Evaluation of Creatinine-Based Methods for Estimating the Urine Volume of Lactating and Dry Dairy Cows with Special Consideration of Using Spot Urine Samples
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Animals
2.2. Sample Collection
2.3. Analyses
2.4. Calculation and Statistics
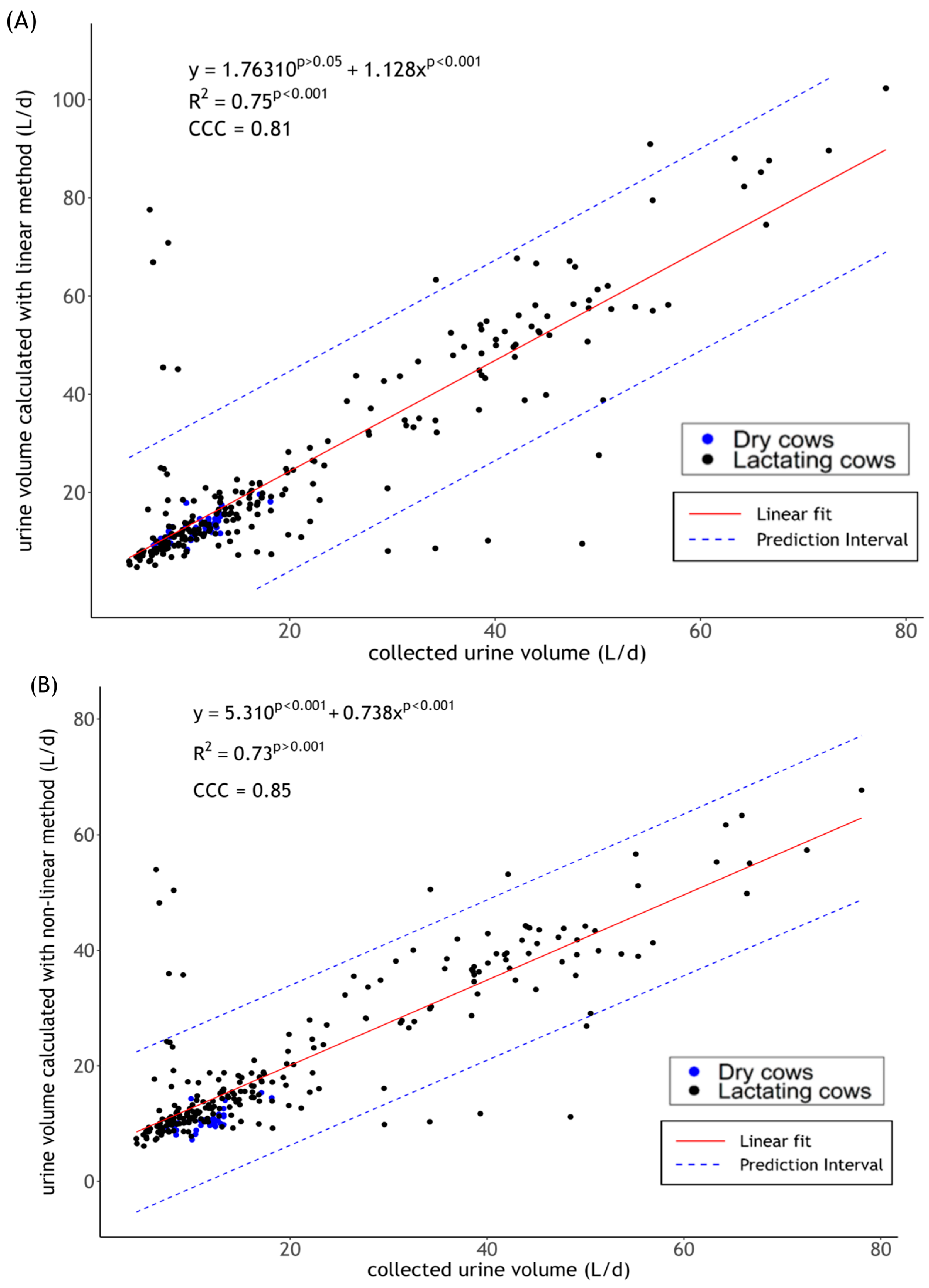
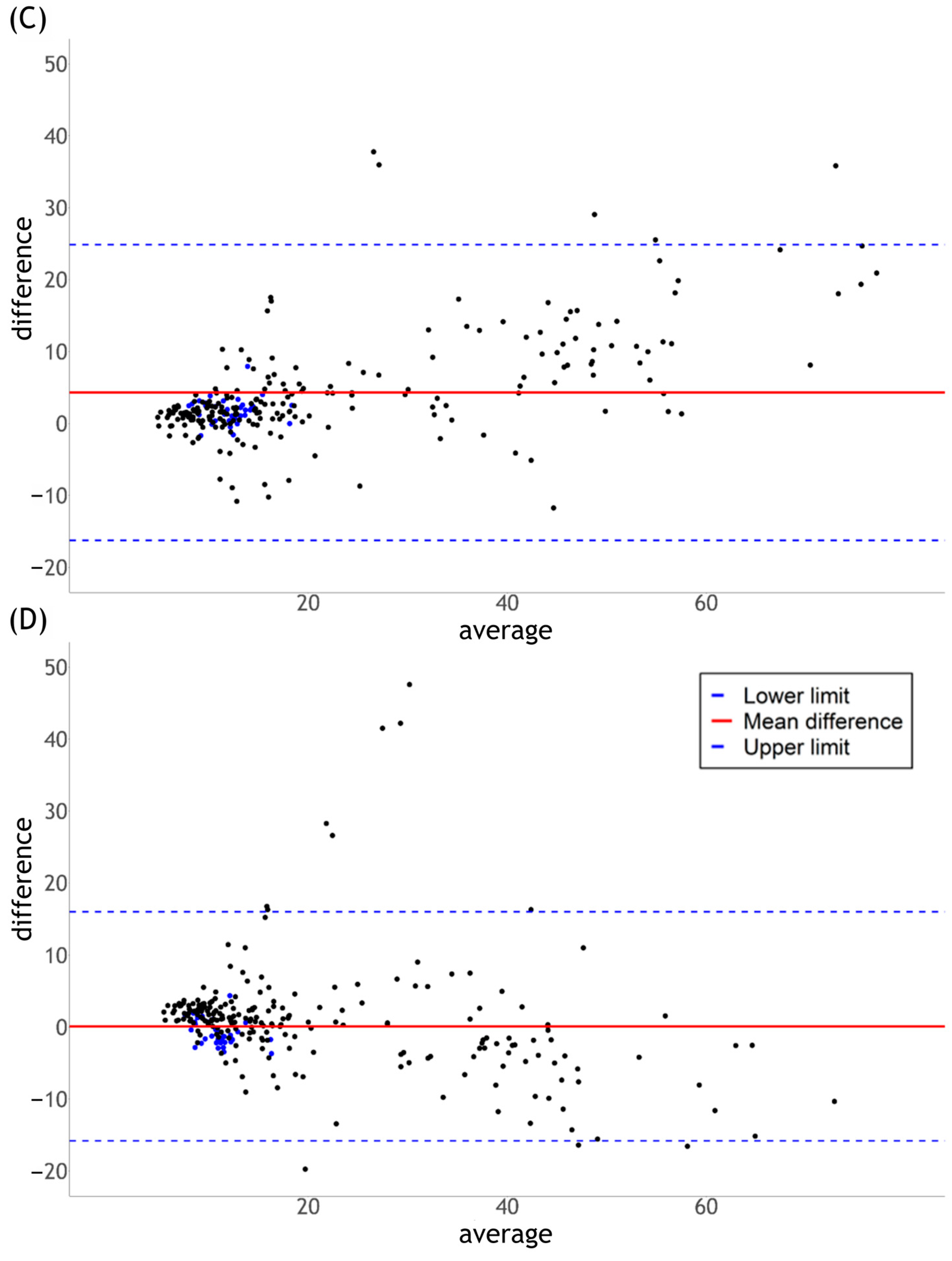
2.4.1. Calculation
2.4.2. Statistics
3. Results
3.1. Comparison of Cr Concentrations Between Urine Pool and Urine Spot Samples
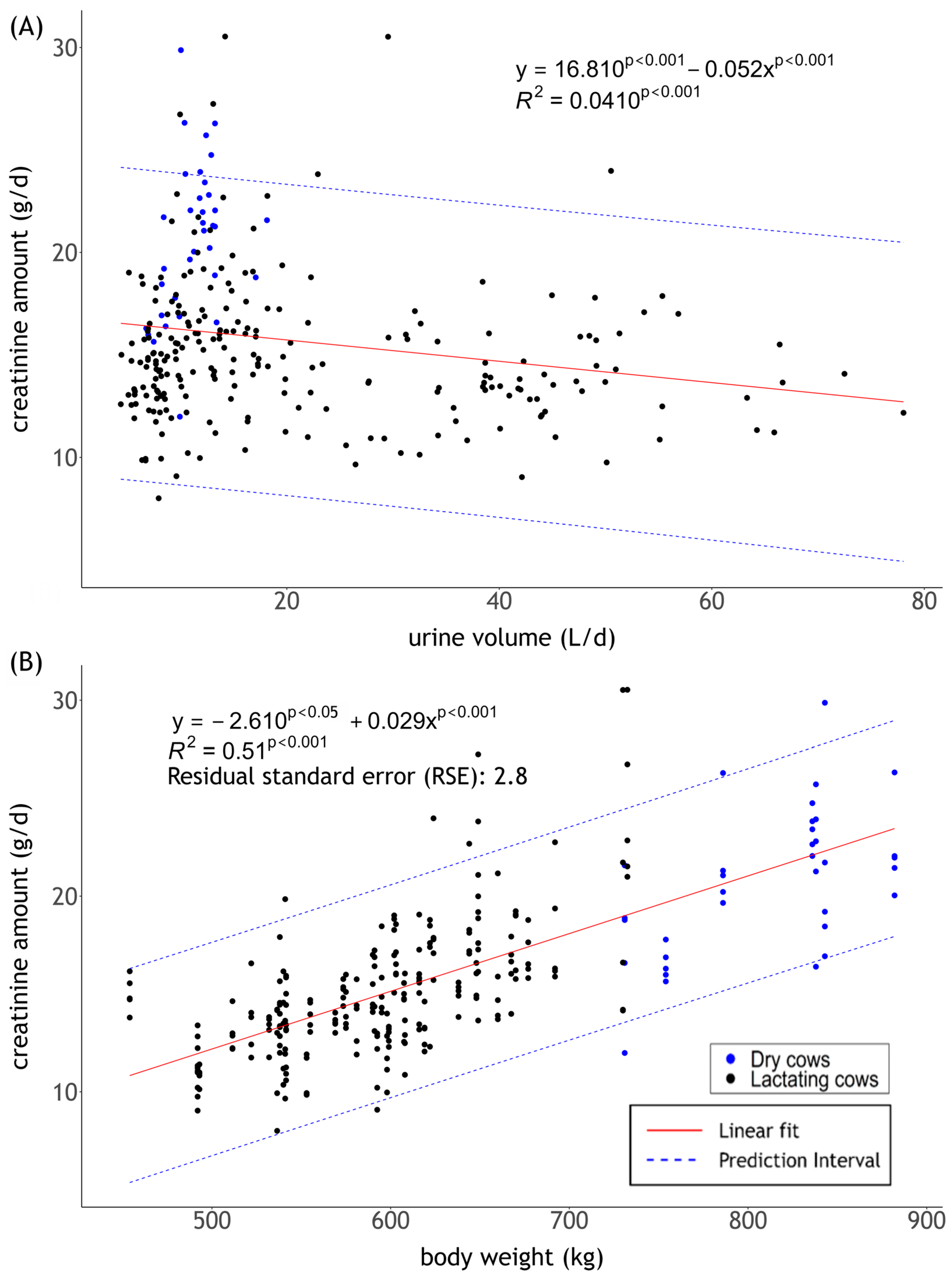
3.2. Cr Amount in Relation to UV and BW
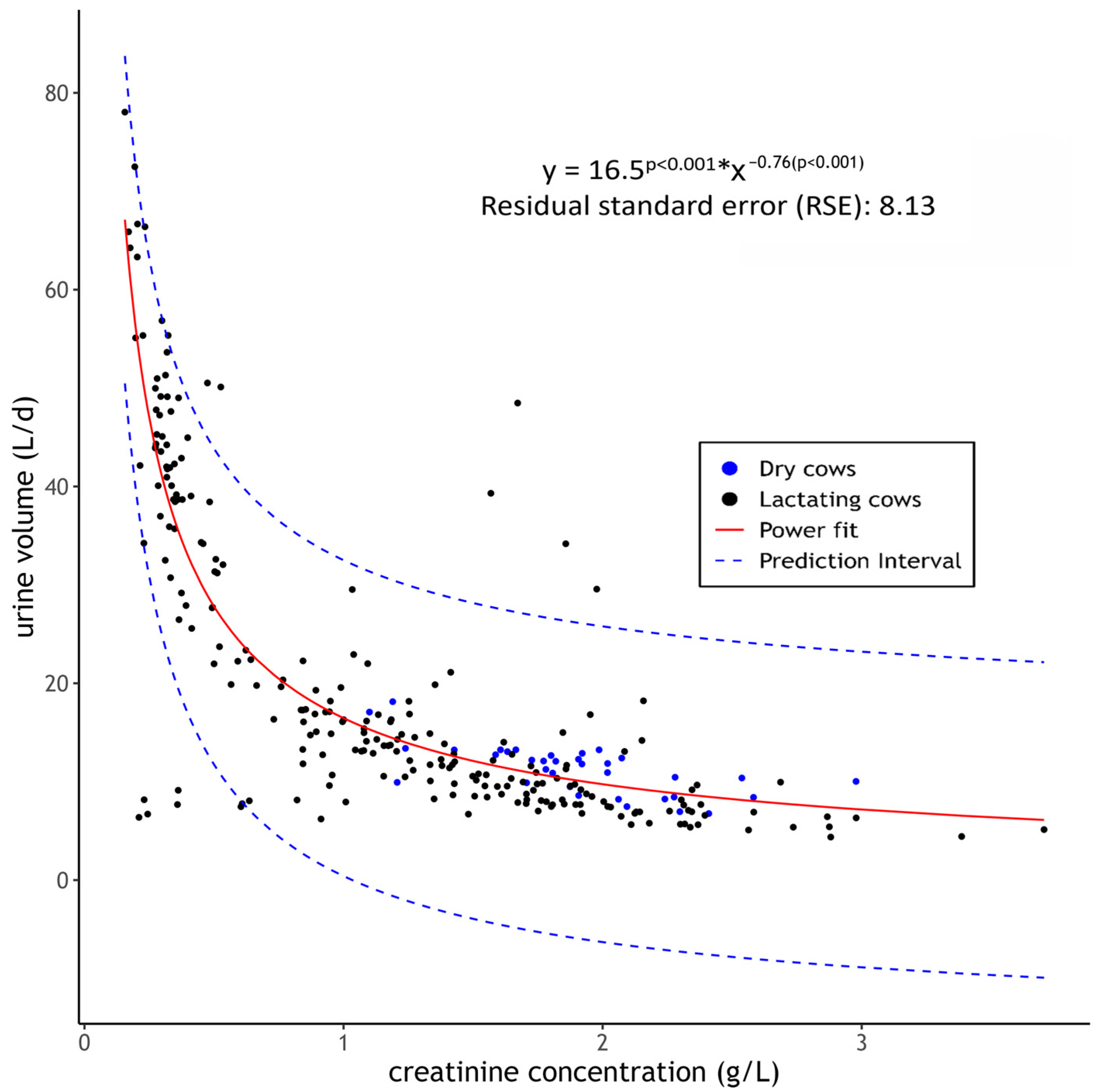
3.3. UV in Correlation to Cr Concentration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIC | Akaike Information Criterion |
| BIC | Bayesian Information Criterion |
| BW | Body Weight |
| CCC | Concordance Correlation Coefficient |
| Cr | Creatinine |
| LOOCV | Leave-One-Out Cross-Validation |
| N | Nitrogen |
| RSE | Residual Standard Error |
| SD | Standard deviation |
| U | Urea |
References
- Korevaar, H. The nitrogen balance on intensive Dutch dairy farms—A review. Livest. Prod. Sci. 1992, 31, 17–27. [Google Scholar] [CrossRef]
- Sorley, M.; Casey, I.; Styles, D.; Merino, P.; Trindade, H.; Mulholland, M.; Zafra, C.R.; Keatinge, R.; Le Gallj, A.; O’Brien, D.; et al. Factors influencing the carbon footprint of milk production on dairy farms with different feeding strategies in western Europe. J. Clean. Prod. 2024, 435, 140104. [Google Scholar] [CrossRef]
- Bundesregierung. Deutsche Nachhaltigkeitsstrategie: Weiterentwicklung 2021; Bundesregierung, Presse-und Informationsamt der Bundesregierung, Ed.; Bundesregierung: Berlin, Germany, 2021.
- Umweltbundesamt. Ammoniakemissionen in der Landwirtschaft Mindern. Gute Fachliche Praxis; Kuratorium für Technik und Bauwesen in der Landwirtschaft e.V.: Dessau-Roßlau, Germany, 2021; p. 2021. [Google Scholar]
- Tamminga, S. Nutrition management of dairy cows as a contribution to pollution control. J. Dairy Sci. 1992, 75, 345–357. [Google Scholar] [CrossRef]
- Castillo, A.R.; Kebreab, E.; Beever, D.E.; France, J. A review of efficiency of nitrogen utilisation in lactating dairy cows and its relationship with environmental pollution. J. Anim. Feed Sci. 2000, 9, 1–32. [Google Scholar] [CrossRef]
- Spanghero, M.; Kowalski, Z.M. Updating analysis of nitrogen balance experiments in dairy cows. J. Dairy Sci. 2021, 104, 7725–7737. [Google Scholar] [CrossRef] [PubMed]
- Spek, J.W.; Dijkstra, J.; Van Duinkerken, G.; Bannink, A. A review of factors influencing milk urea concentration and its relationship with urinary urea excretion in lactating dairy cattle. J. Agric. Sci. 2013, 151, 407–423. [Google Scholar] [CrossRef]
- Muck, R.E.; Steenhuis, T.S. Nitrogen losses from manure storages. Agric. Wastes 1982, 4, 41–54. [Google Scholar] [CrossRef]
- Vanvuuren, A.M.; Vanderkoelen, C.J.; Valk, H.; Devisser, H. Effects of partial replacement of ryegrass by low-protein feeds on rumen fermentation and nitrogen loss by dairy cows. J. Dairy Sci. 1993, 76, 2982–2993. [Google Scholar] [CrossRef] [PubMed]
- Aschemann, M.; Lebzien, P.; Hüther, L.; Südekum, K.H.; Dänicke, S. Effect of niacin supplementation on rumen fermentation characteristics and nutrient flow at the duodenum in lactating dairy cows fed a diet with a negative rumen nitrogen balance. Arch. Anim. Nutr. 2012, 66, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Gorniak, T.; Hüther, L.; Meyer, U.; Lebzien, P.; Breves, G.; Südekum, K.H.; Dänicke, S. Digestibility, ruminal fermentation, ingesta kinetics and nitrogen utilisation in dairy cows fed diets based on silage of a brown midrib or a standard maize hybrid. Arch. Anim. Nutr. 2014, 68, 143–158. [Google Scholar] [CrossRef] [PubMed]
- Winter, L.; Meyer, U.; von Soosten, D.; Gorniak, M.; Lebzien, P.; Dänicke, S. Effect of phytase supplementation on rumen fermentation characteristics and phosphorus balance in lactating dairy cows. Ital. J. Anim. Sci. 2015, 14, 8. [Google Scholar] [CrossRef]
- von Soosten, D.M.U.; Hüther, L.; Dänicke, S. Opportunities to reduce the sampling frequency for measurement of duodenal dry matter flow and ruminal microbial crude protein synthesis in dairy cows. In Proceedings of the Society of Nutrition Physiology, Hanover, Germany, 8–10 March 2016; p. 25. [Google Scholar]
- Tebbe, A.W.; Weiss, W.P. Evaluation of creatinine as a urine marker and factors affecting urinary excretion of magnesium by dairy cows. J. Dairy Sci. 2018, 101, 5020–5032. [Google Scholar] [CrossRef] [PubMed]
- Valadares, R.F.D.; Broderick, G.A.; Valadares, S.C.; Clayton, M.K. Effect of replacing alfalfa silage with high moisture corn on ruminal protein synthesis estimated from excretion of total purine derivatives. J. Dairy Sci. 1999, 82, 2686–2696. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.; Martens, H.; Thöne-Reineke, C. Breeding problems relevant to animal welfare in dairy cattle—Interaction between the breeding goal “milk yield” and the increased occurrence of production diseases. Berl. Münch. Tierarztl. Wochenschr. 2021, 134, 1206–1216. [Google Scholar]
- Oltenacu, P.A.; Broom, D.M. The impact of genetic selection for increased milk yield on the welfare of dairy cows. Anim. Welf. 2010, 19, 39–49. [Google Scholar] [CrossRef]
- Drackley, J.K. Biology of dairy cows during the transition period: The final frontier? J. Dairy Sci. 1999, 82, 2259–2273. [Google Scholar] [CrossRef] [PubMed]
- Winkler, J.; Kersten, S.; Valenta, H.; Huether, L.; Meyer, U.; Engelhardt, U.; Dänicke, S. Simultaneous determination of zearalenone, deoxynivalenol and their metabolites in bovine urine as biomarkers of exposure. World Mycotoxin J. 2015, 8, 63–74. [Google Scholar] [CrossRef]
- Signorell, A.E.M.A. DescTools: Tools for Descriptive Statistics, R package version 0.99.48; R Development Core Team: Vienna, Austria, 2023. [Google Scholar]
- Lehnert, B. BlandAltmanLeh: Plots for Bland-Altman Analysis, R package version 0.3.1; R Development Core Team: Vienna, Austria, 2015. [Google Scholar]
- Akaike, H.S.Y. Analysis of cross classified data by AIC. Ann. Inst. Statist. Math. 1978, 30, 185–197. [Google Scholar]
- Schwarz, G. Estimating the dimension of a model. Ann. Stat. 1978, 6, 461–464. [Google Scholar] [CrossRef]
- James, G.D.W.; Hastie, T.; Tibshirani, R. An Introduction to Statistical Learning with Applications in R; Springer: New York, NY, USA, 2013. [Google Scholar]
- Chizzotti, M.L.; Valadares, S.D.; Valadares, R.F.D.; Chizzotti, F.H.M.; Tedeschi, L.O. Determination of creatinine excretion and evaluation of spot urine sampling in Holstein cattle. Livest. Sci. 2008, 113, 218–225. [Google Scholar] [CrossRef]
- de Groot, T.J.H.A. On the constancy of creatinine excretion in the urine of the dairy cow. Br. Vet. J. 1960, 116, 409–418. [Google Scholar] [CrossRef]
- Lee, C.; Morris, D.L.; Dieter, P.A. Validating and optimizing spot sampling of urine to estimate urine output with creatinine as a marker in dairy cows. J. Dairy Sci. 2019, 102, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Borsook, H.J.W.D. The hydrolysis of phosphocreatine and the origin of urinary creatinine. J. Biol. Chem. 1947, 168, 493–510. [Google Scholar] [CrossRef] [PubMed]
- Scheunert, A.A.T. Lehrbuch der Veterinärphysiologie; Paul Parey: Hamburg, Germany, 1987. [Google Scholar]
- Wyss, M.; Kaddurah-Daouk, R. Creatine and creatinine metabolism. Physiol. Rev. 2000, 80, 1107–1213. [Google Scholar] [CrossRef] [PubMed]
- Fürll, M. Stoffwechselkontrollen und Stoffwechselüberwachung bei Rindern. Nutztierpr. Aktuell 2004, 9. Available online: https://www.yumpu.com/de/document/read/10703372/stoffwechselkontrollen-und-stoffwechseluberwachung-bei-rindern (accessed on 1 April 2025).
- Danese, T.; Sabetti, M.C.; Mezzasalma, N.; Simoni, M.; Quintavalla, C.; Righi, F. Does Acidification Affect Urinary Creatinine in Dairy Cattle? Animals 2024, 14, 315. [Google Scholar] [CrossRef] [PubMed]

| Experiment | Grass Silage (%) | Maize Silage (%) | Concentrate (%) | XP (g/kg TS Total Ration) | Cows | BW (kg) | Cr Excreted (g/d) | Cr Concentration (g/L) | UV (L) | UV Calculated with Linear Method (L) | UV Calculated with Non-Linear Method (L) | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0 | 60 | 40 | 132 | 5 | 590 ± 29 | 13.1 ± 2.5 | 1.6 ± 0.7 | 9.1 ± 2.7 | 12.3 ± 4.5 | 12.8 ± 3.6 | [11] |
| 2 | 0 | 60 | 40 | 135 | 6 | 588 ± 28 | 15 ± 2.4 | 1.7 ± 0.8 | 13 ± 10.5 | 14.9 ± 12.2 | 14.5 ± 9.3 | [11] |
| 3 | 0 | 60 | 40 | 139 | 7 | 626 ± 45 | 15.9 ± 1.7 | 1.4 ± 0.6 | 13.8 ± 8 | 15.9 ± 9.1 | 14.8 ± 7 | [11] |
| 4 | 0 | 70 | 30 | 134 | 6 | 554 ± 39 | 12.6 ± 2.7 | 1 ± 0.7 | 20.9 ± 13.2 | 32.7 ± 34.4 | 26.5 ± 19.9 | [13] |
| 5 | 0 | 70 | 30 | 131 | 6 | 512 ± 33 | 13.1 ± 2 | 1.1 ± 0.7 | 19.9 ± 14.5 | 25 ± 21.5 | 22.9 ± 14.7 | [13] |
| 6 | 0 | 70 | 30 | 132 | 6 | 564 ± 54 | 13.9 ± 2.5 | 0.6 ± 0.5 | 33.2 ± 18.2 | 40.5 ± 23.7 | 32.7 ± 16.3 | [13] |
| 7 | 0 | 70 | 30 | 142 | 5 | 668 ± 42 | 17.3 ± 4.6 | 0.9 ± 0.6 | 30.9 ± 19.3 | 38.4 ± 27.7 | 27 ± 16.3 | [12] |
| 8 | 0 | 70 | 30 | 142 | 6 | 640 ± 47 | 18.8 ± 3.9 | 1 ± 0.8 | 30.9 ± 20 | 32.2 ± 22.8 | 24.6 ± 14.4 | [12] |
| 9 | 50 | 50 | 0 | 108 | 7 ‡ | 810 ± 51 | 20.7 ± 3.7 | 1.9 ± 0.4 (1.9 ± 0.4) | 11.3 ± 2.6 | 12.88 ± 2.6 | 10.5 ± 1.9 | [14] |
| ‡ Dry cows | Values in parentheses represent spot samples |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padberg, K.; Meyer, U.; von Soosten, D.; Billenkamp, F.; Hüther, L.; Unruh, C.; Visscher, C.; Dänicke, S. Evaluation of Creatinine-Based Methods for Estimating the Urine Volume of Lactating and Dry Dairy Cows with Special Consideration of Using Spot Urine Samples. Ruminants 2025, 5, 31. https://doi.org/10.3390/ruminants5030031
Padberg K, Meyer U, von Soosten D, Billenkamp F, Hüther L, Unruh C, Visscher C, Dänicke S. Evaluation of Creatinine-Based Methods for Estimating the Urine Volume of Lactating and Dry Dairy Cows with Special Consideration of Using Spot Urine Samples. Ruminants. 2025; 5(3):31. https://doi.org/10.3390/ruminants5030031
Chicago/Turabian StylePadberg, Katharina, Ulrich Meyer, Dirk von Soosten, Fabian Billenkamp, Liane Hüther, Christin Unruh, Christian Visscher, and Sven Dänicke. 2025. "Evaluation of Creatinine-Based Methods for Estimating the Urine Volume of Lactating and Dry Dairy Cows with Special Consideration of Using Spot Urine Samples" Ruminants 5, no. 3: 31. https://doi.org/10.3390/ruminants5030031
APA StylePadberg, K., Meyer, U., von Soosten, D., Billenkamp, F., Hüther, L., Unruh, C., Visscher, C., & Dänicke, S. (2025). Evaluation of Creatinine-Based Methods for Estimating the Urine Volume of Lactating and Dry Dairy Cows with Special Consideration of Using Spot Urine Samples. Ruminants, 5(3), 31. https://doi.org/10.3390/ruminants5030031







