Simple Summary
The Addax nasomaculatus is an African antelope currently considered “critically endangered”. But to date, very little is known about the biology of this species. The objective of this study was to determine whether cortisol levels (an indicator of stress) and hair color vary by sex, body location, and season over an entire year in zoo-housed addax. Hair samples were taken from the animals in autumn, winter, spring, and summer. Hair cortisol levels in addax were higher in males than in females, with these differences being more evident during spring. Cortisol levels in the hair varied according to the seasons, with maximum values observed in summer. Hair color parameters also varied between seasons. The fur was blacker and yellower in winter and whiter in summer. The males had darker and greener hair color than the females. Sex- and season-determined variations in hair cortisol levels and hair color appear to be important elements to consider in relation to thermal comfort and animal welfare in addax, with warmer seasons highlighted as critical.
Abstract
The Addax nasomaculatus is an African antelope currently considered “critically endangered”. There is little scientific knowledge about the species, which could potentially aid in species conservation. The objective of this study was to determine how hair cortisol concentration and hair color vary according to sex, body site, and season across a whole year in zoo-housed addax. The addax population was housed at Parque Lecocq Zoo, Uruguay. Hair samples were taken from the shoulder and rump in autumn, winter, spring, and summer. Hair cortisol concentration in addax varied according to sex, with males exhibiting a higher hair cortisol concentration than females (1.03 ± 0.05 pg/mg vs. 0.91 ± 0.05 pg/mg, p = 0.016, respectively), with these differences being more significant in the spring (p < 0.0001). Hair cortisol concentration varied across seasons (p < 0.0001), with maximum values observed in summer. The color parameters (L*, a*, and b*) varied across seasons (p < 0.0001). The coat was blacker and yellower in winter and whiter in summer. Males had darker and greener hair color than females (p < 0.05). Sex and season-driven variation in hair cortisol concentration and hair color seem to be important elements to consider in relation to thermal comfort and animal welfare in addax, highlighting the warmer seasons as critical.
1. Introduction
Addax nasomaculatus is an African antelope currently considered extinct in some countries, such as Egypt and Algeria. According to the International Union for Conservation of Nature [1], the addax is globally regarded as “critically endangered”. Despite this designation, there are significant knowledge gaps concerning species biology. Today, the species is predominantly found in captivity rather than in the wild (International Studbooks for Rare Species of Wild Animals in Captivity) [2]. Understanding fundamental aspects of addax biology could facilitate a deeper understanding of the species and enhance welfare and conservation efforts, particularly in captive settings.
According to Broom [3], “The welfare of an individual is its state as regards its attempts to cope with its environment”. Therefore, animals that best adapt to their environment show better welfare conditions. Among the tools to objectively evaluate how animals adapt to their environment, those linked to the stress response play an important role. Stress is defined as a ‘state of homeostatic imbalance’ [4] induced by a stimuli or noxious event, frequently defined as a “stressor” [5]. A crux aspect of the stress response includes activation of the hypothalamic–pituitary–adrenal axis, resulting in cortisol release from the adrenal gland into the circulatory system [6,7]. According to the duration of this phenomenon, stressors can be classified as acute (single or intermittent event of short duration) and chronic (continuous or repeated prolonged exposures over time) [8]. Chronic stress has gained significant relevance in recent years due to its impact on animal welfare and health. Among the known, objective indicators of chronic stress, hair cortisol concentration has been shown to be an important stress assessment tool in numerous ruminant (bovines [9,10]; sheep [11,12,13]) and monogastric species (dogs [14,15]; cats [16,17]; non-human-primates [18,19]). The advantages of using hair cortisol as an indicator of chronic stress include (i) the feasibility of obtaining information from the last two or three months since sample collection; (ii) the collection of a non-invasive sample; (iii) the preservation of hair samples for long periods of time; and (iv) low coefficients of variation [14,20,21].
Among the factors that affect the concentration of cortisol in hair, differences according to sex, body site, hair color, and season of the year have been reported (see reviews: [21,22,23]). For example, in dairy cattle, the concentration of cortisol was higher in white than black hair and higher in hair from the tail than that obtained from the shoulder [10]. In chimpanzees, higher concentrations of cortisol were also reported in white hair than in black hair [24]. However, other studies report no association between hair cortisol concentration and hair color [23,25]. Regarding sex, in certain species like the coyote [26] and the black bear [27], males have exhibited higher hair cortisol concentrations than females. Conversely, in brown bears [28], polar bears [29], and non-human primates [18,30], females showed greater hair cortisol concentrations compared to males. Therefore, the effect of sex on hair cortisol concentrations varies across species. Furthermore, reports on how seasons of the year impact hair cortisol concentration are scarce. In chipmunks, hair cortisol concentrations were found to be higher in samples collected in summer than in spring [31]. However, in dogs [32] and pigs [33], hair cortisol concentrations were higher during winter compared to summer. Beyond the aforementioned individual effects on hair cortisol concentration, interactions between sex, season, body site, and hair color must be considered. For example, Bethge et al. [34] reported a significant increase in hair cortisol levels in males from early wet to early dry seasons (mating season), while no seasonal differences were reported within the reproductive cycle in females. In this sense, it is relevant to characterize how these factors interact to impact hair cortisol concentration within a given species from an environmental perspective. To the best of our knowledge, the use of hair cortisol as a tool for assessing welfare in wild ruminants is limited. One of the few reports available shows a positive association between hair cortisol concentration and aggressive behaviors in Dorcas gazelles [35]. However, hair cortisol concentration remains unmeasured in the addax antelope, especially in the context of hair color, sex, body site, and season.
Parque Lecocq Zoo, Montevideo, Uruguay, has an addax population of approximately 36 individuals in captive conditions, and we recently reported that male behavior varies with seasons in this addax population [36]. In summer, males are more frequently seen lying and consuming water and less frequently engaged in ruminating than in other seasons. Based on this observation, we suggested that these differences might reflect behavioral strategies linked to thermal stress. As such, our hypothesis is that hair cortisol concentrations in addax vary seasonally, with peak concentrations occurring in summer. Furthermore, building from data on other species, we hypothesize that hair cortisol concentrations and hair color vary according to sex and body site. Therefore, the objective of this study was to determine how hair cortisol concentration and hair color vary with sex, body site, and seasons of the year in Addax nasomaculatus antelopes maintained in captivity.
2. Materials and Methods
Animal care and procedures were approved by the Comisión Honoraria de Experimentación Animal, Universidad de la República, Uruguay (CEUAFVET-1077, 95 CHEA).
2.1. Animals, Location and Temperature and Humidity Index
The study was conducted between October 2021 and August 2022, based on the addax antelope population housed at the Parque Lecocq Zoo (Montevideo, Uruguay; 34°47′ S, 56°20′ O). The average air temperature (°C) and relative humidity (%) were obtained from the automatic meteorological station of the Instituto Nacional de Investigación Agropecuaria, sede Las Brujas, Uruguay. Daytime temperatures during data collection were (minimum–maximum) as follows: winter (7.5–12.3 °C), spring (12.8–20.5 °C), summer (19.4–27.2 °C), and autumn (9.5–15.75 °C). The temperature humidity index (THI) was calculated according to The National Research Council formula as follows: THI = (1.8 × T + 32) − [(0.55 − 0.0055 × R) × (1.8 × T − 26)] [37].
At the beginning of the study, the addax population had 36 individuals, composed of adult females, adult males, yearlings, and fawns (age < 1 year old)]. For this study, 1 adult female group (n = 15; age = 8.8 ± 3.8, mean ± SD) and 5 adult male groups (n = 11; age = 9.2 ± 4.2, mean ± SD) were used. All groups were located in independent paddocks of a similar size (~0.25 ha each), within an area of approximately 1.4 ha. Each paddock had trees, feeders, and a water reservoir, and was covered by pasture. Paddocks were also connected to a roofed shelter (25 m2, which they had free access to during the night) with handling facilities, including a squeeze. Animals grazed on pasture and were supplemented with a fixed amount of alfalfa hay and a ration formulated for antelopes daily. All individuals were identified by ear tags and microchips. Animals were habituated to the presence of humans. For each individual and each season, hair samples were taken and hair color was determined as described below.
2.2. Hair Sampling and Cortisol Extraction and Determination
Procedures followed the methods reported by Tallo-Parra et al. [38]. Hair samples were collected from the shoulder and rump (Figure 1). Briefly, animals were handled in a restraint device (squeeze), to which they were accustomed since it was used for the zoo’s own veterinary procedures. The procedure took 11.9 ± 0.3 min and was not invasive or painful. Except for procedures for this study, animals were not handled during the rest of the experimental period. For hair collection, an electric hair clipper (A6 slim, Oster, USA) with a 0.2 mm trimmer size was used. Each area was approximately 10 × 10 cm. All samples were individually stored in zip-lock plastic bags and were maintained at room temperature and in the dark until laboratory processing.
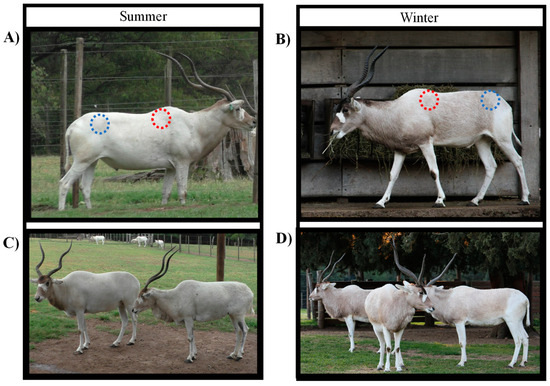
Figure 1.
Images of male (A,B) and female (C,D) Addax nasomaculatus in the summer (A,C) and winter (B,D) seasons housed at the Parque Lecocq zoo, Montevideo, Uruguay. Red dotted circles indicate the shoulder region and blue dotted circles indicate the rump region from which hair samples were obtained.
Approximately 50 mg of hair was placed in a 15 mL conical falcon tube and washed by adding 2.5 mL of isopropanol (2-propanol 99.5%, Sharlab), undergoing shaking at 1800 r.p.m. for 2.5 min. The supernatant was separated by decantation. This procedure was repeated twice, completing a total of three washes. Subsequently, the hair samples were dried for ~36 h at room temperature. Once dried, samples were cut into <2 mm length pieces using a hair clipper, and 50 mg of hair was weighed and placed in a 2 mL Eppendorf tube. To extract cortisol, 1.5 mL of pure methanol was added to each tube, which was shaken at 100 rpm for 18 h at 30 °C. Subsequently, the samples were centrifuged at 7000× g for 2 min, and 0.750 mL of the supernatant was transferred to a new 2 mL Eppendorf tube, which was kept in an oven at 38 °C for 24 h. Once the methanol was completely evaporated, the dry extracts were reconstituted with 0.2 mL EIA buffer provided by the EIA test kit (Cortisol ELISA EQUIPMENT; Neogen® Corporation, Ayr, UK) and each tube was shaken for 30 s. Immediately after finishing the aforementioned procedure, samples were stored at −20 °C until analysis. Hair cortisol concentrations were determined using cortisol EIA detection kits (Neogen® Corporation Europe, Ayr, UK). For low (0.8 pg/mL) and high concentration controls (4 pg/mL), intra-assay correlation coefficients were less than 10% and the inter-assay coefficients of variation were less than 13%.
2.3. Hair Color
Hair color was determined using a colorimeter (Minolta CR10, Minolta Camera Co., Osaka 541, Japan). According to the recommendations of the Commision Internationales de I’Éclairage [39], the results are reported as follows: Lightness (L*): from 10 = blackness to 100 = whiteness; yellowness (b*): from blue (negative value) to yellow (positive value); redness (a*): from green (negative value) to red (positive value). Color was recorded in triplicate for the rump and shoulder.
2.4. Statistical Analysis
Data were analyzed using the GLIMMIX procedure of SAS OnDemand for Academics (v. 3.1.0, SAS Institute Inc., Cary, NC, USA). A repeated-measure analysis of variance was performed for each variable. The type of distribution of the variable was included in the model of the GLIMMIX procedure. Hair cortisol concentration had a lognormal distribution, and color parameters had a normal (Gaussian) distribution. The fixed effects for hair cortisol concentration and hair color parameters were sex, season, body site, and their interactions. The individual was considered a random effect. Differences were considered significant with p values ≤ 0.05. A trend was considered when p values were between 0.05 and 0.10. The results are expressed as the mean ± standard error of the mean (SEM).
3. Results
3.1. Hair Cortisol Concentration
Males had a higher hair cortisol concentration than females (1.03 ± 0.05 pg/mg vs. 0.91 ± 0.05 pg/mg, p = 0.016, respectively). There was a significant effect of season (p < 0.0001) on hair cortisol concentration: the lowest values were recorded in autumn, increasing with maximum concentrations in summer (Figure 2). There was a significant interaction between sex and season (p < 0.0001). In spring, males had higher hair cortisol concentrations than females (Figure 2). In males, hair cortisol concentration increased from winter to spring (p < 0.0001), while concentrations did not differ between these seasons in females. In addition, cortisol concentrations increased from spring to summer for females (p < 0.0001), but not for males. No significant effects on hair cortisol concentrations were observed in terms of body site (p = 0.22), interactions between body site and sex (p = 0.57), or the combined effect of body site, sex, and season (p = 0.11).
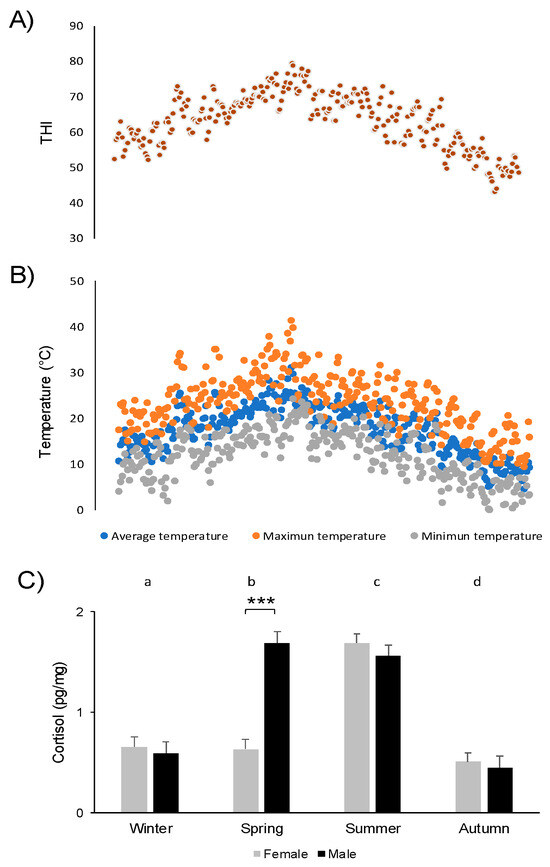
Figure 2.
Changes in THI (temperature and humidity index) (A), maximum, average, and minimum temperature (B), and hair cortisol concentration (mean ± sem) (C) in female and male Addax nasomaculatus according to season of the year. Different subscript letters denote statistical differences between seasons (p < 0.05). Asterisks represent significant differences (p < 0.0001) between sexes within season.
3.2. Hair Color Parameters
The effects of sex, body site, and season on hair color parameters are shown in Table 1. The parameters L* and a* were affected by sex, with females showing higher values than males (Table 1). Sex did not affect the parameter b* of hair color.

Table 1.
Colorimetric parameters (L*, b*, and a*) in hair according to sex, body site, and season in Addax nasomaculatus kept in captivity in Uruguay.
Body site did not affect any color parameters, although all three parameters varied across seasons (Table 1). For the L* parameter, the lowest value was found in winter and the highest values in summer and spring, with no differences between them (Table 1). The highest value of the b* parameter was found in winter compared with the remaining seasons, with no differences among them (Table 1). For the a* parameter, the highest value was recorded in spring and the lowest in autumn (Table 1).
There was a significant interaction between sex and season for the parameter L* (p = 0.006), which was evidenced by higher values in females than in males during autumn (76.01 ± 0.73 vs. 71.09 ± 0.85, p = 0.001), without differences between sexes in other seasons. There was a significant interaction between sex and season for the parameter b* (p = 0.03), which was evidenced by higher values in winter than in autumn in females (13.68 ± 0.29 vs. 11.18 ± 0.32, p < 0.0001, respectively), but not in males.
The a* parameter varied with the interaction between sex and season (p < 0.0001). Its value was higher in females than in males during spring (−1.54 ± 0.59 vs. −7.47 ± 0.62, p < 0.0001, respectively), without differences between sexes in other seasons.
There was no significant effect of the interaction between sex and body site for any of the three color parameters. For the parameters L* and a*, there was no interaction between season and body site, or between season, sex, and body site.
For the b* parameter, there was an interaction between season and body site (p = 0.0167). In the rump, the b* parameter decreased from winter to spring (13.62 ± 0.34 vs. 11.05 ± 0.34, p < 0.0001, respectively), while it did not differ between these seasons in the shoulder. This parameter presented a significant triple interaction between season, sex, and body site (p = 0.0009). In = spring, unlike other seasons, the b* value was higher in the shoulder than in the rump in females, but it did not differ in males (Figure 3). Furthermore, in the rump, males had a higher b* value than females during spring, unlike the other seasons, where no differences were found between sexes and body site (Figure 3).
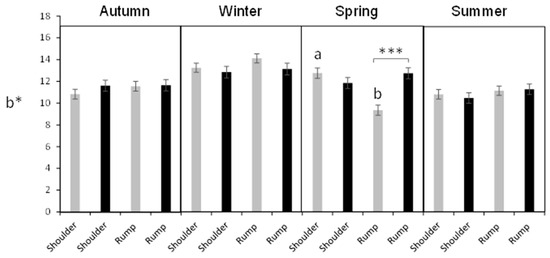
Figure 3.
Hair colorimetric parameter b* (mean ± sem) in female (gray bars) and male (black bars) Addax nasomaculatus according to season and the body site. Asterisks represent differences (p < 0.001) between sexes, for the same season and body site. Different subscript letters denote differences between body sites for the same sex (p < 0.001).
4. Discussion
This is the first study to assess hair cortisol concentration and hair color in Addax nasomaculatus antelope. Both variables varied mainly according to sex and season of the year, and hair color tones (parameters) varied differently between sex and season. Hair cortisol concentration is used as a tool to assess chronic stress in animals [13,20,40]. Hence, the observed cortisol changes, coupled with variations in hair color across seasons, sexes, and body sites, may constitute important parameters in assessing the welfare of this species.
Hair cortisol concentrations varied between sexes, with higher values in male than female addax. This observation coincides with previous reports on feral horses [41], Egyptian mongoose [42], Siberian flying squirrels [43], and free-ranging black bears [27]. However, females had higher hair cortisol concentrations than males in marmots [44], captive corral-housed baboons [45], snowshoe hares [46], common marmosets [47], Rocky Mountain goats [48], pigs [49], and Polar bears [29]. Meanwhile, some studies did not find differences in hair cortisol concentrations between sexes (e.g., Guinea baboons [30]; Canada lynx [50]). Thus, it appears that the relationship between hair cortisol concentration and sex is species-specific, which highlights the importance of characterizing the effects of sex on hair cortisol levels within a single species. Another factor to consider that may also help explain the differences between different studies and species in the effect of sex and hair cortisol concentrations is the reproductive status of the animals. For example, Medill et al. [41] found that females possessing offspring had higher levels of hair cortisol than those without. Moreover, other studies evaluated the reproductive status of females (i.e., pregnancy or lactating condition) in relation to the reproductive season. However, these studies found no effect of reproductive status on hair cortisol concentrations [42,47,48]. The other studies did not evaluate the effect of reproductive status on hair cortisol concentrations [27,29,30,43,44,45,46,49,50]. Although females were not pregnant in our study, when compared to males, we excluded the possible effect of females cycling on hair cortisol concentrations. Furthermore, within the same sexual group, social factors such as hierarchy could influence hair variables. At the same time, these results constitute a foundation for the further exploration of sex-based differences in cortisol production and stress responses in addax.
Trajectories for hair cortisol concentration across seasons differed for each sex, with spring emerging as a key season for these differences. As shown in Figure 2, cortisol in males increased markedly between winter and spring and remained stable between spring and summer. Meanwhile, in females, cortisol increased later than in males, reaching a peak in the summer. The higher levels of hair cortisol in males observed in spring could be associated with seasonal variations in behavior. Hair cortisol concentration reflects conditions experienced two or three months before sampling [14,21]. Therefore, the hair cortisol concentrations of hair collected in spring would reflect the conditions experienced in winter and early spring. According to Villagrán et al. [36], a higher frequency of aggressive and marking behaviors occurs in winter in addax males in the same population. Therefore, the increase in cortisol observed in spring in males (but not in females) could be associated with increased intrasexual competition that occurred in the previous season.
Overall (for both sexes), hair cortisol concentrations peaked in summer, which may be associated with environmental conditions and behavioral patterns. As shown in Figure 2, in summer, both temperature and THI increased and reached maximum values. In the spring and summer, male addax are seen lying and consuming water more frequently and standing and ruminating less frequently than in other seasons [36]. At the same latitudes, dairy cattle [37,51,52], show similar locomotor and feeding patterns in response to thermal stress taking place in summer. It seems that, despite the multiple differences between addax and dairy cattle (e.g., native habitat, human selection pressure), both bovidae species share basic behavioral strategies to cope with heat stress. Therefore, seasonal patterns of hair cortisol concentrations, together with the behavioral traits specifically described in males [36], suggest that summer is a critical season concerning animal welfare. This is an interesting observation considering that addax belongs to desertic African areas characterized by high temperatures and that the species has been raised in Uruguay for more than 30 years. In addition, it is known that dipterans such as Musca domestica and Stomoxys calcitrans generate an important stress response in ruminants [53,54,55]. At Parque Lecocq Zoo, Pérez-Sarasqueta [56] described the highest abundance of both diptera between spring and late summer, which coincides with the highest hair cortisol concentrations. Therefore, heat stress and external parasites, including diptera, would represent major environmental factors explaining the seasonal pattern of stress response reported herein. Thus, this study highlights hair cortisol concentrations as a valuable indicator of chronic stress in addax, which allowed us to further explain the seasonal variation and identify differences between sexes.
In relation to hair color, the most consistent effects were season and sex, and they both impacted color parameters in different ways. Regardless of sex and body site, the three color parameters varied with season. The coat was generally blacker and yellower in winter and whiter in summer, i.e., L* and b* parameters -, while extreme intensities of green and red (i.e., the a* parameter) were recorded in autumn and spring. These changes in coat color pattern objectively measured as a function of season in addax coincide with previous descriptive observations. It has been reported that during summer, the fur of the addax is white, while in winter, it has a grayer cover and longer hair [57]. It has been suggested that a whiter coating helps addax reflect the radiant heat and light sun during summer [57]. Similar patterns of seasonal color changes were associated with thermoregulation in other species [58,59]. To the best of our knowledge, the way seasonal variations in coat color are associated with thermoregulation is still unknown in ruminants.
Regardless of season and body site, female hair was whiter (higher L* values) and more reddish (higher a* values) than that of males. Meanwhile, when considering the season, female hair was whiter (L*) in autumn and more reddish (a*) in spring. Furthermore, unlike males, female hair was more yellow (b*) in winter than in autumn. In summary, males were darker (mainly in autumn) and less reddish (mainly in spring) than females.
As far as we know, differences in hair color between sexes have not been previously evaluated in ruminant species. Caro and Mallarino [60] mention in their review that in mammals, hair color is mainly influenced by social factors (such as position within the social hierarchy) and hormonal levels. Because, in our experimental setting, addax were housed in same-sex groups, the influence of hierarchy in explaining hair color differences between genders may not be relevant. However, these differences could be associated with different endocrine status between males and females [60]. Female (progesterone and estrogen) and male (testosterone) sexual steroids play a differential role in the activity of melanocytes [61], which may determine differences in hair color. Coincidentally, in several deer species, coat darkening may occur [62] and an increase in growth and activity of the apocrine and sebaceous glands can be associated with an increase in testosterone concentrations around the reproductive season [63]. Duncan and Goldman [64] reported that castration in both female and male hamsters affected hair color, while chronic administration of testosterone in castrated males attenuated hair color changes induced by photoperiod. Additionally, estrogen increased hair pigmentation in female mice [65]. In 40-year-old (or younger) humans, men have lower L* values (darker hair) than women of the same age [66]. Therefore, given the information gathered from ruminants and other species, it is possible to speculate that differences in hair color between sexes in addax could be associated with differences in hormonal production, melanocyte activity, and/or skin secretion. Furthermore, it is important to consider the potential role of sun-bleaching on coat color during the different seasons, particularly the increase in white following summer and spring. Although the experimental design of this study does not allow inferences in this regard, it is important to take into account the possible role of sun exposure on hair color.
5. Conclusions
In conclusion, hair cortisol concentration and hair color in addax varied mainly according to sex and season of the year. Males had higher hair cortisol concentrations than females in spring and high cortisol concentrations were evident in both sexes during summer. Males had darker and greener hair color than females. Coats were blacker and yellower in winter and whiter in summer and ranged from a greener shade in autumn to a redder tone in spring. The changes in hair cortisol concentration and hair color according to sex and season reported in this study are important factors to consider as markers of animal welfare for this species, highlighting the warmer seasons as critical periods in the year.
Author Contributions
J.P.D. and M.V. had the original idea. J.P.D., M.E.B., S.B., A.P., S.S., C.E., A.P.-S. and M.V. participated in handling animals and obtaining hair samples and determining color. M.E.B., S.B., J.P.D. and M.V. participated in the preparation of the samples prior to the determination of cortisol in hair. A.C.M. and P.P. performed the determination of cortisol in hair. J.P.D. statistically analyzed the data and wrote the first version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support was provided by Comisión Sectorial de Investigación Científica (CSIC, Proyecto I + D 2020), Project ID: 216, Universidad de la República, Uruguay.
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee (Animal care and procedures were approved by the Comisión Honoraria de Ex-perimentación Animal, Universidad de la República, Uruguay (CEUAFVET-1077, 95 CHEA).
Data Availability Statement
All data supporting reported results can be shared upon request directly to our corresponding authors.
Acknowledgments
The authors acknowledge Luciana Pacheco, Sofía Garay, Victoria Rohrer, and Andrea Bein for helping in data collection and Álvaro Modernell, Francisco Gutiérrez, and Valeria Da Silva (Sistema Departamental de Zoológicos, Intendencia Municipal de Montevideo, Montevideo, Uruguay) for caring for the animals and supporting the conduct of the study. We are especially grateful to Andres Gomez (Department of Animal Science, University of Minnesota, USA) for English language review of manuscript.
Conflicts of Interest
None of the authors have any financial or personal relationship that could inappropriately influence in the content of the paper.
References
- The IUCN Red List of Threatened Species. 2016. Available online: https://www.iucnredlist.org/ (accessed on 5 February 2024).
- International Studbooks for Rare Species of Wild Animals in Captivity. 2018. Available online: https://www.waza.org/priorities/conservation/waza-international-studbooks/ (accessed on 5 February 2024).
- Broom, D.M. Indicators of poor welfare. Br. Vet. J. 1986, 142, 524–526. [Google Scholar] [CrossRef] [PubMed]
- Sapolsky, R.M. Social status and health in humans and other animals. Annu. Rev. Anthropol. 2004, 33, 393–418. [Google Scholar] [CrossRef]
- Damián, J.P.; Bausero, M.; Bielli, A. Acute stress, hypothalamic-hypophyseal-gonadal axis and testicular function—A review. Ann. Anim. Sci. 2015, 15, 31–50. [Google Scholar] [CrossRef]
- Sapolsky, R.M.; Romero, L.M.; Munck, A.U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000, 21, 55–89. [Google Scholar] [CrossRef] [PubMed]
- Korte, S.M.; Koolhaas, J.M.; Wingfield, J.C.; McEwen, B.S. The Darwinian concept of stress: Benefits of allostasis and costs of allostatic load and the trade-offs in health and disease. Neurosci. Biobehav. Rev. 2005, 29, 3–38. [Google Scholar] [CrossRef] [PubMed]
- Pacák, K.; Palkovits, M. Stressor specificity of central neuroendocrine responses: Implications for stress-related disorders. Endocr. Rev. 2001, 22, 502–548. [Google Scholar] [CrossRef] [PubMed]
- Comin, A.; Peric, T.; Corazzin, M.; Veronesi, M.C.; Meloni, T.; Zufferli, V.; Cornacchia, G.; Prandi, A. Hair cortisol as a marker of hypothalamic-pituitary-adrenal axis activation in Friesian dairy cows clinically or physiologically compromised. Livest. Sci. 2013, 152, 36–41. [Google Scholar] [CrossRef]
- Burnett, T.A.; Madureira, A.M.; Silper, B.F.; Nadalin, A.; Tahmasbi, A.; Veira, D.M.; Cerri, R.L. Short communication: Factors affecting hair cortisol concentrations in lactating dairy cows. J. Dairy Sci. 2014, 97, 7685–7690. [Google Scholar] [CrossRef]
- Stubsjøen, S.M.; Bohlin, J.; Dahl, E.; Knappe-Poindecker, M.; Fjeldaas, T.; Lepschy, M.; Palme, R.; Langbein, J.; Ropstad, E. Assessment of chronic stress in sheep (part I): The use of cortisol and cortisone in hair as non-invasive biological markers. Small Rumin. Res. 2015, 132, 25–31. [Google Scholar] [CrossRef]
- Stubsjøen, S.M.; Sørheim, K.; Chincarini, M.; Bohlin, J.; Brunberg, E.; Fuchs, B.; Palme, R.; Grøva, L. Exploring hair cortisone concentration as a novel tool to assess chronic stress in sheep with tick-borne fever. Small Rumin. Res. 2018, 164, 110–119. [Google Scholar] [CrossRef]
- Poudel, S.; Fike, J.H.; Pent, G.J. Hair cortisol as a measure of chronic stress in ewes grazing either hardwood silvopastures or open pastures. Agronomy 2022, 12, 1566. [Google Scholar] [CrossRef]
- Mesarcova, L.; Kottferova, J.; Skurkova, L.; Leskova, L.; Kmecova, N. Analysis of cortisol in dog hair-a potential biomarker of chronic stress: A review. Vet. Med. 2017, 62, 363–376. [Google Scholar] [CrossRef]
- Sundman, A.S.; Van Poucke, E.; Svensson Holm, A.C.; Faresjö, Å.; Theodorsson, E.; Jensen, P.; Roth, L.S.V. Long-term stress levels are synchronized in dogs and their owners. Sci. Rep. 2019, 9, 7391. [Google Scholar] [CrossRef] [PubMed]
- Contreras, E.T.; Vanderstichel, R.; Hovenga, C.; Lappin, M.R. Evaluation of hair and nail cortisol concentrations and associations with behavioral, physical, and environmental indicators of chronic stress in cats. J. Vet. Intern. Med. 2021, 35, 2662–2672. [Google Scholar] [CrossRef] [PubMed]
- Wojtaś, J. Hair cortisol levels in cats with and without behavioural problems. J. Feline Med. Surg. 2023, 25, 1098612X221150624. [Google Scholar] [CrossRef] [PubMed]
- Dettmer, A.M.; Novak, M.A.; Meyer, J.S.; Suomi, S.J. Population density-dependent hair cortisol concentrations in rhesus monkeys (Macaca mulatta). Psychoneuroendocrinology 2014, 42, 59–67. [Google Scholar] [CrossRef]
- Rakotoniaina, J.H.; Kappeler, P.M.; Kaesler, E.; Hämäläinen, A.M.; Kirschbaum, C.; Kraus, C. Hair cortisol concentrations correlate negatively with survival in a wild primate population. BMC Ecol. 2017, 17, 30. [Google Scholar] [CrossRef]
- Lee, D.Y.; Kim, E.; Choi, M.H. Technical and clinical aspects of cortisol as a biochemical marker of chronic stress. BMB Rep. 2015, 48, 209–216. [Google Scholar] [CrossRef]
- Heimbürge, S.; Kanitz, E.; Otten, W. The use of hair cortisol for the assessment of stress in animals. Gen. Comp. Endocrinol. 2019, 270, 10–17. [Google Scholar] [CrossRef]
- Burnard, C.; Ralph, C.; Hynd, P.; Hocking, E.J.; Tilbrook, A. Hair cortisol and its potential value as a physiological measure of stress response in human and non-human animals. Anim. Prod. Sci. 2017, 57, 401–414. [Google Scholar] [CrossRef]
- Ghassemi Nejad, J.; Kim, B.W.; Lee, B.H.; Sung, K.I. Coat and hair color: Hair cortisol and serotonin levels in lactating Holstein cows under heat stress conditions. Anim. Sci. J. 2017, 88, 190–194. [Google Scholar] [CrossRef]
- Yamanashi, Y.; Morimura, N.; Mori, Y.; Hayashi, M.; Suzuki, J. Cortisol analysis of hair of captive chimpanzees (Pan troglodytes). Gen. Comp. Endocrinol. 2013, 194, 55–63. [Google Scholar] [CrossRef]
- Nedić, S.; Pantelić, M.; Vranješ-Đurić, S.; Jovanović, L.; Čebulj-Kadunc, N.; Kobal, S.; Snoj, T.; Kirovski, D. Cortisol concentrations in hair, blood and milk of Holstein and Busha cattle. Slov. Vet. Res. 2017, 54, 163–172. [Google Scholar] [CrossRef]
- Schell, C.J.; Young, J.K.; Lonsdorf, E.V.; Mateo, J.M.; Santymire, R.M. Investigation of techniques to measure cortisol and testosterone concentrations in coyote hair. Zoo Biol. 2017, 36, 220–225. [Google Scholar] [CrossRef]
- Lafferty, D.J.; Laudenslager, M.L.; Mowat, G.; Heard, D.; Belant, J.L. Sex, Diet, and the Social Environment: Factors Influencing Hair Cortisol Concentration in Free-Ranging Black Bears (Ursus americanus). PLoS ONE 2015, 10, e0141489. [Google Scholar] [CrossRef]
- Cattet, M.; Macbeth, B.J.; Janz, D.M.; Zedrosser, A.; Swenson, J.E.; Dumond, M.; Stenhouse, G.B. Quantifying long-term stress in brown bears with the hair cortisol concentration: A biomarker that may be confounded by rapid changes in response to capture and handling. Conserv. Physiol. 2014, 1, cou026. [Google Scholar] [CrossRef]
- Bechshøft, T.Ø.; Sonne, C.; Dietz, R.; Born, E.W.; Novak, M.A.; Henchey, E.; Meyer, J.S. Cortisol levels in hair of East Greenland polar bears. Sci. Total Environ. 2011, 409, 831–834. [Google Scholar] [CrossRef]
- Fourie, N.H.; Brown, J.L.; Jolly, C.J.; Phillips-Conroy, J.E.; Rogers, J.; Bernstein, R.M. Sources of variation in hair cortisol in wild and captive non-human primates. Zoology 2016, 119, 119–125. [Google Scholar] [CrossRef]
- Martin, J.G.; Réale, D. Animal temperament and human disturbance: Implications for the response of wildlife to tourism. Behav. Process. 2008, 77, 66–72. [Google Scholar] [CrossRef]
- Roth, L.S.; Faresjö, Å.; Theodorsson, E.; Jensen, P. Hair cortisol varies with season and lifestyle and relates to human interactions in German shepherd dogs. Sci. Rep. 2016, 6, 19631. [Google Scholar] [CrossRef]
- Bacci, M.L.; Nannoni, E.; Govoni, N.; Scorrano, F.; Zannoni, A.; Forni, M.; Martelli, G.; Sardi, L. Hair cortisol determination in sows in two consecutive reproductive cycles. Reprod. Biol. 2014, 14, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Bethge, J.; Fietz, J.; Razafimampiandra, J.C.; Ruthsatz, K.; Dausmann, K.H. Season and reproductive activity influence cortisol levels in the Malagasy primate Lepilemur edwardsi. J. Exp. Zool. A. Ecol. Integr. Physiol. 2022, 337, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Salas, M.; Temple, D.; Abáigar, T.; Cuadrado, M.; Delclaux, M.; Enseñat, C.; Almagro, V.; Martínez-Nevado, E.; Quevedo, M.Á.; Carbajal, A.; et al. Aggressive behavior and hair cortisol levels in captive Dorcas gazelles (Gazella dorcas) as animal-based welfare indicators. Zoo Biol. 2016, 35, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Villagrán, M.; Ceva, M.; Machiñena, A.; Perdomo, M.; Berro, L.; Echaides, C.; Damián, J.P. The environment matters: Season and female contact affect the behavior of captive Addax nasomaculatus male antelope. Acta Ethol. 2023, 26, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Morales-Piñeyrúa, J.T.; Damián, J.P.; Banchero, G.; Blache, D.; Sant’Anna, A.C. Metabolic profile and productivity of dairy Holstein cows milked by a pasture-based automatic milking system during early lactation: Effects of cow temperament and parity. Res. Vet. Sci. 2022, 147, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Tallo-Parra, O.; Manteca, X.; Sabes-Alsina, M.; Carbajal, A.; Lopez-Bejar, M. Hair cortisol detection in dairy cattle by using EIA: Protocol validation and correlation with faecal cortisol metabolites. Animal 2015, 9, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- CIE. CIE, Commision Internationale d l’Éclairage, Colourimetry; Publ. No. 15; Bureau Central CIEA: Vienna, Austria, 1976. [Google Scholar]
- Sharma, A.; Umapathy, G.; Kumar, V.; Phillips, C.J.C. Hair Cortisol in Sheltered Cows and Its Association with Other Welfare Indicators. Animals 2019, 9, 248. [Google Scholar] [CrossRef]
- Medill, S.A.; Janz, D.M.; McLoughlin, P.D. Hair Cortisol Concentrations in Feral Horses and the Influence of Physiological and Social Factors. Animals 2023, 13, 2133. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, A.; Bailey, L.; Bandeira, V.; Dehnhard, M.; Fonseca, C.; de Sousa, L.; Jewgenow, K. Age, sex and storage time influence hair cortisol levels in a wild mammal population. PLoS ONE 2019, 14, e0221124. [Google Scholar] [CrossRef]
- Santangeli, A.; Wistbacka, R.; Morosinotto, C.; Raulo, A. Hair cortisol concentration in Siberian flying squirrels is unrelated to landscape and social factors. Naturwissenschaften 2019, 106, 29. [Google Scholar] [CrossRef]
- Zenth, F.; Corlatti, L.; Giacomelli, S.; Saleri, R.; Cavalli, V.; Andrani, M.; Donini, V. Hair cortisol concentration as a marker of long-term stress: Sex and body temperature are major determinants in wild-living Alpine marmots. Mamm. Biol. 2022, 102, 2083–2089. [Google Scholar] [CrossRef]
- Lutz, C.K.; Meyer, J.S.; Novak, M.A. Hair cortisol in captive corral-housed baboons. Gen. Comp. Endocrinol. 2021, 302, 113692. [Google Scholar] [CrossRef]
- Lavergne, S.G.; Peers, M.J.L.; Mastromonaco, G.; Majchrzak, Y.N.; Nair, A.; Boutin, S.; Boonstra, R. Hair cortisol as a reliable indicator of stress physiology in the snowshoe hare: Influence of body region, sex, season, and predator-prey population dynamics. Gen. Comp. Endocrinol. 2020, 294, 113471. [Google Scholar] [CrossRef]
- Garber, P.A.; McKenney, A.; Bartling-John, E.; Bicca-Marques, J.C.; De la Fuente, M.F.; Abreu, F.; Schiel, N.; Souto, A.; Phillips, K.A. Life in a harsh environment: The effects of age, sex, reproductive condition, and season on hair cortisol concentration in a wild non-human primate. PeerJ 2020, 8, e9365. [Google Scholar] [CrossRef]
- Dulude-de Broin, F.; Côté, S.D.; Whiteside, D.P.; Mastromonaco, G.F. Faecal metabolites and hair cortisol as biological markers of HPA-axis activity in the Rocky mountain goat. Gen. Comp. Endocrinol. 2019, 280, 147–157. [Google Scholar] [CrossRef]
- Bergamin, C.; Comin, A.; Corazzin, M.; Faustini, M.; Peric, T.; Scollo, A.; Gottardo, F.; Montillo, M.; Prandi, A. Cortisol, DHEA, and Sexual Steroid Concentrations in Fattening Pigs’ Hair. Animals 2019, 9, 345. [Google Scholar] [CrossRef] [PubMed]
- Terwissen, C.V.; Mastromonaco, G.F.; Murray, D.L. Influence of adrenocorticotrophin hormone challenge and external factors (age, sex, and body region) on hair cortisol concentration in Canada lynx (Lynx canadensis). Gen. Comp. Endocrinol. 2013, 194, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Grille, L.; Adrien, M.L.; Olmos, M.; Chilibroste, P.; Damián, J.P. Diet change from a system combining total mixed ration and pasture to confinement system (total mixed ration) on milk production and composition, blood biochemistry and behavior of dairy cows. Anim. Sci. J. 2019, 90, 1484–1494. [Google Scholar] [CrossRef] [PubMed]
- Pons, M.V.; Adrien, M.L.; Mattiauda, D.A.; Breijo, M.A.; Meikle, A.; Chilibroste, P.; Damián, J.P. Welfare of dairy cows in mixed feeding systems under two different conditions of confinement: Behavioural, biochemical and physiological indicators. App. Anim. Behav. Sci. 2023, 265, 105995. [Google Scholar] [CrossRef]
- Baldacchino, F.; Muenworn, V.; Desquesnes, M.; Desoli, F.; Charoenviriyaphap, T.; Duvallet, G. Transmission of pathogens by Stomoxys flies (Diptera, Muscidae): A review. Parasite 2013, 20, 26. [Google Scholar] [CrossRef]
- Vitela-Mendoza, I.; Cruz-Vázquez, C.; Solano-Vergara, J.; Orihuela-Trujillo, A. Short communication: Relationship between serum cortisol concentration and defensive behavioral responses of dairy cows exposed to natural infestation by stable fly, Stomoxys calcitrans. J. Dairy Sci. 2016, 99, 9912–9916. [Google Scholar] [CrossRef]
- Rochon, K.; Hogsette, J.A.; Kaufman, P.E.; Olafson, P.U.; Swiger, S.L.; Taylor, D.B. Stable fly (Diptera: Muscidae)—Biology, management, and research needs. J. Integr. Pest. Manag. 2021, 12, 38. [Google Scholar] [CrossRef]
- Pérez-Sarasqueta, A.L. Abundancia y Dinámica Poblacional de Mosca Doméstica (Musca domestica) y Mosca de los Establos (Stomoxys calcitrans) en dos Ecosistemas en el sur de Uruguay. Master’s Thesis, Facultad de Veterinaria, Universidad de la República, Montevideo, Uruguay, 2021. [Google Scholar]
- Krausman, P.R.; Casey, A.L. Addax nasomaculatus. Mamm. Species 2007, 807, 1–4. [Google Scholar] [CrossRef]
- Walsberg, G.E. Thermal effects of seasonal coat change in three subarctic mammals. J. Therm. Biol. 1991, 16, 291–296. [Google Scholar] [CrossRef]
- Stuart-Fox, D.; Newton, E.; Clusella-Trullas, S. Thermal consequences of colour and near-infrared reflectance. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372, 20160345. [Google Scholar] [CrossRef]
- Caro, T.; Mallarino, R. Coloration in Mammals. Trends Ecol. Evol. 2020, 35, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Grymowicz, M.; Rudnicka, E.; Podfigurna, A.; Napierala, P.; Smolarczyk, R.; Smolarczyk, K.; Meczekalski, B. Hormonal Effects on Hair Follicles. Int. J. Mol. Sci. 2020, 21, 5342. [Google Scholar] [CrossRef]
- Bubenik, G.A.; Bubenik, A.B. Seasonal variations in hair pigmentation of white-tailed deer and their relationship to sexual activity and plasma testosterone. J. Exp. Zool. 1985, 235, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Ebling, F.J. The effects of cyproterone acetate and oestradiol upon testosterone stimulated sebaceous activity in the rat. Acta Endocrinol. 1973, 72, 361–365. [Google Scholar] [CrossRef]
- Duncan, M.J.; Goldman, B.D. Hormonal regulation of the annual pelage color cycle in the Djungarian hamster, Phodopus sungorus. II. Role of prolactin. J. Exp. Zool. 1984, 230, 97–103. [Google Scholar] [CrossRef]
- Hirobe, T.; Kiuchi, M.; Wakamatsu, K.; Ito, S. Estrogen increases hair pigmentation in female recessive yellow mice. Zool. Sci. 2010, 27, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Itou, T.; Ito, S.; Wakamatsu, K. Effects of Aging on Hair Color, Melanosomes, and Melanin Composition in Japanese Males and Their Sex Differences. Int. J. Mol. Sci. 2022, 23, 14459. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).