Alkaline Phosphatase (ALP) in Non-Cow Milk and Dairy Products: A Review of Current Evidence and Future Trends
Abstract
1. Introduction
2. Methodology
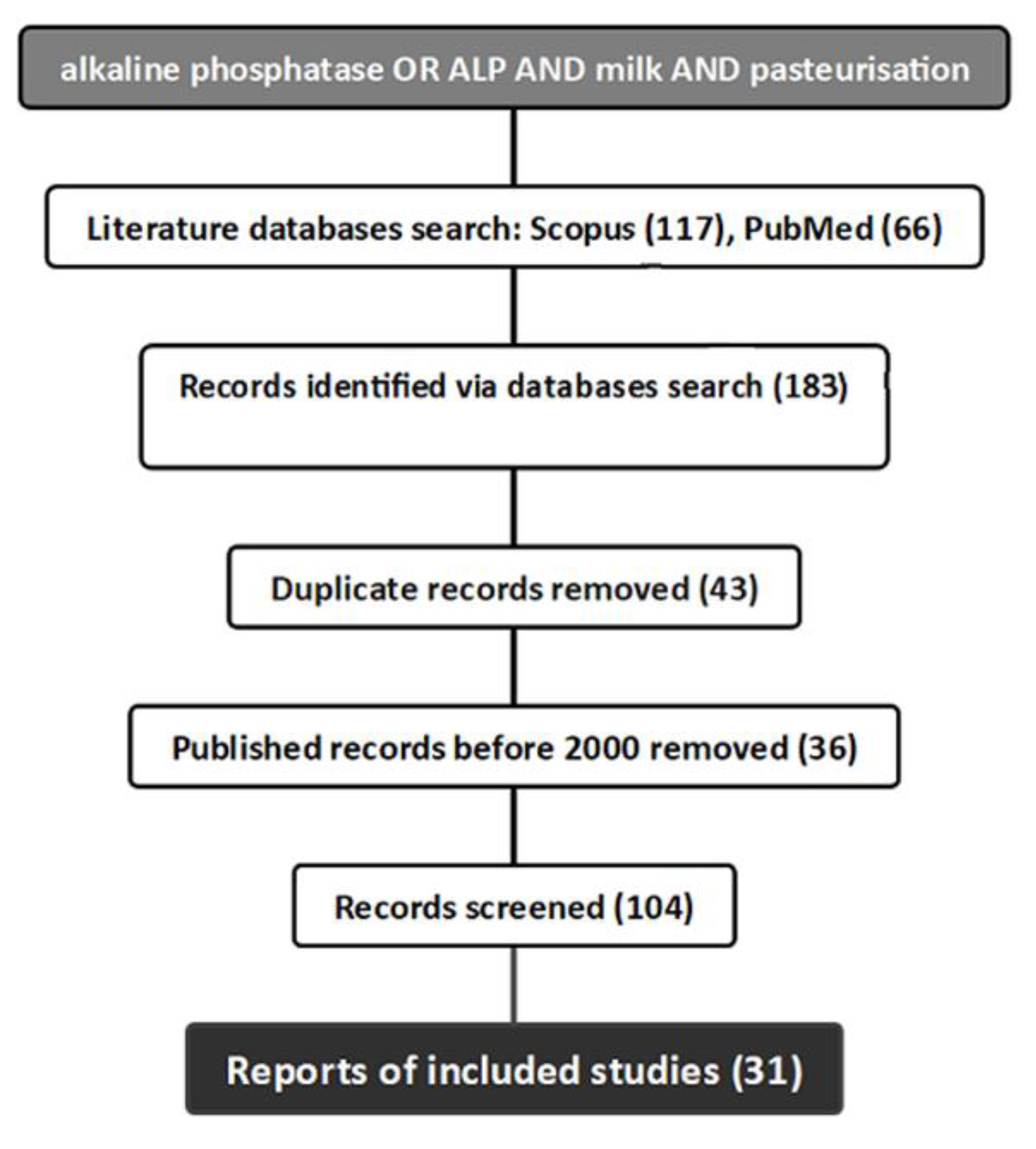
2.1. Search Strategy and Data Sources
2.2. Inclusion/Exclusion Criteria
2.3. Study Selection and Data Extraction
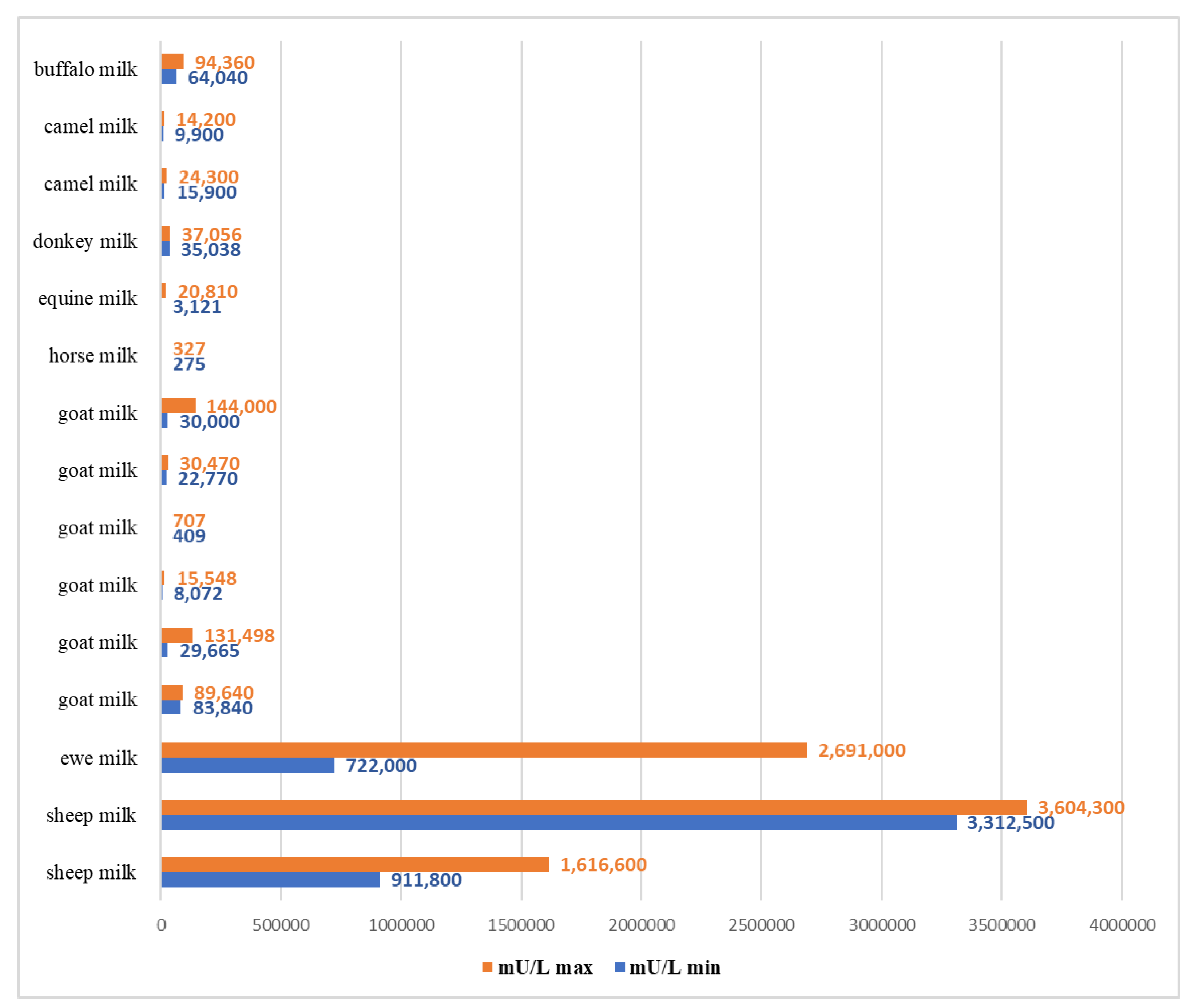
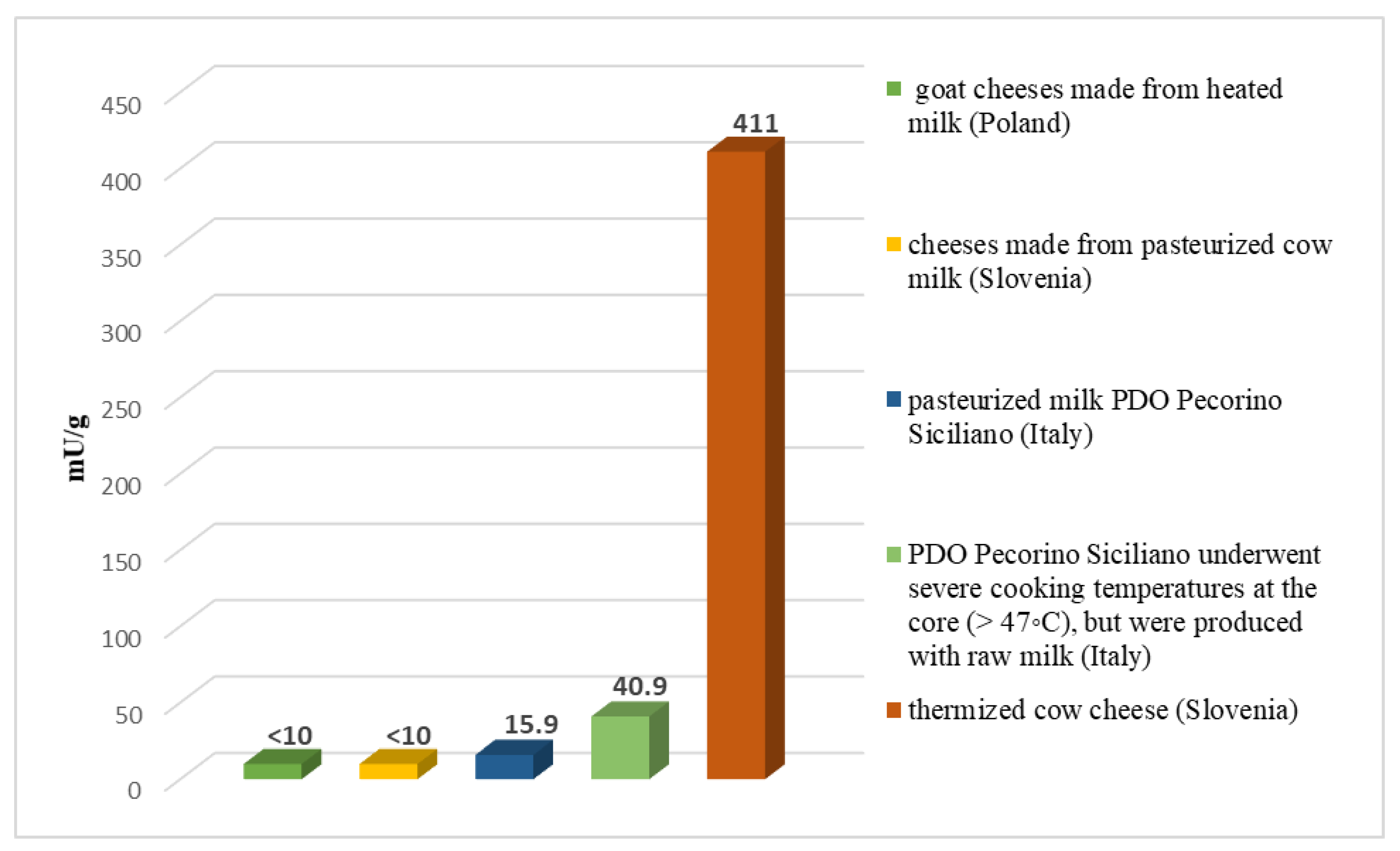
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gerosa, S.; Skoet, J. Milk Availability: Trends in Production and Demand and Medium-Term Outlook; ESA Working Papers; 289000; Food and Agriculture Organization of the United Nations, Agricultural Development Economics Division (ESA): Rome, Italy, 2012. [Google Scholar]
- Faye, B.; Konuspayeva, G. The Sustainability Challenge to the Dairy Sector–The Growing Importance of Non-Cattle Milk Production Worldwide. Int. Dairy J. 2012, 24, 50–56. [Google Scholar] [CrossRef]
- FAO.org. Available online: https://www.fao.org/faolex/results/details/en/c/LEX-FAOC087031/ (accessed on 23 July 2022).
- Ritota, M.; Di Costanzo, M.G.; Mattera, M.; Manzi, P. New Trends for the Evaluation of Heat Treatments of Milk. J. Anal. Methods Chem. 2017, 2017, e1864832. [Google Scholar] [CrossRef]
- Rankin, S.A.; Christiansen, A.; Lee, W.; Banavara, D.S.; Lopez-Hernandez, A. Invited Review: The Application of Alkaline Phosphatase Assays for the Validation of Milk Product Pasteurization. J. Dairy Sci. 2010, 93, 5538–5551. [Google Scholar] [CrossRef]
- Suzuki, U.; Yoshimura, K.; Takaishi, M. About the Enzyme “Phytase”, Which Splits “Anhydro-Oxy-Methylene Diphosphoric Acid”. Bull. Coll. Agric. Tokyo Imp. Univ. 1907, 7, 503–512. [Google Scholar]
- Shakeel-ur-Rehman; Fleming, C.M.; Farkye, N.Y.; Fox, P.F. Indigenous Phosphatases in Milk. In Advanced Dairy Chemistry—1 Proteins: Part A/Part B; Fox, P.F., McSweeney, P.L.H., Eds.; Springer: Boston, MA, USA, 2003; pp. 523–543. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA); Clawin-Rädecker, I.; De Block, J.; Egger, L.; Willis, C.; Da Silva Felicio, M.T.; Messens, W. The Use of Alkaline Phosphatase and Possible Alternative Testing to Verify Pasteurisation of Raw Milk, Colostrum, Dairy and Colostrum-Based Products. EFSA J. Eur. Food Saf. Auth. 2021, 19, e06576. [Google Scholar] [CrossRef]
- Kosikowski, F.V. Enzyme Behavior and Utilization in Dairy Technology. J. Dairy Sci. 1988, 71, 557–573. [Google Scholar] [CrossRef]
- Claeys, W.L.; Van Loey, A.M.; Hendrickx, M.E. Kinetics of Alkaline Phosphatase and Lactoperoxidase Inactivation, and of Beta-Lactoglobulin Denaturation in Milk with Different Fat Content. J. Dairy Res. 2002, 69, 541–553. [Google Scholar] [CrossRef]
- Vega-Warner, A.V.; Wang, C.-H.; Smith, D.M.; Ustunol, Z. Milk Alkaline Phosphatase Purification and Production of Polyclonal Antibodies. J. Food Sci. 1999, 64, 601–605. [Google Scholar] [CrossRef]
- Rademacher, B.; Hinrichs, J. Effects of High Pressure Treatment on Indigenous Enzymes in Bovine Milk: Reaction Kinetics, Inactivation and Potential Application. Int. Dairy J. 2006, 16, 655–661. [Google Scholar] [CrossRef]
- Lorenzen, P.C.; Martin, D.; Clawin-Rädecker, I.; Barth, K.; Knappstein, K. Activities of Alkaline Phosphatase, γ-Glutamyltransferase and Lactoperoxidase in Cow, Sheep and Goat’s Milk in Relation to Heat Treatment. Small Rumin. Res. 2010, 89, 18–23. [Google Scholar] [CrossRef]
- Marchand, S.; Merchiers, M.; Messens, W.; Coudijzer, K.; Block, J. Thermal Inactivation Kinetics of Alkaline Phosphatase in Equine Milk. Int. Dairy J. 2009, 19, 763–767. [Google Scholar] [CrossRef]
- Raynal-Ljutovac, K.; Park, Y.W.; Gaucheron, F.; Bouhallab, S. Heat Stability and Enzymatic Modifications of Goat and Sheep Milk. Small Rumin. Res. 2007, 68, 207–220. [Google Scholar] [CrossRef]
- Tziboula-Clarke, A. Goat Milk. Encycl. Dairy Sci. 2003, 2, 1270–1279. [Google Scholar]
- Vamvakaki, A.-N.; Zoidou, E.; Moatsou, G.; Bokari, M.; Anifantakis, E. Residual Alkaline Phosphatase Activity after Heat Treatment of Ovine and Caprine Milk. Small Rumin. Res. 2006, 65, 237–241. [Google Scholar] [CrossRef]
- Harding, F.; Garry, E. Collaborative Evaluation of a Fluorometric Method for Measuring Alkaline Phosphatase Activity in Cow’s, Sheep’s, and Goat’s Milk. J. Food Prot. 2005, 68, 1047–1053. [Google Scholar] [CrossRef]
- Moatsou, G. Indigenous Enzymatic Activities in Ovine and Caprine Milks. Int. J. Dairy Technol. 2010, 63, 16–31. [Google Scholar] [CrossRef]
- Scintu, M.F.; Daga, E.; Ledda, A. Evaluation of Spectrophotometric and Fluorometric Methods for Alkaline Phosphatase Activity Determination in Ewe’s Milk. J. Food Prot. 2000, 63, 1258–1261. [Google Scholar] [CrossRef]
- Salter, R.S.; Fitchen, J. Evaluation of a Chemiluminescence Method for Measuring Alkaline Phosphatase Activity in Whole Milk of Multiple Species and Bovine Dairy Drinks: Interlaboratory Study. J. AOAC Int. 2006, 89, 1061–1070. [Google Scholar] [CrossRef]
- Klotz, V.; Hill, A.; Warriner, K.; Griffiths, M.; Odumeru, J. Assessment of the Colorimetric and Fluorometric Assays for Alkaline Phosphatase Activity in Cow’s, Goat’s, and Sheep’s Milk. J. Food Prot. 2008, 71, 1884–1888. [Google Scholar] [CrossRef]
- Jakób, A.; Bryjak, J.; Wojtowicz, H.; Illeová, V.; Annus, J.; Polakovič, M. Inactivation Kinetics of Food Enzymes during Ohmic Heating. Food Chem. 2010, 123, 369–376. [Google Scholar] [CrossRef]
- Albillos, S.M.; Reddy, R.; Salter, R. Evaluation of Alkaline Phosphatase Detection in Dairy Products Using a Modified Rapid Chemiluminescent Method and Official Methods. J. Food Prot. 2011, 74, 1144–1154. [Google Scholar] [CrossRef] [PubMed]
- Dumitrascu, L.; Nicoleta, S.; Stanciu, S.; Rapeanu, G. Inactivation Kinetics of Alkaline Phosphatase from Different Species of Milk Using Quinolyl Phosphate as a Substrate. Food Sci. Biotechnol. 2014, 23, 1773–1778. [Google Scholar] [CrossRef]
- Sevastou, A.; Tragoulias, S.S.; Kalogianni, D.P.; Christopoulos, T.K. Mix-and-Read Method for Assessment of Milk Pasteurization Using a Smartphone or a Common Digital Camera. Anal. Bioanal. Chem. 2020, 412, 5663–5669. [Google Scholar] [CrossRef]
- Moatsou, G.; Moschopoulou, E.; Zoidou, E.; Kamvysi, A.; Liaskou, D.; Tsigkou, V.; Sakkas, L. Changes in Native Whey Protein Content, Gel Formation, and Endogenous Enzyme Activities Induced by Flow-Through Heat Treatments of Goat and Sheep Milk. Dairy 2021, 2, 410–421. [Google Scholar] [CrossRef]
- Faría, R.J.F.; García, U.; García, A.; Tovar, V. A Efficiency of Pasteurization the Goat Milk in Cheesemaking Miniplant. Rev. Cient. Fac. Cienc. Vet. Univ. Zulia 2000, 10, 119–123. [Google Scholar]
- Sharma, S.K.; Sehgal, N.; Kumar, A. Dry-Reagent Strips for Testing Milk Pasteurization. LWT -Food Sci. Technol. 2003, 36, 567–571. [Google Scholar] [CrossRef]
- Sharma, R.; Kaur, S.; Rajput, Y.; Kumar, R. Activity and Thermal Stability of Indigenous Enzymes in Cow, Buffalo and Goat Milk. Milchwissenschaft 2009, 64, 173–175. [Google Scholar]
- Rola, J.; Sosnowski, M. Determination of Alkaline Phosphatase Activity in Goat Milk and Cheese by Fluorimetric Method as a Verification of Efficacy of Pasteurisation Process. Bull. Vet. Inst. Pulawy 2011, 55, 705–708. [Google Scholar]
- Ziobro, G.C.; McElroy, K.M. Fluorometric Detection of Active Alkaline Phosphatase and Gamma-Glutamyl Transferase in Fluid Dairy Products from Multiple Species. J. Food Prot. 2013, 76, 892–898. [Google Scholar] [CrossRef]
- Sosnowski, M.; Rola, J.G.; Osek, J. Alkaline Phosphatase Activity and Microbiological Quality of Heat-Treated Goat Milk and Cheeses. Small Rumin. Res. 2016, 136, 132–136. [Google Scholar] [CrossRef]
- Lombardi, P.; Avallone, L.; d’Angelo, A.; Mor, T.; Bogin, E. Buffalo-Milk Enzyme Levels, Their Sensitivity to Heat Inactivation, and Their Possible Use as Markers for Pasteurization. J. Food Prot. 2000, 63, 970–973. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, L.S.B.; Verma, N. A Three Step Approach for the Purification of Alkaline Phosphatase from Non-Pasteurized Milk. J. Food Sci. Technol. 2015, 52, 3140–3146. [Google Scholar] [CrossRef] [PubMed]
- Lakra, S.; Jadhav, V.J.; Garg, S.R. Development of a Chromatographic Method for the Determination of Alkaline Phosphatase Activity in Pasteurized Milk. Food Anal. Methods 2016, 9, 2002–2009. [Google Scholar] [CrossRef]
- Jadhav, V.J.; Badgujar, P.C. Development of a HPLC Fluorescence Method for Determining Efficacy of Milk Pasteurization. Food Anal. Methods 2021, 14, 260–267. [Google Scholar] [CrossRef]
- Giacometti, F.; Bardasi, L.; Merialdi, G.; Morbarigazzi, M.; Federici, S.; Piva, S.; Serraino, A. Shelf Life of Donkey Milk Subjected to Different Treatment and Storage Conditions. J. Dairy Sci. 2016, 99, 4291–4299. [Google Scholar] [CrossRef] [PubMed]
- Wernery, U.; Maier, U.; Johnson, B.; George, R.M.; Braun, F. Comparative Study on Different Enzymes Evaluating Heat Treatment of Dromedary Milk. Milchwissenschaft 2006, 61, 281–285. [Google Scholar]
- Wernery, U.; Fischbach, S.; Johnson, B.; Jose, S. Evaluation of Alkaline Phosphatase (ALP), γ-Glutamyl Transferase (GGT) and Lactoperoxidase (LPO) Activities for Their Suitability as Markers of Camel Milk Heat Inactivation. Milchwissenschaft 2008, 63, 265–267. [Google Scholar]
- Lorenzen, P.; Wernery, R.; Johnson, B.; Jose, S.; Wernery, U. Evaluation of Indigenous Enzymes in Raw and Pasteurised Camel Milk. Small Rumin. Res. 2011, 97, 79–82. [Google Scholar] [CrossRef]
- Yoshitomi, K. Alkaline Phosphatase Activity in Cheeses Measured by Fluorometry. Int. J. Food Sci. Technol. 2004, 39, 349–353. [Google Scholar] [CrossRef]
- Hodošček, L.; Rupnik, S.; Ahčin, K.; Biasizzo, M. Alkaline Phosphatase Activity in Slovenian Cheese Made from Pasteurized, Thermized or Raw Milk. Acta Agric. Slov. 2012, 100, 265–268. [Google Scholar]
- Todaro, M.; Lo Presti, V.; Macaluso, A.; Alleri, M.; Licitra, G.; Chiofalo, V. Alkaline Phosphatase Survey in Pecorino Siciliano PDO Cheese. Foods Basel Switz. 2021, 10, 1648. [Google Scholar] [CrossRef] [PubMed]
- Malissiova, E.; Alexandraki, M.; Manouras, A. Preliminary Data on the Suitability of Alkaline Phosphatase Use as Pasteurization Indicator for Donkey Milk. Acta Vet. Eurasia 2022. [Google Scholar] [CrossRef]
- Tsiamita, A.; Valiakos, G.; Natsaridis, N.; Fotiadou, S.; Manouras, A.; Malissiova, E. Preliminary Results on the Comparative Evaluation of Alkaline Phosphatase Commercial Tests Efficiency in Non-Cow Milk Pasteurization. BioTech 2022, 11, 39. [Google Scholar] [CrossRef] [PubMed]
- Ying, C.; Wang, H.-T.; Hsu, J.-T. Relationship of Somatic Cell Count, Physical, Chemical and Enzymatic Properties to the Bacterial Standard Plate Count in Dairy Goat Milk. Livest. Prod. Sci. 2002, 74, 63–77. [Google Scholar] [CrossRef]
- Persson, Y.; Larsen, T.; Nyman, A.-K. Variation in Udder Health Indicators at Different Stages of Lactation in Goats with No Udder Infection. Small Rumin. Res. 2014, 116, 51–56. [Google Scholar] [CrossRef][Green Version]
- Katsoulos, P.D.; Christodoulopoulos, G.; Minas, A.; Karatzia, M.A.; Pourliotis, K.; Kritas, S.K. The Role of Lactate Dehydrogenase, Alkaline Phosphatase and Aspartate Aminotransferase in the Diagnosis of Subclinical Intramammary Infections in Dairy Sheep and Goats. J. Dairy Res. 2010, 77, 107–111. [Google Scholar] [CrossRef]
- Narenji Sani, R.; Hajigolikhani, B.; Ahmadi-Hamedani, M.; Kafshdouzan, K. Diagnostic Evaluation of Milk Lactate Dehydrogenase and Alkaline Phosphatase Activities by Receiver Operating Characteristic Analysis Curve in Early Lactation of Ewes with Subclinical Mastitis. Vet. Res. Forum 2018, 9, 343–348. [Google Scholar] [CrossRef]
- Sharma, R.S.; Ganguli, N.C. Purification and Properties of Reactivated Alkaline Phosphatase from Buffalo Milk. Milchwissenschaft 1974, 29, 79–84. [Google Scholar]
- Patil, M.P.; Nagvekar, A.S.; Ingole, S.D.; Bharucha, S.V.; Palve, V.T. Somatic Cell Count and Alkaline Phosphatase Activity in Milk for Evaluation of Mastitis in Buffalo. Vet. World 2015, 8, 363–366. [Google Scholar] [CrossRef]
- Izadi, A.; Khedmat, L.; Mojtahedi, S.Y. Nutritional and Therapeutic Perspectives of Camel Milk and Its Protein Hydrolysates: A Review on Versatile Biofunctional Properties. J. Funct. Foods 2019, 60, 103441. [Google Scholar] [CrossRef]
- Aspri, M.; Economou, N.; Papademas, P. Donkey Milk: An Overview on Functionality, Technology, and Future Prospects. Food Rev. Int. 2017, 33, 316–333. [Google Scholar] [CrossRef]


| Year | Title | Country | Reference |
|---|---|---|---|
| Sheep milk | |||
| 2000 | Evaluation of spectrophotometric and fluorometric methods for alkaline phosphatase activity determination in ewe’s milk | Italy | [20] |
| 2005 | Collaborative evaluation of a fluorometric method for measuring alkaline phosphatase activity in cow’s, sheep’s, and goat’s milk | UK, USA | [18] |
| 2006 | Evaluation of a chemiluminescence method for measuring alkaline phosphatase activity in whole milk of multiple species and bovine dairy drinks: Interlaboratory study | USA | [21] |
| 2006 | Residual alkaline phosphatase activity after heat treatment of ovine and caprine milk | Greece | [17] |
| 2007 | Heat stability and enzymatic modifications of goat and sheep milk | France, USA | [15] |
| 2008 | Assessment of the colorimetric and fluorometric assays for alkaline phosphatase activity in cow’s, goat’s, and sheep’s milk | Canada | [22] |
| 2010 | Inactivation kinetics of food enzymes during ohmic heating | Slovak | [23] |
| 2010 | Activities of alkaline phosphatase, γ-glutamyltransferase and lactoperoxidase in cow, sheep and goat’s milk in relation to heat treatment | Germany | [13] |
| 2011 | Evaluation of Alkaline Phosphatase Detection in Dairy Products Using a Modified Rapid Chemiluminescent Method and Official Methods | USA | [24] |
| 2014 | Inactivation kinetics of alkaline phosphatase from different species of milk using quinolyl phosphate as a substrate | Romania | [25] |
| 2020 | Mix-and-read method for assessment of milk pasteurization using a smartphone or a common digital camera | Greece | [26] |
| 2021 | Changes in Native Whey Protein Content, Gel Formation, and Endogenous Enzyme Activities Induced by Flow-Through Heat Treatments of Goat and Sheep Milk | Greece | [27] |
| Goat milk | |||
| 2000 | Efficiency of Pasteurization the Goat Milk in Cheesemaking Miniplant | Venezouela | [28] |
| 2003 | Dry-reagent strips for testing milk pasteurization | India | [29] |
| 2005 | Collaborative evaluation of a fluorometric method for measuring alkaline phosphatase activity in cow’s, sheep’s, and goat’s milk | UK, USA | [18] |
| 2006 | Evaluation of a chemiluminescence method for measuring alkaline phosphatase activity in whole milk of multiple species and bovine dairy drinks: Interlaboratory study | USA | [21] |
| 2006 | Residual alkaline phosphatase activity after heat treatment of ovine and caprine milk | Greece | [17] |
| 2007 | Heat stability and enzymatic modifications of goat and sheep milk | France, USA | [15] |
| 2008 | Assessment of the colorimetric and fluorometric assays for alkaline phosphatase activity in cow’s, goat’s, and sheep’s milk | Canada | [22] |
| 2009 | Activity and thermal stability of indigenous enzymes in cow, buffalo and goat milk | India | [30] |
| 2010 | Inactivation kinetics of food enzymes during ohmic heating | Slovak | [23] |
| 2010 | Activities of alkaline phosphatase, γ-glutamyltransferase and lactoperoxidase in cow, sheep and goat’s milk in relation to heat treatment | Germany | [13] |
| 2011 | Evaluation of alkaline phosphatase detection in dairy products using a modified rapid chemiluminescent method and official methods | USA | [24] |
| 2011 | Determination of alkaline phosphatase activity in goat milk and cheese by fluorimetric method as a verification of efficacy of pasteurisation process | Poland | [31] |
| 2013 | Fluorometric detection of active alkaline phosphatase and gamma-glutamyl transferase in fluid dairy products from multiple species | USA | [32] |
| 2014 | Inactivation kinetics of alkaline phosphatase from different species of milk using quinolyl phosphate as a substrate | Romania | [25] |
| 2016 | Alkaline phosphatase activity and microbiological quality of heat-treated goat milk and cheeses | Poland | [33] |
| 2021 | The use of alkaline phosphatase and possible alternative testing to verify pasteurisation of raw milk, colostrum, dairy and colostrum-based products | EU | [8] |
| 2021 | Changes in Native Whey Protein Content, Gel Formation, and Endogenous Enzyme Activities Induced by Flow-Through Heat Treatments of Goat and Sheep Milk | Greece | [27] |
| Buffalo milk | |||
| 2000 | Buffalo-milk enzyme levels, their sensitivity to heat inactivation, and their possible use as markers for pasteurization | Italy, Israel | [34] |
| 2003 | Dry-reagent strips for testing milk pasteurization | India | [29] |
| 2006 | Evaluation of a chemiluminescence method for measuring alkaline phosphatase activity in whole milk of multiple species and bovine dairy drinks: Interlaboratory study | USA | [21] |
| 2009 | Activity and thermal stability of indigenous enzymes in cow, buffalo and goat milk | India | [30] |
| 2015 | A three step approach for the purification of alkaline phosphatase from non-pasteurized milk | India | [35] |
| 2016 | Development of a Chromatographic Method for the Determination of Alkaline Phosphatase Activity in Pasteurized Milk | India | [36] |
| 2021 | The use of alkaline phosphatase and possible alternative testing to verify pasteurisation of raw milk, colostrum, dairy and colostrum-based products | EU | [8] |
| 2021 | Development of a HPLC Fluorescence Method for Determining Efficacy of Milk Pasteurization | India | [37] |
| Equid milk | |||
| 2009 | Thermal inactivation kinetics of alkaline phosphatase in equine milk | Belgium | [14] |
| 2013 | Fluorometric detection of active alkaline phosphatase and gamma-glutamyl transferase in fluid dairy products from multiple species | USA | [32] |
| 2016 | Shelf life of donkey milk subjected to different treatment and storage conditions | Italy | [38] |
| 2021 | The use of alkaline phosphatase and possible alternative testing to verify pasteurisation of raw milk, colostrum, dairy and colostrum-based products | EU | [8] |
| Camel milk | |||
| 2006 | Comparative study on different enzymes evaluating heat treatment of dromedary milk | n/a | [39] |
| 2008 | Evaluation of alkaline phosphatase (ALP), γ-glutamyl transferase (GGT) and lactoperoxidase (LPO) activities for their suitability as markers of camel milk heat inactivation | n/a | [40] |
| 2011 | Evaluation of indigenous enzymes in raw and pasteurised camel milk | Germany | [41] |
| 2021 | The use of alkaline phosphatase and possible alternative testing to verify pasteurisation of raw milk, colostrum, dairy and colostrum-based products | EU | [8] |
| Non-cow dairy products | |||
| 2000 | Efficiency of Pasteurization the Goat Milk in Cheesemaking Miniplant | Venezouela | [28] |
| 2004 | Alkaline Phosphatase Activity in Cheeses Measured by Fluorometry | USA | [42] |
| 2006 | Evaluation of a chemiluminescence method for measuring alkaline phosphatase activity in whole milk of multiple species and bovine dairy drinks: Interlaboratory study | USA | [21] |
| 2009 | Thermal inactivation kinetics of alkaline phosphatase in equine milk | Belgium | [14] |
| 2011 | Evaluation of alkaline phosphatase detection in dairy products using a modified rapid chemiluminescent method and official methods | USA | [24] |
| 2011 | Determination of alkaline phosphatase activity in goat milk and cheese by fluorimetric method as a verification of efficacy of pasteurisation process | Poland | [31] |
| 2012 | Alkaline phosphatase activity in Slovenian cheese made from pasteurized, thermized or raw milk | Slovenia | [43] |
| 2013 | Fluorometric detection of active alkaline phosphatase and gamma-glutamyl transferase in fluid dairy products from multiple species | USA | [32] |
| 2016 | Alkaline phosphatase activity and microbiological quality of heat-treated goat milk and cheeses | Poland | [33] |
| 2021 | The use of alkaline phosphatase and possible alternative testing to verify pasteurisation of raw milk, colostrum, dairy and colostrum-based products | ΕU | [8] |
| 2021 | Changes in Native Whey Protein Content, Gel Formation, and Endogenous Enzyme Activities Induced by Flow-Through Heat Treatments of Goat and Sheep Milk | Greece | [27] |
| 2021 | Alkaline Phosphatase Survey in Pecorino Siciliano PDO Cheese | Italy | [44] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malissiova, E.; Fotiadou, S.; Tzereme, A.; Cheimona, D.; Soultani, G.; Maisoglou, I.; Manouras, A. Alkaline Phosphatase (ALP) in Non-Cow Milk and Dairy Products: A Review of Current Evidence and Future Trends. Ruminants 2022, 2, 435-447. https://doi.org/10.3390/ruminants2040030
Malissiova E, Fotiadou S, Tzereme A, Cheimona D, Soultani G, Maisoglou I, Manouras A. Alkaline Phosphatase (ALP) in Non-Cow Milk and Dairy Products: A Review of Current Evidence and Future Trends. Ruminants. 2022; 2(4):435-447. https://doi.org/10.3390/ruminants2040030
Chicago/Turabian StyleMalissiova, Eleni, Stamatia Fotiadou, Anastasia Tzereme, Dimitra Cheimona, Georgia Soultani, Ioannis Maisoglou, and Athanasios Manouras. 2022. "Alkaline Phosphatase (ALP) in Non-Cow Milk and Dairy Products: A Review of Current Evidence and Future Trends" Ruminants 2, no. 4: 435-447. https://doi.org/10.3390/ruminants2040030
APA StyleMalissiova, E., Fotiadou, S., Tzereme, A., Cheimona, D., Soultani, G., Maisoglou, I., & Manouras, A. (2022). Alkaline Phosphatase (ALP) in Non-Cow Milk and Dairy Products: A Review of Current Evidence and Future Trends. Ruminants, 2(4), 435-447. https://doi.org/10.3390/ruminants2040030