Natural Feed Additives in Sub-Saharan Africa: A Systematic Review of Efficiency and Sustainability in Ruminant Production
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
- Studies involving ruminant animals.
- Use of natural feed additives, such as plant extracts, herbs, essential oils, organic acids, probiotics, enzymes, clay, seaweed, or tannins.
- Only additives derived from natural sources were considered.
- Studies reporting on production efficiency outcomes.
- Studies reporting on sustainability indicators.
- Research conducted within countries in Sub-Saharan Africa
- Studies published in English
- Originally published research articles
- Studies were excluded based on the following criteria:
- Studies focusing on non-ruminant species.
- Research conducted outside Sub-Saharan Africa.
- Use of synthetic or chemical feed additives, such as antibiotics, synthetic vitamins, or hormonal growth promoters.
- Studies where the nature of the additive cannot be determined or is not natural.
- Studies not reporting on production efficiency or sustainability-related outcomes.
- Nutritional composition or feed formulation studies without animal performance data.
- Revisions, books, short communications, congress papers, review articles, and letters.
2.2. Assessment of Risk of Bias
3. Results
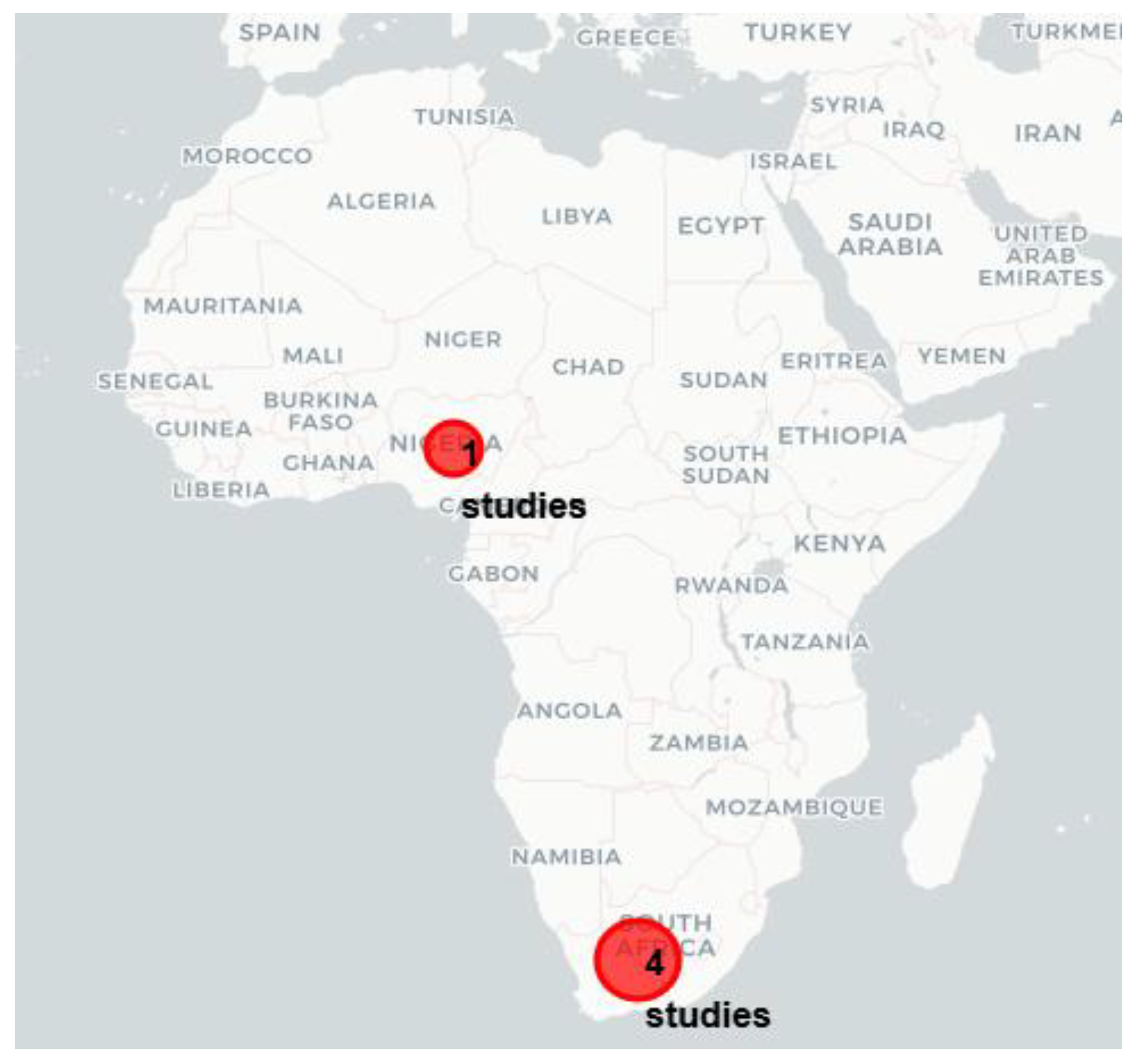
3.1. Characteristics of the Included Studies
3.2. Synthesised Findings
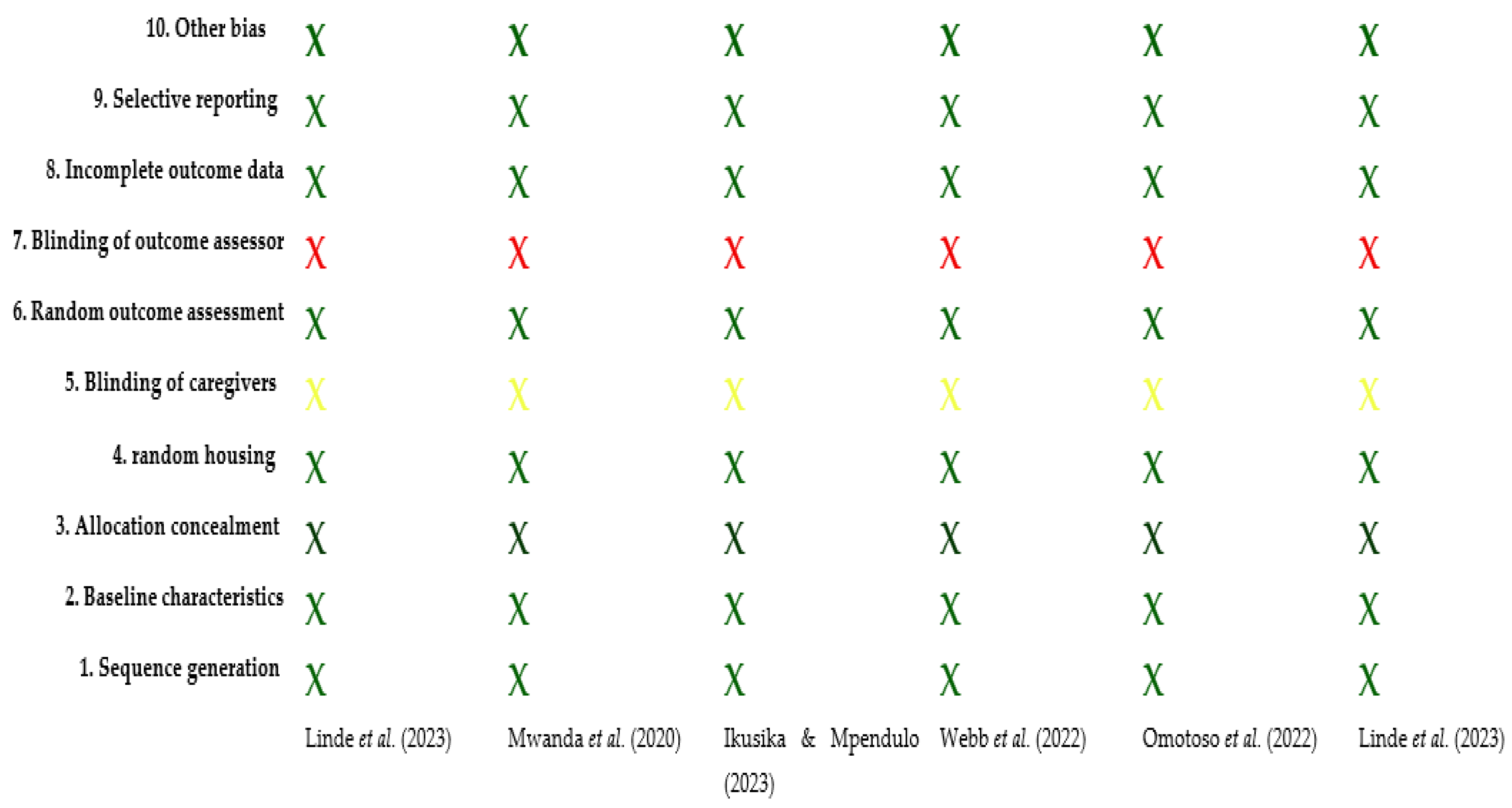
3.3. Assessment of Risk of Bias
4. Discussion
4.1. Efficiency of Natural Feed Additives
4.1.1. Fossil Shell Flour
4.1.2. Curcuma Longa (Turmeric Rhizome Powder)
4.1.3. Probiotic (EO) and Essential Oil (PRO)
4.1.4. Azadirachta indica and Moringa oleifera Leaf Extracts
4.2. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Erdaw, M.M. Contribution, prospects and trends of livestock production in sub-Saharan Africa: A review. Int. J. Agric. Sustain. 2023, 21, 2247776. [Google Scholar] [CrossRef]
- Ayantunde, A.A.; Duncan, A.J.; Van Wijk, M.T.; Thorne, P. Role of herbivores in sustainable agriculture in Sub-Saharan Africa. Animal 2018, 12, s199–s209. [Google Scholar] [CrossRef] [PubMed]
- Anim-Jnr, A.S.; Sasu, P.; Bosch, C.; Mabiki, F.P.; Frimpong, Y.O.; Emmambux, M.N.; Greathead, H.M.R. Sustainable small ruminant production in low-and middle-income African countries: Harnessing the potential of agroecology. Sustainability 2023, 15, 15326. [Google Scholar] [CrossRef]
- Katiku, P.N.; Kimitei, R.K.; Korir, B.K.; Muasya, T.K.; Chengole, J.M.; Ogillo, B.P.; Munyasi, J.W.; Karimi, S.K. Value chain assessment of small ruminant production, challenges and opportunities: The case of southern rangelands of Kenya. Livest. Res. Rural. Dev. 2013, 25, 1. [Google Scholar]
- Alemayehu, K. Value chain assessment of beef cattle production and marketing in Ethiopia: Challenges and opportunities of linking smallholder farmers to the markets. Livest. Res. Rural Dev. 2011, 23, 255–265. [Google Scholar]
- Mueller, B.; Acero, F.; Estruch, E. Creating Employment Potential in Small-Ruminant Value Chains in the Ethiopian Highlands; FAO: Rome, Italy, 2017. [Google Scholar]
- Stroebel, A. Socio-Economic Complexities of Smallholder Resource-Poor Ruminant Livestock Production Systems in Sub-Saharan Africa. Ph.D. Thesis, University of the Free State, Bloemfontein, South Africa, 2004. [Google Scholar]
- Cooke, A.S.; Machekano, H.; Gwiriri, L.C.; Tinsley, J.H.; Silva, G.M.; Nyamukondiwa, C.; Safalaoh, A.; Morgan, E.R.; Lee, M.R. The nutritional feed gap: Seasonal variations in ruminant nutrition and knowledge gaps in relation to food security in Southern Africa. Food Secur. 2024, 17, 73–100. [Google Scholar] [CrossRef]
- Wang, J.; Deng, L.; Chen, M.; Che, Y.; Li, L.; Zhu, L.; Chen, G.; Feng, T. Phytogenic feed additives as natural antibiotic alternatives in animal health and production: A review of the literature of the last decade. Anim. Nutr. 2024, 17, 244–264. [Google Scholar] [CrossRef]
- Honan, M.; Feng, X.; Tricarico, J.M.; Kebreab, E. Feed additives as a strategic approach to reduce enteric methane production in cattle: Modes of action, effectiveness and safety. Anim. Prod. Sci. 2021, 62, 1303–1317. [Google Scholar] [CrossRef]
- Chisoro, P.; Jaja, I.F.; Assan, N. Incorporation of local novel feed resources in livestock feed for sustainable food security and circular economy in Africa. Front. Sustain. 2023, 4, 1251179. [Google Scholar] [CrossRef]
- World Commission on Environment and Development (WCED). Our Common Future; Oxford University Press: Oxford, UK, 1987. [Google Scholar]
- Food and Agriculture Organisation of the United Nations (FAO). Building a Common Vision for Sustainable Food and Agriculture: Principles and Approaches; FAO: Rome, Italy, 2014; Available online: https://www.fao.org/3/i3940e/i3940e.pdf (accessed on 12 April 2025).
- Hossain, M.S.; Small, B.C.; Kumar, V.; Hardy, R. Utilisation of functional feed additives to produce cost—effective, eco-friendly aquafeeds high in plant—based ingredients. Rev. Aquac. 2024, 16, 121–153. [Google Scholar] [CrossRef]
- Arsène, M.M.; Davares, A.K.; Andreevna, S.L.; Vladimirovich, E.A.; Carime, B.Z.; Marouf, R.; Khelifi, I. Probiotics are used in animal feeding for safe production and as potential alternatives to antibiotics. Vet. World 2021, 14, 319. [Google Scholar] [CrossRef]
- Bąkowski, M.; Kiczorowska, B. Probiotic microorganisms and herbs in ruminant nutrition as natural modulators of health and production efficiency–a review. Ann. Anim. Sci. 2021, 21, 3–28. [Google Scholar] [CrossRef]
- Kumar, K.; Dey, A.; Rose, M.K.; Dahiya, S.S. Impact of dietary phytogenic composite feed additives on immune response, antioxidant status, methane production, growth performance, and nutrient utilisation of buffalo (Bubalus bubalis) calves. Antioxidants 2022, 11, 325. [Google Scholar] [CrossRef] [PubMed]
- Swelum, A.A.; Hashem, N.M.; Abdelnour, S.A.; Taha, A.E.; Ohran, H.; Khafaga, A.F.; El-Tarabily, K.A.; Abd El-Hack, M.E. Effects of phytogenic feed additives on the reproductive performance of animals. Saudi J. Biol. Sci. 2021, 28, 5816–5822. [Google Scholar] [CrossRef] [PubMed]
- Parkunan, T.; Bharti, M.K.; Govindasamy, T.; Kumar, M.; Ramasamy, D.K.; Mahesh, M.S. Herbal Feed Additives and Supplements for a Sustainable Ruminant Production. In Feed Additives and Supplements for Ruminants; Springer Nature Singapore: Singapore, 2024; pp. 197–234. [Google Scholar]
- Kelly, L.; Kebreab, E. Recent advances in feed additives have the potential to mitigate enteric methane emissions from ruminant livestock. J. Soil. Water Conserv. 2023, 78, 111–123. [Google Scholar] [CrossRef]
- Balehegn, M.; Ayantunde, A.; Amole, T.; Njarui, D.; Nkosi, B.D.; Müller, F.L.; Meeske, R.; Tjelele, T.J.; Malebana, I.M.; Madibela, O.R.; et al. Forage conservation in sub-Saharan Africa: Review of experiences, challenges, and opportunities. Agron. J. 2022, 114, 75–99. [Google Scholar] [CrossRef]
- Palangi, V.; Lackner, M. Management of enteric methane emissions in ruminants using feed additives: A review. Animals 2022, 12, 3452. [Google Scholar] [CrossRef]
- Tsiplakou, E.; Pitino, R.; Manuelian, C.L.; Simoni, M.; Mitsiopoulou, C.; De Marchi, M.; Righi, F. Plant feed additives as natural alternatives to the use of synthetic antioxidant vitamins in livestock animal products yield, quality, and oxidative status: A review. Antioxidants 2021, 10, 780. [Google Scholar] [CrossRef]
- Ogutu, F.O.; Okiko, G.; Wanjala, G.; Luvitaa, S.; Obong’o, B.O.; Vriesekoop, F.; Munialo, C.D. Unlocking the potential of plant-based foods in sub-Saharan Africa: A review of the opportunities and challenges. Int. J. Food Sci. Technol. 2024, 59, 5326–5342. [Google Scholar] [CrossRef]
- Sejian, V.; Silpa, M.V.; Lees, A.M.; Krishnan, G.; Devaraj, C.; Bagath, M.; Anisha, J.P.; Reshma Nair, M.R.; Manimaran, A.; Bhatta, R.; et al. Opportunities, challenges, and ecological footprint of sustaining small ruminant production in the changing climate scenario. Agroecol. Footpr. Manag. Sustain. Food Syst. 2020, Dec. 17, 365–396. [Google Scholar]
- Rethlefsen, M.L.; Kirtley, S.; Waffenschmidt, S.; Ayala, A.P.; Moher, D.; Page, M.J.; Koffel, J.B. PRISMA-S: An extension to the PRISMA statement for reporting literature searches in systematic reviews. Syst. Rev. 2021, 10, 39. [Google Scholar] [CrossRef]
- Ikusika, O.O.; Mpendulo, C.T.; Zindove, T.J.; Okoh, A.I. Effect of varying inclusion levels of fossil shell flour on growth performance, water intake, digestibility, and N retention in Dohne-Merino wethers. Animals 2019, 9, 565. [Google Scholar] [CrossRef] [PubMed]
- Mwanda, L.; Ikusika, O.O.; Mpendulo, C.T.; Okoh, A.I. Effects of fossil shell flour supplementation on heat tolerance of Dohne Merino rams. Vet. Anim. Sci. 2020, 10, 100133. [Google Scholar] [CrossRef] [PubMed]
- Ikusika, O.O.; Mpendulo, C.T. Feed preference, body condition scoring, and growth performance of Dohne Merino ram fed varying levels of fossil shell flour. Open Agric. 2023, 8, 20220161. [Google Scholar] [CrossRef]
- Webb, E.C.; Hassen, A.; Olaniyi, M.O.; Pophiwa, P. Effect of dietary inclusion of Azadirachta indica and Moringa oleifera leaf extracts on the carcass quality and fatty acid composition of lambs fed high forage total mixed rations. Animals 2022, 12, 2039. [Google Scholar] [CrossRef]
- Omotoso, O.B.; Adebisi, A.A.; Olufemi-Amodu, B.; Fajemisin, A.N. Effect of feeding frequency of Curcuma longa supplemented diets on nutrient intake, growth performance, and rumen fermentation characteristics of goats. Acta Fytotech. Zootech. 2022, 25, 185–193. [Google Scholar] [CrossRef]
- Linde, D.A.; Schokker, D.; du Toit, C.J.; Ramkilawon, G.D.; van Marle-Köster, E. The effect of a Bacillus probiotic and essential oils compared to an ionophore on the rumen microbiome composition of feedlot cattle. Animals 2023, 13, 2927. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; De Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Khan, F.M. Escherichia coli (E. coli) as an Indicator of Faecal Contamination in Water: A Review, 2020. Available online: https://www.preprints.org/frontend/manuscript/a5908f9835becdb1afd0d5de71116d3e/download_pub (accessed on 12 April 2025).
- Hewlings, S.J.; Kalman, D.S. Curcumin: A review of its effects on human health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Harikumar, K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune, and neoplastic diseases. Int. J. Biochem. Cell Biol. 2009, 41, 40–59. [Google Scholar] [CrossRef]
- Lin, X.; Bai, D.; Wei, Z.; Zhang, Y.; Huang, Y.; Deng, H.; Huang, X. Curcumin attenuates oxidative stress in RAW264. 7 cells by increasing the activity of antioxidant enzymes and activating the Nrf2-Keap1 pathway. PLoS ONE 2019, 14, e0216711. [Google Scholar]
- Ramírez-Mendoza, A.A.; Ramírez-Herrera, M.A.; Cortez-Álvarez, C.R.; Nery-Flores, S.D.; Tejeda-Martínez, A.R.; Romero-Prado, M.M.D.J.; Mendoza-Magaña, M.L. Curcumin modifies the activity of plasmatic antioxidant enzymes and the hippocampal oxidative profile in rats upon acute and chronic exposure to ozone. Molecules 2022, 27, 4531. [Google Scholar] [CrossRef]
- Celi, P. Oxidative stress in ruminants. In Studies on Veterinary Medicine; Humana Press: Totowa, NJ, USA, 2011; pp. 191–231. [Google Scholar]
- Liu, K.; Zhang, Y.; Yu, Z.; Xu, Q.; Zheng, N.; Zhao, S.; Wang, J. Ruminal microbiota–host interaction and its effect on nutrient metabolism. Anim. Nutr. 2021, 7, 49–55. [Google Scholar] [CrossRef]
- Lungu, K.; Afolayan, A.J. Moringa oleifera Lam. in animal production: A review. S. Afr. J. Anim. Sci. 2024, 54, 1–13. [Google Scholar]
- Tshabalala, N.B.; Nhlapho, S.; Marambire, L.; Ngxwala, N.N. A review of Moringa oleifera in livestock nutrition: Current status and prospects. Trop. Anim. Health Prod. 2020, 52, 3327–3336. [Google Scholar]

| Database | Search Terms (String) | Filters Applied |
|---|---|---|
| Scopus | (“natural feed additives” OR “plant additives” OR “plant-based additives”) AND (“ruminant” OR “cattle” OR “sheep” OR “goats”) | Document Type: Article; Language: English |
| Web of Science | ||
| ScienceDirect | ||
| EBSCOhost (Academic Search Ultimate) |
| Study (Author, Year) | Country | Study Design | Animal Type | Sample Size | Intervention (Type and Dose) | Comparator | Duration | Purpose | Key Outcomes | Main Findings |
|---|---|---|---|---|---|---|---|---|---|---|
| Omotoso et al. (2022) [31] | Nigeria | CRD | WAD doe goats | 20 | Curcuma longa (10 g TRP/day) | Concentrated diet | 56 days | Enhance nutrient intake, growth, and rumen fermentation | ↑ ADG, ↓ FCR, ↑ feed intake | Daily turmeric dosing improved performance; intermittent dosing was less effective |
| Webb et al. (2022) [30] | South Africa | CRBD | Dohne Merino wether lambs | 40 | A. indica and M. oleifera leaf extracts (50 mg/kg feed) | Monensin | 23 weeks | Improve carcass quality and FA profile | No significant effect on carcass traits (p > 0.05) | Moringa showed numerically leaner carcasses; no statistical difference was observed |
| Linde et al. (2023) [32] | CRD | Bonsmara weaners | 48 | Probiotic (2.75 g/d), EO (3.21 g/d), monensin (0.3 g/d) | 120 days | Modify rumen microbiome and improve feed efficiency | ↔ ADG, ↔ DFI, ↓ FCR (PRO vs. MON) | PRO and EO matched monensin for growth, with PRO showing superior FCR (p < 0.05) | ||
| Ikusika et al. (2019) [27] | Dohne-Merino wethers | 16 | FSF at 0%, 2%, 4%, and 6% of DM | 40:60 concentrate: hay | 105 days | Assess growth, digestibility, and N retention | ↑ feed intake, ↑ ADG, ↓ FCR, ↓ water intake | FSF improved growth and efficiency dose-dependently; 4% was most effective | ||
| Mwanda et al. (2020) [28] | Dohne Merino rams | 24 | FSF at 0, 20, 40, 60 g/kg | 100 days | Improve heat tolerance and stress resistance | ↑ weight gain, ↑ feed efficiency, ↑ water intake | 40–60 g/kg FSF improved growth and stress resilience under heat | |||
| Ikusika & Mpendulo (2023) [29] | Dohne Merino rams | FSF at 0, 20, 40, 60 g/kg | 90 days | Evaluate feed preference, growth, and BCS | ↑ intake, ↑ BCS, ↑ preference score | 60 g/kg FSF is most preferred; improved BCS and performance |
| Natural Feed Additive | Ruminant Livestock | Recommended Dosage | The Effect of Natural Feed Additives | References |
|---|---|---|---|---|
| Fossil shell flour | Dohne-Merino wethers | Inclusion of FSF at 4% of DM | Improvements were in dry matter intake (DMI), feed efficiency, average daily gain, total weight gain, nitrogen retention, and the apparent digestibility of most nutrients | Ikusika et al. (2019) [27] |
| Dohne-Merino rams | 40 g of FSF/kg | Enhances growth performance and contributes to the mitigation of heat stress | Mwanda et al. (2020) [28] | |
| Enhances feed intake, body condition score, and feeding behaviour by increasing the palatability and overall acceptability of the diet | Ikusika & Mpendulo (2023) [29] | |||
| Azadirachta indica and Moringa oleifera Leaf Extracts | Dohne-Merino wether lambs | 50 mg/kg of feed | No statistically significant effects on carcass traits (p > 0.05), though meat % ↑ and fat % ↓ numerically | Webb et al. (2022) [30] |
| Curcuma longa | West African Dwarf breed of doe goats | TRP at 10 g of TRP/300 g of feed | Improved goats’ performance | Omotoso et al. (2022) [31] |
| Probiotics: Bacillus subtilis and Bacillus licheniformis (3.2 × 109 CFU/g) Essential oils: eugenol (17%), capsicum (7%), and cinnamaldehyde (11%). | Bonsmara weaners | probiotic (2.75 g/animal/day), or essential oils (3.21 g/animal/day | ADG and feed intake were statistically comparable to monensin, but FCR was significantly better with PRO, indicating functional equivalence with superior efficiency | Linde et al. (2023) [32] |
| Bias Domain | Ikusika et al. (2019) [27] | Mwanda et al. (2020) [28] | Ikusika & Mpendulo (2023) [29] | Webb et al. (2022) [30] | Omotoso et al. (2022) [31] | Linde et al. (2023) [32] |
|---|---|---|---|---|---|---|
| Low | Low | Low | Low | Low | Low |
| Low | Low | Low | Low | Low | Low |
| Low | Low | Low | Low | Low | Low |
| Low | Low | Low | Low | Low | Low |
| Yellow | Yellow | Yellow | Yellow | Yellow | Yellow |
| Low | Low | Low | Low | Low | Low |
| High | High | High | High | High | High |
| Low | Low | Low | Low | Low | Low |
| Low | Low | Low | Low | Low | Low |
| Low | Low | Low | Low | Low | Low |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ntsongota, Z.; Ikusika, O.O.; Mpendulo, T.C. Natural Feed Additives in Sub-Saharan Africa: A Systematic Review of Efficiency and Sustainability in Ruminant Production. Ruminants 2025, 5, 36. https://doi.org/10.3390/ruminants5030036
Ntsongota Z, Ikusika OO, Mpendulo TC. Natural Feed Additives in Sub-Saharan Africa: A Systematic Review of Efficiency and Sustainability in Ruminant Production. Ruminants. 2025; 5(3):36. https://doi.org/10.3390/ruminants5030036
Chicago/Turabian StyleNtsongota, Zonaxolo, Olusegun Oyebade Ikusika, and Thando Conference Mpendulo. 2025. "Natural Feed Additives in Sub-Saharan Africa: A Systematic Review of Efficiency and Sustainability in Ruminant Production" Ruminants 5, no. 3: 36. https://doi.org/10.3390/ruminants5030036
APA StyleNtsongota, Z., Ikusika, O. O., & Mpendulo, T. C. (2025). Natural Feed Additives in Sub-Saharan Africa: A Systematic Review of Efficiency and Sustainability in Ruminant Production. Ruminants, 5(3), 36. https://doi.org/10.3390/ruminants5030036






