1. Introduction
The world’s human population is constantly growing; it is estimated that it will reach 8.5 billion people by the end of the year 2030 and 9.7 billion by 2050 [
1]. This population has an ancient dependence on obtaining high-quality protein in the diet, derived mainly from ruminant animal products [
2]. However, in recent decades, ruminant production has suffered counterattacks, attributed to deforestation due to the need for more land for grazing [
3]. Furthermore, ruminant production is attacked by environmentalists, since they are also considered the most polluting production animals due to the generation of methane in their digestive processes [
4]. Additionally, safe food supporters attempted to disqualify ruminant products due to their higher content of saturated and trans fatty acids, which can be correlated with metabolic and heart diseases [
5].
In this sense, ruminant producers are adopting management practices that improve this productive chain, such as the use of intensive systems instead of grazing systems. In this system, there is a tendency to reduce the supply of commonly used ingredients (such as ground corn and soybean meal) because the price of these constantly increases [
6] due to the competition between the use of these ingredients in ruminants, non-ruminants, and human feeding [
7,
8]. For these reasons, the use of alternative feeds (by-products and waste) is promoted because their use reduces diet costs and offers a relevant nutritional contribution [
9,
10,
11,
12,
13,
14,
15,
16].
However, some unconventional feeds contain antinutritional or toxic factors that affect animal performance, such as ricin in castor bean meal [
11,
17]. Due to this, the evaluation of these feeds in in vivo trials is limited. For this reason, in vitro methods can be used, which have a high reliability and reproducibility level due to the similarity with the ruminal environment characteristics [
18,
19]. Additionally, all ingredients or diets need to be analyzed to determine their nutritional value for animals.
To mimic the rumen environment, the in vitro method uses ruminal inoculum obtained from cannulated animals. However, the use of cannulated animals has been criticized due to practical problems, ethical reasons, animal welfare, and the long-term maintenance costs [
18,
20]. Therefore, the search for alternative inoculum is essential to reduce the use of cannulated animals. Among the options evaluated, the rumen content of cattle slaughtered in a commercial slaughterhouse emerges as a potential alternative [
21]. Nevertheless, to validate an alternative inoculum, it would be necessary to evaluate and process a large number of variables, resulting in the increased cost of this method [
22]. In this sense, the multivariate analysis technique can be used to characterize or evaluate divergences from quantitative or qualitative variables [
23]. With this technique, it is possible to reduce the number of variables and determine the variables that effectively influenced the trial to reduce costs in similar studies.
In this context, this study aimed to determine the reliability of the ruminal inoculum obtained from cattle slaughtered in a commercial slaughterhouse for the in vitro analysis of alternative feeds for ruminants through multivariate analysis.
2. Materials and Methods
2.1. Location and Ethical Considerations
The experiment was conducted on the São Gonçalo dos Campos experimental farm belonging to the Federal University of Bahia, located in the municipality of São Gonçalo dos Campos, BA, Brazil (12°23′57.51″ S and 38°52′44.66″ W). All procedures were previously approved by the Ethics Committee on Animal Use (CEUA) at the Federal University of Bahia (Approval number: 17/2014).
2.2. Rumen Content Collection and Inoculum Preparation—Cannulated Animals
This trial involved five crossbreed male cattle with an approximate average body weight of 320 ± 9.4 kg (mean ± standard deviation) and provided with ruminal cannula. The animals were housed in 3 × 6 m individual stalls provided with individual feeders and drinkers. Fifteen days before the beginning of collection were used to prepare the animals (adaptation period). In this period, the animals were treated against internal and external parasites (Ranger LA, Ivermectin 3.5%, dose 1 mL per 50 kg of body weight, Salvador, Brazil) and adapted to a total mixed ratio diet to meet the maintenance requirements of the animals (102.1 g/kg of crude protein (CP)/kg of dry matter (DM) and 337.48 Mcal of digestible energy/kg DM). The forage (Tifton-85 hay-Cynodon sp.)–concentrate (ground corn, soybean meal, urea, mineral mix) proportion used was 40:60. The animals were fed twice a day, at 09:00 and 17:00 h.
To collect the rumen content, the animals were led to a suitable containment area. Thermal containers (5 L capacity) with hot water (40 °C) and CO2 were used to simulate the temperature and anaerobic conditions of the ruminal environment. The cap of the cannula was removed, and the solid (approximately 1.6 kg from 8 points including the cranial, dorsal, ventral, and caudal part of the rumen) and liquid (approximately 1.5 L collected with a vacuum pump) rumen content were collected directly to the same thermal container (without water) with the continuous use of CO2. Immediately, the thermal containers were transported to the laboratory for sample processing (20 min walking distance).
In the laboratory, the rumen content samples were strained (using 4 layers of cheesecloth) directly into a 500 mL graduated cylinder (40 °C) inside a fume hood with continuous use of CO2. The contents were immediately poured into the preheated (40 °C) vessels (400 mL/flask) of the Ankom DaisyII incubator. A 15 mL subsample was collected to verify the pH and temperature of the ruminal inoculum.
2.3. Rumen Content Collection and Inoculum Preparation—Slaughtered Animals
To obtain this inoculum, the rumen content of five to seven cattle slaughtered in a commercial slaughterhouse was collected. The origin, breed, weight, and feeding management of the animals were unknown. After slaughter, the rumen of the animals (randomly distributed) was directed to an internal organ washing room (approximately 15 min between animal death and rumen content collection). The rumen was opened, and the exposed content (approximately 1.5 L) was collected immediately (maximum of 7 min between collections). This was stored in bottles preheated at 39 °C, with the continuous addition of CO2. Immediately, the thermal containers were transported to the laboratory for sample processing (25 min driving distance). The procedure for obtaining the ruminal inoculum in the laboratory was as described above.
2.4. Experimental Design, Diets, and In Vitro Analysis
A completely randomized design was used to compare the two sources of ruminal inoculum. For this, sixteen diets with different ingredients (
Table 1) and chemistry compositions (
Table 2) were incubated. The byproducts used in the formulation of the diets were palm kernel cake (PKC), crude glycerin (CG), licuri cake (LC), and castor bean meal (CBM). The number that follows the name of the diet refers to the level of inclusion of the byproduct in the respective diet. The forage–concentrate proportion of the PKC diets was 35:65, CG diets was 50:50, LC diets was 40:60, and CBM diets was 50:50. The forage used in all diets was Tifton-85 (
Cynodon sp.) hay. This variation was used to avoid experimentation bias.
The in vitro DM (IVDMD) and in vitro neutral detergent fiber (IVNDFD) digestibility were measured using two artificial rumens (Ankom
®, Daisy
II Incubator, Ankom Technológic Corp., Macedon, NY, USA), following the procedures described by Holden [
24]. For this, 0.5 g of each diet (n = 3) was placed into TNT (100 g/m) bags, which were cut and sealed (5 × 5 cm) according to Casali et al. [
25]. Two bags without samples (blank observation) were also used. Twenty-six TNT bags (24 with a sample and 2 blank) were placed into jars (totaling 8 jars, previously heated to 40 °C, with the continuous addition of CO
2), totaling 104 bags/machine. Immediately, 1600 mL of buffer solution and 400 mL of ruminal inoculum were added [
20]. The incubation process was carried out at 39 °C for 48 h. Subsequently, 40 mL of HCl (6N) and 8 g of pepsin (Sigma 1:10,000; dissolved in 34 mL of distilled H
2O) were added and incubated for an additional 24 h. Afterwards, the bags were washed with tap water until the water ran clean, pre-dried in forced-air ovens (55 °C for 12 h), dried at 105 °C for 16 h, and weighed. Thereafter, the dried bags were analyzed to determine NDF content [
26]. The incubation process was repeated 10 times, totaling 2080 bags analyzed.
To determine the ruminal fermentation parameters, two Daisy
II Fermenter incubators (39 °C) were used (Totaling 8 jars; previously heated to 40 °C; continuously addition of CO
2). For each jar (caps were fitted with three track taps), 10 g of sample was weighed, and 1600 mL of buffer solution and 400 mL of ruminal inoculum were added (4 diets per incubation and two ruminal inoculum sources). At 0, 2, 4, 6, and 8 h after the start of incubation, a sample of 30 mL of buffered ruminal fluid was collected and stored; 10 mL was used to determine rumen ammonia nitrogen [
27], 10 mL was used to determine volatile fatty acids [
28], and 10 mL was used to determine pH and protozoa [
29].
2.5. Chemical Analysis of Ingredients and Diets
Samples of ingredients and diets were collected and stored at −20 °C. Afterward, the samples were dried in a forced-air oven at a temperature of 55 °C for 72 h. The samples were ground in a Wiley mill (model 0.48, Marconi, Piracicaba, Brazil) using 1 and 2 mm screens.
Samples were analyzed for dry matter (DM; 930.15), ether extract (EE; 920.39), crude protein (CP; 976.05), and ash (942.05) following the methods of the Association of Official Analytical Chemists [
30]. Neutral detergent fiber was assayed with a heat-stable amylase and expressed exclusive of residual ash and protein (NDFap), acid detergent fiber was expressed exclusive of residual ash and protein (ADF), and lignin contents were estimated according to the methods described by Van Soest et al. [
26]. The cellulose and hemicellulose fractions were estimated from the determined values of NDF, ADF, and lignin. Non-fibrous carbohydrates (NFC) were estimated according to Hall [
31] equations.
2.6. Chemical Analysis of Ingredients and Diets
To estimate the IVDMD and IVNDFD, the following formula was used:
where W
1 is the weight of the empty bags, W
2 is the weight of the sample, W
3 is the final weight of the bag after incubation, W
4 is the blank correction, and W
5 is the final weight of the bag after analyzing the NDF.
The data obtained from the variables correlated with ruminal fermentation, obtained from the analysis of sixteen diets using two different sources of ruminal inoculum (
Table 3), were subjected to different multivariate analyzes to determine the reliability of the ruminal inoculum of animals slaughtered in commercial slaughterhouses compared to that obtained from cannulated animals.
The analysis of relative contribution within the total variation was carried out using the Singh method [
32], resulting in the discarding of variables with a contribution of less than 1%.
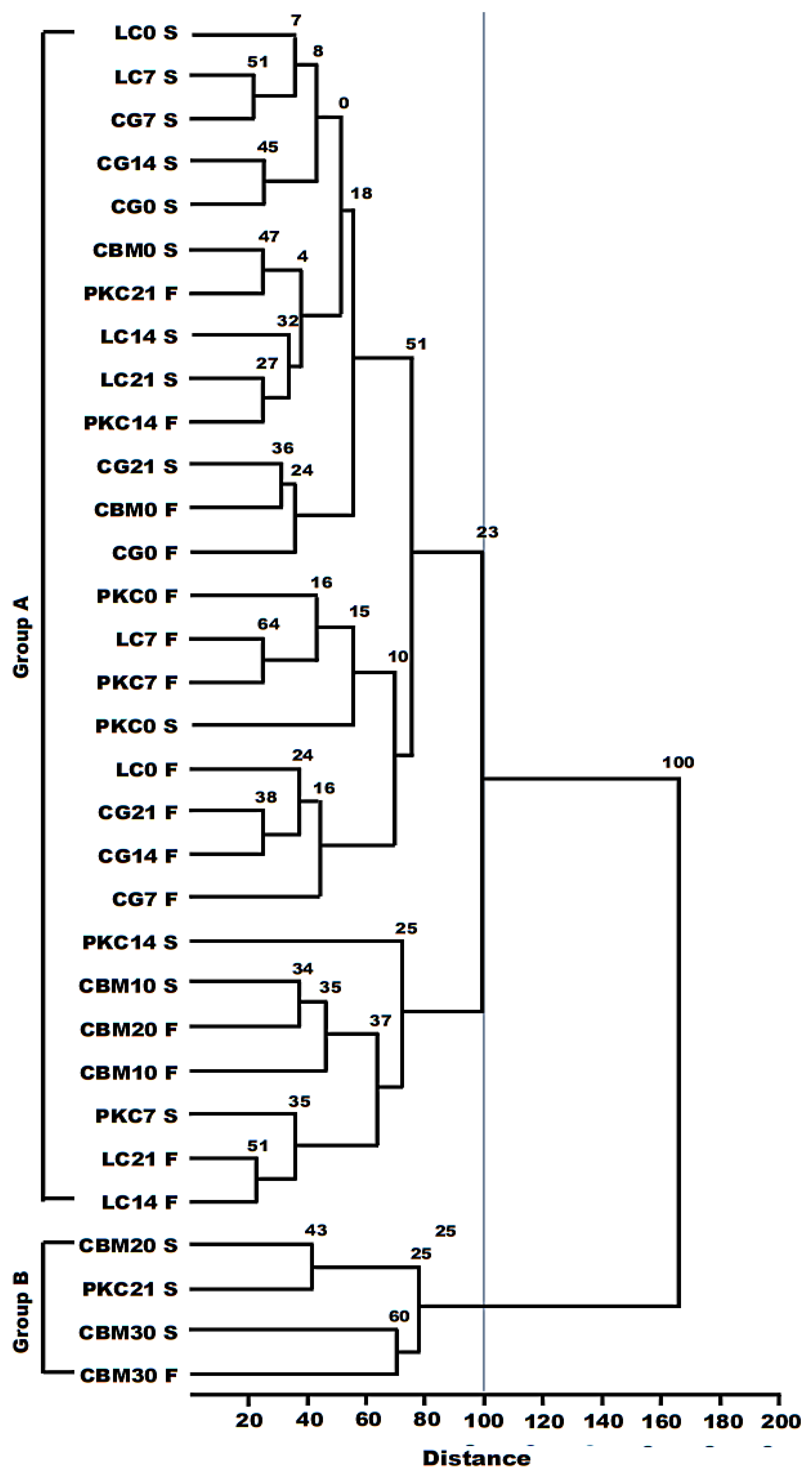
From the data, a divergence analysis was carried out between the diets for quantitative variables. The matrix obtained was based on the Euclidean distance, using the Tocher’s optimization method considered the criterion that the average inter-group distance should be higher than the average intra-group distance. Furthermore, the hierarchical average clustering between groups was obtained using the UPGMA hierarchical method (unweighted pair group method with arithmetic mean). From this analysis, a dendrogram was generated with the groups with the greatest similarity. The consistency of the clusters using the hierarchical method was verified using the cophenetic correlation coefficient (CCC) with 1000 permutations.
Principal component analysis is performed to synthesize the data obtained into a set of variables with a smaller number of linear combinations of orthogonal variables that are called principal components. In this way, this analysis sequentially minimizes the variation of the data. The new condensed loadings show the relative importance (weighting) of the original data in explaining the variation in the observed data. Furthermore, to define the names of the principal components, the eigenvalues in each component (magnitude of relative contribution or relative importance of the characteristics) was observed. The multivariate statistical procedures were performed using the computer program Genes [
33]. In the principal component analysis, the first components that together explained at least 80% of the total variation were selected for discussion [
34].
4. Discussion
The ruminal inoculum is essential for in vitro fermentation trials, but it also represents the greatest source of variation in these analysis systems [
18]. Cannulated cattle are generally used to obtain the ruminal inoculum, and even with this method, it is recommended that the animals be adapted to specific diets. According to Camacho et al. [
35], the rumen microbiology of animals not adapted to diets containing concentrate and forage can be a source of variation for the in vitro analysis of feed digestibility. To avoid sources of variation that affect the ruminal inoculum, sampling should be similar in time and collection method as described by Fortina et al. [
21]. Using univariate analysis methods, Alba et al. [
20] concluded that the rumen inoculum of cattle in slaughterhouses with unknown data, such as feeding, management, sex, breed, and others, can be used as a potential substitute for rumen inoculum obtained from cannulated cattle. However, the authors found some differences, as did other authors who used other sources of ruminal inoculum [
36]. Therefore, we believe that multivariate analysis can help us find what is or are the factors that must be taken into account when using this source of rumen inoculum.
For this reason, in the current study, sixteen nutritionally different diets were used to evaluate the viability of ruminal inoculum from slaughtered cattle compared to ruminal inoculum from cannulated cattle (inoculum commonly used in in vitro evaluation of cattle feed). To determine the viability of this alternative ruminal inoculum, nine variables correlated with ruminal fermentation were analyzed through a multivariate analysis. The variables correlated with the ruminal fermentation analyzed were IVDMD, IVNDFD, rumen ammonia nitrogen, pH, protozoa content, acetate–propionate ratio, and concentrations of acetate, propionate, and butyrate. The statistic evaluation of these variables was carried out using multivariate analysis methods to determine the viability of the ruminal inoculum from cattle slaughtered at commercial slaughterhouse and to determine if the limiting variable in the in vitro evaluation is the source of the ruminal inoculum or a different variable.
According to the relative contribution [
32] of the variables correlated with ruminal fermentation, the IVDMD, IVNDFD, and acetic acid production influence 90.19% of the determination of the viability of the source of ruminal inoculum from slaughtered cattle. It is important to note that these variables are highly correlated with each other and with the NDF content of the diet. The content and characteristics of NDF present in the diet affect its digestibility and this directly influences the digestibility of DM [
37,
38]. Furthermore, the amount and digestibility of NDF are directly responsible for the increase or decrease in acetate production [
39,
40]. In an extensive review, Foster et al. [
36] observed that even if all the variables of the animals used as a source of ruminal inoculum were controlled, the cell wall components of the feed are the main components that could affect the digestibility of dry matter or organic matter in in vitro studies. When equine feces were used as an alternative inoculum source in in vitro trials, significant differences between ruminal inoculum sources were observed for IVDMD and IVNDFD [
41]. The objective of the principal component analysis is to reduce the number of variables found in the relative contribution method (Singh method) to a smaller number of orthogonal variables that represent the greatest degree of variation in the study. In the current study, two variables, represented by IVNDFD and acetate production, were found to account for the greatest degree of variability. Therefore, it can be inferred that the fiber content of the evaluated diets was limiting to determine the viability of the ruminal inoculum from slaughtered cattle.
To corroborate our theory, the grouping of the diets via the Tocher optimization method and the dendrogram obtained via the hierarchical grouping between groups method separated the diets with the highest level of lignin into different groups. It is also possible to observe, in the dendrogram, a distance of 60% between the CBM30 S and CBM30 F samples. Therefore, the indigestibility characteristics of the fiber present in the diets were the most responsible in determining the viability of the ruminal inoculum obtained from slaughtered cattle.
The quality and source of the ruminal inoculum directly influence the in vitro evaluation of feed for cattle. The inoculum can be significantly affected by the feed offered to the animals, which can result in differences in the populations of microorganisms in the rumen [
18,
42,
43,
44]. This variation affects the digestibility of the nutritional components of the diet.
Therefore, the apparent digestibility of the diet and NDF is higher when the ruminal inoculum comes from cannulated cattle supplied with mixed feed (forage and concentrate) compared to the inoculum from animals fed only pasture or hay [
45,
46,
47].
The lignin content of the diet has a negative correlation of up to 93% with NDF digestibility, promoted by the interaction between the carbohydrate fractions of the cell wall and the phenolic components of lignin (coumaric and ferulic acids) [
48]. Among these components, ferulic acid has the greatest negative influence on digestibility [
49].
This lignin component is found in smaller amounts in green forages and in higher concentrations in concentrated components or in dry forages [
50]. Brachiaria brizantha grass has ferulic acid concentrations between 2.58 and 4.43 g/kg DM [
51]. The concentration of this lignin component is 13.9 g/kg DM in Tifton 85 hay [
52] and 22.4 g/kg DM in corn bran [
50].
The CBM, PKC, LC, and GB diets have castor bean meal, palm kernel cake, licuri cake, and crude glycerin as different ingredients, respectively. Licuri does not contain ferulic acid in its composition [
53], while castor bean meal and palm kernel cake have ferulic acid as one of the main phenolic compounds in their composition [
54,
55].
Ferulic acid can be degraded by some ruminal microorganisms through different mechanisms, as well as non-oxidative decarboxylation, demethylation, b-oxidation, coenzyme A-independent deacetylation, side-chain reduction, and direct deacetylation [
49]. However, a prior adaptation of ruminal microorganisms to this compound is possible, so its degradability is significant. As observed in Chiaravalli et al. [
43] and Kilama et al. [
56], lignin is the most important component of NDF or non-degradable NDF that can affect the digestibility of the dry matter or organic matter of foods used in feeding ruminants.
In the current experiment, the cannulated cattle were fed with diets containing concentrate while the diet of the slaughtered cattle was unknown. However, in the region where the commercial slaughterhouse is located (Bahía, Brazil), more than 90% of the cattle are managed in an extensive system [
57]. In this sense, it is possible to infer that the animals did not receive concentrate supplementation in the diet and were fed predominantly with pasture. Therefore, it is possible to affirm that for the source of ruminal inoculum from slaughter cattle to be viable for the in vitro study of diets with concentrates for cattle, the donor animals must be adapted with diets with concentrates.