Simple Summary
Nutrition during gestation plays a pivotal role in influencing the growth, development, and overall performance of cattle offspring. This study examined whether supplementing pregnant Nellore heifers with additional protein and energy could positively influence the growth and development of their calves. The study compared two strategies: only mineral supplementation (Non-Programmed) versus additional protein-energy supplementation (Fetal Programmed, FP). The results showed that cows in the FP group maintained better body condition throughout pregnancy, which could have contributed to early advantages for their calves. At birth and in the first few months of life, calves from supplemented dams were heavier and had improved morphological traits, particularly in rump width and length at 45 days old. However, as they grew under the same management conditions, these differences diminished, and at slaughter, there was no significant difference in body weight, carcass traits, or growth performance between the groups. This suggests that while prenatal nutrition can influence early development, long-term effects may depend more on postnatal management and environmental conditions. Understanding how maternal nutrition affects cattle performance can help refine feeding strategies to enhance beef production efficiency.
Abstract
Fetal programming suggests that maternal nutrition during gestation influences offspring growth, development, and productivity. This study evaluated the effects of prenatal protein-energy supplementation on the lifetime performance of Nellore cattle. Twenty-eight nulliparous heifers were inseminated and assigned to one of two groups: Non-Programmed; receiving only mineral supplementation; or Fetal Programmed (FP); receiving additional protein-energy supplementation throughout gestation. Cows in the FP group maintained significantly better body condition score during gestation (p < 0.01), and their calves exhibited greater body weight (BW) during the first 56 days (p < 0.05) and a tendency to grow to a greater BW up to 250 days (p < 0.10) in addition to improved morphological traits, such as increased rump width and length at 45 days of age (p ≤ 0.02). However, these advantages were not sustained in later growth stages, as no significant differences were observed in final body weight, ultrasound carcass traits, or overall feedlot performance. These findings suggest that while prenatal nutrition can influence early developmental traits, its long-term impact on offspring performance may be limited under consistent postnatal management. Nonetheless, the limited sample size, combined with the absence of molecular data and individual feed intake and efficiency measurements, constrains a more comprehensive interpretation of the programming effects on offspring performance. Further research is needed to explore the molecular mechanisms of fetal programming, particularly its epigenetic effects and interactions with postnatal nutrition, to optimize strategies for improving the efficiency and sustainability of beef cattle.
1. Introduction
Fetal programming (FP) establishes a connection between prenatal nutrition and long-term effects in offspring [1]. During gestation, both inadequate and excessive maternal nutrition can profoundly impact the structural development of organs, compromise prenatal and neonatal health, and influence growth, development, and feed efficiency in livestock [2,3,4]. These effects are not confined to a single generation, as FP is linked to transgenerational phenomena driven by stable, inheritable changes in gene expression [5]. Such changes, often referred to as epigenetic modifications, occur without altering the DNA sequence and are mediated by processes such as chromatin remodeling, DNA methylation, histone modifications, and mechanisms involving small non-coding RNAs, which lead to phenotypic variations across generations [6,7].
In beef cattle, primary myogenesis begins during the first two months of gestation, whereas secondary myogenesis occurs between the second and seventh months. Muscle fiber formation is exclusive to the fetal phase and does not occur postnatally [8]. During primary myogenesis, a limited number of muscle fibers are formed, indicating that maternal nutrition at this stage has a relatively minor effect on skeletal muscle development. However, most muscle fibers are formed during secondary myogenesis, and any disruption during this critical phase results in irreversible consequences for the offspring [3,4,9,10]. Maternal undernutrition in ruminants has been associated with adverse outcomes such as increased neonatal mortality, intestinal and respiratory dysfunction, metabolic disorders, slower postnatal growth rates, reduced meat quality, and lower feed efficiency [11]. Therefore, prenatal nutrition plays a pivotal role in the beef industry, influencing several physiological aspects throughout an animal’s lifespan [12].
Numerous studies have demonstrated the significant influence of maternal nutrition on muscle development, adipose tissue formation [13,14,15], body weight [16,17], and other economically important traits [18]. Despite significant advancements, research on the long-term effects of maternal nutrition on offspring performance remains limited, particularly in tropical cattle breeds such as Nellore [19]. Furthermore, few studies have comprehensively examined the impact of maternal phenotypes, such as body weight and body condition score, which are essential for understanding dams’ energy metabolism and its role in shaping offspring development and performance. In this study, we hypothesized that fetal programming through prenatal nutrition influences beef cattle performance. Accordingly, the objective was to evaluate the effects of fetal programming through different supplementation strategies in Nellore steers and to investigate the associations between prenatal nutritional interventions and offspring growth performance and carcass traits from the prenatal stage through finishing.
2. Materials and Methods
2.1. Animals and Experimental Design
This study was approved by the Ethics Committee on Animal Use of the Faculty of Animal Science and Food Engineering at the University of São Paulo under protocol number 1843241117.
A total of 28 nulliparous Nellore heifers (26 ± 2 months of age) were artificially inseminated at fixed time intervals (FTAI) using sexed semen from a single bull. Pregnancy was confirmed 30 days post-FTAI. The heifers were selected and randomly assigned to treatments according to body weight, and body condition score, measured at the time of insemination in order to keep the batches as homogeneous as possible.
The heifers were maintained on pastures of Urochloa brizantha cv. Marandu, equipped with feeding troughs for supplementation and water troughs. Two treatments were applied: NP, Non-Programmed: received mineral supplementation at 0.3 g/kg of the average body weight of the group; FP, Fetal Programmed: received protein-energy supplementation at 5 g/kg of the average body weight of the group throughout gestation (level of supplementation that meets the dams’ nutritional demands). The maternal diet compositions and nutritional contents were previously detailed by Cracco et al. [20] and are shown in Table 1.

Table 1.
Composition and nutritional content of maternal supplements.
Postpartum, protein-energy supplementation was discontinued, and all animals were managed in a single pasture (with Marandu grass) under identical environmental, nutritional, and health management conditions. Thus, treatments were applied exclusively during gestation, and all calves and dams were uniformly managed after parturition.
A total of 28 male calves remained with their dams until weaning at eight months of age. After weaning, the calves were raised on pasture for 10 months before entering the finishing phase in feedlot. During the rearing period, the animals were supplemented with protein-energy supplements to facilitate the transition to the feedlot. At the start of the finishing period, the animals were adapted for 14 days to the new finishing diet and to the facilities and management in the feedlot. The finishing phase lasted 97 days and during this period the animals were fed a total mixed ration twice a day with a 70/30 concentrate/volume ratio, after which the animals were slaughtered at 21 months of age.
2.2. Dams Phenotypes Evaluation
Ultrasound measurements, BW, and BCS were monitored in the heifers during the pre-gestational period, at 4, 6, and 9 months of gestation, and postpartum. Ultrasound measurements included longissimus muscle area (LMA), subcutaneous fat thickness (SFT), and rump fat thickness (RFT).
Ultrasound evaluations were performed using an Aloka SSD-500 ultrasound machine equipped with a 17.2 cm linear transducer operating at a frequency of 3.5 MHz (Aloka Co., Ltd., Wallingford, CT, USA). To enhance the coupling between the transducer and the animal skin, vegetable oil was applied as a medium for ultrasonic wave conduction [21].
Carcass ultrasound scans were conducted by a technician certified by the Ultrasound Guidelines Council following the methodology outlined by the Beef Improvement Federation [22]. FT was measured at the intersection of the Biceps femoris and Gluteus medius muscles between the ilium and ischium, whereas SFT and LMA were measured between the 12th and 13th ribs.
To accommodate the curvature of the dorso-lumbar region during SFT and LMA measurements, a silicone acoustic guide was used to adapt the transducer. The collected images were stored on a notebook hard drive for further analysis. The data were processed using the Lince® software (version 1.0, M&S Consultoria Agropecuária Ltda., Pirassununga, SP, Brazil) to generate specific measurements for each trait.
2.3. Offspring Phenotypes Evaluation
All animals were weighed periodically from birth to slaughter using a Coimma® digital scale to monitor weight gain. Weights were recorded every 30 days.
The same ultrasound measurements used for the heifers were applied to their progeny. Carcass ultrasounds were carried out every 3 months during rearing and every 28 days during finishing. These measurements included longissimus muscle area (LMA), subcutaneous fat thickness (SFT), and rump fat thickness (RFT), which were obtained using the same standardized methodologies and procedures.
2.4. Statistical Analyses
All individual evaluation results were generated using the “lm” function in the R statistical environment, considering treatment as a fixed effect. Residuals were tested for normality using the Shapiro–Wilk test and for homoscedasticity using Levene’s test [23].
For time-dependent analyses, the “lmer” function from the lme4 package was used to analyze repeated measures over time. A linear mixed-effects regression model was applied, considering each experimental unit as a random effect and treatment, time, and their interactions as fixed effects. Residuals were tested for normality using the Shapiro–Wilk test and for homoscedasticity using the Breusch–Pagan test.
For ordinal variables, such as cow scores, the ordinal package was used to fit an ordinal regression model with random effects, applying the same fixed and random effects structure as in the mixed-effects regression model. Both models were compared based on their AIC values, with the model having the lowest AIC chosen as the preferred model. The ordinal model showed a better balance between goodness-of-fit and model complexity, which is largely attributed to the nature of the dependent variable being qualitative and ordinal, rather than quantitative. The ordinal model is specifically designed to handle such data, making it a more appropriate choice for this analysis.
3. Results
3.1. Body Condition Score of Dams
No significant differences were observed in BCS between treatments during the early months of gestation (p > 0.05). However, at 6 and 9 months of gestation, cows in the NP treatment group exhibited greater BCS variation than those in the FP treatment group (p < 0.01).
3.2. Body Weight and Ultrasound Measurements of Dams
FP treatment increased BW from the 6th month of gestation compared to the NP group (p < 0.01). Additionally, FP-treated cows exhibited greater LMA, STF, and RTF starting from the 4th month of gestation compared to NP cows (p ≤ 0.01). Postpartum, FP cows maintained a significantly higher BW than NP cows (p < 0.01), as detailed in Table 2. All variables, including BW and ultrasound-derived measurements, were significantly influenced by time, treatment, and the interaction between treatment and time (p < 0.01).

Table 2.
Body weight and morphology of cows under different maternal nutrition strategies.
Figure 1 highlights differences in BCS, with cows in the FP group maintaining higher scores, whereas those in the control group experienced a decline in condition over time. The BCS differences at 6 months (p < 0.01) and 9 months (p < 0.01) underscore the positive impact of FP treatment on maintaining body condition during critical gestational stages.
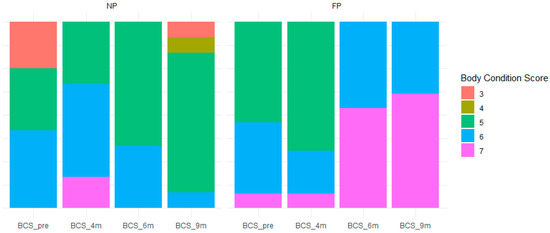
Figure 1.
Variation in body condition score across maternal nutrition strategies. The figure illustrates the variation in body condition score between the groups. NP (Non-Programmed) refers to heifers receiving mineral supplementation at 0.3 g/kg of body weight (BW), while FP (Fetal Programmed) represents heifers supplemented with protein-energy at 5 g/kg of BW.
3.3. Offspring Phenotypes Measurements
Calf weight analysis revealed significant differences between the NP and FP groups during the first 56 days of life (p < 0.05), with FP calves exhibiting higher weights than NP calves, as detailed in Table 3. Although these differences diminished over time, a trend toward greater weight gain in the FP group persisted up to 250 days (p < 0.10). No significant differences in final weight at slaughter were observed between the treatment groups (p > 0.05), nor were there significant differences in weight progression over time between treatments. However, both groups showed a consistent weight gain trajectory over time (p < 0.01), independent of the nutritional strategy.

Table 3.
Body weight from birth to slaughter of calves under different maternal nutrition strategies.
3.4. Ultrasound Measurements
No significant differences were observed in the ultrasound measurements of calves across the maternal supplementation strategies (Table 4), nor were there significant differences between treatments over time (p > 0.05). However, over time, an increase in the measured areas was noted (p < 0.01).

Table 4.
Ultrasound evaluation of calves under different maternal nutrition strategies.
3.5. Morphological Measurements
At 45 days of age, significant differences were observed between the NP and FP groups in certain morphological traits, likely reflecting enhanced skeletal growth during fetal life, particularly influenced by maternal supplementation during the critical window of bone formation, when most muscle fibers are formed. As shown in Table 5, the rump width and length were higher in the FP group than in the NP group (p ≤ 0.02). Additionally, rib depth and body length tended to be greater in the FP group (p < 0.10). However, at most time points, the morphological traits did not differ significantly between the treatment groups (p > 0.05). No significant interaction between treatment and time was observed (p > 0.05). Nevertheless, a significant increase in morphological measurements over time was noted (p < 0.01), indicating normal growth progression in both groups.

Table 5.
Morphological measurements of calves under different maternal nutrition strategies.
4. Discussion
We hypothesized that fetal programming through protein-energy supplementation during gestation influences offspring performance in beef cattle. Our results indicate that maternal nutrition influenced calf weight up to 250 days and had a short-term effect on certain early-life morphological traits, such as rump width and length at 45 days of age; however, these differences were not sustained throughout the growth period.
The significant differences observed in early skeletal development align with previous studies showing that maternal nutrition during gestation influences neonatal morphology [3,4]. Our results suggest that maternal supplementation throughout gestation may enhance fetal growth trajectories, particularly during secondary myogenesis, which occurs between the second and seventh months of gestation [3], thereby contributing to increased birth weight and improved early carcass traits. However, the absence of long-term effects suggests that postnatal factors, including uniform management and nutrition, may have mitigated the initial benefits of fetal programming.
The increased rump width and length and the tendency for increased rib depth and body length in the FP group are consistent with some results in the literature [24,25]. On the other hand, the lack of statistical significance at later stages suggests that compensatory growth mechanisms may have minimized early morphological advantages, reducing the effects of prenatal nutritional interventions [24,25,26].
Additionally, despite the initial impact on morphological traits, the treatments showed no significant differences in ultrasound parameters or overall growth performance. This contrasts with studies that have reported lasting effects of fetal programming on body composition and feed efficiency [16,17,18]. This may be attributed to the moderate nature of the nutritional intervention, which provided sufficient nutrients to support fetal development [27,28] without imposing extreme dietary restrictions or excesses. In contrast, other studies have applied extreme interventions, such as overfeeding at 1.5 times the maintenance requirements [29] or restricting intake to 65% of the nutritional requirements during early gestation [30]. Under the conditions of the present study, maternal supplementation may not lead to substantial alterations in offspring growth trajectories beyond the early stages of life. Nevertheless, the absence of feed intake and efficiency measurements in the present study constrains the interpretation of potential programming effects on nutrient utilization. Future research should incorporate these parameters to elucidate how prenatal nutritional strategies shape postnatal metabolic efficiency and influence long-term productive performance in ruminants.
Interestingly, maternal supplementation significantly affected the BW and BCS of the dams during gestation, particularly from the 6th to 9th months of pregnancy. This finding is consistent with previous studies [31,32], which demonstrated that prenatal nutritional strategies contribute to improved maternal energy reserves, thereby enhancing postpartum recovery and lactational performance. Maintaining an adequate BCS during gestation may play a crucial role in optimizing milk production and early calf development, potentially mitigating direct effects on birth weight and postnatal performance [32,33]. However, since calves were raised under uniform postnatal conditions, any potential advantage of improved milk yield may have been counteracted by later environmental factors.
The lack of significant differences in ultrasound-measured carcass traits such as LMA and SFT suggests that maternal nutrition had minimal influence on offspring adipogenesis. Given that adipogenesis primarily occurs in the final months of gestation and continues postnatally [3], the fetal nutritional environment in our study may not have strongly modulated fat deposition. This aligns with findings in sheep, where fetal programming effects on carcass traits were only observed under extreme maternal nutritional conditions [34]. However, our findings contrast with those of previous studies in cattle, which reported significant effects of maternal nutrition on adipose tissue development and fat deposition [3,35].
From a practical standpoint, these results suggest that enhancing maternal supplementation throughout gestation can improve maternal body condition and support early offspring weight and morphology without necessarily altering long-term performance. While previous research has highlighted potential benefits of fetal programming on economically relevant traits [33,36], our findings emphasize that postnatal management plays a crucial role in determining ultimate growth and carcass outcomes. Future studies should explore the molecular mechanisms underlying these effects, particularly the epigenetic regulation and metabolic programming.
Overall, this study contributes to the growing body of literature on fetal programming in beef cattle by demonstrating that while maternal nutrition influences early skeletal development and dam body condition, its effects on long-term offspring performance remain limited under typical pasture-based systems. These findings highlight the need for further research to determine whether specific maternal dietary interventions combined with targeted postnatal nutrition strategies can enhance the sustainability and efficiency of beef production.
5. Conclusions
This study offers one of the first validations of fetal programming effects in tropical beef cattle by assessing the long-term outcomes in the offspring. Our findings suggest that protein-energy supplementation during gestation in Nellore cows influences early developmental traits but does not lead to significant long-term differences in offspring performance. These findings highlight the relevance of maternal nutritional strategies during gestation and underscore the importance of postnatal management in shaping productive outcomes. Future research should investigate the molecular mechanisms underlying these effects, focusing on epigenetic regulation, including DNA methylation profiling, miRNA and long non-coding RNA expression, and their roles in metabolic programming and tissue development.
Author Contributions
Conceptualization, M.H.d.A.S.; methodology, A.C.F. and G.H.G.P.; formal analysis, G.H.G.P. and G.d.V.P.; investigation, A.C.F., B.C.T.P., É.F. and F.J.S.J.; writing—original draft preparation, G.d.V.P. and A.T.N.; writing—review and editing, G.d.V.P. and A.T.N.; supervision, M.H.d.A.S.; project administration, M.H.d.A.S.; funding acquisition, M.H.d.A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the São Paulo Research Foundation (FAPESP), grant number (17/12105-2), and the National Council for Scientific and Technological Development (CNPq), grant number 307593/2021-5.
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee on Animal Use of the Faculty of Animal Science and Food Engineering at the University of São Paulo, protocol code 1843241117, approved in 03 September 2018.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Du, M.; Zhao, J.X.; Yan, X.; Huang, Y.; Nicodemus, L.V.; Yue, W.; McCormick, R.J.; Zhu, M.J. Fetal muscle development, mesenchymal multipotent cell differentiation, and associated signaling pathways. J. Anim. Sci. 2011, 89, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Vautier, A.N.; Cadaret, C.N. Long-Term Consequences of Adaptive Fetal Programming in Ruminant Livestock. Front. Anim. Sci. 2022, 3, 778440. [Google Scholar] [CrossRef]
- Du, M.; Tong, J.; Zhao, J.; Underwood, K.R.; Zhu, M.; Ford, S.P.; Nathanielsz, P.W. Fetal programming of skeletal muscle development in ruminant animals. J. Anim. Sci. 2010, 88, E51–E60. [Google Scholar] [CrossRef]
- Du, M.; Ford, S.P.; Zhu, M.J. Optimizing livestock production efficiency through maternal nutritional management and fetal developmental programming. Anim. Front. 2017, 7, 5–11. [Google Scholar] [CrossRef]
- Ji, Y.; Wu, Z.; Dai, Z.; Wang, X.; Li, J.; Wang, B.; Wu, G. Fetal and neonatal programming of postnatal growth and feed efficiency in swine. J. Anim. Sci. Biotechnol. 2017, 8, 764–778. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Z.; Li, D.; Li, N.; Dindot, S.V.; Satterfield, M.C.; Bazer, F.W.; Wu, G.Y. Nutrition, epigenetics, and metabolic syndrome. Antioxid. Redox Signal. 2012, 17, 282–301. [Google Scholar] [CrossRef]
- Feeney, A.; Nilsson, E.; Skinner, M.K. Epigenetics and transgenerational inheritance in domesticated farm animals. J. Anim. Sci. Biotechnol. 2014, 5, 48. [Google Scholar] [CrossRef]
- Mikovic, J.; Lamon, S. The effect of maternal metabolic status on offspring health: A role for skeletal muscle? J. Physiol. 2018, 596, 5079. [Google Scholar] [CrossRef]
- Ithurralde, J.; Genovese, P.; Abud, M.J.; López-Pérez, Á.; Pérez-Clariget, R.; Bielli, A. Maternal undernutrition affects secondary myogenesis in a muscle-dependent way across the major muscles of 70-day old ovine fetuses. Small Rumin. Res. 2020, 191, 106174. [Google Scholar] [CrossRef]
- Picard, B.; Lefaucheur, L.; Berri, C.; Duclos, M.J. Muscle fibre ontogenesis in farm animal species. Reprod. Nutr. Dev. 2002, 42, 415–431. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Wallace, J.M.; Spencer, T.E. Board-invited review: Intrauterine growth retardation: Implications for the animal sciences. J. Anim. Sci. 2006, 84, 2316–2337. [Google Scholar] [CrossRef] [PubMed]
- Funston, R.N.; Larson, D.M.; Vonnahme, K.A. Effects of maternal nutrition on conceptus growth and offspring performance: Implications for beef cattle production. J. Anim. Sci. 2010, 88, E205–E215. [Google Scholar] [CrossRef]
- Polizel, G.H.G.; de Francisco Strefezzi, R.; Cracco, R.C.; Fernandes, A.C.; Zuca, C.B.; Castellar, H.H.; Baldin, G.C.; de Almeida Santana, M.H. Effects of different maternal nutrition approaches on weight gain and on adipose and muscle tissue development of young bulls in the rearing phase. Trop. Anim. Health Prod. 2021, 53, 536. [Google Scholar] [CrossRef]
- Costa, T.C.; Gionbelli, M.P.; Duarte, M.d.S. Fetal programming in ruminant animals: Understanding the skeletal muscle development to improve meat quality. Anim. Front. 2021, 11, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Otomaru, K.; Oshima, K.; Goto, Y.; Oshima, I.; Muroya, S.; Saneshima, R.; Nagao, Y.; Kinoshita, A. Effects of low and high levels of maternal nutrition consumed for the entirety of gestation on the development of muscle, adipose tissue, bone, and the organs of Wagyu cattle fetuses. Anim. Sci. J. 2021, 92, e13600. [Google Scholar] [CrossRef] [PubMed]
- Cracco, R.C.; Ruy, I.M.; Polizel, G.H.G.; Fernandes, A.C.; Furlan, É.; Baldin, G.C.; Chagas Santos, G.E.; de Almeida Santana, M.H. Evaluation of Maternal Nutrition Effects in the Lifelong Performance of Male Beef Cattle Offspring. Vet. Sci. 2023, 10, 443. [Google Scholar] [CrossRef]
- Valiente, S.L.; Rodríguez, A.M.; Long, N.M.; Quintans, G.; Miccoli, F.E.; Lacau-Mengido, I.M.; Maresca, S. Age at First Gestation in Beef Heifers Affects Fetal and Postnatal Growth, Glucose Metabolism and IGF1 Concentration. Animals 2021, 11, 3393. [Google Scholar] [CrossRef]
- Polizel, G.H.G.; Cançado, F.A.; Dias, E.; Fernandes, A.C.; Cracco, R.C.; Carmona, B.T.; Castellar, H.; Daiana Poleti, M.; de Almeida Santana, M.H. Effects of Different Prenatal Nutrition Strategies on the Liver Metabolome of Bulls and Its Correlation with Body and Liver Weight. Metabolites 2022, 12, 441. [Google Scholar] [CrossRef]
- Martins, T.S.; Sanglard, L.M.P.; Silva, W.; Chizzotti, M.L.; Rennó, L.N.; Serão, N.V.L.; Silva, F.F.; Guimarães, S.E.F.; Ladeira, M.M.; Dodson, M.V.; et al. Molecular Factors Underlying the Deposition of Intramuscular Fat and Collagen in Skeletal Muscle of Nellore and Angus Cattle. PLoS ONE 2015, 10, e0139943. [Google Scholar] [CrossRef]
- Cracco, R.C.; Alexandre, P.A.; Polizel, G.H.G.; Fernandes, A.C.; de Almeida Santana, M.H. Evaluation of Muscle Long Non-Coding RNA Profile during Rearing and Finishing Phase of Bulls Subjected to Different Prenatal Nutritional Strategies. Animals 2024, 14, 652. [Google Scholar] [CrossRef]
- Santana, M.H.A.; Ventura, R.V.; Utsunomiya, Y.T.; Neves, H.H.R.; Alexandre, P.A.; Oliveira Junior, G.A.; Gomes, R.C.; Bonin, M.N.; Coutinho, L.L.; Garcia, J.F.; et al. A genomewide association mapping study using ultrasound-scanned information identifies potential genomic regions and candidate genes affecting carcass traits in Nellore cattle. J. Anim. Breed. Genet. 2015, 132, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Beef Improvement Federation. Guidelines for Uniform Beef Improvement Program; Beef Improvement Federation: Prairie, MS, USA, 2018. [Google Scholar]
- R Development Core Team. R. A language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2008. [Google Scholar]
- Forsén, T.; Eriksson, J.G.; Tuomilehto, J.; Osmond, C.; Barker, D.J. Growth In Utero and During Childhood Among Women Who Develop Coronary Heart Disease: Longitudinal Study. BMJ 1999, 319, 1403–1407. [Google Scholar] [CrossRef]
- Keogh, K.; Waters, S.M.; Kelly, A.K.; Kenny, D.A. Feed restriction and subsequent realimentation in Holstein Friesian bulls: I. Effect on animal performance; muscle, fat, and linear body measurements; and slaughter characteristics. J. Anim. Sci. 2015, 93, 3578–3589. [Google Scholar] [CrossRef] [PubMed]
- Hornick, J.L.; Van Eenaeme, C.; Gérard, O.; Dufrasne, I.; Istasse, L. Mechanisms of reduced and compensatory growth. Domest. Anim. Endocrinol. 2000, 19, 121–132. [Google Scholar] [CrossRef]
- da Silva, A.G.; Paulino, M.F.; Detmann, E.; Fernandes, H.J.; da Silva Amorim, L.; Ortega, R.E.M.; de Carvalho, V.V.; da Costa Lima, J.A.; de Moura, F.H.; Benevides Monteiro, M.; et al. Energetic-protein supplementation in the last 60 days of gestation improves performance of beef cows grazing tropical pastures. J. Anim. Sci. Biotechnol. 2017, 8, 78. [Google Scholar] [CrossRef]
- Nascimento, K.B.; Galvão, M.C.; Meneses, J.A.M.; Ramírez-Zamudio, G.D.; Pereira, D.G.; Paulino, P.V.R.; Casagrande, D.R.; Gionbelli, T.R.S.; Ladeira, M.M.; Duarte, M.S.; et al. Maternal protein supplementation during mid-gestation improves offspring performance and metabolism in beef cows. J. Anim. Sci. 2024, 102, skae058. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.S.; Paulino, P.V.R.; Nascimento, C.S.; Botelho, M.E.; Martins, T.S.; Filho, S.C.V.; Dodson, M.V.; Guimarães, S.E.F.; Du, M. Maternal overnutrition enhances mRNA expression of adipogenic markers and collagen deposition in skeletal muscle of beef cattle fetuses. J. Anim. Sci. 2014, 92, 3846–3854. [Google Scholar] [CrossRef]
- Noya, A.; Casasús, I.; Ferrer, J.; Sanz, A. Long-Term Effects of Maternal Subnutrition in Early Pregnancy on Cow-Calf Performance, Immunological and Physiological Profiles during the Next Lactation. Animals 2019, 9, 936. [Google Scholar] [CrossRef]
- Stalker, L.A.; Adams, D.C.; Klopfenstein, T.J.; Feuz, D.M.; Funston, R.N. Effects of pre- and postpartum nutrition on reproduction in spring calving cows and calf feedlot performance. J. Anim. Sci. 2006, 84, 2582–2589. [Google Scholar] [CrossRef]
- Warner, J.M.; Martin, J.L.; Hall, Z.C.; Kovarik, L.M.; Hanford, K.J.; Rasby, R.J. The effects of supplementing beef cows grazing cornstalk residue with a dried distillers grain based cube on cow and calf performance. Prof. Anim. Sci. 2011, 27, 540–546. [Google Scholar] [CrossRef]
- Rodrigues, L.M.; Schoonmaker, J.P.; Resende, F.D.; Siqueira, G.R.; Rodrigues MacHado Neto, O.; Gionbelli, M.P.; Ramalho Santos Gionbelli, T.; Ladeira, M.M. Effects of protein supplementation on Nellore cows’ reproductive performance, growth, myogenesis, lipogenesis and intestine development of the progeny. Anim. Prod. Sci. 2020, 61, 371–380. [Google Scholar] [CrossRef]
- Sartori, E.D.; Sessim, A.G.; Brutti, D.D.; Lopes, J.F.; McManus, C.M.; Barcellos, J.O.J. Fetal programming in sheep: Effects on pre- and postnatal development in lambs. J. Anim. Sci. 2020, 98, skaa294. [Google Scholar] [CrossRef] [PubMed]
- Underwood, K.R.; Tong, J.F.; Price, P.L.; Roberts, A.J.; Grings, E.E.; Hess, B.W.; Means, W.J.; Du, W. Nutrition during mid to late gestation affects growth, adipose tissue deposition, and tenderness in cross-bred beef steers. Meat Sci. 2010, 86, 588–593. [Google Scholar] [CrossRef]
- Brasil I de, G.; Naves, A.C.; Macedo, I.M.; Teixeira, R.C.; Viu MA de, O.; Lopes, D.T.; Gambarini, L.M. Energy-protein supplementation before and after parturition of Nellore primiparous cows in the Brazilian tropical savannah. Res. Soc. Dev. 2021, 10, e14710313231. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).