Simple Summary
Different vegetable oils have been tested in ruminant diets to increase the energy concentration of the diet and improve the lipid quality of milk and meat. However, when we add vegetable oil to the diet, we change the ruminal fermentation pattern, which directly interferes with sheep’s digestive behavior and selectivity. Furthermore, the increase in calories in the diet can change animals’ thermoregulation, especially in tropical environments. In our study, it was evidenced that sheep showed better diet selectivity in flocks in which the oil used was canola oil with higher monounsaturated fatty acid content and that the canola oil + cashew nut shell liquid blend increased dietary energy content, improving selectivity due to a lower concentration of polyunsaturated fatty acids intake.
Abstract
This research evaluated the effects of energy supplementation on sheep’s feeding behavior, feed preference, and thermoregulatory responses using technical cashew nutshell liquid (CNSL) and different vegetable oils with different unsaturated fatty acid (UFA) compositions. The experiment was completely randomized with five treatments: a mixture of CNSL (0.5%) + vegetable oils [canola (high in monounsaturated fatty acids—MUFA), and corn, soybean, sunflower, or cottonseed oil (high in polyunsaturated fatty acids-PUFA) at 1.5%] based on total diet dry matter, with eight replications. Forty uncastrated male sheep, with an average initial BW of 24.44 ± 1.5 kg, were evaluated for 70 days. The CNSL + vegetable oil blend did not affect DM and neutral detergent fiber (aNDF) intake (p > 0.05). However, diets with canola oil resulted in higher SFA intake (p < 0.05) than other oils. The canola oil + CNSL blend led to a higher intake of UFA and MUFA and lower PUFA intake than other oil blends (p < 0.05). Sheep fed canola oil ruminated fewer boli per day than those fed soybean and sunflower oils. Using three sieves (pef1.18) reflected in higher sheep aNDF intake. Respiratory frequency and surface temperature of sheep were lower before feeding than 3 h after, without effects of the type of oil. Higher serum creatinine and cholesterol levels were observed in sheep fed CNSL with corn and canola oils compared to other oils. Serum calcium was lower in sheep fed CNSL with soybean and canola compared to sunflower and corn. Including CNSL with vegetable oils with different FA compositions did not affect physiological and thermographic variables. However, sheep showed better diet selectivity and lower bolus rumination with higher MUFA (canola oil) content. Including CNSL with canola oil in sheep diets is recommended, as it increases dietary energy content, enhances diet selectivity, reduces PUFA intake, and does not impact animal health.
1. Introduction
Growing sheep is a category with high nutrient requirements, particularly protein and energy. The efficiency of the digestive process is related to rumen fermentation and microbial activity, which, depending on the diet, can represent a loss of gross energy from the feed ingested (which can vary between 6% and 12%) and worsen the animal’s performance [1]. A widely used strategy to increase dietary energy is using vegetable oils, which improve nutrient use efficiency, especially energy nutrients [2].
In semi-arid regions, environmental conditions such as high temperatures, relative humidity, and solar radiation can lead to discomfort and stress in animals, significantly affecting livestock production. The complexity of the thermal environment surrounding animals means that the exchange of thermal energy between them and their surroundings is influenced by a range of physiological and environmental factors, which are interconnected in various ways [3,4,5,6]. Consequently, heat stress is one of the primary constraints on animal production, underscoring the importance of maintaining production activities within a thermal comfort zone to optimize animal performance [4]. To help animals in this stressful situation, lipid supplementation has been highlighted as an efficient nutritional practice since lipids are 2.25 times more energetic than carbohydrates. In addition, they have a lower caloric increase when compared to carbohydrates and proteins, making them an excellent dietary option, especially during the hottest periods of the year [7]. Increasing fatty acid intake can increase energy efficiency, as the direct deposition of dietary fatty acids in animal tissues replaces the metabolic steps of converting carbohydrates or short-chain fatty acids (SCFA) [8,9]. However, a very important aspect of fatty acid metabolism in the rumen is that it does not contribute to the growth of rumen microbial protein. This must, therefore, be considered when adjusting energy and protein [10].
Fat is a concentrated energy source, and energy production is linked to the length of the carbon chain of each fatty acid, as well as the presence of double bonds in unsaturated fatty acids. When sheep ingest fat, it is metabolized and generates energy, part of which is released as heat [11]. Animals that eat diets with higher energy levels result in reduced feed intake and fermentation heat, consequently losing less energy and improving feed conversion of the animals, and this is even more important in tropical environments where temperatures are quite high [12]. Thus, adding fat to the diets of growing and finishing sheep generally improves feed efficiency, increasing weight gain and influencing carcass measurements [11,13].
Several sources of lipids can be added to ruminant diets, including vegetable oils that influence the rumen biohydrogenation process and reduce the caloric increase in the diet, improving the animal’s ability to withstand high daily temperatures, especially in semi-arid zones. To maximize these benefits, the liquid from the cashew nutshell (Anacardium occidentale), known as CNSL, a byproduct and waste product, can be used to feed ruminants.
The CNSL, a phenolic lipid, primarily consists of anacardic acid, cardanol, and cardol, long-chain unsaturated phenols [14]. Including technical CNSL in ruminant diets has proven antioxidant and bactericidal activity, offering potential applicability in animal production [15,16]. This addition can result in changes to the fermentation profile in the rumen environment, including the mitigation of enteric methane, leading to improvements in production performance and nutrient metabolism [17]. In vitro studies conducted by Watanabe et al. [18] demonstrated that the application of CNSL reduced methane production by 70% and increased propionate production by 44%. This could also contribute to thermal comfort, as metabolic production is a factor in thermal stress. Shinkai et al. [19] observed a rise in rumen propionate production and a 38% reduction in methane emissions in cows fed a diet of hay and concentrate (60:40 ratio) with the inclusion of 4 g of CNSL per 100 kg of body weight. However, its composition can cause astringency due to phenolic compounds and alter the selectivity of animals, making it necessary to evaluate it. Both Ramos et al. [16] and Araújo [17] recommended a maximum level of 0.75% CNSL in sheep diets, given the risk of toxicity and astringency negatively affecting nutrient intake and digestibility. Compton [20] evaluated levels up to 300 ppm of CNSL in cows’ diets and found no impact on the rumen fermentation profile and apparent nutrient digestibility. However, studies have reported positive effects from including of vegetable oils [21] and CNSL in sheep diets, such as no change in intake and no interference in physiological and thermographic variables [17,20].
Thus, this study tested the hypothesis that energy supplementation with CNSL associated with vegetable oils of the UFA compositions will change the feeding behavior and feed preference and contribute to the thermoregulation of sheep. Therefore, intake and factors related to ingestive behavior and selectivity were evaluated, as well as serum and physiological parameters of sheep raised in a confinement system in a semi-arid region.
2. Materials and Methods
The study took place in the semi-arid region of Brazil, classified as having a Bsh (hot semi-arid) climate according to the Köppen climate classification, with an average temperature of 28 °C, ranging from a minimum of 22.4 °C to a maximum of 33.5 °C. The historical average annual rainfall is 996.6 ± 300.0 mm, with precipitation primarily occurring between December and May, and 43% falling between March and April [22].
2.1. Animals, Experimental Design, Diets, and Facilities
Forty uncastrated male Santa Ines × Dorper crossbred sheep, approximately three months old with an initial average weight of 24.44 ± 1.5 kg, were utilized in this study. The animals were allocated in a randomized complete design with five treatments, each combining 0.5% CNSL of the total diet dry matter (DM) with 1.5% DM of different vegetable oils (soybean, corn, cottonseed, sunflower, and canola) across eight experimental units.
At the start of the experiment, all sheep were weighed, identified, vaccinated against clostridium and rabies, dewormed, and housed individually in wooden stalls elevated 0.5 m above the ground. These stalls, measuring 1.3 × 1.5 m2 and equipped with feeders and drinkers, were in an open shed with an asbestos cement roof, concrete central floor, and wooden slats measuring 16 m long and 6 m wide. The shed had a central aisle of 1.8 m wide and a ceiling height of 2.5 m. The experimental period was 70 days, with the first 15 days dedicated to acclimating the sheep to their new environment, management practices, and diets, followed by 55 days of sampling and data collection.
The experimental diets had a roughage-to-concentrate ratio of 40:60 in a total mixed ration, formulated to meet requirements for an average daily weight gain of 250 g [1]. Sorghum silage served as the roughage, while the concentrate consisted of soybean meal, ground corn, mineral salt, and the specified treatments combining 0.5% CNSL and 1.5% of various vegetable oils (canola, corn, soybean, sunflower, or cottonseed) based on total diet DM (Table 1). The animals were fed twice daily at 8:00 and 15:00, with intake measured daily to maintain 10% leftovers. Water was provided ad libitum.

Table 1.
Proportion of ingredients and chemical composition of experimental diets.
To extract the cashew nutshell liquid (CNSL), the shells were steam-heated to 80 °C and then pressed, yielding CNSL and a residual cake [23]. A thermomechanical process was used for solvent extraction, heating the CNSL to warm the raw nuts to around 190 °C. At this temperature, the outer shell breaks, releasing alkylphenols from the porous mesocarp, and the inner shell is removed, allowing the recovery of the nuts [24].
The extracted CNSL underwent decarboxylation to eliminate CO2 and moisture by heating it to 140 °C with continuous stirring [23]. This heat treatment decarboxylates anacardic acid to form cardanol, resulting in technical CNSL [24], used in this study. The decarboxylated CNSL was then filtered through a filter press and stored.
2.2. Determination of Chemical Composition of Diets and Ingredients
Samples of ingredients, diet, and leftovers were pre-dried in a forced air circulation oven at 55 °C for 72 h before being ground into 1.0 mm particles using a Willey-type knife mill. The chemical composition was analyzed according to the standards of [25], determining dry matter (method 934.01), ash (method 942.05), crude protein (method 968.06), and ether extract (method 920.39).
Neutral detergent fiber (NDF) and acid detergent fiber (ADF) were measured following the procedure by Van Soest [26], with modifications for non-woven fabric [27], using thermostable amylase (Sigma A3306; Sigma-Aldrich, Steinheim, Germany) and expressed exclusive of residual ash (aNDF). Lignin was determined using method 973.18 [25], involving treatment of the ADF residue with 72% sulfuric acid. Neutral and acid detergent insoluble protein levels were determined according to methodology of Licitra et al. [28]. Non-fiber carbohydrates (NFC) were calculated based on Mertens [29], considering aNDF corrected for ash and protein. Metabolizable energy (ME) was calculated using Weiss [30] methodology, based on total digestible nutrients (TDN) contents, using the following formula: TDN = %DCP + %DaNDF + %DEE × 2.25 + %DNFC; 1 kg TDN = 4409 kcal DE; ME = DE × 0.82.
2.3. Determination of Fatty Acid Composition in the Oils of the Diets
A quick and efficient method for extracting total lipids was employed [31]. The fatty acid profile of the oil samples (Table 2) was analyzed via gas chromatography following the preparation of fatty acid esters according to Hartman and Lago’s [32] procedure. The analysis utilized a Thermo Scientific Focus gas chromatograph (Thermo Electron SpA®, Milan, Italy) equipped with a Supelco® Analytical SPTM-2560 capillary column, measuring 100 m × 0.25 mm × 0.20 µm (Supelco® Inc., Bellefonte, PA, USA). Both the injector and detector were set at 250 °C. The column temperature started at 140 °C for 5 min, then increased to 220 °C at a rate of 4 °C/min and was held at this final temperature for 10 min. The split ratio was set to 1:50, with an injection volume of 1.2 μL. The fatty acid results are presented as a relative percentage for each, derived by internal normalization of the chromatographic peak areas. The fatty acids were identified by comparing the relative retention times of sample FAME peaks with those of standards. A mixture of 37 FAMEs (standard 47885-U, TraceCERT®, Sigma Aldrich; Merck KGaA, Darmstadt, Germany) was used as the reference standard.

Table 2.
Composition of the main fatty acids identified in the vegetable oils in the diets.
2.4. Intake and Ingestive Behavior
The animals were weighed at the beginning (initial body weight) and end (final body weight) of the experiment and every 15 days to adjust consumption. Nutrient intake was calculated by subtracting the amount of each nutrient found in the leftovers from the total amount offered in the diet, with results expressed in grams per day (g/d). The animals’ feeding behavior was observed twice during the experimental period, on the 30th and 50th days, with observations recorded every 10 min over 24 h using the scan sampling method outlined by Martin and Bateson [33]. Trained observers, working in shifts, collected data on the time the animals spent eating, ruminating, or being inactive, positioning themselves strategically to avoid influencing the animals’ behavior. Night-time observations were conducted using artificial lighting.
To estimate the average number of boluses ruminated per day, the number of chews per bolus, and the chewing time per bolus, evaluations were carried out in four periods: from 8:00 to 10:00, 12:00 to 14:00, 16:00 to 18:00, and 20:00 to 22:00 over the 24 h ingestive behavior assessment period, using digital chronometers as described by Bürger et al. [34]. Feeding and rumination efficiencies for dry matter (DM) and neutral detergent fiber (aNDF) in kg/h were calculated by dividing the intake of each nutrient by the total feeding and rumination times, respectively. Total chewing time was also recorded. Eating and ruminating efficiency rate and the total number and time of rumen boluses in chewings were calculated according to Polli et al. [35].
2.5. Diet Selectivity
Each animal’s feed trough was weighed and sampled on the 41st and 43rd days of the experimental period, at 12 and 24 h post-feeding, respectively, for dry matter (DM) analysis and particle size. A 250 g sample was collected, and particle size was assessed using the sieve particle stratification method with the “Penn State Particle Separator—PSPS”, as designed by Lammers et al. [36] and modified by Kononoff et al. [37]. This separator comprises three sieves (1.18 mm, 8 mm, and 19 mm) that divide the samples into four fractions according to particle size. The physical effectiveness factor of fiber (pef) was calculated in two ways: pef8, based on the sum of the percentages of particles larger than 8 mm [36], and pef1.18, based on the sum of the percentages of particles larger than 1.18 mm [29,37]. The physically effective neutral detergent fiber (NDFpe) was determined by multiplying the sample’s neutral detergent fiber content by the previously calculated pef. The chemical composition of the effectively consumed diet was estimated by dividing the intake of each nutrient by the intake of DM and multiplying by 100.
2.6. Environmental Variables
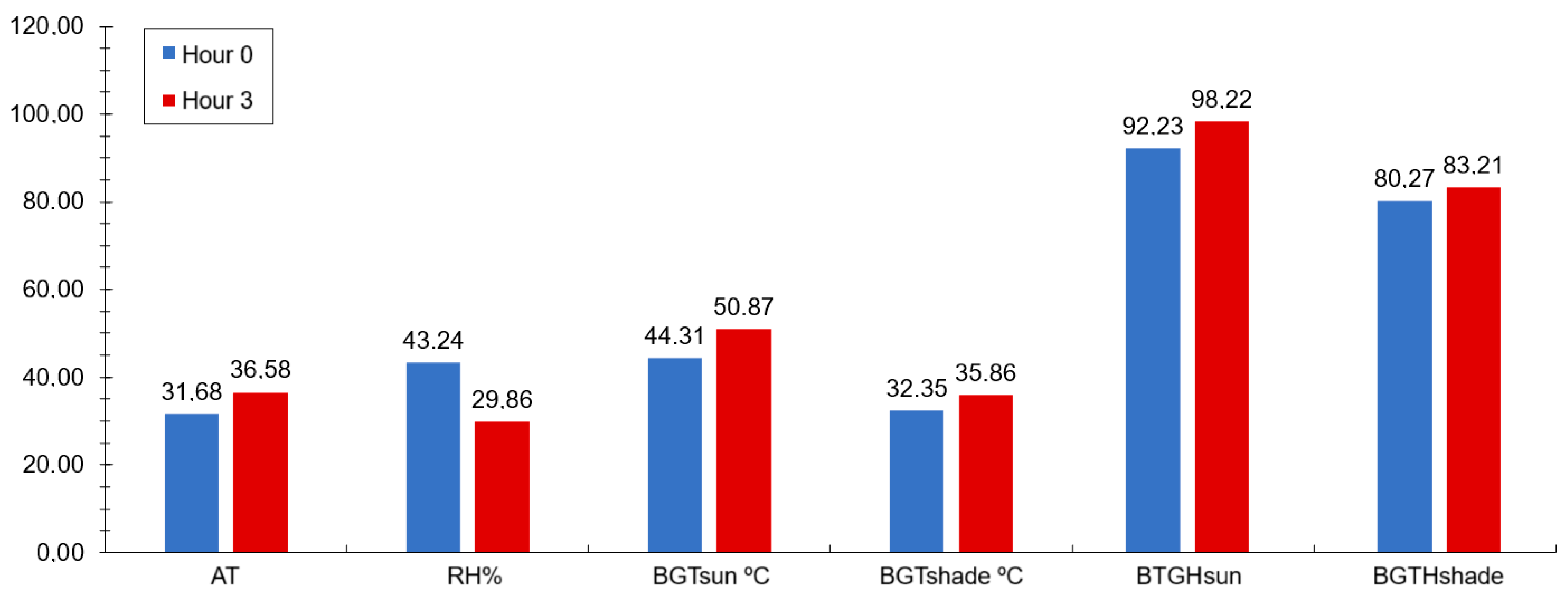
During the experimental period, the climatological data were recorded using a datalogger (HOBO® brand, model U12-013, Onset, São Paulo, Brazil) attached to two black globes, installed in the sunny and shaded environments, at a similar height to the animals, which was programmed to record the environmental data every hour for 24 h, throughout the experimental period, using the environmental data to calculate the black globe temperature and humidity index (BGHI) through the following formula: BGHI = [Tbg + (0.36 × Tdp) + 41.5], described by Buffington et al. [38], where Tbg is the black globe temperature and Tdp is the dew point temperature (Figure 1).
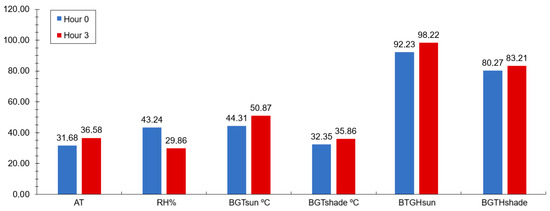
Figure 1.
Averages of meteorological data: environmental temperature (AT), relative humidity (RH), black globe temperature in the sun (BGTsun), black globe temperature in the shade (BGTshade), black globe temperature and humidity index in the sun (BTGHsun) and in the shade (BTGHshade), before (0 h or at feeding moment) and 3 h after the feeding. (SEM = 7.86).
2.7. Physiological Variables of the Sheep
Physiological data were gathered on the 15th and 35th days of the experiment, before and three hours after the sheep were offered their diets, denoted as hour 0 (7:00 to 14:00) and hour 3 (10:00 to 17:00) post-feeding.
The respiratory rate was assessed by placing a flexible stethoscope on the right thoracic region, counting the number of respiratory movements for 30 s, and multiplying by two to obtain the rate per minute. Rectal temperature was measured using a veterinary clinical thermometer capable of registering temperatures up to 44 °C, inserted directly into the animal’s rectum for two minutes, with results expressed in degrees Celsius (°C).
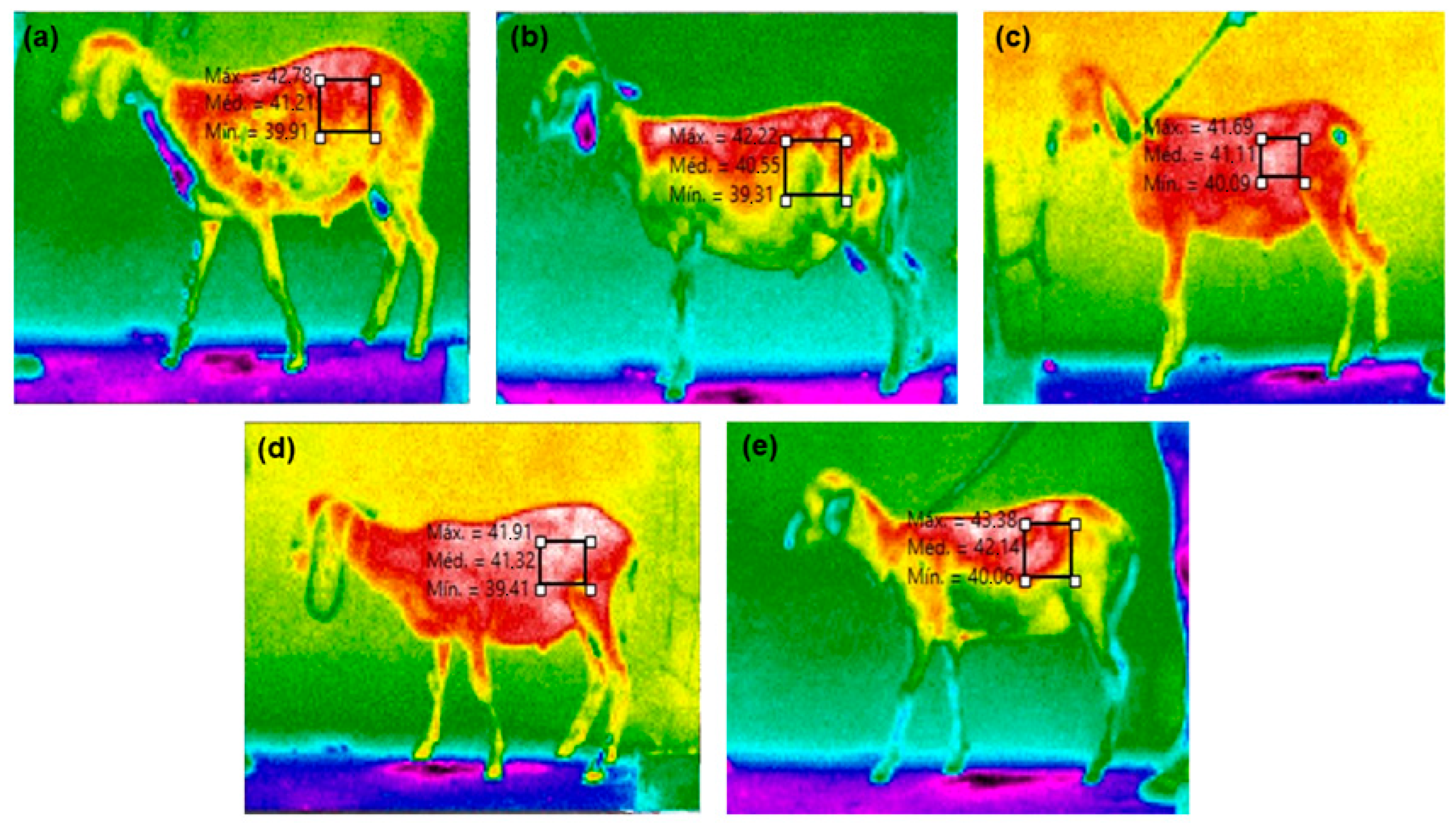
Surface temperature (ST) was captured using an infrared thermographic camera (Fluke Serie-Ti 25) with automatic calibration and an emissivity of 0.98, as recommended by the manufacturer for biological tissues. Thermograms of both the right and left sides of the animals were taken and analyzed using Smartview software (version 4.3, Copyright© Fluke Corporation, Norwich, UK, 2006–2017) to derive thermographic variables (maximum, minimum, and average surface temperatures) selected from the sheep’s right and left flanks (Figure 2).
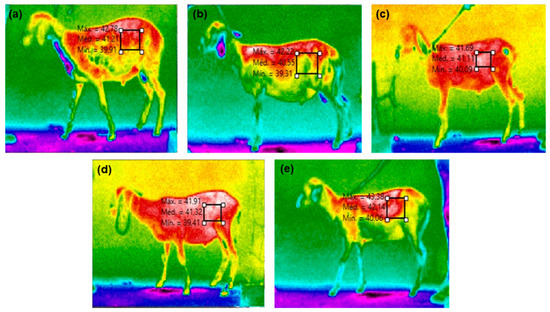
Figure 2.
Thermographic variables (maximum, minimum, and medium surface temperatures) selected in the left flank region of the sheep fed diets containing mixtures of 0.5% of cashew nut shell liquid (of total DM) and 1.5% of soybean (a), cottonseed (b), sunflower (c), corn (d), or (e) canola oil (of total DM).
2.8. Serum Metabolites
To evaluate serum parameters, 5.0 mL of blood was drawn from the animals on days 15 and 35 via jugular venipuncture and placed into pre-labeled tubes with a vacuum system (Becton, Dickson and Co., São Paulo, SP, Brazil). A No. 16 catheter (Medical Supply®, São Paulo, Brazil) was gently inserted into each animal to aid collection and ensure animal comfort. The samples were left at room temperature until clot formation occurred. They were centrifuged (Centrifuge 90-1 model, Coleman®, São Paulo, Brazil) at 2500× g for five minutes to separate the blood serum. For subsequent analysis, serum samples were stored at −20 °C in Eppendorf® tubes (Sigma-Aldrich, São Paulo, Brazil).
Biochemical test kits were employed to quantify serum metabolites, including total protein, cholesterol, triglycerides, albumin, aspartate aminotransferase (AST), gamma-glutamyl transferase (GGT), urea, and creatinine using the Cobas® C111 automated biochemical analyzer (Roche, Ludwigsburg, Germany), utilizing enzymatic or colorimetric kinetic assays. Electrolyte levels were analyzed using an automated analyzer (Max Ion-Medmax, Shenzhen, China) via the direction selectivity method for calcium, phosphorus, and magnesium minerals.
2.9. Statistical Analysis
Intake, ingestive behavior, and serum metabolite data underwent analysis following the principles of a randomized complete design, utilizing the MIXED procedure [39]. Collection dates were treated as repeated measurements over time. Each animal served as the experimental unit for all variables examined, as described by the statistical model:
where Yij = value corresponding to the observation of repetition ii of treatment j; μ = overall mean; Oj = effect of treatment j (where j represents CNSL + canola or soybean or corn or cottonseed or sunflower oil); eij = random error associated with the observation.
Yijk = μ + Oj + eijk
The physiological variables of sheep were analyzed under a completely randomized design, employing a split-plot arrangement. Treatments (vegetable oil inclusion + CNSL blend) were allocated to plots, while collection times were assigned to subplots, with eight replications, based on the statistical model:
where Yijk = value corresponding to the observation of repetition ii in treatment j and at collection time kk; μ = overall mean; Oj = effect of treatment j (where j represents CNSL + canola or soybean or corn or cottonseed or sunflower oil); Tk = effect of collection time kk (before and 3 h after feeding); OTjk = interaction of the effects of treatment j with collection time kk; eijk = random error associated with the observation
Yijk = μ + Oj + Tk + OTjk + eijk
Treatment means were compared using Tukey’s test, with significance determined at p < 0.05.
3. Results
The CNSL and unsaturated-fatty-acid-rich vegetable oil blend did not affect (p > 0.05) the composition of the diet effectively consumed by the sheep, as shown in Table 3. Similarly, the intake of dry matter and aNDF and the duration spent on ruminating, feeding, and idling by the sheep remained unaffected (p > 0.05) by incorporating different vegetable oils high in unsaturated fatty acids alongside CNSL.

Table 3.
Composition of effectively intake diet of sheep containing mixtures of CNSL (0.5% of total DM) and different vegetable oils rich in unsaturated fatty acids (1.5% of total DM).
However, diets containing canola oil (MUFA) promoted a higher intake of SFA (p < 0.0001) compared to other vegetable oils. In contrast, sheep fed with cottonseed oil and CNSL blend intake had higher SFA than other vegetable oils (p < 0.0001). Sheep fed with canola oil (MUFA) and CNSL blend presented a higher intake of the UFA and MUFA compared to cottonseed oil and CNSL mixture and lower PUFA intake compared to other vegetable oils (p < 0.0001). Sheep fed with sunflower or corn oil presented higher PPUFA intake and similar intake of UFA. Cottonseed and corn oil diets promoted similar sheep intake of the MUFA (p < 0.0001).
The diets did not affect the intake efficiencies of DM and aNDF, rumination rates of DM and aNDF, amount of DM/bolus, and chewing time (p > 0.05). However, the number of boluses ruminated daily was higher (p = 0.044) in treatments containing CNSL associated with soybean or sunflower oils (Table 4) compared to CNSL associated with canola oil.

Table 4.
Intake and ingestive behavior of sheep fed diets containing mixtures of CNSL (0.5% of total DM) and different vegetable oils rich in unsaturated fatty acids (1.5% of total DM).
Regarding the selectivity of the diets by the sheep, when the diets were offered 12 h after feeding, the amount of particles retained on the 19 mm sieve (p = 0.043) was higher for the diet containing the CNSL and sunflower oil mixture, while in the 8 mm sieve (p = 0.012), it was higher for the diet with CNSL and soybean oil mixture, and in the 1.8 mm sieve (p = 0.035), it was higher for the diet with CNSL and canola oil (Table 5).

Table 5.
Particle size distribution, physical effectiveness factors (pef), and physically effective neutral detergent fiber (NDFpe) content of the diets containing mixtures of CNSL (0.5% of total DM) and different vegetable oils rich in unsaturated fatty acids (1.5% of total DM).
There was an effect of the diets (p = 0.034) on the effectiveness, using only two sieves (pef8), reflected in higher NDFpe8 intake (p = 0.055) for the diet containing the CNSL and sunflower oil compared to the diet with CNSL and canola oil.
There was no effect (p > 0.05) for the base sieves, pef1.18, and the intake of NDFpe1.18. In the samples taken 24 h after feeding, there was an effect on the number of particles retained only on the 8 mm sieve (p = 0.036), with greater retention in the diet with CNSL and soybean oil mixture compared to the diet with CNSL and canola oil, and on the amount of particles retained on the base of the sieve (p = 0.006), which was inversely proportional. Regarding effectiveness, there was an effect (p = 0.044) using three sieves (pef1.18), which was reflected in the higher NDFpe1.18 intake (p = 0.045) for the diet containing the CNSL and soybean oil mixture when compared to the diet with CNSL and canola oil.
There was no effect of interaction between the combination of CNSL and different vegetable oils rich in unsaturated fatty acids and the collection time of the sheep’s physiological variables (p > 0.05).
The combination of CNSL with vegetable oils rich in unsaturated fatty acids did not affect (p > 0.05) the rectal temperature and right surface temperature of the sheep before (0 h) and 3 h after feeding (Table 6). There was also no effect of diet on rectal and surface temperatures before (0 h) and 3 h after feeding (p > 0.05). However, the average right surface temperature, without considering time, computed by the camera, shows that the fermentation heat was higher (p < 0.05) in the animals fed CNSL mixed with sunflower or corn oil (Figure 2) when compared to CNSL mixed with canola oil, with which the animals had the lowest average surface temperature. When comparing times (0 and 3), the respiratory rate was lower in sheep fed CNSL mixed with sunflower or corn oil (p = 0.0099) than in animals fed diets with CNSL and soybean, cottonseed, or canola oil.

Table 6.
Physiological and thermographic parameters according to the time of evaluation of sheep fed diets containing mixtures of CNSL (0.5% of total DM) and different vegetable oils rich in unsaturated fatty acids (1.5% of total DM).
The diets did not exert any discernible effect (p > 0.05) on the serum concentrations of albumin (ALB), total protein (TP), urea (URE), triglycerides (TRI), aspartate aminotransferase (AST), gamma glutamyl transferase (GGT), and the minerals phosphorus (P) and magnesium (Mg), as outlined in Table 7. However, higher serum creatinine concentrations were observed in sheep fed CNSL mixed with corn or canola oil (p = 0.007) compared to animals fed CNSL mixed with sunflower oil. Animals fed the CNSL with canola oil had higher serum cholesterol concentrations (p = 0.050) than those fed the diet with CNSL and sunflower oil. Serum calcium concentrations were higher in sheep (p = 0.001) fed the CNSL with sunflower or corn oil than in sheep fed CNSL and soybean or canola oil.

Table 7.
Blood metabolites in sheep fed diets containing mixtures of CNSL (0.5% of total DM) and different vegetable oils rich in unsaturated fatty acids (1.5% of total DM).
4. Discussion
Cottonseed oil had the highest concentration of SFA (24.3%), followed by sunflower oil (17.1%) and corn oil (16.2%), while canola oil had the highest concentration of MUFA (63.7%), which was much higher than the other oils. Corn oil (59.5%) and sunflower oil (54.15%) had the highest concentrations of PUFA. Consequently, despite the intense similarity in composition, the diets allowed differences in the consumption of different fatty acids (FA). Sheep fed canola oil, which was more concentrated in MUFA than other oils, showed lower consumption of SFA and PUFA and higher consumption of UFA and total MUFA, which promoted differences in rumen fermentation.
The microbial ecosystem in the rumen responds to variations in diet type and composition, which are main factors influencing microbial activity and function in the rumen [14,15,16,17]. Therefore, even though the diets were very similar in overall composition (Table 1), altering only the oils’ fatty acid (FA) composition demonstrated that the FA saturation level was sufficient to modify the animals’ feeding behavior. This highlights the significance of comprehending the intricate populations of ruminal microbes, their interplay, and their responses to diverse dietary compositions [40]. Additionally, it is crucial to elucidate responses to various dietary FA compositions based on proposed differences in lipid metabolism [41].
The similarity between diets in terms of fiber, energy, and protein content may explain the intake of aNDF and DM and, consequently, the times of the activities related to ingestive behavior since dietary fiber is one of the main factors that interferes with feeding and rumination activities. In addition, vegetable oils rich in unsaturated fatty acids were mixed with CNSL in the same proportion and in quantities that did not cause negative effects on the digestive process. Several studies using vegetable oils in sheep diets [42,43,44] have shown that DM and aNDF intake can be affected by the addition of oil sources to the diet. However, the extent of this interference depends on both the oil source and the level at which it is added to the feed. Most studies confirm that the use of lipids has little or no effect on the activities of the microbial flora and the other characteristics of the rumen environment as long as it does not exceed 7% of the total DM of the diet.
However, adding CNSL to the oils reduced its astringent effect since DM intakes were above 1300 g/d. This can be explained by the fact that the diets contained 0.5% CNSL in the total DM, which is a level below the recommendation of Ramos et al. [16] and Araújo et al. [17], who indicated a maximum of 0.75% CNSL in sheep diets, given the risk of toxicity and astringency, which negatively affects intake and digestibility of nutrients. The combination of oil and CNSL could be an interesting alternative to increase the energy content of the diet without affecting intake.
Despite not affecting rumination time and intake, the inclusion of the CNSL mixed with soybean and sunflower oils increased the number of boluses ruminated (No./day), which may be associated with the distribution of particles, especially in the 1.8 mm sieves (Table 3), indicating a greater amount of concentrate in the leftovers, and consequently allowing more selection of the fiber fractions [45].
Particle size analysis tries to determine the actual frequency distribution of the particles according to the size and selection of the animal’s diet. The particle size distribution of the diets offered to the sheep 12 h after feeding showed that the animals fed the mixture of CNSL and canola oil had higher intake of larger particles (pef8), corresponding to a higher intake of NDFpe8. In the distribution of particles twenty-four hours after feeding, the animals fed the CNSL and canola oil blend continued to consume more of the roughage and rejected the concentrate, with a greater volume of particles at the base, confirmed by a higher intake of NDFpe1.18. The selection of the diet by the animals that consumed CNSL mixed with canola oil can be attributed to the direct action on the rumen caused by the higher composition of unsaturated fatty acids in canola oil, causing a reduction in intake due to the loss of palatability of the diet and the change in rumen fermentation [46,47].
The combination of CNSL with various oils did not induce changes in the physiological variables, suggesting that the oil type in the diet does not necessarily correlate with its effectiveness in enhancing animal thermal comfort. In regions like semi-arid areas, temperatures often surpass the thermal comfort zone for small ruminants during the hottest hours of the day, prompting them to seek shade, reduce dry matter intake (DMI), and increase water consumption. In such conditions, diets with higher energy density can aid animals by enabling quicker eating and reducing fermentation rates.
Studies by Gomes et al. [48], Neiva et al. [49], and Nobre et al. [7] have reported similar environmental conditions, with temperatures exceeding the thermal comfort zone, especially from midday onward. The average BGHI (Black Globe Humidity Index) values reflect a correlation between feeding time and environmental factors, with the highest averages occurring three hours post-feeding, indicating elevated environmental temperatures that expose animals, even those in confinement, to thermal discomfort.
The BGHI levels for sheep and goats categorize heat stress severity: values below 82 indicate no heat stress, while values above 82 and below 86 denote severe heat stress, and those above 86 signify extremely severe heat stress. The climate data and BGHI indicate that the animals experienced extremely severe heat stress. Despite these adverse environmental conditions, physiological variables displayed average rectal temperatures (RT), typically ranging from 38.3 °C to 39.9 °C in sheep.
Respiratory rate (RR) values can quantify heat stress severity, with ranges indicating low, medium–high, and high stress levels for ruminants. The observed high RR values suggest medium–high stress levels in the study animals, likely influenced by the elevated BGHI values, mainly three hours post-feeding. Sheep fed CNSL mixed with sunflower and corn oil exhibited lower RR values, possibly due to a lesser calorie increase from their diet. These oils contain higher amounts of linoleic acid, which can inhibit fat synthesis by lysogenic enzymes.
Thermographic measurements of the left flank surface temperature (ST) suggest higher fermentation heat in animals fed CNSL mixed with sunflower or corn oil, resulting in elevated RR values. Conversely, sheep fed CNSL mixed with canola oil displayed the lowest RR values, likely due to reduced biohydrogenation and less heat production during rumen fermentation. This indicates that fermentation heat correlates more strongly with unsaturation levels and double bond presence in the diet’s fat content rather than the amount of vegetable fat consumed.
Flank ST is also significantly correlated with climatic parameters, particularly environmental temperature. Previous studies have highlighted positive correlations between physiological parameters and thermographic measurements, indicating thermography’s efficacy as a thermal comfort indicator. These correlations are attributed to the efficiency of thermoregulation mechanisms, which rely on the thermal gradient between the animal’s body and its surroundings. A more significant thermal gradient facilitates heat dissipation through conduction, convection, and radiation, enhancing thermal comfort.
Plasma creatinine concentrations show the efficiency of glomerular filtration, with total excretion by the kidneys. High levels of this metabolite suggest impaired kidney function [50]. Among the treatments, creatinine concentrations in the sheep blood were below the reference values (1.2–1.9 mg/dL) for the sheep [51], indicating low physical activity and low proteolysis by muscle tissues, which reflects the confinement. Creatinine concentrations in the body are dependent on muscle mass and endogenous nitrogen utilization [52].
For cholesterol levels, the range considered normal for sheep is 50–76 mg/dL [50,51] and can be influenced by the diet, intake, age, and physiological state of the animals. The reduction in the concentration of cholesterol in the blood of the sheep in treatments that used CNSL associated with soybean, cottonseed, sunflower, and corn oil in comparison to canola oil may be related to the composition of polyunsaturated fatty acids (Table 2). According to Brzozowska and Oprządek [53], PUFA and non-esterified PUFA appear to be more potent inhibitors of intake than monounsaturated and esterified PUFA, respectively. These data indicate that the use of lipid sources known to be rich in PUFA and non-esterified can influence dry matter intake. Despite the similar DMI, animals that consumed canola oil continued to consume more of the roughage and rejected the concentrate, with a greater volume of particles at the bottom, as observed in the sieves (Table 4). This may have been due to the FA composition of the oils since canola oil had a lower amount of PUFA (32%) while the other oils had more than 50% PUFA. Therefore, the more selective roughage diet may have contributed to the increase in blood cholesterol.
5. Conclusions
Including CNSL combined with different vegetable oils rich in unsaturated fatty acids in the diet of confined sheep did not affect intake or physiological and thermographic variables, which were more susceptible to weather conditions and the time of day when the data were recorded. The mixture of CNSL and canola oil caused the sheep to select the roughage over the concentrate and increased the animals’ serum cholesterol compared to the other oils. Combining canola oil with CNSL reduced PUFA intake and minimized the effects of phenolic compounds (CNSL) on the animal’s health but showed greater rejection to concentrate with this supplementation.
Author Contributions
É.L.G.A.: conceptualization, data curation, formal analyses, investigation, writing—original draft; K.H.d.O.S.d.L. and Y.C.S.B.: data curation, formal analyses; J.M.P.F. and B.B.d.S.: methodology, supervision; J.P.F.d.O. and M.A.F.: formal analyses, software, validation; A.F.d.M.V.: data curation, formal analyses, investigation; R.L.O.: investigation, funding acquisition, supervision; L.R.B.: conceptualization, project administration, supervision, funding acquisition, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This study was partially supported by the National Council for Scientific and Technological Development (Brazil) agency from the PQ-2023 (Grant #306920/2023-9) and MCTIC/CNPq (Grant #406734/2022-4).
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee of Ethics Committee for Animal Experimentation Use (protocol code 55/2022, 1 November 2022).
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets that support the findings of this study are available from https://doi.org/10.17632/tj6yzp9rzy.1, accessed on 6 June 2024.
Acknowledgments
This research was supported by the Research Support Foundation of the State of Paraiba and the Federal University of Campina Grande through facilities support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- National Research Council-NRC. Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids, and New World Camelids; The National Academies Press: Washington, DC, USA, 2007. [Google Scholar] [CrossRef]
- Oliveira, F.d.S.; Fernandes Neto, V.d.P.; Nascimento e Silva, M.N.d.; Cardoso, F.S.; Costa, A.P.R. Effect of heat stress on physiological and biochemical parameters of sheep raised in tropical climate. Pubvet 2012, 6, 1359. [Google Scholar] [CrossRef]
- Maia, A.S.C.; Nascimento, S.T.; Nascimento, C.C.N.; Gebremedhin, K.G. Thermal equilibrium of goats. J. Therm. Biol. 2016, 58, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Sejian, V.; Bagath, M.; Krishnan, G.; Rashamol, V.P.; Pragna, P.; Devaraj, C.; Bhatta, R. Genes for resilience to heat stress in small ruminants: A review. Small Rum. Res. 2019, 173, 42–53. [Google Scholar] [CrossRef]
- Santos, M.L.P.; Dada, J.M.V.; Muniz, P.C.; Nunes-Zotti, M.L.A.; Barros, F.R.O.d.; Vieira, F.M.C. Physiological responses of Santa Inês x Dorper ewes and lambs to thermal environment of silvopasture and open pasture systems. Small Rum. Res. 2021, 205, 106–565. [Google Scholar] [CrossRef]
- Mascarenhas, N.M.H.; Furtado, D.A.; Fonsêca, V.F.C.; Souza, B.B.d.; Oliveira, A.G.; Morais, F.T.L.; Silva, R.d.S.; Silva, M.R.d.; Batista, L.F.; Dornelas, K.C.; et al. Thermal stress index for native sheep. J. Therm. Biol. 2023, 115, 103607. [Google Scholar] [CrossRef] [PubMed]
- Nobre, I.S.; Souza, B.B.; Marques BA, A.; Azevedo, A.M.; Araújo, R.P.; Gomes TL, S.; Batista, L.F.; Silva, G.A. Evaluation of Levels of fat protected and concentrate on productive performance and sheep thermoregulation. Rev. Bras. de Saúde e Produção Anim. 2016, 17, 116–126. [Google Scholar] [CrossRef]
- Baldwin, R.L.; Smith, N.E.; Taylor, J.; Sharp, M. Manipulating metabolic parameters to improve growth rate and milk secretion. J. Anim. Sci. 1980, 51, 1416–1428. [Google Scholar] [CrossRef]
- Jin, Y.; Jiang, B.; Wang, H. Growth performance, meat quality and lipid metabolism in finishing lambs fed diets containing rumen-unprotected and rumen-protected betaine. Italian. J. Anim. Sci. 2021, 20, 2041–2050. [Google Scholar] [CrossRef]
- Tedeschi, L.O.; Cannas, A.; Fox, D.G. A nutrition mathematical model to account for dietary supply and requirements of energy and other nutrients for domesticated small ruminants: The development and evaluation of the Small Ruminant Nutrition System. Small Rum. Res. 2010, 89, 174–184. [Google Scholar] [CrossRef]
- Palmquist, D.L. The role of dietary fats in efficiency of ruminants. J. Nutr. 1994, 124 (Suppl. S8), 1377S–1382S. [Google Scholar] [CrossRef]
- Behan, A.A.; Loh, T.C.; Fakurazi, S.; Kaka, U.; Kaka, A.; Samsudin, A.A. Effects of Supplementation of Rumen Protected Fats on Rumen Ecology and Digestibility of Nutrients in Sheep. Animals 2019, 9, 400. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Dai, C.; Li, J.; Huang, P.; Li, Y.; Ding, X.; Huang, J.; Hussain, T.; Yang, H. Effects of dietary energy on growth performance, carcass characteristics, serum biochemical index, and meat quality of female Hu lambs. Anim. Nutr. 2020, 6, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Himejima, M.; Kubo, I. Antibacterial agents from the cashew Anacardium occidentale (Anacardiaceae) nut shell oil. J. Agric. Food Chem. 1991, 39, 418–421. [Google Scholar] [CrossRef]
- Osmari, M.P.; Matos, L.F.d.; Salab, B.L.; Diaz, T.G.; Giotto, F.M. Cashew nut shell liquid: Characteristics and applicability in animal production. Pubvet 2015, 9, 143–149. [Google Scholar]
- Ramos, L.M.G.; Bezerra, L.R.; Oliveira, J.P.F.d.; Souza, M.P.d.; Silva, A.L.d.; Sales, E.P.; Mazzetto, S.E.; Pereira Filho, J.M.; Oliveira, R.L. Effects of feeding growing-finishing lambs with cashew nut shell liquid on the growth performance, physicochemical attributes, lipid peroxidation and sensorial parameters of burger. Small Ruminant Res. 2021, 202, 106–468. [Google Scholar] [CrossRef]
- Araújo, D.; Araújo, M.; Silva, S.; Pereira Filho, J.; Parente, M.; Oliveira, R.; Mazzetto, S.; Oliveira, J.; Edvan, R.; Bezerra, L. Effect of technical cashew nut shell liquid on growth, physicochemical and fatty acid composition of lamb meat. Small Rum. Res. 2023, 227, 107070. [Google Scholar] [CrossRef]
- Watanabe, Y.; Suzuki, R.; Koike, S.; Nagashima, K.; Mochizuki, M.; Forester, R.J.; Kobayashi, Y. In vitro evaluation of cashew nut shell liquid as a methane-enhancing agent for ruminants. J. Dairy Sci. 2010, 93, 5258–5267. [Google Scholar] [CrossRef]
- Shinkai, T.; Enishi, O.; Mitsumori, M.; Higuchi, K.; Kobayashi, Y.; Takenaka, A.; Nagashima, K.; Mochizuki, M.; Kobayashi, Y. Mitigation of methane production from cattle by feeding cashew nut shell liquid. J. Dairy Sci. 2012, 95, 5308–5316. [Google Scholar] [CrossRef]
- Compton, C.; Peña, O.M.; Hikita, C.; Watanabe, T.; Jenkins, T.C.; Lascano, G.J.; Aguerre, M.J. Effects of cashew nut shell extract on ruminal fermentation and nutrient digestibility under continuous culture. Ruminants 2023, 3, 92–99. [Google Scholar] [CrossRef]
- Lup, F.; Pop, I.M.; Simeanu, D.; Vicas, S.; Simeanu, C.; Mierlita, D. Research regarding fatty acid profile and health lipid indices in the lambs meat of employing feed supplemented with different vegetable oils. Rev. Chim. 2018, 69, 222–227. [Google Scholar] [CrossRef]
- Instituto Nacional de Meteorologia. Banco de Dados Meteorológicos para Ensino e Pesquisa; INMET/BDMEP: Brasília, DF, Brazil, 2023.
- Lubi, M.C.; Thachil, E.T. Cashew nut shell liquid (CNSL)-a versatile monomer for polymer synthesis. Des. Monomers Polym. 2000, 3, 123–153. [Google Scholar] [CrossRef]
- Mazzetto, S.E.; Lomonaco, D.; Mele, G. Cashew nut oil: Opportunities and challenges in the context of sustainable industrial development. Quím. Nova 2009, 32, 732–741. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists—AOAC. Official Methods of Analysis, 19th ed.; AOAC International: Gaithersburg, MD, USA, 2012. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Senger, C.C.D.; Kozloski, G.V.; Bonnecarrère Sanchez, L.M.; Mesquita, F.R.; Alves, T.P.; Castagnino, D.S. Evaluation of autoclave procedures for fibre analysis in forage and concentrate feedstuffs. Anim. Feed Sci. Technol. 2008, 146, 169–174. [Google Scholar] [CrossRef]
- Licitra, G.; Hernandez, T.M.; Van Soest, P.J. Standardization of procedures for nitrogen fractionation of ruminant feeds. Anim. Feed Sci. Technol. 1996, 57, 347–358. [Google Scholar] [CrossRef]
- Mertens, D.R. Creating a system for meeting the fiber requirements of dairy cows. J. Dairy Sci. 1997, 80, 1463–1481. [Google Scholar] [CrossRef]
- Weiss, W.P. Energy Prediction Equations for Ruminant Feeds. In Cornell Nutrition Conference Feed Manufactures; Proceedings; Cornell University: Ithaca, NY, USA, 1999; pp. 176–185. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Hartman, L.; Lago, R.C. Rapid preparation of fatty acid methyl esters from lipids. Lab. Pract. 1973, 22, 475–481. [Google Scholar]
- Martin, P.; Bateson, P. Measuring Behaviour, 2nd ed.; Cambridge University Press: Cambridge, UK, 1993; Volume 222. [Google Scholar]
- Bürger, P.J.; Pereira, J.C.; Queiroz, A.C.d.; Silva, J.F.C.d.; Valadares Filho, S.d.C.; Cecon, P.R.; Casali, A.D.P. Ingestive behavior in Holstein calves fed diets with different concentrate levels. Rev. Bras. Zootec. 2000, 29, 236–242. [Google Scholar] [CrossRef]
- Polli, V.A.; Restle, J.; Senna, D.B.; Almeida, S.R.S.d. Aspectos relativos à ruminação de bovinos e bubalinos em regime de confinamento. Rev. Bras. Zootec. 1996, 25, 987–993. [Google Scholar]
- Lammers, B.P.; Buckmaster, D.R.; Heinrichs, A.J. A simple method for the analysis of particle sizes of forage and total mixed rations. J. Dairy Sci. 1996, 79, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Kononoff, P.J.; Heinrichs, A.J.; Buckmaster, D.R. Modification of Penn State forage and total mixed ration particle separator and the effects of moisture content on its measurements. J. Dairy Sci. 2003, 86, 1858–1863. [Google Scholar] [CrossRef]
- Buffington, D.E.; Collazo-Arocho, A.; Canton, G.H.; Pitt, D. Black Globe-humidity index (BGHI) as Comfort Equation for Dziry Cows. Trans. Asae 1981, 24, 0711–0714. [Google Scholar] [CrossRef]
- Statistical Analysis System, S.A.S. User’s Guide: Statistics; SAS Institute Inc.: Cary, NC, USA, 2002. [Google Scholar]
- Maia MR, G.; Chaudhary, L.C.; Figueres, L.; Wallace, R.J. Metabolism of polyunsaturated fatty acids and their toxicity to the microflora of the rumen. Antonie van Leeuwenhoek 2007, 91, 303–314. [Google Scholar] [CrossRef]
- Shingfield, K.J.; Bernard, L.; Leroux, C.; Chilliard, Y. Role of trans fatty acids in the nutritional regulation of mammary lipogenesis in ruminants. Animal 2010, 4, 1140–1166. [Google Scholar] [CrossRef] [PubMed]
- Francisco, A.; Dentinho, M.T.; Alves, S.P.; Portugal, P.V.; Fernandes, F.; Sengo, S.; Jerónimo, E.; Oliveira, M.A.; Costa, P.; Sequeira, A.; et al. Growth performance, carcass and meat quality of lambs supplemented with increasing levels of a tanniferous bush (Cistus ladanifer L.) and vegetable oils. Meat Sci. 2015, 100, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Diógenes, L.; Bezerra, L.; Pereira Filho, J.; Silva Junior, J.; Oliveira, J.; Moura, J.; Barbosa, A.; Souza, M.; Sousa, S.; Pereira, E.; et al. Effects of the dietary inclusion of buriti oil on lamb performance, carcass traits, digestibility, nitrogen balance, ingestive behavior and blood metabolites. Animals 2020, 10, 1973. [Google Scholar] [CrossRef]
- de Oliveira Maia Parente, M.; Rocha, K.S.; Bessa, R.J.B.; Parente, H.N.; de Moura Zanine, A.; Machado, N.A.F.; de Brito Lourenço Júnior, J.; Bezerra, L.R.; Landim, A.V.; Alves, S.P. Effects of the dietary inclusion of babassu oil or buriti oil on lamb performance, meat quality and fatty acid composition. Meat Sci. 2020, 160, 107971. [Google Scholar] [CrossRef]
- Ackermans, N.L.; Martin, L.F.; Hummel, J.; Müller, D.W.H.; Clauss, M.; Hatt, J.-M. Feeding selectivity for diet abrasiveness in sheep and goats. Small Rum. Res. 2019, 175, 160–164. [Google Scholar] [CrossRef]
- Nguyen, D.V.; Malau-Aduli, B.S.; Nichols, P.D.; Malau-Aduli, A.E. Growth performance and carcass characteristics of Australian prime lambs supplemented with pellets containing canola oil or flaxseed oil. Anim. Prod. Sci. 2017, 58, 2100–2108. [Google Scholar] [CrossRef]
- Lima, P.R.; Apdini, T.; Freire, A.S.; Santana, A.S.; Moura LM, L.; Nascimento JC, S.; Rodrigues RT, S.; Dijkstra, J.; Garcez Neto, A.F.; Queiroz, M.A.Á.; et al. Dietary supplementation with tannin and soybean oil on intake, digestibility, feeding behavior, ruminal protozoa and methane emission in sheep. Anim. Feed Sci. Technol. 2019, 249, 10–17. [Google Scholar] [CrossRef]
- Gomes, C.A.V.; Furtado, D.A.; Medeiros, A.N.; Silva, D.S.; Pimenta Filho, E.C.; Lima Junior, V. Effect of thermal ambient and feed supplementation levels on physiologic parameters of Moxotó goats. Rev. Bras. de Eng. Agrícola e Ambient. 2008, 12, 213–219. [Google Scholar] [CrossRef]
- Neiva, J.N.; Teixeira, M.; Turco, H.N.; Oliveira, S.M.; Moura, A.D. Effects of environmental stress on physiological parameters of feedlot sheep in the Northeast of Brazil. Rev. Bras. Zootec. 2004, 33, 668–678. [Google Scholar] [CrossRef]
- Kaneko, J.J.; Harve, J.W.; Bruss, M.L. Clinical Biochemistry of Domestic Animals, 6th ed.; Academic Press: San Diego, CA, USA, 2008. [Google Scholar] [CrossRef]
- Meyer, D.J.; Harvey, J.W. Veterinary Laboratory Medicine: Interpretation and Diagnosis; WB Saunders: St. Louis, MO, USA, 2004. [Google Scholar]
- Caldeira, R.M.; Belo, A.T.; Santos, C.C.; Vasquez, M.I.; Portugal, A.V. The effect of long-term feed restriction and over-nutrition on body condition score, blood metabolites and hormonal profiles in ewes. Small Rum. Res. 2007, 68, 233–241. [Google Scholar] [CrossRef]
- Brzozowska, A.M.; Oprządek, J. Metabolism of fatty acids in tissues and organs of the ruminants-a review. Anim. Sci. Pap. Rep. 2016, 34, 211–219. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).