Early Exposure to Polyphenol-Rich Sugarcane Extract (PRSE) Mitigates Aging While Enhancing Thermotolerance in C. elegans
Abstract
1. Introduction
2. Materials and Methods
2.1. Polyphenol-Rich Sugarcane Extract and Treatment
2.2. Escherichia coli OP50 Culture Conditions and Maintenance of C. elegans
2.3. Lifespan Assay
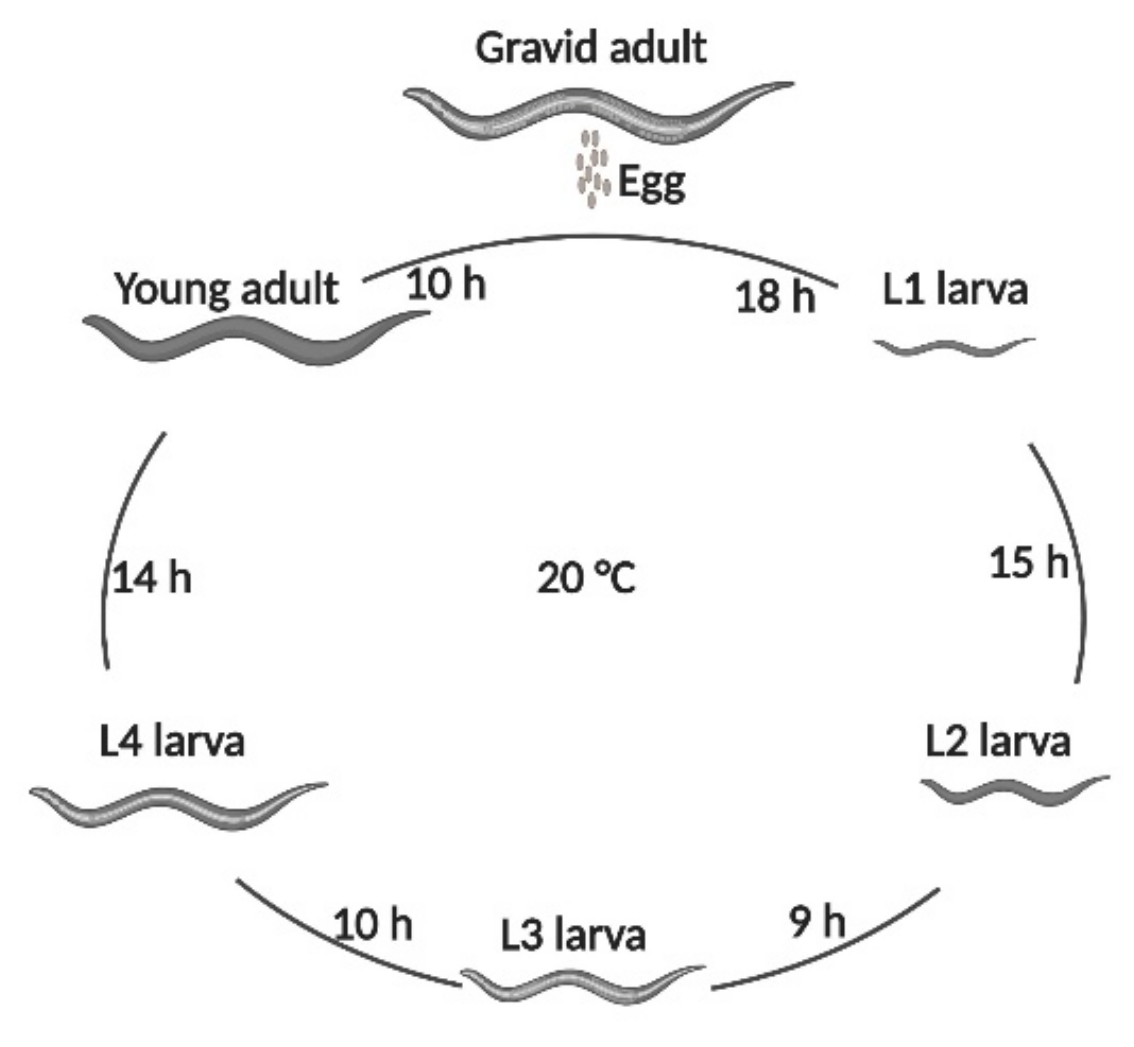
2.4. Timing of Exposure to PRSE
2.5. Thermotolerance Assay
2.6. Statistical Analysis
3. Results
3.1. Supplementation of PRSE Extends C. elegans Lifespan
3.2. PRSE Supplement Intervention Beginning at Early Stages Extends the Lifespan of C. elegans
3.3. Supplementation of PRSE at Early Stage Extends Lifespan through the Insulin/IGF-1 Signaling (IIS) Pathway
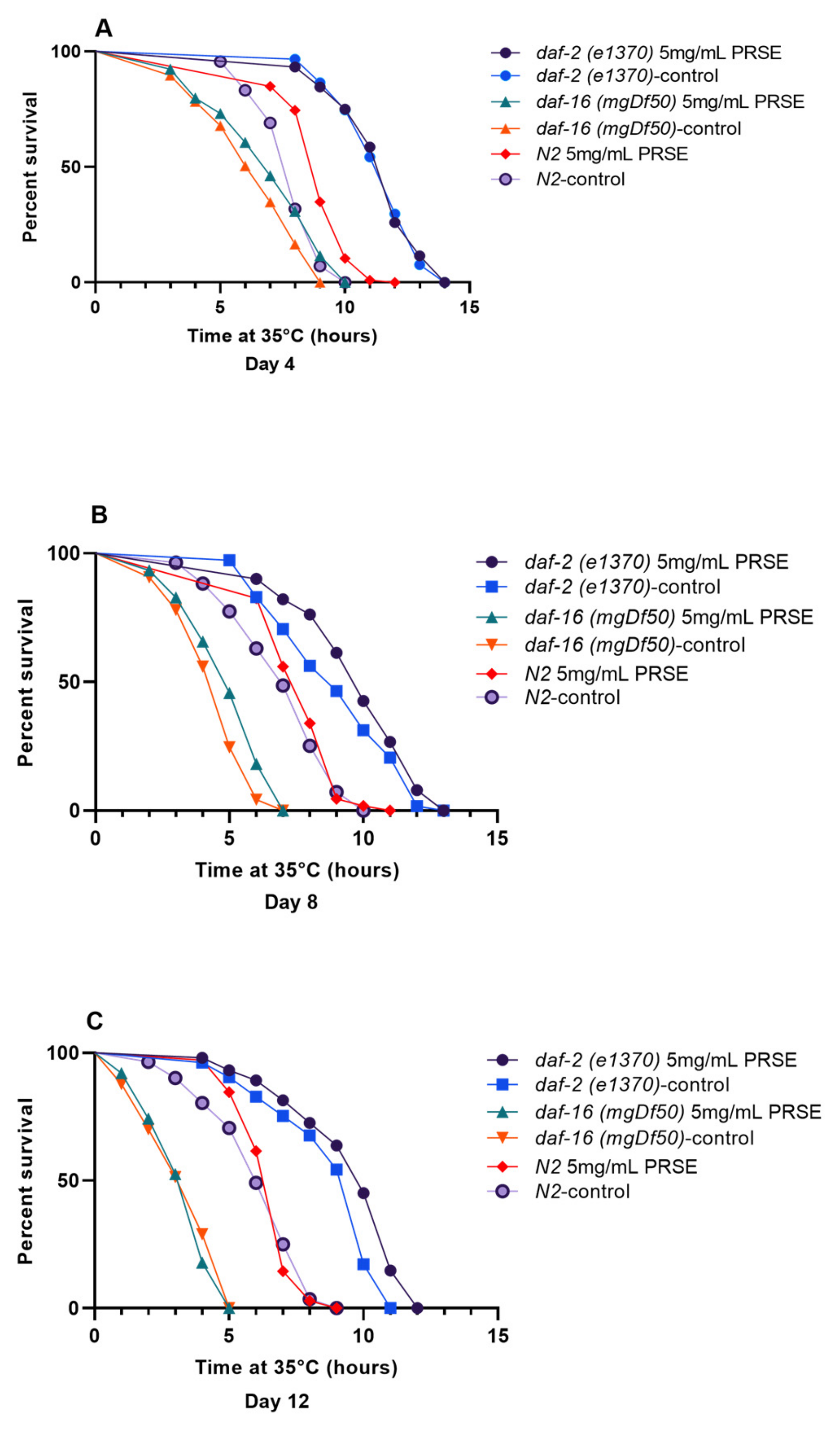
3.4. PRSE Supplementation Moderates Thermotolerance in C. elegans Nematodes
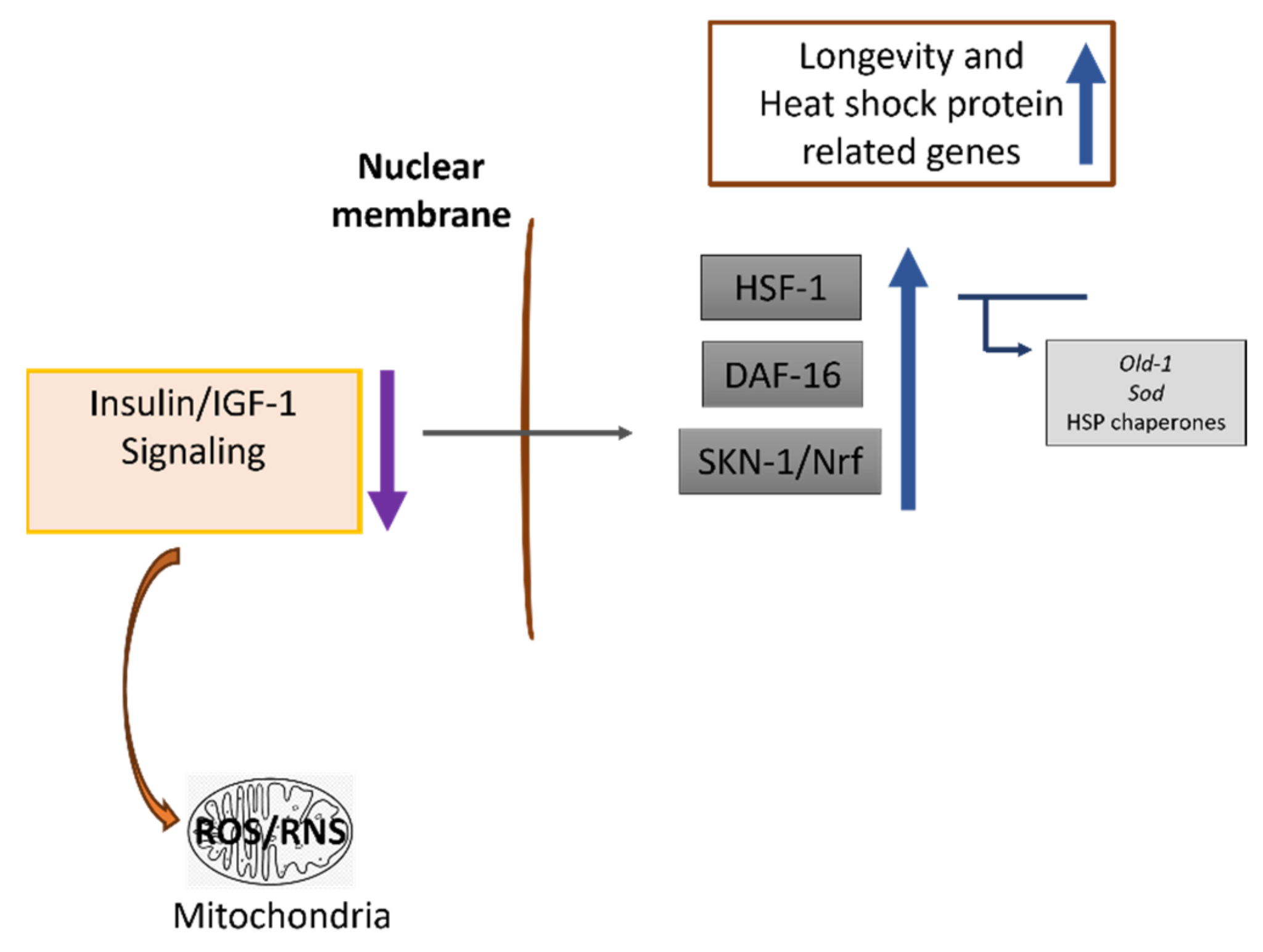
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization WHO Home Page. Available online: https://www.who.int/data/gho/data/themes/noncommunicable-diseases (accessed on 30 August 2023).
- Budreviciute, A.; Damiati, S.; Sabir, D.K.; Onder, K.; Schuller-Goetzburg, P.; Plakys, G.; Katileviciute, A.; Khoja, S.; Kodzius, R. Management and Prevention Strategies for Non-Communicable Diseases (NCDs) and Their Risk Factors. Front. Public Health 2020, 8, 574111. [Google Scholar] [CrossRef]
- Queen, N.J.; Hassan, Q.N.; Cao, L. Improvements to Healthspan Through Environmental Enrichment and Lifestyle Interventions: Where Are We Now? Front. Neurosci. 2020, 14, 605. [Google Scholar] [CrossRef]
- Couteur, D.G.L.; Barzilai, N. New Horizons in Life Extension, Healthspan Extension and Exceptional Longevity. Age Ageing 2022, 51, afac156. [Google Scholar] [CrossRef]
- Moskalev, A.; Chernyagina, E.; Kudryavtseva, A.; Shaposhnikov, M. Geroprotectors: A Unified Concept and Screening Approaches. Aging Dis. 2017, 8, 354. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, C. The First Long-Lived Mutants: Discovery of the Insulin/IGF-1 Pathway for Ageing. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 9–16. [Google Scholar] [CrossRef]
- Ruetenik, A.; Barrientos, A. Dietary Restriction, Mitochondrial Function and Aging: From Yeast to Humans. Biochim. Biophys. Acta BBA Bioenerg. 2015, 1847, 1434–1447. [Google Scholar] [CrossRef] [PubMed]
- Lacerda, D.C.; Urquiza-Martínez, M.V.; Manhaes-de-Castro, R.; Visco, D.B.; Derosier, C.; Mercado-Camargo, R.; Torner, L.; Toscano, A.E.; Guzmán-Quevedo, O. Metabolic and Neurological Consequences of the Treatment with Polyphenols: A Systematic Review in Rodent Models of Noncommunicable Diseases. Nutr. Neurosci. 2022, 25, 1680–1696. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- El Gharras, H. Polyphenols: Food Sources, Properties and Applications—A Review: Nutraceutical Polyphenols. Int. J. Food Sci. Technol. 2009, 44, 2512–2518. [Google Scholar] [CrossRef]
- Ji, J.; Yang, X.; Flavel, M.; Shields, Z.P.; Neoh, J.; Bowen, M.-L.; Kitchen, B. Age-Deterring and Skin Care Function of a Polyphenol Rich Sugarcane Concentrate. Cosmetics 2020, 7, 30. [Google Scholar] [CrossRef]
- Zhang, S.; Li, F.; Zhou, T.; Wang, G.; Li, Z. Caenorhabditis elegans as a Useful Model for Studying Aging Mutations. Front. Endocrinol. 2020, 11, 554994. [Google Scholar] [CrossRef] [PubMed]
- Mudd, N.; Liceaga, A.M. Caenorhabditis elegans as an In Vivo Model for Food Bioactives: A Review. Curr. Res. Food Sci. 2022, 5, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Munasinghe, M.; Almotayri, A.; Thomas, J.; Heydarian, D.; Jois, M. Early Exposure Is Necessary for the Lifespan Extension Effects of Cocoa in C. Elegans. Nutr. Metab. Insights 2021, 14, 117863882110294. [Google Scholar] [CrossRef] [PubMed]
- Deseo, M.A.; Elkins, A.; Rochfort, S.; Kitchen, B. Antioxidant Activity and Polyphenol Composition of Sugarcane Molasses Extract. Food Chem. 2020, 314, 126180. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Yang, X.; Flavel, M.; Shields, Z.P.-I.; Kitchen, B. Antioxidant and Anti-Diabetic Functions of a Polyphenol-Rich Sugarcane Extract. J. Am. Coll. Nutr. 2019, 38, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Porta-de-la-Riva, M.; Fontrodona, L.; Villanueva, A.; Cerón, J. Basic Caenorhabditis elegans Methods: Synchronization and Observation. J. Vis. Exp. 2012, 10, e4019. [Google Scholar] [CrossRef]
- Sutphin, G.L.; Kaeberlein, M. Measuring Caenorhabditis elegans Life Span on Solid Media. J. Vis. Exp. 2009, 12, 1152. [Google Scholar] [CrossRef]
- Banse, S.A.; Lucanic, M.; Sedore, C.A.; Coleman-Hulbert, A.L.; Plummer, W.T.; Chen, E.; Kish, J.L.; Hall, D.; Onken, B.; Presley, M.P.; et al. Automated Lifespan Determination across Caenorhabditis Strains and Species Reveals Assay-Specific Effects of Chemical Interventions. GeroScience 2019, 41, 945–960. [Google Scholar] [CrossRef]
- Vergara-Irigaray, N.; Riesen, M.; Piazza, G.; Bronk, L.F.; Driessen, W.H.P.; Edwards, J.K.; Arap, W.; Pasqualini, R.; Gramotnev, D.K.; Wang, W.; et al. Lab-on-a-Chip for Studies in C. Elegans. In Encyclopedia of Nanotechnology; Bhushan, B., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 1181–1192. ISBN 978-90-481-9750-7. [Google Scholar]
- Herndon, L.A.; Schmeissner, P.J.; Dudaronek, J.M.; Brown, P.A.; Listner, K.M.; Sakano, Y.; Paupard, M.C.; Hall, D.H.; Driscoll, M. Stochastic and Genetic Factors Influence Tissue-Specific Decline in Ageing C. Elegans. Nature 2002, 419, 808–814. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Strange, K.C. Elegans: Methods and Applications; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Feng, S.; Luo, Z.; Tao, B.; Chen, C. Ultrasonic-Assisted Extraction and Purification of Phenolic Compounds from Sugarcane (Saccharum officinarum L.) Rinds. LWT Food Sci. Technol. 2015, 60, 970–976. [Google Scholar] [CrossRef]
- Kadam, U.S.; Ghosh, S.B.; De, S.; Suprasanna, P.; Devasagayam, T.P.A.; Bapat, V.A. Antioxidant Activity in Sugarcane Juice and Its Protective Role against Radiation Induced DNA Damage. Food Chem. 2008, 106, 1154–1160. [Google Scholar] [CrossRef]
- Vaiserman, A.M. Early-Life Nutritional Programming of Longevity. J. Dev. Orig. Health Dis. 2014, 5, 325–338. [Google Scholar] [CrossRef]
- Duque-Guimarães, D.; Ozanne, S. Early Nutrition and Ageing: Can We Intervene? Biogerontology 2017, 18, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, M.D.; Lupu, D.S. Nutritional Influence on Epigenetics and Effects on Longevity. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Analysis & Policy Observatory Home Page. Available online: https://apo.org.au/node/108431 (accessed on 21 April 2023).
- Kim, S.Y.; Webb, A.E. Neuronal Functions of FOXO/DAF-16. Nutr. Healthy Aging 2017, 4, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Uno, M.; Tani, Y.; Nono, M.; Okabe, E.; Kishimoto, S.; Takahashi, C.; Abe, R.; Kurihara, T.; Nishida, E. Neuronal DAF-16-to-Intestinal DAF-16 Communication Underlies Organismal Lifespan Extension in C. elegans. iScience 2021, 24, 102706. [Google Scholar] [CrossRef]
- Lin, K.; Hsin, H.; Libina, N.; Kenyon, C. Regulation of the Caenorhabditis elegans Longevity Protein DAF-16 by Insulin/IGF-1 and Germline Signaling. Nat. Genet. 2001, 28, 139–145. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, A.V.; Leal-Tassias, A.; Rodríguez-Sánchez, N.; Piquer-Gil, M.; Martorell, P.; Genovés, S.; Acosta, C.; Burks, D.; Ramón, D.; Mullor, J.L. Use of Medaka Fish as Vertebrate Model to Study the Effect of Cocoa Polyphenols in the Resistance to Oxidative Stress and Life Span Extension. Rejuvenation Res. 2018, 21, 323–332. [Google Scholar] [CrossRef]
- Shakeri, M.; Cottrell, J.J.; Wilkinson, S.; Le, H.H.; Suleria, H.A.R.; Warner, R.D.; Dunshea, F.R. A Dietary Sugarcane-Derived Polyphenol Mix Reduces the Negative Effects of Cyclic Heat Exposure on Growth Performance, Blood Gas Status, and Meat Quality in Broiler Chickens. Animals 2020, 10, 1158. [Google Scholar] [CrossRef]
- Surco-Laos, F.; Dueñas, M.; González-Manzano, S.; Cabello, J.; Santos-Buelga, C.; González-Paramás, A.M. Influence of Catechins and Their Methylated Metabolites on Lifespan and Resistance to Oxidative and Thermal Stress of Caenorhabditis elegans and Epicatechin Uptake. Food Res. Int. 2012, 46, 514–521. [Google Scholar] [CrossRef]
- Volovik, Y.; Moll, L.; Marques, F.C.; Maman, M.; Bejerano-Sagie, M.; Cohen, E. Differential Regulation of the Heat Shock Factor 1 and DAF-16 by Neuronal Nhl-1 in the Nematode C. elegans. Cell Rep. 2014, 9, 2192–2205. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, T.; Sakamoto, K. Quercetin Enhances Motility in Aged and Heat-Stressed Caenorhabditis elegans Nematodes by Modulating Both HSF-1 Activity, and Insulin-like and P38-MAPK Signalling. PLoS ONE 2020, 15, e0238528. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Choe, K.P. Characterization of Skn-1/Wdr-23 Phenotypes in Caenorhabditis elegans; Pleiotrophy, Aging, Glutathione, and Interactions with Other Longevity Pathways. Mech. Ageing Dev. 2015, 149, 88–98. [Google Scholar] [CrossRef]
- Wu, P.-S.; Yen, J.-H.; Kou, M.-C.; Wu, M.-J. Luteolin and Apigenin Attenuate 4-Hydroxy-2-Nonenal-Mediated Cell Death through Modulation of UPR, Nrf2-ARE and MAPK Pathways in PC12 Cells. PLoS ONE 2015, 10, e0130599. [Google Scholar] [CrossRef]
- Fang, Z.; Chen, Y.; Wang, G.; Feng, T.; Shen, M.; Xiao, B.; Gu, J.; Wang, W.; Li, J.; Zhang, Y. Evaluation of the Antioxidant Effects of Acid Hydrolysates from Auricularia auricular Polysaccharides Using a Caenorhabditis elegans Model. Food Funct. 2019, 10, 5531–5543. [Google Scholar] [CrossRef]
- Kaletsky, R.; Lakhina, V.; Arey, R.; Williams, A.; Landis, J.; Ashraf, J.; Murphy, C.T. The C. elegans Adult Neuronal IIS/FOXO Transcriptome Reveals Adult Phenotype Regulators. Nature 2016, 529, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.T. Insulin/Insulin-like Growth Factor Signaling in C. elegans. WormBook 2013, 26, 1–43. [Google Scholar] [CrossRef]
- Pinkston-Gosse, J.; Kenyon, C. DAF-16/FOXO Targets Genes That Regulate Tumor Growth in Caenorhabditis elegans. Nat. Genet. 2007, 39, 1403–1409. [Google Scholar] [CrossRef]
- Zečić, A.; Braeckman, B.P. DAF-16/FoxO in Caenorhabditis elegans and Its Role in Metabolic Remodeling. Cells 2020, 9, 109. [Google Scholar] [CrossRef]
- Wilson, M.A.; Shukitt-Hale, B.; Kalt, W.; Ingram, D.K.; Joseph, J.A.; Wolkow, C.A. Blueberry Polyphenols Increase Lifespan and Thermotolerance in Caenorhabditis elegans. Aging Cell 2006, 5, 59–68. [Google Scholar] [CrossRef]
- McColl, G.; Rogers, A.N.; Alavez, S.; Hubbard, A.E.; Melov, S.; Link, C.D.; Bush, A.I.; Kapahi, P.; Lithgow, G.J. Insulin-like Signaling Determines Survival during Stress via Posttranscriptional Mechanisms in C. elegans. Cell Metab. 2010, 12, 260–272. [Google Scholar] [CrossRef]
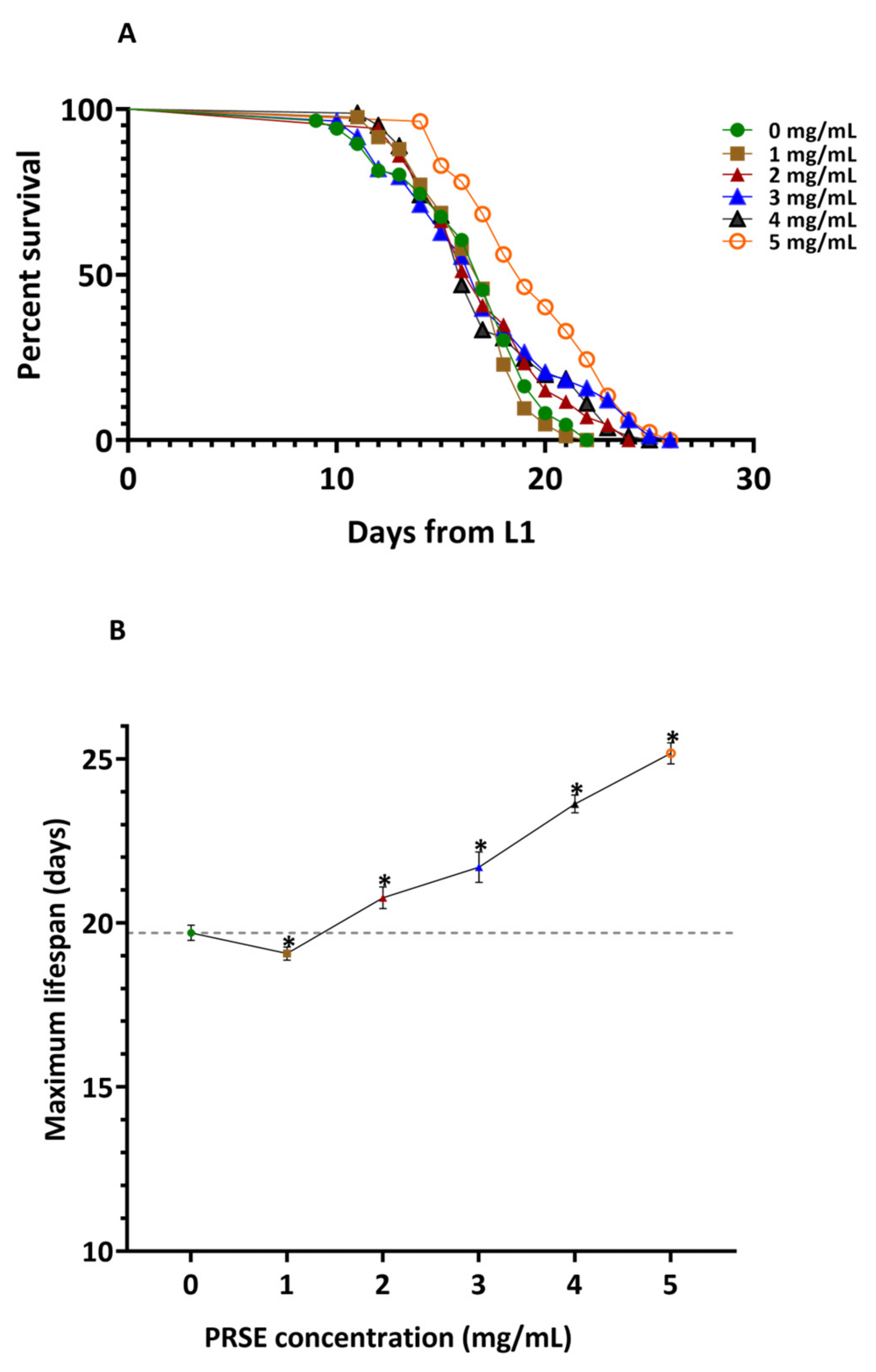


| PRSE Concentration (mg/mL) | Sample Size (n) | Mean Lifespan (Days) ± SEM | % Extension Compared to Control | Median Lifespan (Days) ± SE | % Extension Compared to Control | Maximum Lifespan(Days) ± SEM |
|---|---|---|---|---|---|---|
| 0 | 86 | 16.49 ± 0.36 | 17 ± 0.35 | 19.70 ± 0.23 | ||
| 1 | 83 | 16.65 ± 0.28 | 0.98 | 17 ± 0.31 | 0.00 | 19.07 ± 0.20 * |
| 2 | 87 | 17.12 ± 0.35 | 3.81 | 17 ± 0.41 | 0.00 | 20.77 ± 0.33 * |
| 3 | 83 | 17.12 ± 0.46 | 3.83 | 17 ± 0.34 | 0.00 | 21.70 ± 0.46 * |
| 4 | 81 | 17.15 ± 0.38 | 4.00 | 17 ± 0.32 | 0.00 | 23.63 ± 0.27 * |
| 5 | 82 | 19.48 ± 0.37 * | 18.12 * | 19 ± 0.69 * | 11.76 * | 25.17 ± 0.32 * |
| Timing of Exposure to PRSE | Sample Size (n) | Mean Lifespan (Days) ± SEM | % Extension Compared to Control | Median Lifespan (Days) ± SE | % Extension Compared to Control | Maximum Lifespan (Days) ± SEM |
|---|---|---|---|---|---|---|
| No PRSE | 94 | 16.39 ± 0.35 | 18 ± 0.49 | 19.80 ± 0.23 | ||
| L1 | 91 | 18.14 ± 0.45 * | 10.67 * | 18 ± 0.57 | 0.00 | 22.97 ± 0.37 * |
| L1 + 15 h | 97 | 20.37 ± 0.33 * | 24.26 * | 20 ± 0.35 * | 11.1 * | 24.40 ± 0.18 * |
| L1 + 24 h | 99 | 16.61 ± 0.36 | 1.36 | 18 ± 0.65 | 0.00 | 20.70 ± 0.25 * |
| L1 + 34 h | 97 | 16.94 ± 0.35 | 3.32 | 18 ± 0.34 | 0.00 | 20.40 ± 0.20 * |
| L1 + 48 h | 98 | 17.05 ± 0.28 | 4.01 | 18 ± 0.22 | 0.00 | 19.70 ± 0.17 |
| Strain/Treatment | Sample Size (n) | Mean Lifespan (Days) ± SEM | % Reduction Compared to Control | Median Lifespan (Days) ± SE | % Reduction Compared to Control |
|---|---|---|---|---|---|
| daf-16 (mgDf50) I 0 mg/mL-control | 80 | 18.55 ± 0.35 | 19 ± 0.55 | ||
| daf-16 (mgDf50) I 5 mg/mL | 87 | 17.76 ± 0.40 | −4.27 | 18 ± 0.58 | −5.26 |
| daf-2 (e1370) III 0 mg/mL-control | 98 | 25.82 ± 0.63 | 26 ± 0.93 | ||
| daf-2 (e1370) III 5 mg/mL | 100 | 25.33 ± 0.68 | −1.88 | 25 ± 1.11 | −3.85 |
| Strain | Treatment | Day | Sample Size (n) | Mean Survival ± SEM | % Extension/Reduction Compared to Control | Median Survival ± SE | % Extension/Reduction Compared to Control |
|---|---|---|---|---|---|---|---|
| N2 | Control | 4 | 113 | 7.87 ± 0.12 | 8.00 ± 0.12 | ||
| PRSE | 106 | 9.06 ± 0.12 * | 15.1 * | 9.00 ± 0.12 * | 12.5 * | ||
| N2 | Control | 8 | 111 | 7.06 ± 0.18 | 7.00 ± 0.25 | ||
| PRSE | 109 | 7.79 ± 0.12 * | 10.3 * | 8.00 ± 0.21 * | 14.3 * | ||
| N2 | Control | 12 | 112 | 6.15 ± 0.16 | 6.00 ± 0.21 | ||
| PRSE | 104 | 6.61 ± 0.10 * | 7.4 * | 7.00 ± 0.08 * | 16.7 * | ||
| daf-2 (e1370) | Control | 4 | 118 | 11.49 ± 0.14 | 12 ± 0.19 | ||
| PRSE | 104 | 11.49 ± 0.16 | −0.01 | 12 ± 0.13 | 0 | ||
| daf-2 (e1370) | Control | 8 | 112 | 9.07 ± 0.21 | 9 ± 0.38 | ||
| PRSE | 101 | 9.87 ± 0.20 * | 8.82 * | 10 ± 0.15* | 11.11 * | ||
| daf-2 (e1370) | Control | 12 | 105 | 8.84 ± 0.21 | 10 ± 0.20 | ||
| PRSE | 102 | 9.58 ± 0.21 * | 8.38 * | 10 ± 0.26 | 0 | ||
| daf-16 (MgDf50) | Control | 4 | 115 | 6.37 ± 0.18 | 7 ± 0.27 | ||
| PRSE | 104 | 6.94 ± 0.21 * | 8.92 * | 7 ± 0.33 | 0 | ||
| daf-16 (MgDf50) | Control | 8 | 118 | 4.53 ± 0.12 | 5 ± 0.13 | ||
| PRSE | 105 | 5.06 ± 0.15 * | 11.54 * | 5 ± 0.20 | 0 | ||
| daf-16 (MgDf50) | Control | 12 | 107 | 3.39 ± 0.13 | 4 ± 0.21 | ||
| PRSE | 101 | 3.37 ± 0.12 | −0.5 | 4 ± 0.14 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heydarian, D.; Flavel, M.; Munasinghe, M.; Almotayri, A.; Jois, M.; Thomas, J. Early Exposure to Polyphenol-Rich Sugarcane Extract (PRSE) Mitigates Aging While Enhancing Thermotolerance in C. elegans. J. Ageing Longev. 2024, 4, 15-27. https://doi.org/10.3390/jal4010002
Heydarian D, Flavel M, Munasinghe M, Almotayri A, Jois M, Thomas J. Early Exposure to Polyphenol-Rich Sugarcane Extract (PRSE) Mitigates Aging While Enhancing Thermotolerance in C. elegans. Journal of Ageing and Longevity. 2024; 4(1):15-27. https://doi.org/10.3390/jal4010002
Chicago/Turabian StyleHeydarian, Deniz, Matthew Flavel, Mihiri Munasinghe, Abdullah Almotayri, Markandeya Jois, and Jency Thomas. 2024. "Early Exposure to Polyphenol-Rich Sugarcane Extract (PRSE) Mitigates Aging While Enhancing Thermotolerance in C. elegans" Journal of Ageing and Longevity 4, no. 1: 15-27. https://doi.org/10.3390/jal4010002
APA StyleHeydarian, D., Flavel, M., Munasinghe, M., Almotayri, A., Jois, M., & Thomas, J. (2024). Early Exposure to Polyphenol-Rich Sugarcane Extract (PRSE) Mitigates Aging While Enhancing Thermotolerance in C. elegans. Journal of Ageing and Longevity, 4(1), 15-27. https://doi.org/10.3390/jal4010002








