Abstract
This study investigated potential carry-over effects of increased growth rates prior to breeding at seven months of age on mammary glands of two-year-old ewes bearing one or two lambs, and examined the association between ewe mammary structures and the growth of their progeny. Ewe live weight and mammary ultrasound measures were recorded at 119 days of pregnancy, 29 days of lactation (L29), and weaning of the progeny (L79) in 64 two-year-old ewes selected from two treatments. The heavy group (n = 32) was preferentially fed prior to their first breeding at seven months of age, achieving an average live weight of 47.9 ± 0.38 kg. The control group (n = 32) weighed an average of 44.9 ± 0.49 kg at breeding. Lambs (n = 74) were weighed at birth, L29 and L79. Udder ultrasound measures did not differ (p > 0.10) between treatments, indicating no carry-over effects of treatments on mammary glands of two-year-old ewes. The association between ultrasound measures and lamb growth seemed to differ depending on lamb birth rank. More research is needed to further investigate these associations and determine whether ultrasonography could be used to identify ewes whose progeny would have greater growth rates based on birth rank.
1. Introduction
Higher growth rates between weaning and puberty can have detrimental effects on mammary gland development and milk production in ewe lambs [1,2,3,4,5,6]. While the exact mechanism is unknown, it is possible that these effects are mediated through a reduction in mammary parenchyma and an accumulation of fat in the mammary fat pad [1,4]. The allometric phase of mammary gland development in ewe lambs occurs between two and five months of age [1,7]. During this phase, parenchymal development will determine future development of the alveoli and, subsequently, milk production [4,5,6]. Increased post-weaning growth rates result in the earlier attainment of puberty in ewe lambs, which has been reported to interrupt the allometric phase [8,9]. Further, Villeneuve et al. [10] reported that ewe lambs rearing a single lamb with increased growth rates between weaning and subsequent breeding at seven months of age produced less milk over two lactations than those that had slower growth rates. Little is known, however, about potential carry-over effects of an increased growth rate of ewe lambs between weaning and their first breeding at seven months of age on the mammary gland of ewes and its internal structures.
Mammary gland cistern size during lactation is positively associated with milk production of both dairy [11,12] and non-dairy ewes [13]. In Haslin et al. [14], the depth of the mammary gland cistern in pregnancy and week three of lactation was positively correlated with milk yield in the third week of lactation of ewe lambs rearing single lambs. In Haslin et al. [15], single-lamb growth to weaning was positively associated with parenchymal depth in pregnancy and gland cistern size in early lactation. Meanwhile, growth to weaning of single lambs was negatively associated with mammary fat pad depth in the seventh week of lactation and positively associated with depth of the fat pad at weaning [14]. Currently, no data have been published on the associations between mammary gland ultrasound measures of older non-dairy ewes and the growth of their lambs. In addition, the associations between internal mammary gland structures and twin-lamb growth rates to weaning are unknown.
The first objective of the present study was to investigate the potential carry-over effects of increased growth rates between weaning and breeding at seven months of age on the mammary gland during the second pregnancy and lactation of two-year-old ewes bearing one or two lambs. The second objective was to investigate the association between mammary internal structures of two-year-old ewes and lamb growth to weaning. It was hypothesised that the mammary gland cistern size of two-year-old ewes would be positively associated with lamb growth to weaning.
2. Materials and Methods
The Massey University Animal Ethics Committee approved all animal handling procedures (MUAEC-17/16). The experiment was conducted at Massey University Keeble Farm (latitude: 40°24′03″ N, longitude: 175°35′51″ E), 5 km south of Palmerston North, New Zealand.
2.1. Experimental Design
The overall experimental design was previously described in Haslin et al. [16]. Briefly, twin-born Romney ewe lambs (n = 270) were allocated at weaning, at approximately 86 days of age, to one of two treatments so that live weight at weaning did not differ between treatments (28.6 kg ± 0.16; 3 January 2018). Post-weaning, the “Heavy” group (n = 135), which was preferentially fed until breeding (10 May 2018), achieved an average live weight of 47.9 ± 0.38 kg. The “Control” group (n = 135) achieved an average of 44.9 ± 0.49 kg at breeding. The difference in live weight was achieved by differing herbage allowances on either a ryegrass (Lolium perenne L.) and white-clover-based (Trifolium repens L.) sward or lucerne (Medicago sativa L.), and a cereal-based concentrate feed (CP 10.5%, NDF 17.6%, ADF 7.1%, and ME 12.8 MJ/kg DM). Individual ewe intakes were not measured. Both treatments were managed as one mob and grazed on ryegrass/white clover pasture under commercial New Zealand grazing conditions from the first breeding onwards. Their first set of lambs were weaned at approximately 100 days of lactation (17 January 2019).
Both treatments were rebred at 18 months of age (P0; 29 April 2019) to Romney rams for 34 days at a ratio of 1:60. The number of ewes pregnant and the number of fetuses conceived were determined at pregnancy diagnosis using trans-abdominal ultrasound (P93; 25 July 2019). Romney two-year-old ewes from each treatment were selected at pregnancy diagnosis based on two criteria (P93; heavy, n = 32, 62.5 ± 1.13 kg and control, n = 32, 65.3 ± 1.15 kg; lsmeans ± s.e.m.; Figure 1). Ewes were selected if their udder was examined with ultrasound as a ewe lamb and they had weaned a lamb as a ewe lamb (heavy, n = 23 and control, n = 23) or if they weaned a lamb as a ewe lamb and were diagnosed pregnant at P93 as a two-year-old ewe (heavy, n = 9 and control, n = 9) to enable the inclusion of more single- and twin-bearing two-year-old ewes (Figure 1). Both treatments included single- (heavy, n = 15 and control, n = 14), twin- (heavy, n = 16 and control, n = 16), and triplet-bearing ewes (heavy, n = 1 and control, n = 2). At P134, two-year-old ewes from both treatments were randomly assigned to one of the four lambing paddocks (average stocking rate 13.8 ewes/ha). Ewes from both treatments were present in each lambing paddock. The lactation period was deemed to have begun (L1) after the first lamb had been born (20 September 2019)). The lambing period lasted for 22 days (20 September 2019) to 12 October 2019). Ewes whose lamb died before weaning were excluded from the remainder of this experiment.
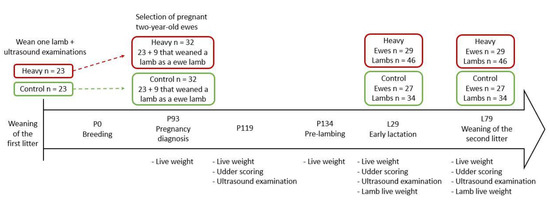
Figure 1.
Experimental design and animal measurements of the present experiment.
From breeding (P0) to weaning (L79), both treatments were managed as a single cohort and rotationally grazed on ryegrass/white clover pasture under commercial New Zealand grazing conditions. The pre-grazing pasture masses during pregnancy and lactation were on average 2209 ± 167 and 1592 ± 63 kg DM/ha, respectively. Ewes were supplemented with approximately 0.5 kg/ewe/day of grass baleage from P0 to P25 (CP 11.5%, NDF 52.9%, and ADF 31.4%) and with approximately 200 g/ewe/day of a cereal-based supplement (CP 9.7%, NDF 14.6%, and ADF 4.4%) from P0 to P44.
2.2. Animal Measurements
Live weights of the two-year-old ewes were recorded at 93, 119, and 134 days of pregnancy (P93, P119, and P134), 29 days of lactation (L29), and weaning (L79) (Figure 1). Twice-daily lambing observations were conducted between P134 and L29, during which lambs were tagged and their sex, birth weight, date of birth, dam number, and lambing paddock were recorded within 18 h of birth. All lambs were treated the same, irrespective of their dam treatments. Lambs were weighed again at an average of 29 ± 5 (L29) and 79 ± 5 days of age (L79) (Figure 1).
2.2.1. Udder Score and Morphology
Udder scores and measures of udder morphology were detailed in Haslin et al. [15]. Briefly, ewe udder scores and morphological measures were performed at P119, L29, and L79 (Figure 1). Palpation of both udder halves and teats, and an assessment of udder depth and symmetry were included in the udder scores [17].
Udder circumference (UC, cm) and the height of each udder half (cm) were included in morphological measurements. Udder volume (UV, cm3) was calculated according to Ayadi et al. [18]:
where UV = udder volume (cm3); π = 3.14159; R = radius (cm); UH = udder height (cm); UC = udder circumference (cm).
R = UC/2π
UV= π × R2 × UH
2.2.2. Ultrasound Examination
The ultrasonographic examination method used was described in Haslin et al. [15]. Ultrasound examinations were performed at P119, L29, and L79 by a single operator. At L29 and L79, ultrasound examinations were not conducted for ewes whose lambs had died (heavy, n = 3 and control, n = 3). At L29 and L79, ewes were separated from their lambs four hours prior to the examination to enable the mammary gland to accumulate milk [13,19]. Ewes were placed in a sitting position. Ultrasound examinations were performed with an ultrasound scanner fitted with a linear transducer with a 5.0–10.0 MHz imaging frequency (Mindray Digital Ultrasonic Diagnostic Imaging System DP6600 vet with 75L38EA, ShenZhen, China). The transducer was applied on the external base of the teats at an angle of 30° from the caudal–cranial axis with an inclination of 45° in relation to the teat [20].
A minimum of three images were saved from each udder half. One image per udder half was used for image processing, which had a suitable resolution per udder half and showed the mammary parenchyma, fat pad, gland cistern, and limit between the mammary gland and the abdominal wall [15,21,22]. Udder halves with a palpation score of 4 or 5 at any time point (P119, L29, or L79) were considered “abnormal” [17]. To ensure that only images of healthy (“normal”) udders were processed, images from “abnormal” udder halves were excluded (heavy = 4 ewes with 1 half each and 7 ewes with both udder halves at L79, and control = 2 ewes with 1 half each and 6 ewes with both udder halves at L79).
The ImageJ software [23] was used to process the selected images. The smallest and largest demarcation (abdominal wall) were assessed as the total depth of mammary gland conservative (MTc) and generous (MTg), respectively [22]. The parenchyma (PAR), fat pad (FP), and gland cistern (GC) depths, as well as MTc and MTg, were estimated at the widest point for each compartment using the straight tracer, the skin layer was excluded. These depths were expressed in millimetres. The templates created by Haslin et al. [15] were used to standardize the assessment of each compartment.
2.3. Statistical Analysis
Statistical analyses were conducted using SAS v9.4 (SAS Institute Inc., Cary, NC, USA) and RStudio v1.2. (RStudio Team, PBC, Boston, MA, USA). Ewes that died, (control n = 2), whose lambs died prior to weaning (heavy, n = 3 single-bearing ewes, and control, n = 1 single- and n = 2 twin-bearing ewes), and ewes that gave birth to triplet lambs (heavy, n = 1 and control, n = 2) were excluded from the analyses. The final data set included 28 ewes in the heavy group (12 single- and 16 twin-bearing ewes) and 25 ewes in the control group (13 single- and 12 twin-bearing ewes), and a total of 269 images.
Growth of ewes in each of the treatments included in this experiment from weaning to their first breeding at seven months of age and growth from birth to L79 were analysed using a linear mixed model. The model for growth of ewes included treatment (heavy vs. control) as a fixed effect and ewe date of birth as a covariate. The model for growth from birth to L79 included treatment, birth rank (1 or 2), and sex of the lamb as fixed effects, and lambing date as a covariate.
Ewe live weight at P119, L29, and L79, and lamb growth from birth to L29 and from L29 to L79 were analysed using linear mixed models with repeated measures. The model for ewe live weight included treatment, time point (P119, P134, L29, and L79), pregnancy rank (single or twin), and a two-way interaction between treatment and time point as fixed effects and lambing date was included as a covariate. The model for lamb growth included treatment, time (birth to L29 and L29 to L79), birth rank, and sex of the lamb as fixed effects and a two-way interaction between treatment and time, ewe as a random effect, and lambing date as a covariate.
The proportion of “abnormal” udder halves (udder palpation scores of 4 or 5) and udder symmetry (yes/no) were analysed using a generalised linear model allowing for repeated measures, assuming a binomial distribution and using a logit transformation. Both models included treatment, time point (P119, L29, and L79), pregnancy rank and a two-way interaction between treatment and time point as fixed effects, and lambing date as a covariate. The model for the proportion of abnormal also included udder half (right vs. left) as a fixed effect.
Udder depth score was analysed using a generalised linear model, allowing for repeated measures, assuming a Poisson distribution, and was log-transformed. Treatment, time point, pregnancy rank, and two-way interactions between treatment and time point as well as treatment and pregnancy rank were included as fixed effects, and lambing date as a covariate.
General linear mixed models were used to analyse UC, UH, UV, PAR, GC, FP, MTc, and MTg with repeated measures. The models included treatment, time point, pregnancy rank and two-way interactions between treatment and time point, and treatment and pregnancy rank as fixed effects, and lambing date as a covariate. The models for FP, MTc, UH, GC, PAR, and MTg also included udder halves and their two-way interaction, and ewe as a random effect.
Ultrasound, morphological measures, ewe live weight, and lamb growth for treatments were pooled together as no differences (p > 0.10) between treatments were identified. The residuals of the average FP, GC, MTc, PAR, MTg, and UH measures of both udder halves, UC, UV, and ewe live weight at P119, L29, and L79, lamb growth from birth to L29, L29 to L79, and birth to L79 were generated using general mixed models as undertaken by Haslin et al. [15]. Ewe live weight, UH, MTg, UV, and UC were adjusted for treatment and lambing date. In the model, MTc, FP, GC, and PAR per udder were adjusted for treatment, lambing date and MTg. Lamb growth was adjusted for treatment, sex of the lamb, and lambing date. Linear associations between the residuals of lamb growth (birth to L29, L29 to L79, and birth to L79), udder morphology (UH, UV, and UC), ultrasound measures (GC, MTc, FP, PAR, and MTg), and ewe live weight at each time point (P119, L29, and L79) were tested using Pearson correlations, as used by Haslin et al. [15].
2.3.1. Selection of the Predictive Variables
General linear mixed models, conducted with RStudio v1.2. (RStudio Team, PBC, Boston, MA, USA, Packages “lme4” and “performance”), were used to analyse growth from birth to L29, L29 to L79, and birth to L79. To examine the individual correlation between each predictive variable and lamb growth, Pearson correlations were calculated and each predictive variable that was correlated with growth of lambs (p ≤ 0.20) was included in the multiple regression model [24]. High collinearity (>0.80 [24]) was assessed between all selected predictive variables and if detected, one of the two predictive variables was selected on the basis of biological relevance to be included in the models. General linear models were used to test individual associations between lamb growth and two-way interactions between selected predictive variables, resulting in the following equations:
Lamb growth from birth to L29 = br + UC at P119 + GC at L29 + PAR at L29 + UH at L29.
Lamb growth from birth to L79 = br + FP at P119 + GC at P119 + UC at P119 + GC at L29 + PAR at L29 + UH at L29 + UH at L29 × UC at L79 + GC at L79 + MTc at L79 + UH at L79 + UC at L79.
Where br = birth rank (single or twin) and UH at L29 × UC at L79 is the interaction between UH at L29 and UC at L79.
2.3.2. Backward Manual Predictive Variable Elimination
To select the best model explaining the variation in the growth of lambs, backward manual eliminations were performed by removing variables with p > 0.10. Confounding effects were assessed for each non-significant (p > 0.10) variable that was removed by calculating the changes in the model coefficients. If the changes in the coefficients were greater than 20% [24], the predictive variable was included in the regression models. Ewe was included as a random effect in each model. The proportion of the variance explained by the predictive variables (i.e., marginal coefficient of determination) was estimated using Nakagawa and Schielzeth [25].
To calculate the effects of the depth of mammary internal structures related to lamb growth based on the multiple regression analyses on lamb growth and live weight, values of the depth of mammary structures in the average and the 90th percentile were used.
3. Results
3.1. Ewe Live Weight and Lamb Growth
Ewes in the heavy group had greater growth rates between their weaning and first breeding at seven months of age than control ewes (p < 0.05; 149 ± 4.2 g/d vs. 136 ± 4.4 g/d, respectively). At their second breeding, ewe live weight did not differ (p > 0.10) between treatments at any time point (Figure 2). At P119 and P134, twin-bearing ewes were heavier (p < 0.01) than single-bearing ewes (70.0 ± 0.95 kg vs. 64.6 ± 1.00 kg at P119 and 73.1 ± 0.94 kg vs. 67.6 ± 0.99 kg at P134, respectively), irrespective of treatment. Ewe live weight did not differ (p > 0.10) between single- and twin-bearing ewes at L29 and L79.
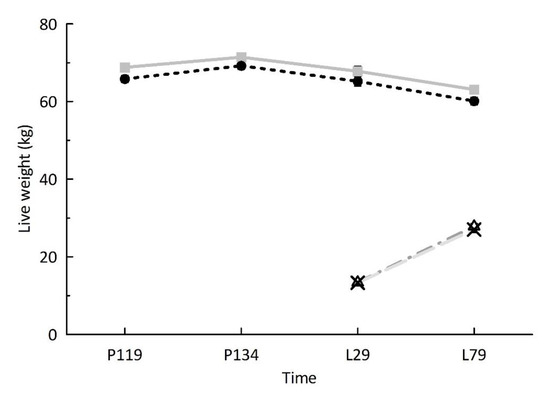
Figure 2.
Live weight (±SEM) of ewes in the control (n = 25; grey square and solid line) and the heavy groups (n = 28; black circle and dotted line), and live weight of the progeny born to two-year-old ewes in the control (n = 34; triangle and dash–dotted line) and the heavy groups (n = 40; black cross and long dashed line). P119, day 119 of pregnancy; P134, day 134 of pregnancy; L29, day 29 of lactation; L79, weaning.
Lamb live weight did not differ between treatments (p > 0.10) at birth (control = 7.0 ± 0.30 vs. heavy = 6.8 ± 0.29), L29, or L79 (Figure 2). Lamb growth rates from birth to L29, L29 to L79, and from birth to L79 did not differ (p > 0.10) between treatments (data not shown). Single lambs had greater (p < 0.001) growth rates than twin lambs from birth to L29 (singles 289 ± 16 g/d vs. twins 219 ± 14 g/d), L29 to L79 (singles 288 ± 12 g/d vs. twins 234 ± 11 g/d), and birth to L79 (singles 288 ± 9.5 g/d vs. twins 232 ± 8.7 g/d). Female lambs had greater (p < 0.05) growth rates from birth to L79 than male lambs (268 ± 8.0 g/d vs. 253 ± 8.0 g/d, respectively).
3.2. Udder Score and Morphology
The proportion of abnormal udder palpation scores and ewes with asymmetric udders did not differ (p > 0.10) between treatments or pregnancy ranks (data not shown). There were no abnormal teat palpation scores at any time point (p > 0.10). The proportion of abnormal udder palpation scores was greater (p < 0.05) at L79 than at P119 and L29 (Table 1). The proportion of ewes with asymmetric udders was greater (p < 0.05) at L29 and L79 than at P119 (Table 1). Scores of udder depth did not differ (p > 0.10) between treatments (data not shown), but were greater (p < 0.001) at P119 than L29, which was greater than L79 (p < 0.001; Table 1). At P119 and L29, udder depth scores were greater (p < 0.01) for single-bearing ewes than twin-bearing ewes (mean (95% confidence interval); 5.0 (4.9–5.0) vs. 4.4 (4.2–4.6) at P119 and 4.2 (4.0–4.4) vs. 3.9 (3.8–4.0) at L29, respectively), but did not differ at L79 (p > 0.10).

Table 1.
Effect of time (P119, L29, and L79) on the proportion (95% confidence intervals) of abnormal udder palpation scores 1, asymmetric udders (asymmetry), and least square means (95% confidence intervals) of udder depth score of two-year-old ewes.
Udder height (UH), UC, and UV did not differ (p > 0.10) between treatments at any time point (data not shown). At P119, UH of the right half was greater (p < 0.05) than the left half (5.6 ± 0.14 vs. 5.3 ± 0.15, respectively), but udder halves did not differ (p > 0.10) at L29 or L79. At L29, UH, UC, and UV were greater (p < 0.01) than P119 and L79 which was greater (p < 0.01) than P119 (Table 2). At P119, UH, UC, and UV were greater (p < 0.01) for twin-bearing ewes than single-bearing ones (Table 2). At L29, UH and UV were greater (p < 0.05) for twin-bearing ewes than single-bearing ewes (Table 2). At L79, UH, UC, and UV did not differ by birth rank (p > 0.10; Table 2).

Table 2.
Effect of time (P119, L29, and L79) and pregnancy rank (single or twin) on udder height (UH), udder volume (UV), udder circumference (UC), the depths of the mammary gland cistern (GC), mammary parenchyma (PAR) and the fat pad (FP), and total depths of mammary gland generous (MTg) and conservative (MTc) of two-year-old ewes. Least square means ± s.e.m.
3.3. Ultrasound Measures
The depth of PAR, GC, MTg, FP, and MTc did not differ (p > 0.10) by udder half or treatment at any time point (data not shown). At P119, PAR was greater (p < 0.01) for twin-bearing ewes than single-bearing ewes, but did not differ (p > 0.10) between pregnancy rank at L29 or L79 (Table 2). Pregnancy rank had no effect (p > 0.10) on GC, MP, MTg, or MTc at any time (Table 2). At P119, GC was smaller than L29 or L79 (p < 0.001; Table 2). At L29, PAR, MTc, and MTg were greater (p < 0.05) than L79, which was in turn greater (p < 0.05) than P119 (Table 3). At L79, FP was smaller (p < 0.01) than P119 (Table 2).

Table 3.
Correlation coefficients of residuals of lamb growth from birth to early lactation (birth to L29), early lactation to weaning (L29 to L79), and birth to weaning (birth to L79), with udder height (UH) in late pregnancy (P119), udder circumference (UC) at weaning (L79), volume (UV) at P119, depth of the mammary fat pad (FP) at L79, and the total depth of the mammary gland generous (MTg) at P119 and L79 of two-year-old ewes.
3.4. Correlations between Growth of the Lambs and Udder Measures
Growth from birth to L29 was negatively associated with FP at L79 (p < 0.05; Table 3). Growth from L29 to L79 was negatively associated (p < 0.05) with UV and UH from the left udder half at P119 and positively correlated with UC, FP, and MTg at L79 (p < 0.05; Table 3). Growth from birth to L79 was negatively associated (p < 0.05) with UH of both udder halves and UV at P119 and was positively associated with UC, UV, and MTg at L79 (p < 0.05; Table 3). Non-significant (p > 0.10) correlations are presented in Table S1.
3.5. Prediction of the Growth of Lambs Using Udder Measures
The model for growth from birth to early lactation (L29) explained 21.7% of the variation in lamb growth rates and included birth rank, UH and GC at L29 and the random effect of ewe (Table 4). The difference between a ewe with an average GC and a ewe with a GC at L29 in the 90th percentile was 13.3 mm (Table 5), resulting in a 26.6 g/d difference in growth from birth to L29.

Table 4.
Regression coefficients (±s.e.m.) of the average of morphological (UC, UH) and ultrasound (PAR, FP, GC, MTc) measurements in late pregnancy (P119), early lactation (L29), and at weaning (L79), and of the random effect of ewe (ewe) on lamb growth (g/d) from birth to early lactation (birth to L29) and birth to weaning (birth to L79).

Table 5.
Descriptive statistics of the depth of the parenchyma (PAR), gland cistern (GC), and mammary fat pad (FP) in late pregnancy (P119), early lactation (L29), and at weaning (L79), irrespective of treatment.
The best model for growth from birth to L79 explained 52.9% of the variation in growth rates and included birth rank, FP and UC at P119, GC and PAR at L29, GC, MTc, UH, and UC at L79, the interaction between UH at L29 and UC at L79, and the random effect of ewe (Table 4). The difference between a ewe with an average GC at L29 and a ewe with a GC in the 90th percentile was 13.3 mm (Table 5), resulting in a −30.6 g/d difference in growth to L79. The difference between a ewe with an average PAR at L29 and a ewe with a PAR in the 90th percentile was 8.6 mm (Table 5), resulting in a −3.1 g/d difference in growth to L79.
4. Discussion
4.1. Difference between Treatments and Pregnancy Rank
The dimensions of the mammary glands and internal structures of two-year-old ewes bearing either single or twin lambs and lamb weaning weights did not differ between treatments. These findings are consistent with Haslin et al. [15,26] that indicated that increased growth rates prior to ewe lambs’ first breeding at seven months of age had no effect on ultrasound measures and morphology of the mammary gland during pregnancy and lactation nor subsequent lamb weaning weights. Villeneuve et al. [10], however, reported that two-year-old ewes with increased growth rates prior to their first breeding produced less milk in their second lactation and had lighter single lambs at weaning than ewes with slower growth rates. There were greater differences between ewe growth rates (76 to 82 g/d) and greater magnitude in growth (223 to 305 g/d) in the study of Villeneuve et al. [10] than in the current experiment (8 g/d difference and 136 to 148 g/d of magnitude), which may explain the difference in findings. The small difference in ewe growth rates and its lower magnitude in the present experiment may have limited the identification of potential carry-over effects on ewe mammary glands. In addition, Hue-Beauvais et al. [6] indicated that diets with a high fat content can be detrimental for mammary gland development. Although ewes in the present experiment were complemented with concentrate feed prior to both breeding periods [16], their diet was pasture-based and contained less fat than diets in previous studies, which mainly used concentrate feed [1,4,10,27]. The present results suggest that there were no carry-over effects of increased growth rates between weaning and breeding at seven months of age on the morphology and dimensions of the internal structures of the mammary gland of two-year-old ewes. Ultrasound imaging, however, enables only the udder dimensions to be visualised [15,22] and echo-textural characteristics [28,29] of the mammary internal structures to be assessed. Further investigations may be warranted to determine the effect of increasing growth rates prior to the first breeding on the cellular development and function of the ewe mammary gland, particularly if greater live weight differences than seen in the present experiment can be achieved.
This is the first experiment that has examined the dimensions of mammary internal structures of twin- and single-bearing ewes using ultrasound. In late pregnancy and early lactation, udder dimensions were greater for twin- than single-bearing ewes. This result is consistent with previous findings that reported that twin-bearing ewes had larger udders than single-bearing ewes [30,31,32]. In addition, in late pregnancy in the current experiment, parenchymal depth was greater for twin- than in single-bearing ewes. The mammary parenchyma includes the milk secretory cells and the ductal network [33,34]. Hence, a larger parenchyma in late pregnancy could indicate a higher number of epithelial cells and the potential for greater milk yield. Twin-bearing ewes produce approximately 30 to 50% more milk than single-bearing ewes [35], which would support this suggestion. Interestingly, the depth of the gland cistern did not differ between single- and twin-bearing ewes even though gland cistern size has been shown to be positively correlated with milk production [12,13,36]. This result could be due a greater suckling frequency of twin than single lambs [37], thus not requiring a larger gland cistern as milking removal is more frequent. As twin-bearing ewes produce more milk than single-bearing ewes [35,38], along with having a larger parenchyma, it would be expected that they would also have larger cisterns. The tissues and structure of the mammary gland were shown to change during lactation depending on the number of lambs reared; this is known as mammary plasticity [39]. More research is needed to determine the relationship between ewe mammary gland cistern size and lamb suckling frequency, which can be detected using an accelerometer [40].
4.2. Udder Growth between Pregnancy and Weaning
The proportion of abnormal udder scores was greater at weaning than during late pregnancy or early lactation. This result may be due to the start of involution which involves important tissue remodelling in the mammary gland and is initiated by an accumulation of milk in the mammary gland [41]. Udder morphological measures were the greatest in early lactation and greater at weaning than in late pregnancy, which likely indicated that there was some degree of milk production still occurring at weaning. These results were consistent with previous findings, which reported that udder volume greatly increases between late pregnancy and the first week of lactation, which is followed by a progressive decrease until total involution between 30 and 60 days after weaning [12,32,38,42]. Udder height, circumference, and volume were greater than udder morphological measures at weaning in non-dairy ewe lambs [26]. This time of weaning, which was earlier in the present study (79 days of lactation) than in Haslin et al. [26] (100 days of lactation), could explain this difference in udder size as the stage of mammary involution differed [41]. The changes over time of morphological and ultrasound measures of the mammary gland in the present experiment were consistent with normal mammary gland growth during pregnancy and lactation.
4.3. Predictions of Growth of Lambs Using Udder Morphological and Ultrasound Measures
The variation in lamb growth rate explained by ewe udder measures was moderate and greater from birth to weaning (53%) than birth to early lactation (22%). The variation explained in lamb growth rate to weaning was greater than that reported in Haslin et al. [14] and [15] in ewe lambs, but still only explained half of the variation in lamb growth to weaning. The differences with the findings in Haslin et al. [15] may be due to the age of the lambs when weaning measures were recorded. Lambs were approximately 100 days of age in Haslin et al. [15] compared with 79 days of age in the current experiment. As lambs grow, their milk intake decreases and intake of solid food increases, therefore, lambs in the current experiment would have been more dependent on milk. The decrease in milk intake and increase in pasture intake in lamb nutrition lead to the start of involution in the mammary gland, which changes the parenchyma and fat pad [41]. Moreover, the predicted lamb growth rates included twin lambs, which are known to have slower growth rates than single lambs [31]. Predictions of lamb growth using ultrasound measures, therefore, may differ between single and twin lambs and may explain the differences with Haslin et al. [14] and [15], which included only single lambs. The differences observed could also be due to the age of the ewes. In Haslin et al. [14,15], ultrasound examinations were performed on ewe lambs. Ewes have been reported to produce less milk during their first than second lactation [10,43], which may also result in differences as ewes age. Lamb growth is affected by different factors, including milk production and composition and solid feed quantity and quality [44]. Although mammary ultrasound measures were positively associated with milk production in ewe lambs [14] and mature ewes [12,13], these measures do not provide information on milk composition or the lamb’s herbage intake, which may explain why only half of the variation in lamb growth could be explained.
In the current experiment, it was hypothesised that the gland cistern size of two-year-old ewes would be positively associated with the growth of lambs to weaning. Gland cistern depth in early lactation was positively correlated with predicted growth of lambs to early lactation, but was negatively associated with growth of lambs to weaning. The difference in growth of lambs to early lactation between ewes with average and large gland cisterns was moderate (less than 900 g in early lactation), which was lower than that reported in Haslin et al. [15]. The negative association between the gland cistern in early lactation and lamb growth to weaning indicates that ewes with larger cisterns had lambs with slower growth rates to weaning than ewes with smaller cisterns, resulting in a difference of 2.4 kg in lamb weaning weight (79 days of lactation) in the present experiment. This finding contrasts with results reported in Haslin et al. [15] for ewe lambs, that the gland cistern was positively correlated with growth of lambs to weaning. This negative association could be due to the inclusion of both single and twin lambs in the regression model. Milk drains, and is stored in, the gland cistern between suckling events [45]. Gland cistern size has been positively associated with milk production, indicating that ewes with larger cisterns had a greater milk yield than ewes with smaller cisterns [12,13,36]. It is also known that twin-bearing ewes produce more milk than single-bearing ewes [35] and that twin lambs have slower growth rates than single lambs [31,46]. Hence, it would be expected that twin-bearing ewes would have larger cisterns than single-bearing and twin lambs with slower growth rates than single lambs. Similarly, parenchymal depth in early lactation was negatively associated with predicted growth of lambs to weaning, resulting in a difference in lamb weaning weight of less than 300 g. Further investigations are needed on the different associations between single- and twin-lambs growth rates to weaning and the depth of the gland cisterns. This knowledge would help determine whether ultrasound could be used to identify ewes for increased lamb growth rates based on birth ranks.
5. Conclusions
No carry-over effects of increased growth rates prior to breeding at seven months of age were observed on the morphology and dimensions of internal structures of the mammary gland of two-year-old ewes during their second lactation. This experiment was the first to compare mammary glands of twin- and single-bearing ewes using ultrasound. There was a positive relationship between gland cistern depth in early lactation and growth of lambs to early lactation but the relationship between gland cistern depth in early lactation and growth of lambs to weaning was negative. Further investigations are warranted to investigate the associations between mammary ultrasound measures and growth rates of lambs depending on their birth rank and to determine whether ultrasonography could be used as a method for farmers to identify ewes that would have lambs with greater growth rates based on their birth ranks.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ruminants1020006/s1, Table S1: Correlation coefficients of residuals of growth of lambs from birth to early lactation (birth to L29), early lactation to weaning (L29 to L79), and birth to weaning (birth to L79) with udder height (UH), volume (UV), circumference (UC), live weight (Ewe LW), depth of the mammary parenchyma (PAR), gland cistern (GC), and the total depth of the mammary gland generous (MTg) and conservative (MTc) of two-year-old ewes in late-pregnancy (P119), early lactation (L29), and at weaning (L79).
Author Contributions
Conceptualization, methodology, investigation, resources and visualization, formal analysis, writing—review and editing, and project administration, E.H., P.R.K., R.A.C.-T., S.T.M. and H.T.B.; software, writing—original draft preparation, and data curation, E.H.; supervision, R.A.C.-T., P.R.K., S.T.M. and H.T.B.; funding acquisition, H.T.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Beef + Lamb New Zealand and Massey University.
Institutional Review Board Statement
The experiment and all animal handling procedures were approved by the Massey University Animal Ethics Committee (MUAEC-17/16).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this experiment are available within the article.
Acknowledgments
The authors wish to acknowledge Dean Burnham, Geoff Purchas, Keebles Farm staff, and the helpers for their technical assistance, and Beef + Lamb New Zealand and Massey University for funding this study.
Conflicts of Interest
The authors declare no conflict of interest. There are no relevant financial or non-financial competing interest to report. The funders had no role in the design of the study, in the collection, analysis, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
References
- Johnsson, I.D.; Hart, I.C. Pre-pubertal mammogenesis in the sheep 1. The effects of level of nutrition on growth and mammary development in female lambs. Anim. Sci. 1985, 41, 323–332. [Google Scholar] [CrossRef]
- Hovey, R.C.; McFadden, T.B.; Akers, R.M. Regulation of mammary gland growth and morphogenesis by the mammary fat pad: A species comparison. J. Mammary Gland. Biol. Neoplasia 1999, 4, 53–68. [Google Scholar] [CrossRef]
- Tolman, B.; McKusick, B.C. The Effect of Growth Rate on Mammary Gland Development in Ewe Lambs: A Review. In Proceedings of the Dairy Sheep Symposium, Eau Claire, WI, USA, 1 November 2001. [Google Scholar]
- Villeneuve, L.; Cinq-Mars, D.; Lacasse, P. Effects of restricted feeding of prepubertal ewe lambs on growth performance and mammary gland development. Animal 2010, 4, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Berryhill, G.E.; Trott, J.E.; Derpinghaus, A.L.; Hovey, R.C. Triennal Lactation Symposium/Bolfa: Dietary regulation of allometric ductal growth in the mammary glands. J. Anim. Sci. 2017, 95, 5664–5674. [Google Scholar] [CrossRef] [PubMed]
- Hue-Beauvais, C.; Faulconnier, Y.; Charlier, M.; Leroux, C. Nutritional Regulation of Mammary Gland Development and Milk Synthesis in Animal Models and Dairy Species. Genes 2021, 12, 523. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.R. Mammary gland growth in sheep. J. Anim. Sci. 1975, 41, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.J.; Capuco, A.V.; Ross, D.A.; Lintault, L.M.; Van Amburgh, M.E. Developmental and nutritional regulation of the prepubertal heifer mammary gland: I. Parenchyma and fat pad mass and composition. J. Dairy Sci. 2006, 89, 4289–4297. [Google Scholar] [CrossRef]
- Van Amburgh, M.E.; Soberon, F.; Meyer, M.J.; Molano, R.A. Symposium review: Integration of postweaning nutrient requirements and supply with composition of growth and mammary development in modern dairy heifers. J. Dairy Sci. 2019, 102, 3692–3705. [Google Scholar] [CrossRef]
- Villeneuve, L.; Cinq-Mars, D.; Lacasse, P. Effects of restricted feeding of prepubertal ewe lambs on reproduction and lactation performances over two breeding seasons. Animal 2010, 4, 1997–2003. [Google Scholar] [CrossRef]
- Nudda, A.; Pulina, G.; Vallebella, R.; Bencini, R.; Enne, G. Ultrasound technique for measuring mammary cistern size of dairy ewes. J. Dairy Res. 2000, 67, 101–106. [Google Scholar] [CrossRef]
- Rovai, M.; Caja, G.; Such, X. Evaluation of udder cisterns and effects on milk yield of dairy ewes. J. Dairy Sci. 2008, 91, 4622–4629. [Google Scholar] [CrossRef]
- Caja, G.; Such, X.; Ruberte, J.; Carretero, A.; Navarro, M. The use of ultrasonography in the study of mammary gland cisterns during lactation in sheep. Proc. Eur. Soc. Anim. Prod. 1999, 95, 91–93. [Google Scholar]
- Haslin, E.; Corner-Thomas, R.A.; Kenyon, P.R.; Peterson, S.W.; Morris, S.T.; Blair, H.T. Associations among Mammary Ultrasound Measurements, Milk Yield of Non-Dairy Ewe Lambs and the Growth of Their Single Lambs. Animals 2021, 11, 2052. [Google Scholar] [CrossRef]
- Haslin, E.; Corner-Thomas, R.A.; Kenyon, P.R.; Molenaar, A.J.; Morris, S.T.; Blair, H.T. Mammary Gland Structures Are Not Affected by an Increased Growth Rate of Yearling Ewes Post-Weaning but Are Associated with Growth Rates of Singletons. Animals 2021, 11, 884. [Google Scholar] [CrossRef]
- Haslin, E.; Corner-Thomas, R.A.; Kenyon, P.R.; Pettigrew, E.J.; Hickson, R.E.; Morris, S.T.; Blair, H.T. Effects of heavier live weight of ewe lambs at mating on fertility, lambing percentage, subsequent live weight and the performance of their progeny. N. Z. J. Agric. Res. 2020, 1–15. [Google Scholar] [CrossRef]
- Griffiths, K.J.; Ridler, A.L.; Compton, C.W.R.; Corner-Thomas, R.A.; Kenyon, P.R. Investigating associations between lamb survival to weaning and dam udder and teat scores. N. Z. Vet. J. 2019, 67, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Ayadi, M.; Such, X.; Ezzehizi, N.; Zouari, M.; Najar, T.; M’Rad, M.B.; Casals, R. Relationship between mammary morphology traits and milk yield of Sicilo-Sarde dairy sheep in Tunisia. Small Rumin. Res. 2011, 96, 41–45. [Google Scholar] [CrossRef]
- Ruberte, J.; Carretero, A.; Fernandez, M.; Navarro, M.; Caja, G.; Kirchner, F.; Such, X. Ultrasound mammography in the lactating ewe and its correspondence to anatomical section. Small Rumin. Res. 1994, 13, 199–204. [Google Scholar] [CrossRef]
- Albino, R.L.; Marcondes, M.I.; Akers, R.M.; Detmann, E.; Carvalho, B.C.; Silva, T.E. Mammary gland development of dairy heifers fed diets containing increasing levels of metabolisable protein: Metabolisable energy. J. Dairy Res. 2015, 82, 113–120. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Albino, R.L.; Guimarães, S.E.F.; Daniels, K.M.; Fontes, M.M.S.; Machado, A.F.; Dos Santos, G.B.; Marcondes, M.I. Mammary gland ultrasonography to evaluate mammary parenchymal composition in prepubertal heifers. J. Dairy Sci. 2017, 100, 1588–1591. [Google Scholar] [CrossRef]
- Molenaar, A.J.; Maclean, P.H.; Gilmour, M.L.; Draganova, I.G.; Symes, C.W.; Margerison, J.K.; McMahon, C.D. Effect of whole-milk allowance on liveweight gain and growth of parenchyma and fat pads in the mammary glands of dairy heifers at weaning. J. Dairy Sci. 2020, 103, 5061–5069. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, T.; Rasband, W.S. ImageJ User Guide—IJ 1.46. Available online: imagej.nih.gov/ij/docs/guide/ (accessed on 20 June 2019).
- Dohoo, I.R.; Martin, W.; Stryhn, H.E. (Eds.) Model-Building Strategies. In Veterinary Epidemiologic Research; AVC Inc.: Charlottetown, PEI, Canada, 2003; pp. 317–334. [Google Scholar]
- Nakagawa, S.; Schielzeth, H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 2013, 4, 133–142. [Google Scholar] [CrossRef]
- Haslin, E.; Corner-Thomas, R.A.; Kenyon, P.R.; Morris, S.T.; Blair, H.T. Impacts of a heavier live weight at breeding on the morphology of mammary glands of non-dairy ewe lambs. Anim. Prod. Sci. 2021, 61, 165. [Google Scholar]
- Umberger, S.H.; Goode, L.; Caruolo, E.V.; Harvey, R.W.; Britt, J.H.; Linnerud, A.C. Effects of accelerated growth during rearing on reproduction and lactation in ewes lambing at 13 to 15 months of age. Theriogenology 1985, 23, 555–564. [Google Scholar] [CrossRef]
- Barbagianni, M.S.; Gouletsou, P.G.; Valasi, I.; Petridis, I.G.; Giannenas, I.; Fthenakis, G.C. Ultrasonographic findings in the ovine udder during lactogenesis in healthy ewes or ewes with pregnancy toxaemia. J. Dairy Res. 2015, 82, 293–303. [Google Scholar] [CrossRef]
- Murawski, M.; Schwarz, T.; Jamieson, M.; Ahmadi, B.; Bartlewski, P.M. Echotextural characteristics of the mammary gland during early lactation in two breeds of sheep varying in milk yields. Anim. Reprod. 2019, 16, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.R.; Hughson, G.; Farquhar, P.A.; Rattray, P.V. The relationship between the degree of udder development and milk production in Coopworth ewes. Proc. N. Z. Soc. Anim. Prod. 1980, 40, 163–165. [Google Scholar]
- Kenyon, P.R.; van der Linden, D.S.; Blair, H.T.; Morris, S.T.; Jenkinson, C.M.C.; Peterson, S.W.; Mackenzie, D.D.S.; Firth, E.C. Effects of dam size and nutritional plane during pregnancy on lamb performance to weaning. Small Rumin. Res. 2011, 97, 21–27. [Google Scholar] [CrossRef]
- Davis, S.R. Triennial Lactation Symposium/Bolfa: Mammary growth during pregnancy and lactation and its relationship with milk yield. J. Anim. Sci. 2017, 95, 5675–5688. [Google Scholar] [CrossRef] [PubMed]
- Akers, R.M. Overview of Mammary Development. In Lactation and the Mammary Gland; Akers, R.M., Ed.; Blackwell Publishing Company: Ames, IA, USA, 2002; Chapter 1; pp. 3–44. [Google Scholar]
- Pourlis, A. Ovine mammary morphology and associations with milk production, milkability and animal selection. Small Rumin. Res. 2020, 184, 106009. [Google Scholar] [CrossRef]
- Nowak, R.; Porter, R.-H.; Blache, D.; Dwyer, C.M. Behaviour and the Welfare of the Sheep. In The Welfare of Sheep; Dwyer, C.M., Philips, C., Eds.; Animal Welfare; Springer: Edinburgh, UK, 2008; Chapter 3; Volume 6, pp. 81–134. [Google Scholar]
- Castillo, V.; Such, X.; Caja, G.; Salama, A.A.K.; Albanell, E.; Casals, R. Changes in alveolar and cisternal compartments induced by milking interval in the udder of dairy ewes. J. Dairy Sci. 2008, 91, 3403–3411. [Google Scholar] [CrossRef] [PubMed]
- Hinch, G.N. The sucking behaviour of triplet, twin and single lambs at pasture. Appl. Anim. Behav. Sci. 1989, 22, 39–48. [Google Scholar] [CrossRef]
- Lérias, J.R.; Hernández-Castellano, L.E.; Suárez-Trujillo, A.; Castro, N.; Pourlis, A.; Almeida, A.M. The mammary gland in small ruminants: Major morphological and functional events underlying milk production—A review. J. Dairy Res. 2014, 81, 304–318. [Google Scholar] [CrossRef]
- Boutinaud, M.; Hervé, L.; Quesnel, H.; Lollivier, V.; Finot, L.; Dessauge, F.; Chanat, E.; Lacasse, P.; Charton, C.; Guinard-Flament, J. Review: The cellular mechanisms underlying mammary tissue plasticity during lactation in ruminants. Animal 2019, 13, s52–s64. [Google Scholar] [CrossRef]
- Kuźnicka, E.; Gburzyński, P. Automatic detection of suckling events in lamb through accelerometer data classification. Comput. Electron. Agric. 2017, 138, 137–147. [Google Scholar] [CrossRef]
- Petridis, I.G.; Fthenakis, G.C. Mammary involution and relevant udder health management in sheep. Small Rumin. Res. 2019, 181, 66–75. [Google Scholar] [CrossRef]
- Tatarczuch, L.; Philip, C.; Lee, C.S. Involution of the sheep mammary gland. J. Anat. 1997, 190, 405–416. [Google Scholar] [CrossRef]
- Morgan, J.E.; Fogarty, N.M.; Nielsen, S.; Gilmour, A.R. Milk yield and milk composition from grazing primiparous non-dairy crossbred ewes. Aust. J. Agric. Res. 2006, 57, 377–387. [Google Scholar] [CrossRef]
- Danso, A.S.; Morel, P.C.H.; Kenyon, P.R.; Blair, H.T. Brief communication: Effect of early life diet on lamb growth and organ development. Proc. N. Z. Soc. Anim. Prod. 2014, 74, 205–208. [Google Scholar]
- Akers, R.M. Mammary Development, Anatomy, and Physiology. In Lactation and the Mammary Gland; Akers, R.M., Ed.; Blackwell Publishing Company: Ames, IA, USA, 2002; Chapter 2; pp. 45–65. [Google Scholar]
- Torres-Hernandez, G.; Hohenboken, W. Relationships between ewe milk production and composition and preweaning lamb weight gain. J. Anim. Sci. 1980, 50, 597–603. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).