Abstract
Selecting high-immune-responding cattle benefits the individual animal and the herd. To assess factors that have a role in determining the immune status of cattle, this study used 55 weaned bull and 57 weaned heifer Brahman calves. Antibody-mediated immune response (AMIR) was determined by using a vaccine-specific IgG, enzyme-linked immunosorbent assay (ELISA) in response to cattle receiving the Salmonella Newport Extract vaccine. Cell-mediated immune response (CMIR) was determined by using a subcutaneous (neck) sensitization dose of Candida albicans (CA) with Quil-A adjuvant on Day 0. On Day 14, caudal skinfold thickness (SFT) was measured using Harpenden calipers prior to the intradermal injection of CA into the skinfold, and on Day 15, the injection site SFT was measured again. The response was determined by using the difference in SFT from Day 15 (post-injection) and Day 14 (pre-injection). In weaned Brahman calves, AMIR was not influenced by sex; however, there was sexual dimorphism associated with CMIR, in that bull calves had a greater response than heifers (p < 0.05). Our studies demonstrate that weaned Brahman calves can be separated into AMIR and CMIR classes and that AMIR and CMIR should be investigated further as selection tools in beef cattle production.
1. Introduction
Immune competence phenotyping could be a tool to select healthier cattle [1]. The immune system is divided into two categories: the innate immune system and the adaptive immune system [2,3]. Specifically, innate immunity is the body’s nonspecific defense mechanism triggered when an antigen appears, whereas adaptive immunity utilizes specific antigen recognition systems and creates immunological memory after initial exposure. Cell-mediated immunity includes the activation of phagocytes, antigen-specific cytotoxic T-lymphocytes, and the release of cytokines in response to an antigen. Antibody-mediated immunity utilizes the production of antibodies by B-lymphocytes to bind to specific antigens.
High-immune-responding dairy cattle have increased growth efficiency or reduced infection incidence [4,5]. Specifically, high immune responders also have an improved response to vaccination, higher colostrum quality, and increased disease resistance in cows. Animals with a high cell-mediated immune response (CMIR) and antibody-mediated immune response (AMIR) tend to have significantly higher cortisol concentrations, suggesting that high-immune-responding animals have a higher stress response or a higher basal serum concentration. Cortisol concentration can vary, with mild stressful conditions such as exercise and handling enhancing the immune system and its response, while long-term and chronic stressors suppress the immune response [6]. In contrast, an inverse relationship between stress response and immune competence was identified in a prospective study of yearling Holstein-Friesian and Holstein-Friesian x Jersey heifers maintained under pasture conditions [7].
Temperament, defined as the reactivity or fear response to humans, has also been evaluated regarding the immune system [8]. Temperament has been statistically associated with measures of health, stress, and growth. For example, calves with a nervous temperament have a higher cortisol concentration at weaning, a reduced serum concentration of IgM, lower average daily gain, and increased morbidity when compared with calmer calves [9].
Health management programs developed to mitigate the immunosuppressive effects of weaning and post-weaning transportation stressors often focus on boosting antibody titers [10,11,12,13]. The objective of the present study was to provide an initial comparison of both the antibody- and cellular-mediated immune responses of weaned bull and heifer calves of a tropically adapted breed of cattle. Specifically, the objective of this study was to determine if sex, body weight, body condition score, or weaning temperament influenced the AMIR and CMIR of Brahman calves that were weaned for 70 days. In the present study, the temperament score was greater in heifer calves, whereas body weight was greater in bull calves. The AMIR did not differ between heifer and bull calves; however, there was sexual dimorphism in CMIR, with the bull calves having a greater response than the heifer calves.
2. Materials and Methods
2.1. Experimental Design
All experimental procedures were in compliance with the Guide for the Care and Use of Agricultural Animals in Research and Teaching [14] and approved by the Institutional Animal Care and Use Committee of Texas A&M AgriLife Research (AUP-#2015–019A). Fifty-five bull calves and fifty-seven heifer calves from the registered purebred Brahman research herd located at the Texas A&M AgriLife Research Center in Overton, TX (approximately 32°16′15.00″ N; 94°58′20.99″ W), were used in this study. At 28 days prior to weaning and at the time of weaning (195 days of age), each calf received the standard regimen of vaccinations to protect against bacterial and viral pathogens in accordance with the herd health protocol. The standard health protocol for the calf crop included immunization with the Fortress 8 (Pfizer, Exton, PA, USA), Clostridial, and Titanium 5 (Diamond Animal Health, Des Moines, IA, USA) respiratory complex vaccines. The temperament of each calf was determined at weaning. Temperament score [15,16] was an average of exit velocity (EV) and pen score (PS). Exit velocity is an objective measurement that records the rate as meters per second (m/s) at which cattle exit a working chute [15,17]. Pen score [18] is a subjective measurement in which cattle are separated into small groups of three to five, and their reactivity to a human observer is scored on a scale of 1 (calm, docile, approachable) to 5 (aggressive, volatile, crazy).
The AMIR evaluation was determined by using responses to the Salmonella Newport extract vaccine, as it was a novel vaccine for the herd of research cattle. To evaluate CMIR, Candida albicans with Quil-A was used as an adjuvant because of their effectiveness in inducing an inflammatory response [19]. Caudal tail-fold thickness following a local cellular immune challenge was used because it is a quantitative characteristic for analyzing CMIR [20]. The present study evaluating factors affecting AMIR and CMIR based on novel stimuli began 70 days post-weaning (i.e., Day 0) to allow for the completion of the immune response to routine vaccines administered 28 days prior to weaning and on the day of weaning. On Day 0 of this study of factors affecting AMIR and CMIR, body weight and body condition score were recorded prior to blood collection and immunization with a commercial vaccine not previously used on this research herd. On Day 0, whole blood samples (2 × 10 mL) were collected via jugular venipuncture in VACUTAINER® tubes (BD, Franklin Lakes, NJ, USA) to harvest serum for subsequent determination of cortisol and IgG concentrations. After blood collection on Day 0, the calves received (1) in the right neck 2 mL of Salmonella Newport Extract vaccine with siderophore receptor and porin protein (SRP) technology (Zoetis, Florham Park, NJ, USA) and (2) in the left neck a subcutaneous (neck) sensitization dose of 25 × 103 protein nitrogen units (PNUs) of Candida albicans (CA; Greer Labs, Lenoir, NC, USA) with 750 µg of Quil-A adjuvant (InvivoGen, San Diego, CA, USA). On Day 14, caudal skinfold thickness (SFT) was measured using spring-loaded calipers (Harpenden skinfold calipers; Creative Health Products Inc., Ann Arbor, MI, USA) prior to the intradermal injection of 5 × 103 PNU CA into the skinfold. On Day 15, the injection site SFT was measured, and blood serum samples were collected. Response was determined by using the difference in SFT from Day 15 (post-injection) and Day 14 (pre-injection). Blood samples collected between 8 A.M. and 11 A.M. on Day 0 and Day 15 were allowed to clot overnight at 4 °C before centrifugation for 30 min at 2675× g, and the harvested serum was stored at −80 °C until used to determine serum concentrations of cortisol and IgG.
2.2. Cortisol
Serum cortisol concentrations were determined with a single-antibody RIA that utilized polypropylene tubes coated with cortisol antiserum (MP Biomedicals, Orangeburg, NY, USA), as previously described [21]. The cortisol antiserum cross-reactivity with steroids was aldosterone, 0.03%; corticosterone, 0.94%; deoxycorticosterone, 0.26%; progesterone, 0.02%; and estradiol, 0.01%. The radiolabeled tracer was 125I cortisol. Radioactivity was detected by using an automatic gamma counter (COBRA II Auto-Gamma, PerkinElmer, Inc., Shelton, CT, USA) for 2 min, and counts per minute were converted into ng/mL using a microcomputer assay analysis program (Assay Zap; Biosoft, Cambridge, UK). All serum samples were analyzed in duplicate 25 μL aliquots within a single assay, with an intra-assay coefficient of variation (CV) of 4.9%.
2.3. Evaluation of AMIR
Serum samples were analyzed for Salmonella Newport Extract vaccine-specific IgG with a double sandwich, enzyme-linked immunosorbent assay, as previously described [22]. In brief, 96-well plates were coated with the Salmonella Newport Extract vaccine (Zoetis, Florham Park, NJ, USA) dissolved in a carbonate buffer 1:4 dilution, and all plates were blocked with milk. Serum samples and controls were diluted to 1:700. Positive and negative controls were obtained by pooling serum samples pre-immunization (negative control) and serum samples obtained on D 15 (positive control). All controls and samples were added to the plate in triplicate, allowing 15 animals (Day 0 and Day 15) to be run per plate. Horseradish-conjugated sheep anti-bovine IgG was used as the secondary antibody and was diluted to 1:8000. SureBlue™ TMB 1-Component Microwell Peroxidase Substrate was used for color development (VWR Corp., Missouri City, TX, USA). Absorbance was read at 450 nm using an automated microplate reader (Synergy H1, BioTek Instruments, Winooski, VT, USA). To calculate AMIR for each animal, the following equation was used: (Day 15 average/Positive control average)—(Day 0 average/Positive control average). The absorbance of each set of triplicates was averaged. Calves were then categorized into the following AMIR classes within sex: (1) low: ½ SD less than the mean AMIR; (2) intermediate: within ½ SD of the mean AMIR; and (3) High: greater than ½ SD of the mean AMIR.
2.4. Evaluation of CMIR
The degree of cell-mediated immune response was determined by using the difference in SFT from Day 15 (post-injection) and Day 14 (pre-injection). Calves were then categorized into the following CMIR classes within sex: (1) low: ½ SD less than the mean CMIR; (2) intermediate: within ½ SD of the mean CMIR; and (3) high: greater than ½ SD of the mean CMIR.
2.5. Statistical Analysis
Data were analyzed using the MIXED procedure of SAS [23] to evaluate the effects of calf sex, weaning temperament, and their interactions on CMIR, CMIR class, AMIR, and AMIR class. Sire was a random variable. Data are presented as unadjusted means +/− the standard error. Specific comparisons were made using Fisher’s Protected Least Significant Difference, with p < 0.05 considered significant. Pearson’s correlation coefficients were also calculated to characterize relationships between pairs of variables including weight, temperament, AMIR response class, and CMIR response class.
3. Results and Discussion
3.1. Temperament Score at Weaning Differed between Bull and Heifer Calves
In this study, the heifer calves were more temperamental than the bull calves. The weaning temperament score of heifers was greater (p < 0.01) than that of bulls (Figure 1), as the exit velocity and pen score were greater, respectively, for heifers (2.6 ± 0.1 m/s and 2.4 ± 0.2) than for bulls (2.3 ± 0.1 m/s and 1.7 ± 0.1). Within cohorts of either Bos taurus or Bos indicus cattle, heifers have been reported to be more temperamental and stress-responsive than steers and bulls based on assessments of behavioral and physiological variables [21,24,25,26,27,28].
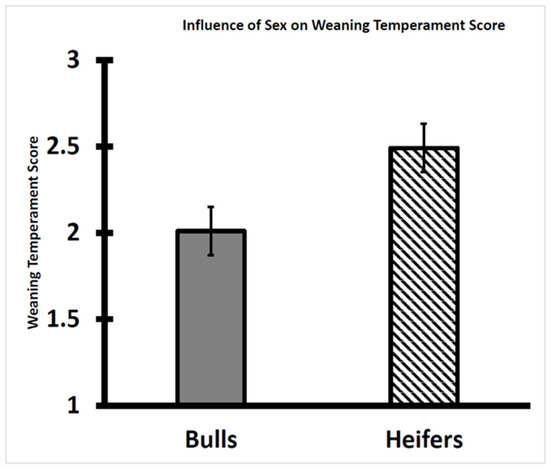
Figure 1.
Influence of sex classification on temperament score of Brahman bull (n = 55) and heifer (n = 57) calves at weaning. Heifer calves had a greater weaning temperament score than the bull calves (p < 0.01). The temperament score (TS = pen score + exit velocity/2) was used for assignment to 1 of 3 temperament classes (calm; intermediate; temperamental).
3.2. Assessment of AMIR in Bull and Heifer Calves
The Brahman bull and heifer calves were categorized as depicted, respectively, by Figure 2A,B into three clear response classes at two weeks after initial immunization with the Salmonella Newport Extract vaccine. The three response classes are designated as low, intermediate, and high AMIR within the bull and heifer groups. The screening of dairy cow and heifer populations to identify individuals with low and high adaptive immune responses has been accomplished by using primary antigens such as hen egg white lysozyme [29] or tetanus toxoid [4].
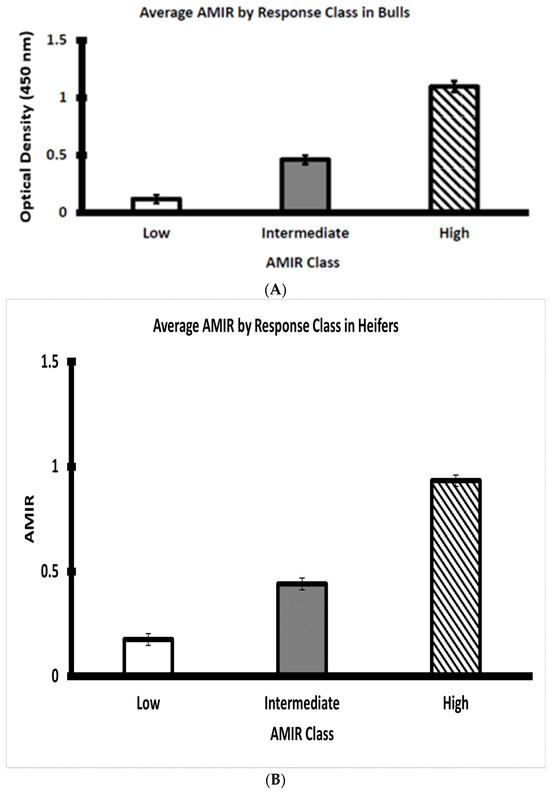
Figure 2.
(A) Average AMIR of weaned Brahman bull calves by response class (n = 22 low, 17 intermediate, and 16 high bulls). (B) Average AMIR of weaned Brahman heifer calves by response class (n = 17 low, 26 intermediate, and 14 high heifers).
Within each AMIR response class for bulls and heifers, the temperament score at weaning was plotted to visualize potential relationships with antibody production in response to the Salmonella vaccine. Temperament scores did not differ between the three AMIR response classes with bulls (Figure 3A) or heifers (Figure 3B). An assessment of the influence of temperament on AMIR in bulls and heifers has not been reported, although several studies report contradictory associations between cortisol concentration and AMIR and CMIR, as discussed in a subsequent section of this article.
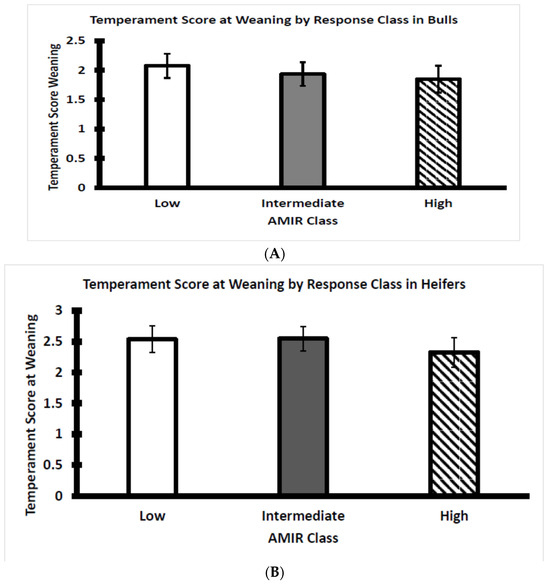
Figure 3.
(A) Average temperament score of Brahman bull calves at weaning by AMIR response class (n = 22 low, 17 intermediate, and 16 high bulls; p > 0.05). (B) Average temperament score of Brahman heifer calves at weaning by AMIR response class (n = 17 low, 26 intermediate, and 14 high heifers; p > 0.05).
Although temperament score was greater (p < 0.05) for the heifer calves than the bull calves (Figure 1), the AMIR did not differ (p > 0.05) by sex classification (Figure 4). A lack of an effect of sex classification on AMIR in response to the Salmonella Newport vaccine used in this study of Brahman beef cattle at 8–9 months of age is consistent with the observation that immunization with hen egg white lysozyme does not differ between Holstein-Friesian and Norwegian Red-Holstein crossbred bulls and heifers at 6 months of age [30].
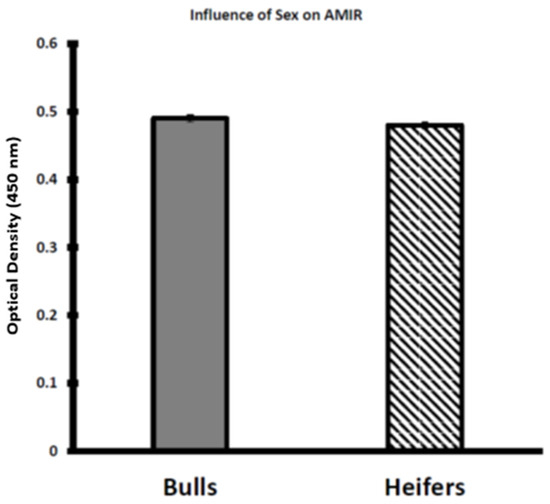
Figure 4.
Influence of sex classification on antibody-mediated immune response (AMIR) in weaned Brahman calves. AMIR was not different between bull (n = 55) and heifer (n = 57) calves (p > 0.05).
3.3. Assessment of CMIR in Bull and Heifer Calves
The Brahman bull and heifer calves were categorized as depicted, respectively, by Figure 5A,B into three clear response classes at 24 h after the second exposure to Candida albicans. The three response classes are designated as low, intermediate, and high CMIR within the bull and heifer groups. This CMIR ranking of both beef bulls and beef heifers conforms with the findings of Heriazon et al. [29], who standardized the use of Candida albicans challenges to elicit a delayed-type hypersensitivity reaction (i.e., increased skinfold thickness) in dairy cows.
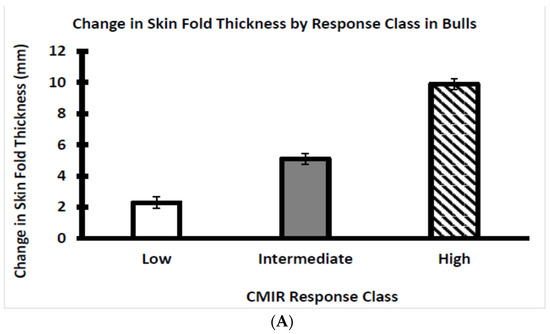
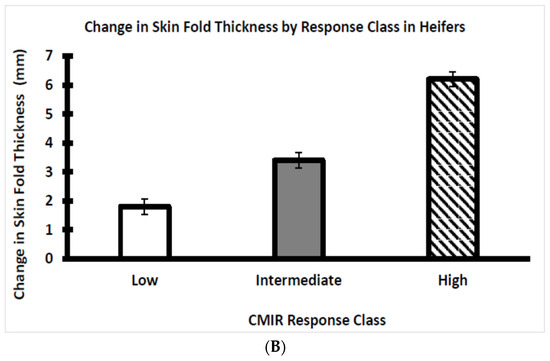
Figure 5.
(A) Average CMIR of weaned Brahman bull calves by CMIR response class (n = 19 low, 19 intermediate, and 17 high bulls). (B) Average CMIR of weaned Brahman heifer calves by CMIR response class (n = 20 low, 16 intermediate, and 21 high heifers).
Within each CMIR response class for bulls and heifers, the temperament score at weaning was plotted to visualize potential relationships with skinfold thickness in response to the challenge with Candida albicans. Temperament scores did not differ between the three CMIR response classes with bulls (Figure 6A) or heifers (Figure 6B). Analogous to the situation for AMIR, an assessment of the influence of temperament on CMIR in bulls and heifers has not been reported, although it has been reported that serum cortisol either is not related to CMIR or is positively related to CMIR [4,31], as discussed in a subsequent section of this article.
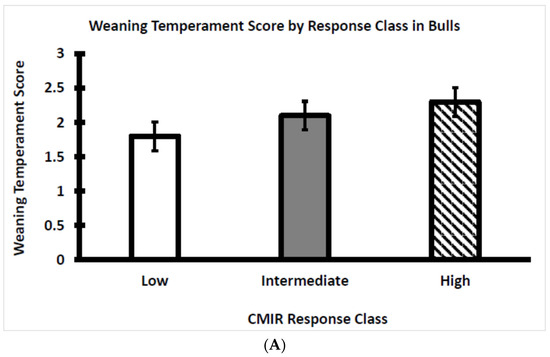
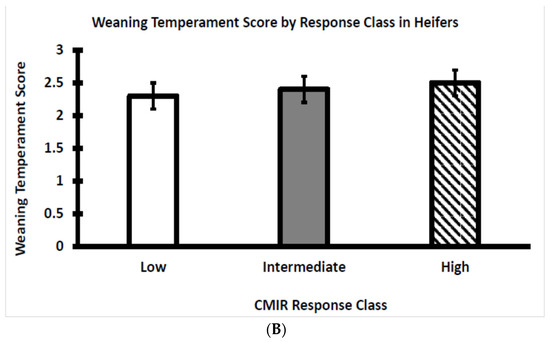
Figure 6.
(A) Average temperament score of Brahman bull calves at weaning by CMIR response class (n = 22 low, 17 intermediate, and 16 high bulls; p > 0.05). (B) Average temperament score of Brahman heifer calves at weaning by CMIR response class (n = 20 low, 16 intermediate, and 21 high heifers; p > 0.05).
The CMIR did differ by sex classification as the change in skinfold thickness was greater (p < 0.05) in bulls than in heifers (Figure 7). Previously, it was reported that sex classification did not influence CMIR in Holstein bulls and heifers that were 3-to-6 months younger than the Brahman bulls and heifers evaluated as reported herein [30]. Whether these disparate observations are related to breed or age differences remains to be determined.
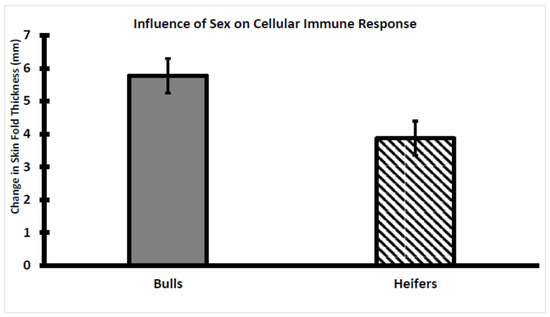
Figure 7.
The influence of sex classification on cell-mediated immune response (CMIR) in weaned Brahman calves. CMIR was greater in bull (n = 55) than in heifer (n = 57) calves (p < 0.05).
3.4. AMIR and CMIR in Relation to Body Condition Score, Cortisol, and Temperament
There were no significant relationships between AMIR or CMIR classes and growth traits, serum cortisol, or temperament (p > 0.05) (Figure 8, Figure 9, Figure 10 and Figure 11). On Day 0 of the study, body weight was greater (p < 0.05) in bull calves (195 ± 5 kg) than heifer calves (173 ± 5 kg), which is consistent with findings by Browning et al. [32]. The body condition score (on a nine-point scale) on Day 0 was similar for bulls (5.5 ± 0.08) and heifers (5.3 ± 0.09). No significant statistical associations were identified between either AMIR or CMIR with earlier-in-life growth traits (i.e., average daily gain from birth to weaning; weaning weight). Specifically, body weight and the body condition score at the start of the study were not associated (p > 0.10) with subsequent AMIR or CMIR in these bulls or heifers. In the response class data (high, intermediate, low), there were no significant effects on growth or temperament traits regarding AMIR.
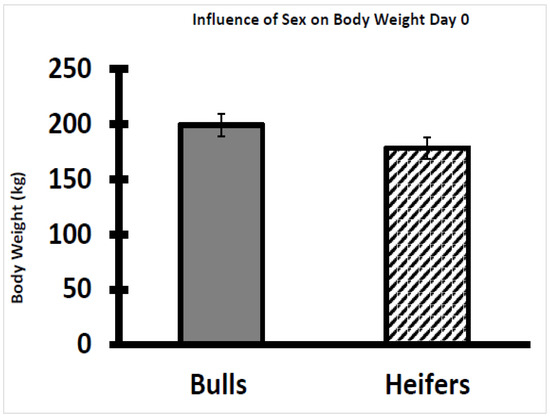
Figure 8.
Influence of sex on body weight of weaned Brahman bull and heifer calves on Day 0. Weaned bull calves had greater body weight on Day 0 than the heifer calves (p < 0.01).
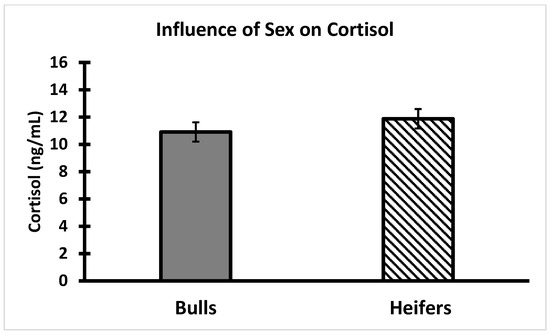
Figure 9.
Influence of sex classification on serum cortisol in weaned Brahman calves. Serum cortisol was not different between bull (n = 55) and heifer (n = 57) calves (p > 0.05).
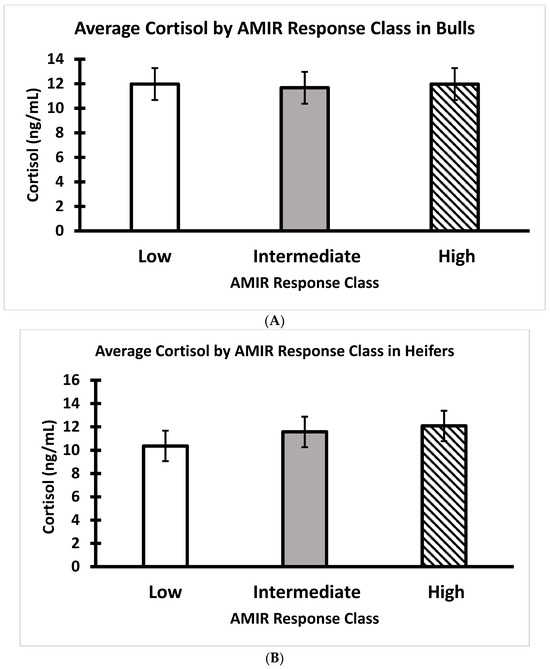
Figure 10.
(A) Average serum cortisol of Brahman bull calves at weaning by AMIR response class (n = 22 low, 17 intermediate, and 16 high bulls; p > 0.05). (B) Average serum cortisol of Brahman heifer calves at weaning by AMIR response class (n = 17 low, 26 intermediate, and 14 high heifers; p > 0.05).
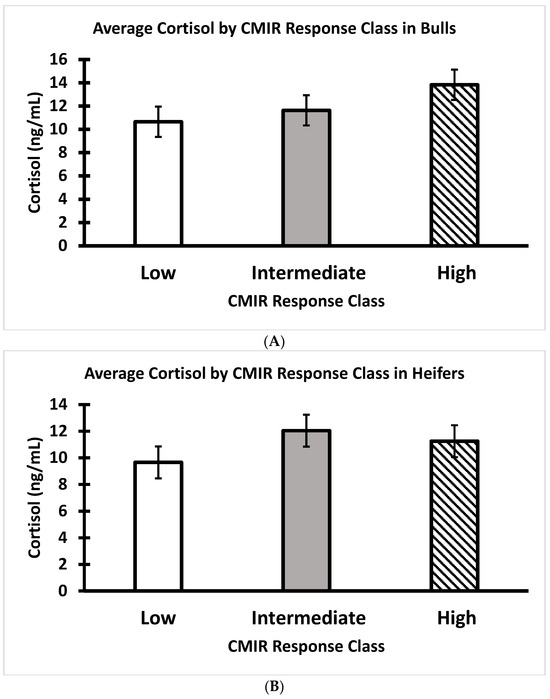
Figure 11.
(A) Average serum cortisol of Brahman bull calves at weaning by CMIR response class. (n = 19 low, 19 intermediate, and 17 high bulls; p > 0.05). (B) Average serum cortisol of Brahman heifer calves at weaning by CMIR response class (n = 20 low, 16 intermediate, and 21 high heifers; p > 0.05).
There was sexual dimorphism associated with CMIR, in that bulls had a greater response than heifers. Conversely, for AMIR, there was no difference between bull and heifer calves. In this study, the heifer calves were more temperamental than the bull calves. Bos indicus cattle breeds have been reported to be more temperamental than Bos taurus breeds, with females being more temperamental than males [24]. Bulls having a greater CMIR could be due to females having a decreased response that is related to their increased temperament scores. Sexual dimorphism was previously reported for the neuroendocrine and immune responses of Brahman heifers and bulls following transportation stress at 8-to-9 months of age. Specifically, the endocrine and immune responses of heifers exceeded those observed in bulls after challenges with corticotropin-releasing hormone [33] and lipopolysaccharide [34]. Future research will clarify potential sex-specific immune–endocrine interactions that coordinate the metabolism and health of heifers, bulls, and steers.
The likelihood of the successful selection of livestock for immune responsiveness was demonstrated in a porcine model [35]. The experimental approach involved the identification and selection of animals with an enhanced general immune response, which was assessed by combining measures of the animal’s antibody- and cell-mediated adaptive immune responses. To compare animals based on CMIR, Candida albicans was used to induce a type 1 immune response bias. It was determined that Candida albicans with Quil-A and hen egg white lysosome were antigen/adjuvant combinations capable of inducing CMIR and AMIR, respectively, without interfering with diagnostic tests [29,36]. The tail skinfold was determined as the ideal injection site for CMIR evaluation because the neck was significantly more sensitive [29].
In our study, the Salmonella Newport Extract vaccine was used for the determination of AMIR because it was a novel vaccine for this research herd. Salmonella can be a devastating problem to the dairy and beef industries and is a significant foodborne pathogen that has been reported to be increasing in incidence by the USDA. Vaccination is one of the best prevention methods for the control of Salmonella. The herd-wide benefits of the Salmonella Newport Extract vaccine include increased milk yield and increased protection from Salmonella infection [22].
The ability to separate bull and heifer calves based on their adaptive immune responses is supported by several studies. However, factors such as calf age at vaccination, the persistent prevalence of maternal antibodies, the type of vaccine, adjuvants or lack thereof, and the location of the vaccination play a role in an individual calf’s response to the vaccine [37]. This indicates that differences in antibody response after vaccination would be expected, as calves can range from having high-to-low antibody titers regardless of the sex of the calf [37,38]. Our results with weaned Brahman calves concur with earlier findings in dairy cattle in that high- and low-AMIR and -CMIR animals can be identified [4,39].
Thompson-Crispi et al. [38] found a negative correlation between AMIR and CMIR in Holstein cattle (r = −0.13; p > 0.05). In our study, the correlation between AMIR and CMIR was r = 0.01 for the heifers and r = 0.30 (p < 0.05) for the bulls. The greater correlation in bull calves could be related to the sexual dimorphism seen for CMIR in the bull calves. The negative genetic correlation between AMIR and CMIR observed in studies of several species could be due to cytokines that may promote CMIR and tend to inhibit AMIR, and vice versa. These observations highlight the necessity of gathering important biological information to further enhance the progress that can be achieved by selecting sires and dams based on their immune traits. This approach promises to provide broad-based disease resistance to a multitude of organisms [40,41,42,43].
The benefits of selecting for high-immune-responding cattle have been evaluated in various studies. One of the advantages of selecting for AMIR and CMIR is the low-to-moderate heritability associated with these traits [15,39,40,44]. Another benefit of high-immune-responding animals that has been seen in dairy cattle is an association between improved responses to vaccination and a decreased occurrence of disease. For example, cows that ranked higher in AMIR and CMIR had decreased instances of mastitis, metritis, and other illnesses [40]. The concept of immune resistance (an appropriate balancing of the beneficial aspects of antibody response and the adverse aspects of inflammatory responses) has recently been suggested as a key homeostatic mechanism to protect humans against pathogenic respiratory viruses [45]. A biometric approach to reduce morbidity and mortality to enteric and respiratory diseases in Bos taurus dairy and beef cattle is of increasing relevance to animal welfare and production efficiency [1,46,47]. The improvement seen in the health, productivity, and reproductive aspects of dairy cattle by selecting for enhanced immunity is something that is of increasing importance in the beef cattle industry [48]. Our data should encourage the further consideration and study of AMIR and CMIR for use as selection tools to further improve the health, productivity, and well-being of Brahman cattle. This is of imminent pertinence in view of the future increased dependence on heat-tolerant livestock to cope with the stressors that will be encountered should climate change progress as predicted.
4. Conclusions
The objective of this study was to provide an initial comparison of the immune responses of weaned bull and heifer calves of a tropically adapted breed of beef cattle. Our study, conducted in a research herd of registered, purebred Brahman cattle, demonstrated that weaned Brahman calves can be separated by AMIR and CMIR classes and that AMIR and CMIR should be investigated further as selection tools in beef cattle production. The heifer calves had higher weaning temperament scores than the bull calves, while the bull calves had greater body weights than the heifers. There was no difference between sexes regarding AMIR, but there was sexual dimorphism in CMIR, with the bull calves having a greater response than the heifers. The evidence discovered regarding the factors influencing antibody- and cellular-mediated immune responses can ultimately be used to modify beef cattle management practices in order to (1) improve breeding stock by culling low-immune-responding individuals and breeding moderate-to-high responders, (2) minimize the negative influences of illness on production that are increased in low-immune-responding cattle, and (3) enhance the immune functions and overall health of cattle.
Author Contributions
Conceptualization, R.D.R. and T.H.W.J.; investigation, C.L.Y., R.D.R. and T.H.W.J.; resources, R.D.R. and T.H.W.J.; data curation and analyses, C.L.Y., D.G.R., R.D.R. and T.H.W.J.; writing—original draft preparation, C.L.Y.; writing—review and editing, C.L.Y., D.G.R., R.D.R. and T.H.W.J.; supervision, project administration, and funding acquisition, R.D.R. and T.H.W.J. All authors have read and agreed to the published version of the manuscript.
Funding
Stipend support for C.L.Y. was provided by (1) an Excellence Fellowship from Texas A&M University’s College of Agriculture and Life Sciences and (2) assistantships from the Department of Animal Science and Texas A&M AgriLife Research. This work was supported in part by USDA Formula Animal Health G-9084 (R.D.R., T.H.W.J.) and USDA Hatch Projects H-9022 (T.H.W.J.), S1086 (D.G.R.), W4112 (R.D.R.), and W4173 (T.H.W.J.). Texas A&M AgriLife Research at College Station (T.H.W.J.) and Overton (R.D.R.). contributed support.
Institutional Review Board Statement
The animal study protocol was approved by the Texas A&M AgriLife Research Agricultural Animal Care and Use Committee (Animal Use Protocol 2015–019A). All procedures were conducted in compliance with the Guide for the Care and Use of Agricultural Animals in Research and Teaching [14].
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available upon reasonable request.
Acknowledgments
The authors thank D. Neuendorff, A. Lewis, D. Law, and T. Garcia for assistance with animal management and sample collection. The technical advice of J. Bray, C. Long, W. Mwangi, N. Cohen, and L. Berghman of Texas A&M University is appreciated.
Conflicts of Interest
The authors declare no conflict of interest. The funders and supporters had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
References
- Emmam, M.; Livernois, A.; Paibomesai, M.; Atalla, H.; Mallard, B. Genetic and epigenetic regulation of immune response and resistance to infectious diseases in domestic ruminants. Vet. Clin. Food Anim. 2019, 35, 405–429. [Google Scholar] [CrossRef]
- Tizard, I.R. Veterinary Immunology: An Introduction, 10th ed.; Elsevier/Saunders: Philadelphia, PA, USA, 2016; ISBN 9780323523493. [Google Scholar]
- Abbas, A.K.; Lichtman, A.H.; Pillai, S. Basic Immunology: Functions and Disorders of the Immune System, 6th ed.; Elsevier/Saunders: Philadelphia, PA, USA, 2019; ISBN 9788131259573. [Google Scholar]
- Aleri, J.W.; Hine, B.C.; Pyman, M.F.; Mansell, P.D.; Wales, W.J.; Mallard, B.; Fisher, A.D. Assessing adaptive immune response phenotypes in Australian Holstein-Friesian heifers in a pasture-based production system. J. Anim. Sci. 2015, 93, 3713–3721. [Google Scholar]
- Thompson-Crispi, K.A.; Miglior, F.; Mallard, B.A. Incidence rates of clinical mastitis among Canadian Holsteins classified as high, average, or low immune responders. Clin. Vaccine Immunol. 2013, 20, 106–112. [Google Scholar] [CrossRef]
- Hines, M.T.; Ii, H.C.S.; Bayly, W.M.; Leroux, A.J. Exercise and immunity: A review with emphasis on the horse. J. Veter Intern. Med. 1996, 10, 280–289. [Google Scholar] [CrossRef]
- Aleri, J.; Hine, B.; Pyman, M.; Mansell, P.; Wales, W.; Mallard, B.; Stevenson, M.; Fisher, A. Associations between immune competence, stress responsiveness, and production in Holstein-Friesian and Holstein-Friesian × Jersey heifers reared in a pasture-based production system in Australia. J. Dairy Sci. 2019, 102, 3282–3294. [Google Scholar] [CrossRef] [PubMed]
- Fordyce, G.; Dodt, R.; Wythes, J. Cattle temperaments in extensive beef herds in northern Queensland: Factors affecting temperament. Aust. J. Exp. Agric. 1988, 28, 683–687. [Google Scholar] [CrossRef]
- Fell, L.R.; Colditz, I.G.; Walker, K.H.; Watson, D.L. Associations between temperament, performance and immune function in cattle entering a commercial feedlot. Aust. J. Exp. Agric. 1999, 39, 795–802. [Google Scholar] [CrossRef]
- Texas Cooperative Extension. Value Added Calf (VAC)–Vaccination Programs. 2005. Available online: https:animalscience.tamu.edu/wp-content/uploads/sites/14/2012/04/beef-vac-vaccination.pdf (accessed on 28 June 2023).
- Richeson, J.T.; Beck, P.A.; Gadberry, M.S.; Gunter, S.A.; Hess, T.W.; Hubbell, D.S.; Jones, C. Effects of on-arrival versus delayed modified live virus vaccination on health, performance, and serum infectious bovine rhinotracheitis titers of newly received beef calves. J. Anim. Sci. 2008, 86, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Galyean, M.L.; Duff, G.C.; Rivera, J.D. Galyean appreciation club review: Revisiting nutrition and health of newly received cattle—What have we learned in the last 15 years? J. Anim. Sci. 2022, 100, skac067. [Google Scholar] [CrossRef] [PubMed]
- Matty, J.M.; Reddout, C.; Adams, J.; Major, M.; Lalman, D.; Biggs, R.; Salak-Johnson, J.L.; Beck, P.A. The effects of respiratory vaccine type and timing on antibody titers, immunoglobulins, and growth performance in pre- and post-weaned beef calves. Vet. Sci. 2023, 10, 37. [Google Scholar] [CrossRef]
- FASS. Guide for Care and Use of Agricultural Animals in Research and Teaching, 3rd ed.; Federation of Animal Science Societies: Champaign, IL, USA, 2010. [Google Scholar]
- Curley, K.O., Jr.; Paschal, J.C.; Welsh, T.H., Jr.; Randel, R.D. Exit velocity as a measurement of cattle temperament is repeatable and associated with serum concentration of cortisol in Brahman bulls. J. Anim. Sci. 2006, 84, 3100–3103. [Google Scholar] [CrossRef] [PubMed]
- King, D.; Pfeiffer, C.S.; Randel, R.; Welsh, T.; Oliphint, R.; Baird, B.; Curley, K.; Vann, R.; Hale, D.; Savell, J. Influence of animal temperament and stress responsiveness on the carcass quality and beef tenderness of feedlot cattle. Meat Sci. 2006, 74, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Burrow, H.M.; Seifert, G.W.; Corbet, N.J. A new technique for measuring temperament in cattle. Proc. Aust. Soc. Anim. Prod. 1988, 17, 154–157. [Google Scholar]
- Hammond, A.C.; Olson, T.A.; Chase, C.C., Jr.; Bowers, E.J.; Randel, R.D.; Murphy, C.N.; Vogt, D.W.; Tewolde, A. Heat tolerance in two tropically adapted Bos taurus breeds, Senepol and Romosinuano, compared with Brahman, Angus, and Hereford cattle in Florida. J. Anim. Sci. 1996, 74, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, S.; Begley, N.; Schaeffer, L.; Burnside, E.; Mallard, B. Antibody and cell-mediated immune responses and survival between Holstein and Norwegian Red x Holstein Canadian calves. J. Dairy Sci. 2011, 94, 1576–1585. [Google Scholar] [CrossRef] [PubMed]
- Hernández, A.; Quinton, M.; Miglior, F.; Mallard, B.A. Genetic parameters of dairy cattle immune response traits. In Proceedings of the 8th World Congress on Genetics Applied to Livestock Production, Belo Horizonte, Minas Gerais, Brazil, WCGALP, Belo Horizonte, Brazil, 13–18 August 2006; pp. 15–18. [Google Scholar]
- Littlejohn, B.P.; Price, D.M.; Banta, J.P.; Lewis, A.W.; Neuendorff, D.A.; Carroll, J.A.; Vann, R.C.; Welsh, T.H., Jr.; Randel, R.D. Prenatal transportation stress alters temperament and serum cortisol concentrations in suckling Brahman calves. J. Anim. Sci. 2016, 94, 602–609. [Google Scholar] [CrossRef]
- Hermesch, D.R.; Thomson, D.U.; Loneragan, G.H.; Renter, D.R.; White, B.J. Effects of a commercially available vaccine against Salmonella enterica serotype Newport on milk production, somatic cell count and shedding of Salmonella organisms in female dairy cattle with no clinical signs of salmonellosis. Am. J. Ver. Res. 2008, 69, 1229–1234. [Google Scholar] [CrossRef]
- SAS. SAS/STAT User’s Guide, Version 9.3; SAS Institute: Cary, NC, USA, 2011. [Google Scholar]
- Voisinet, B.D.; Grandin, T.; Tatum, J.D.; O’Connor, S.F.; Struthers, J.J. Feedlot cattle with calm temperaments have higher average daily gains than cattle with excitable temperaments. J. Anim. Sci. 1997, 75, 892–896. [Google Scholar] [CrossRef]
- Shrode, R.R.; Hammack, S.P. Chute behavior of yearling beef cattle. J. Anim. Sci. 1971, 33, 193. [Google Scholar]
- Tennessen, T.; Price, M.A.; Berg, R.T. Comparative responses of bulls and steers to transportation. Can. J. Anim. Sci. 1984, 64, 333–338. [Google Scholar] [CrossRef]
- Lay, D.C., Jr.; Friend, T.H.; Grissom, K.K.; Bowers, C.L.; Mal, M.E. Effects of freeze or hot-iron branding of Angus calves on some physiological and behavioral indicators of stress. Appl. Anim. Behav. Sci. 1992, 33, 137–147. [Google Scholar] [CrossRef]
- Hoppe, S.; Brandt, H.R.; König, S.; Erhardt, G.; Gauly, M. Temperament traits of beef calves measured under field conditions and their relationships to performance. J. Anim. Sci. 2010, 88, 1982–1989. [Google Scholar] [CrossRef]
- Heriazon, A.; Yager, J.A.; Sears, W.; Mallard, B.A. Induction of delayed-type hypersensitivity and interferon-gamma to Candida albicans and anti-hen-egg white lysozyme antibody as phenotypic markers of enhanced bovine immune response. Vet. Immunol. Immunopathol. 2009, 129, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Begley, N.; Buckley, F.; Burnside, E.; Schaeffer, L.; Pierce, K.; Mallard, B. Immune responses of Holstein and Norwegian Red × Holstein calves on Canadian dairy farms. J. Dairy Sci. 2009, 92, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Aleri, J.W.; Hine, B.C.; Pyman, M.F.; Mansell, P.D.; Wales, W.J.; Mallard, B.; Fisher, A.D. An assessment of immune and stress responsiveness in Holstein Friesian cows selected for high and low feed conversion efficiency. Anim. Prod. Sci. 2016, 57, 244–251. [Google Scholar] [CrossRef]
- Browning, R., Jr.; Leite-Browning, M.L.; Neuendorff, D.A.; Randel, R.D. Preweaning growth of Angus-(Bos taurus), Brahman-(Bos indicus), and Tuli-(Sanga) sired calves and reproductive performance of their Brahman dams. J. Anim. Sci. 1995, 73, 2558–2563. [Google Scholar] [CrossRef]
- Hulbert, L.E.; Carroll, J.A.; Ballou, M.A.; Burdick, N.C.; Dailey, J.W.; Caldwell, L.C.; Loyd, A.N.; Vann, R.C.; Welsh, T.H., Jr.; Randel, R.D. Sexually dimorphic stress and pro-inflammatory cytokine responses to an intravenous corticotropin-releasing hormone challenge in Brahman cattle following transportation. Innate Immun. 2013, 19, 378–387. [Google Scholar] [CrossRef]
- Carroll, J.A.; Sanchez, N.C.B.; Hulbert, L.E.; Ballou, M.A.; Dailey, J.W.; Caldwell, L.C.; Vann, R.C.; Welsh, T.H., Jr.; Randel, R.D. Sexually dimorphic innate immunological responses of pre-pubertal Brahman cattle following an intravenous lipopolysaccharide challenge. Vet. Immunol. Immunopathol. 2015, 166, 108–115. [Google Scholar] [CrossRef]
- Wilkie, B.; Mallard, B. Selection for high immune response: An alternative approach to animal health maintenance? Vet. Immunol. Immunopathol. 1999, 72, 231–235. [Google Scholar] [CrossRef]
- Romani, L. Innate and adaptive immunity in Candida albicans infections and saprophytism. J. Leukocyte Biol. 2000, 68, 175–179. [Google Scholar] [CrossRef]
- Chamorro, M.F.; Woolums, A.; Walz, P.H. Vaccination of calves against common respiratory viruses in the face of maternally derived antibodies (IFOMA). Anim. Health Res. Rev. 2016, 17, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, J.; Fulton, R.W.; Burge, L.J.; DuBois, W.R.; Payton, M. Passively transferred immunity in newborn calves, rate of antibody decay, and effect on subsequent vaccination with modified live virus vaccine. Bov. Pract. 2019, 35, 47–55. [Google Scholar] [CrossRef]
- Wagter, L.; Mallard, B.; Wilkie, B.; Leslie, K.; Boettcher, P.; Dekkers, J. A quantitative approach to classifying Holstein cows based on antibody responsiveness and its relationship to peripartum mastitis occurrence. J. Dairy Sci. 2000, 83, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Thompson-Crispi, K.; Sewalem, A.; Miglior, F.; Mallard, B. Genetic parameters of adaptive immune response traits in Canadian Holsteins. J. Dairy Sci. 2012, 95, 401–409. [Google Scholar] [CrossRef]
- Biozzi, G.; Mouton, D.; Heumann, A.M.; Bouthillier, Y.; Stiffel, C.; Mevel, J.C. Genetic analysis of antibody responsiveness to sheep erythrocytes in crosses between lines of mice selected for high or low antibody synthesis. Am. J. Immunol. 1979, 36, 427–438. [Google Scholar]
- Mouton, D.; Bouthillier, Y.; Mevel, J.; Biozzi, G. Genetic selection for antibody responsiveness in mice: Further evidence for inverse modification of macrophage catabolic activity without alteration of the expression of T-cell-mediated immunity. Eur. J. Immunol. 1984, 135, 173–186. [Google Scholar] [CrossRef]
- Sarker, N.; Tsudzuki, M.; Nishibori, M.; Yamamoto, Y. Direct and correlated response to divergent selection for serum immunoglobulin M and G levels in chickens. Poult. Sci. 1999, 78, 1–7. [Google Scholar] [CrossRef]
- Abdel-Azim, G.; Freeman, A.; Kehrli, M., Jr.; Kelm, S.; Burton, J.; Kuck, A.; Schnell, S. Genetic basis and risk factors for infectious and noninfectious diseases in US Holsteins. J. Dairy Sci. 2005, 88, 1199–1207. [Google Scholar] [CrossRef]
- Ahuja, S.K.; Manoharan, M.S.; Lee, G.C.; McKinnon, L.R.; Meunier, J.A.; Steri, M.; Harper, N.; Fiorillo, E.; Smith, A.M.; Restrepo, M.I.; et al. Immune resilience despite inflammatory stress promotes longevity and favorable health outcomes including resistance to infection. Nat. Commun. 2023, 14, 3286. [Google Scholar] [CrossRef]
- Gifford, C.A.; Holland, B.P.; Mills, R.L.; Maxwell, C.L.; Farney, J.K.; Terrill, S.J.; Step, D.L.; Richards, C.J.; Robles, L.O.B.; Krehbiel, C.R. Growth and development symposium: Impacts of inflammation on cattle growth and carcass merit. J. Anim. Sci. 2012, 90, 1438–1451. [Google Scholar] [CrossRef]
- Cockrum, R.R.; Speidel, S.E.; Salak-Johnson, J.L.; Chase, C.C.L.; Peel, R.K.; Weaber, R.L.; Loneragan, G.H.; Wagner, J.J.; Boddhireddy, P.; Thomas, M.G.; et al. Genetic parameters estimated at receiving for circulating cortisol, immunoglobulin G, interleukin 8, and incidence of bovine respiratory disease in feedlot beef steers. J. Anim. Sci. 2016, 94, 2770–2778. [Google Scholar] [CrossRef] [PubMed]
- Husseini, N.; Beard, S.C.; Hodgins, D.C.; Barnes, C.; Chik, E.; Mallard, B.A. Immuno-phenotyping of Canadian beef cattle: Adaptation of the high immune response methodology for utilization in beef cattle. Transl. Anim. Sci. 2022, 6, txac006. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).