A Novel Direct-Fed Microbial Impacts Growth Performance and Supports Overall Health of Feedlot Cattle
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Arrival Processing
2.2.1. Treatment Allocation
- (1)
- Negative control—(CON; no DFM feeding)
- (2)
- Direct-fed microbial—feeding of 50 mg/head per day of a DFM containing L. animalis 506, P. freudenreichii 507, B. licheniformis 809, and B. subtilis 597 (BDP; BOVAMINE DEFEND® Plus; Chr Hansen, Milwaukee, WI, USA).
2.2.2. Feed, Housing, and Water
2.2.3. Interim Weight
2.2.4. Harvest
2.2.5. Carcass Outcomes and Liver Scoring
2.2.6. Economic Analysis
2.2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yoon, I.; Stern, M. Influence of direct-fed microbials on ruminal microbial fermentation and performance of ruminants—A Review. Asian-Australas. J. Anim. Sci. 1995, 8, 533–555. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine. Nutrient Requirements of Beef Cattle: Eighth Revised Edition; The National Academies Press: Washington, DC, USA, 2016. [Google Scholar] [CrossRef]
- Hanford, K.J.; Kreikemeier, W.M.; Ware, D. The effects of Bovamine on feedlot performance of finishing cattle: A meta-analysis. J. Anim. Sci. 2011, 89 (Suppl. S1), 258. [Google Scholar]
- Dick, K.J.; Duff, G.C.; Limesand, W.S.; Cuno, S.P.; Knudson, D.K.; McMurphy, C.P.; Hall, L.W.; Bernal-Rigoli, J.C.; Marchello, J.A. Effects of a direct-fed microbial on digestive-tract morphology of Holstein bull calves and performance and carcass characteristics of Holstein. Prof. Anim. Sci. 2013, 29, 107–115. [Google Scholar] [CrossRef]
- Dias, B.G.C.; Santos, F.A.P.; Meschiatti, M.; Brixner, B.M.; Almeida, A.A.; Queiroz, O.; Cappellozza, B.I. Effects of feeding different probiotic types on metabolic, performance, and carcass responses of Bos indicus feedlot cattle offered a high-concentrate diet. J. Anim. Sci. 2022, 100, skac289. [Google Scholar] [CrossRef] [PubMed]
- Cappellozza, B.I.; Copani, G.; Boll, E.J.; Queiroz, O. Supplementation of the direct-fed microbial Enterococcus faecium 669 impacts performance of pre-weaning dairy calves. JDS Commun. 2023, 4, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, O.; Cappellozza, B.I.; Capern, L.C.; Schutz, J.S.; Cull, C.A.; Queiroz, O.; Copani, G. A Novel Direct-Fed Microbial for Beef Cattle Has a Supportive Effect against Clostridium perfringens In Vitro and In Vivo. Ruminants 2023, 3, 189–201. [Google Scholar] [CrossRef]
- Lopez, A.M.; Sarturi, J.O.; Johnson, B.J.; Woerner, D.R.; Henry, D.D.; Ciriaco, F.M.; Silva, K.G.S.; Rush, C.J. Effects of bacterial direct-fed microbial combinations on beef cattle growth performance, feeding behavior, nutrient digestibility, ruminal morphology, and carcass characteristics. J. Anim. Sci. 2023, 102, skae004. (In Progress) [Google Scholar] [CrossRef] [PubMed]
- Brashears, M.M.; Galyean, M.L.; Loneragan, G.H.; Mann, J.E.; Killinger-Mann, K. Prevalence of Escherichia coli O157:H7 and performance by beef feedlot cattle given Lactobacillus direct fed microbials. J. Food Prot. 2003, 66, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Krehbiel, C.R.; Rust, S.R.; Zhang, G.; Gilliland, S.E. Bacterial direct fed microbials in ruminant diets: Performance response and mode of action. J. Anim. Sci. 2003, 81, 120–132. [Google Scholar] [CrossRef]
- Younts-Dahl, S.M.; Osborn, G.D.; Galyean, M.L.; Rivera, J.D.; Loneragan, G.H.; Brashears, M.M. Reduction of Escherichia coli O157 in finishing beef cattle by various doses of Lactobacillus acidophilus in direct fed microbials. J. Food Prot. 2005, 68, 6–10. [Google Scholar] [CrossRef]
- Ban, Y.; Guan, L.L. Implication and challenges of direct-fed microbial supplementation to improve ruminant production and health. J. Anim. Sci. Biotechnol. 2021, 12, 109. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.; Krehbiel, C. Current and Future Status of Practical Applications: Beef Cattle; Springer: New York, NY, USA, 2012. [Google Scholar] [CrossRef]
- Nagaraja, T.G.; Newbold, C.J.; Van Nevel, C.J.; Demeyer, D.I. Manipulation of ruminal fermentation. In The Rumen Microbial Ecosystem; Springer: Dordrecht, The Netherlands, 1997; pp. 523–632. [Google Scholar]
- Retta, K. Role of probiotics in rumen fermentation and animal performance: A review. Int. J. Livest. Prod. 2016, 7, 24–32. [Google Scholar]
- EElam, N.A.; Gleghorn, J.F.; Rivera, J.D.; Galyean, M.L.; Defoor, P.J.; Brashears, M.M.; Younts-Dahl, S.M. Effects of live cultures of Lactobacillus acidophilus (strains NP45 and NP51) and Propionibacterium freudenreichii on performance, carcass, and intestinal characteristics, and Escherichia coli strain O157 shedding of finishing beef steers. J. Anim. Sci. 2003, 81, 2686–2698. [Google Scholar] [CrossRef] [PubMed]
- Raeth-Knight, M.; Linn, J.; Jung, H. Effect of Direct-Fed Microbials on performance, diet digestibility, and rumen characteristics of Holstein Dairy Cows. J. Dairy Sci. 2007, 90, 1802–1809. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, J.T.; Galyean, M.L. Nutritional recommendations of feedlot consulting nutritionists: The 2007 Texas Tech University survey1. J. Anim. Sci. 2007, 85, 2772–2781. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.; Calle, M.A.; Ribeiro, F.R.; Pond, A.R.; Kreikemeier, W.M.; McDonald, A.; Johnson, E.G.; Edmonds, M.D.; Loneragan, G.H.; Brashears, M.M.; et al. Effect of Direct-Fed Microbial Supplementation on Pathogenic Escherichia coli Fecal Shedding, Live Performance, and Carcass Characteristics in Feedlot Steers. Open J. Anim. Sci. 2020, 10, 683–705. [Google Scholar] [CrossRef]
- Green, D.H.; Wakeley, P.R.; Page, A.; Barnes, A.; Baccigalupi, L.; Ricca, E.; Cutting, S.M. Characterization of Two Bacillus Probiotics. Appl. Environ. Microbiol. 1999, 65, 4288–4291. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Wang, J.; Deng, L. Effects of Bacillus subtilis natto on milk production, rumen fermentation and ruminal microbiome of dairy cows. Animal 2013, 7, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Qiao, G.H.; Shan, A.S.; Ma, N.; Ma, Q.Q.; Sun, Z.W. Effect of supplemental Bacillus cultures on rumen fermentation and milk yield in Chinese Holstein cows. J. Anim. Physiol. Anim. Nutr. 2009, 94, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Deng, K.D.; Xiao, Y.; Ma, T.; Tu, Y.; Diao, Q.Y.; Chen, Y.H.; Jiang, J.J. Ruminal fermentation, nutrient metabolism, and methane emissions of sheep in response to dietary supplementation with Bacillus licheniformis. Anim. Feed Sci. Technol. 2018, 241, 38–44. [Google Scholar] [CrossRef]
- Hoa, N.T.; Baccigalupi, L.; Huxham, A.; Smertenko, A.; Van, P.H.; Ammendola, S.; Ricca, E.; Cutting, S.M. Characterization of Bacillus species used for oral bacteriotherapy and bacterio-prophylaxis of gastrointestinal disorders. Appl. Environ. Microbiol. 2000, 66, 5241–5247. [Google Scholar] [CrossRef]
- Maruta, K.; Miyazaki, H.; Masuda, S.; Takahashi, M.; Marubashi, T.; Tadano, Y.; Takahashi, H. Exclusion of intestinal pathogens by continuous feeding with Bacillus subtilis C-3102 and its influence on the intestinal microflora in broilers. Anim. Sci. Technol. 1996, 67, 273–280. [Google Scholar]
- Jiraphocakul, S.; Sullivan, T.W.; Shahani, K.M. Influence of a Dried Bacillus subtilis Culture and Antibiotics on Performance and Intestinal Microflora in Turkeys. Poult. Sci. 1990, 69, 1966–1973. [Google Scholar] [CrossRef] [PubMed]
- Perry, T.W.; Purkhiser, E.D.; Beeson, W.M. Effects of Supplemental Enzymes on Nitrogen Balance, Digestibility of Energy and Nutrients and on Growth and Feed Efficiency of Cattle. J. Anim. Sci. 1966, 25, 760–764. [Google Scholar] [CrossRef]
- Phuoc, T.L.; Jamikorn, U. Effects of probiotic supplement (Bacillus subtilis and Lactobacillus acidophilus) on feed efficiency, growth performance, and microbial population of weaning rabbits. Asian-Australas. J. Anim. Sci. 2017, 30, 198. [Google Scholar] [CrossRef]
- Sadat Hoseini Madani, N.; Adorian, T.J.; Ghafari Farsani, H.; Hoseinifar, S.H. The effects of dietary probiotic Bacilli (Bacillus subtilis and Bacillus licheniformis) on growth performance, feed efficiency, body composition and immune parameters of whiteleg shrimp (Litopenaeus vannamei) post larvae. Aquac. Res. 2018, 49, 1926–1933. [Google Scholar] [CrossRef]
- Ferrari, E.; Jarnagin, A.S.; Schmidt, B.F. Commercial Production of Extracellular Enzymes. In Bacillus Subtilis and Other Gram-Positive Bacteria; Sonenshein, A.L., Hoch, J.A., Losick, R., Eds.; Wiley: Hoboken, NJ, USA, 1993. [Google Scholar] [CrossRef]
- Pan, L.; Harper, K.; Queiroz, O.; Copani, G.; Cappellozza, B.I. Effects of a Bacillus-based direct-fed microbial on in vitro nutrient digestibility of forage and high-starch concentrate substrates. Transl. Anim. Sci. 2022, 6, txac067. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.S.; Krehbiel, C.R.; Galyean, M.L.; Remmenga, M.D.; Peters, J.P.; Hibbard, B.; Robinson, J.; Moseley, W.M. Evaluation of models of acute and subacute acidosis on dry matter intake, ruminal fermentation, blood chemistry, and endocrine profiles of beef steers. J. Anim. Sci. 2000, 78, 3155–3168. [Google Scholar] [CrossRef]
- Swinney-Floyd, D.; Gardner, B.A.; Owens, F.N.; Rehberger, T.; Parrott, T. Effect of inoculation with either strain P-63 alone or in combination with Lactobacillus acidophilus LA53545 on performance of feedlot cattle. J. Anim. Sci. 1999, 77 (Suppl. 1), 77. [Google Scholar]
- Peterson, R.E.; Klopfenstein, T.J.; Erickson, G.E.; Folmer, J.; Hinkley, S.; Moxley, R.A.; Smith, D.R. Effect of Lactobacillus acidophilus strain NP51 on Escherichia coli O157:H7 fecal shedding and finishing performance in beef feedlot cattle. J. Food Prot. 2007, 70, 287–291. [Google Scholar] [CrossRef]
- Lawrence, M.; Polukis, S.; Barnard, A.M.; Miller, M.A.; Kung, L.; Gressley, T.F. Evaluating the effects of Lactobacillus animalis and Propionibacterium freudenreichii on performance and rumen and fecal measures in lactating dairy cows. J. Dairy Sci. 2021, 104, 4119–4133. [Google Scholar] [CrossRef] [PubMed]
- Bernardeau, M.; Lehtinen, M.J.; Forssten, S.D.; Nurminen, P. Importance of the gastrointestinal life cycle of Bacillus for probiotic functionality. J. Food Sci. Technol. 2017, 54, 2570–2584. [Google Scholar] [CrossRef]
- Schallmey, M.; Sigh, A.; Ward, O.P. Developments in the use of Bacillus species for industrial production. Can. J. Microbiol. 2004, 50, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Cappellozza, B.I.; Joergensen, J.N.; Copani, G.; Bryan, K.A.; Fantinati, P.; Bodin, J.-C.; Malek Khahi, M.; NinoDeGuzman, C.; Arriola, K.G.; Lima, L.O.; et al. Evaluation of a Bacillus-based direct-fed microbial probiotic on in vitro rumen gas production and nutrient digestibility of different feedstuffs and total mixed rations. Transl. Anim. Sci. 2023, 7, txad044. [Google Scholar] [CrossRef] [PubMed]
- Luise, D.; Bosi, P.; Raff, L.; Amatucci, L.; Virdis, S.; Trevisi, P. Bacillus spp. probiotic strains as a potential tool for limiting the use of antibiotics and improving the growth and health of pigs and chickens. Front. Microbiol. 2022, 13, 801827. [Google Scholar] [CrossRef]
- Cordeiro, M.; Melo, N.; Oliveira, A.; Cappellozza, B.I.; Bernardes, T. The probiotic containing Bacillus licheniformis and Bacillus subtilis improves feed efficiency in beef cattle. J. Anim. Sci. 2023, 101 (Suppl. S3), skad281.519. [Google Scholar]
- Cooper, R.J.; Klopfenstein, T.J.; Stock, R.A.; Milton, C.T.; Herold, D.W.; Parrott, J.C. Effects of imposed feed intake variation on acidosis and performance of finishing steers. J. Anim. Sci. 1999, 77, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Theurer, M.E.; Fox, J.T.; McCarty, T.M.; McCollum, R.M.; Jones, T.M.; Simpson, J.; Martin, T. Evaluation of the reticulorumen pH throughout the feeding period for beef feedlot steers maintained in a commercial feedlot and its association with liver abscesses. J. Am. Vet. Med. Assoc. 2021, 259, 899–908. [Google Scholar] [CrossRef]
- Nagaraja, T.G.; Titgemeyer, E.C. Ruminal acidosis in beef cattle: The current microbiological and nutritional outlook. J. Dairy Sci. 2007, 90, E17–E38. [Google Scholar] [CrossRef] [PubMed]
- Amachawadi, R.G.; Nagaraja, T.G. First report of anaerobic isolation of Salmonella enterica from liver abscesses of feedlot cattle. J. Clin. Microbiol. 2015, 53, 3100–3101. [Google Scholar] [CrossRef] [PubMed]
- Elfenbein, J.R.; Endicott-Yazdani, T.; Porwollik, S.; Bogomolnaya, L.M.; Cheng, P.; Guo, J.; Zheng, Y.; Yang, H.J.; Talamantes, M.; Shields, C.; et al. Novel determinants of intestinal colonization of Salmonella enterica serotype Typhimurium identified in bovine enteric infection. Infect. Immun. 2013, 81, 4311–4320. [Google Scholar] [CrossRef]
- Fox, S.M. Probiotics: Intestinal inoculants for production animals. Vet. Med. 1988, 83, 806–830. [Google Scholar]
- McAllister, T.A.; Beauchemin, K.A.; Alazzeh, A.Y.; Baah, J.; Teather, R.M.; Stanford, K. The use of direct fed microbials to mitigate pathogens and enhance production in cattle. Can. J. Anim. Sci. 2011, 91, 193–211. [Google Scholar] [CrossRef]
- Buntyn, J.O.; Schmidt, T.; Nisbet, D.; Callaway, T. The Role of Direct-Fed Microbials in Conventional Livestock Production. Annu. Rev. Anim. Biosci. 2016, 4, 335–355. [Google Scholar] [CrossRef]
- Theurer, M.E.; Amachawadi, R.G. Antimicrobial and Biological Methods to Control Liver Abscesses. Vet. Clin. Food Anim. Pract. 2022, 38, 383–394. [Google Scholar] [CrossRef]
| Ration | |||
| Ingredient | Starter | Intermediate | Finish † |
| Steam flaked corn | 32.0 | 53.4 | 76.4 |
| Wet distillers grain | 8.5 | 8.6 | 8.5 |
| Ground alfalfa hay | 47.6 | 25.3 | 9.4 |
| Corn steep | 8.2 | 7.1 | 0.0 |
| Fat | 0.0 | 1.4 | 1.4 |
| Liquid finisher § | 3.7 | 4.2 | 4.3 |
| Calculated nutrients | |||
| Dry matter, % | 70.69 | 69.83 | 70.84 |
| NEm, Mcal/kg | 1.72 | 2.00 | 2.17 |
| NEg, Mcal/kg | 1.10 | 1.35 | 1.51 |
| Crude protein, % | 17.25 | 15.19 | 13.83 |
| Non-protein nitrogen, % | 2.52 | 2.47 | 2.55 |
| Crude fat, % | 3.13 | 4.81 | 5.47 |
| Crude fiber, % | 18.02 | 10.97 | 6.51 |
| Calcium, % | 1.20 | 1.00 | 0.78 |
| Phosphorus, % | 0.36 | 0.41 | 0.37 |
| Potassium, % | 1.46 | 1.04 | 0.61 |
| Variable | CON 1 | BDP 2 | p-Value | ||||
|---|---|---|---|---|---|---|---|
| No. Calves (Pens) | 811 (12) | 814 (12) | - | ||||
| Enrollment weight, kg | 370.5 | ± | 8.42 | 370.6 | ± | 8.42 | 0.92 |
| Interim body weight, kg | 530.3 | ± | 9.56 | 533.6 | ± | 9.56 | 0.06 |
| ADG, kg * | 1.90 | ± | 0.04 | 1.95 | ± | 0.04 | 0.06 |
| ADG, kg † | 1.96 | ± | 0.03 | 2.00 | ± | 0.03 | 0.06 |
| F:G * | 5.39 | ± | 0.12 | 5.19 | ± | 0.12 | 0.06 |
| F:G † | 5.26 | ± | 0.10 | 5.09 | ± | 0.10 | 0.05 |
| Average dry matter intake, kg | 10.22 | ± | 0.20 | 10.14 | ± | 0.20 | 0.30 |
| BRD first treatment, % | 2.75 | ± | 0.62 | 2.86 | ± | 0.64 | 0.89 |
| BRD second treatment, % | 0.37 | ± | 0.21 | 0.12 | ± | 0.12 | 0.34 |
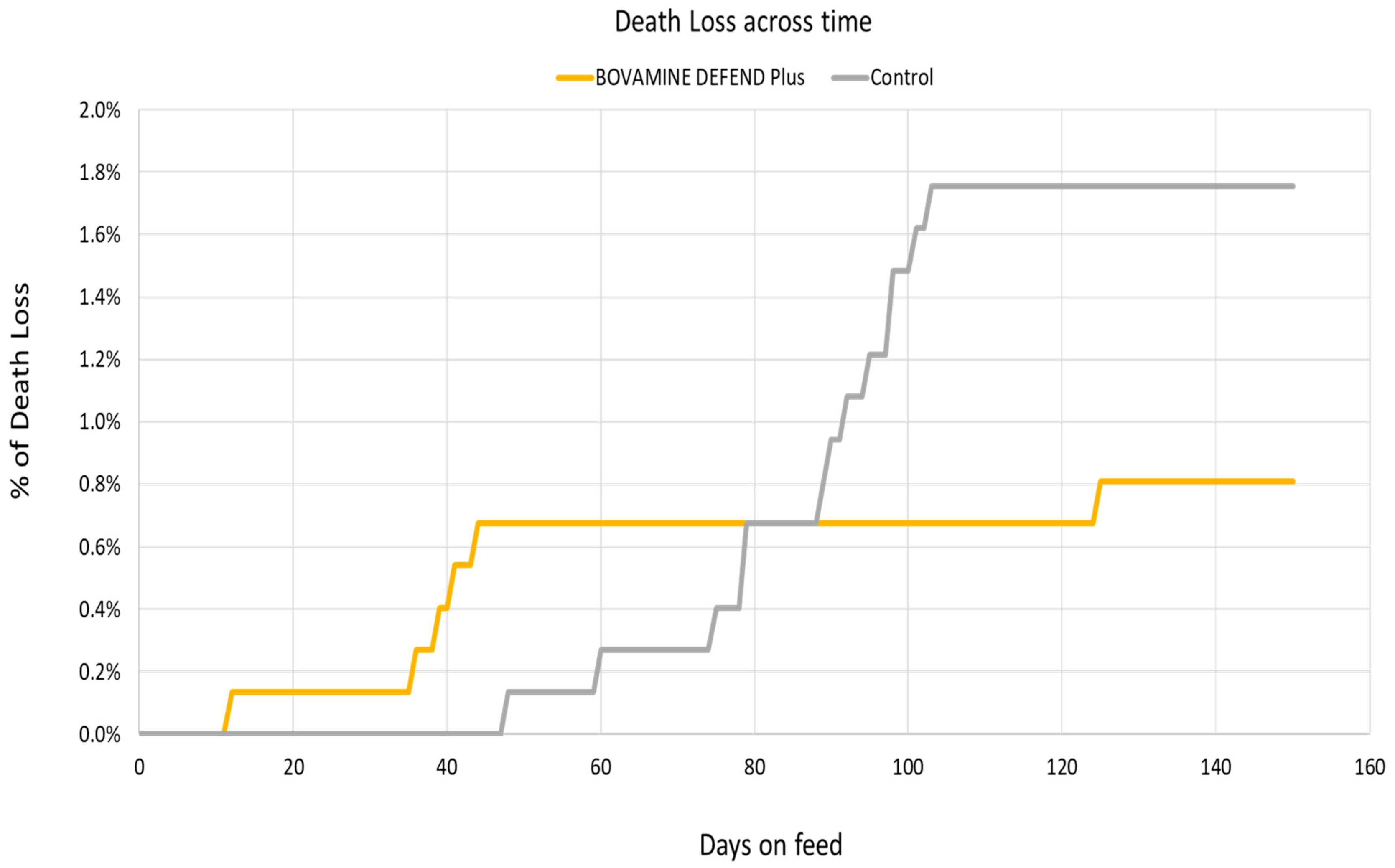
| Overall mortality, % | 0.58 | ± | 0.33 | 0.48 | ± | 0.28 | 0.76 |
| Variable | CON 1 | BDP 2 | p-Value | ||||
|---|---|---|---|---|---|---|---|
| BRD first treatment, % | 0.11 | ± | 0.15 | 0.21 | ± | 0.27 | 0.42 |
| BRD second treatment, % | 0.12 | ± | 0.12 | 0.12 | ± | 0.12 | 0.99 |
| Overall mortality, % | 0.72 | ± | 0.41 | 0.10 | ± | 0.11 | 0.07 |
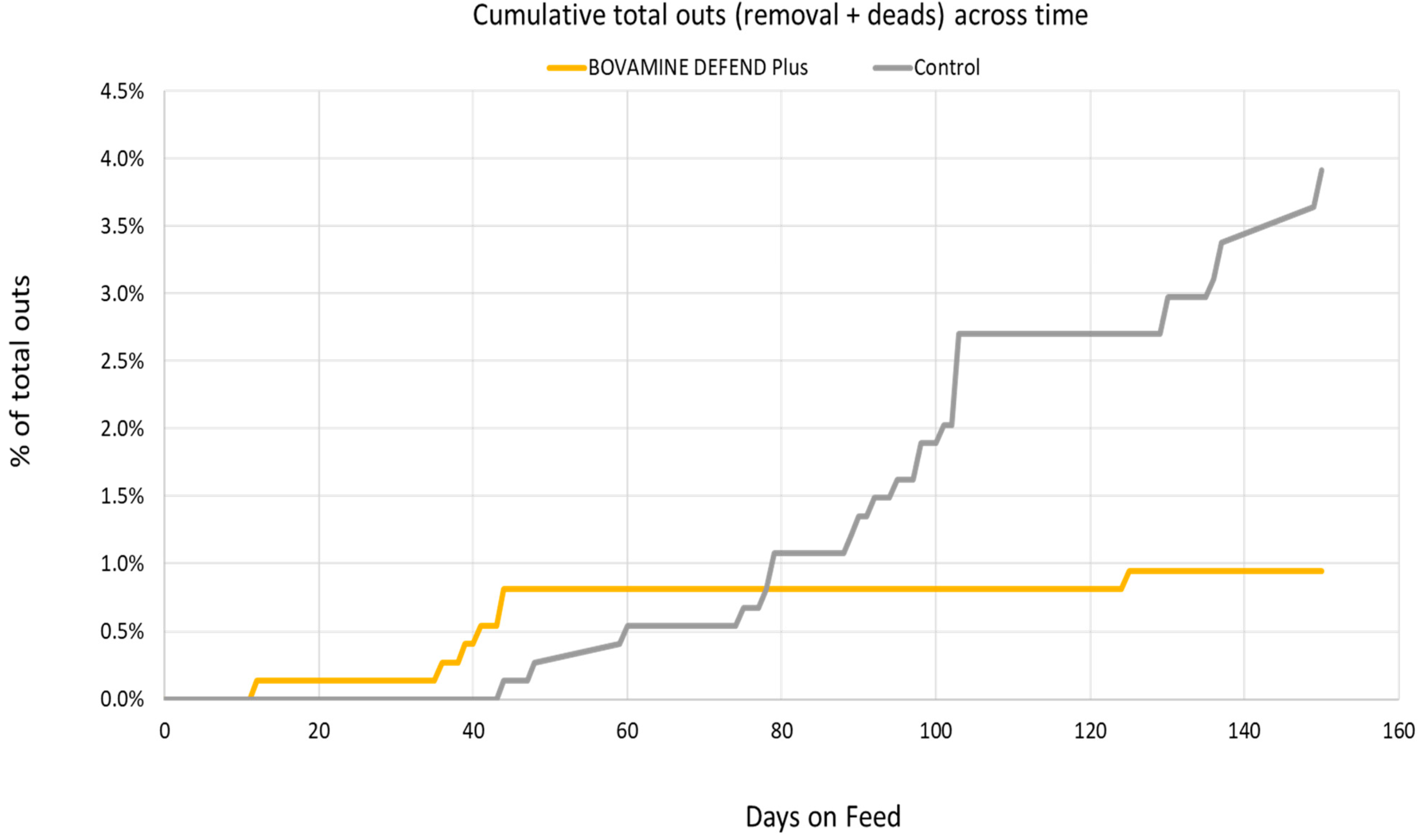
| Total outs (deads + removals §), % | 1.61 | ± | 0.56 | 0.11 | ± | 0.12 | 0.01 |
| Variable | CON 1 | BDP 2 | p-Value | ||||
|---|---|---|---|---|---|---|---|
| BRD first treatment, % | 2.84 | ± | 0.69 | 3.17 | ± | 0.75 | 0.68 |
| BRD second treatment, % | 0.49 | ± | 0.25 | 0.25 | ± | 0.17 | 0.42 |
| Overall mortality, % | 1.18 | ± | 0.52 | 0.54 | ± | 0.29 | 0.11 |
| BRD mortality, % | 0.37 | ± | 0.21 | 0.12 | ± | 0.12 | 0.34 |
| Digestive mortality, % | 0.46 | ± | 0.45 | 0.12 | ± | 0.15 | 0.21 |
| Other mortality, % | 0.47 | ± | 0.33 | 0.31 | ± | 0.23 | 0.52 |
| Removals, § % | 0.99 | ± | 0.35 | 0.12 | ± | 0.12 | 0.05 |
| Total outs (deads + removals), % | 2.23 | ± | 0.69 | 0.74 | ± | 0.32 | 0.01 |
| Variable | CON 1 | BDP 2 | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Final body weight, kg ¶ | 576.2 | ± | 6.30 | 577.8 | ± | 6.30 | 0.53 |
| ADG, kg * | 1.47 | ± | 0.04 | 1.54 | ± | 0.04 | 0.06 |
| ADG, kg † | 1.55 | ± | 0.03 | 1.56 | ± | 0.03 | 0.51 |
| F:G * | 7.08 | ± | 0.14 | 6.71 | ± | 0.14 | 0.05 |
| F:G † | 6.86 | ± | 0.12 | 6.63 | ± | 0.12 | 0.06 |
| Average dry matter intake, kg | 10.39 | ± | 0.18 | 10.33 | ± | 0.18 | 0.42 |
| Cost of gain §, $/45.5 kg * | 159.83 | ± | 3.23 | 151.87 | ± | 3.23 | 0.06 |
| Cost of gain §, $/45.5 kg † | 155.02 | ± | 2.66 | 150.20 | ± | 2.65 | 0.08 |
| Variable | CON 1 | BDP 2 | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Hot carcass weight, kg | 358.90 | ± | 3.39 | 359.80 | ± | 3.39 | 0.53 |
| Dressing percent §, % | 62.30 | ± | 0.20 | 62.23 | ± | 0.20 | 0.54 |
| Ribeye area, in 2 | 12.51 | ± | 0.16 | 12.48 | ± | 0.16 | 0.54 |
| Backfat, in | 0.54 | ± | 0.02 | 0.55 | ± | 0.02 | 0.81 |
| Marbling score | 464.94 | ± | 12.31 | 465.90 | ± | 12.31 | 0.84 |
| Quality grade distribution | 0.61 | ||||||
| Prime, % | 1.48 | ± | 0.48 | 1.64 | ± | 0.53 | |
| Upper 2/3 choice, % | 34.24 | ± | 6.13 | 36.47 | ± | 6.27 | |
| Lower 1/3 choice, % | 52.38 | ± | 3.75 | 51.03 | ± | 4.12 | |
| Select, % | 11.60 | ± | 2.95 | 10.59 | ± | 2.74 | |
| Standard, % | 0.30 | ± | 0.14 | 0.27 | ± | 0.13 | |
| Yield grade | 3.28 | ± | 0.09 | 3.31 | ± | 0.09 | 0.36 |
| Yield grade distribution | 0.38 | ||||||
| 1, % | 1.55 | ± | 0.46 | 1.41 | ± | 0.42 | |
| 2, % | 28.16 | ± | 4.80 | 26.37 | ± | 4.63 | |
| 3, % | 59.44 | ± | 3.00 | 60.43 | ± | 2.68 | |
| 4, % | 10.44 | ± | 2.34 | 11.34 | ± | 2.52 | |
| 5, % | 0.41 | ± | 0.17 | 0.45 | ± | 0.18 | |
| Liver abnormalities | 0.52 | ||||||
| Normal, % | 83.32 | ± | 2.21 | 84.50 | ± | 2.11 | |
| A−, % | 8.72 | ± | 1.18 | 8.16 | ± | 1.13 | |
| A, % | 1.26 | ± | 0.32 | 1.17 | ± | 0.30 | |
| A+, % | 2.32 | ± | 0.47 | 2.15 | ± | 0.44 | |
| Other †, % | 4.35 | ± | 0.78 | 4.01 | ± | 0.73 | |
| Total liver abscesses, % | 14.81 | ± | 1.26 | 10.41 | ± | 1.07 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guimaraes, O.; Preedy, G.; Fox, J.T.; Cappellozza, B.I.; Davis, T.C.; Schutz, J.S.; Theurer, M.E. A Novel Direct-Fed Microbial Impacts Growth Performance and Supports Overall Health of Feedlot Cattle. Ruminants 2024, 4, 267-279. https://doi.org/10.3390/ruminants4020019
Guimaraes O, Preedy G, Fox JT, Cappellozza BI, Davis TC, Schutz JS, Theurer ME. A Novel Direct-Fed Microbial Impacts Growth Performance and Supports Overall Health of Feedlot Cattle. Ruminants. 2024; 4(2):267-279. https://doi.org/10.3390/ruminants4020019
Chicago/Turabian StyleGuimaraes, Octavio, Garrett Preedy, J. Trent Fox, Bruno I. Cappellozza, Ty C. Davis, Jennifer S. Schutz, and Miles E. Theurer. 2024. "A Novel Direct-Fed Microbial Impacts Growth Performance and Supports Overall Health of Feedlot Cattle" Ruminants 4, no. 2: 267-279. https://doi.org/10.3390/ruminants4020019
APA StyleGuimaraes, O., Preedy, G., Fox, J. T., Cappellozza, B. I., Davis, T. C., Schutz, J. S., & Theurer, M. E. (2024). A Novel Direct-Fed Microbial Impacts Growth Performance and Supports Overall Health of Feedlot Cattle. Ruminants, 4(2), 267-279. https://doi.org/10.3390/ruminants4020019






