Detection of Anaplasma phagocytophilum in Ovine Serum Samples—A Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Samples and Serological Examination
2.2. Molecular Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stuen, S.; Granquist, E.G.; Silaghi, C. Anaplasma phagocytophilum—A widespread multi-host pathogen with highly adaptive strategies. Front. Cell. Infect. Microbiol. 2013, 3, 31. [Google Scholar] [CrossRef] [PubMed]
- Rymaszewska, A.; Grenda, S. Bacteria of the genus Anaplasma—Characteristics of Anaplasma and their vectors: A review. Vet. Med. 2008, 53, 573–584. [Google Scholar] [CrossRef]
- Henniger, T.; Henniger, P.; Grossmann, T.; Distl, O.; Ganter, M.; von Loewenich, F.D. Congenital infection with Anaplasma phagocytophilum in a calf in Northern Germany. Acta Vet. Scand. Suppl. 2013, 55, 38. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.; Simon, D.; Escobar, H.M.; Soller, J.T.; Bullerdiek, J.; Beelitz, P.; Pfister, K.; Nolte, I. Anaplasma phagocytophilum in dogs in Germany. Zoonoses Public Health 2007, 54, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Kohn, B.; Silaghi, C.; Galke, D.; Arndt, G.; Pfister, K. Infections with Anaplasma phagocytophilum in dogs in Germany. Res. Vet. Sci. 2011, 91, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Hamel, D.; Bondarenko, A.; Silaghi, C.; Nolte, I.; Pfister, K. Seroprevalence and bacteraemia of Anaplasma phagocytophilum in cats from Bavaria and Lower Saxony (Germany). Berl. Munch. Tierarztl. Wochenschr. 2012, 125, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, I.; Kohn, B.; Müller, E. Anaplasma phagocytophilum in domestic cats from Germany, Austria and Switzerland and clinical/laboratory findings in 18 PCR-positive cats (2008–2020). J. Feline Med. Surg. 2021, 24, 290–297. [Google Scholar] [CrossRef] [PubMed]
- von Loewenich, F.D.; Stumpf, G.; Baumgarten, B.U.; Röllinghoff, M.; Dumler, J.S.; Bogdan, C. A case of equine granulocytic ehrlichiosis provides molecular evidence for the presence of pathogenic Anaplasma phagocytophilum (HGE agent) in Germany. Eur. J. Clin. Microbiol. Infect. Dis. 2003, 22, 303–305. [Google Scholar] [CrossRef]
- Silaghi, C.; Liebisch, G.; Pfister, K. Genetic variants of Anaplasma phagocytophilum from 14 equine granulocytic anaplasmosis cases. Parasites Vectors 2011, 4, 161. [Google Scholar] [CrossRef]
- Silaghi, C.; Nieder, M.; Sauter-Louis, C.; Knubben-Schweizer, G.; Pfister, K.; Pfeffer, M. Epidemiology, genetic variants and clinical course of natural infections with Anaplasma phagocytophilum in a dairy cattle herd. Parasites Vectors 2018, 11, 20. [Google Scholar] [CrossRef]
- Tegtmeyer, P.; Ganter, M.; von Loewenich, F.D. Simultaneous infection of cattle with different Anaplasma phagocytophilum variants. Ticks Tick-Borne Dis. 2019, 10, 1051–1056. [Google Scholar] [CrossRef]
- Langenwalder, D.B.; Schmidt, S.; Gilli, U.; Pantchev, N.; Ganter, M.; Silaghi, C.; Aardema, M.L.; von Loewenich, F.D. Genetic characterization of Anaplasma phagocytophilum strains from goats (Capra aegagrus hircus) and water buffalo (Bubalus bubalis) by 16S rRNA gene, ankA gene and multilocus sequence typing. Ticks Tick-borne Dis. 2019, 10, 101267. [Google Scholar] [CrossRef] [PubMed]
- Bauer, B.U.; Raileanu, C.; Tauchmann, O.; Fischer, S.; Ambros, C.; Silaghi, C.; Ganter, M. Anaplasma phagocytophilum and Anaplasma ovis-Emerging pathogens in the German sheep population. Pathogens 2021, 10, 1298. [Google Scholar] [CrossRef] [PubMed]
- Scharf, W.; Schauer, S.; Freyburger, F.; Petrovec, M.; Schaarschmidt-Kiener, D.; Liebisch, G.; Runge, M.; Ganter, M.; Kehl, A.; Dumler, J.S.; et al. Distinct host species correlate with Anaplasma phagocytophilum ankA gene clusters. J. Clin. Microbiol. 2011, 49, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Matei, I.A.; Estrada-Pena, A.; Cutler, S.J.; Vayssier-Taussat, M.; Varela-Castro, L.; Potkonjak, A.; Zeller, H.; Mihalca, A.D. A review on the eco-epidemiology and clinical management of human granulocytic anaplasmosis and its agent in Europe. Parasites Vectors 2019, 12, 599. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y.; Ni, D.; Li, Q.; Yu, Y.; Yu, X.-j.; Wan, K.; Li, D.; Liang, G.; Jiang, X.; et al. Nosocomial transmission of human granulocytic anaplasmosis in China. JAMA 2008, 300, 2263–2270. [Google Scholar] [CrossRef] [PubMed]
- Dugat, T.; Lagrée, A.-C.; Maillard, R.; Boulouis, H.-J.; Haddad, N. Opening the black box of Anaplasma phagocytophilum diversity: Current situation and future perspectives. Front. Cell. Infect. Microbiol. 2015, 5, 61. [Google Scholar] [CrossRef]
- Kauffmann, M.; Rehbein, S.; Hamel, D.; Lutz, W.; Heddergott, M.; Pfister, K.; Silaghi, C. Anaplasma phagocytophilum and Babesia spp. in roe deer (Capreolus capreolus), fallow deer (Dama dama) and mouflon (Ovis musimon) in Germany. Mol. Cell. Probes 2017, 31, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Stuen, S. Haemoparasites in small ruminants in European countries: Challenges and clinical relevance. Small Rumin. Res. 2016, 142, 22–27. [Google Scholar] [CrossRef]
- Gokce, H.I.; Woldehiwet, Z. Differential haematological effects of tick-borne fever in sheep and goats. J. Vet. Med. 1999, 46, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Grøva, L.; Olesen, I.; Steinshamn, H.; Stuen, S. Prevalence of Anaplasma phagocytophilum infection and effect on lamb growth. Acta Vet. Scand. Suppl. 2011, 53, 30. [Google Scholar] [CrossRef] [PubMed]
- Stuen, S.; Bergström, K.; Palmer, E. Reduced weight gain due to subclinical Anaplasma phagocytophilum (formerly Ehrlichia phagocytophila) infection. Exp. Appl. Acarol. 2002, 28, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Sargison, N.; Edwards, G. Tick infestations in sheep in the UK. In Pract. 2009, 31, 58–65. [Google Scholar] [CrossRef]
- Daniel, R.G.; Carson, A.; Evans, C.; Cookson, R.; Wessels, M. Pathological observations of tick-borne fever and intercurrent bacterial infections in lambs. Vet. Rec. Case Rep. 2016, 4, e000367. [Google Scholar] [CrossRef]
- Stuen, S.; Bergström, K.; Petrovec, M.; Van de Pol, I.; Schouls Leo, M. Differences in clinical manifestations and haematological and serological responses after experimental infection with genetic variants of Anaplasma phagocytophilum in sheep. Clin. Vaccine Immunol. 2003, 10, 692–695. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.J.; Birtles, R.J.; Radford, A.D.; Woldehiwet, Z. Recurrent bacteraemia in sheep infected persistently with Anaplasma phagocytophilum. J. Comp. Pathol. 2012, 147, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Dreher, U.M.; de la Fuente, J.; Hofmann-Lehmann, R.; Meli, M.L.; Pusterla, N.; Kocan, K.M.; Woldehiwet, Z.; Braun, U.; Regula, G.; Staerk, K.D.C.; et al. Serologic cross-reactivity between Anaplasma marginale and Anaplasma phagocytophilum. Clin. Vaccine Immunol. 2005, 12, 1177–1183. [Google Scholar] [CrossRef]
- Hartelt, K.; Oehme, R.; Frank, H.; Brockmann, S.O.; Hassler, D.; Kimmig, P. Pathogens and symbionts in ticks: Prevalence of Anaplasma phagocytophilum (Ehrlichia spp.), Wolbachia spp., Rickettsia spp., and Babesia spp. in Southern Germany. Int. J. Med. Microbiol. Suppl. 2004, 293, 86–92. [Google Scholar] [CrossRef]
- Hildebrandt, A.; Krämer, A.; Sachse, S.; Straube, E. Detection of Rickettsia spp. and Anaplasma phagocytophilum in Ixodes ricinus ticks in a region of Middle Germany (Thuringia). Ticks Tick-borne Dis. 2010, 1, 52–56. [Google Scholar] [CrossRef]
- May, K.; Strube, C. Prevalence of Rickettsiales (Anaplasma phagocytophilum and Rickettsia spp.) in hard ticks (Ixodes ricinus) in the city of Hamburg, Germany. Parasitol. Res. 2014, 113, 2169–2175. [Google Scholar] [CrossRef] [PubMed]
- Rubel, W.; Schoneberg, C.; Wolf, A.; Ganter, M.; Bauer, B.U. Seroprevalence and risk factors of Anaplasma spp. in German small ruminant flocks. Animals 2021, 11, 2793. [Google Scholar] [CrossRef]
- Foggie, A. Studies on the infectious agent of tick-borne fever in sheep. J. Pathol. 1951, 63, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Lepidi, H.; Bunnell, J.E.; Martin, M.E.; Madigan, J.E.; Stuen, S.; Dumler, J.S. Comparative pathology and immunohistology associated with clinical illness after Ehrlichia phagocytophila-group infections. Am. J. Trop. Med. Hyg. 2000, 62, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Shabana, I.I.; Alhadlag, N.M.; Zaraket, H. Diagnostic tools of caprine and ovine anaplasmosis: A direct comparative study. BMC Vet. Res. 2018, 14, 165. [Google Scholar] [CrossRef]
- Torina, A.; Galindo, R.C.; Vicente, J.; Di Marco, V.; Russo, M.; Aronica, V.; Fiasconaro, M.; Scimeca, S.; Alongi, A.; Caracappa, S.; et al. Characterization of Anaplasma phagocytophilum and A. ovis infection in a naturally infected sheep flock with poor health condition. Trop. Anim. Health Prod. 2010, 42, 1327–1331. [Google Scholar] [CrossRef] [PubMed]
- Silaghi, C.; Santos, A.S.; Gomes, J.; Christova, I.; Matei, I.A.; Walder, G.; Domingos, A.; Bell- Sakyi, L.; Sprong, H.; von Loewenich, F.D.; et al. Guidelines for the Direct Detection of Anaplasma spp. in Diagnosis and Epidemiological Studies. Vector-Borne Zoonotic Dis. 2017, 17, 12–22. [Google Scholar] [CrossRef]
- Kawahara, M.; Rikihisa, Y.; Lin, Q.; Isogai, E.; Tahara, K.; Itagaki, A.; Hiramitsu, Y.; Tajima, T. Novel genetic variants of Anaplasma phagocytophilum, Anaplasma bovis, Anaplasma centrale, and a novel Ehrlichia spp. in wild deer and ticks on two major islands in Japan. Appl. Environ. Microbiol. 2006, 72, 1102–1109. [Google Scholar] [CrossRef]
- Kiilerich, A.M.; Christensen, H.; Thamsborg, S.M. Anaplasma phagocytophilum in Danish sheep: Confirmation by DNA sequencing. Acta Vet. Scand. Suppl. 2009, 51, 55. [Google Scholar] [CrossRef] [PubMed]
- Massung, R.F.; Slater, K.; Owens, J.H.; Nicholson, W.L.; Mather, T.N.; Solberg, V.B.; Olson, J.G. Nested PCR assay for detection of granulocytic Ehrlichiae. J. Clin. Microbiol. 1998, 36, 1090–1095. [Google Scholar] [CrossRef] [PubMed]
- Parvizi, O.; El-Adawy, H.; Melzer, F.; Roesler, U.; Neubauer, H.; Mertens-Scholz, K. Seroprevalence and molecular detection of bovine anaplasmosis in Egypt. Pathogens 2020, 9, 64. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.; Prufer, T.L.; Schoneberg, C.; Campe, A.; Runge, M.; Ganter, M.; Bauer, B.U. Prevalence of Coxiella burnetii in German sheep flocks and evaluation of a novel approach to detect an infection via preputial swabs at herd-level. Epidemiol. Infect. 2020, 148, e75. [Google Scholar] [CrossRef] [PubMed]
- Sirigireddy, K.R.; Ganta, R.R. Multiplex detection of Ehrlichia and Anaplasma species pathogens in peripheral blood by real-time reverse transcriptase-polymerase chain reaction. J. Mol. Diagn. 2005, 7, 308–316. [Google Scholar] [CrossRef]
- Tappe, J.; Strube, C. Anaplasma phagocytophilum and Rickettsia spp. infections in hard ticks (Ixodes ricinus) in the city of Hanover (Germany). Ticks Tick-Borne Dis. 2013, 4, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Gorman, J.K.; Hoar, B.R.; Nieto, N.C.; Foley, J.E. Evaluation of Anaplasma phagocytophilum infection in experimentally inoculated sheep and determination of Anaplasma spp. seroprevalence in 8 free-ranging sheep flocks in California and Oregon. Am. J. Vet. Res. 2012, 73, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Tumwebaze, M.A.; Byamukama, B.; Tayebwa, D.S.; Byaruhanga, J.; Angwe, M.K.; Galon, E.M.; Liu, M.; Lee, S.-H.; Ringo, A.E.; Adjou Moumouni, P.F.; et al. First molecular detection of Babesia ovis, Theileria spp., Anaplasma spp., and Ehrlichia ruminantium in goats from Western Uganda. Pathogens 2020, 9, 895. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, Z.; Niu, Q.; Liu, J.; Han, R.; Guan, G.; Li, Y.; Liu, G.; Luo, J.; Yin, H. Anaplasma phagocytophilum in sheep and goats in Central and Southeastern China. Parasites Vectors 2016, 9, 593. [Google Scholar] [CrossRef][Green Version]
- Dumler, J.S.; Bakken, J.S. Human granulocytic ehrlichiosis in Wisconsin and Minnesota: A frequent infection with the potential for persistence. J. Infect. Dis. 1996, 173, 1027–1030. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pancholi, P.; Kolbert, C.P.; Mitchell, P.D.; Reed, K.D.; Dumler, J.S.; Bakken, J.S.; Telford, S.R., III; Persing, D.H. Ixodes dammini as a potential vector of human granulocytic ehrlichiosis. J. Infect. Dis. 1995, 172, 1007–1012. [Google Scholar] [CrossRef]
- Chmielewska-Badora, J.; Zwolinski, J.; Cisak, E.; Wojcik-Fatla, A.; Buczek, A.; Dutkiewicz, J. Prevalence of Anaplasma phagocytophilum in Ixodes ricinus ticks determined by polymerase chain reaction with two pairs of primers detecting 16S rRNA and ankA genes. Ann. Agric. Environ. Med. 2007, 14, 281–285. [Google Scholar] [PubMed]
- Fingerle, V.; Munderloh, U.G.; Liegl, G.; Wilske, B. Coexistence of Ehrlichiae of the Phagocytophila group with Borrelia burgdorferi in Ixodes ricinus from Southern Germany. Med. Microbiol. Immunol. 1999, 188, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.G.; Ko, S.; Kim, Y.-J.; Yang, H.-J.; Lee, H.; Shin, N.-S.; Choi, K.-S.; Chae, J.-S. New genetic variants of Anaplasma phagocytophilum and Anaplasma bovis from Korean water deer (Hydropotes inermis argyropus). Vector Borne Zoonotic Dis. 2011, 11, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Dunning Hotopp, J.C.; Lin, M.; Madupu, R.; Crabtree, J.; Angiuoli, S.V.; Eisen, J.A.; Seshadri, R.; Ren, Q.; Wu, M.; Utterback, T.R.; et al. Comparative genomics of emerging human ehrlichiosis agents. PLoS Genet. 2006, 2, e21. [Google Scholar] [CrossRef]
- Rymaszewska, A. PCR for detection of tick-borne Anaplasma phagocytophilum pathogens: A review. Vet. Med. 2011, 56, 529–536. [Google Scholar] [CrossRef]
- Massung, R.F.; Slater, K.G. Comparison of PCR assays for detection of the agent of human granulocytic ehrlichiosis, Anaplasma phagocytophilum. J. Clin. Microbiol. 2003, 41, 717–722. [Google Scholar] [CrossRef]
- Gethmann, J.; Hoffmann, B.; Kasbohm, E.; Süss, J.; Habedank, B.; Conraths, F.J.; Beer, M.; Klaus, C. Research paper on abiotic factors and their influence on Ixodes ricinus activity—Observations over a two-year period at several tick collection sites in Germany. Parasitol. Res. 2020, 119, 1455–1466. [Google Scholar] [CrossRef] [PubMed]
- Schulz, M.; Mahling, M.; Pfister, K. Abundance and seasonal activity of questing Ixodes ricinus ticks in their natural habitats in Southern Germany in 2011. J. Vector Ecol. 2014, 39, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Hamzah, K.J.; Hasso, S.A. Molecular prevalence of Anaplasma phagocytophilum in sheep from Iraq. Open Vet. J. 2019, 9, 238–245. [Google Scholar] [CrossRef]
- Zhang, Y.; Lv, Y.; Zhang, F.; Zhang, W.; Wang, J.; Cui, Y.; Wang, R.; Jian, F.; Zhang, L.; Ning, C. Molecular and phylogenetic analysis of Anaplasma spp. in sheep and goats from six provinces of China. J. Vasc. Surg. Cases 2016, 17, 523–529. [Google Scholar] [CrossRef]
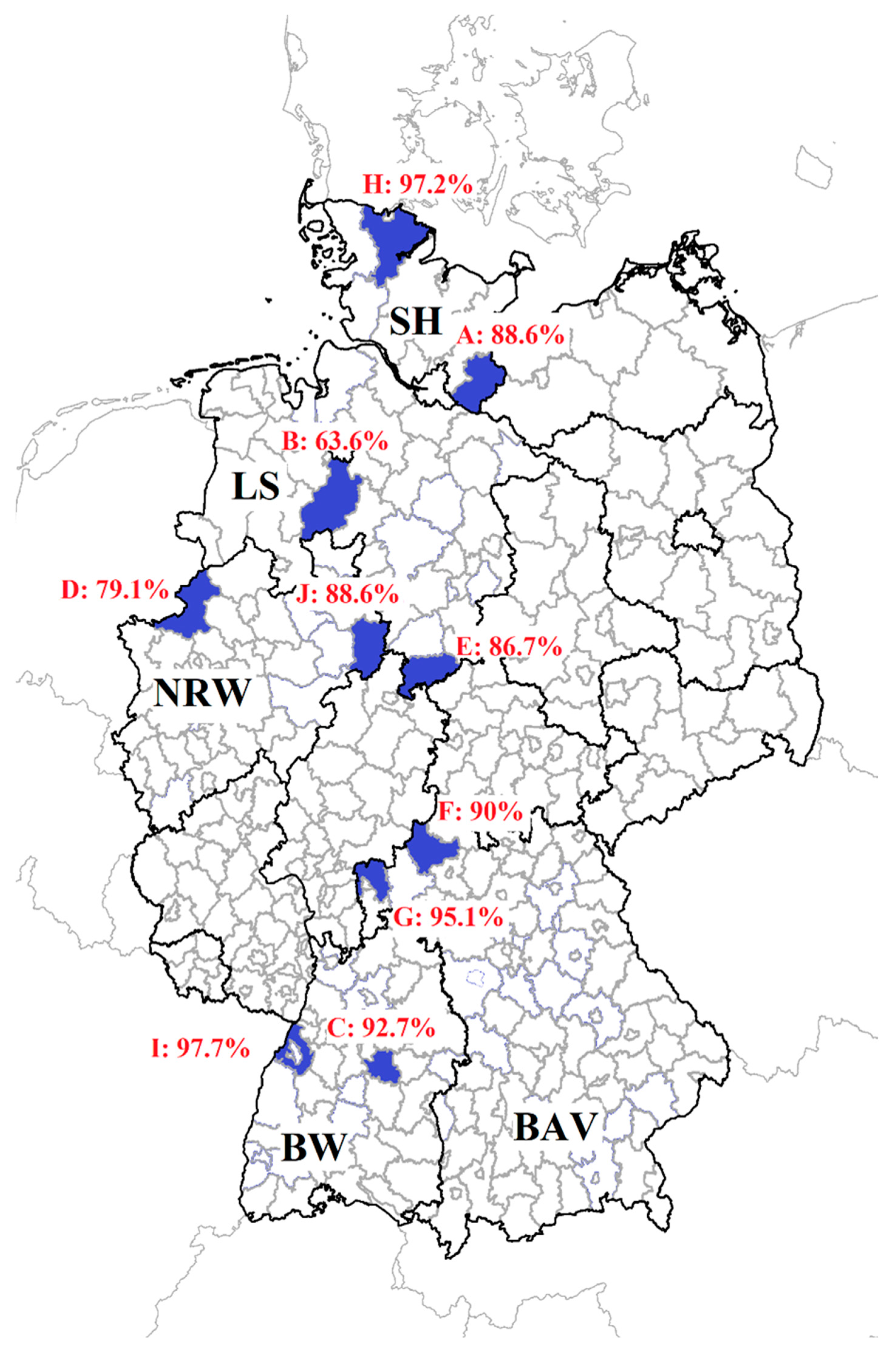
| Flock ID | Number of Sampled Sheep per Flock | Number of Anaplasma spp. Antibody Positive Sheep (cELISA) | Number of Anaplasma phagocytophilum Positive Sheep (Real-Time PCR) |
|---|---|---|---|
| A | 44 | 39 | 2 |
| B | 44 | 28 | 0 |
| C | 41 | 38 | 0 |
| D | 43 | 34 | 0 |
| E | 30 | 26 | 0 |
| F | 40 | 36 | 0 |
| G | 41 | 39 | 0 |
| H | 36 | 35 | 0 |
| I | 44 | 43 | 0 |
| J | 44 | 39 | 0 |
| Total | 407 | 357 | 2 |
| Reagents | Sequence (5′ to 3′) and/or Trade Name | Reaction Mix |
|---|---|---|
| Master mix | TaqMan™, ABsolute Blue QPCR low Rox, (Thermo Fisher Scientific, Inc., Waltham, MA, USA) | 12.5 µL combined with 2.14 µL of nuclease-free water (Carl Roth GmbH + Co. KG, Karlsruhe, Germany) |
| Probe | 6-FAM-TTG CTA TAA AGA ATA ATT AGT GGC AGA CG- MGBNFQ TaqMan™, liquid 1xTE, 100 pmol/µL, (Thermo Fisher Scientific, Inc.) | 0.06 µL (10 μM) |
| Forward primer | 5′ CTC AGA ACG AAC GCT GG, desalt, (Sigma-Aldrich, Inc., St. Louis, MO, USA), TaqMan™ | 0.15 μL (50 μM) |
| Reverse primer | 5′ CAT TTC TAG TGG CTA TCC C, desalt, (Sigma-Aldrich, Inc.), TaqMan™ | 0.15 μL (50 μM) |
| Animal and ID | Age (Years) | cELISA (Inhibition %) | 16S rRNA Real-Time PCR (Cq) |
|---|---|---|---|
| Ewe 42 | 5 | 61.4 | 39.92/negative |
| Ewe 43 | 3 | 35.9 | 40.56/43.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubel, W.; Ganter, M.; Bauer, B.U. Detection of Anaplasma phagocytophilum in Ovine Serum Samples—A Retrospective Study. Ruminants 2022, 2, 351-359. https://doi.org/10.3390/ruminants2030024
Rubel W, Ganter M, Bauer BU. Detection of Anaplasma phagocytophilum in Ovine Serum Samples—A Retrospective Study. Ruminants. 2022; 2(3):351-359. https://doi.org/10.3390/ruminants2030024
Chicago/Turabian StyleRubel, Wiebke, Martin Ganter, and Benjamin Ulrich Bauer. 2022. "Detection of Anaplasma phagocytophilum in Ovine Serum Samples—A Retrospective Study" Ruminants 2, no. 3: 351-359. https://doi.org/10.3390/ruminants2030024
APA StyleRubel, W., Ganter, M., & Bauer, B. U. (2022). Detection of Anaplasma phagocytophilum in Ovine Serum Samples—A Retrospective Study. Ruminants, 2(3), 351-359. https://doi.org/10.3390/ruminants2030024





