Abstract
Nematophagous fungi are a biological control tool used against gastrointestinal nematodes in livestock. These fungi prey on free-living larvae in faeces and could be affected by active drugs excreted post-treatment. This study aimed to determine in vitro and under environmental conditions the effect of the following anthelmintics on the fungus Duddingtonia flagrans: ivermectin, levamisole, albendazole, fenbendazole and ricobendazole. The in vitro effect of anthelmintics on fungal growth and predatory capacity was assessed in corn meal agar and coprocultures, respectively. Ivermectin (1, 2 and 10 ppm), fenbendazole (0.027, 0.054 and 1 ppm) and albendazole (1 ppm) significantly affected fungal development. The fungal efficacy against L3 was high in the control and levamisole coprocultures but decreased significantly in the presence of albendazole, fenbendazole, ricobendazole and ivermectin. The impact of levamisole on D. flagrans was further assessed under environmental conditions in autumn and winter; the fungal efficacy measured in faecal pats and the surrounding herbage was not affected by levamisole at any time. This study shows that using albendazole, fenbendazole, ricobendazole or ivermectin may compromise fungal activity, as these drugs affect the free-living stages of nematodes in faeces, but levamisole can be safely considered in parasite control strategies involving D. flagrans and anthelmintic treatments.
1. Introduction
Gastrointestinal nematodes (GIN) affecting cattle represent one of the major health and economic hurdles for grass-fed livestock production. One useful tool to tackle this problem is the use of nematophagous fungi, such as Duddingtonia flagrans, as biological control agents against these parasites [1]. Thus, biological control becomes a component of an integrated control strategy to improve animal health as well as the sustainability of cattle production. D. flagrans acts on the free-living larvae of GIN in the environment, specifically in faecal masses. Although anthelmintic drugs act on parasitic stages inside the host, most of them are eliminated in faeces in their active form, thus potentially interfering with the fungal effect on the free-living larval stages. This means that this possible reaction of the fungi to anthelmintics should be considered when planning an integrated parasite control programme [2]. In vitro studies have shown that D. flagrans is susceptible to most widely used anthelmintics and have determined either the minimum inhibitory concentration (MIC) for fungal growth or the effective middle concentrations (EC50) inhibiting spore germination [3,4]. But going a step further, there is a lack of information on how the active forms of anthelmintics impact the fungal activity when eliminated in faeces, which is the substrate where the nematophagous fungi exert their biological control capabilities on GIN. Therefore, it is necessary to fill this information gap if both GIN control measures—biological control and anthelmintic treatments—are to be used in conjunction. The aim of this study was to determine, both in vitro and under environmental conditions, the effect of the most commonly used anthelmintics on the nematophagous fungus D. flagrans.
2. Materials and Methods
2.1. Fungal Material
The local isolate D. flagrans 03/99 was used for the study [5]. This isolate is maintained at the Laboratorio de Parasitología y Enfermedades Parasitarias, Facultad de Ciencias Veterinarias, UNCPBA, Tandil. Fresh fungal chlamydospores were recovered from enriched Saboraud agar cultures that had been incubated for 28 days at 27 °C [6] and were kept at 4 °C until used.
2.2. Anthelmintics
The active ingredients of the following anthelmintics were used: levamisole (LEV), albendazole (ABZ), fenbendazole (FBZ), ricobendazole (RBZ) and ivermectin (IVM). Pure reference standards (97–99% purity) of ABZ, RBZ and FBZ were purchased from Toronto Chemicals Research Inc. (Toronto, ON, Canada), and IVM and LVM were obtained from Sigma Chemical Co. (St. Louis, MO, USA). The active ingredients were used diluted in methanol or dimethylsulfoxide (DMSO) at concentrations either found in faeces after their administration [7,8,9,10] or previously studied [3] (see Section 2.4).
2.3. In Vitro Effect of Anthelmintics on D. flagrans Predatory Capability in Faecal Media
Faeces from organically raised calves harbouring a GIN natural infection were used for this step; the faeces had a GIN egg count of 208 eggs per gram. A modification of a standard technique [11] was used to set up groups of 10 coprocultures each for each anthelmintic diluted in methanol at the following concentrations: LEV, 1 ppm; ABZ, 1 ppm; FBZ, 1 ppm; RBZ, 1 ppm; and IVM, 2 ppm. Chlamydospores of D. flagrans were added to the cultures at a concentration of 11,000 chlamydospores/g faeces. Groups of coprocultures as positive controls were cultures with anthelmintics (+), diluent (-) and chlamydospores (-); cultures with anthelmintics (-), diluent (+) and chlamydospores (-); and cultures with anthelmintics (-), diluent (-) and chlamydospores (+). Coprocultures without the addition of any agent represented the negative control. All coprocultures were set up at the same time using 10 g of faeces each and incubated at room temperature (20–27 °C) for 14 days. After third-stage larvae (L3) were recovered from cultures, they were kept at 4 ºC for posterior counting and identification [12].
2.4. In Vitro Effect of Anthelmintics on D. flagrans Growth
This phase was conducted to determine the growth of D. flagrans in the presence of anthelmintics and comprised two assays. The first assay coincided with the fungal predatory capability assay described above; thus, the same concentrations of anthelmintics listed in Section 2.3 were used. In view of the results obtained (see Section 3.2), a second assay was set up further testing different concentrations of most of the anthelmintics, as follows: ABZ, 0.027 and 0.054 ppm (corresponding to the MIC of ABZ for fungal growth [3]); FBZ, 0.027 and 0.054 ppm, and RBZ, 2.77 ppm (corresponding to the maximum concentration of the metabolite albendazole sulphoxide (ABZ.SO) in faeces after oral treatment with ABZ [9]); and IVM, 1 and 10 ppm (corresponding to IVM concentration in faeces—as ppm of dry weight—after subcutaneous and pour-on administration [7,8]). The 1 ppm LEV concentration remained unchanged given that higher concentrations are not detected in faeces after its administration to cattle.
For both assays, each anthelmintic diluted in methanol was added to 2% corn meal agar (CMA) in sufficient quantities so the final concentrations in the agar were the required ones described above. The agar was then poured on 9 cm Petri dishes. One cm2 of CMA containing fresh D. flagrans mycelium was then added on the centre (assay 1) or on the border (assay 2) of each dish. CMA plates with D. flagrans and without anthelmintics were used as control of fungal growth while plates with fungus and methanol were used as control of the diluent. The diluent DMSO was also tested in assay 2 to determine its possible use in the next steps and future studies. A total of 84 (12 per group, assay 1) and 110 Petri dishes (10 per group, assay 2) were incubated at 27 °C, and the radial growth of the fungus was recorded every 24 h along two axes marked on the plates using a digital Vernier calliper until the mycelia in the control group reached the edge of the plates. The daily growth rate was then estimated as continuous growth in millimetres per day using the following formula: Total length in n days/(n days—days of lag phase) [13]. The lag phase is defined as the first phase of a fungal growth curve, where little or no observable growth occurs [14]. Those plates where bacterial contamination developed at any stage of the incubation period were discarded from the analysis.
2.5. Effect of LEV on D. flagrans in Faeces under Environmental Conditions
The results obtained from the in vitro studies detailed above (see results in Section 3.1 and Section 3.2) plus those from a previous study [15] were the basis for this phase. The aim was to determine whether treating calves with LEV would interfere with the biological control process occurring inside the faecal mass under natural environmental conditions. The study took place on an experimental plot of the Facultad de Ciencias Veterinarias, UNCPBA, Tandil, in the two seasonal occasions of late summer–early autumn and winter, given that these are the two most critical points in the development of gastrointestinal nematodosis in calves under local grazing conditions. Faeces containing GIN eggs were collected from naturally infected calves from an organic family-run farm. After thoroughly mixing the faeces, egg counts were estimated in triplicate, resulting in an average of 160 and 200 eggs per gram in autumn and winter, respectively. In each seasonal study, faeces were then divided in the following four groups of ten 500 g faecal pats each: (1) D. flagrans + LEV diluted in DMSO, (2) D. flagrans + DMSO (as control of the diluent), (3) D. flagrans (as positive control), and (4) control with no added agents (as negative control). Chlamydospores of D. flagrans were added to faeces in groups 1, 2 and 3 at a concentration of 11,000 chlamydospores/g faeces. LEV diluted in DMSO was added to group 1 at a concentration of 1 ppm, while DMSO in the amount of 1 mL/kg faeces was added to group 2.
The 40 faecal pats were deposited each season as described in an earlier report [15] on a parasite-free pasture composed of red clover (Trifolium pratense), white clover (T. repens), orchardgrass (Dactylis glomerata) and perennial ryegrass (Lolium perenne). Six weeks post-deposition, each faecal pat was carefully picked up, weighed and baermmanised to extract the L3 following an established technique [16]. Herbage was collected under and around each faecal pat as described earlier [17] to recover the L3 migrated from the pats. Recovery of the L3 was achieved by washing the herbage in a cement mixer for ten minutes, followed by sieving the washing liquid through a nylon sieve, mesh size 20 μm, where L3 and small debris were retained. The L3 were then separated from debris by baermannisation overnight at room temperature. The L3 recovered from faecal pats and herbage were kept at 4 °C until counted and identified [12].
2.6. Statistical Analysis
Data analysis was performed using the software GraphPad Prism 9.4.1 for Windows, GraphPad Software, San Diego, CA, USA, www.graphpad.com. Data were subjected to non-parametric one-way ANOVA followed by Dunn’s multiple comparison tests with confidence level 0.05.
The inhibition of fungal growth was calculated using the following formula: Inhibition % = 100 – ((average daily growth rate of treated group/average daily growth rate control group) × 100).
The reduction in L3 numbers was calculated using the following formula: Reduction % = 100 – ((average L3 of treated group/average L3 control group) × 100).
3. Results
3.1. In Vitro Effect of Anthelmintics on D. flagrans Predatory Capability in Faecal Media
The reduction in the numbers of L3 obtained from coprocultures due to the predatory activity of D. flagrans was significant in those cultures containing the fungus alone (91.8%, p < 0.001), the fungus and LEV (90.1%, p < 0.001) and the fungus and methanol (89.1%, p < 0.001). None or negligible numbers of L3 were obtained from cultures with ABZ, FBZ, RBZ or IVM, either from those containing D. flagrans or the controls without fungus (Figure 1); therefore, the effect of these four anthelmintics on fungal predation could not be assessed.
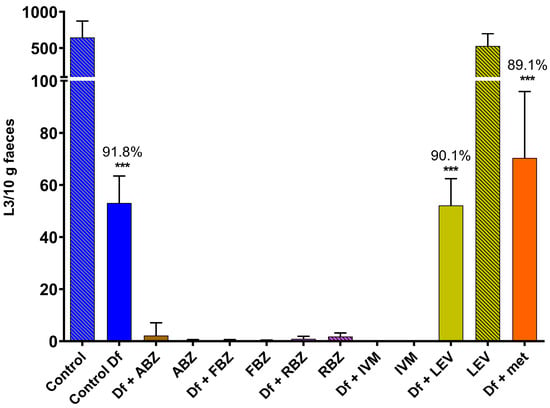
Figure 1.
Numbers of L3 recovered from coprocultures containing GIN eggs to which chlamydospores of D. flagrans and/or anthelmintics diluted in methanol had been added. Each bar represents the mean (n = 10) with its standard deviation, expressed as L3/10 g faeces. ***: p < 0.001; Df: D. flagrans; ABZ: albendazole; FBZ: fenbendazole; RBZ: ricobendazole; IVM: ivermectin; LEV: levamisole; met: methanol.
The prevalence of GIN genera in those groups where L3 were found was 58.5–77.6% for Ostertagia, 6.6–25.3% for Cooperia, 9.9–14.4% for Haemonchus, 0.7–4.4% for Trichostrongylus and 0.2–2.1% for Oesophagostomum. This genera prevalence was not altered between controls and anthelmintic- or methanol-added groups.
3.2. In Vitro Effect of Anthelmintics on D. flagrans Growth
The D. flagrans mycelia in all plates of the control group reached the edge of the plates by day 9 in assay 1 (Figure 2) and by day 11 in assay 2 (Figure 3). In both assays the rate of fungal growth was very similar for the control, methanol, RBZ and LEV groups. The fungus exposed to IVM grew significantly slower than the control (p < 0.01–p < 0.05) in all the tested concentrations. FBZ not only significantly delayed (p < 0.0001–p < 0.001) the growth of D. flagrans in all concentrations tested but fully inhibited mycelial growth in 75%, 60% and 40% of the plates for 1, 0.054 and 0.027 ppm, respectively. ABZ only affected the mycelial growth at 1 ppm (p < 0.0001) (Figure 2). The addition of DMSO to the CMA plates did not alter the rate of fungal growth (Figure 3).
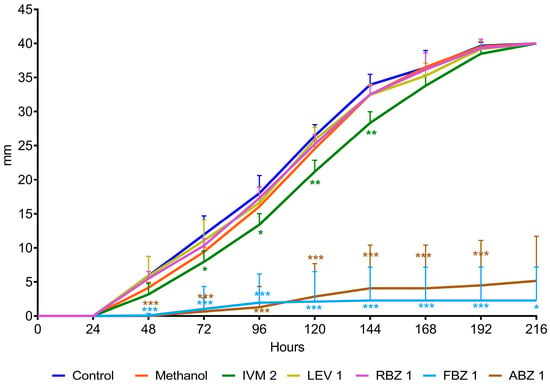
Figure 2.
Radial growth of D. flagrans in CMA plates containing anthelmintics diluted in methanol (assay 1). Each point shows the mean (n = 12) and its standard deviation, expressed as mm of mycelial growth every 24 h. *: p < 0.05; **: p < 0.01; ***: p < 0.001. Control: plates with fungus and without anthelmintics; Methanol: plates with fungus and only the diluent; IVM 2: ivermectin 2 ppm; LEV 1: levamisole 1 ppm; RBZ 1: ricobendazole 1 ppm; FBZ 1: fenbendazole 1 ppm; ABZ 1: albendazole 1 ppm.
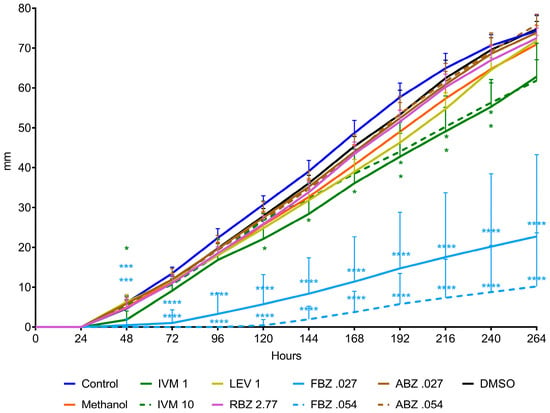
Figure 3.
Radial growth of D. flagrans in CMA plates containing anthelmintics diluted in methanol (assay 2). Each point shows the mean (n = 10) and its standard deviation, expressed as mm of mycelial growth every 24 h. *: p < 0.05; **: p < 0.01; ***: p < 0.001; ****: p < 0.0001. Control: plates with fungus and without anthelmintics; Methanol: plates with fungus and only the diluent; IVM 1: ivermectin 1 ppm; IVM 10: ivermectin 10 ppm; LEV 1: levamisole 1 ppm; RBZ 2.77: ricobendazole 2.77 ppm; FBZ0.027: fenbendazole 0.027 ppm; FBZ 0.054: fenbendazole 0.054 ppm; ABZ 0.027: albendazole 0.027 ppm; ABZ 0.054: albendazole 0.054 ppm; DMSO: plates with fungus and dimethylsulfoxide.
The average growth rate of D. flagrans exposed to the different active ingredients of the tested anthelmintics is presented in Figure 4 for assay 1 and Figure 5 for assay 2. In assay 1, the drugs that significantly reduced the fungal growth in comparison with the control group were IVM 2 ppm (11.3%, p = 0.0210), ABZ 1 ppm (85.9%, p < 0.0001), and FBZ (93.9%, p < 0.0001). In assay 2, the fungal growth was significantly reduced by IVM 1 ppm (21.8%, p = 0.0148), IVM 10 ppm (20.3%, p = 0.0193), FBZ 0.027 ppm (71.4%, p < 0.0001) and FBZ 0.054 ppm (81.3.7%, p < 0.0001).
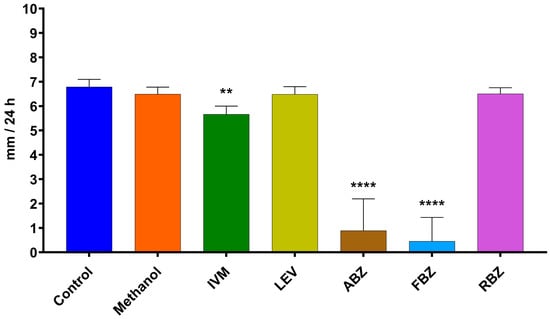
Figure 4.
Average daily growth rate of D. flagrans growing in CMA plates containing anthelmintics diluted in methanol (assay 1). Each bar represents the mean (n = 12) with its standard deviation, expressed as mm/24 h. **: p < 0.001; ****: p < 0.0001. Control: plates with fungus and without anthelmintics; Methanol: plates with fungus and only the diluent; IVM: ivermectin 2 ppm; LEV: levamisole 1 ppm; ABZ: albendazole 1 ppm; FBZ: fenbendazole 1 ppm; RBZ: ricobendazole 1 ppm.
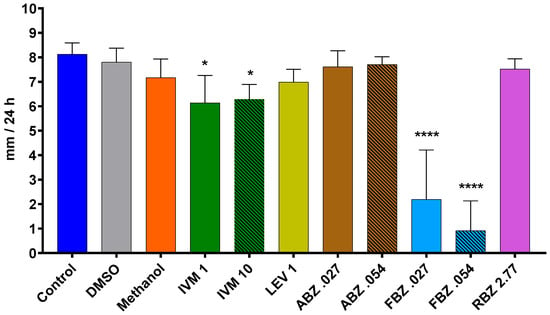
Figure 5.
Average daily growth rate of D. flagrans growing in CMA plates containing anthelmintics diluted in methanol (assay 2). Each bar represents the mean (n = 10) with its standard deviation, expressed as mm/24 h. *: p < 0.05; ****: p < 0.0001. Control: plates with fungus and without anthelmintics; DMSO: plates with fungus and dimethylsulfoxide; Methanol: plates with fungus and only the diluent; IVM 1: ivermectin 1 ppm; IVM 10: ivermectin 10 ppm; LEV 1: levamisole 1 ppm; ABZ 0.027: albendazole 0.027 ppm; ABZ 0.054: albendazole 0.054 ppm; FBZ 0.027: fenbendazole 0.027 ppm; FBZ 0.054: fenbendazole 0.054 ppm; RBZ 2.77: ricobendazole 2.77 ppm.
3.3. Effect of LEV on D. flagrans in Faeces under Environmental Conditions
The numbers of L3 recovered from faecal pats and herbage revealed that the presence of LEV and DMSO in faeces did not affect the viability or the nematophagous capabilities of D. flagrans (Table 1). The range of fungal efficacy, whether or not in the presence of the anthelmintic and/or the diluent, was 63.64–90.91% in faeces and 74.75–100% in herbage during autumn, while in winter the fungal efficacy was 97.81–100% in faeces and 94.59–100% in herbage.

Table 1.
Numbers of L3 recovered from faeces (expressed as L3/g faeces) and herbage (expressed as L3/kg dry matter (DM)) 6 weeks post deposition of faecal pats in autumn and winter. Each number represents the mean (n = 10), with standard deviations shown in brackets. Df: D. flagrans; DMSO: dimethylsulfoxide; LEV: levamisole; Red. %: reduction percentage of L3.
No differences were detected between groups within each seasonal study with regard to the weight of faecal pats after 6 weeks in the environment (range: 131–146 g and 45–75 g in autumn and winter, respectively) or the weight of dry grass from the herbage samples (range: 0.18–0.20 kg and 0.18–0.31 kg in autumn and winter, respectively).
4. Discussion
The results from this study advance the knowledge on the action of anthelmintics on the nematophagous fungus D. flagrans, thus helping to elucidate whether synthetic anthelmintics can be used in livestock production enterprises where biological control is applied.
In relation to the effect of anthelmintics on the fungal predatory capacity, the larval reductions obtained in coprocultures with D. flagrans when in the presence of LEV compared to the control groups show that the fungus was not affected by either this anthelmintic or the diluent used. The larval reductions of >90% coincide with previous in vitro reports that used the fungus at the same concentration as in the present study [18]. Likewise, increasing concentrations of LEV of 1.25 to 12,500 ppm did not show any harmful effect on chlamydospore germination [4]. On the other hand, any possible effect of either IVM or the different benzimidazoles (BDZ) tested could not be determined in coprocultures given the ovicidal and larvicidal activity of BDZ [19] and IVM [20], respectively. Other authors [21] have determined that BDZ affect Ostertagia ostertagi eggs in lower concentrations than the ones used in the present study. Similarly, an ex vivo evaluation of thiabendazole at a concentration of 0.1 mg/g faeces (100 ppm) in animals experimentally infected with Trichostrongylus colubriformis that received D. flagrans failed to determine the effect of the drug on the fungus due to lack of larval development in the anthelmintic-treated groups [22].
The rate of mycelial radial growth in the control groups is in agreement with previous studies [23,24], where different isolates of D. flagrans incubated at 30 °C grew 50–60 mm per week. In this study, it was seen that the fungal growth was not affected by the two diluents tested, i.e., methanol and DMSO, which is contrary to earlier observations [22], where DMSO reportedly impaired the fungal growth.
The mycelial growth of D. flagrans in the agar plates was also not affected by LEV. Although the MIC of LEV for D. flagrans growth was previously determined at 0.117 ppm [3], the much higher concentration used in the present study (1 ppm) did not show any inhibitory effect. Another study [25] reported that a massive concentration of 22,300 ppm of LEV inhibited the growth of two other nematophagous fungi, Arthrobotrys conoides and A. robusta; however, there are several methodological issues that cast doubts on their results, not least that the water-insoluble anthelmintics tested were incorrectly diluted in water. Additionally, the inhibitory effects of 22,300 ppm of LEV would seldom have any practical significance, since such concentration cannot be detected in faeces given that urine is the main elimination route for this drug [10].
The BDZ used in the control of GIN are derivatives of thiabendazole (TBZ), which is not only a parasiticide but a fungicide as well [26]. Thus, it is expected that BDZ have an impact on the growth and development of nematophagous fungi. Concentrations as low as 1 ppm of TBZ significantly reduced the mycelial growth of D. flagrans in agar Sabouraud, while total growth inhibition was recorded at 10 ppm [22]. Another benzimidazolic fungicide tested, carbendazim, inhibited the growth of the egg-parasitic fungus Pochonia suchlasporia at concentrations of 0.5 to 4.5 ppm [27,28]. Regarding those BDZ used as anthelmintics for livestock, other studies have shown that 160 ppm of ABZ inhibited the growth of three egg-parasitic fungal species [29], while at 0.5 ppm of the same drug, P. suchlasporia developed after an initial lag phase of 120 h and reached 78 mm by day 29 [28]. The drugs ABZ and RBZ (ABZ.SO) were detected in faeces up to 72 h after lambs were treated with ABZ at a dose rate of 10 mg/kg bw; the Cmax concentrations were 7.7 ppm (recorded at 9 h post-treatment) and 2.8 ppm (recorded at 7.5 h post-treatment) for ABZ and RBZ, respectively [9]. In the present study, ABZ at 1 ppm significantly impaired the mycelial growth of D. flagrans, while RBZ at 1 and 2.77 ppm did not diminish fungal development. This could be attributed to the fact that, as an ABZ metabolite, RBZ shows less affinity for the β-tubulin protein and is less active than ABZ [30]. The lower concentrations of ABZ tested (0.027 and 0.054 ppm) had no detrimental effect on fungal growth despite a previous study recording the minimum inhibitory concentration of this drug for D. flagrans as 0.031 ppm [3]. On the other hand, the fungal growth was either significantly reduced or completely inhibited in the presence of FBZ at the three concentrations tested (0.027, 0.057 and 1 ppm). A hypothetical explanation for this different effect could be that FBZ has a closer chemical structure to TBZ—a strong fungicide—in comparison to ABZ. Alternatively, the differences in the antifungal activity of drugs in the same chemical group could simply be a matter of different molecular structures, as described in relation to some phytopathogenic fungi, where the EC50 was much lower for FBZ than ABZ [31]. Regardless the reason, the results obtained in this study are in accordance with those from a more recent in vitro study showing that FBZ was the anthelmintic drug most affecting D. flagrans [4].
Thus, it is difficult to envisage the use of this anthelmintic within an integrated parasite control approach involving biological control as well. Nevertheless, solid information from in vivo studies is needed to shed more light on this.
One of the most widely used anthelmintics is IVM, which, in turn, is the one that has generated the highest levels of anthelmintic resistance and is the most ecotoxic [7,32,33,34]. The active ingredient of this drug appears in faeces in concentrations of 2 ppm when administered subcutaneously or up to 10 ppm after pour-on administration [7,8,35,36]. Therefore, the in vitro growth-inhibiting action of IVM at 160 ppm previously recorded against three fungal species [29] would not affect the fungi in the substrate where biological control takes place. The fungal growth rate in the present study was 11.3 to 21.8% slower than the control but was never fully inhibited at concentrations of 1, 2 and 10 ppm. This contrasts sharply with a previously reported MIC for the mycelial growth of D. flagrans of 0.5 ppm of IVM [3]. In agreement with the present results, abamectin—another member of the avermectin family—used at 1 ppm reduced the mycelial growth of some species of entomopathogenic fungi by 16–36% [37]. In vitro assays of Rhabditis spp., a nematode causing otitis in cattle, showed that the combined treatment of D. flagrans plus IVM was less effective (43.2%) than the treatment with the fungus alone (64.2%) [38]. All these results appear to indicate that a strategy of simultaneous use of nematophagous fungi and IVM would not be advisable against GIN in cattle. However, as stated above for FBZ, results from in vivo studies should be conducted to elucidate the matter further.
The effect of LEV on D. flagrans was determined in early autumn and winter under environmental conditions, given that these are the most challenging seasons for the local grazing cattle production systems in relation to the risk of clinical helminthoses [39]. The larval reduction effect of the fungus was 64–98% in faecal pats and 89–95% on the surrounding herbage. These results are similar to earlier ones [15] showing the high predatory capacity of the fungus in both faeces (79–95% efficacy) and pasture (80–100% and 88% efficacy). This study indicates that the combined used of the D. flagrans with LEV would not negatively affect the biological control of GIN. A field study using a combination of administration of D. flagrans and selective targeted treatments in sheep showed that biological plus chemical control of GIN was highly effective compared to chemical control alone [40]. In view of this and the results obtained under natural conditions in the present study, similar results with such an association could be expected in grazing cattle systems.
Regarding the simultaneous use of nematophagous fungi and BDZ, two separate in vivo studies in small ruminants fed fungal chlamydospores showed that D. flagrans did not appear in faeces before 96 h post-treatment with ABZ [39] or thiabendazole [22]. Likewise, the detection in faeces of this fungus and the egg-parasitic fungus Verticillium chamydosporium was not possible when the plasmatic concentration of RBZ (ABZ.SO) was 1 ppm or higher [41,42].
In a context of natural parasitic reinfections (e.g., animals treated and left grazing on the same GIN-infected pasture) an effective treatment with LEV (a drug with very low blood persistence [10] would mean that no GIN eggs would appear in faeces until 2–3 weeks post-treatment, so the fungus could only start exerting its nematophagous activity at that time. Likewise, under field conditions of GIN susceptible to IVM, the appearance of GIN eggs would not be expected in faeces for 6–9 weeks post-treatment if IVM is administered subcutaneously at a dose rate of 200 μg/kg BW [43]. Therefore, it would be advisable to wait until that time to start the fungal administration to the animals. It must be kept in mind that the timing between anthelmintic treatments (especially with LEV, ABZ and RBZ) and the start (or re-start) of administration of fungal chlamydospores would be much shorter—even simultaneously combined—in a context of anthelmintic resistance, when GIN eggs are present in faeces after a failed treatment. The above timing recommendations should be followed if the whole herd is in need of anthelmintic treatment, as it would be in the presence of clinical parasitosis or when the faecal egg count-based monitoring indicates the need for treatment. Should this be the case, the resumption of fungal administration would depend on the epidemiological context of the disease as well as the grazing conditions, e.g., whether the animals could still be at risk of L3 build-up on pasture in the weeks to come and whether the herbage availability is at its lowest. On the other hand, under a targeted selective treatment programme, when just a few animals are treated with anthelmintics, there would be no need to interrupt the administration of fungal chlamydospores to the whole herd. Therefore, the use of anthelmintics in any farm where the biological control agent is applied must be strategic and will depend on the time of the year (or grazing season), the parasitic burden—as indirectly monitored by faecal egg counts or other indicators—and the potential appearance of clinical parasitosis.
Lastly, any attempt to design an integrated parasite control programme by including different management strategies such as biological control, anthelmintic treatment, rotational grazing, etc. must take into consideration the epidemiology of the GIN present in a particular geographical area and, as well, must be accompanied by a regular monitoring regime of faecal egg counts. It must be remembered that the foundation of an integrated parasite control programme is the combination of control strategies with herd management practices so as to rationalise the need for using conventional anthelmintics in order to preserve their efficacy in the long run as well as reducing the risk of residues in animal products and the risks of ecotoxicity.
5. Conclusions
The simultaneous combination of D. flagrans and anthelmintics does not seem feasible. This is principally because, if GIN are susceptible to the chosen drug, there are not free-living larvae in faeces immediately after the anthelmintic treatment. Likewise, BDZ and IVM are still active after their faecal excretion, thus affecting the development of the nematophagous fungus. However, the use of biological control and anthelmintic treatments under an integrated parasite control system is possible. The correct timing between anthelmintic treatment and fungal administration should be observed to obtain the best results.
Author Contributions
Conceptualization, S.Z., C.S. and S.F.; methodology, S.Z., F.S. and S.F.; formal analysis, S.Z. and S.F.; investigation, S.Z., F.S., L.I., I.G., P.D., L.C., M.J., C.S. and S.F.; resources, C.S. and S.F.; data curation, S.F., S.Z., F.S. and C.S.; writing—original draft preparation, S.Z.; writing—review and editing, S.Z., F.S., L.I., C.S. and S.F.; visualization, S.Z., F.S. and S.F.; supervision, S.F. and C.S.; project administration, C.S. and S.F.; funding acquisition, C.S. and S.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Agencia Nacional de Promoción Científica y Tecnológica, Fondo para la Investigación Científica y Tecnológica, grant number PICT-2017-4030.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors thank Santiago Leeson for allowing access to his family-run farm to collect faeces from the herd. Gisele Bernat is thanked for her laboratory assistance. Peter Purslow is also thanked for his language editing help with the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Araújo, J.V.; Braga, F.R.; Mendoza-de-Gives, P.; Paz-Silva, A.; Vilela, V.L.R. Recent advances in the control of helminths of domestic animals by helminthophagous fungi. Parasitologia 2021, 1, 168–176. [Google Scholar] [CrossRef]
- Szewc, M.; De Waal, T.; Zintl, A. Biological methods for the control of gastrointestinal nematodes. Vet. J. 2021, 268, 105602. [Google Scholar] [CrossRef]
- Vieira, J.N.; Maia Filho, F.S.; Ferreira, G.F.; Mendes, J.F.; Gonçalves, C.L.; Villela, M.M.; Pereira, D.I.B.; Nascente, P.S. In vitro susceptibility of nematophagous fungi to antiparasitic drugs: Interactions and implications for biological control. Brazilian J. Biol. 2016, 77, 476–479. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, N.; Gong, P.; Li, J.; Wang, X.; Li, X.; Wang, F.; Cai, K.; Zhang, X. In vitro assays on the susceptibility of four species of nematophagous fungi to anthelmintics and chemical fungicides/antifungal drug. Lett. Appl. Microbiol. 2021, 73, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Saumell, C.A.; Fernández, A.S.; Fusé, L.A.; Rodríguez, M.; Sagüés, M.F.; Iglesias, L.E. Nematophagous fungi from decomposing cattle faeces in Argentina. Rev. Iberoam. Micol. 2015, 32, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Sagüés, M.F.; Fusé, L.A.; Iglesias, L.E.; Moreno, F.C.; Saumell, C.A. Optimization of production of chlamydospores of the nematode-trapping fungus Duddingtonia flagrans in solid culture media. Parasitol. Res. 2012, 112, 1047–1051. [Google Scholar] [CrossRef]
- Iglesias, L.E.; Saumell, C.A.; Fernández, A.S.; Fusé, L.A.; Lifschitz, A.L.; Rodríguez, E.M.; Steffan, P.E.; Fiel, C.A. Environmental impact of ivermectin excreted by cattle treated in autumn on dung fauna and degradation of faeces on pasture. Parasitol. Res. 2006, 100, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Sommer, C.; Steffansen, B. Changes with time after treatment in the concentrations of ivermectin in fresh cow dung and in cow pats aged in the field. Vet. Parasitol. 1993, 48, 67–73. [Google Scholar] [CrossRef]
- Prchal, L.; Podlipná, R.; Lamka, J.; Dědková, T.; Skálová, L.; Vokřál, I.; Lecová, L.; Vaněk, T.; Szotáková, B. Albendazole in environment: Faecal concentrations in lambs and impact on lower development stages of helminths and seed germination. Environ. Sci. Pollut. Res. 2016, 23, 13015–13022. [Google Scholar] [CrossRef]
- Baggot, J.D.; McKellar, Q.A. The absorption, distribution and elimination of anthelmintic drugs: The role of pharmacokinetics. J. Vet. Pharmacol. Ther. 1994, 17, 409–419. [Google Scholar] [CrossRef]
- Fiel, C.; Steffan, P.; Ferreyra, D. Diagnóstico de las Parasitosis más Frecuentes de los Rumiantes: Técnicas de Diagnóstico e Interpretación de los Resultados; Abad Benjamín: Tandil, Argentina, 2011; ISBN 9789873315022. [Google Scholar]
- Niec, R. Cultivo e identificación de larvas infectantes de nematodes gastrointestinales del bovino y ovino. Man. Técnico 1968, 3, 1–37. [Google Scholar]
- Fernández, A.S.; Larsen, M.; Wolstrup, J.; Grønvold, J.; Nansen, P.; Bjørn, H. Growth rate and trapping efficacy of nematode-trapping fungi under constant and fluctuating temperatures. Parasitol. Res. 1999, 85, 661–668. [Google Scholar] [CrossRef]
- Griffin, D.H. Fungal Physiology, 2nd ed.; Wiley-Liss: New York, NY, USA, 1994. [Google Scholar]
- Fernández, S.; Zegbi, S.; Sagües, F.; Iglesias, L.; Guerrero, I.; Saumell, C. Trapping behaviour of Duddingtonia flagrans against gastrointestinal nematodes of cattle under year-round grazing conditions. Pathogens 2023, 12, 401. [Google Scholar] [CrossRef] [PubMed]
- Grønvold, J. Rain splash dispersal of third-stage larvae of Cooperia spp. (trichostrongylidae). J. Parasitol. 1984, 70, 924–926. [Google Scholar] [CrossRef] [PubMed]
- Fernández, A.S.; Larsen, M.; Nansen, P.; Grønvold, J.; Henriksen, S.A.; Wolstrup, J. Effect of the nematode-trapping fungus Duddingtonia flagrans on the free-living stages of horse parasitic nematodes: A plot study. Vet. Parasitol. 1997, 73, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Zegbi, S.; Sagües, F.; Saumell, C.; Guerrero, I.; Iglesias, L.; Fernández, S. In vitro efficacy of different concentrations of Duddingtonia flagrans on varying egg densities of gastrointestinal nematodes of cattle. Exp. Parasitol. 2021, 230, 108156. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, R. In vitro and in vivo studies on the ovicidal activity of fenbendazole. Res. Vet. Sci. 1978, 25, 263–265. [Google Scholar] [CrossRef]
- Miller, J.E.; Morrison, D.G. Effect of fenbendazole and ivermectin on development of strongylate nematode eggs and larvae in calf feces. Vet. Parasitol. 1992, 43, 265–270. [Google Scholar] [CrossRef]
- Borgsteede, F.H.M.; Geerts, S.; de Deken, R.; Kumar, V.; Brandt, J. Studies on an Ostertagia ostertagi strain suspected to be resistant to benzimidazoles. Vet. Parasitol. 1992, 41, 85–92. [Google Scholar] [CrossRef]
- Paraud, C.; Pors, I.; Chartier, C. Activity of Duddingtonia flagrans on Trichostrongylus colubriformis larvae in goat feces and interaction with a benzimidazole treatment. Small Rumin. Res. 2004, 55, 199–207. [Google Scholar] [CrossRef]
- Grønvold, J.; Nansen, P.; Henriksen, S.A.; Larsen, M.; Wolstrup, J.; Bresciani, J.; Rawat, H.; Fribert, L. Induction of traps by Ostertagia ostertagi larvae, chlamydospore production and growth rate in the nematode-trapping fungus Duddingtonia flagrans. J. Helminthol. 1996, 70, 291–297. [Google Scholar] [CrossRef]
- Wang, B.B.; Zhang, N.; Gong, P.T.; Li, J.H.; Yang, J.; Zhang, H.B.; Zhang, X.C.; Cai, K.Z. Morphological variability, molecular phylogeny, and biological characteristics of the nematophagous fungus Duddingtonia flagrans. J. Basic Microbiol. 2019, 59, 645–657. [Google Scholar] [CrossRef]
- Araújo, J.V.; Santos, M.A.; Ferraz, S. Efeito de drogas anti-helmínticas sobre o crescimento de fungos nematófagos do gênero Arthrobotrys. Nat. São Paulo 1995, 20, 157–163. [Google Scholar]
- Townsend, L.B.; Wise, D.S. The synthesis and chemistry of certain anthelmintic benzimidazoles. Parasitol. Today 1990, 6, 107–112. [Google Scholar] [CrossRef]
- Sanyal, P.K.; Sumbria, D.; Pal, S.; Mandal, S.C. Factors affecting growth of a new isolate of egg parasitic fungus Pochonia suchlasporia on in vitro culture media. J. Vet. Parasitol. 2012, 26, 99–103. [Google Scholar]
- Wahane, N.; Sanyal, P.; Kumar, D.; Pal, S.; Bisen, S.; Baghel, K. In vitro screening of egg parasitic fungus Pochonia suchlasporia for its possible use as biocontrol agent against fasciolosis and amphistomosis in ruminants. Ind. J. Small Rum. 2014, 20, 66–68. [Google Scholar]
- Ferreira, G.F.; Freitas, T.M.; Gonçalves, C.L.; Mendes, J.F.; Vieira, J.N.; Villareal, J.P.; Nascente, P.S. Antiparasitic drugs: In vitro tests against nematophagous fungi. Braz. J. Biol. 2016, 76, 990–993. [Google Scholar] [CrossRef]
- Lubega, G.W.; Prichard, R.K. Interaction of benzimidazole anthelmintics with Haemonchus contortus tubulin: Binding affinity and anthelmintic efficacy. Exp. Parasitol. 1991, 73, 203–213. [Google Scholar] [CrossRef]
- An, J.X.; Ma, Y.; Zhao, W.B.; Hu, Y.M.; Wang, Y.R.; Zhang, Z.J.; Luo, X.F.; Zhang, B.Q.; Ding, Y.Y.; Liu, Y.Q. Drug Repurposing strategy ii: From approved drugs to agri-fungicide leads. J. Antibiot. 2023, 76, 131–182. [Google Scholar] [CrossRef]
- Cristel, S.; Fiel, C.; Anziani, O.; Descarga, C.; Cetrá, B.; Romero, J.; Fernández, S.; Entrocasso, C.; Lloberas, M.; Medus, D.; et al. Anthelmintic resistance in grazing beef cattle in central and northeastern areas of Argentina. Vet. Parasitol. Reg. Stud. Reports 2017, 9, 25–28. [Google Scholar] [CrossRef]
- Kaplan, R.M.; Vidyashankar, A.N. An Inconvenient truth: Global worming and anthelmintic resistance. Vet. Parasitol. 2012, 186, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Yeates, G.W.; Dimander, S.O.; Waller, P.J.; Höglund, J. Environmental impact on soil nematodes following the use of the ivermectin sustained-release bolus or the nematophagous fungus Duddingtonia flagrans to control nematode parasites of cattle in Sweden. Acta Agric. Scand. Sect. A Anim. Sci. 2002, 52, 233–242. [Google Scholar] [CrossRef]
- Moreno-Morales, J.C.; Andrade-Becerra, R.J.; Pulido-Medellín, M.O. Cuantificación de ivermectina eliminada en materia fecal de novillos tratados. Cienc. Agric. 2015, 12, 97. [Google Scholar] [CrossRef]
- Suarez, V.H.; Lifschitz, A.L.; Sallovitz, J.M.; Lanusse, C.E. Effects of ivermectin and doramectin faecal residues on the invertebrate colonization of cattle dung. J. Appl. Entomol. 2003, 127, 481–488. [Google Scholar] [CrossRef]
- Asi, M.R.; Bashir, M.H.; Afzal, M.; Ashfaq, M.; Sahi, S.T. Compatibility of entomopathogenic fungi, Metarhizium anisopliae and Paecilomyces fumosoroseus with selective insecticides. Pakistan J. Bot. 2010, 42, 4207–4214. [Google Scholar]
- Ferraz, C.M.; Sobral, S.A.; Senna, C.C.; Junior, O.F.; Moreira, T.F.; Tobias, F.L.; de Freitas Soares, F.E.; Geniêr, H.L.A.; Vilela, V.L.R.; Lima, J.A.C.; et al. Combined use of ivermectin, dimethyl sulfoxide, mineral oil and nematophagous fungi to control Rhabditis spp. Vet. Parasitol. 2019, 275, 108924. [Google Scholar] [CrossRef] [PubMed]
- Fiel, C.; Steffan, P.; Entrocasso, C. Epidemiología e impacto productivo de nematodos en la Pampa Húmeda. In Enfermedades Parasitarias de Importancia Clínica y Productiva en Rumiantes. Fundamentos Epidemiológicos Para su Diagnóstico y Control; Fiel, C., Nari, A., Eds.; Editorial Hemisferio Sur, SRL: Montevideo, Uruguay, 2013; pp. 29–58. [Google Scholar]
- Vilela, V.L.R.; Feitosa, T.F.; Braga, F.R.; Vieira, V.D.; de Lucena, S.C.; de Araújo, J.V. Control of sheep gastrointestinal nematodes using the combination of Duddingtonia flagrans and levamisole hydrochloride 5%. Rev. Bras. Parasitol. Vet. 2018, 27, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, P.K.; Chauhan, J.B.; Mukhopadhyaya, P.N. Implications of fungicidal effects of benzimidazole compounds on Duddingtonia flagrans in integrated nematode parasite management in livestock. Vet. Res. Commun. 2004, 28, 375–385. [Google Scholar] [CrossRef]
- Singh, R.K.; Sanyal, P.K.; Patel, N.K.; Sarkar, A.K.; Santra, A.K.; Pal, S.; Mandal, S.C. Fungus–benzimidazole interactions: A prerequisite to deploying egg-parasitic fungi Paecilomyces lilacinus and Verticillium chlamydosporium as biocontrol agents against fascioliasis and amphistomiasis in ruminant livestock. J. Helminthol. 2010, 84, 123–131. [Google Scholar] [CrossRef]
- Entrocasso, C.; Parra, D.; Vottero, D.; Farias, M.; Uribe, L.F.; Ryan, W.G. Comparison of the persistent activity of ivermectin, abamectin, doramectin and moxidectin in cattle. Vet. Rec. 1996, 138, 91–92. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).