Cytokine Profiling and Puberty Enhancement Post Altrenogest Feeding in Prepubertal Murrah Buffalo (Bubalus bubalis) Heifers
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Place of Study
2.2. Selection and Management of Prepubertal Buffaloes
2.3. Study Design
2.4. Observations and Sampling
2.4.1. Blood Collection
2.4.2. Ultrasonographic Observations
2.4.3. Pregnancy Diagnosis
2.4.4. Hormone Estimation
2.5. Statistical Analysis
3. Results
3.1. Estradiol 17-β
3.2. Progesterone
3.3. Anti-Müllerian Hormone
3.4. Interferon-γ (IFNγ)
3.5. Interleukin-1 (IL1)
3.6. Interleukin-6 (IL6)
3.7. Interleukin-13 (IL13)
3.8. Transforming Growth Factor-β (TGFβ)
3.9. Tumor Necrosis Factor-α (TNFα)
3.10. Size of Largest Follicle
3.11. Pregnancy Outcome and Cyclicity
3.12. Karl Pearson Correlation Coefficient within Hormones, Cytokines, and Follicular Size
3.12.1. Altrenogest Group
3.12.2. Controlled Internal Drug Release (CIDR) Group
3.12.3. Co-Synch Group
4. Discussion
4.1. Estradiol 17β
4.2. Progesterone
4.3. Anti-Müllerian Hormone
4.4. Interferon-γ (IFNγ)
4.5. Interleukin-1 (IL1)
4.6. Interleukin-6 (IL6)
4.7. Interleukin-13 (IL13)
4.8. Transforming Growth Factor-β (TGFβ)
4.9. Tumor Necrosis Factor-α (TNFα)
4.10. Size of Largest Follicle
4.11. Pregnancy Outcome and Cyclicity
4.12. Karl Pearson Correlation Coefficient between Hormones, Cytokines, and Follicular Size
4.12.1. Altrenogest Supplementation Group
4.12.2. CIDR Group
4.12.3. Co-Synch Group
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Minervino, A.H.H.; Zava, M.; Vecchio, D.; Borghese, A. Bubalus bubalis: A short story. Front. Vet. Sci. 2020, 7, 570413. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Singh, P.; Goyal, R.K.; Hb, S.; Kumhar, B.L. Existing housing and feeding management practices of buffaloes in Firozabad district of Uttar Pradesh, India. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1831–1838. [Google Scholar] [CrossRef]
- Vasantha, S.K.I.; Kona, S.S.R. Physiology of puberty in females: A review. Int. J. Vet. Sci. Anim. Hus. 2016, 2, 23–26. [Google Scholar]
- Gupta, S.K.; Singh, P.; Shinde, K.P.; Lone, S.M.; Kumar, N.; Kumar, A. Strategies for attaining early puberty in cattle and buffalo: A review. Agric. Rev. 2016, 37, 160–167. [Google Scholar] [CrossRef]
- Orsi, N.M.; Tribe, R.M. Cytokine networks and the regulation of uterine function in pregnancy and parturition. J. Neuroendocrinol. 2008, 4, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Arsenescu, R.; Arsenescu, V.; De Villiers, W.J. TNFα and the development of the neonatal immune system: Implications for inhibitor use in pregnancy. Off. J. Am. Col. Gastroenterol. 2011, 4, 559–562. [Google Scholar] [CrossRef] [PubMed]
- Witkin, S.S.; Linhares, I.M.; Bongiovanni, A.M.; Herway, C.; Skupski, D. Unique alterations in infection-induced immune activation during pregnancy. BJOG Int. J. Obst. Gynaecol. 2011, 2, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Knight, P.G.; Glister, C. TGFβ superfamily members and ovarian follicle development. Reproduction 2006, 2, 191–206. [Google Scholar] [CrossRef]
- Wu, R.; Van der Hoek, K.H.; Ryan, N.K.; Norman, R.J.; Robker, R.L. Macrophage contributions to ovarian function. Hum. Reprod. 2004, 12, 119–133. [Google Scholar] [CrossRef]
- Tarrant, J.M. Blood cytokines as biomarkers of in vivo toxicity in preclinical safety assessment: Considerations for their use. Toxicol. Sci. 2010, 1, 4–16. [Google Scholar] [CrossRef]
- Casazza, K.; Hanks, L.J.; Alvarez, J.A. Role of various cytokines and growth factors in pubertal development. Cytokines Growth Mediat. Phys. Act. Child. Dur. Puberty 2010, 55, 14–31. [Google Scholar] [CrossRef]
- Jost, A.; Vigier, B.; Prépin, J.; Perchellet, J.P. Studies on sex differentiation in mammals. In Proceedings of the 1972 Laurentian Hormone Conference, Mont Tremblant, QC, Canada, 27 August–1 September 1972; Academic Press: Cambridge, MA, USA, 1973; pp. 1–41. [Google Scholar] [CrossRef]
- Durlinger, A.; Visser, J.; Themmen, A. Regulation of ovarian function: The role of anti-Mullerian hormone. Reproduction 2002, 5, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Gigli, I.; Cushman, R.A.; Wahl, C.M.; Fortune, J.E. Evidence for a role for anti-Müllerian hormone in the suppression of follicle activation in mouse ovaries and bovine ovarian cortex grafted beneath the chick chorioallantoic membrane. Mol. Reprod. Dev. 2005, 4, 480–488. [Google Scholar] [CrossRef]
- Durlinger, A.L.; Gruijters, M.J.; Kramer, P.; Karels, B.; Kumar, T.R.; Matzuk, M.M.; Themmen, A.P. Anti-Mullerian hormone attenuates the effects of FSH on follicle development in the mouse ovary. Endocrinology 2001, 11, 4891–4899. [Google Scholar] [CrossRef]
- Hinglak, S. Studies on Reducing Age at First Calving by Oral Progesterone Feeding in Puzbertal Murrah Buffalo Heifers. Master’s Thesis, Guru Angad Dev Veterinary and Animal Sciences University, Ludhiana, India, 2013. [Google Scholar]
- Abouel-Ghaitb, H. Progesterone-based hormonal treatments to induce and synchronize the onset of puberty in buffalo-heifers. Kafrelsheikh Vet. Med. J. 2021, 1, 20–25. [Google Scholar] [CrossRef]
- Naseer, Z.; Ahmad, N.; Khan, M.I.R.; Ahmad, E.; Tahir, M.Z.; Singh, J. Effect of GnRH and estradiol benzoate on follicular wave emergence, estrus, ovulation and pregnancy rate in CIDR treated Nili-Ravi buffaloes. J. Anim. Plant Sci. 2012, 3, 142–146. [Google Scholar]
- Aulakh, A.S.; Singh, P.; Singh, G.; Dhindsa, S.S.; Honparkhe, M.; Kaur, A. Effect of altrenogest feeding in prepubertal buffalo heifers vis-a-vis plasma estrogen-progesterone levels and biochemical profile. Int. J. Curr. Microbiol. Appl. Sci. 2020, 8, 2337–2347. [Google Scholar] [CrossRef]
- Mingoti, G.Z.; Neves, T.V.; Silva, J.C.B.; Oliveira, L.O.F.; Nogueira, E. Use of Melengestrol Acetate in nutritional blocks for heifers under extensive pastures. Anim. Reprod. 2018, 3, 316. [Google Scholar]
- Anitha, A.; Rao, K.S.; Suresh, J.; Moorthy, P.S.; Reddy, Y.K. A body condition score (BCS) system in Murrah buffaloes. Buff. Bull. 2011, 31, 79–99. [Google Scholar]
- Pierson, R.A.; Ginther, O.J. Ultrasonographic appearance of the bovine uterus during the estrous cycle. J. Am. Vet. Med. 1987, 8, 995–1001. [Google Scholar]
- Roy, K.S.; Prakash, B.S. Changes in endocrine profiles during ovsynch and ovsynch plus norprolac treatment in Murrah buffalo heifers at hot summer season. Trop. Anim. Health Prod. 2009, 41, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Haldar, A.; Prakash, B.S. Growth hormone-releasing factor (GRF) induced growth hormone advances puberty in female buffaloes. Anim. Reprod. Sci. 2006, 92, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Rico, C.; Fabre, S.; Médigue, C.; Clemente, N.D.; Clément, F.; Bontoux, M.; Monniaux, D. Anti-Müllerian hormone is an endocrine marker of ovarian gonadotropin-responsive follicles and can help to predict superovulatory responses in the cow. Biol. Reprod. 2009, 1, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Ireland, J.J.; Smith, G.W.; Scheetz, D.; Jimenez-Krassel, F.; Folger, J.K.; Ireland, J.L.H.; Evans, A.C.O. Does size matter in females? An overview of the impact of the high variation in the ovarian reserve on ovarian function and fertility, utility of anti-Müllerian hormone as a diagnostic marker for fertility and causes of variation in the ovarian reserve in cattle. Reprod. Fertil. Dev. 2010, 1, 1–14. [Google Scholar] [CrossRef]
- Weenen, C.; Laven, J.S.; Von Bergh, A.R.; Cranfield, M.; Groome, N.P.; Visser, J.A.; Themmen, A.P. Anti-Müllerian hormone expression pattern in the human ovary: Potential implications for initial and cyclic follicle recruitment. Mol. Hum. Reprod. 2004, 2, 77–83. [Google Scholar] [CrossRef]
- Carlsson, I.B.; Scott, J.E.; Visser, J.A.; Ritvos, O.; Themmen, A.P.N.; Hovatta, O. Anti-Müllerian hormone inhibits initiation of growth of human primordial ovarian follicles in vitro. Hum. Reprod. 2006, 9, 2223–2227. [Google Scholar] [CrossRef] [PubMed]
- Pellatt, L.; Rice, S.; Dilaver, N.; Heshri, A.; Galea, R.; Brincat, M.; Mason, H.D. Anti-Müllerian hormone reduces follicle sensitivity to follicle-stimulating hormone in human granulosa cells. Fertil. Steril. 2011, 5, 1246–1251. [Google Scholar] [CrossRef] [PubMed]
- Monniaux, D.; Drouilhet, L.; Rico, C.; Estienne, A.; Jarrier, P.; Touzé, J.L.; Fabre, S. Regulation of anti-Müllerian hormone production in domestic animals. Reprod. Fertil. Dev. 2012, 1, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nature 1994, 6505, 425–432. [Google Scholar] [CrossRef]
- Yeung, E.H.; Sundaram, R.; Ghassabian, A.; Xie, Y.; Buck Louis, G. Parental obesity and early childhood development. Pediatrics 2017, 139, e20161459. [Google Scholar] [CrossRef]
- Simon, L.; Spiewak, K.A.; Ekman, G.C. Regulation and action of interferon-γ in the mouse testis. Endocrinology 2010, 151, 1470–1481. [Google Scholar]
- Huber, R.; Pietsch, D.; Günther, J.; Welz, B.; Vogt, N.; Brand, K. Regulation of monocyte differentiation by specific signaling modules and associated transcription factor networks. Cell. Mol. Life Sci. 2014, 71, 63–92. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, A.; Payne, D.W.; Packman, J.N.; Andreani, C.L.; Resnick, C.E.; Hernandez, E.R.; Adashi, E.Y. Cytokine-mediated regulation of ovarian function: Interleukin-1 inhibits gonadotropin-induced androgen biosynthesis. Endocrinology 1991, 129, 1250–1256. [Google Scholar] [CrossRef] [PubMed]
- Fukuoka, M.; Mori, T.; Taii, S.; Yasuda, K. Interleukin-1 inhibits luteinization of porcine granulosa cells in culture. Endocrinology 1988, 122, 367–369. [Google Scholar] [CrossRef] [PubMed]
- Barak, V.; Yanai, P.; Treves, A.J.; Roisman, I.; Simon, A.; Laufer, N. Interleukin-1: Local production and modulation of human granulosa luteal cells steroidogenesis. Fertil. Steril. 1992, 58, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Gottschall, P.E.; Katsuura, G.; Hoffmann, S.T.; Arimura, A. Interleukin 1: An inhibitor of luteinizing hormone receptor formation in cultured rat granulosa cells. FASEB J. 1988, 9, 2492–2496. [Google Scholar] [CrossRef] [PubMed]
- Fukuoka, M.; Yasuda, K.; Emi, N.; Fujiwara, H.; Iwai, M.; Takakura, K.; Mori, T. Cytokine modulation of progesterone and estradiol secretion in cultures of luteinized human granulosa cells. J. Clin. Endocrinol. Metab. 1992, 75, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Polan, M.L.; Daniele, A.; Kuo, A. Gonadal steroids modulate human monocyte interleukin-1 (IL1) activity. Fertil. Steril. 1998, 49, 964–968. [Google Scholar] [CrossRef]
- Hurwitz, A.; Dushnik, M.; Solomon, H.; Ben-Chetrit, A.; Finci-Yeheskel, Z.; Milwidsky, A.; Yagel, S. Cytokine-mediated regulation of rat ovarian function: Interleukin-1 stimulates the accumulation of a 92-kilodalton gelatinase. Endocrinology 1993, 132, 2709–2714. [Google Scholar] [CrossRef]
- Ben-Shlomo, I.; Adashi, E.Y.; Payne, D.W. The morphogenic/cytotoxic and prostaglandin-stimulating activities of interleukin-1 beta in the rat ovary are nitric oxide independent. J. Clin. Investig. 1994, 94, 1463–1469. [Google Scholar] [CrossRef]
- Ghodsi, M.; Hojati, V.; Attaranzade, A.; Saifi, B. A Cross-sectional study on the follicular fluid concentration of some interleukins and clinical factors in polycystic ovary syndrome patients. Int. J. Womens Health Reprod. Sci. 2021, 9, 124–129. [Google Scholar] [CrossRef]
- Kollmann, Z.; Schneider, S.; Fux, M.; Bersinger, N.A.; von Wolff, M. Gonadotrophin stimulation in IVF alters the immune cell profile in follicular fluid and the cytokine concentrations in follicular fluid and serum. Hum. Reprod. 2017, 32, 820–831. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, F.; Kawano, Y.; Kosay Hasan, Z.; Narahara, H.; Miyakawa, I. The clinical role of interleukin-6 and interleukin-6 soluble receptor in human follicular fluids. Clin. Exp. Med. 2003, 3, 27–31. [Google Scholar] [CrossRef]
- Qin, L.; Xu, W.; Li, X.; Meng, W.; Hu, L.; Luo, Z.; Li, S. Differential expression profile of immunological cytokines in local ovary in patients with polycystic ovarian syndrome: Analysis by flow cytometry. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 197, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Bou Nemer, L.; Shi, H.; Carr, B.R.; Word, R.A.; Bukulmez, O. Effect of single-dose ibuprofen on follicular fluid levels of interleukins in poor responders undergoing in vitro fertilization. Syst. Biol. Reprod. Med. 2019, 65, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Maeda, A.; Goto, Y.; Matsuda-Minehata, F.; Cheng, Y.; Inoue, N.; Manabe, N. Changes in expression of interleukin-6 receptors in granulosa cells during follicular atresia in pig ovaries. J. Reprod. Dev. 2007, 53, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; de Matos, D.G.; Fan, H.Y.; Shimada, M.; Palmer, S.; Richards, J.S. Interleukin-6: An autocrine regulator of the mouse cumulus cell-oocyte complex expansion process. Endocrinology 2009, 150, 3360–3368. [Google Scholar] [CrossRef] [PubMed]
- Ripley, D.; Shoup, B.; Majewski, A.; Chegini, N. Differential expression of interleukins IL13 and IL15 in normal ovarian tissue and ovarian carcinomas. Gynecol. Oncol. 2004, 92, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.S.; Russell, D.L.; Ochsner, S.; Hsieh, M.; Doyle, K.H.; Falender, A.E.; Sharma, S.C. Novel signaling pathways that control ovarian follicular development, ovulation, and luteinization. Recent Prog. Horm. Res. 2002, 57, 195–220. [Google Scholar] [CrossRef]
- Chang, H.; Brown, C.W.; Matzuk, M.M. Genetic analysis of the mammalian transforming growth factor-β superfamily. Endocr. Rev. 2002, 23, 787–823. [Google Scholar] [CrossRef]
- Kristensen, S.G.; Rasmussen, A.; Byskov, A.G.; Andersen, C.Y. Isolation of pre-antral follicles from human ovarian medulla tissue. Hum. Reprod. 2011, 26, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Pangas, S.A.; Matzuk, M.M. The TGF-beta Family in the Reproductive Tract. Cold Spring Harb. Monogr. Ser. 2008, 50, 861. [Google Scholar]
- Chen, Y.G. Endocytic regulation of TGFβ signaling. Cell Res. 2009, 19, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Cavaillon, J.M.; Adib-Conquy, M.; Fitting, C.; Adrie, C.; Payen, D. Cytokine cascade in sepsis. Scand. J. Infect. Dis. 2003, 35, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Bemelmans, M.H.A.; Van Tits, L.J.H.; Buurman, W.A. Tumor necrosis factor: Function, release and clearance. Crit. Rev. Immunol. 1996, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pampori, Z.A.; Pandita, S. Age-and sex-related variability in physiological and immune responses to endotoxin challenge in Murrah buffaloes (Bubalus bubalis). J. Appl. Anim. Res. 2015, 43, 1–9. [Google Scholar] [CrossRef][Green Version]
- Azmi, T.I.; O’shea, J.D. Mechanism of deletion of endothelial cells during regression of the corpus luteum. Lab. Investig. J. Tech. Met. Path. 1984, 51, 206–217. [Google Scholar]
- Evans, A.C.O.; Adams, G.P.; Rawlings, N.C. Endocrine and ovarian follicular changes leading up to the first ovulation in prepubertal heifers. J. Reprod. Fertil. 1994, 100, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Imwalle, D.B.; Patterson, D.J.; Schillo, K.K. Effects of melengestrol acetate on onset of puberty, follicular growth, and patterns of luteinizing hormone secretion in beef heifers. Biol. Reprod. 1998, 58, 1432–1436. [Google Scholar] [CrossRef]
- Aulakh, A.S. Effect of Altrenogest Feeding vis-à-vis Ovsynch Regimen in Prepubertal Buffalo Heifers. Master’s Thesis, Guru Angad Dev Veterinary and Animal Sciences University, Ludhiana, India, 2020. [Google Scholar]
- Squires, E.L.; Heesemann, C.P.; Webel, S.K.; Shideler, R.K.; Voss, J.L. Relationship of altrenogest to ovarian activity, hormone concentrations and fertility of mares. J. Anim. Sci. 1983, 56, 901–910. [Google Scholar] [CrossRef]
- Davis, D.L.; Stevenson, J.S.; Schmidt, W.E. Scheduled Breeding of Gilts after Estrous Synchronization with Altrenogest. J. Anim. Sci. 1985, 60, 599–602. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Clarence, E.F. The Use of Altrenogest to Control Reproductive Function in Beef Cattle. Ph.D. Dissertation, Louisiana State University, Baton Rouge, LA, USA, 2004. [Google Scholar]
- Tauck, S.A.; Wilkinson, J.R.C.; Olsen, J.R.; Janitell, J.N.; Berardinelli, J.G. Comparison of controlled internal drug release device and melengesterol acetate as progestin sources in an estrous synchronization protocol for beef heifers. Theriogenology 2007, 68, 162–167. [Google Scholar] [CrossRef]
- Ferguson, C.E.; Kesler, D.; Godke, R. The use of altrenogest for estrus synchronization in yearling beef heifer. Trends Anim. Vet. Sci. 2010, 2, 1–4. [Google Scholar]
- Grégoire, A.; Allard, A.; Huamán, E.; León, S.; Silva, R.M.; Buff, S.; Joly, T. Control of the estrous cycle in guinea-pig (Cavia porcellus). Theriogenology 2012, 78, 842–847. [Google Scholar] [CrossRef]
- Ziecik, A.J.; Drzewiecka, K.; Gromadzka-Hliwa, K.; Klos, J.; Witek, P.; Knapczyk-Stwora, K.; Kaczmarek, M.M. Altrenogest affects the development and endocrine milieu of ovarian follicles in prepubertal and mature gilts. Biol. Reprod. 2020, 103, 1069–1084. [Google Scholar] [CrossRef] [PubMed]
- Szekeres-Bartho, J.; Wegmann, T.G. A progesterone-dependent immunomodulatory protein alters the Th1Th2 balance. J. Reprod. Immun. 1996, 31, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Piccinni, M.P.; Giudizi, M.G.; Biagiotti, R.; Beloni, L.; Giannarini, L.; Sampognaro, S.; Livi, C. Progesterone favors the development of human T helper cells producing Th2-type cytokines and promotes both IL-4 production and membrane CD30 expression in established Th1 cell clones. J. Immunol. 1995, 155, 128–133. [Google Scholar] [CrossRef]
- Oertelt-Prigione, S.; Regitz-Zagrosek, V. Progesterone: A critical hormone in modulating the immune response. In Female Immune Response; Springer: Berlin/Heidelberg, Germany, 2009; pp. 151–166. [Google Scholar]
- Singh, P.M.; Garg, K. Interferon-γ and progesterone levels in women with luteal phase deficiency: A comparative study. J. Hum. Reprod. Sci. 2014, 7, 247–252. [Google Scholar]
- Dewailly, D.; Andersen, C.Y.; Balen, A.; Broekmans, F.; Dilaver, N.; Fanchin, R.; Anderson, R.A. The physiology and clinical utility of anti-Müllerian hormone in women. Hum. Reprod. 2014, 20, 370–385. [Google Scholar] [CrossRef]
- Visser, J.A.; Themmen, A.P. Anti-Müllerian hormone and folliculogenesis. Mol. Cell. Endocrinol. 2005, 234, 81–86. [Google Scholar] [CrossRef]
- Sengupta, J.; Lalitkumar, P.G.; Najwa, A.R.; Ghosh, D.; Saha, S.; Gupta, S.K. Impact of locally released interferon-γ on ovarian steroidogenesis and corpus luteum function in the rat. Mol. Cell. Endocrinol. 2013, 372, 21–30. [Google Scholar]
- Ledee-Bataille, N.; Olivennes, F.; Lefaix, J.L.; Chaouat, G.; Frydman, R.; Delanian, S. Combined treatment by pentoxifylline and tocopherol for recipient women with a thin endometrium enrolled in an oocyte donation programme. Hum. Reprod. 2002, 17, 1249–1253. [Google Scholar] [CrossRef] [PubMed]
- Fanchin, R.; Schonäuer, L.M.; Righini, C.; Guibourdenche, J.; Frydman, R.; Taieb, J. Serum anti-Müllerian hormone is more strongly related to ovarian follicular status than serum inhibin B, estradiol, FSH and LH on day 3. Hum. Reprod. 2003, 18, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Cope, A.P.; Liblau, R.S. T-cell therapy in the treatment of autoimmune disease. Immunology 2008, 123, 6–14. [Google Scholar]
- Dinarello, C.A. Immunological and inflammatory functions of the interleukin-1 family. Ann. Rev. Immunol. 2009, 27, 519–550. [Google Scholar] [CrossRef] [PubMed]
- Grunig, G.; Warnock, M.; Wakil, A.E.; Venkayya, R.; Brombacher, F.; Rennick, D.M.; Corry, D.B. Requirement for IL13 independently of IL-4 in experimental asthma. Science 1998, 282, 2261–2263. [Google Scholar] [CrossRef] [PubMed]
- Petraglia, F.; Florio, P.; Nappi, C.; Genazzani, A.R. Peptide signaling in human placenta and membranes: Autocrine, paracrine, and endocrine mechanisms. Endocr. Rev. 1996, 17, 156–186. [Google Scholar] [CrossRef][Green Version]
- Kuperman, D.A.; Huang, X.; Koth, L.L.; Chang, G.H.; Dolganov, G.M.; Zhu, Z.; Erle, D.J. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat. Med. 2002, 8, 885–889. [Google Scholar] [CrossRef]
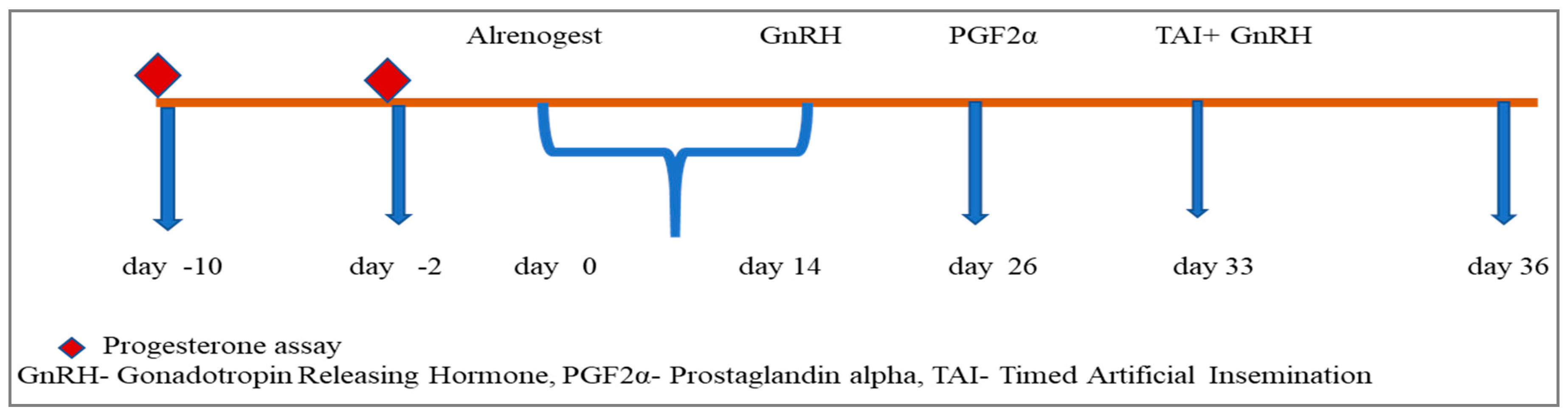
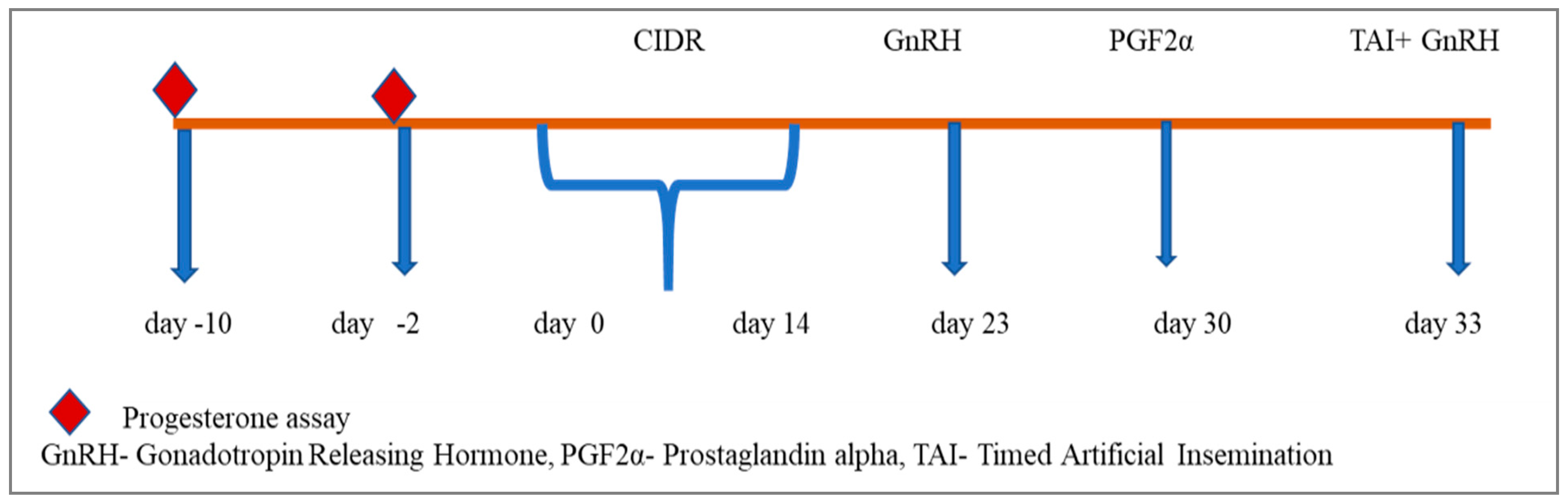
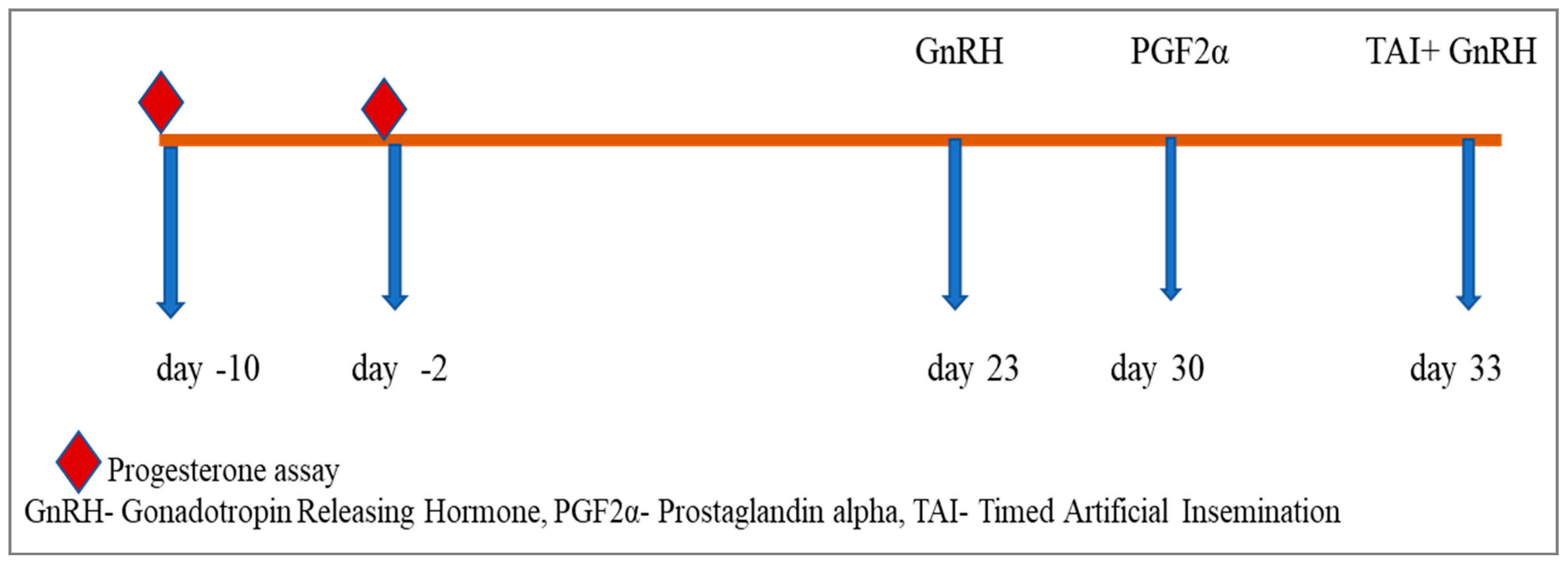
| Group | Number of Heifers (n) | Treatment |
|---|---|---|
| Group I | 6 | Altrenogest @ 0.044 mg/kg/day/animal for 14 days plus modified Co-Synch (Figure 1) |
| Group II | 6 | CIDR plus Co-Synch (Figure 2) |
| Group III | 6 | Co-Synch (Figure 3) |
| Day of Sampling (Day) | Treatment | Group 1 | Group 2 | Group 3 (Control) |
|---|---|---|---|---|
| 0 | Altrenogest/CIDR | Day 0 | Day 0 | Day 0 |
| 7 | Day 7 | Day 7 | Day 7 | |
| 14 | Day 14 | Day 14 | Day 14 | |
| 23 | Co-synch | - | Day 0 | Day 0 |
| 26 | Day 0 | - | - | |
| 30 | - | Day 7 | Day 7 | |
| 33 | Day 7 | Day 9 | Day 9 | |
| 36 | Day 9 | - | - |
| Period | Day | Altrenogest (Group 1) | CIDR (Group 2) | Co-Synch (Group 3) | p-Value |
|---|---|---|---|---|---|
| Day of P4 treatment | 0 (start of P4 treatment) | 16.57 ± 2.05 | 15.09 ± 2.47 | 17.45 ± 0.98 | 0.691 |
| 7 | 18.52 ± 1.15 | 18.35 ± 1.22 | 17.02 ± 1.03 | 0.604 | |
| 14 | 19.26 ± 0.72 | 16.26 ± 1.79 | 17.8 ± 1.29 | 0.313 | |
| p-value | 0.361 | 0.126 | 0.640 | ||
| During Co-synch | 0 (start of Co-synch) | 20.11 a ± 0.36 | 20.07 ± 0.35 | 15.83 ± 3.19 | 0.210 |
| 7 | 19.77 a ± 0.34 | 19.6 ± 0.55 | 19.77 ± 0.57 | 0.961 | |
| 9 | 14.41 b ± 1.97 | 16.2 ± 3.14 | 19.94 ± 0.44 | 0.213 | |
| p-value | 0.024 | 0.303 | 0.304 |
| Period | Day | Altrenogest (Group 1) | CIDR (Group 2) | Co-Synch (Group 3) | p-Value |
|---|---|---|---|---|---|
| Day of P4 treatment | 0 (start of P4 treatment) | 0.68 ab ± 0.06 | 0.68 ± 0.10 | 0.68 ± 0.09 | 0.998 |
| 7 | 0.59 bB ± 0.15 | 2.66 A ± 1.00 | 0.81 B ± 0.12 | 0.048 | |
| 14 | 0.97 a ± 0.15 | 1.03 ± 0.18 | 1.21 ± 0.29 | 0.706 | |
| p-value | 0.043 | 0.064 | 0.096 | ||
| During Co-synch | 0 (Start of Co-synch | 0.63 ± 0.16 | 0.96 ab ± 0.36 | 0.88 ab ± 0.23 | 0.664 |
| 7 | 1.33 ± 0.36 | 1.37 a ± 0.20 | 1.91 a ± 0.39 | 0.386 | |
| 9 | 1.17 A ± 0.18 | 0.46 bB ± 0.08 | 0.51 bB ± 0.10 | 0.002 | |
| p-value | 0.159 | 0.041 | 0.007 |
| Period | Day | Altrenogest (Group 1) | CIDR (Group 2) | Co-Synch (Group 3) | p-Value |
|---|---|---|---|---|---|
| Day of P4 treatment | 0 | 1.61 b ± 0.41 | 12.4 ± 6.15 | 11.24 ± 4.03 | 0.177 |
| 7 | 2.73 b ± 0.51 | 11.63 ± 6.37 | 36.38 ± 29.78 | 0.397 | |
| 14 | 6.60 a ± 1.17 | 10.23 ± 4.23 | 31.85 ± 25.89 | 0.463 | |
| p-value | 0.001 | 0.910 | 0.507 | ||
| During Co-synch | 0 | 4.61 ± 0.61 | 13.96 ± 6.74 | 34.28 ± 26.85 | 0.427 |
| 7 | 5.07 ± 0.95 | 12.02 ± 5.17 | 32.9 ± 26.87 | 0.451 | |
| 9 | 4.68 ± 1.08 | 11.23 ± 4.21 | 7.76 ± 3.25 | 0.360 | |
| p-value | 0.909 | 0.737 | 0.374 |
| Period | Day | Altrenogest (Group 1) | CIDR (Group 2) | Co-Synch (Group 3) | p-Value |
|---|---|---|---|---|---|
| Days of P4 treatment | 0 (Start of P4 treatment) | 227.23 ± 31.83 | 347.67 ± 85.28 | 207.12 ± 55.29 | 0.249 |
| 7 | 202.14 ± 23.82 | 255.48 ± 68.02 | 170.83 ± 35.39 | 0.446 | |
| 14 | 237.03 ± 47.15 | 342.22 ± 73.24 | 202.78 ± 43.09 | 0.220 | |
| p-value | 0.789 | 0.066 | 0.628 | ||
| During Co-synch | 0 (Start of Co-synch) | 204.92 ± 20.2 | 324.46 ± 63.5 | 205.07 ab ± 20.32 | 0.084 |
| 7 | 382.12 ± 85.01 | 296.78 ± 66.16 | 269.05 a ± 38.47 | 0.469 | |
| 9 | 203.82 ± 30.26 | 296.7 ± 59.79 | 166.96 b ± 24.47 | 0.105 | |
| p-value | 0.059 | 0.683 | 0.033 |
| Period | Day | Altrenogest (Group 1) | CIDR (Group 2) | Co-Synch (Group 3) | p-Value |
|---|---|---|---|---|---|
| Day of P4 treatment | 0 (Start of P4 treatment) | 717.68 ± 298.27 | 742.49 ± 159.75 | 392.41 ± 148.93 | 0.452 |
| 7 | 742.89 ± 199.48 | 604.83 ± 120.69 | 706.03 ± 202.09 | 0.853 | |
| 14 | 684.37 ± 197.41 | 536.46 ± 116.87 | 412.39 ± 83.11 | 0.415 | |
| p-value | 0.960 | 0.505 | 0.282 | ||
| During Co-synch | 0 (Start of Co-synch) | 382.31 b ± 123.55 | 370.57 ± 67.56 | 438.02 ± 169.26 | 0.923 |
| 7 | 755.16 aA ± 195.41 | 339.81 B ± 112.94 | 231.12 B ± 70.39 | 0.038 | |
| 9 | 723.14 aA ± 232.52 | 279.01B ± 84.73 | 229.04 B ± 77.33 | 0.024 | |
| p-value | 0.043 | 0.727 | 0.327 |
| Period | Day | Altrenogest (Group 1) | CIDR (Group 2) | Co-Synch (Group 3) | p-Value |
|---|---|---|---|---|---|
| Days of P4 treatment | 0 (Start of P4 treatment) | 138.61 ± 41.45 | 103.07 ± 48.04 | 55.47 ± 14.58 | 0.320 |
| 7 | 170.17 ± 60.18 | 137.77 ± 46.80 | 123.35 ± 49.20 | 0.813 | |
| 14 | 172.84 ± 37.35 | 101.29 ± 48.80 | 56.43 ± 21.84 | 0.122 | |
| p-value | 0.788 | 0.820 | 0.316 | ||
| During Co-synch | 0 (Start of Co-synch) | 152.22 ± 54.79 | 119.34 ab ± 33.00 | 53.17 ± 15.26 | 0.205 |
| 7 | 127.89 ± 28.67 | 69.30 b ± 16.99 | 51.20 ± 16.51 | 0.057 | |
| 9 | 160.38 ± 37.55 | 163.03 a ± 38.61 | 109.6 ± 39.41 | 0.556 | |
| p-value | 0.823 | 0.012 | 0.148 |
| Period | Day | Altrenogest (Group 1) | CIDR (Group 2) | Co-Synch (Group 3) | p-Value |
|---|---|---|---|---|---|
| Days of P4 treatment | 0 (Start of P4 treatment) | 60.36 ± 30.27 | 69.22 ± 20.39 | 46.89 ± 11.42 | 0.775 |
| 7 | 50.44 ± 15.18 | 93.42 ± 24.3 | 60.26 ± 23.34 | 0.354 | |
| 14 | 40.37 ± 9.06 | 84.09 ± 25.17 | 37.93 ± 11.92 | 0.129 | |
| p-value | 0.568 | 0.124 | 0.620 | ||
| During Co-synch | 0 (Start of Co-Synch) | 37.83 ± 18.78 | 64.72 ± 6.78 | 42.25 ± 10.09 | 0.316 |
| 7 | 64.11 ± 23.91 | 56.45 ± 8.22 | 40.30 ± 13.50 | 0.594 | |
| 9 | 86.84 ± 21.66 | 45.17 ± 2.90 | 48.69 ± 21.65 | 0.217 | |
| p-value | 0.155 | 0.068 | 0.782 |
| Period | Day | Altrenogest (Group 1) | CIDR (Group 2) | Co-Synch (Group 3) | p-Value |
|---|---|---|---|---|---|
| Days of P4 treatment | 0 (Start of P4 treatment) | 17.25 ± 7.04 | 36.15 ± 6.24 | 23.31 ± 4.39 | 0.108 |
| 7 | 22.27 ± 6.63 | 44.51 ± 2.95 | 32.46 ± 7.32 | 0.057 | |
| 14 | 21.00 B ± 6.05 | 43.07 A ± 2.51 | 34.52 AB ± 7.29 | 0.044 | |
| p-value | 0.721 | 0.195 | 0.251 | ||
| During Co-synch | 0 (Start of Co-synch) | 23.76 ± 6.13 | 32.08 ± 5.35 | 30.44 ± 8.63 | 0.668 |
| 7 | 16.51 ± 6.25 | 34.23 ± 6 | 32.97 ± 4.74 | 0.080 | |
| 9 | 19.48 ± 8.44 | 35.06 ± 3.2 | 26.67 ± 6.01 | 0.245 | |
| p-value | 0.753 | 0.901 | 0.803 |
| Period | Day | Altrenogest (Group 1) | CIDR (Group 2) | Co-Synch (Group 3) | p-Value |
|---|---|---|---|---|---|
| Days of P4 treatment | 0 (Start of P4 treatment) | 19.85 ± 2.47 | 29.18 ± 3.96 | 27.92 ± 1.61 | 0.072 |
| 7 | 18.67 B ± 2.73 | 32.68 A ± 2.83 | 26.91 AB ± 4.62 | 0.039 | |
| 14 | 22.32 B ± 1.95 | 33.67 A ± 3.14 | 24.10 B ± 2.75 | 0.018 | |
| p-value | 0.451 | 0.452 | 0.548 | ||
| During Co-synch | 0 (Start of Co-synch) | 17.75 B ± 2.34 | 29.81 A ± 2.50 | 23.08 AB ± 2.44 | 0.011 |
| 7 | 20.55 ± 2.26 | 23.9 ± 2.36 | 22.41 ± 4.43 | 0.760 | |
| 9 | 20.78 ± 3.94 | 25.15 ± 3.36 | 19.72 ± 1.86 | 0.459 | |
| p-value | 0.653 | 0.297 | 0.699 |
| Period | Day | Altrenogest (Group 1) | CIDR (Group 2) | Co-Synch (Group 3) | p-Value |
|---|---|---|---|---|---|
| Day of P4 treatment | 0 (Start of P4 treatment) | 3.08 b ± 0.33 | 3.00 ± 0.45 | 2.71 b ± 0.36 | 0.768 |
| 7 | 6.83 aA ± 0.70 | 7.17 A ± 0.79 | 4.00 abB ± 0.52 | 0.009 | |
| 14 | 8.17 a ± 0.70 | 6.67 ± 1.41 | 6.83 a ± 0.75 | 0.527 | |
| p-value | <0.001 | 0.093 | 0.010 | ||
| During Co-synch | 0 (Start of Co-synch) | 5.83 b ± 0.48 | 4.67 ± 0.92 | 4.17 b ± 0.60 | 0.248 |
| 7 | 9.00 a ± 1.00 | 11.00 ± 3.11 | 6.17 a ± 0.60 | 0.234 | |
| 9 | 10.83 a ± 1.82 | 8.75 ± 1.26 | 8.50 a ± 1.52 | 0.520 | |
| p-value | 0.018 | 0.135 | 0.008 |
| Variable | AMH | Estradiol 17-β | IFNγ | IL1 | IL6 | IL13 | Progesterone | TGFβ | TNFα | Follicle Size |
|---|---|---|---|---|---|---|---|---|---|---|
| AMH | 1 | 0.049 | 0.103 | −0.046 | 0.012 | 0.007 | 0.272 | 0.158 | 0.134 | 0.132 |
| Estradiol 17-β | 0.049 | 1 | 0.036 | 0.001 | 0.023 | −0.073 | −0.173 | 0.211 | −0.264 | −0.172 |
| IFNγ | 0.103 | 0.036 | 1 | 0.164 | 0.188 | 0.251 | 0.626 ** | −0.020 | 0.123 | 0.168 |
| IL1 | −0.046 | 0.001 | 0.164 | 1 | 0.098 | 0.182 | 0.118 | 0.015 | −0.088 | −0.022 |
| IL6 | 0.012 | 0.023 | 0.188 | 0.098 | 1 | −0.175 | 0.055 | 0.010 | 0.202 | −0.109 |
| IL13 | 0.007 | −0.073 | 0.251 | 0.182 | −0.175 | 1 | 0.306 | −0.022 | −0.031 | 0.150 |
| Progesterone | 0.272 | −0.173 | 0.626 ** | 0.118 | 0.055 | 0.306 | 1 | 0.069 | 0.150 | 0.246 |
| TGFβ | 0.158 | 0.211 | −0.020 | 0.015 | 0.010 | −0.022 | 0.069 | 1 | 0.236 | −0.191 |
| TNF-α | 0.134 | −0.264 | 0.123 | −0.088 | 0.202 | −0.031 | 0.150 | 0.236 | 1 | −0.210 |
| Follicle size | 0.132 | −0.172 | 0.168 | −0.022 | −0.109 | 0.150 | 0.246 | −0.191 | −0.210 | 1 |
| Variable | AMH | Estradiol 17-β | IFNγ | IL1 | IL6 | IL13 | Progesterone | TGFβ | TNFα | Follicle Size |
|---|---|---|---|---|---|---|---|---|---|---|
| AMH | 1 | −0.307 | 0.673 ** | −0.120 | −0.176 | −0.181 | −0.247 | 0.463 ** | −0.342 * | −0.067 |
| Estradiol 17-β | −0.307 | 1 | −0.348 * | 0.072 | 0.314 | 0.229 | 0.184 | −0.168 | 0.269 | 0.197 |
| IFNγ | 0.673 ** | −0.348 * | 1 | −0.167 | −0.252 | 0.017 | −0.454 ** | 0.439 ** | −0.373 * | −0.117 |
| IL1 | −0.120 | 0.072 | −0.167 | 1 | 0.168 | 0.082 | 0.001 | 0.089 | 0.466 ** | −0.291 |
| IL6 | −0.176 | 0.314 | −0.252 | 0.168 | 1 | 0.322 | 0.118 | 0.139 | 0.483 ** | 0.062 |
| IL13 | −0.181 | 0.229 | 0.017 | 0.082 | 0.322 | 1 | 0.315 | 0.188 | 0.507 ** | 0.101 |
| Progesterone | −0.247 | 0.184 | −0.454 ** | 0.001 | 0.118 | 0.315 | 1 | −0.096 | 0.243 | 0.095 |
| TGFβ | 0.463 ** | −0.168 | 0.439 ** | 0.089 | 0.139 | 0.188 | −0.096 | 1 | 0.169 | −0.058 |
| TNFα | −0.342 * | 0.269 | −0.373 * | 0.466 ** | 0.483 ** | 0.507 ** | 0.243 | 0.169 | 1 | −0.034 |
| Follicle size | −0.067 | 0.197 | −0.117 | −0.291 | 0.062 | 0.101 | 0.095 | −0.058 | −0.034 | 1 |
| Variable | AMH | Estradiol 17-β | IFNγ | IL1 | IL6 | IL13 | Progesterone | TGFβ | TNFα | Follicle Size |
|---|---|---|---|---|---|---|---|---|---|---|
| AMH | 1 | −0.255 | −0.047 | 0.256 | 0.026 | −0.093 | 0.181 | 0.408 * | −0.041 | −0.168 |
| Estradiol 17-β | −0.255 | 1 | 0.007 | −0.231 | −0.044 | 0.035 | 0.017 | −0.199 | −0.049 | 0.351 * |
| IFNγ | −0.047 | 0.007 | 1 | 0.083 | −0.080 | 0.061 | −0.029 | 0.173 | 0.392 * | −0.076 |
| IL1 | 0.256 | −0.231 | 0.083 | 1 | −0.034 | −0.202 | −0.100 | −0.023 | 0.391 * | −0.210 |
| IL6 | 0.026 | −0.044 | −0.08 | −0.034 | 1 | 0.394 * | −0.349 * | 0.015 | 0.035 | −0.286 |
| IL13 | −0.093 | 0.035 | 0.061 | −0.202 | 0.394 * | 1 | −0.150 | 0.377 * | 0.265 | −0.034 |
| Progesterone | 0.181 | 0.017 | −0.029 | −0.100 | −0.349 * | −0.15 | 1 | −0.053 | −0.250 | 0.249 |
| TGFβ | 0.408 * | −0.199 | 0.173 | −0.023 | 0.015 | 0.377 * | −0.053 | 1 | 0.012 | 0.131 |
| TNFα | −0.041 | −0.049 | 0.392 * | 0.391 * | 0.035 | 0.265 | −0.250 | 0.012 | 1 | −0.278 |
| Follicle size | −0.168 | 0.351 * | −0.076 | −0.210 | −0.286 | −0.034 | 0.249 | 0.131 | −0.278 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haridas, S.S.; Singh, P.; Ratta, N.S.; Singh, C.; Honparkhe, M. Cytokine Profiling and Puberty Enhancement Post Altrenogest Feeding in Prepubertal Murrah Buffalo (Bubalus bubalis) Heifers. Ruminants 2025, 5, 24. https://doi.org/10.3390/ruminants5020024
Haridas SS, Singh P, Ratta NS, Singh C, Honparkhe M. Cytokine Profiling and Puberty Enhancement Post Altrenogest Feeding in Prepubertal Murrah Buffalo (Bubalus bubalis) Heifers. Ruminants. 2025; 5(2):24. https://doi.org/10.3390/ruminants5020024
Chicago/Turabian StyleHaridas, Sneha Swapna, Prahlad Singh, Navdeep Singh Ratta, Chanchal Singh, and Mrigank Honparkhe. 2025. "Cytokine Profiling and Puberty Enhancement Post Altrenogest Feeding in Prepubertal Murrah Buffalo (Bubalus bubalis) Heifers" Ruminants 5, no. 2: 24. https://doi.org/10.3390/ruminants5020024
APA StyleHaridas, S. S., Singh, P., Ratta, N. S., Singh, C., & Honparkhe, M. (2025). Cytokine Profiling and Puberty Enhancement Post Altrenogest Feeding in Prepubertal Murrah Buffalo (Bubalus bubalis) Heifers. Ruminants, 5(2), 24. https://doi.org/10.3390/ruminants5020024







