CrossRef]
- Hoffmann, T.; O’Dowd, C.D.; Seinfeld, J.H. Iodine oxide homogeneous nucleation: An explanation for coastal new particle production. Geophys. Res. Lett. 2001, 28, 1949–1952. [Google Scholar] [CrossRef]
- Saiz-Lopez, A.; Shillito, J.A.; Coe, H.; Plane, J.M.C. Measurements and modelling of I2, IO, OIO, BrO and NO3 in the mid-latitude marine boundary layer. Atmos. Chem. Phys. 2003, 6, 1513–1528. [Google Scholar] [CrossRef]
- He, X.-C.; Tham, Y.J.; Dada, L.; Wang, M.; Finkenzeller, H.; Stolzenburg, D.; Iyer, S.; Simon, M.; Kürten, A.; Shen, J.; et al. Role of iodine oxoacids in atmospheric aerosol nucleation. Science 2021, 371, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.C.G.; Lewis, T.R.; Blitz, M.A.; Plane, J.M.C.; Kumar, M.; Francisco, J.S.; Saiz-Lopez, A. A gas-to-particle conversion mechanism helps to explain atmospheric particle formation through clustering of iodine oxides. Nat. Commun. 2020, 11, 4521. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, D. The distribution and transformations of iodine in the environment. Environ. Int. 1984, 10, 321–339. [Google Scholar] [CrossRef]
- Moreda-Piñeiro, A.; Romarís-Hortas, V.; Bermejo-Barrera, P. A review on iodine speciation for environmental, biological and nutrition fields. J. Anal. At. Spectrom. 2011, 26, 2107–2152. [Google Scholar] [CrossRef]
- Bowley, H.E. Iodine Dynamics in the Terrestrial Environment. Ph.D. Thesis, University of Nottingham, Nottingham, UK, 2013. [Google Scholar]
- Saunders, R.W.; Kumar, R.; Macdonald, S.M.; Plane, J.M.C. Insights into the Photochemical Transformation of Iodine in Aqueous Systems: Humic Acid Photosensitized Reduction of Iodate. Environ. Sci. Technol. 2012, 46, 11854–11861. [Google Scholar] [CrossRef]
- Fuge, R.; Johnson, C.C. Iodine and human health, the role of environmental geochemistry and diet, a review. Appl. Geochem. 2015, 63, 282–302. [Google Scholar] [CrossRef]
- Von Glasow, R.; Hughes, C. Biogeochemical Cycles: Bromine. Encycl. Atmos. Sci. 2014, 42, 194–200. [Google Scholar]
- Saiz-Lopez, A.; von Glasow, R. Reactive halogen chemistry in the troposphere. Chem. Soc. Rev. 2012, 41, 6448–6472. [Google Scholar] [CrossRef]
- Öberg, G. The natural chlorine cycle—Fitting the scattered pieces. Appl. Microbiol. Biotechnol. 2002, 58, 565–581. [Google Scholar] [CrossRef]
- Graedel, T.E.; Keene, W.C. The Budget and Cycle of Earth’s Natural Chlorine. Pure Appl. Chem. 1996, 68, 1689–1697. [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Klein, E.M.; Vengosh, A. Global Biogeochemical Cycle of Fluorine. Glob. Biogeochem. Cycles 2020, 34, e2020GB006722. [Google Scholar] [CrossRef]
- Barnum, T.P.; Cheng, Y.; Hill, K.A.; Lucas, L.N.; Carlson, H.K.; Coates, J.D. Identification of a parasitic symbiosis between respiratory metabolisms in the biogeochemical chlorine cycle. ISME J. 2020, 14, 1194–1206. [Google Scholar] [CrossRef] [PubMed]
- Eychenne, R.; Chérel, M.; Haddad, F.; Guérard, F.; Gestin, J.-F. Overview of the Most Promising Radionuclides for Targeted Alpha Therapy: The “Hopeful Eight”. Pharmaceutics 2021, 13, 906. [Google Scholar] [CrossRef] [PubMed]
- Fraústo da Silva, J.J.R.; Williams, R.J.P. The Biological Chemistry of the Elements—The Inorganic Chemistry of Life; Clarendon Press: Oxford, UK, 1991. [Google Scholar]
- Youngblut, M.D.; Wang, O.; Barnum, T.P.; Coates, J.D. (Per)chlorate in Biology on Earth and Beyond. Annu. Rev. Microbiol. 2016, 70, 435–457. [Google Scholar] [CrossRef]
- Ingraham, L.L.; Meyer, D.L. Biochemistry of Dioxygen. In Biochemistry of the Elements; Frieden, E., Ed.; Plenum Press: New York, NY, USA, 1985; Volume 4. [Google Scholar]
- Villafañe, F. Where Is Ozone in the Frost Diagram? J. Chem. Educ. 2009, 86, 432. [Google Scholar] [CrossRef][Green Version]
- Lu, Z.; Jenkyns, H.; Rickaby, R.E. Iodine to calcium ratios in marine carbonate as a paleo-redox proxy during oceanic anoxic events. Geology 2010, 38, 1107–1110. [Google Scholar] [CrossRef]
- Lu, W.; Ridgwell, A.; Thomas, E.; Hardisty, D.S.; Luo, G.; Algeo, T.J.; Saltzman, M.R.; Gill, B.C.; Shen, Y.; Ling, H.-F.; et al. Late inception of a resiliently oxygenated upper ocean. Science 2018, 361, 174–177. [Google Scholar] [CrossRef]
- Lu, Z.; Lu, W.; Rickaby, R.E.M.; Thomas, E. Earth History of Oxygen and the iprOxy; Cambridge University Press: Cambridge, UK, 2020. [Google Scholar]
- Gkotsi, D.S.; Dhaliwal, J.; McLachlan, M.M.; Mulholand, K.R.; Goss, R.J. Halogenases: Powerful tools for biocatalysis (mechanisms applications and scope). Curr. Opin. Chem. Biol. 2018, 43, 119–126. [Google Scholar] [CrossRef]
- Ludewig, H.; Molyneux, S.; Ferrinho, S.; Guo, K.; Lynch, R.; Gkotsi, D.S.; Goss, R.J. Halogenases: Structures and functions. Curr. Opin. Struct. Biol. 2020, 65, 51–60. [Google Scholar] [CrossRef]
- Shaw, P.D.; Hager, L.P. Biological Chlorination. IV. Peroxidative Nature of Enzymatic Chlorination1. J. Am. Chem. Soc. 1959, 81, 6527–6528. [Google Scholar]
- Sundaramoorthy, M.; Terner, J.; Poulos, T.L. The crystal structure of chloroperoxidase: A heme peroxidase–cytochrome P450 functional hybrid. Structure 1995, 3, 1367–1378. [Google Scholar] [CrossRef]
- Loughran, N.B.; O’Connor, B.; Ó’Fágáin, C.; O’Connell, M.J. The phylogeny of the mammalian heme peroxidases and the evolution of their diverse functions. BMC Evol. Biol. 2008, 8, 101. [Google Scholar] [CrossRef] [PubMed]
- de Boer, E.; van Kooyk, Y.; Tromp, M.; Plat, H.; Wever, R. Bromoperoxidase from Ascophyllum nodosum: A novel class of enzymes containing vanadium as a prosthetic group? Biochim. Biophys. Acta (BBA) Protein Struct. Mol. Enzym. 1986, 869, 48–53. [Google Scholar] [CrossRef]
- Leblanc, C.; Vilter, H.; Fournier, J.-B.; Delage, L.; Potin, P.; Rebuffet, E.; Michel, G.; Solari, P.; Feiters, M.; Czjzek, M. Vanadium haloperoxidases: From the discovery 30 years ago to X-ray crystallographic and V K-edge absorption spectroscopic studies. Co-ord. Chem. Rev. 2015, 301, 134–146. [Google Scholar] [CrossRef]
- Held, A.M.; Halko, D.J.; Hurst, J.K. Mechanisms of chlorine oxidation of hydrogen peroxide. J. Am. Chem. Soc. 1978, 100, 5732–5740. [Google Scholar] [CrossRef]
- Kanofsky, J.R.; Hoogland, H.; Wever, R.; Weiss, S.J. Singlet oxygen production by human eosinophils. J. Biol. Chem. 1988, 263, 9692–9696. [Google Scholar] [CrossRef]
- Everett, R.R.; Butler, A. Bromide-assisted hydrogen peroxide disproportionation catalyzed by vanadium bromoperoxidase: Absence of direct catalase activity and implications for the catalytic mechanism. Inorg. Chem. 1989, 28, 393–395. [Google Scholar] [CrossRef]
- Wiesner, W.; van Pée, K.H.; Lingens, F. Purification and characterization of a novel bacterial non-heme chloroperoxidase from Pseudomonas pyrrocinia. J. Biol. Chem. 1988, 263, 13725–13732. [Google Scholar] [CrossRef]
- Hecht, H.; Sobek, H.; Haag, T.; Pfeifer, O.; Van Pée, K.-H.; Van Pée, K.-H. The metal-ion-free oxidoreductase from Streptomyces aureofaciens has an α/β hydrolase fold. Nat. Genet. 1994, 1, 532–537. [Google Scholar] [CrossRef]
- China, H.; Okada, Y.; Ogino, H. Production mechanism of active species on the oxidative bromination following perhydrolase activity. J. Phys. Org. Chem. 2016, 29, 84–91. [Google Scholar] [CrossRef]
- Kirk, O.; Conrad, L.S. Metal-Free Haloperoxidases: Fact or Artifact? Angew. Chem. Int. Ed. Engl. 1999, 38, 977–979. [Google Scholar] [CrossRef]
- Schofield, R.M.S.; Bailey, J.; Coon, J.J.; Devaraj, A.; Garrett, R.W.; Goggans, M.S.; Hebner, M.G.; Lee, B.S.; Lee, D.; Lovern, N.; et al. The homogenous alternative to biomineralization: Zn- and Mn-rich materials enable sharp organismal “tools” that reduce force requirements. Sci. Rep. 2021, 11, 17481. [Google Scholar] [CrossRef] [PubMed]
- Schofield, R.M.S.; Niedbala, J.C.; Nesson, M.H.; Tao, Y.; Shokes, J.E.; Scott, R.A.; Latimer, M.J. Br-rich tips of calcified crab claws are less hard but more fracture resistant: A comparison of mineralized and heavy-element biological materials. J. Struct. Biol. 2009, 166, 272–287. [Google Scholar] [CrossRef] [PubMed]
- Birkedal, H.; Khan, R.K.; Slack, N.; Broomell, C.; Lichtenegger, H.C.; Zok, F.; Stucky, G.D.; Waite, J.H. Halogenated Veneers: Protein Cross-Linking and Halogenation in the Jaws of Nereis, a Marine Polychaete Worm. ChemBioChem 2006, 7, 1392–1399. [Google Scholar] [CrossRef]
- Park, H.B.; Lam, Y.C.; Gaffney, J.P.; Weaver, J.C.; Krivoshik, S.R.; Hamchand, R.; Pieribone, V.; Gruber, D.F.; Crawford, J.M. Bright Green Biofluorescence in Sharks Derives from Bromo-Kynurenine Metabolism. iScience 2019, 19, 1291–1336. [Google Scholar] [CrossRef]
- Arakawa, Y.; Akiyama, Y.; Furukawa, H.; Suda, W.; Amachi, S. Growth Stimulation of Iodide-Oxidizing α-Proteobacteria in Iodide-Rich Environments. Microb. Ecol. 2012, 63, 522–531. [Google Scholar] [CrossRef]
- Suzuki, M.; Eda, Y.; Ohsawa, S.; Kanesaki, Y.; Yoshikawa, H.; Tanaka, K.; Muramatsu, Y.; Yoshikawa, J.; Sato, I.; Fujii, T.; et al. Iodide Oxidation by a Novel Multicopper Oxidase from the Alphaproteobacterium Strain Q-1. Appl. Environ. Microbiol. 2012, 78, 3941–3949. [Google Scholar] [CrossRef]
- Amachi, S.; Muramatsu, Y.; Akiyama, Y.; Miyazaki, K.; Yoshiki, S.; Hanada, S.; Kamagata, Y.; Ban-Nai, T.; Shinoyama, H.; Fujii, T. Isolation of Iodide-Oxidizing Bacteria from Iodide-Rich Natural Gas Brines and Seawaters. Microb. Ecol. 2005, 49, 547–557. [Google Scholar] [CrossRef]
- Gozlan, R.S.; Margalith, P. Iodide Oxidation byPseudomonas iodooxidans. J. Appl. Bacteriol. 1974, 37, 493–499. [Google Scholar] [CrossRef]
- Li, H.-P.; Daniel, B.; Creeley, D.; Grandbois, R.; Zhang, S.; Xu, C.; Ho, Y.-F.; Schwehr, K.A.; Kaplan, D.I.; Santschi, P.H.; et al. Superoxide Production by a Manganese-Oxidizing Bacterium Facilitates Iodide Oxidation. Appl. Environ. Microbiol. 2014, 80, 2693–2699. [Google Scholar] [CrossRef]
- Dairi, T.; Nakano, T.; Aisaka, K.; Katsumata, R.; Hasegawa, M. Cloning and Nucleotide Sequence of the Gene Responsible for Chlorination of Tetracycline. Biosci. Biotechnol. Biochem. 1995, 59, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Keller, S.; Wage, T.; Hohaus, K.; Hölzer, M.; Eichhorn, E.; Van Pée, K.-H. Purification and Partial Characterization of Tryptophan 7-Halogenase (PrnA) from Pseudomonas fluorescens. Angew. Chem. Int. Ed. 2000, 39, 2300–2302. [Google Scholar] [CrossRef]
- Mori, S.; Pang, A.H.; Thamban Chandrika, N.; Garneau-Tsodikova, S.; Tsodikov, O.V. Unusual substrate and halide versatility of phenolic halogenase PltM. Nat. Commun. 2019, 10, 1255. [Google Scholar] [CrossRef] [PubMed]
- Podzelinska, K.; Latimer, R.; Bhattacharya, A.; Vining, L.C.; Zechel, D.L.; Jia, Z. Chloramphenicol biosynthesis: The structure of CmlS, a flavin-dependent halogenase showing a covalent flavin-aspartate bond. J. Mol. Biol. 2010, 397, 316–331. [Google Scholar] [CrossRef]
- Fisher, B.F.; Snodgrass, H.M.; Jones, K.A.; Andorfer, M.C.; Lewis, J.C. Site-Selective C–H Halogenation Using Flavin-Dependent Halogenases Identified via Family-Wide Activity Profiling. ACS Central Sci. 2019, 5, 1844–1856. [Google Scholar] [CrossRef]
- Gkotsi, D.S.; Ludewig, H.; Sharma, S.V.; Connolly, J.A.; Dhaliwal, J.; Wang, Y.; Unsworth, W.P.; Taylor, R.J.K.; McLachlan, M.M.W.; Shanahan, S.; et al. A marine viral halogenase that iodinates diverse substrates. Nat. Chem. 2019, 11, 1091–1097. [Google Scholar] [CrossRef]
- Vaillancourt, F.H.; Yin, J.; Walsh, C.T. SyrB2 in syringomycin E biosynthesis is a nonheme FeII α-ketoglutarate- and O2-dependent halogenase. Proc. Natl. Acad. Sci. USA 2005, 102, 10111–10116. [Google Scholar] [CrossRef]
- Blasiak, L.C.; Vaillancourt, F.H.; Walsh, C.T.; Drennan, C.L. Crystal structure of the non-haem iron halogenase SyrB2 in syringomycin biosynthesis. Nature 2006, 440, 368–371. [Google Scholar] [CrossRef]
- Galonić, D.P.; Barr, E.W.; Walsh, C.T.; Bollinger, J.M.; Krebs, C. Two interconverting Fe(IV) intermediates in aliphatic chlorination by the halogenase CytC3. Nat. Chem. Biol. 2007, 3, 113–116. [Google Scholar] [CrossRef]
- Wong, S.D.; Srnec, M.; Matthews, M.L.; Liu, L.V.; Kwak, Y.; Park, K.; Iii, C.B.B.; Alp, E.E.; Zhao, J.; Yoda, Y.; et al. Elucidation of the Fe(iv)=O intermediate in the catalytic cycle of the halogenase SyrB2. Nature 2013, 499, 320–323. [Google Scholar] [CrossRef]
- Mitchell, A.J.; Zhu, Q.; Maggiolo, A.O.; Ananth, N.R.; Hillwig, M.L.; Liu, X.; Boal, A.K. Structural basis for halogenation by iron- and 2-oxo-glutarate-dependent enzyme WelO5. Nat. Chem. Biol. 2016, 12, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Hillwig, M.L.; Zhu, Q.; Ittiamornkul, K.; Liu, X. Discovery of a Promiscuous Non-Heme Iron Halogenase in Ambiguine Alkaloid Biogenesis: Implication for an Evolvable Enzyme Family for Late-Stage Halogenation of Aliphatic Carbons in Small Molecules. Angew. Chem. Int. Ed. Engl. 2016, 55, 5780–5784. [Google Scholar] [CrossRef] [PubMed]
- Hillwig, M.; Liu, X. A new family of iron-dependent halogenases acts on freestanding substrates. Nat. Chem. Biol. 2014, 10, 921–923. [Google Scholar] [CrossRef] [PubMed]
- DuBois, J.L.; Ojha, S. Production of Dioxygen in the Dark: Dismutases of Oxyanions. Met. Ions Life Sci. 2014, 15, 45–87. [Google Scholar]
- Kengen, S.W.M.; Rikken, G.B.; Hagen, W.R.; van Ginkel, C.G.; Stams, A. Purification and Characterization of (Per)Chlorate Reductase from the Chlorate-Respiring Strain GR-1. J. Bacteriol. 1999, 181, 6706–6711. [Google Scholar] [CrossRef]
- Sun, S.Q.; Chen, S.L. How does Mo-dependent perchlorate reductase work in the decomposition of oxyanions? Dalton Trans. 2019, 48, 5683–5691. [Google Scholar] [CrossRef]
- Amachi, S.; Kawaguchi, N.; Muramatsu, Y.; Tsuchiya, S.; Watanabe, Y.; Shinoyama, H.; Fujii, T. Dissimilatory Iodate Reduction by Marine Pseudomonas sp. Strain SCT. Appl. Environ. Microbiol. 2007, 73, 5725–5730. [Google Scholar] [CrossRef]
- Tamai, N.; Ishii, T.; Sato, Y.; Fujiya, H.; Muramatsu, Y.; Okabe, N.; Amachi, S. Bromate Reduction by Rhodococcus sp. Br-6 in the Presence of Multiple Redox Mediators. Environ. Sci. Technol. 2016, 50, 10527–10534. [Google Scholar] [CrossRef]
- Maymó-Gatell, X.; Tandoi, V.; Gossett, J.M.; Zinder, S.H. Characterization of an H2-utilizing enrichment culture that reductively dechlorinates tetrachloroethene to vinyl chloride and ethene in the absence of methanogenesis and acetogenesis. Appl. Environ. Microbiol. 1995, 61, 3928–3933. [Google Scholar] [CrossRef]
- Maymó-Gatell, X.; Chien, Y.-T.; Gossett, J.M.; Zinder, S.H. Isolation of a Bacterium That Reductively Dechlorinates Tetrachloroethene to Ethene. Science 1997, 276, 1568–1571. [Google Scholar] [CrossRef]
- Schubert, T.; Adrian, L.; Sawers, R.G.; Diekert, G. Organohalide respiratory chains: Composition, topology and key enzymes. FEMS Microbiol. Ecol. 2018, 94, fiy035. [Google Scholar] [CrossRef] [PubMed]
- Bommer, M.; Kunze, C.; Fesseler, J.; Schubert, T.; Diekert, G.; Dobbek, H. Structural basis for organohalide respiration. Science 2014, 346, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Dolfing, J. Energetic Considerations in Organohalide Respiration. In Organohalide-Respiring Bacteria; Adrian, L., Löffler, F.E., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 31–48. [Google Scholar]
- Yang, Y.; Sanford, R.; Yan, J.; Chen, G.; Cápiro, N.L.; Li, X.; Löffler, F.E. Roles of Organohalide-Respiring Dehalococcoidia in Carbon Cycling. mSystems 2020, 5, e00757-19. [Google Scholar] [CrossRef]
- Atashgahi, S.; Häggblom, M.M.; Smidt, H. Organohalide respiration in pristine environments: Implications for the natural halogen cycle. Environ. Microbiol. 2018, 20, 934–948. [Google Scholar] [CrossRef] [PubMed]
- Chekan, J.R.; Lee, G.Y.; El Gamal, A.; Purdy, T.N.; Houk, K.N.; Moore, B.S. Bacterial Tetrabromopyrrole Debrominase Shares a Reductive Dehalogenation Strategy with Human Thyroid Deiodinase. Biochemistry 2019, 58, 5329–5338. [Google Scholar] [CrossRef]
- Temme, H.R.; Carlson, A.; Novak, P.J. Presence, Diversity, and Enrichment of Respiratory Reductive Dehalogenase and Non-respiratory Hydrolytic and Oxidative Dehalogenase Genes in Terrestrial Environments. Front. Microbiol. 2019, 10, 1258. [Google Scholar] [CrossRef] [PubMed]
- Fetzner, S. Bacterial dehalogenation. Appl. Microbiol. Biotechnol. 1998, 50, 633–657. [Google Scholar] [CrossRef]
- Ang, T.-F.; Maiangwa, J.; Salleh, A.B.; Normi, Y.M.; Leow, T.C. Dehalogenases: From Improved Performance to Potential Microbial Dehalogenation Applications. Molecules 2018, 23, 1100. [Google Scholar] [CrossRef]
- Cavina, L.; Van Der Born, D.; Klaren, P.H.M.; Feiters, M.C.; Boerman, O.C.; Rutjes, F.P.J.T. Design of Radioiodinated Pharmaceuticals: Structural Features Affecting Metabolic Stability towards in Vivo Deiodination. Eur. J. Org. Chem. 2017, 2017, 3387–3414. [Google Scholar] [CrossRef]
- Macdonald, T.L.; Anders, M.W. Chemical Mechanisms of Halocarbon Metabolism. CRC Crit. Rev. Toxicol. 1983, 11, 85–120. [Google Scholar] [CrossRef]
- Cnubben, N.H.; Vervoort, J.; Boersma, M.G.; Rietjens, I.M. The effect of varying halogen substituent patterns on the cytochrome-P450 catalyzed dehalogenation of 4-halogenated anilines to 4-aminophenol metabolites. Biochem. Pharmacol. 1995, 49, 1235–1248. [Google Scholar] [CrossRef]
- Sinsheimer, J.E.; Wang, T.; Röder, S.; Shum, Y.Y. Mechanisms for biodehalogenation of iodocompounds. Biochem. Biophys. Res. Commun. 1978, 83, 281–286. [Google Scholar] [CrossRef]
- Zhang, C.; Kenny, J.R.; Le, H.; Deese, A.; Ford, K.A.; Lightning, L.K.; Fan, P.W.; Driscoll, J.P.; Halladay, J.S.; Hop, C.E.; et al. Novel mechanism for dehalogenation and glutathione conjugation of dihalogenated anilines in human liver microsomes: Evidence for ipso glutathione addition. Chem. Res. Toxicol. 2011, 24, 1668–1677. [Google Scholar] [CrossRef] [PubMed]
- Messerschmidt, A.; Prade, L.; Wever, R. Implications for the Catalytic Mechanism of the Vanadium-Containing Enzyme Chloroperoxidase from the Fungus Curvularia inaequalis by X-Ray Structures of the Native and Peroxide Form. Biol. Chem. 1997, 378, 309–315. [Google Scholar] [CrossRef]
- Dutzler, R.; Campbell, E.B.; MacKinnon, R. Gating the Selectivity Filter in ClC Chloride Channels. Science 2003, 300, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Skitchenko, R.K.; Usoltsev, D.; Uspenskaya, M.; Kajava, A.V.; Guskov, A. Census of halide-binding sites in protein structures. Bioinformatics 2020, 36, 3064–3071. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.M.; Berden, G.; Oomens, J.; Williams, E.R. Halide anion binding to Gly(3), Ala(3) and Leu(3). Int. J. Mass Spectrom. 2015, 377, 440–447. [Google Scholar] [CrossRef]
- O’Hagan, D.; Schaffrath, C.; Cobb, S.L.; Hamilton, J.T.; Murphy, C.D. Biosynthesis of an organofluorine molecule—A fluorinase enzyme has been discovered that catalyses carbon-fluorine bond formation. Nature 2002, 416, 279. [Google Scholar]
- Cadicamo, C.D.; Courtieu, J.; Deng, H.; Meddour, A.; O’Hagan, D. Enzymatic fluorination in Streptomyces cattleya takes place with an inversion of configuration consistent with an SN2 reaction mechanism. Chembiochem 2004, 5, 685–690. [Google Scholar] [CrossRef]
- Healy, E.F. The effect of desolvation on nucleophilic halogenase activity. Comput. Theor. Chem. 2011, 964, 91–93. [Google Scholar] [CrossRef]
- Vincent, M.A.; Hillier, I.H. The solvated fluoride anion can be a good nucleophile. Chem. Commun. 2005, 5902–5903. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Robinson, D.A.; McEwan, A.R.; O’Hagan, D.; Naismith, J.H. Mechanism of Enzymatic Fluorination in Streptomyces cattleya. J. Am. Chem. Soc. 2007, 129, 14597–14604. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Huang, F.; Deng, H.; Schaffrath, C.; Spencer, J.B.; O’Hagan, D.; Naismith, J.H. Crystal structure and mechanism of a bacterial fluorinating enzyme. Nature 2004, 427, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Sooklal, S.A.; Mpangase, P.T.; Tomescu, M.-S.; Aron, S.; Hazelhurst, S.; Archer, R.H.; Rumbold, K. Functional characterisation of the transcriptome from leaf tissue of the fluoroacetate-producing plant, Dichapetalum cymosum, in response to mechanical wounding. Sci. Rep. 2020, 10, 20539. [Google Scholar] [CrossRef]
- Eustaquio, A.; Pojer, F.; Noel, J.P.; Moore, B.S. Discovery and characterization of a marine bacterial SAM-dependent chlorinase. Nat. Chem. Biol. 2008, 4, 69–74. [Google Scholar] [CrossRef]
- Beer, L.L.; Moore, B.S. Biosynthetic Convergence of Salinosporamides A and B in the Marine Actinomycete Salinispora tropica. Org. Lett. 2007, 9, 845–848. [Google Scholar] [CrossRef]
- Wuosmaa, A.M.; Hager, L.P. Methyl Chloride Transferase: A Carbocation Route for Biosynthesis of Halometabolites. Science 1990, 249, 160–162. [Google Scholar] [CrossRef]
- Ohsawa, N.; Tsujita, M.; Morikawa, S.; Itoh, N. Purification and Characterization of a Monohalomethane-producing Enzyme S-adenosyl-L-methionine: Halide Ion Methyltransferase from a Marine Microalga, Pavlova pinguis. Biosci. Biotechnol. Biochem. 2001, 65, 2397–2404. [Google Scholar] [CrossRef]
- Schmidberger, J.W.; James, A.B.; Edwards, R.H.; Naismith, J.H.; O’Hagan, D. Halomethane Biosynthesis: Structure of a SAM-Dependent Halide Methyltransferase from Arabidopsis thaliana. Angew. Chem. Int. Ed. 2010, 49, 3646–3648. [Google Scholar] [CrossRef]
- Paul, C.; Pohnert, G. Production and role of volatile halogenated compounds from marine algae. Nat. Prod. Rep. 2011, 28, 186–195. [Google Scholar] [CrossRef]
- Küpper, F.C.; Miller, E.P.; Andrews, S.J.; Hughes, C.; Carpenter, L.; Meyer-Klaucke, W.; Toyama, C.; Muramatsu, Y.; Feiters, M.C.; Carrano, C.J. Emission of volatile halogenated compounds, speciation and localization of bromine and iodine in the brown algal genome model Ectocarpus siliculosus. JBIC J. Biol. Inorg. Chem. 2018, 23, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Amachi, S. Microbial Contribution to Global Iodine Cycling: Volatilization, Accumulation, Reduction, Oxidation, and Sorption of Iodine. Microbes Environ. 2008, 23, 269–276. [Google Scholar] [CrossRef] [PubMed]
- WMO. Scientific Assessment of Ozone Depletion: 1994; WMO: Geneva, Switzerland, 1995. [Google Scholar]
- WMO. Scientific Assessment of Ozone Depletion: 1998; WMO: Geneva, Switzerland, 1999. [Google Scholar]
- Cicerone, R.J.; Walters, S.; Liu, S.C. Non-linear response of stratospheric ozone column to chlorine injections. J. Geophys. Res. -Ocean. 1983, 88, 3647–3661. [Google Scholar] [CrossRef]
- Farman, J.C.; Gardiner, B.G.; Shanklin, J.D. Large losses of total ozone in Antarctica reveal seasonal ClOx/NOx interaction. Nature 1985, 315, 207–210. [Google Scholar] [CrossRef]
- Solomon, S. The discovery of the Antarctic ozone hole. Nature 2019, 575, 46–47. [Google Scholar] [CrossRef]
- Barrie, L.A.; Bottenheim, J.W.; Schnell, R.; Crutzen, P.J.; Rasmussen, R.A. Ozone destruction and photochemical reactions at polar sunrise in the lower Arctic atmosphere. Nature 1988, 334, 138–141. [Google Scholar] [CrossRef]
- Crutzen, P.J. The influence of nitrogen oxides on the atmospheric ozone content. Q. J. R. Meteorol. Soc. 1970, 96, 320–325. [Google Scholar] [CrossRef]
- Molina, M.J.; Rowland, F.S. Stratospheric sink for chlorofluoromethanes: Chlorine atom-catalysed destruction of ozone. Nat. 1974, 249, 810–812. [Google Scholar] [CrossRef]
- McNeill, V.F. Obituary Mario Molina (1943–2020). Nature 2020, 587, 193. [Google Scholar] [CrossRef]
- Prather, M.J.; Blake, D.R.F. Sherwood Rowland (1927–2012). Nature 2012, 484, 168. [Google Scholar] [CrossRef]
- Prather, M.J.; McElroy, M.B.; Wofsy, S.C. Reductions in ozone at high concentrations of stratospheric halogens. Nature 1984, 312, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.G.; Brune, W.H.; Proffitt, M.H. Ozone destruction by chlorine radicals within the Antarctic vortex: The spatial and temporal evolution of ClO-O3anticorrelation based on in situ ER-2 data. J. Geophys. Res. Earth Surf. 1989, 94, 11465–11479. [Google Scholar] [CrossRef]
- Gareau, B.J. A critical review of the successful CFC phase-out versus the delayed methyl bromide phase-out in the Montreal Protocol. Int. Environ. Agreem. -Politics Law Econ. 2010, 10, 209–231. [Google Scholar] [CrossRef]
- Jovanovic, D.; Lukinovic, M.; Vitosevic, Z. Environment and health—Thirty years of successful implementation of the Montreal protocol. Srpski Arhiv Za Celokupno Lekarstvo 2019, 147, 492–496. [Google Scholar] [CrossRef]
- Chipperfield, M.P.; Bekki, S.; Dhomse, S.; Harris, N.R.P.; Hassler, B.; Hossaini, R.; Steinbrecht, W.; Thiéblemont, R.; Weber, M. Detecting recovery of the stratospheric ozone layer. Nature 2017, 549, 211–218. [Google Scholar] [CrossRef]
- Weatherhead, E.C.; Andersen, S. The search for signs of recovery of the ozone layer. Nature 2006, 441, 39–45. [Google Scholar] [CrossRef]
- Koenig, T.K.; Baidar, S.; Campuzano-Jost, P.; Cuevas, C.A.; Dix, B.; Fernandez, R.P.; Guo, H.; Hall, S.R.; Kinnison, D.; Nault, B.A.; et al. Quantitative detection of iodine in the stratosphere. Proc. Natl. Acad. Sci. USA 2020, 117, 1860–1866. [Google Scholar] [CrossRef]
- Simpson, W.R.; Brown, S.S.; Saiz-Lopez, A.; Thornton, J.A.; von Glasow, R. Tropospheric Halogen Chemistry: Sources, Cycling, and Impacts. Chem. Rev. 2015, 115, 4035–4062. [Google Scholar] [CrossRef]
- Sherwen, T.; Evans, M.J.; Carpenter, L.J.; Andrews, S.J.; Lidster, R.T.; Dix, B.; Koenig, T.K.; Sinreich, R.; Ortega, I.; Volkamer, R.; et al. Iodine’s impact on tropospheric oxidants: A global model study in GEOS-Chem. Atmos. Chem. Phys. 2016, 16, 1161–1186. [Google Scholar] [CrossRef]
- Sherwen, T.; Evans, M.J.; Sommariva, R.; Hollis, L.D.J.; Ball, S.M.; Monks, P.S.; Reed, C.; Carpenter, L.J.; Lee, J.D.; Forster, G.; et al. Effects of halogens on European air-quality. Faraday Discuss. 2017, 200, 75–100. [Google Scholar] [CrossRef]
- Saiz-Lopez, A.; Fernandez, R.P.; Ordóñez, C.; Kinnison, D.E.; Martín, J.C.G.; Lamarque, J.-F.; Tilmes, S. Iodine chemistry in the troposphere and its effect on ozone. Atmos. Chem. Phys. 2014, 14, 13119–13143. [Google Scholar] [CrossRef]
- Saiz-Lopez, A.; Plane, J.M.C.; Baker, A.R.; Carpenter, L.; von Glasow, R.; Martin, J.C.G.; McFiggans, G.; Saunders, R.W. Atmospheric Chemistry of Iodine. Chem. Rev. 2011, 112, 1773–1804. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Díaz, J.D.; Shimabuku, K.K.; Ma, J.; Enumah, Z.O.; Pignatello, J.J.; Mitch, W.A.; Dodd, M.C. Sunlight-Driven Photochemical Halogenation of Dissolved Organic Matter in Seawater: A Natural Abiotic Source of Organobromine and Organoiodine. Environ. Sci. Technol. 2014, 48, 7418–7427. [Google Scholar] [CrossRef] [PubMed]
- Read, K.; Mahajan, A.; Carpenter, L.; Evans, M.J.; Faria, B.V.E.; Heard, D.; Hopkins, J.; Lee, J.D.; Moller, S.; Lewis, A.; et al. Extensive halogen-mediated ozone destruction over the tropical Atlantic Ocean. Nature 2008, 453, 1232–1235. [Google Scholar] [CrossRef]
- von Glasow, R.; von Kuhlmann, R.; Lawrence, M.G.; Platt, U.; Crutzen, P.J. Impact of reactive bromine chemistry in the troposphere. Atmos. Chem. Phys. 2004, 4, 2481–2497. [Google Scholar] [CrossRef]
- Bloss, W.J.; Lee, J.D.; Johnson, G.P.; Sommariva, R.; Heard, D.; Saiz-Lopez, A.; Plane, J.M.C.; McFiggans, G.; Coe, H.; Flynn, M.; et al. Impact of halogen monoxide chemistry upon boundary layer OH and HO2 concentrations at a coastal site. Geophys. Res. Lett. 2005, 32, L06814. [Google Scholar] [CrossRef]
- Sander, R.; Rudich, Y.; Von Glasow, R.; Crutzen, P.J. The role of BrNO3 in marine tropospheric chemistry: A model study. Geophys. Res. Lett. 1999, 26, 2857–2860. [Google Scholar] [CrossRef]
- Carpenter, L.; Macdonald, S.M.; Shaw, M.D.; Kumar, R.; Saunders, R.W.; Parthipan, R.; Wilson, J.; Plane, J. Atmospheric iodine levels influenced by sea surface emissions of inorganic iodine. Nat. Geosci. 2013, 6, 108–111. [Google Scholar] [CrossRef]
- Carpenter, L.J.; Chance, R.J.; Sherwen, T.; Adams, T.J.; Ball, S.M.; Evans, M.J.; Hepach, H.; Hollis, L.D.; Hughes, C.; Jickells, T.D.; et al. Marine iodine emissions in a changing world. Proc. R. Soc. A 2021, 477, 20200824. [Google Scholar] [CrossRef]
- Legrand, M.; McConnell, J.R.; Preunkert, S.; Arienzo, M.; Chellman, N.; Gleason, K.; Sherwen, T.; Evans, M.J.; Carpenter, L. Alpine ice evidence of a three-fold increase in atmospheric iodine deposition since 1950 in Europe due to increasing oceanic emissions. Proc. Natl. Acad. Sci. USA 2018, 115, 12136–12141. [Google Scholar] [CrossRef]
- Cuevas, C.A.; Maffezzoli, N.; Corella, J.P.; Spolaor, A.; Vallelonga, P.; Kjær, H.A.; Simonsen, M.F.; Winstrup, M.; Vinther, B.; Horvat, C.; et al. Rapid increase in atmospheric iodine levels in the North Atlantic since the mid-20th century. Nat. Commun. 2018, 9, 1452. [Google Scholar] [CrossRef] [PubMed]
- Keller, J.M.; Boynton, W.V.; Karunatillake, S.; Baker, V.R.; Dohm, J.M.; Evans, L.G.; Finch, M.J.; Hahn, B.C.; Hamara, D.K.; Janes, D.M.; et al. Equatorial and midlatitude distribution of chlorine measured by Mars Odyssey GRS. J. Geophys. Res. Earth Surf. 2006, 112. [Google Scholar] [CrossRef]
- Wyngaarden, J.B.; Wright, B.M.; Ways, P. The Effect of certain anions upon the accumulation and retention of iodide by the thyroid gland. Endocrinology 1952, 50, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Davila, A.F.; Willson, D.; Coates, J.D.; McKay, C.P. Perchlorate on Mars: A chemical hazard and a resource for humans. Int. J. Astrobiol. 2013, 12, 321–325. [Google Scholar] [CrossRef]
- Channer, D.; de Ronde, C.; Spooner, E. The Cl−Br−I− composition of ∼3.23 Ga modified seawater: Implications for the geological evolution of ocean halide chemistry. Earth Planet. Sci. Lett. 1997, 150, 325–335. [Google Scholar] [CrossRef]
- Burgess, R.; Goldsmith, S.L.; Sumino, H.; Gilmour, J.D.; Marty, B.; Pujol, M.; Konhauser, K.O. Archean to Paleoproterozoic seawater halogen ratios recorded by fluid inclusions in chert and hydrothermal quartz. Am. Miner. 2020, 105, 1317–1325. [Google Scholar] [CrossRef]
- Lu, Z.; Hoogakker, B.; Hillenbrand, C.-D.; Zhou, X.; Thomas, E.; Gutchess, K.M.; Lu, W.; Jones, L.; Rickaby, R.E.M. Oxygen depletion recorded in upper waters of the glacial Southern Ocean. Nat. Commun. 2016, 7, 11146. [Google Scholar] [CrossRef]
- Pohl, A.; Lu, Z.; Lu, W.; Stockey, R.G.; Elrick, M.; Li, M.; André, D.; Shen, Y.; He, R.; Finnegan, S.; et al. Reorganization of ocean circulation and oxygenation during late Ordovician glaciation. Nat. Geosci. 2021, in press. [Google Scholar]
- Hoogakker, B.A.A.; Lu, Z.; Umling, N.; Jones, L.; Zhou, X.; Rickaby, R.E.M.; Thunell, R.; Cartapanis, O.; Galbraith, E. Glacial expansion of oxygen-depleted seawater in the eastern tropical Pacific. Nature 2018, 562, 410–413. [Google Scholar] [CrossRef]
- Thilly, C.H.; Vanderpas, J.B.; Bebe, N.; Ntambue, K.; Contempre, B.; Swennen, B.; Moreno-Reyes, R.; Bourdoux, P.; Delange, F. Iodine deficiency, other trace elements, and goitrogenic factors in the etiopathogeny of iodine deficiency disorders (IDD). Biol. Trace Element Res. 1992, 32, 229. [Google Scholar] [CrossRef]
- Chatin, A. Recherches sur l’iode des eaux douces; de la présence de ce corps dans les plantes at les animaux terrestes. C. R. Acad. Sci. 1851, 31, 280–283. [Google Scholar]
- Zimmermann, M.B. Research on Iodine Deficiency and Goiter in the 19th and Early 20th Centuries. J. Nutr. 2008, 138, 2060–2063. [Google Scholar] [CrossRef] [PubMed]
- Baumann, F. Ueber das normale Vorkommen von Jod im Thierkörper. Z. Phys. Chem. 1896, 21, 319–330. [Google Scholar] [CrossRef][Green Version]
- Roos, E. Ueber die Wirkung des Thyrojodins. Biol. Chem. 1897, 22, 18–61. [Google Scholar] [CrossRef][Green Version]
- Yarrington, C.D.; Pearce, E.N. Dietary Iodine in Pregnancy and Postpartum. Clin. Obstet. Gynecol. 2011, 54, 459–470. [Google Scholar] [CrossRef]
- Pal, G. Endocrine Physiology. In Textbook of Medical Physiology; Ahuja Publishing House: New Delhi, India, 2007; p. 346. [Google Scholar]
- Hetzel, B.; Dunn, J. The iodine deficiency disorders: Their nature and prevention. Annu. Rev. Nutr. 1989, 9, 21–38. [Google Scholar] [CrossRef]
- Porterfield, S.P.; White, B.A. Endocrine Physiology; Mosby: London, UK, 2007. [Google Scholar]
- Delange, F. The Disorders Induced by Iodine Deficiency. Thyroid 1994, 4, 107–128. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Shan, Z.; Teng, W. Effects of Increased Iodine Intake on Thyroid Disorders. Endocrinol. Metab. 2014, 29, 240–247. [Google Scholar] [CrossRef]
- Zimmermann, M.B.; Gizak, M.; Abbott, K.; Andersson, M.; Lazarus, J.H. Iodine deficiency in pregnant women in Europe. Lancet Diabetes Endocrinol. 2015, 3, 672–674. [Google Scholar] [CrossRef]
- Solomon, B.L.; Evaul, J.E.; Burman, K.D.; Wartofsky, L. Remission rates with antithyroid drug therapy: Continuing influence of iodine intake? Ann. Intern. Med. 1987, 107, 510–512. [Google Scholar] [CrossRef]
- Blasco, B.; Rios, J.; Cervilla, L.; Sánchez-Rodrigez, E.; Ruiz, J.; Romero, L. Iodine biofortification and antioxidant capacity of lettuce: Potential benefits for cultivation and human health. Ann. Appl. Biol. 2008, 152, 289–299. [Google Scholar] [CrossRef]
- Venturi, S.; Venturi, M. Iodide, thyroid and stomach carcinogenesis: Evolutionary story of a primitive antioxidant? Eur. J. Endocrinol. 1999, 140, 371–372. [Google Scholar] [CrossRef] [PubMed]
- Eskin, B.; Stadel, B. Dietary iodine and cancer risk. Lancet 1976, 308, 807–808. [Google Scholar] [CrossRef]
- Ghent, W.R.; Eskin, B.A.; Low, D.E.; Hill, L.P. Iodine replacement in fibrocystic disease of the breast. Can. J. Surg. 1993, 36, 453–460. [Google Scholar] [PubMed]
- Parkin, D.M.; Whelan, S.L.; Ferlay, J.; Teppo, L.; Thomas, D.B. Cancer Incidence in Five Continents. In Lyon: International Agency for Research on Cancer; World Health Organization Scientific Publications: Geneva, Switzerland, 1997; Volume 8, pp. 66–68. [Google Scholar]
- Funahashi, H.; Imai, T.; Tanaka, Y.; Tobinaga, J.; Wada, M.; Morita, T.; Yamada, F.; Tsukamura, K.; Oiwa, M.; Kikumori, T.; et al. Suppressive effect of iodine on DMBA-induced breast tumor growth in the rat. J. Surg. Oncol. 1996, 61, 209–213. [Google Scholar] [CrossRef]
- Carpena, X.; Vidossich, P.; Schroettner, K.; Calisto, B.M.; Banerjee, S.; Stampler, J.; Soudi, M.; Furtmüller, P.G.; Rovira, C.; Fita, I.; et al. Essential Role of Proximal Histidine-Asparagine Interaction in Mammalian Peroxidases. J. Biol. Chem. 2009, 284, 25929–25937. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, N.; Sharma, S.; Singh, S.B.; Kaur, P.; Bhushan, A.; Srinivasan, A.; Singh, T.P. Crystal Structure of Lactoperoxidase at 2. 4 Å Resolution. J. Mol. Biol. 2008, 376, 1060–1075. [Google Scholar] [CrossRef]
- Ruf, J.; Carayon, P. Structural and functional aspects of thyroid peroxidase. Arch. Biochem. Biophys. 2006, 445, 269–277. [Google Scholar] [CrossRef]
- McCall, A.S.; Cummings, C.F.; Bhave, G.; Vanacore, R.; Page-McCaw, A.; Hudson, B.G. Bromine Is an Essential Trace Element for Assembly of Collagen IV Scaffolds in Tissue Development and Architecture. Cell 2014, 157, 1380–1392. [Google Scholar] [CrossRef]
- Tin, A.; Nadkarni, G.; Evans, A.M.; Winkler, C.A.; Bottinger, E.; Rebholz, C.M.; Sarnak, M.J.; Inker, L.A.; Levey, A.S.; Lipkowitz, M.S.; et al. Serum 6-Bromotryptophan Levels Identified as a Risk Factor for CKD Progression. J. Am. Soc. Nephrol. 2018, 29, 1939–1947. [Google Scholar] [CrossRef]
- Mayeno, A.N.; Curran, A.J.; Roberts, R.L.; Foote, C.S. Eosinophils Preferentially Use Bromide to Generate Halogenating Agents. J. Biol. Chem. 1989, 264, 5660–5668. [Google Scholar] [CrossRef]
- Wu, W.; Chen, Y.; D’Avignon, A.; Hazen, S.L. 3-Bromotyrosine and 3,5-Dibromotyrosine Are Major Products of Protein Oxidation by Eosinophil Peroxidase: Potential Markers for Eosinophil-Dependent Tissue Injury in Vivo. Biochemistry 1999, 38, 3538–3548. [Google Scholar] [CrossRef] [PubMed]
- Sabir, M.; Tan, Y.Y.; Aris, A.; Mani, A.R. The role of endogenous bromotyrosine in health and disease. Free Radic. Res. 2019, 53, 1019–1034. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.R.; McTamney, P.M.; Adler, J.M.; LaRonde-LeBlanc, N.; Rokita, S.E. Crystal Structure of Iodotyrosine Deiodinase, a Novel Flavoprotein Responsible for Iodide Salvage in Thyroid Glands. J. Biol. Chem. 2009, 284, 19659–19667. [Google Scholar] [CrossRef]
- Bianco, A.C.; Larsen, P.R. Cellular and Structural Biology of the Deiodinases. Thyroid 2005, 15, 777–786. [Google Scholar] [CrossRef]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The Halogen Bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef]
- Auffinger, P.; Hays, F.A.; Westhof, E.; Ho, P.S. Halogen bonds in biological molecules. Proc. Natl. Acad. Sci. USA 2004, 101, 16789–16794. [Google Scholar] [CrossRef]
- Bayse, C.A.; Rafferty, E.R. Is Halogen Bonding the Basis for Iodothyronine Deiodinase Activity? Inorg. Chem. 2010, 49, 5365–5367. [Google Scholar] [CrossRef]
- World Health Organization. Iodine Deficiency in Europe: A Continuing Public Health Problem; WHO: Geneva, Switzerland, 2007. [Google Scholar]
- World Health Organization, Nutrition Unit. Recommended Iodine Levels in Salt and Guidelines for Monitoring Their Adequacy and Effectiveness; WHO: Geneva, Switzerland, 1996. [Google Scholar]
- Crawford, B.A.; Cowell, C.T.; Emder, P.J.; Learoyd, D.L.; Chua, E.L.; Sinn, J.; Jack, M.M. Iodine toxicity from soy milk and seaweed ingestion is associated with serious thyroid dysfunction. Med J. Aust. 2010, 193, 413–415. [Google Scholar] [CrossRef]
- Müssig, K.; Thamer, C.; Bares, R.; Lipp, H.P.; Häring, H.U.; Gallwitz, B. Iodine-induced thyrotoxicosis after ingestion of kelp-containing tea. J. Gen. Intern. Med. 2006, 21, C11–C14. [Google Scholar] [CrossRef]
- Nishiyama, S.; Mikeda, T.; Okada, T.; Nakamura, K.; Kotani, T.; Hishinuma, A. Transient Hypothyroidism or Persistent Hyperthyrotropinemia in Neonates Born to Mothers with Excessive Iodine Intake. Thyroid 2004, 14, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Müssig, K. Iodine-induced toxic effects due to seaweed consumption. Compr. Handb. Iodine 2009, 897–908. [Google Scholar]
- Palmieri, N.; Forleo, M.B. The potential of edible seaweed within the western diet. A segmentation of Italian consumers. Int. J. Gastron. Food Sci. 2020, 20, 100202. [Google Scholar] [CrossRef]
- Teas, J.; Pino, S.; Critchley, A.; Braverman, L.E. Variability of iodine content in common commercially available edible seaweeds. Thyroid 2004, 14, 836–841. [Google Scholar] [CrossRef]
- Lüning, K.; Mortensen, L. European aquaculture of sugar kelp (Saccharina latissima) for food industries: Iodine content and epiphytic animals as major problems. Bot. Mar. 2015, 58, 449–455. [Google Scholar] [CrossRef]
- Nitschke, U.; Stengel, D.B. Quantification of iodine loss in edible Irish seaweeds during processing. J. Appl. Phycol. 2016, 28, 3527–3533. [Google Scholar] [CrossRef]
- Nagataki, S. The Average of Dietary Iodine Intake due to the Ingestion of Seaweeds is 1.2 mg/day in Japan. Thyroid 2008, 18, 667–668. [Google Scholar] [CrossRef]
- Al-Adilah, H.; Peters, A.F.; Al-Bader, D.; Raab, A.; Akhdhar, A.; Feldmann, J.; Küpper, F.C. Iodine and fluorine concentrations in seaweeds of the Arabian Gulf identified by morphology and DNA barcodes. Bot. Mar. 2020, 63, 509–519. [Google Scholar] [CrossRef]
- Saenko, G.N.; Kravtsova, Y.Y.; Ivanenko, V.V.; Sheludko, S.I. Concentration of iodine and bromine by plants in the seas of Japan and Okhotsk. Mar. Biol. 1978, 47, 243–250. [Google Scholar] [CrossRef]
- Pavelka, S.; Vobecký, M.; Babicky, A. Halogen speciation in the rat thyroid: Simultaneous determination of bromine and iodine by short-term INAA. J. Radioanal. Nucl. Chem. Artic. 2008, 278, 575–579. [Google Scholar] [CrossRef]
- Romarís–Hortas, V.; García-Sartal, C.; Barciela-Alonso, M.D.C.; Domínguez-González, R.; Moreda-Piñeiro, A.; Bermejo-Barrera, P. Bioavailability study using an in-vitro method of iodine and bromine in edible seaweed. Food Chem. 2011, 124, 1747–1752. [Google Scholar] [CrossRef]
- Buzalaf, M.A.R.; Pessan, J.P.; Honorio, H.M.; Ten Cate, J.M. Mechanisms of action of fluoride for caries control. Monogr. Oral. Sci. 2011, 22, 97–114. [Google Scholar] [PubMed]
- Jenkins, G.N. The mechanism of action of fluoride in reducing caries incidence. Int. Dent. J. 1967, 17, 552. [Google Scholar]
- Voynar, A. Biological Role of Microelements in Human and Animal Organism; Vysshaya Shokola: Moscow, Russia, 1960. [Google Scholar]
- Krishnamachari, K.A. Skeletal fluorosis in humans: A review of recent progress in the understanding of the disease. Prog. Food Nutr. Sci. 1986, 10, 279–314. [Google Scholar] [PubMed]
- Li, S.; Smith, K.D.; Davis, J.H.; Gordon, P.B.; Breaker, R.R.; Strobel, S.A. Eukaryotic resistance to fluoride toxicity mediated by a widespread family of fluoride export proteins. Proc. Natl. Acad. Sci. USA 2013, 110, 19018–19023. [Google Scholar] [CrossRef]
- Yoo, J.I.; Seppälä, S.; O’Malley, M.A. Engineered fluoride sensitivity enables biocontainment and selection of genetically-modified yeasts. Nat. Commun. 2020, 11, 5459. [Google Scholar] [CrossRef]
- Wood, J.M.; Kennedy, F.S.; Wolfe, R.S. Reaction of multihalogenated hydrocarbons with free and bound reduced vitamin B12. Biochemistry 1968, 7, 1707–1713. [Google Scholar] [CrossRef]
- Carpenter, L.J.; Malin, G.; Liss, P.S.; Küpper, F.C. Novel biogenic iodine-containing trihalomethanes and other short-lived halocarbons in the coastal east Atlantic. Glob. Biogeochem. Cycles 2000, 14, 1191–1204. [Google Scholar] [CrossRef]
- Paul, N.A.; De Nys, R.; Steinberg, P. Chemical defence against bacteria in the red alga Asparagopsis armata: Linking structure with function. Mar. Ecol. Prog. Ser. 2006, 306, 87–101. [Google Scholar] [CrossRef]
- Küpper, F.C.; Leblanc, C.; Meyer-Klaucke, W.; Potin, P.; Feiters, M.C. Different speciation for bromine in brown and red algae, revealed by in vivo X-ray absorption spectroscopic studies. J. Phycol. 2014, 50, 652–664. [Google Scholar] [CrossRef]
- McAllister, T.A.; Cheng, K.-J.; Okine, E.K.; Mathison, G.W. Dietary, environmental and microbiological aspects of methane production in ruminants. Can. J. Anim. Sci. 1996, 76, 231–243. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2007: The Physical Science Basis. In Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L., Eds.; Cambridge University Press: Cambridge, UK, 2007; p. 1009. [Google Scholar]
- Roque, B.M.; Salwen, J.K.; Kinley, R.; Kebreab, E. Inclusion of Asparagopsis armata in lactating dairy cows’ diet reduces enteric methane emission by over 50 percent. J. Clean. Prod. 2019, 234, 132–138. [Google Scholar] [CrossRef]
- Roque, B.M.; Venegas, M.; Kinley, R.D.; de Nys, R.; Duarte, T.L.; Yang, X.; Kebreab, E. Red seaweed (Asparagopsis taxiformis) supplementation reduces enteric methane by over 80 percent in beef steers. PLoS ONE 2021, 16, e0247820. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Norman, H.C.; Kinley, R.D.; Laurence, M.; Wilmot, M.; Bender, H.; de Nys, R.; Tomkins, N. Asparagopsis taxiformis decreases enteric methane production from sheep. Anim. Prod. Sci. 2018, 58, 681–688. [Google Scholar] [CrossRef]
- Roque, B.M.; Brooke, C.G.; Ladau, J.; Polley, T.; Marsh, L.J.; Najafi, N.; Pandey, P.; Singh, L.; Kinley, R.; Salwen, J.K.; et al. Effect of the macroalgae Asparagopsis taxiformis on methane production and rumen microbiome assemblage. Anim. Microbiome 2019, 1, 3. [Google Scholar] [CrossRef]
- Machado, L.; Magnusson, M.; Paul, N.A.; de Nys, R.; Tomkins, N. Effects of Marine and Freshwater Macroalgae on In Vitro Total Gas and Methane Production. PLoS ONE 2014, 9, e85289. [Google Scholar] [CrossRef]
- Machado, L.; Magnusson, M.; Paul, N.; Kinley, R.D.; de Nys, R.; Tomkins, N. Dose-response effects of Asparagopsis taxiformis and Oedogonium sp. on in vitro fermentation and methane production. J. Appl. Phycol. 2015, 28, 1443–1452. [Google Scholar] [CrossRef]
- Machado, L.; Magnusson, M.; Paul, N.A.; Kinley, R.; De Nys, R.; Tomkins, N. Identification of bioactives from the red seaweed Asparagopsis taxiformis that promote antimethanogenic activity in vitro. J. Appl. Phycol. 2016, 28, 3117–3126. [Google Scholar] [CrossRef]
- Machado, L.; Tomkins, N.; Magnusson, M.; Midgley, D.J.; de Nys, R.; Rosewarne, C.P. In Vitro Response of Rumen Microbiota to the Antimethanogenic Red Macroalga Asparagopsis taxiformis. Microb. Ecol. 2017, 75, 811–818. [Google Scholar] [CrossRef]
- Zhu, P.; Li, D.; Yang, Q.; Su, P.; Wang, H.; Heimann, K.; Zhang, W. Commercial cultivation, industrial application, and potential halocarbon biosynthesis pathway of Asparagopsis sp. Algal Res. 2021, 56, 102319. [Google Scholar] [CrossRef]
- Küpper, F.C.; Schweigert, N.; Gall, E.A.; Legendre, J.-M.; Vilter, H.; Kloareg, B. Iodine uptake in Laminariales involves extracellular, haloperoxidase-mediated oxidation of iodide. Planta 1998, 207, 163–171. [Google Scholar] [CrossRef]
- Truesdale, V.W. The biogeochemical effect of seaweeds upon close-to natural concentrations of dissolved iodate and iodide in seawater—Preliminary study with Laminaria digitata and Fucus serratus. Estuarine, Coast. Shelf Sci. 2008, 78, 155–165. [Google Scholar] [CrossRef]
- Carrano, M.W.; Yarimizu, K.; Gonzales, J.L.; Cruz-López, R.; Edwards, M.S.; Tymon, T.M.; Küpper, F.C.; Carrano, C.J. The influence of marine algae on iodine speciation in the coastal ocean. ALGAE 2020, 35, 167–176. [Google Scholar] [CrossRef]
- Tymon, T.M.; Miller, E.P.; Gonzales, J.L.; Raab, A.; Küpper, F.C.; Carrano, C.J. Some aspects of the iodine metabolism of the giant kelp Macrocystis pyrifera (Phaeophyceae). J. Inorg. Biochem. 2017, 177, 82–88. [Google Scholar] [CrossRef]
- Chance, R.; Baker, A.R.; Küpper, F.C.; Hughes, C.; Kloareg, B.; Malin, G. Release and transformations of inorganic iodine by marine macroalgae. Estuar. Coast. Shelf Sci. 2009, 82, 406–414. [Google Scholar] [CrossRef]
- Gonzales, J.; Tymon, T.; Küpper, F.C.; Edwards, M.S.; Carrano, C.J. The potential role of kelp forests on iodine speciation in coastal seawater. PLoS ONE 2017, 12, e0180755. [Google Scholar]
- Carrano, M.W.; Carrano, C.J.; Edwards, M.S.; Al-Adilah, H.; Fontana, Y.; Sayer, M.D.; Katsaros, C.; Raab, A.; Feldmann, J.; Küpper, F.C. Laminaria kelps impact iodine speciation chemistry in coastal seawater. Estuar. Coast. Shelf Sci. 2021, 262, 107531. [Google Scholar] [CrossRef]
- Bartsch, I.; Wiencke, C.; Bischof, K.; Buchholz, C.; Buck, B.H.; Eggert, A.; Feuerpfeil, P.; Hanelt, D.; Jacobsen, S.; Karez, R.; et al. The genus Laminaria sensu lato: Recent insights and developments. Eur. J. Phycol. 2008, 43, 1–86. [Google Scholar] [CrossRef]
- Leblanc, C.; Colin, C.; Cosse, A.; Delage, L.; La Barre, S.; Morin, P.; Fiévet, B.; Voiseux, C.; Ambroise, Y.; Verhaeghe, E.; et al. Iodine transfers in the coastal marine environment: The key role of brown algae and of their vanadium-dependent haloperoxidases. Biochimie 2006, 88, 1773–1785. [Google Scholar] [CrossRef]
- Gall, E.A.; Küpper, F.C.; Kloareg, B. A survey of iodine content in Laminaria digitata. Bot. Mar. 2004, 47, 30–37. [Google Scholar] [CrossRef]
- Eschle Ueber den Jodgehalt einiger Algenarten. Z. Physiol. Chem. 1897, 23, 30–37. [CrossRef][Green Version]
- Golenkin, M. Algologische Notizen; 1. Das Vorkommen von freiem Iod bei Bonnemaisonia asparagoides. Bull. Société Impériale Nat. Moscou 1894, 8, 257–270. [Google Scholar]
- Sauvageau, C. Sur quelques algues floridées renfermant de l’iode à l’état libre. Bull. Stn. Biol. Arcachon 1925, 22, 3–43. [Google Scholar]
- Kylin, H. Über das Vorkommen von Jodiden, Bromiden und Jodidoxydasen bei Meeresalgen. Hoppe-Seyler’s Z. Physiol. Chem. 1929, 186, 50–84. [Google Scholar] [CrossRef]
- Dangeard, P. Sur le dégagement de l’iode chez les algues marines. Comptes Rendus Hebd. Séances Académie Sci. 1928, 186, 892–894. [Google Scholar]
- Tong, W.; Chaikoff, I.L. Metabolism of 131I by the marine alga, Nereocystis luetkeana. J. Biol. Chem. 1955, 215, 473–484. [Google Scholar] [CrossRef]
- Baily, N.A.; Kelly, S. Iodine Exchange in Ascophyllum. Biol. Bull. 1955, 109, 13–20. [Google Scholar] [CrossRef]
- Shaw, T. The mechanism of iodine accumulation by the brown sea weed Laminaria digitata. The uptake of 131I. Proc. Roy. Soc. Lond. B 1959, 150, 356–371. [Google Scholar]
- Shaw, T.I. The mechanism of iodine accumulation by the brown sea weed Laminaria digitata. II. Respiration and iodide uptake. Proc. Roy. Soc. Lond. B 1960, 152, 109–117. [Google Scholar]
- Huang, R.-J.; Thorenz, U.R.; Kundel, M.; Venables, D.S.; Ceburnis, D.; Ho, K.F.; Chen, J.; Vogel, A.L.; Küpper, F.C.; Smyth, P.P.A.; et al. The seaweeds Fucus vesiculosus and Ascophyllum nodosum are significant contributors to coastal iodine emissions. Atmospheric Chem. Phys. 2013, 13, 5255–5264. [Google Scholar] [CrossRef]
- Verhaeghe, E.F.; Fraysse, A.; Guerquin-Kern, J.-L.; Wu, T.-D.; Devès, G.; Mioskowski, C.; Leblanc, C.; Ortega, R.; Ambroise, Y.; Potin, P. Microchemical imaging of iodine distribution in the brown alga Laminaria digitata suggests a new mechanism for its accumulation. JBIC J. Biol. Inorg. Chem. 2007, 13, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Kapanna, A.N.; Sitakara Rao, V. Iodine content of marine algae from Gujarat coast. J. Sci. Ind. Res. B Phys. Sci. 1962, 21, 559–560. [Google Scholar]
- Palmer, C.J.; Anders, T.L.; Carpenter, L.J.; Küpper, F.C.; McFiggans, G.B. Iodine and Halocarbon Response of Laminaria digitata to Oxidative Stress and Links to Atmospheric New Particle Production. Environ. Chem. 2005, 2, 282–290. [Google Scholar] [CrossRef]
- Küpper, F.C.; Carpenter, L.J.; Leblanc, C.; Toyama, C.; Uchida, Y.; Maskrey, B.H.; Robinson, J.; Verhaeghe, E.F.; Malin, G.; Luther, G.W., III; et al. In vivo Speciation studies and antioxidant properties of bromine in Laminaria digitata reinforce the significance of iodine accumulation for kelps. J. Exp. Bot. 2013, 64, 2653–2664. [Google Scholar] [CrossRef] [PubMed]
- Leri, A.; Mayer, L.M.; Thornton, K.R.; Northrup, P.; Dunigan, M.R.; Ness, K.J.; Gellis, A.B. A marine sink for chlorine in natural organic matter. Nat. Geosci. 2015, 8, 620–624. [Google Scholar] [CrossRef]
- Theiler, R.; Cook, J.C.; Hager, L.P.; Siuda, J.F. Halohydrocarbon Synthesis by Bromoperoxidase. Science 1978, 202, 1094–1096. [Google Scholar] [CrossRef]
- Beissner, R.S.; Guilford, W.J.; Coates, R.M.; Hager, L.P. Synthesis of brominated heptanones and bromoform by a bromoperoxidase of marine origin. Biochemistry 1981, 20, 3724–3731. [Google Scholar] [CrossRef]
- Leri, A.C.; Dunigan, M.R.; Wenrich, R.L.; Ravel, B. Particulate organohalogens in edible brown seaweeds. Food Chem. 2018, 272, 126–132. [Google Scholar] [CrossRef]
- Leri, A.; Marcus, M.A.; Myneni, S.C. X-ray spectromicroscopic investigation of natural organochlorine distribution in weathering plant material. Geochim. Cosmochim. Acta 2007, 71, 5834–5846. [Google Scholar] [CrossRef]
- Reina, R.G.; Leri, A.C.; Myneni, S.C.B. Cl K-edge X-ray Ssectroscopic investigation of enzymatic formation of organochlorines in weathering plant material. Environ. Sci. Technol. 2004, 38, 783–789. [Google Scholar] [CrossRef]
- Leri, A.C.; Hakala, A.; Marcus, M.A.; Lanzirotti, A.; Reddy, C.M.; Myneni, S.C.B. Natural organobromine in marine sediments: New evidence of biogeochemical Br cycling. Glob. Biogeochem. Cycles 2010, 24. [Google Scholar] [CrossRef]
- Küpper, F.C.; Müller, D.G.; Peters, A.F.; Kloareg, B.; Potin, P. Oligoalginate recognition and oxidative burst play a key role in natural and induced resistance of sporophytes of laminariales. J. Chem. Ecol. 2002, 28, 2057–2081. [Google Scholar] [CrossRef] [PubMed]
- Potin, P. Oxidative Burst and Related Responses in Biotic Interactions of Algae. In Algal Chemical Ecology; Springer: Berlin/Heidelberg, Germany, 2008; pp. 245–271. [Google Scholar]
- de Oliveira, L.S.; Tschoeke, D.A.; Magalhães Lopes, A.C.R.; Sudatti, D.B.; Meirelles, P.M.; Thompson, C.C.; Pereira, R.C.; Thompson, F.L. Molecular mechanisms for microbe recognition and defense by the red seaweed Laurencia dendroidea. Msphere 2017, 2, e00094-17. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, F.; Hoppe, H.G.; Friedlander, M. Bacterial induction and inhibition of a fast necrotic response in Gracilaria conferta (Rhodophyta). J. Appl. Phycol. 1997, 9, 277–285. [Google Scholar] [CrossRef]
- Weinberger, F.; Friedlander, M.; Hoppe, H.-G. Oligoagars elicit a Physiological Response in Gracilaria conferta (Rhodophyta). J. Phycol. 1999, 35, 747–755. [Google Scholar] [CrossRef]
- Weinberger, F.; Friedlander, M. Endogenous and exogenous elicitors of a hypersensitive response in Gracilaria conferta (Rhodophyta). J. Appl. Phycol. 2000, 12, 139–145. [Google Scholar] [CrossRef]
- Weinberger, F.; Friedlander, M. Response of Gracilaria conferta (Rhodophyta) to oligoagars results in defense against agar-degrading epiphytes. J. Phycol. 2000, 36, 1079–1086. [Google Scholar] [CrossRef]
- Bouarab, K.; Potin, P.; Weinberger, F.; Correa, J.; Kloareg, B. The Chondrus crispus-Acrochaete operculata host-pathogen association, a novel model in glycobiology and applied phycopathology. J. Appl. Phycol. 2001, 13, 185–193. [Google Scholar] [CrossRef]
- Weinberger, F.; Richard, C.; Kloareg, B.; Kashman, Y.; Hoppe, H.-G.; Friedlander, M. Structure-activity relationships of oligoagar elicitors toward Gracilaria conferta (Rhodophyta). J. Phycol. 2001, 37, 418–426. [Google Scholar] [CrossRef]
- Weinberger, F.; Pohnert, G.; Kloareg, B.; Potin, P. A signal released by an enclophytic attacker acts as a substrate for a rapid defensive reaction of the red alga Chondrus crispus. Chembiochem 2002, 3, 1260–1263. [Google Scholar] [CrossRef]
- Weinberger, F.; Leonardi, P.; Miravalles, A.; Correa, J.A.; Lion, U.; Kloareg, B.; Potin, P. Dissection of two distinct defense-related responses to agar oligosaccharides in Gracilaria chilensis (Rhodophyta) and Gracilaria conferta (Rhodophyta). J. Phycol. 2005, 41, 863–873. [Google Scholar] [CrossRef]
- Küpper, F.C.; Kloareg, B.; Guern, J.; Potin, P. Oligoguluronates Elicit an Oxidative Burst in the Brown Algal Kelp Laminaria digitata. Plant Physiol. 2001, 125, 278–291. [Google Scholar] [CrossRef] [PubMed]
- Küpper, F.C.; Gaquerel, E.; Boneberg, E.-M.; Morath, S.; Salaün, J.-P.; Potin, P. Early events in the perception of lipopolysaccharides in the brown alga Laminaria digitata include an oxidative burst and activation of fatty acid oxidation cascades. J. Exp. Bot. 2006, 57, 1991–1999. [Google Scholar] [CrossRef] [PubMed]
- Zambounis, A.; Gaquerel, E.; Strittmatter, M.; Salaun, J.-P.; Potin, P.; Küpper, F.C. Prostaglandin A2 triggers a strong oxidative burst in Laminaria: A novel defense inducer in brown algae? ALGAE 2012, 27, 21–32. [Google Scholar] [CrossRef]
- Küpper, F.C.; Gaquerel, E.; Cosse, A.; Adas, F.; Peters, A.F.; Müller, D.G.; Kloareg, B.; Salaün, J.-P.; Potin, P. Free Fatty Acids and Methyl Jasmonate Trigger Defense Reactions in Laminaria digitata. Plant Cell Physiol. 2009, 50, 789–800. [Google Scholar] [CrossRef]
- Collén, J.; Ekdahl, A.; Abrahamsson, K.; Pedersén, M. The involvement of hydrogen peroxide in the production of volatile halogenated compounds by Meristiella gelidium. Phytochemistry 1994, 36, 1197–1202. [Google Scholar] [CrossRef]
- Pedersen, M.; Collen, J.; Abrahamsson, K.; Ekdahl, A. Production of halocarbons from seaweeds: An oxidative stress reaction? Sci. Mar. 1996, 60, 257–263. [Google Scholar]
- Weinberger, F.; Coquempot, B.; Forner, S.; Morin, P.; Kloareg, B.; Potin, P. Different regulation of haloperoxidation during agar oligosaccharide-activated defence mechanisms in two related red algae, Gracilaria sp. and Gracilaria chilensis. J. Exp. Bot. 2007, 58, 4365–4372. [Google Scholar] [CrossRef]
- Küpper, F.C.; Maier, I.; Müller, D.G.; Goer, S.L.D.; Guillou, L. Phylogenetic affinities of two eukaryotic pathogens of marine macroalgae, Eurychasma dicksonii (Wright) Magnus and Chytridium polysiphoniae Cohn. Cryptogam. Algol. 2006, 27, 165–184. [Google Scholar]
- Sekimoto, S.; Beakes, G.W.; Gachon, C.M.; Müller, D.G.; Küpper, F.C.; Honda, D. The Development, Ultrastructural Cytology, and Molecular Phylogeny of the Basal Oomycete Eurychasma dicksonii, Infecting the Filamentous Phaeophyte Algae Ectocarpus siliculosus and Pylaiella littoralis. Protist 2008, 159, 299–318. [Google Scholar] [CrossRef]
- Strittmatter, M.; Grenville-Briggs, L.J.; Breithut, L.; Van West, P.; Gachon, C.M.; Küpper, F.C. Infection of the brown alga Ectocarpus siliculosus by the oomycete Eurychasma dicksonii induces oxidative stress and halogen metabolism. Plant Cell Environ. 2016, 39, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Wever, R.; Tromp, M.G.M.; Krenn, B.E.; Marjani, A.; Van Tol, M. Brominating activity of the seaweed Ascophyllum nodosum: Impact on the biosphere. Environ. Sci. Technol. 1991, 25, 446–449. [Google Scholar] [CrossRef]
- Scott, R. Observations on the Iodo-Amino-Acids of Marine Algae using Iodine-131. Nature 1954, 173, 1098–1099. [Google Scholar] [CrossRef]
- Klemperer, H.G. The accumulation of iodide by Fucus ceranoides. Biochem. J. 1957, 67, 381–390. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Manley, S.L. Micronutrient uptake and translocation by Macrocystis pyrifera (Phaeophyta). J. Phycol. 1984, 20, 192–201. [Google Scholar] [CrossRef]
- Manley, S.L.; Dastoor, M.N. Methyl halide (CH3X) production from the giant kelp, Macrocystis, and estimates of global CH3X production by kelp. Limnol. Oceanogr. 1987, 32, 709–715. [Google Scholar] [CrossRef]
- Manley, S.L.; Dastoor, M.N. Methyl iodide (CH3I) production by kelp and associated microbes. Mar. Biol. 1988, 98, 477–482. [Google Scholar] [CrossRef]
- Manley, S.L.; Goodwin, K.; North, W.J. Laboratory production of bromoform, methylene bromide, and methyl-iodide by macroalgae and distribution in nearshore Southern California waters. Limnol. Oceanogr. 1992, 37, 1652–1659. [Google Scholar] [CrossRef]
- Konotchick, T.; Dupont, C.L.; Valas, R.E.; Badger, J.H.; Allen, A.E. Transcriptomic analysis of metabolic function in the giant kelp, Macrocystis pyrifera, across depth and season. New Phytol. 2013, 198, 398–407. [Google Scholar] [CrossRef]
- Goodwin, K.D.; North, W.J.; Lidstrom, M.E. Production of bromoform and dibromomethane by Giant Kelp: Factors affecting release and comparison to anthropogenic bromine sources. Limnol. Oceanogr. 1997, 42, 1725–1734. [Google Scholar] [CrossRef]
- Stutz, J.; Jobson, B.T.; Sumner, A.L. Impact of Reactive Halogen Species on the Air Quality in California Coastal Areas; Final Report: CRC A-62-1/2 & ARB #05-307; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Finley, B.D.; Saltzman, E.S. Observations of Cl2, Br2, and I2 in coastal marine air. J. Geophys. Res. Atmos. 2008, 113. [Google Scholar] [CrossRef]
- Butler, A.; Sandy, M. Mechanistic considerations of halogenating enzymes. Nature 2009, 460, 848–854. [Google Scholar] [CrossRef]
- Graham, M.H.; Vasquez, J.A.; Buschmann, A.H. Global ecology of the giant kelp Macrocystis: From ecotypes to ecosystems. In Oceanography and Marine Biology; Gibson, R.N., Atkinson, R.J.A., Gordon, J.D.M., Eds.; Crc Press-Taylor & Francis Group: Boca Raton, FL, USA, 2007; Volume 45, pp. 39–88. [Google Scholar]
- Camargo, J.A. Fluoride toxicity to aquatic organisms: A review. Chemosphere 2002, 50, 251–264. [Google Scholar] [CrossRef]
- Tromp, M.; Van, T.T.; Wever, R. Reactivation of vanadium bromoperoxidase; inhibition by metallofluoric compounds. Biochim. Biophys. Acta (BBA) Protein Struct. Mol. Enzym. 1991, 1079, 53–56. [Google Scholar] [CrossRef]
- Ma, L.; Li, Y.; Meng, L.; Deng, H.; Li, Y.; Zhang, Q.; Diao, A. Biological fluorination from the sea: Discovery of a SAM-dependent nucleophilic fluorinating enzyme from the marine-derived bacterium Streptomyces xinghaiensis NRRL B24674. RSC Adv. 2016, 6, 27047–27051. [Google Scholar] [CrossRef]
- Huang, S.; Ma, L.; Tong, M.H.; Yu, Y.; O’Hagan, D.; Deng, H. Fluoroacetate biosynthesis from the marine-derived bacterium Streptomyces xinghaiensis NRRL B-24674. Org. Biomol. Chem. 2014, 12, 4828–4831. [Google Scholar] [CrossRef] [PubMed]
- Carter-Franklin, J.N.; Butler, A. Vanadium Bromoperoxidase-Catalyzed Biosynthesis of Halogenated Marine Natural Products. J. Am. Chem. Soc. 2004, 126, 15060–15066. [Google Scholar] [CrossRef]
- Young, E.G.; Langille, W.M. The occurrence of inorganic elements in marine algae of the Atlantic provinces of Canada. Can. J. Bot. 1958, 36, 301–310. [Google Scholar] [CrossRef]
- Mohamed, W.E.-D.; Hamad, M.T.M.H.; Kamel, M.Z. Application of statistical response surface methodology for optimization of fluoride removal efficiency by Padina sp. alga. Water Environ. Res. 2020, 92, 1080–1088. [Google Scholar] [CrossRef]
- Babu, A.N.; Reddy, D.S.; Kumar, G.S.; Ravindhranath, K.; Mohan, G.V.K. Sequential synergetic sorption analysis of Gracilaria Rhodophyta biochar toward aluminum and fluoride: A statistical optimization approach. Water Environ. Res. 2019, 92, 880–898. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Srebnik, M. Natural halogenated fatty acids: Their analogues and derivatives. Prog. Lipid Res. 2002, 41, 315–367. [Google Scholar] [CrossRef]
- Todd, J.S.; Proteau, P.J.; Gerwick, W.H. Egregiachlorides A-C: New chlorinated oxylipins from the marine brown alga Egregia menziesii. Tetrahedron Lett. 1993, 34, 7689–7692. [Google Scholar] [CrossRef]
- Kousaka, K.; Ogi, N.; Akazawa, Y.; Fujieda, M.; Yamamoto, Y.; Takada, Y.; Kimura, J. Novel Oxylipin Metabolites from the Brown Alga Eisenia bicyclis. J. Nat. Prod. 2003, 66, 1318–1323. [Google Scholar] [CrossRef] [PubMed]
- Jesus, A.; Correia-Da-Silva, M.; Afonso, C.; Pinto, M.; Cidade, H. Isolation and Potential Biological Applications of Haloaryl Secondary Metabolites from Macroalgae. Mar. Drugs 2019, 17, 73. [Google Scholar] [CrossRef]
- Harper, M.K. Introduction to the Chemical Ecology of Marine Natural Products. In Marine Chemical Ecology; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Dembitsky, V.M. Biogenic Iodine and Iodine-Containing Metabolites. Nat. Prod. Commun. 2006, 1, 1934578X0600100210. [Google Scholar] [CrossRef]
- Wang, B.-G.; Gloer, J.B.; Ji, N.-Y.; Zhao, J.-C. Halogenated Organic Molecules of Rhodomelaceae Origin: Chemistry and Biology. Chem. Rev. 2013, 113, 3632–3685. [Google Scholar] [CrossRef]
- Gribble, G.W. Naturally Occurring Organohalogen Compounds—A Survey. J. Nat. Prod. 1992, 55, 1353–1395. [Google Scholar] [CrossRef]
- Gribble, G.W. Natural Organohalogens: Many More Than You Think! J. Chem. Educ. 1994, 71, 907–911. [Google Scholar] [CrossRef]
- Gribble, G.W. The diversity of naturally produced organohalogens. Chemosphere 2003, 52, 289–297. [Google Scholar] [CrossRef]
- Gribble, G.W. Natural organohalogens: A new frontier for medicinal agents? J. Chem. Educ. 2004, 81, 1441–1449. [Google Scholar] [CrossRef]
- Gribble, G.W. A recent survey of naturally occurring organohalogen compounds. Environ. Chem. 2015, 12, 396–405. [Google Scholar] [CrossRef]
- Gribble, G.W. Biological Activity of Recently Discovered Halogenated Marine Natural Products. Mar. Drugs 2015, 13, 4044–4136. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, K.H.M.; Guaratini, T.; Barros, M.; Falcão, V.R.; Tonon, A.P.; Lopes, N.; Campos, S.; Torres, M.; Souza, A.D.O.; Colepicolo, P.; et al. Metabolites from algae with economical impact. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2007, 146, 60–78. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2009, 26, 170–244. [Google Scholar] [CrossRef] [PubMed]
- Agatsuma, Y.; Seki, T.; Kurata, K.; Taniguchi, K. Instantaneous effect of dibromomethane on metamorphosis of larvae of the sea urchins Strongylocentrotus nudus and Strongylocentrotus intermedius. Aquaculture 2006, 251, 549–557. [Google Scholar] [CrossRef]
- Wright, J.T.; De Nys, R.; Poore, A.G.B.; Steinberg, P.D. Chemical defense in a marine alga: Heritability and the potential for selection by herbivores. Ecology 2004, 85, 2946–2959. [Google Scholar] [CrossRef]
- Steinberg, P.D.; De Nys, R. Chemical mediation of colonisation of seaweed surfaces. J. Phycol. 2002, 38, 621–629. [Google Scholar] [CrossRef]
- Dworjanyn, S.A.; De Nys, R.; Steinberg, P.D. Chemically mediated antifouling in the red alga Delisea pulchra. Mar. Ecol. Prog. Ser. 2006, 318, 153–163. [Google Scholar] [CrossRef][Green Version]
- Lane, A.; Stout, E.P.; Lin, A.-S.; Prudhomme, J.; Le Roch, K.; Fairchild, C.R.; Franzblau, S.; Hay, M.; Aalbersberg, W.; Kubanek, J. Antimalarial Bromophycolides J−Q from the Fijian Red Alga Callophycus serratus. J. Org. Chem. 2009, 74, 2736–2742. [Google Scholar] [CrossRef]
- Lane, A.L.; Nyadong, L.; Galhena, A.S.; Shearer, T.L.; Stout, E.P.; Parry, R.M.; Kwasnik, M.; Wang, M.D.; Hay, M.E.; Fernandez, F.M.; et al. Desorption electrospray ionization mass spectrometry reveals surface-mediated antifungal chemical defense of a tropical seaweed. Proc. Natl. Acad. Sci. USA 2009, 106, 7314–7319. [Google Scholar] [CrossRef]
- Paradas, W.C.; Salgado, L.T.; Sudatti, D.B.; Crapez, M.A.; Fujii, M.T.; Coutinho, R.; Pereira, R.C.; Filho, G.M.A. Induction of halogenated vesicle transport in cells of the red seaweed Laurencia obtusa. Biofouling 2010, 26, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Dworjanyn, S.A.; De Nys, R.; Steinberg, P.D. Localisation and surface quantification of secondary metabolites in the red alga Delisea pulchra. Mar. Biol. 1999, 133, 727–736. [Google Scholar] [CrossRef]
- Young, D.N.; Howard, B.M.; Fenical, W. Subcellular localization of brominated secondary metabolites in the red alga Laurencia snyderae. J. Phycol. 1980, 16, 182–185. [Google Scholar] [CrossRef]
- Paul, N.A.; Cole, L.; De Nys, R.; Steinberg, P.D. Ultrastructure of the gland cells of the red alga Asparagopsis armata (Bonnemaisoniaceae). J. Phycol. 2006, 42, 637–645. [Google Scholar] [CrossRef]
- Murphy, C.; Moore, R.M.; White, R.L. Peroxidases from marine microalgae. J. Appl. Phycol. 2000, 12, 507–513. [Google Scholar] [CrossRef]
- Winter, J.; Moore, B.S. Exploring the Chemistry and Biology of Vanadium-dependent Haloperoxidases. J. Biol. Chem. 2009, 284, 18577–18581. [Google Scholar] [CrossRef]
- Wever, R.; Hemrika, W. Vanadium haloperoxidases. In Handbook of Metalloproteins; Messerschmidt, A., Huber, R., Poulos, T., Wieghardt, K., Eds.; John Wiley & Sons: Chichester, UK, 2001; pp. 1417–1428. [Google Scholar]
- Butler, A.; Walker, J.V. Marine haloperoxidases. Chem. Rev. 1993, 93, 1937–1944. [Google Scholar] [CrossRef]
- Wever, R. Structure and function of vanadium haloperoxidases. In Vanadium—Biochemical and Molecular Biological Approaches; Michibata, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 95–126. [Google Scholar]
- Blasiak, L.C.; Drennan, C.L. Structural Perspective on Enzymatic Halogenation. Acc. Chem. Res. 2008, 42, 147–155. [Google Scholar] [CrossRef]
- van Pe, K.H.; Dong, C.; Flecks, S.; Naismith, J.; Patallo, E.P.; Wage, T. Biological halogenation has moved far beyond haloperoxidases. In Advances in Applied Microbiology; Elsevier Academic Press Inc.: San Diego, CA, USA, 2006; Volume 59, pp. 127–157. [Google Scholar]
- Vilter, H. Vanadium-dependent haloperoxidases. In Vanadium and Its Role in Life; Sigel, H., Sigel, A., Eds.; Marcel Dekker: New York, NY, USA, 1995; pp. 325–362. [Google Scholar]
- Butler, A. Mechanistic considerations of the vanadium haloperoxidases. Co-ord. Chem. Rev. 1999, 187, 17–35. [Google Scholar] [CrossRef]
- Butler, A. Vanadium haloperoxidases. Curr. Opin. Chem. Biol. 1998, 2, 279–285. [Google Scholar] [CrossRef]
- Carter-Franklin, J.N.; Parrish, J.D.; Tschirret-Guth, R.A.; Little, A.R.D.; Butler, A. Vanadium Haloperoxidase-Catalyzed Bromination and Cyclization of Terpenes. J. Am. Chem. Soc. 2003, 125, 3688–3689. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.; Carter-Franklin, J.N. The role of vanadium bromoperoxidase in the biosynthesis of halogenated marine natural products. Nat. Prod. Rep. 2004, 21, 180–188. [Google Scholar] [CrossRef]
- Messerschmidt, A.; Wever, R. X-ray structure of a vanadium-containing enzyme: Chloroperoxidase from the fungus Curvularia inaequalis. Proc. Natl. Acad. Sci. USA 1996, 93, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Colin, C.; Leblanc, C.; Wagner, E.; Delage, L.; Leize-Wagner, E.; Van Dorsselaer, A.; Kloareg, B.; Potin, P. The Brown Algal Kelp Laminaria digitata Features Distinct Bromoperoxidase and Iodoperoxidase Activities. J. Biol. Chem. 2003, 278, 23545–23552. [Google Scholar] [CrossRef]
- Vilter, H. Peroxidases from Phaeophyceae IV. Fractionation and Location of Peroxidase Isoenzymes in Ascophyllum nodosum (L.) Le Jol. Bot. Mar. 1983, 26, 451–455. [Google Scholar] [CrossRef]
- Vilter, H. Peroxidases from phaeophyceae: A vanadium(V)-dependent peroxidase from Ascophyllum nodosum. Phytochemistry 1984, 23, 1387–1390. [Google Scholar] [CrossRef]
- Weyand, M.; Hecht, H.-J.; Kieß, M.; Liaud, M.-F.; Vilter, H.; Schomburg, D. X-ray structure determination of a vanadium-dependent haloperoxidase from Ascophyllum nodosum at 2.0 Å resolution. J. Mol. Biol. 1999, 293, 595–611. [Google Scholar] [CrossRef]
- Feiters, M.C.; Leblanc, C.; Küpper, F.C.; Meyer-Klaucke, W.; Michel, G.; Potin, P. Bromine is an Endogenous Component of a Vanadium Bromoperoxidase. J. Am. Chem. Soc. 2005, 127, 15340–15341. [Google Scholar] [CrossRef]
- Itoh, N.; Izumi, Y.; Yamada, H. Characterization of nonheme type bromoperoxidase in Corallina pilulifera. J. Biol. Chem. 1986, 261, 5194–5200. [Google Scholar] [CrossRef]
- Itoh, N.; Izumi, Y.; Yamada, H. Purification of bromoperoxidase from Corallina pilulifera. Biochem. Biophys. Res. Commun. 1985, 131, 428–435. [Google Scholar] [CrossRef]
- Itoh, N.; Izumi, Y.; Yamada, H. Characterization of nonheme iron and reaction mechanism of bromoperoxidase in Corallina pilulifera. J. Biol. Chem. 1987, 262, 11982–11987. [Google Scholar] [CrossRef]
- Wever, R.; Plat, H.; de Boer, E. Isolation procedure and some properties of the bromoperoxidase from the seaweed Ascophyllum nodosum. Biochim. Biophys. Acta (BBA) Protein Struct. Mol. Enzym. 1985, 830, 181–186. [Google Scholar] [CrossRef]
- Sheffield, D.; Harry, T.; Smith, A.; Rogers, L. Purification and characterization of the vanadium bromoperoxidase from the macroalga Corallina officinalis. Phytochemistry 1992, 32, 21–26. [Google Scholar] [CrossRef]
- Shimonishi, M.; Kuwamoto, S.; Inoue, H.; Wever, R.; Ohshiro, T.; Izumi, Y.; Tanabe, T. Cloning and expression of the gene for a vanadium-dependent bromoperoxidase from a marine macro-alga, Corallina pilulifera. FEBS Lett. 1998, 428, 105–110. [Google Scholar] [CrossRef]
- Vreeland, V.; Ng, K.L.; Epstein, L. cDNA sequence and active recombinant vanadium bromoperoxidase from Fucus embryos. Mol. Biol. Cell 1998, 9, 180A. [Google Scholar]
- Ohshiro, T.; Nakano, S.; Takahashi, Y.; Suzuki, M.; Izumi, Y. Occurrence of bromoperoxidase in the marine green macro-alga, Ulvella lens, and emission of volatile brominated methane by the enzyme. Phytochemistry 1999, 52, 1211–1215. [Google Scholar] [CrossRef]
- Isupov, M.; Dalby, A.; Brindley, A.A.; Izumi, Y.; Tanabe, T.; Murshudov, G.N.; Littlechild, J.A. Crystal structure of dodecameric vanadium-dependent bromoperoxidase from the red algae Corallina officinalis. J. Mol. Biol. 2000, 299, 1035–1049. [Google Scholar] [CrossRef]
- Suthiphongchai, T.; Boonsiri, P.; Panijpan, B. Vanadium-dependent bromoperoxidases from Gracilaria algae. J. Appl. Phycol. 2007, 20, 271–278. [Google Scholar] [CrossRef]
- Manley, S.L.; Barbero, P.E. Physiological constraints on bromoform (CHBr3) production by Ulva lactuca (Chlorophyta). Limnol. Oceanogr. 2001, 46, 1392–1399. [Google Scholar] [CrossRef]
- Littlechild, J.; Rodriguez, E.G.; Isupov, M. Vanadium containing bromoperoxidase—Insights into the enzymatic mechanism using X-ray crystallography. J. Inorg. Biochem. 2009, 103, 617–621. [Google Scholar] [CrossRef]
- Fournier, J.-B.; Rebuffet, E.; Delage, L.; Grijol, R.; Meslet-Cladière, L.; Rzonca, J.; Potin, P.; Michel, G.; Czjzek, M.; Leblanc, C. The Vanadium Iodoperoxidase from the Marine Flavobacteriaceae Species Zobellia galactanivorans Reveals Novel Molecular and Evolutionary Features of Halide Specificity in the Vanadium Haloperoxidase Enzyme Family. Appl. Environ. Microbiol. 2014, 80, 7561–7573. [Google Scholar] [CrossRef] [PubMed]
- Gribble, G.W. Naturally occuring organohalogen compounds—A comprehensive survery. In Progress in the Chemistry of Organic Natural Products; Springer: Cham, Switzerland, 1996; pp. 1–423. [Google Scholar]
- Ballschmiter, K. Pattern and sources of naturally produced organohalogens in the marine environment: Biogenic formation of organohalogens. Chemosphere 2003, 52, 313–324. [Google Scholar] [CrossRef]
- Rasmussen, R.A.; Khalil, M.A.K.; Gunawardena, R.; Hoyt, S.D. Atmospheric methyl iodide (CH3I). J. Geophys. Res. Ocean. 1982, 87, 3086–3090. [Google Scholar] [CrossRef]
- Class, T.; Ballschmiter, K. Chemistry of organic traces in air. J. Atmos. Chem. 1988, 6, 35–46. [Google Scholar] [CrossRef]
- Itoh, N. Volatile halogenated compounds from marine algae; Their formation mechanisms and geochemical aspects. Recent Res. Devel. Phytochem. 1997, 1, 309–327. [Google Scholar]
- Butler, J.H. Better budgets for methyl halides? Nature 2000, 403, 260–261. [Google Scholar] [CrossRef]
- de Jong, E.; Field, J.A. Sulfur tuft and turkey tail: Biosynthesis and Biodegradation of Organohalogens by Basidiomycetes. Annu. Rev. Microbiol. 1997, 51, 375–414. [Google Scholar] [CrossRef]
- Rezanka, T.; Spižek, J. Griseofulvin and other biologically active halogen containing compounds from fungi. Elsevier 2005, 32, 471–547. [Google Scholar]
- Gribble, G.W. Recently Discovered Naturally Occurring Heterocyclic Organohalogen Compounds. Heterocycles 2012, 84, 157–207. [Google Scholar] [CrossRef]
- Lovelock, J.E. Natural halocarbons in the air and in the sea. Nature 1975, 256, 193–194. [Google Scholar] [CrossRef]
- Scarratt, M.; Moore, R. Production of methyl chloride and methyl bromide in laboratory cultures of marine phytoplankton. Mar. Chem. 1996, 54, 263–272. [Google Scholar] [CrossRef]
- Richter, U.; Wallace, D.W.R. Production of methyl iodide in the tropical Atlantic Ocean. Geophys. Res. Lett. 2004, 31. [Google Scholar] [CrossRef]
- Karlsson, A.; Auer, N.; Schulz-Bull, D.; Abrahamsson, K. Cyanobacterial blooms in the Baltic—A source of halocarbons. Mar. Chem. 2008, 110, 129–139. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2006, 23, 26–78. [Google Scholar] [CrossRef] [PubMed]
- Erickson, K.L. Constituents of Laurencia. Mar. Nat. Prod. Chem. Biol. Perspect. 1983, 5, 131–257. [Google Scholar]
- Roche, J.; Yagi, Y. Sur la fixation de l’iode radioactif par les algues et sur les constituants iodés des Laminaires. Comptes Rendus Société Biol. Paris 1952, 146, 642–645. [Google Scholar]
- Kelly, S.; Baily, N.A. The uptake of radioactive iodine by Ascophyllum. Biol. Bull. 1951, 100, 188–190. [Google Scholar] [CrossRef]
- Kelly, S. Respiration and iodine uptake in Ascophyllum. Biol. Bull. 1953, 104, 138–145. [Google Scholar] [CrossRef]
- Nightingale, P.; Malin, G.; Liss, P.S. Production of chloroform and other low molecular-weight halocarbons by some species of macroalgae. Limnol. Oceanogr. 1995, 40, 680–689. [Google Scholar] [CrossRef]
- Lim, Y.-K.; Phang, S.-M.; Rahman, N.A.; Sturges, W.T.; Malin, G. Halocarbon emissions from marine phytoplankton and climate change. Int. J. Environ. Sci. Technol. 2017, 14, 1355–1370. [Google Scholar] [CrossRef]
- Laturnus, F.; Giese, B.; Wiencke, C.; Adams, F.C. Low-molecular-weight organoiodine and organobromine compounds released by polar macroalgae—The influence of abiotic factors. Anal. Bioanal. Chem. 2000, 368, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Berg, W.W.; Heidt, L.E.; Pollock, W.; Sperry, P.D.; Cicerone, R.J.; Gladney, E.S. Brominated organic species in the Arctic atmosphere. Geophys. Res. Lett. 1984, 11, 429–432. [Google Scholar] [CrossRef]
- Fogelqvist, E. Carbon tetrachloride, tetrachloroethylene, 1, 1, 1-trichloroethane and bromoform in Arctic seawater. J. Geophys. Res. Ocean. 1985, 90, 9181–9193. [Google Scholar] [CrossRef]
- Moore, R.M.; Zafiriou, O.C. Photochemical production of methyl iodide in seawater. J. Geophys. Res. Earth Surf. 1994, 99, 16415–16420. [Google Scholar] [CrossRef]
- Brownell, D.K.; Moore, R.M.; Cullen, J.J. Production of methyl halides by Prochlorococcus and Synechococcus. Glob. Biogeochem. Cycles 2010, 24. [Google Scholar] [CrossRef]
- Williams, J.; Gros, V.; Atlas, E.; Maciejczyk, K.; Batsaikhan, A.; Schöler, H.F.; Forster, C.; Quack, B.; Yassaa, N.; Sander, R.; et al. Possible evidence for a connection between methyl iodide emissions and Saharan dust. J. Geophys. Res. Earth Surf. 2007, 112. [Google Scholar] [CrossRef]
- Sturges, W.T.; Cota, G.F.; Buckley, P.T. Vertical profiles of bromoform in snow, sea ice, and seawater in the Canadian Arctic. J. Geophys. Res. Ocean. 1997, 102, 25073–25083. [Google Scholar] [CrossRef]
- Keng, F.S.-L.; Phang, S.-M.; Rahman, N.A.; Leedham, E.C.; Hughes, C.; Robinson, A.D.; Harris, N.R.P.; Pyle, J.A.; Sturges, W.T. Volatile halocarbon emissions by three tropical brown seaweeds under different irradiances. J. Appl. Phycol. 2013, 25, 1377–1386. [Google Scholar] [CrossRef]
- Mtolera, M.S.; Collén, J.; Pedersén, M.; Ekdahl, A.; Abrahamsson, K.; Semesi, A.K. Stress-induced production of volatile halogenated organic compounds in Eucheuma denticulatum (Rhodophyta) caused by elevated pH and high light intensities. Eur. J. Phycol. 1996, 31, 89–95. [Google Scholar] [CrossRef]
- Collén, J.; Porcel, B.; Carré, W.; Ball, S.G.; Chaparro, C.; Tonon, T.; Barbeyron, T.; Michel, G.; Noel, B.; Valentin, K.; et al. Genome structure and metabolic features in the red seaweed Chondrus crispus shed light on evolution of the Archaeplastida. Proc. Natl. Acad. Sci. USA 2013, 110, 5247–5252. [Google Scholar] [CrossRef]
- Berg, W.W.; Sperry, P.D.; Rahn, K.A.; Gladney, E.S. Atmospheric bromine in the Arctic. J. Geophys. Res. Ocean. 1983, 88, 6719–6736. [Google Scholar] [CrossRef]
- Fan, S.-M.; Jacob, D.J. Surface ozone depletion in Arctic spring sustained by bromine reactions on aerosols. Nature 1992, 359, 522. [Google Scholar] [CrossRef]
- Scarratt, M.G.; Moore, R.M. Production of chlorinated hydrocarbons and methyl iodide by the red microalga Porphyridium purpureum. Limnol. Oceanogr. 1999, 44, 703–707. [Google Scholar] [CrossRef]
- Hughes, C.; Malin, G.; Nightingale, P.; Liss, P.S. The effect of light stress on the release of volatile iodocarbons by three species of marine microalgae. Limnol. Oceanogr. 2006, 51, 2849–2854. [Google Scholar] [CrossRef]
- Hughes, C.; Sun, S. Light and brominating activity in two species of marine diatom. Mar. Chem. 2016, 181, 1–9. [Google Scholar] [CrossRef]
- Mithoo-Singh, P.K.; Keng, F.S.-L.; Phang, S.-M.; Elvidge, E.C.L.; Sturges, W.T.; Malin, G.; Rahman, N.A. Halocarbon emissions by selected tropical seaweeds: Species-specific and compound-specific responses under changing pH. PeerJ 2017, 5, e2918. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsson, K.; Choo, K.-S.; Pedersén, M.; Johansson, G.; Snoeijs, P. Effects of temperature on the production of hydrogen peroxide and volatile halocarbons by brackish-water algae. Phytochemistry 2003, 64, 725–734. [Google Scholar] [CrossRef]
- Mehrtens, G.; Laturnus, F. Halogenating activity in an Arctic population of brown macroalga Laminaria saccharina (L.) Lamour. Polar Res. 1997, 16, 19–26. [Google Scholar]
- Laturnus, F.; Adams, F.C.; Wiencke, C. Methyl halides from Antarctic macroalgae. Geophys. Res. Lett. 1998, 25, 773–776. [Google Scholar] [CrossRef]
- Atkinson, H.M.; Huang, R.-J.; Chance, R.; Roscoe, H.K.; Hughes, C.; Davison, B.; Schönhardt, A.; Mahajan, A.S.; Saiz-Lopez, A.; Hoffmann, T.; et al. Iodine emissions from the sea ice of the Weddell Sea. Atmos. Chem. Phys. 2012, 12, 11229–11244. [Google Scholar] [CrossRef]
- O’Dowd, C.D.; Jimenez, J.L.; Bahreini, R.; Flagan, R.C.; Seinfeld, J.H.; Hämeri, K.; Pirjola, L.; Kulmala, M.; Jennings, S.G.; Hoffmann, T. Marine aerosol formation from biogenic iodine emissions. Nature 2002, 417, 632–636. [Google Scholar] [CrossRef]
- Kerkweg, A.; Jöckel, P.; Warwick, N.; Gebhardt, S.; Brenninkmeijer, C.A.M.; Lelieveld, J. Consistent simulation of bromine chemistry from the marine boundary layer to the stratosphere—Part 2: Bromocarbons. Atmos. Chem. Phys. 2008, 8, 5919–5939. [Google Scholar] [CrossRef]
- Liang, Q.; Stolarski, R.S.; Kawa, S.R.; Nielsen, J.E.; Douglass, A.R.; Rodriguez, J.M.; Blake, D.R.; Atlas, E.L.; Ott, L.E. Finding the missing stratospheric Br-y: A global modeling study of CHBr3 and CH2Br2. Atmos. Chem. Phys. 2010, 10, 2269–2286. [Google Scholar] [CrossRef]
- Ordóñez, C.; Lamarque, J.-F.; Tilmes, S.; Kinnison, D.E.; Atlas, E.L.; Blake, D.R.; Santos, G.S.; Brasseur, G.; Saiz-Lopez, A. Bromine and iodine chemistry in a global chemistry-climate model: Description and evaluation of very short-lived oceanic sources. Atmos. Chem. Phys. 2012, 12, 1423–1447. [Google Scholar] [CrossRef]
- Warwick, N.J.; Pyle, J.A.; Carver, G.D.; Yang, X.; Savage, N.H.; O’Connor, F.M.; Cox, R.A. Global modeling of biogenic bromocarbons. J. Geophys. Res. -Atmos. 2006, 111, D24305. [Google Scholar] [CrossRef]
- Butler, J.H.; King, D.B.; Lobert, J.M.; Montzka, S.; Yvon-Lewis, S.; Hall, B.D.; Warwick, N.; Mondeel, D.J.; Aydin, M.; Elkins, J.W. Oceanic distributions and emissions of short-lived halocarbons. Glob. Biogeochem. Cycles 2007, 21. [Google Scholar] [CrossRef]
- Carpenter, L.J.; Liss, P.S. On temperate sources of bromoform and other reactive organic bromine gases. J. Geophys. Res. Earth Surf. 2000, 105, 20539–20547. [Google Scholar] [CrossRef]
- Quack, B.; Wallace, D.W.R. Air-sea flux of bromoform: Controls, rates, and implications. Glob. Biogeochem. Cycles 2003, 17. [Google Scholar] [CrossRef]
- Yokouchi, Y.; Hasebe, F.; Fujiwara, M.; Takashima, H.; Shiotani, M.; Nishi, N.; Kanaya, Y.; Hashimoto, S.; Fraser, P.; Toom-Sauntry, D.; et al. Correlations and emission ratios among bromoform, dibromochloromethane, and dibromomethane in the atmosphere. J. Geophys. Res. Earth Surf. 2005, 110. [Google Scholar] [CrossRef]
- Carpenter, L.J. Iodine in the Marine Boundary Layer. Chem. Rev. 2003, 103, 4953–4962. [Google Scholar] [CrossRef]
- Yokouchi, Y.; Osada, K.; Wada, M.; Hasebe, F.; Agama, M.; Murakami, R.; Mukai, H.; Nojiri, Y.; Inuzuka, Y.; Toom-Sauntry, D.; et al. Global distribution and seasonal concentration change of methyl iodide in the atmosphere. J. Geophys. Res. Earth Surf. 2008, 113, D18311. [Google Scholar] [CrossRef]
- Bell, N.; Hsu, L.; Jacob, D.J.; Schultz, M.; Blake, D.R.; Butler, J.H.; King, D.B.; Lobert, J.M.; Maier-Reimer, E. Methyl iodide: Atmospheric budget and use as a tracer of marine convection in global models. J. Geophys. Res. Earth Surf. 2002, 107, e4340. [Google Scholar] [CrossRef]
- Menard, H.W.; Smith, S.M. Hypsometry of ocean basin provinces. J. Geophys. Res. Earth Surf. 1966, 71, 4305–4325. [Google Scholar] [CrossRef]
- Ziska, F.; Quack, B.; Abrahamsson, K.; Archer, S.D.; Atlas, E.; Bell, T.; Butler, J.H.; Carpenter, L.J.; Jones, C.E.; Harris, N.R.P.; et al. Global sea-to-air flux climatology estimates of bromoform, dibromomethane and methyl iodide. Atmos. Chem. Phys. 2013, 13, 8915–8934. [Google Scholar] [CrossRef]
- McFiggans, G.; Coe, H.; Burgess, R.; Allan, J.; Cubison, M.; Alfarra, M.R.; Saunders, R.; Saiz-Lopez, A.; Plane, J.M.C.; Wevill, D.; et al. Direct evidence for coastal iodine particles from Laminaria macroalgae—Linkage to emissions of molecular iodine. Atmospheric Chem. Phys. 2004, 4, 701–713. [Google Scholar] [CrossRef]
- Saiz-Lopez, A.; Plane, J.M.C.; McFiggans, G.; Williams, P.I.; Ball, S.M.; Bitter, M.; Jones, R.L.; Hongwei, C.; Hoffmann, T. Modelling molecular iodine emissions in a coastal marine environment: The link to new particle formation. Atmos. Chem. Phys. 2006, 6, 883–895. [Google Scholar] [CrossRef]
- Hou, X.; Dahlgaard, H.; Nielsen, S. Iodine-129 Time Series in Danish, Norwegian and Northwest Greenland Coast and the Baltic Sea by Seaweed. Estuar. Coast. Shelf Sci. 2000, 51, 571–584. [Google Scholar] [CrossRef]
- Hou, X.; Hansen, V.; Aldahan, A.; Possnert, G.; Lind, O.C.; Lujaniene, G. A review on speciation of iodine-129 in the environmental and biological samples. Anal. Chim. Acta 2009, 632, 181–196. [Google Scholar] [CrossRef]
- Morita, T.; Niwa, K.; Fujimoto, K.; Kasai, H.; Yamada, H.; Nishiutch, K.; Sakamoto, T.; Godo, W.; Taino, S.; Hayashi, Y.; et al. Detection and activity of iodine-131 in brown algae collected in the Japanese coastal areas. Sci. Total Environ. 2010, 408, 3443–3447. [Google Scholar] [CrossRef]
- Manley, S.L.; Lowe, C.G. Canopy-Forming Kelps as California’s Coastal Dosimeter: 131I from Damaged Japanese Reactor Measured in Macrocystis pyrifera. Environ. Sci. Technol. 2012, 46, 3731–3736. [Google Scholar] [CrossRef]
- Lebeau, D.; Leroy, N.; Doizi, D.; Wu, T.-D.; Guerquin-Kern, J.-L.; Perrin, L.; Ortega, R.; Voiseux, C.; Fournier, J.-B.; Potin, P.; et al. Mass spectrometry—Based imaging techniques for iodine-127 and iodine-129 detection and localization in the brown alga Laminaria digitata. J. Environ. Radioact. 2021, 231, 106552. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).