Shining Light on Photosynthesis in the Harmful Dinoflagellate Karenia mikimotoi–Responses to Short-Term Changes in Temperature, Nitrogen Form, and Availability
Abstract
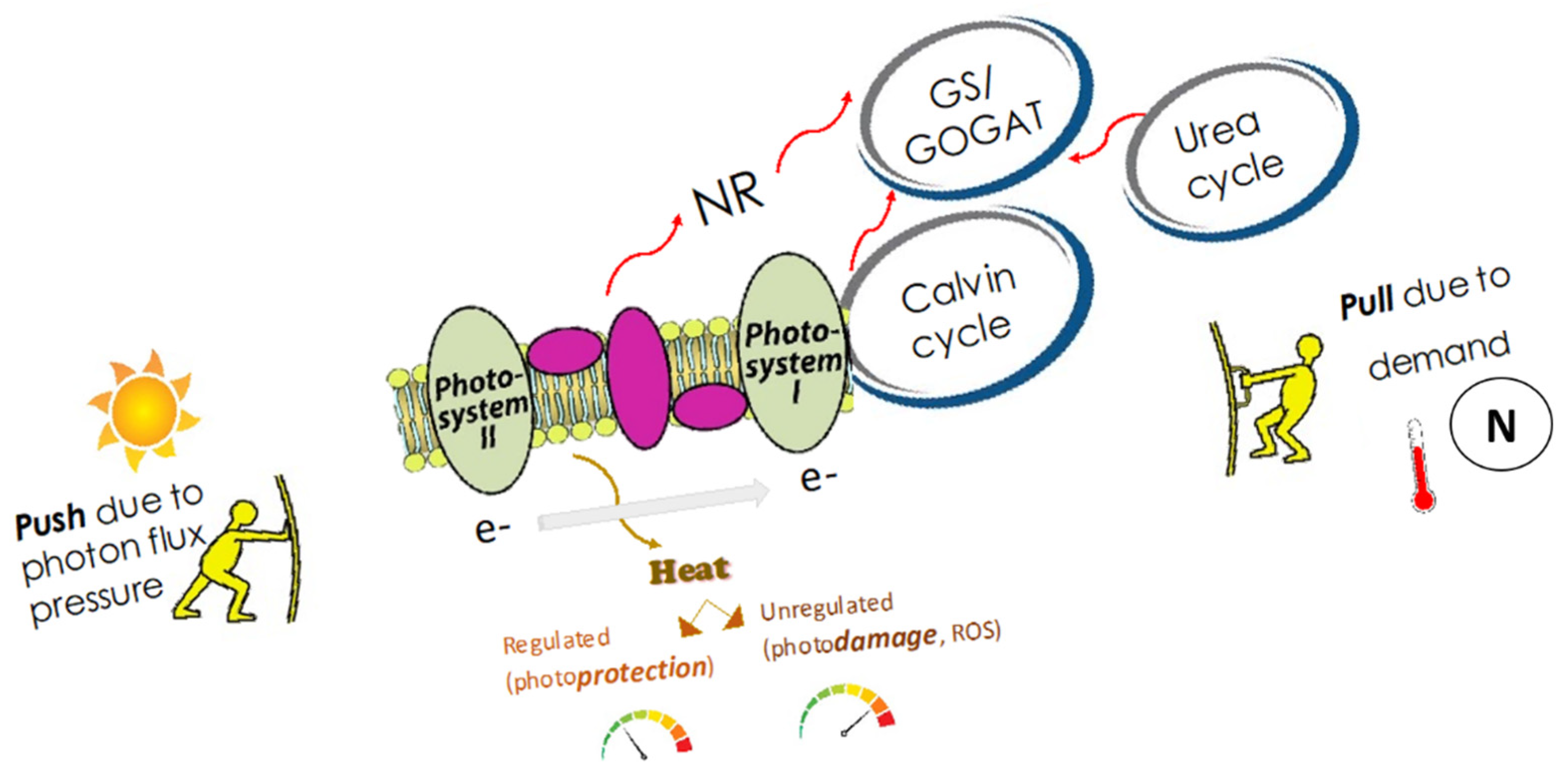
:1. Introduction
2. Materials and Methods
2.1. Algal Culture
2.2. Specific Growth Rate, Nutrient Analysis
2.3. Experimental Setup
2.4. Chl Fluorescence Measurements
2.5. 15N Isotope Analysis
2.6. Statistical Analysis
3. Results
3.1. Initial Cell and Nutrient Conditions
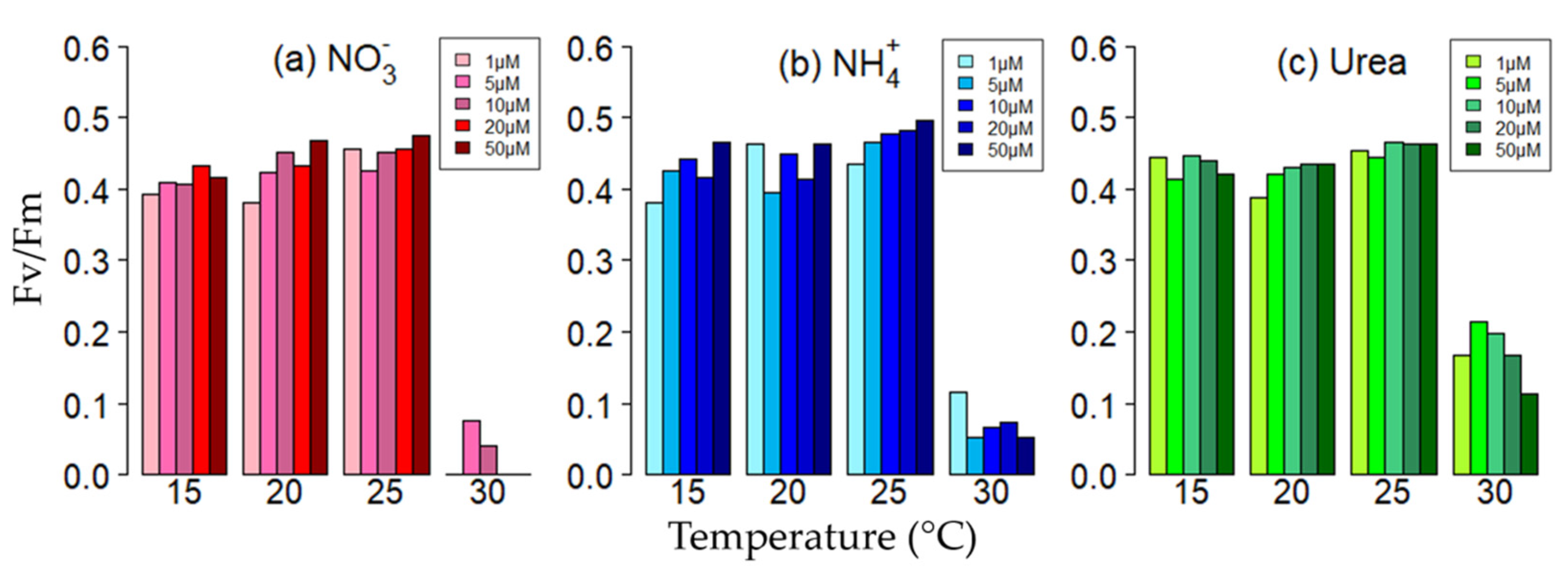
3.2. PSII Quantum Efficiency and Quantum Yields
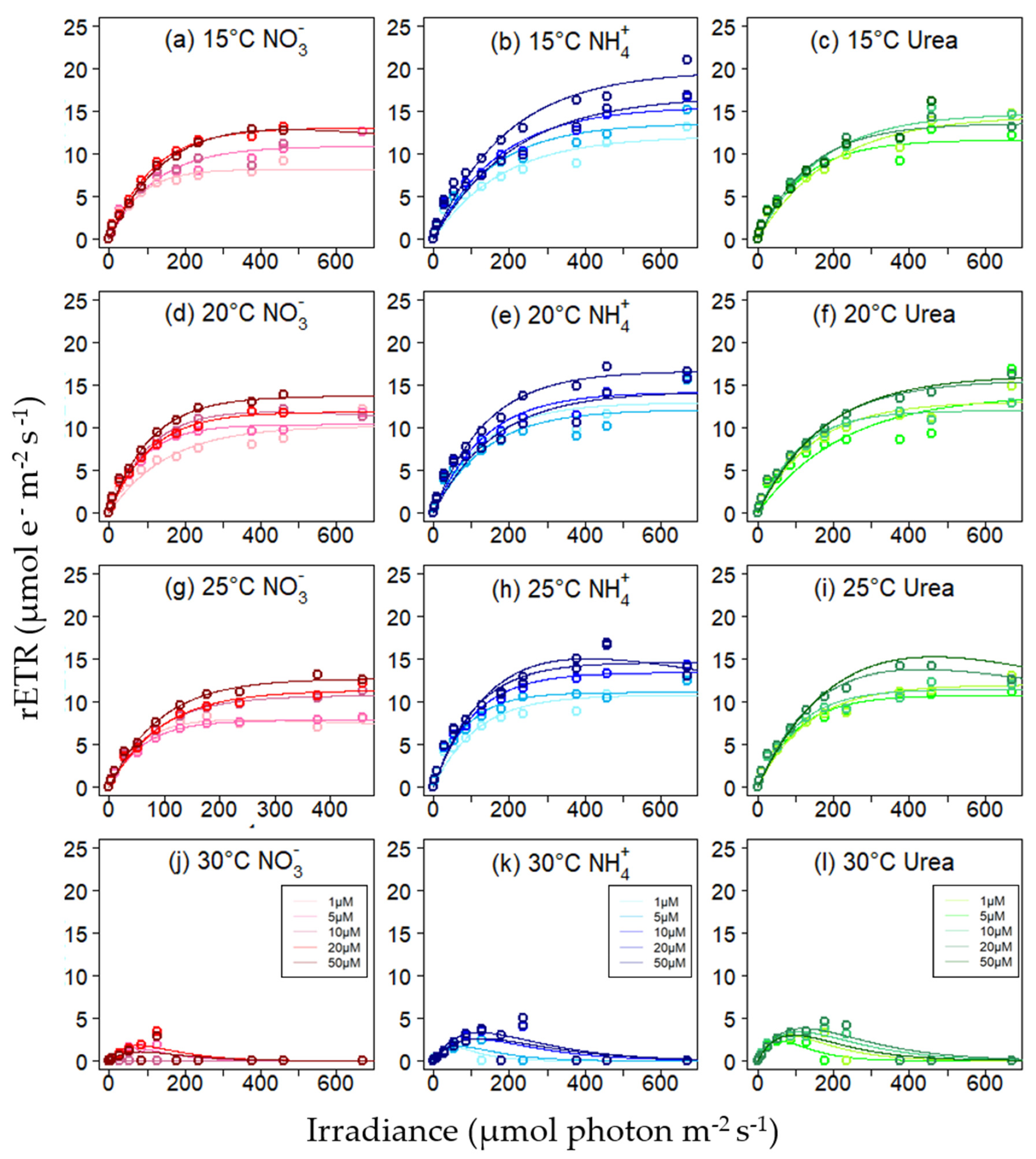
3.3. Relative Electron Transfer Rate
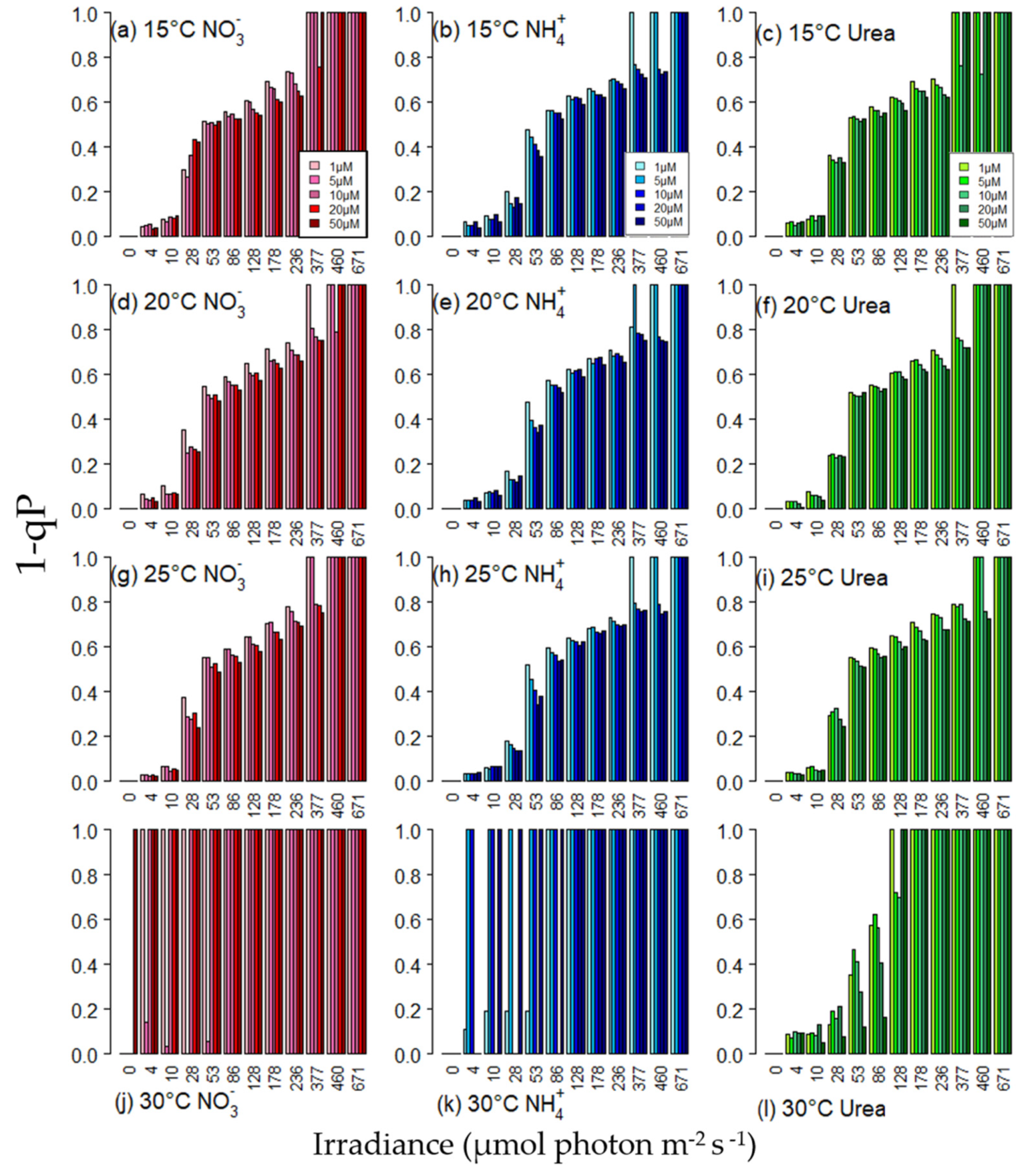
3.4. Excitation Pressure
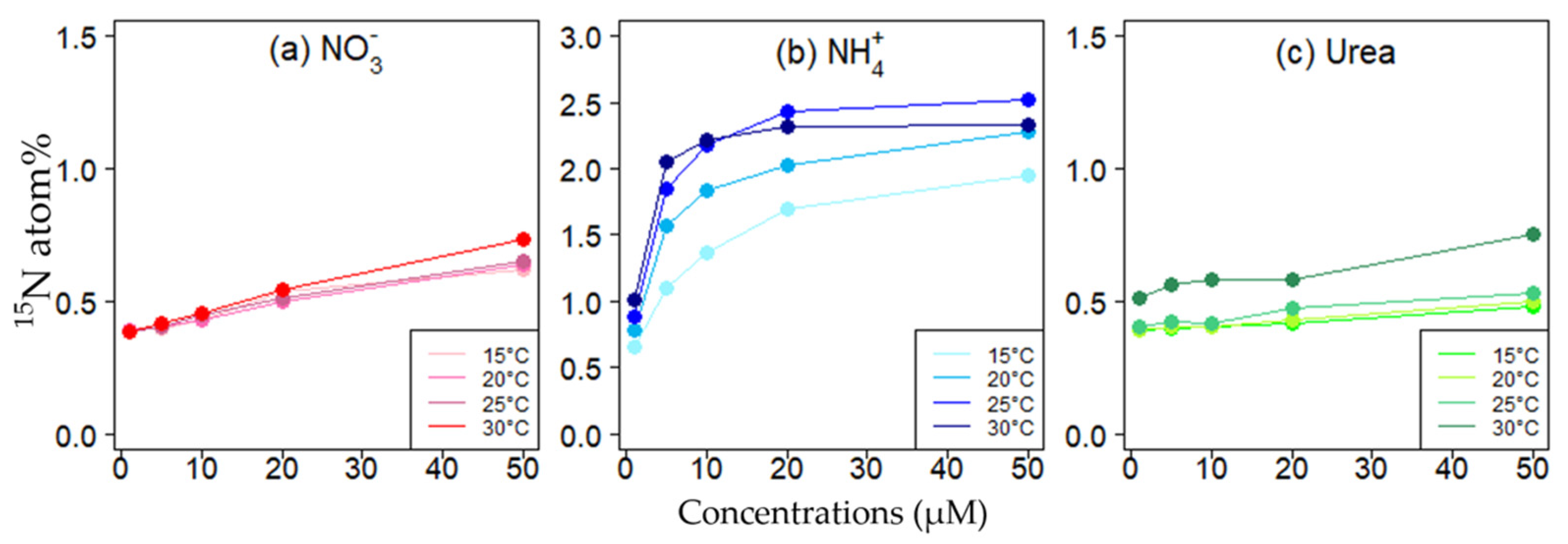
3.5. 15N Isotope Incorporation
4. Discussion
4.1. Temperature, Energy (Light) and Material (N) Addition Effects on Photosynthesis
4.2. N Form Effects on Photosynthesis at 30 °C
4.3. Implications of Temperature Effects for Natural Assemblages
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brand, L.E.; Campbell, L.; Bresnan, E. Karenia: The biology and ecology of a toxic genus. Harmful Algae 2012, 14, 156–178. [Google Scholar] [CrossRef]
- Li, X.; Yan, T.; Yu, R.; Zhou, M. A review of karenia mikimotoi: Bloom events, physiology, toxicity and toxic mechanism. Harmful Algae 2019, 90, 101702. [Google Scholar] [CrossRef] [PubMed]
- Haywood, A.J.; Steidinger, K.A.; Truby, E.W.; Bergquist, P.R.; Bergquist, P.L.; Adamson, J.; MacKenzie, L. Comparative morphology and molecular phylogenetic analysis of three new species of the genus Karenia (Dinophyceae) from New Zealand. J. Phycol. 2004, 40, 165–179. [Google Scholar] [CrossRef]
- Lenes, J.M.; Heil, C.A. A historical analysis of the potential nutrient supply from the N2 fixing marine cyanobacterium Trichodesmium spp. to Karenia brevis blooms in the eastern Gulf of Mexico. J. Plankton Res. 2010, 32, 1421–1431. [Google Scholar] [CrossRef] [Green Version]
- Weisberg, R.H.; Zheng, L.; Liu, Y.; Lembke, C.; Lenes, J.M.; Walsh, J.J. Why no red tide was observed on the West Florida Continental Shelf in 2010. Harmful Algae 2014, 38, 119–216. [Google Scholar] [CrossRef]
- Weisberg, R.H.; He, R. Local and deep-ocean forcing contributions to anomalous water properties on the West Florida Shelf. J. Geophys Res. Oceans 2003, 108, 3184. [Google Scholar] [CrossRef]
- Glibert, P.M.; Wilkerson, F.P.; Dugdale, R.C.; Raven, J.A.; Dupont, C.L.; Leavitt, P.R.; Parker, A.E.; Burkholder, J.M.; Kana, T.M. Pluses and minuses of ammonium and nitrate uptake and assimilation by phytoplankton and implications for productivity and community composition, with emphasis on nitrogen-enriched conditions. Limnol. Oceanogr. 2016, 61, 165–197. [Google Scholar] [CrossRef]
- Gao, Y.; Smith, G.J.; Alberte, R.S. Nitrate reductase from the marine diatom Skeletonema costatum (biochemical and immunological characterization). Plant Physiol. 1983, 103, 1437–1445. [Google Scholar] [CrossRef] [Green Version]
- Kristiansen, S. The temperature optimum of the nitrate reductase assay for marine phytoplankton. Limnol. Oceanogr. 1983, 28, 776–780. [Google Scholar] [CrossRef]
- Lomas, M.W.; Glibert, P.M. Temperature regulation of nitrate uptake: A novel hypothesis about nitrate uptake and reduction in cool-water diatoms. Limnol. Oceanogr. 1999, 44, 556–572. [Google Scholar] [CrossRef]
- Lomas, M.W.; Glibert, P.M. Interactions between NH4+ and NO3− uptake and assimilation: Comparison of diatoms and dinoflagellates at several growth temperatures. Mar. Biol. 1999, 133, 541–551. [Google Scholar] [CrossRef]
- Clayton, J.R., Jr.; Ahmed, S.I. Detection of glutamate synthase (GOGAT) activity in phytoplankton: Evaluation of cofactors and assay optimization. Mar. Ecol. Prog. Ser. 1986, 32, 115–122. [Google Scholar] [CrossRef]
- Fan, C.; Glibert, P.M.; Alexander, J.; Lomas, M.W. Characterization of urease activity in three marine phytoplankton species, Aureococcus anophagefferens, Prorocentrum minimum, and Thalassiosira weissflogii. Mar. Biol. 2003, 142, 949–958. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef] [Green Version]
- Kana, T.M.; Geider, R.J.; Critchley, C. Regulation of photosynthetic pigments in micro-algae by multiple environmental factors: A dynamic balance hypothesis. New Phytol. 1997, 137, 629–638. [Google Scholar] [CrossRef]
- Ras, M.; Steyer, J.; Bernard, O. Temperature effect on microalgae: A crucial factor for outdoor production. Rev. Environ. Sci. Bio. 2013, 12, 153–164. [Google Scholar] [CrossRef] [Green Version]
- Peers, G.; Truong, T.B.; Ostendorf, E.; Busch, A.; Elrad, D.; Grossman, A.R.; Hippler, M.; Niyogi, K.K. An ancient light-harvesting protein is critical for the regulation of algal photosynthesis. Nature 2009, 462, 518–521. [Google Scholar] [CrossRef]
- Perozoni, F.; Beghini, G.; Cazzaniga, S.; Ballottari, M. Clamydomonas reinhardtii LHCSR1 and LHCSR3 proteins involved in photoprotective non-photochemical quenching have different quenching efficiency and different carotenoid affinity. Sci. Rep. 2020, 10, 21957. [Google Scholar] [CrossRef]
- Huang, K.; Feng, Q.; Zhang, Y.; Ou, L.; Cen, J.; Lu, S.; Qi, Y. Comparative uptake and assimilation of nitrate, ammonium, and urea by dinoflagellate Karenia mikimotoi and diatom Skeletonema costatum s.l. in the coastal waters of the East China Sea. Mar. Pollut. Bull. 2020, 155, 111200. [Google Scholar] [CrossRef]
- Zhang, Q.; Rencheng, Y.; Jiangjing, S.; Tian, Y.; Yunfeng, W.; Mingjiang, Z. Will harmful dinoflagellate Karenia mikimotoi grow phagotrophically? Chin. J. Oceanol. Limn. 2011, 29, 849–859. [Google Scholar] [CrossRef]
- Zhang, Q.; Song, J.; Yu, R.; Yan, T.; Wang, Y.; Kong, F.; Zhou, M. Roles of mixotrophy in blooms of different dinoflagellates: Implications from the growth experiment. Harmful Algae 2013, 30, 10–26. [Google Scholar] [CrossRef]
- Heil, C.A.; Dixon, L.K.; Hall, E.; Garrett, M.; Lenes, J.M.; O’Neil, J.M.; Walsh, B.M.; Bronk, D.A.; Killberg-Thoreson, L.; Hitchcock, G.L.; et al. Blooms of Karenia brevis (Davis) G. Hansen & Ø. Moestrup on the West Florida Shelf: Nutrient sources and potential management strategies based on a multi-year regional study. Harmful Algae 2014, 38, 127–140. [Google Scholar]
- Glibert, P.M.; Burkholder, J.M.; Kana, T.M.; Alexander, J.; Skelton, H.; Shilling, C. Grazing by Karenia brevis on Synechococcus enhances its growth rate and may help to sustain blooms. Aquat. Microb. Ecol. 2009, 44, 17–30. [Google Scholar] [CrossRef] [Green Version]
- Jeong, H.J.; Park, J.Y.; Nho, J.H.; Park, M.O.; Ha, J.H.; Seong, K.A.; Jeng, C.; Seong, C.N.; Lee, K.Y.; Yih, W.H. Feeding by red-tide dinoflagellates on the cyanobacterium Synechococcus. Aquat. Microb. Ecol. 2005, 41, 131–143. [Google Scholar] [CrossRef] [Green Version]
- Guillard, R.R. Culture of phytoplankton for feeding marine invertebrates. In Culture of Marine Invertebrate Animals; Smith, W.L., Chanley, M.H., Eds.; Plenum Press: New York, NY, USA, 1975; pp. 29–60. [Google Scholar]
- Schreiber, U.; Bilger, W.; Neubauer, C. Chlorophyll fluorescence as a non-intrusive indicator for rapid assessment of in vivo photosynthesis. In Ecophysiology of Photosynthesis; Schulze, E., Caldwell, M.M., Eds.; Springer: Berlin/Heidelberg, Germany, 1994; pp. 49–70. [Google Scholar]
- Kramer, D.M.; Johnson, G.; Kiirats, O.; Edwards, G.E. New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Photosynth. Res. 2004, 79, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Platt, T.; Gallegos, C.L.; Harrison, W.G. Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton. J. Mar. Res. 1980, 38, 687–701. [Google Scholar]
- Maxwell, K.; Johnson, G. Chlorophyll fluorescence-a practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Glibert, P.M.; Middelburg, J.J.; McClelland, J.W.; Zanden, M.J.V. Stable isotope tracers: Enriching our perspectives and questions on sources, fates, rates, and pathways of major elements in aquatic systems. Limnol. Oceanogr. 2019, 64, 950–981. [Google Scholar] [CrossRef] [Green Version]
- Padfield, D.; Yvon-Durocher, G.; Buckling, A.; Jennings, S.; Yvon-Durocher, G. Rapid evolution of metabolic traits explains thermal adaptation in phytoplankton. Ecol. Lett. 2015, 19, 133–142. [Google Scholar] [CrossRef] [Green Version]
- Shen, A.; Ma, Z.; Jiang, K.; Li, D. Effects of temperature on growth, photophysiology, Rubisco gene expression in Prorocentrum donghaiense and Karenia mikimotoi. Ocean Sci. J. 2016, 51, 581–589. [Google Scholar] [CrossRef]
- Hikosaka, K.; Ishikawa, K.; Borjigidal, A.; Muller, O.; Onoda, Y. Temperature acclimation of photosynthesis: Mechanisms involved in the changes in temperature dependence of photosynthetic rate. J. Exp. Bot. 2006, 57, 291–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, A.Q.; Shi, X.Y.; Ding, Y.Y.; Tang, H.J.; Wang, L.S.; Wang, X.L. Effects of temperature on the growth and nitrate reductase activity of Chaetoceros curvisetus and Karenia mikimotoi. Prog. Biochem. Biophys. 2014, 41, 896–9003. [Google Scholar]
- Dagenais-Bellefeuille, S.; Morse, D. Putting N in dinoflagellates. Front. Microbiol. 2013, 4, 369. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.; Xiao, Y.; Liu, L.; Xie, Y.; Ma, R. Transcriptome responses of the dinoflagellate Karenia mikimotoi driven by nitrogen deficiency. Harmful Algae 2021, 103, 101977. [Google Scholar] [CrossRef]
- Solomon, C.M.; Collier, J.L.; Berg, G.M.; Glibert, P.M. Role of urea in microbial metabolism in aquatic systems: A biochemical and molecular review. Aquat. Microb. Ecol. 2010, 59, 67–88. [Google Scholar] [CrossRef]
- Gentien, P. Bloom dynamics and ecophysiology of the Gymnodinium mikimotoi species complex. In Physiological Ecology of Harmful Algal Blooms; Anderson, D.M., Cembella, A.D., Hallegraeff, G.M., Eds.; Springer: Berlin/Heidelberg, Germany, 1998; pp. 155–173. [Google Scholar]
- Shen, A.; Yuan, X.; Liu, G.; Li, D. Growth interactions between the bloom-forming dinoflagellates Prorocentrum donghaiense and Karenia mikimotoi under different temperature. Thalassas 2014, 30, 33–45. [Google Scholar]
- Eppley, R.W. Temperature and phytoplankton growth in the sea. Fish. Bull. 1972, 70, 1063–1085. [Google Scholar]
- Marañón, E.; Lorenzo, M.P.; Cermeño, P.; Mouriño-Carballido, B. Nutrient limitation suppresses the temperature dependence of phytoplankton metabolic rates. ISME J. 2018, 12, 1836–1845. [Google Scholar] [CrossRef] [Green Version]
- Glibert, P.M.; Harrison, J.; Heil, C.; Seitzinger, S. Escalating worldwide use of urea—A global change contributing to coastal eutrophication. Biogeochemistry 2006, 77, 441–463. [Google Scholar] [CrossRef]



| ANOVA (N form Effects) | ||||
|---|---|---|---|---|
| Fv/Fm | rETRmax | |||
| F(df = 2) | p-value | F (df = 2) | p-value | |
| 15 °C | 1.329 | 0.301 | 4.901 | 0.028 |
| 20 °C | 0.329 | 0.726 | 3.942 | 0.048 |
| 25 °C | 1.361 | 0.293 | 3.238 | 0.075 |
| 30 °C | 25.58 | <0.001 | 12.85 | 0.001 |
| NO3− | NH4+ | Urea | ||||
|---|---|---|---|---|---|---|
| Fv/Fm | rETRmax | Fv/Fm | rETRmax | Fv/Fm | rETRmax | |
| 15 °C | 0.41 ± 0.01 | 11.2 ± 2.0 | 0.43 ± 0.03 | 15.6 ± 3.0 | 0.43 ± 0.02 | 14.1 ± 1.6 |
| 20 °C | 0.43 ± 0.03 | 11.6 ± 1.4 | 0.44 ± 0.03 | 14.0 ± 1.7 | 0.42 ± 0.02 | 14.1 ± 1.7 |
| 25 °C | 0.45 ± 0.02 | 10.1 ± 2.2 | 0.47 ± 0.02 | 13.0 ± 2.0 | 0.46 ± 0.01 | 12.7 ± 1.8 |
| 30 °C | 0.02 ± 0.03 | 0.9 ± 0.8 | 0.07 ± 0.03 | 2.4 ± 0.7 | 0.17 ± 0.04 | 3.1 ± 0.6 |
| ANOVA (temperature effects at 15–25 °C) | ||||||
| F (df = 2) | 3.935 | 0.856 | 3.368 | 1.613 | 7.485 | 1.192 |
| p-value | 0.049 | 0.449 | 0.069 | 0.240 | 0.008 | 0.337 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, S.H.; Glibert, P.M. Shining Light on Photosynthesis in the Harmful Dinoflagellate Karenia mikimotoi–Responses to Short-Term Changes in Temperature, Nitrogen Form, and Availability. Phycology 2022, 2, 30-44. https://doi.org/10.3390/phycology2010002
Ahn SH, Glibert PM. Shining Light on Photosynthesis in the Harmful Dinoflagellate Karenia mikimotoi–Responses to Short-Term Changes in Temperature, Nitrogen Form, and Availability. Phycology. 2022; 2(1):30-44. https://doi.org/10.3390/phycology2010002
Chicago/Turabian StyleAhn, So Hyun (Sophia), and Patricia M. Glibert. 2022. "Shining Light on Photosynthesis in the Harmful Dinoflagellate Karenia mikimotoi–Responses to Short-Term Changes in Temperature, Nitrogen Form, and Availability" Phycology 2, no. 1: 30-44. https://doi.org/10.3390/phycology2010002







