Pseudostichococcus Stands Out from Its Siblings Due to High Salinity and Desiccation Tolerance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cultivation and Inducing Osmolyte Production
2.2. Sorbitol Quantification
2.3. Proline Quantification
2.4. Desiccation and Recovery Experiments
2.5. Statistical Analyses
3. Results
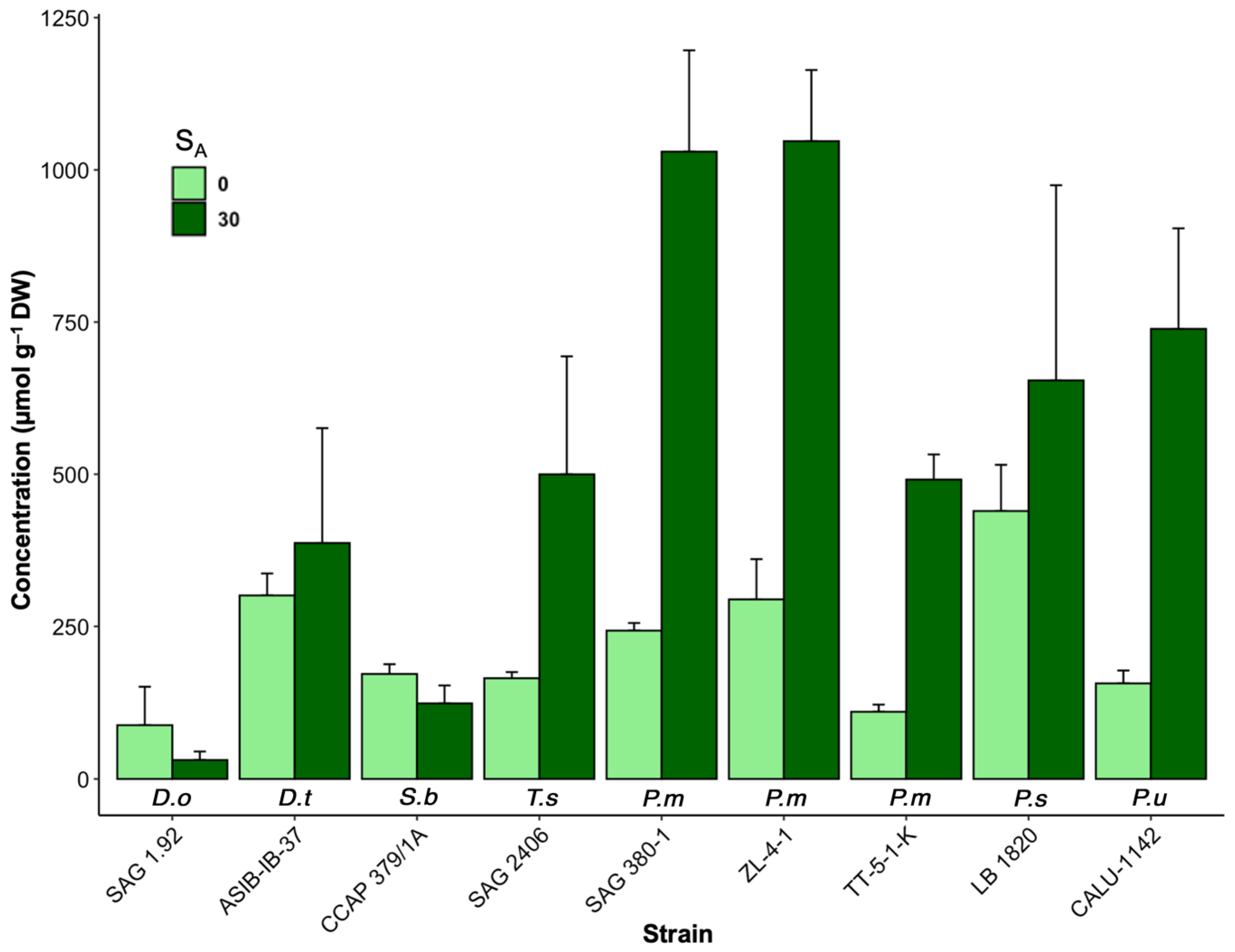
3.1. Changes in Osmolyte Concentrations under Salinity Stress
3.2. Desiccation and Rehydration
4. Discussion
4.1. Effect of Salinity on Proline Production
4.2. Salinity and Desiccation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kirst, G. Salinity Tolerance of Eukaryotic Marine Algae. Annu. Rev. Plant Biol. 1990, 41, 21–53. [Google Scholar] [CrossRef]
- Tavakkoli, E.; Fatehi, F.; Coventry, S.; Rengasamy, P.; McDonald, G.K. Additive Effects of Na+ and Cl− Ions on Barley Growth under Salinity Stress. J. Exp. Bot. 2011, 62, 2189–2203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gasulla, F.; de Nova, P.G.; Esteban-Carrasco, A.; Zapata, J.M.; Barreno, E.; Guéra, A. Dehydration Rate and Time of Desiccation Affect Recovery of the Lichenic Algae Trebouxia Erici: Alternative and Classical Protective Mechanisms. Planta 2009, 231, 195–208. [Google Scholar] [CrossRef] [PubMed]
- González-Hourcade, M.; del Campo, E.M.; Casano, L.M. The Under-Explored Extracellular Proteome of Aero-Terrestrial Microalgae Provides Clues on Different Mechanisms of Desiccation Tolerance in Non-Model Organisms. Microb. Ecol. 2021, 81, 437–453. [Google Scholar] [CrossRef]
- Holzinger, A.; Karsten, U. Desiccation Stress and Tolerance in Green Algae: Consequences for Ultrastructure, Physiological and Molecular Mechanisms. Front. Plant Sci. 2013, 4, 327. [Google Scholar] [CrossRef] [Green Version]
- Tamaru, Y.; Takani, Y.; Yoshida, T.; Sakamoto, T. Crucial Role of Extracellular Polysaccharides in Desiccation and Freezing Tolerance in the Terrestrial Cyanobacterium Nostoc Commune. Appl. Environ. Microbiol. 2005, 71, 7327–7333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Häubner, N.; Schumann, R.; Karsten, U. Aeroterrestrial Microalgae Growing in Biofilms on Facades—Response to Temperature and Water Stress. Microb. Ecol. 2006, 51, 285–293. [Google Scholar] [CrossRef]
- Hellebust, J.A. Mechanisms of Response to Salinity in Halotolerant Microalgae. In Biosalinity in Action: Bioproduction with Saline Water; Springer: Dordrecht, The Netherlands, 1985; pp. 69–81. [Google Scholar]
- Shetty, P.; Gitau, M.M.; Maróti, G. Salinity Stress Responses and Adaptation Mechanisms in Eukaryotic Green Microalgae. Cells 2019, 8, 1657. [Google Scholar] [CrossRef] [Green Version]
- Diehl, N.; Karsten, U.; Bischof, K. Impacts of Combined Temperature and Salinity Stress on the Endemic Arctic Brown Seaweed Laminaria Solidungula, J. Agardh. Polar Biol. 2020, 43, 647–656. [Google Scholar] [CrossRef]
- Kumar, M.; Kumari, P.; Reddy, C.; Jha, B. Salinity and Desiccation Induced Oxidative Stress Acclimation in Seaweeds. Adv. Bot. Res. 2014, 71, 91–123. [Google Scholar]
- Shabala, S. Learning from Halophytes: Physiological Basis and Strategies to Improve Abiotic Stress Tolerance in Crops. Ann. Bot. 2013, 112, 1209–1221. [Google Scholar] [CrossRef] [PubMed]
- van Ginneken, V. Some Mechanism Seaweeds Employ to Cope with Salinity Stress in the Harsh Euhaline Oceanic Environment. Am. J. Plant Sci. 2018, 9, 1191–1211. [Google Scholar] [CrossRef] [Green Version]
- Moore, J.P.; Le, N.T.; Brandt, W.F.; Driouich, A.; Farrant, J.M. Towards a Systems-Based Understanding of Plant Desiccation Tolerance. Trends Plant Sci. 2009, 14, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Muscolo, A.; Sidari, M.; Panuccio, M.R.; Santonoceto, C.; Orsini, F.; Pascale, S.D. Plant Responses in Saline and Arid Environments: An Overview. Eur. J. Plant Sci. Biotechnol. 2011, 5, 1–11. [Google Scholar]
- Munns, R.; James, R.A.; Gilliham, M.; Flowers, T.J.; Colmer, T.D. Tissue Tolerance: An Essential but Elusive Trait for Salt-Tolerant Crops. Funct. Plant Biol. 2016, 43, 1103. [Google Scholar] [CrossRef] [Green Version]
- Schilling, R.K.; Marschner, P.; Shavrukov, Y.; Berger, B.; Tester, M.; Roy, S.J.; Plett, D.C. Expression of the A Rabidopsis Vacuolar H+-pyrophosphatase Gene (AVP 1) Improves the Shoot Biomass of Transgenic Barley and Increases Grain Yield in a Saline Field. Plant Biotechnol. J. 2014, 12, 378–386. [Google Scholar] [CrossRef]
- Hanley, M.E.; Sanders, S.K.D.; Stanton, H.-M.; Billington, R.A.; Boden, R. A Pinch of Salt: Response of Coastal Grassland Plants to Simulated Seawater Inundation Treatments. Ann. Bot. 2019, 125, 267–275. [Google Scholar] [CrossRef]
- Alpert, P. Constraints of Tolerance: Why Are Desiccation-Tolerant Organisms so Small or Rare? J. Exp. Biol. 2006, 209, 1575–1584. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Jiang, J.-G.; Wu, G.-H. Effects of Salinity Changes on the Growth of Dunaliella Salina and Its Isozyme Activities of Glycerol-3-Phosphate Dehydrogenase. J. Agric. Food Chem. 2009, 57, 6178–6182. [Google Scholar] [CrossRef]
- Kaushik, G.; Raza, K. Potential of Novel Dunaliella Salina from Sambhar Salt Lake, India, for Bioremediation of Hexavalent Chromium from Aqueous Effluents: An Optimized Green Approach. Ecotoxicol. Environ. Saf. 2019, 180, 430–438. [Google Scholar]
- Mishra, A.; Mandoli, A.; Jha, B. Physiological Characterization and Stress-Induced Metabolic Responses of Dunaliella Salina Isolated from Salt Pan. J. Ind. Microbiol. Biotechnol. 2008, 35, 1093. [Google Scholar] [CrossRef] [PubMed]
- Carillo, P.; Ciarmiello, L.F.; Woodrow, P.; Corrado, G.; Chiaiese, P.; Rouphael, Y. Enhancing Sustainability by Improving Plant Salt Tolerance through Macro- and Micro-Algal Biostimulants. Biology 2020, 9, 253. [Google Scholar] [CrossRef] [PubMed]
- Das, C.; Naseera, K.; Ram, A.; Meena, R.M.; Ramaiah, N. Bioremediation of Tannery Wastewater by a Salt-Tolerant Strain of Chlorella Vulgaris. J. Appl. Phycol. 2017, 29, 235–243. [Google Scholar] [CrossRef]
- Meng, F.; Liu, D.; Huang, W.; Lei, Z.; Zhang, Z. Effect of Salinity on Granulation, Performance and Lipid Accumulation of Algal-Bacterial Granular Sludge. Bioresour. Technol. Rep. 2019, 7, 100228. [Google Scholar] [CrossRef]
- Sommer, V.; Palm, A.; Schink, A.; Leinweber, P.; Gose, N.; Karsten, U.; Glaser, K. Artificial Biocrust Establishment on Materials of Potash Tailings Piles along a Salinity Gradient. J. Appl. Phycol. 2021, 13, 1–7. [Google Scholar] [CrossRef]
- Sommer, V.; Mikhailyuk, T.; Glaser, K.; Karsten, U. Uncovering Unique Green Algae and Cyanobacteria Isolated from Biocrusts in Highly Saline Potash Tailing Pile Habitats, Using an Integrative Approach. Microorganisms 2020, 8, 1667. [Google Scholar] [CrossRef]
- Sommer, V.; Karsten, U.; Glaser, K. Halophilic Algal Communities in Biological Soil Crusts Isolated from Potash Tailings Pile Areas. Front. Ecol. Evol. 2020, 8, 46. [Google Scholar] [CrossRef] [Green Version]
- Moewus, V.L. Systematische Bestimmung Einzelliger Grüner Algen Auf Grund von Kulturversuchen. Bot. Not. 1951, 4, 287–318. [Google Scholar]
- Proeschold, T.; Darienko, T. The Green Puzzle Stichococcus (Trebouxiophyceae, Chlorophyta): New Generic and Species Concept among This Widely Distributed Genus. Phytotaxa 2020, 441, 113–142. [Google Scholar] [CrossRef]
- Hotter, V.; Glaser, K.; Hartmann, A.; Ganzera, M.; Karsten, U. Polyols and UV-Sunscreens in the Prasiola-Clade (Trebouxiophyceae, Chlorophyta) as Metabolites for Stress Response and Chemotaxonomy. J. Phycol. 2018, 54, 264–274. [Google Scholar] [CrossRef] [Green Version]
- Medwed, C.; Holzinger, A.; Hofer, S.; Hartmann, A.; Michalik, D.; Glaser, K.; Karsten, U. Ecophysiological, Morphological, and Biochemical Traits of Free-Living Diplosphaera Chodatii (Trebouxiophyceae) Reveal Adaptation to Harsh Environmental Conditions. Protoplasma 2021, 258, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Neustupa, J.; Elias, M.; Sejnohova, L. A Taxonomic Study of Two Stichococcus Species (Trebouxiophyceae, Chlorophyta) with a Starch-Enveloped Pyrenoid. Nova Hedwig. 2007, 84, 51–64. [Google Scholar] [CrossRef]
- Bohlin, K. Utkast Till de Gröna Algernas Och Arkegoniaternas Fylogeni; Folded chart; Akademisk Afhandling: Uppsala, Sweden, 1901; p. 43 + iv. [Google Scholar]
- Brown, L.M.; Hellebust, J.A. Some New Taxonomic Characteristics Applied to Stichococcus bacillaris (Chlorophyceae). Can. J. Bot. 1980, 58, 1405–1411. [Google Scholar] [CrossRef]
- Brown, L.M.; Hellebust, J.A. Sorbitol and Proline as Intracellular Osmotic Solutes in the Green Alga Stichococcus bacillaris. Can. J. Bot. 1978, 56, 676–679. [Google Scholar] [CrossRef]
- Ahmad, I.; Hellebust, J.A. The Relationship between Inorganic Nitrogen Metabolism and Proline Accumulation in Osmoregulatory Responses of Two Euryhaline Microalgae. Plant Physiol. 1988, 88, 348–354. [Google Scholar] [CrossRef] [Green Version]
- Van, A.T.; Sommer, V.; Glaser, K. The Ecophysiological Performance and Traits of Genera within the Stichococcus-like Clade (Trebouxiophyceae) under Matric and Osmotic Stress. Microorganisms 2021, 9, 1816. [Google Scholar] [CrossRef]
- Van, A.T.; Karsten, U.; Glaser, K. A Chemosystematic Investigation of Selected Stichococcus-like Organisms (Trebouxiophyta). Algae 2021, 36, 123–135. [Google Scholar] [CrossRef]
- Bischoff, H. Some Soil Algae from Enchanted Rock and Related Algal Species. In Phycological Studies IV; University of Texas Publisher No. 6318: Austin, TX, USA, 1963; Volume 6318, pp. 1–95. [Google Scholar]
- Starr, R.C.; Zeikus, J.A. UTEX—The Culture Collection of Algae at the University of Texas at Austin 1993 List of Cultures 1. J. Phycol. 1993, 29, 1–106. [Google Scholar] [CrossRef]
- Gustavs, L.; Eggert, A.; Michalik, D.; Karsten, U. Physiological and Biochemical Responses of Green Microalgae from Different Habitats to Osmotic and Matric Stress. Protoplasma 2010, 243, 3–14. [Google Scholar] [CrossRef]
- Bačkor, M.; Fahselt, D.; Wu, C.T. Free Proline Content Is Positively Correlated with Copper Tolerance of the Lichen Photobiont Trebouxia Erici (Chlorophyta). Plant Science 2004, 167, 151–157. [Google Scholar] [CrossRef]
- Lee, M.R.; Kim, C.S.; Park, T.; Choi, Y.-S.; Lee, K.-H. Optimization of the Ninhydrin Reaction and Development of a Multiwell Plate-Based High-Throughput Proline Detection Assay. Anal. Biochem. 2018, 556, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Karsten, U.; Herburger, K.; Holzinger, A. Living in Biological Soil Crust Communities of African Deserts—Physiological Traits of Green Algal Klebsormidium Species (Streptophyta) to Cope with Desiccation, Light and Temperature Gradients. J. Plant Physiol. 2016, 194, 2–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schreiber, U.; Bilger, W. Progress in Chlorophyll Fluorescence Research: Major Developments during the Past Years in Retrospect. In Progress in Botany/Fortschritte der Botanik; Springer: Berlin/Heidelberg, Germany, 1993; pp. 151–173. [Google Scholar]
- Genty, B.; Briantais, J.-M.; Baker, N.R. The Relationship between the Quantum Yield of Photosynthetic Electron Transport and Quenching of Chlorophyll Fluorescence. Biochim. Biophys. Acta (BBA)-Gen. Subj. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development for R; RStudio, PBC: Boston, MA, USA, 2020. [Google Scholar]
- Gustavs, L.; Görs, M.; Karsten, U. Polyol Patterns in Biofilm-Forming Aeroterrestrial Green Algae (Trebouxiophyceae, Chlorophyta): Polyols in Aeroterrestrial Trebouxiophyceae. J. Phycol. 2011, 47, 533–537. [Google Scholar] [CrossRef]
- Hayward, J. Studies on the Growth of Stichococcus Bacillaris Naeg in Culture. J. Mar. Biol. Ass. 1974, 54, 261–268. [Google Scholar] [CrossRef]
- Ben Rejeb, K.; Abdelly, C.; Savouré, A. How Reactive Oxygen Species and Proline Face Stress Together. Plant Physiol. Biochem. 2014, 80, 278–284. [Google Scholar] [CrossRef]
- Szabados, L.; Savouré, A. Proline: A Multifunctional Amino Acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Hoekstra, F.A.; Golovina, E.A.; Buitink, J. Mechanisms of Plant Desiccation Tolerance. Trends Plant Sci. 2001, 6, 431–438. [Google Scholar] [CrossRef]
- Gray, D.W.; Lewis, L.A.; Cardon, Z.G. Photosynthetic Recovery Following Desiccation of Desert Green Algae (Chlorophyta) and Their Aquatic Relatives. Plant Cell Environ. 2007, 30, 1240–1255. [Google Scholar] [CrossRef]
- Ritchie, R.J.; Heemboo, M. Trentepohlia sp., a Terrestrial Chlorophyte Growing on Galvanised Iron Lamp Posts. Phycologia 2021, 60, 48–61. [Google Scholar] [CrossRef]
- Pierangelini, M.; Glaser, K.; Mikhailyuk, T.; Karsten, U.; Holzinger, A. Light and Dehydration but Not Temperature Drive Photosynthetic Adaptations of Basal Streptophytes (Hormidiella, Streptosarcina and Streptofilum) Living in Terrestrial Habitats. Microb. Ecol. 2019, 77, 380–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terlova, E.F.; Holzinger, A.; Lewis, L.A. Terrestrial Green Algae Show Higher Tolerance to Dehydration than Do Their Aquatic Sister-Species. Microb. Ecol. 2021, 82, 770–782. [Google Scholar] [CrossRef] [PubMed]
- Holzinger, A.; Kaplan, F.; Blaas, K.; Zechmann, B.; Komsic-Buchmann, K.; Becker, B. Transcriptomics of Desiccation Tolerance in the Streptophyte Green Alga Klebsormidium Reveal a Land Plant-like Defense Reaction. PLoS ONE 2014, 9, e110630. [Google Scholar] [CrossRef] [PubMed]
- Peredo, E.L.; Cardon, Z.G. Shared Up-Regulation and Contrasting down-Regulation of Gene Expression Distinguish Desiccation-Tolerant from Intolerant Green Algae. Proc. Natl. Acad. Sci. USA 2020, 117, 17438–17445. [Google Scholar] [CrossRef]
- Gasulla, F.; del Campo, E.M.; Casano, L.M.; Guéra, A. Advances in Understanding of Desiccation Tolerance of Lichens and Lichen-Forming Algae. Plants 2021, 10, 807. [Google Scholar] [CrossRef]
- Bwapwa, J.; Jaiyeola, A.; Chetty, R. Bioremediation of Acid Mine Drainage Using Algae Strains: A Review. S. Afr. J. Chem. Eng. 2017, 24, 62–70. [Google Scholar] [CrossRef]
- Figler, A.; B-Béres, V.; Dobronoki, D.; Márton, K.; Nagy, S.A.; Bácsi, I. Salt Tolerance and Desalination Abilities of Nine Common Green Microalgae Isolates. Water 2019, 11, 2527. [Google Scholar] [CrossRef] [Green Version]
- Gan, X.; Shen, G.; Xin, B.; Li, M. Simultaneous Biological Desalination and Lipid Production by Scenedesmus Obliquus Cultured with Brackish Water. Desalination 2016, 400, 1–6. [Google Scholar] [CrossRef]


| Strain ID | Species Assignment | Locality and Habitat | Collector/Isolator |
|---|---|---|---|
| SAG 1.92 | Desmococcus olivaceus * | Vienna, Austria; subaerial | W. Vischer, before 1960 |
| ASIB-IB-37 | Deuterostichococcus tetrallantoides * | Dauphin Island, Alabama, USA; soil | T.R. Deason, 1969 |
| SAG 380-1 | Pseudostichococcus monallantoides * | Germany; marine | L. Moewus, 1951 |
| ZL-4-1 | Pseudostichococcus monallantoides | Teutschenthal, Germany; potash tailing heap surroundings | V. Sommer, 2018 |
| TT-5-1-K | Pseudostichococcus monallantoides | Zielitz, Germany; potash tailing heap surroundings | V. Sommer, 2018 |
| LB 1820 | Pseudostichococcus sequoieti * | USA; redwood forest soil | G. Arce, 1971 |
| CALU-1142 | Pseudostichococcus undulates * | Dolomite Mountains, Italy | G. Vinatzer, 1975 |
| CCAP 379/1A | Stichococcus bacillaris * | Likely Switzerland | W. Vischer, before 1936 |
| SAG 2406 | Tritostichococcus solitus * | Northeim, Germany; karstwater stream rock surface | K. Mohr, 2003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van, A.T.; Glaser, K. Pseudostichococcus Stands Out from Its Siblings Due to High Salinity and Desiccation Tolerance. Phycology 2022, 2, 108-119. https://doi.org/10.3390/phycology2010007
Van AT, Glaser K. Pseudostichococcus Stands Out from Its Siblings Due to High Salinity and Desiccation Tolerance. Phycology. 2022; 2(1):108-119. https://doi.org/10.3390/phycology2010007
Chicago/Turabian StyleVan, Anh Tu, and Karin Glaser. 2022. "Pseudostichococcus Stands Out from Its Siblings Due to High Salinity and Desiccation Tolerance" Phycology 2, no. 1: 108-119. https://doi.org/10.3390/phycology2010007
APA StyleVan, A. T., & Glaser, K. (2022). Pseudostichococcus Stands Out from Its Siblings Due to High Salinity and Desiccation Tolerance. Phycology, 2(1), 108-119. https://doi.org/10.3390/phycology2010007






