Current and Future Directions in Immunotherapy for Gastrointestinal Malignancies
Abstract
1. Introduction
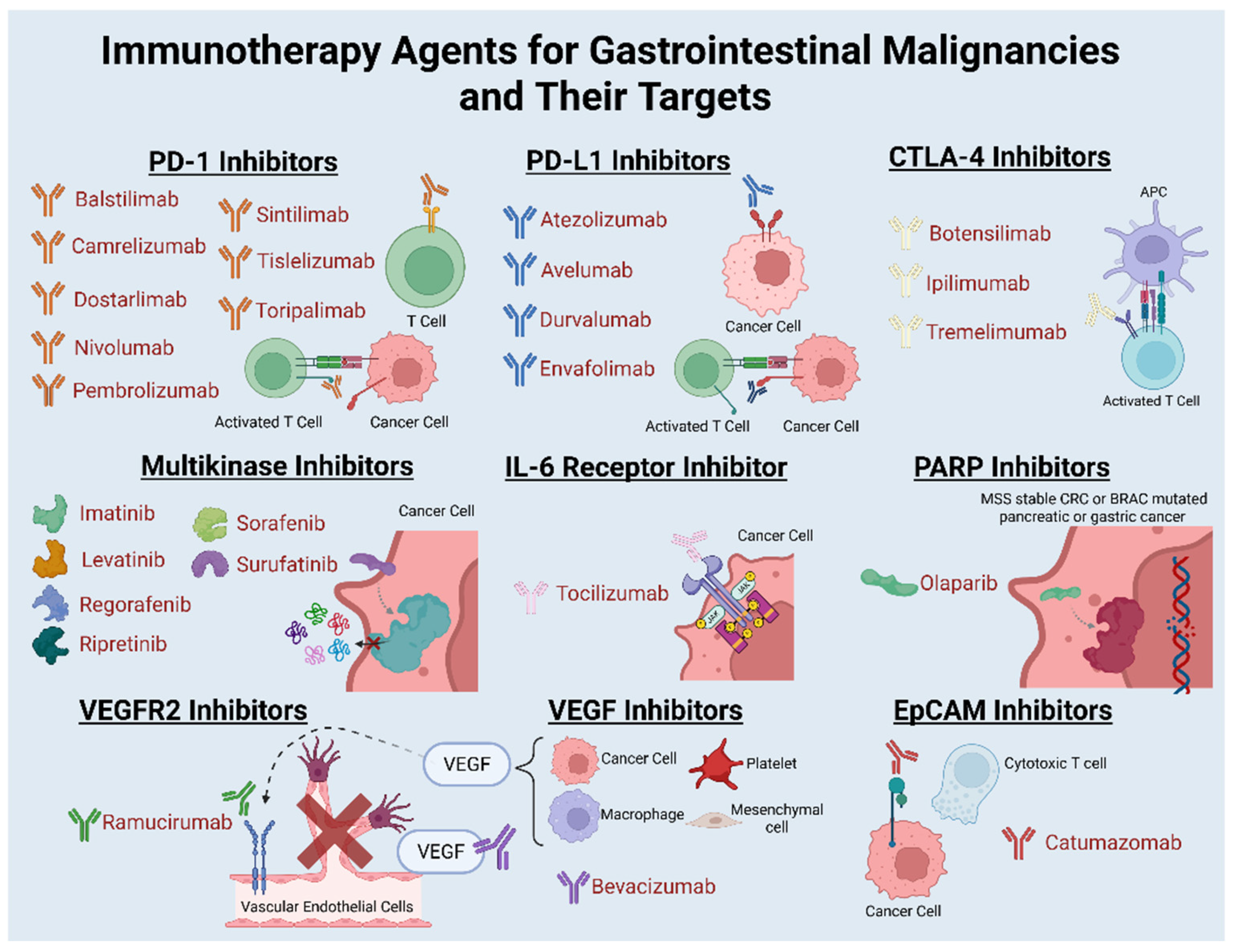
2. Immune Checkpoint Inhibition in Gastrointestinal Malignancies
2.1. Esophageal Cancer
2.2. Gastric Cancer
2.3. Pancreatic Cancer
2.4. Colorectal Cancer
2.5. Hepatocellular Carcinoma
2.6. Biliary Tract Cancers
2.7. Gastrointestinal Stromal Tumor
2.8. Small Bowel Adenocarcinoma
2.9. Neuroendocrine Tumors
2.10. Anal Cancer
2.11. Appendiceal Cancer
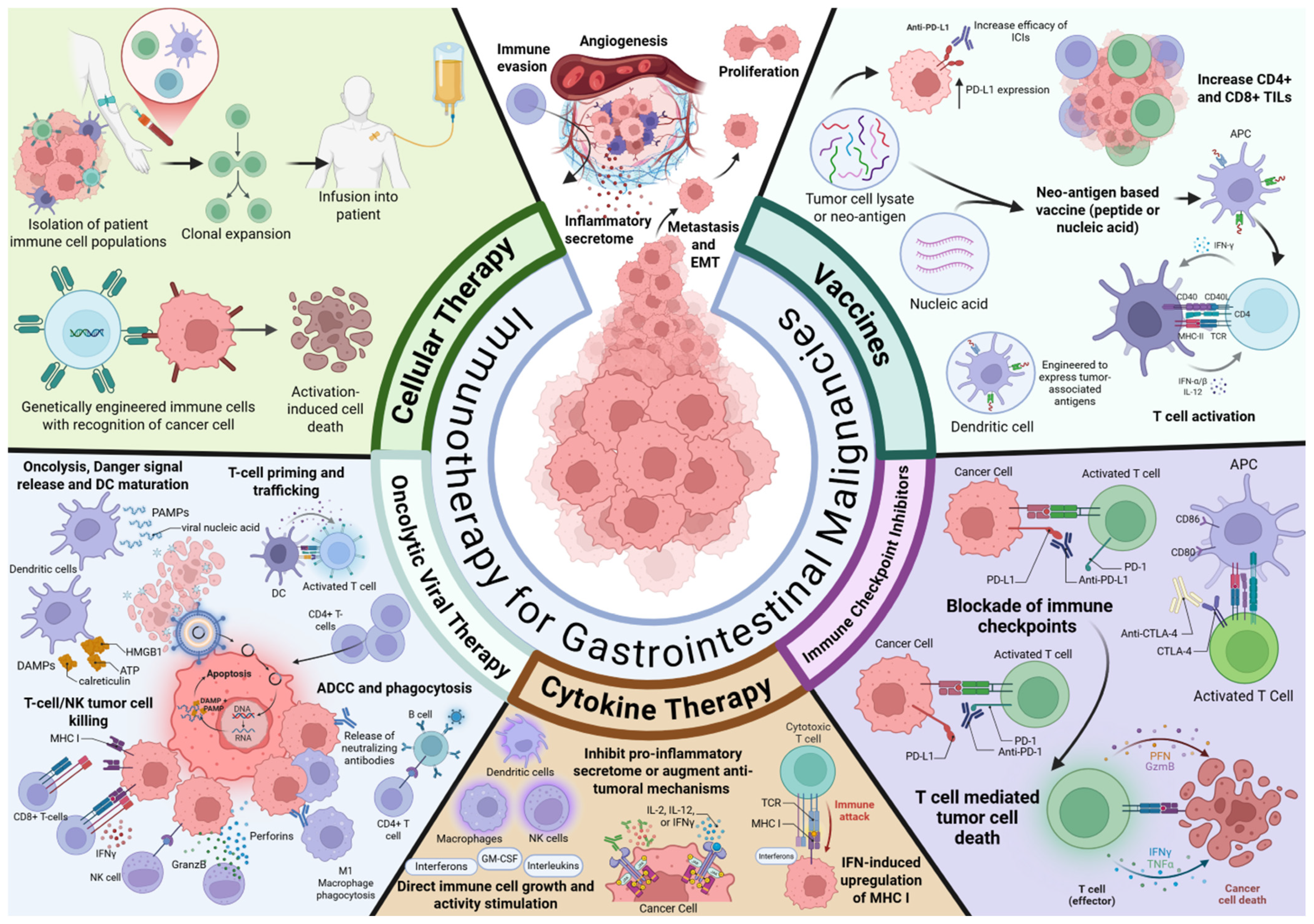
3. Vaccines as Cancer Therapy
3.1. Esophageal Cancer
3.2. Gastric Cancer
3.3. Pancreatic Cancer
3.4. Colorectal Cancer
3.5. Hepatocellular Carcinoma
3.6. Biliary Tract Cancers
3.7. Gastrointestinal Stromal Tumor
4. Cytokine Therapy
4.1. Gastric Cancer
4.2. Pancreatic Cancer
4.3. Colorectal Cancer
4.4. Gastrointestinal Stromal Tumor
4.5. Peritoneal Carcinomatosis
5. Oncolytic Viral Therapy
5.1. Esophageal Cancer
5.2. Pancreatic Cancer
5.3. Colorectal Cancer
5.4. Hepatocellular Carcinoma
5.5. Peritoneal Carcinomatosis
6. Cellular Therapy
6.1. Esophageal Cancer
6.2. Gastric Cancer
6.3. Pancreatic Cancer
6.4. Colorectal Cancer
6.5. Hepatocellular Carcinoma
6.6. Biliary Tract Cancers
6.7. Anal Cancer
6.8. Peritoneal Carcinomatosis
7. Clinical Trials Involving Immunotherapy in Gastrointestinal Malignancies
7.1. Immune Checkpoint Inhibition
7.2. Intraperitoneal Use of Immunotherapy
7.3. Vaccination and Immune Modulation
7.4. Oncolytic Viral Therapy
7.5. Adoptive Cell Therapy
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Burnet, F.M. Immunological aspects of malignant disease. Lancet 1967, 289, 1171–1174. [Google Scholar] [CrossRef]
- Kazemi, M.H.; Sadri, M.; Najafi, A.; Rahimi, A.; Baghernejadan, Z.; Khorramdelazad, H.; Falak, R. Tumor-infiltrating lymphocytes for treatment of solid tumors: It takes two to tango? Front. Immunol. 2022, 13, 1018962. [Google Scholar] [CrossRef]
- McCarthy, P.M.; Valdera, F.A.; Smolinsky, T.R.; Adams, A.M.; O’Shea, A.E.; Thomas, K.K.; Van Decar, S.; Carpenter, E.L.; Tiwari, A.; Myers, J.W.; et al. Tumor infiltrating lymphocytes as an endpoint in cancer vaccine trials. Front. Immunol. 2023, 14, 1090533. [Google Scholar] [CrossRef] [PubMed]
- Qayoom, H.; Sofi, S.; Mir, M.A. Targeting tumor microenvironment using tumor-infiltrating lymphocytes as therapeutics against tumorigenesis. Immunol. Res. 2023, 71, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Wagner, K.; Wolchok, J.D.; Allison, J.P. Novel cancer immunotherapy agents with survival benefit: Recent successes and next steps. Nat. Rev. Cancer 2011, 11, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Teixeira Farinha, H.; Digklia, A.; Schizas, D.; Demartines, N.; Schäfer, M.; Mantziari, S. Immunotherapy for Esophageal Cancer: State-of-the Art in 2021. Cancers 2022, 14, 554. [Google Scholar] [CrossRef]
- Bashash, D.; Zandi, Z.; Kashani, B.; Pourbagheri-Sigaroodi, A.; Salari, S.; Ghaffari, S.H. Resistance to immunotherapy in human malignancies: Mechanisms, research progresses, challenges, and opportunities. J. Cell. Physiol. 2022, 237, 346–372. [Google Scholar] [CrossRef]
- Sathyanarayanan, V.; Neelapu, S.S. Cancer immunotherapy: Strategies for personalization and combinatorial approaches. Mol. Oncol. 2015, 9, 2043–2053. [Google Scholar] [CrossRef]
- Koustas, E.; Trifylli, E.-M.; Sarantis, P.; Papadopoulos, N.; Karapedi, E.; Aloizos, G.; Damaskos, C.; Garmpis, N.; Garmpi, A.; Papavassiliou, K.A.; et al. Immunotherapy as a Therapeutic Strategy for Gastrointestinal Cancer—Current Treatment Options and Future Perspectives. Int. J. Mol. Sci. 2022, 23, 6664. [Google Scholar] [CrossRef]
- Park, R.; Williamson, S.; Kasi, A.; Saeed, A. Immune Therapeutics in the Treatment of Advanced Gastric and Esophageal Cancer. Anticancer. Res. 2018, 38, 5569–5580. [Google Scholar] [CrossRef]
- Phillips, C. First Cancer TIL Therapy Gets FDA Approval for Advanced Melanoma. 2024. Available online: https://www.cancer.gov/news-events/cancer-currents-blog/2024/fda-amtagvi-til-therapy-melanoma (accessed on 11 September 2024).
- Wagner, J.; Wickman, E.; DeRenzo, C.; Gottschalk, S. CAR T Cell Therapy for Solid Tumors: Bright Future or Dark Reality? Mol. Ther. 2020, 28, 2320–2339. [Google Scholar] [CrossRef]
- Kelly, R.J.; Bever, K.; Chao, J.; Ciombor, K.K.; Eng, C.; Fakih, M.; Goyal, L.; Hubbard, J.; Iyer, R.; Kemberling, H.T.; et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immunotherapy for the treatment of gastrointestinal cancer. J. Immunother. Cancer 2023, 11, e006658. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, D.; Allum, W.H.; Stenning, S.P.; Thompson, J.N.; Van de Velde, C.J.H.; Nicolson, M.; Scarffe, J.H.; Lofts, F.J.; Falk, S.J.; Iveson, T.J.; et al. Perioperative Chemotherapy versus Surgery Alone for Resectable Gastroesophageal Cancer. N. Eng. J. Med. 2006, 355, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Al-Batran, S.E.; Homann, N.; Pauligk, C.; Goetze, T.O.; Meiler, J.; Kasper, S.; Kopp, H.; Mayer, F.; Haag, G.M.; Luley, K.; et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): A randomised, phase 2/3 trial. Lancet 2019, 393, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.J.; Ajani, J.A.; Kuzdzal, J.; Zander, T.; Van Cutsem, E.; Piessen, G.; Mendez, G.; Feliciano, J.; Motoyama, S.; Lièvre, A.; et al. Adjuvant Nivolumab in Resected Esophageal or Gastroesophageal Junction Cancer. N. Engl. J. Med. 2021, 384, 1191–1203, Erratum in N. Engl. J. Med. 2023, 388, 672. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Shitara, K.; Moehler, M.; Garrido, M.; Salman, P.; Shen, L.; Wyrwicz, L.; Yamaguchi, K.; Skoczylas, T.; Bragagnoli, A.C.; et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): A randomised, open-label, phase 3 trial. Lancet 2021, 398, 27–40. [Google Scholar] [CrossRef]
- Sun, J.-M.; Shen, L.; Shah, M.A.; Enzinger, P.; Adenis, A.; Doi, T.; Kojima, T.; Metges, J.; Li, Z.; Kim, S.; et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): A randomised, placebo-controlled, phase 3 study. Lancet 2021, 398, 759–771, Erratum in Lancet 2021, 398, 1874. [Google Scholar] [CrossRef]
- Ajani, J.A.; D’Amico, T.A.; Bentrem, D.J.; Cooke, D.; Corvera, C.; Das, P.; Enzinger, P.C.; Enzler, T.; Farjah, F.; Gerdes, H.; et al. Esophageal and Esophagogastric Junction Cancers, Version 2.2023, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2023, 21, 393–422. [Google Scholar] [CrossRef]
- Tieniber, A.D.; Perez, J.E.; Hanna, A.N.; DeMatteo, R.P. Immunotherapy for GI Malignancies, Ready for Prime Time? Ann. Surg. Oncol. 2023, 30, 1787–1793. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, P.; Pang, Q. Immune checkpoint inhibitors for esophageal squamous cell carcinoma: A narrative review. Ann. Transl. Med. 2020, 8, 1193. [Google Scholar] [CrossRef]
- Kono, K.; Nakajima, S.; Mimura, K. Current status of immune checkpoint inhibitors for gastric cancer. Gastric Cancer 2020, 23, 565–578. [Google Scholar] [CrossRef] [PubMed]
- Fashoyin-Aje, L.; Donoghue, M.; Chen, H.; He, K.; Veeraraghavan, J.; Goldberg, K.B.; Keegan, P.; McKee, A.E.; Pazdur, R. FDA Approval Summary: Pembrolizumab for Recurrent Locally Advanced or Metastatic Gastric or Gastroesophageal Junction Adenocarcinoma Expressing PD-L1. Oncologist 2019, 24, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, C.S.; Doi, T.; Jang, R.W.; Muro, K.; Satoh, T.; Machado, M.; Sun, W.; Jalal, S.I.; Shah, M.A.; Metges, J.; et al. Safety and Efficacy of Pembrolizumab Monotherapy in Patients with Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer. JAMA Oncol. 2018, 4, e180013, Erratum in JAMA Oncol. 2019, 5, 579. [Google Scholar] [CrossRef] [PubMed]
- Bang, Y.-J.; Kang, Y.-K.; Catenacci, D.V.; Muro, K.; Fuchs, C.S.; Geva, R.; Hara, H.; Golan, T.; Garrido, M.; Jalal, S.I.; et al. Pembrolizumab alone or in combination with chemotherapy as first-line therapy for patients with advanced gastric or gastroesophageal junction adenocarcinoma: Results from the phase II nonrandomized KEYNOTE-059 study. Gastric Cancer 2019, 22, 828–837. [Google Scholar] [CrossRef]
- Kang, Y.-K.; Boku, N.; Satoh, T.; Ryu, M.; Chao, Y.; Kato, K.; Chung, H.C.; Chen, J.; Muro, K.; Kang, W.K.; et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 390, 2461–2471. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Bendell, J.; Calvo, E.; Kim, J.W.; Ascierto, P.A.; Sharma, P.; Ott, P.A.; Peltola, K.; Jaeger, D.; Evans, J.; et al. CheckMate-032 Study: Efficacy and Safety of Nivolumab and Nivolumab Plus Ipilimumab in Patients with Metastatic Esophagogastric Cancer. J. Clin. Oncol. 2018, 36, 2836–2844, Erratum in J. Clin. Oncol. 2019, 37, 443. [Google Scholar] [CrossRef]
- Hilmi, M.; Bartholin, L.; Neuzillet, C. Immune therapies in pancreatic ductal adenocarcinoma: Where are we now? World J. Gastroenterol. 2018, 24, 2137–2151. [Google Scholar] [CrossRef]
- Dahiya, D.S.; Kichloo, A.; Singh, J.; Albosta, M.; Lekkala, M. Current immunotherapy in gastrointestinal malignancies A Review. J. Investig. Med. 2021, 69, 689–696. [Google Scholar] [CrossRef]
- Kole, C.; Charalampakis, N.; Tsakatikas, S.; Frountzas, M.; Apostolou, K.; Schizas, D. Immunotherapy in Combination with Well-Established Treatment Strategies in Pancreatic Cancer: Current Insights. Cancer Manag. Res. 2022, 14, 1043–1061. [Google Scholar] [CrossRef]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef]
- Ogino, S.; Nosho, K.; Irahara, N.; Meyerhardt, J.A.; Baba, Y.; Shima, K.; Glickman, J.N.; Ferrone, C.R.; Mino-Kenudson, M.; Tanaka, N.; et al. Lymphocytic reaction to colorectal cancer is associated with longer survival, independent of lymph node count, microsatellite instability, and CpG island methylator phenotype. Clin. Cancer Res. 2009, 15, 6412–6420. [Google Scholar] [CrossRef]
- Golshani, G.; Zhang, Y. Advances in immunotherapy for colorectal cancer: A review. Ther. Adv. Gastroenterol. 2020, 13, 1756284820917527. [Google Scholar] [CrossRef]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef]
- Overman, M.J.; McDermott, R.; Leach, J.L.; Lonardi, S.; Lenz, H.; Morse, M.A.; Desai, J.; Hill, A.; Axelson, M.; Moss, R.A.; et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): An open-label, multicentre, phase 2 study. Lancet Oncol. 2017, 18, 1182–1191, Erratum in Lancet Oncol. 2017, 18, 510. [Google Scholar] [CrossRef] [PubMed]
- Andre, T.; Lonardi, S.; Wong, M.; Lenz, H.; Gelsomino, F.; Aglietta, M.; Morse, M.; Van Cutsem, E.; McDermott, R.S.; Hill, A.G.; et al. Nivolumab + ipilimumab combination in patients with DNA mismatch repair-deficient/microsatellite instability-high (dMMR/MSI-H) metastatic colorectal cancer (mCRC): First report of the full cohort from CheckMate-142. J. Clin. Oncol. 2018, 36, 553. [Google Scholar] [CrossRef]
- Cercek, A.; Lumish, M.; Sinopoli, J.; Weiss, J.; Shia, J.; Lamendola-Essel, M.; El Dika, I.H.; Segal, N.; Shcherba, M.; Sugarman, R.; et al. PD-1 Blockade in Mismatch Repair–Deficient, Locally Advanced Rectal Cancer. N. Engl. J. Med. 2022, 386, 2363–2376. [Google Scholar] [CrossRef] [PubMed]
- Ascierto, P.A.; Addeo, R.; Cartenì, G.; Daniele, B.; De Laurentis, M.; Ianniello, G.P.; Morabito, A.; Palmieri, G.; Pepe, S.; Perrone, F.; et al. The role of immunotherapy in solid tumors: Report from the Campania Society of Oncology Immunotherapy (SCITO) meeting, Naples 2014. J. Transl. Med. 2014, 12, 291. [Google Scholar] [CrossRef]
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.; Choo, S.; Trojan, J.; Welling, T.H., 3rd; et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017, 389, 2492–2502. [Google Scholar] [CrossRef]
- DeCarli, K.; Strosberg, J.; Almhanna, K. Immune Checkpoint Inhibitors for Gastrointestinal Malignancies: An Update. Cancers 2022, 14, 4201. [Google Scholar] [CrossRef]
- Yau, T.; Park, J.-W.; Finn, R.S.; Cheng, A.; Mathurin, P.; Edeline, J.; Kudo, M.; Harding, J.J.; Merle, P.; Rosmorduc, O.; et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): A randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022, 23, 77–90. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Sangro, B.; Chan, S.L.; Kelley, R.K.; Lau, G.; Kudo, M.; Sukeepaisarnjaroen, W.; Yarchoan, M.; De Toni, E.N.; Furuse, J.; Kang, Y.K.; et al. Four-year overall survival update from the phase III HIMALAYA study of tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. Ann. Oncol. 2024, 35, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Ricci, A.D.; Brandi, G. Pemigatinib: Hot topics behind the first approval of a targeted therapy in cholangiocarcinoma. Cancer Treat. Res. Commun. 2021, 27, 100337. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.-Y.; He, A.R.; Qin, S.; Chen, L.; Okusaka, T.; Vogel, A.; Kim, J.W.; Suksombooncharoen, T.; Lee, M.A.; Kitano, M.; et al. A phase 3 randomized, double-blind, placebo-controlled study of durvalumab in combination with gemcitabine plus cisplatin (GemCis) in patients (pts) with advanced biliary tract cancer (BTC): TOPAZ-1. J. Clin. Oncol. 2022, 40, 378. [Google Scholar] [CrossRef]
- Speckart, J.; Rasmusen, V.; Talib, Z.; GnanaDev, D.A.; Rahnemai-Azar, A.A. Emerging Therapies in Management of Cholangiocarcinoma. Cancers 2024, 16, 613. [Google Scholar] [CrossRef]
- Li, R.; Liu, Y.; Liu, Y.; Fu, L.; Qi, Q.; Zhang, X.; Cui, Y.; Lu, W.; Li, H. Durvalumab plus gemcitabine/cisplatin/nab-paclitaxel in resectable biliary tract cancer: A phase II, single-arm, open-label study (DurGAP). J. Clin. Oncol. 2024, 42, e16260. [Google Scholar] [CrossRef]
- Naganuma, A.; Sakuda, T.; Murakami, T.; Aihara, K.; Watanuki, Y.; Suzuki, Y.; Shibasaki, E.; Masuda, T.; Uehara, S.; Yasuoka, H.; et al. Microsatellite Instability-high Intrahepatic Cholangiocarcinoma with Portal Vein Tumor Thrombosis Successfully Treated with Pembrolizumab. Int. Med. 2020, 59, 2261–2267. [Google Scholar] [CrossRef]
- Nakamura, M.; Ueno, M.; Hayami, S.; Kawai, M.; Miyamoto, A.; Suzaki, N.; Hironi, S.; Okada, K.; Miyazawa, M.; Kitahata, Y.; et al. Effective Response of Intrahepatic Cholangiocarcinoma to Pembrolizumab: A Case Report. Anticancer. Res. 2020, 40, 4123–4129. [Google Scholar] [CrossRef]
- Czink, E.; Kloor, M.; Goeppert, B.; Fröhling, S.; Uhrig, S.; Weber, T.F.; Meinel, J.; Sutter, C.; Weiss, K.H.; Schirmacher, P.; et al. Successful immune checkpoint blockade in a patient with advanced stage microsatellite-unstable biliary tract cancer. Mol. Case Stud. 2017, 3, a001974. [Google Scholar] [CrossRef]
- Ikeda, Y.; Ono, M.; Ohmori, G.; Ameda, S.; Yamada, M.; Abe, T.; Fujii, S.; Fujita, M.; Maeda, M. Successful pembrolizumab treatment of microsatellite instability-high intrahepatic cholangiocarcinoma: A case report. Clin. Case Rep. 2021, 9, 2259–2263. [Google Scholar] [CrossRef]
- Bang, Y.-J.; Ueno, M.; Malka, D.; Chung, H.C.; Nagrial, A.; Kelley, R.K.; Piha-Paul, S.A.; Ros, W.; Italiano, A.; Nakagawa, K.; et al. Pembrolizumab (pembro) for advanced biliary adenocarcinoma: Results from the KEYNOTE-028 (KN028) and KEYNOTE-158 (KN158) basket studies. J. Clin. Oncol. 2019, 37, 4079. [Google Scholar] [CrossRef]
- Saeed, A.; Park, R.; Al-Jumayli, M.; Al-Rajabi, R.; Sun, W. Biologics, Immunotherapy, and Future Directions in the Treatment of Advanced Cholangiocarcinoma. Clin. Color. Cancer 2019, 18, 81–90. [Google Scholar] [CrossRef]
- LaPelusa, M.; Heumann, T.; Goff, L.; Agarwal, R. Targeted therapies in advanced biliary tract cancers—A narrative review. Chin. Clin. Oncol. 2023, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Lo, J.H.; Agarwal, R.; Goff, L.W.; Heumann, T.R. Immunotherapy in Biliary Tract Cancers: Current Standard-of-Care and Emerging Strategies. Cancers 2023, 15, 3312. [Google Scholar] [CrossRef] [PubMed]
- Kelley, R.K.; Yoo, C.; Finn, R.S.; Furuse, J.; Ren, Z.; Yau, T.; Klümpen, H.; Chan, S.L.; Vogel, A.; Ozaka, M.; et al. Abstract CT008: Pembrolizumab (pembro) in combination with gemcitabine and cisplatin (gem/cis) for advanced biliary tract cancer (BTC): Phase 3 KEYNOTE-966 study. Cancer Res. 2023, 83, CT008. [Google Scholar] [CrossRef]
- Kawamoto, M.; Wada, Y.; Koya, N.; Takama, Y.; Saitsu, H.; Ishizaki, N.; Tabata, M.; Onishi, H.; Nakamura, M.; Morisaki, T. Long-term survival of a patient with recurrent gallbladder carcinoma, treated with chemotherapy, immunotherapy, and surgery: A case report. Surg. Case Rep. 2018, 4, 115. [Google Scholar] [CrossRef]
- Hack, S.P.; Verret, W.; Mulla, S.; Liu, B.; Wang, Y.; Macarulla, T.; Ren, Z.; El-Khoueiry, A.B.; Zhu, A.X. IMbrave 151: A randomized phase II trial of atezolizumab combined with bevacizumab and chemotherapy in patients with advanced biliary tract cancer. Ther. Adv. Med. Oncol. 2021, 13, 175883592110365. [Google Scholar] [CrossRef]
- El-Khoueiry, A.B.; Ren, Z.; Chon, H.; Park, J.O.; Kim, J.W.; Pressiani, T.; Li, D.; Zhukova, L.; Chen, M.; Hack, S.P.; et al. IMbrave151: A phase 2, randomized, double-blind, placebo-controlled study of atezolizumab with or without bevacizumab in combination with cisplatin plus gemcitabine in patients with untreated, advanced biliary tract cancer. J. Clin. Oncol. 2023, 41, 491. [Google Scholar] [CrossRef]
- El-Khoueiry, A.B.; Ren, Z.; Chon, H.J.; Park, J.O.; Kim, J.W.; Pressiani, T.; Li, D.; Zhukova, L.; Zhu, A.X.; Chen, M.; et al. Atezolizumab plus chemotherapy with or without bevacizumab in advanced biliary tract cancer: Results from a randomized proof-of-concept phase II trial (IMbrave151). J. Clin. Oncol. 2024, 42, 435. [Google Scholar] [CrossRef]
- Shi, G.-M.; Huang, X.-Y.; Wu, D.; Sun, H.; Liang, F.; Ji, Y.; Chen, Y.; Yang, G.; Lu, J.; Meng, X.; et al. Toripalimab combined with lenvatinib and GEMOX is a promising regimen as first-line treatment for advanced intrahepatic cholangiocarcinoma: A single-center, single-arm, phase 2 study. Signal Transduct. Target. Ther. 2023, 8, 106. [Google Scholar] [CrossRef]
- Tan, Y.; Trent, J.C.; Wilky, B.A.; Kerr, D.A.; Rosenberg, A.E. Current status of immunotherapy for gastrointestinal stromal tumor. Cancer Gene Ther. 2017, 24, 130–133. [Google Scholar] [CrossRef]
- Li, B.; Chen, H.; Yang, S.; Chen, F.; Xu, L.; Li, Y.; Li, M.; Zhu, C.; Shao, F.; Zhang, X.; et al. Advances in immunology and immunotherapy for mesenchymal gastrointestinal cancers. Mol. Cancer. 2023, 22, 71. [Google Scholar] [CrossRef]
- Blay, J.-Y.; Serrano, C.; Heinrich, M.C.; Zalcberg, J.; Bauer, S.; Gelderblom, H.; Schöffski, P.; Jones, R.L.; Attia, S.; D’Amato, G.; et al. Ripretinib in patients with advanced gastrointestinal stromal tumours (INVICTUS): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2020, 21, 923–934, Erratum in Lancet Oncol. 2020, 21, e341. [Google Scholar] [CrossRef] [PubMed]
- Seifert, A.M.; Zeng, S.; Zhang, J.Q.; Kim, T.S.; Cohen, N.A.; Beckman, M.J.; Medina, B.D.; Maltbaek, J.H.; Loo, J.K.; Crawley, M.H.; et al. PD-1/PD-L1 Blockade Enhances T-cell Activity and Antitumor Efficacy of Imatinib in Gastrointestinal Stromal Tumors. Clin. Cancer Res. 2017, 23, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.; Geva, R.; Gottfried, M.; et al. Efficacy of Pembrolizumab in Patients with Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef]
- Pedersen, K.S.; Foster, N.R.; Overman, M.J.; Boland, P.M.; Kim, S.S.; Arrambide, K.A.; Jaszewski, B.L.; Bekaii-Saab, T.; Graham, R.P.; Welch, J.; et al. ZEBRA: A Multicenter Phase II Study of Pembrolizumab in Patients with Advanced Small-Bowel Adenocarcinoma. Clin. Cancer Res. 2021, 27, 3641–3648. [Google Scholar] [CrossRef]
- Chae, Y.K.; Othus, M.; Patel, S.P.; Zalupski, M.; Kasi, A.; Khalil, M.; Kalyan, A.; Polite, B.; Fenton, S.; Gurung, S.; et al. Abstract 3417: A phase II basket trial of dual anti-CTLA-4 and anti-PD-1 blockade in rare tumors (DART) SWOG S1609: The small bowel tumor cohort. Cancer Res. 2020, 80, 3417. [Google Scholar] [CrossRef]
- Klein, O.; Kee, D.; Markman, B.; Michael, M.; Underhill, C.; Carlino, M.S.; Jackett, L.; Lum, C.; Scott, C.; Nagrial, A.; et al. Immunotherapy of Ipilimumab and Nivolumab in Patients with Advanced Neuroendocrine Tumors: A Subgroup Analysis of the CA209-538 Clinical Trial for Rare Cancers. Clin. Cancer Res. 2020, 26, 4454–4459. [Google Scholar] [CrossRef]
- Patel, S.P.; Mayerson, E.; Chae, Y.K.; Strosberg, J.; Wang, J.; Konda, B.; Hayward, J.; McLeod, C.M.; Chen, H.X.; Sharon, E.; et al. A phase II basket trial of Dual Anti-CTLA-4 and Anti-PD-1 Blockade in Rare Tumors (DART) SWOG S1609: High-grade neuroendocrine neoplasm cohort. Cancer 2021, 127, 3194–3201. [Google Scholar] [CrossRef]
- Al-Toubah, T.; Halfdanarson, T.; Gile, J.; Morse, B.; Sommerer, K.; Strosberg, J. Efficacy of ipilimumab and nivolumab in patients with high-grade neuroendocrine neoplasms. ESMO Open. 2022, 7, 100364. [Google Scholar] [CrossRef]
- Weber, M.M.M.; Apostolidis, L.; Krug, S.; Rinke, A.; Gruen, B.; Michl, P.; Gress, T.M.; Wagner, D.; Roth, W.; Mettler, E.; et al. 1185MO Activity and safety of avelumab alone or in combination with cabozantinib in patients with advanced high grade neuroendocrine neoplasias (NEN G3) progressing after chemotherapy. The phase II, open-label, multicenter AVENEC and CABOAVENEC trials. Ann. Oncol. 2023, 34, S702. [Google Scholar] [CrossRef]
- Fottner, C.; Apostolidis, L.; Krug, S.; Rinke, A.; Grün, B.; Michl, P.; Gress, T.M.; Wagner, D.; Roth, W.; Mettler, E.; et al. Activity and safety of avelumab in high-grade neuroendocrine tumors and poorly differentiated neuroendocrine carcinomas progressive after chemotherapy (AveNEC trial). Clin. Cancer Res. 2025, 31, 860–867. [Google Scholar] [CrossRef]
- Morris, V.K.; Salem, M.E.; Nimeiri, H.; Iqbal, S.; Singh, P.; Ciombor, K.; Polite, B.; Deming, D.; Chan, E.; Wade, J.L.; et al. Nivolumab for previously treated unresectable metastatic anal cancer (NCI9673): A multicentre, single-arm, phase 2 study. Lancet Oncol. 2017, 18, 446–453. [Google Scholar] [CrossRef]
- Lum, C.; Prenen, H.; Body, A.; Lam, M.; Segelov, E. A 2020 update of anal cancer: The increasing problem in women and expanding treatment landscape. Expert. Rev. Gastroenterol. Hepatol. 2020, 14, 665–680. [Google Scholar] [CrossRef]
- Ott, P.A.; Piha-Paul, S.A.; Munster, P.; Pishvaian, M.J.; van Brummelen, E.M.J.; Cohen, R.B.; Gomez-Roca, C.; Ejadi, S.; Stein, M.; Chan, E.; et al. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with recurrent carcinoma of the anal canal. Ann. Oncol. 2017, 28, 1036–1041. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Azad, N.; Chen, Y.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Garrido-Laguna, I.; et al. Anal Carcinoma, Version 2.2023, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2023, 21, 653–677. [Google Scholar] [CrossRef] [PubMed]
- Jayakrishnan, T.; Kamath, S.; Laderian, B.; Krishnamurthi, S. Recent Clinical Advances in Rare Gastrointestinal Tumors. Adv. Oncol. 2023, 3, 179–189. [Google Scholar] [CrossRef]
- Weitz, J.; de Mendoza, T.H.; Tiriac, H.; Lee, J.; Sun, S.; Garg, B.; Patel, J.; Li, K.; Baumgartner, J.; Kelly, K.J.; et al. An Ex Vivo Organotypic Culture Platform for Functional Interrogation of Human Appendiceal Cancer Reveals a Prominent and Heterogenous Immunological Landscape. Clin. Cancer Res. 2022, 28, 4793–4806. [Google Scholar] [CrossRef]
- Al Attar, L.; Truong, P. The Effect of Pembrolizumab in Absence of Programmed Death 1 Receptor. Cureus 2018, 10, e2896. [Google Scholar] [CrossRef]
- Forsythe, S.D.; Erali, R.A.; Sasikumar, S.; Laney, P.; Shelkey, E.; D’Agostino, R., Jr.; Miller, L.D.; Shen, P.; Levine, E.A.; Soker, S.; et al. Organoid Platform in Preclinical Investigation of Personalized Immunotherapy Efficacy in Appendiceal Cancer: Feasibility Study. Clin. Cancer Res. 2021, 27, 5141–5150. [Google Scholar] [CrossRef]
- Nivolumab and Ipilimumab in Mucinous Colorectal and Appendiceal Tumors. Available online: https://clinicaltrials.gov/study/NCT03693846 (accessed on 24 February 2024).
- Hornstein, N.J.; Zeineddine, M.A.; Gunes, B.B.; Pellatt, A.J.; Knafl, M.; Zhu, H.; Willett, A.F.; Yousef, A.; Liu, S.; Sun, R.; et al. Efficacy and Safety of Atezolizumab and Bevacizumab in Appendiceal Adenocarcinoma. Cancer Res. Commun. 2024, 4, 1363–1368. [Google Scholar] [CrossRef] [PubMed]
- Daiko, H.; Marafioti, T.; Fujiwara, T.; Shirakawa, Y.; Nakatsura, T.; Kato, K.; Puccio, I.; Hikichi, T.; Yoshimura, S.; Nakagawa, T.; et al. Exploratory open-label clinical study to determine the S-588410 cancer peptide vaccine-induced tumor-infiltrating lymphocytes and changes in the tumor microenvironment in esophageal cancer patients. Cancer Immunol. Immunother. 2020, 69, 2247–2257. [Google Scholar] [CrossRef] [PubMed]
- Makino, T.; Miyata, H.; Yasuda, T.; Kitagawa, Y.; Muro, K.; Park, J.; Hikichi, T.; Hasegawa, T.; Igarashi, K.; Iguchi, M.; et al. A phase 3, randomized, double-blind, multicenter, placebo-controlled study of S-588410, a five-peptide cancer vaccine as an adjuvant therapy after curative resection in patients with esophageal squamous cell carcinoma. Esophagus 2024, 21, 447–455. [Google Scholar] [CrossRef]
- Wang, C.; Pu, J.; Yu, H.; Liu, Y.; Yan, H.; He, Z.; Feng, X. A Dendritic Cell Vaccine Combined with Radiotherapy Activates the Specific Immune Response in Patients with Esophageal Cancer. J. Immunother. 2017, 40, 71–76. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Okada, K.; Omori, T.; Sugimura, K.; Miyata, H.; Ohue, M.; Kobayashi, S.; Takahashi, H.; Nakano, H.; Mochizuki, C.; et al. Multiple therapeutic peptide vaccines for patients with advanced gastric cancer. Int. J. Oncol. 2017, 50, 1655–1662. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Sugimura, K.; Miyata, H.; Omori, T.; Nakano, H.; Mochizuki, C.; Shimizu, K.; Saito, H.; Ashida, K.; Honjyo, S.; et al. A Pilot Study of Post-Operative Adjuvant Vaccine for Advanced Gastric Cancer. Yonago Acta Med. 2017, 60, 101–105. [Google Scholar] [CrossRef]
- Sundar, R.; Rha, S.Y.; Yamaue, H.; Katsuda, M.; Kono, K.; Kim, H.S.; Kim, C.; Mimura, K.; Kua, L.; Yong, W.P. A phase I/Ib study of OTSGC-A24 combined peptide vaccine in advanced gastric cancer. BMC Cancer 2018, 18, 332. [Google Scholar] [CrossRef]
- de Jesus, V.H.F.; Felismino, T.C.; de Barros e Silva, M.J.; de Souza e Silva, V.; Riechelmann, R.P. Current approaches to immunotherapy in noncolorectal gastrointestinal malignancies. Clinics 2018, 73, e510s. [Google Scholar] [CrossRef]
- Middleton, G.; Silcocks, P.; Cox, T.; Valle, J.; Wadsley, J.; Propper, D.; Coxon, F.; Ross, P.; Madhusudan, S.; Roques, T.; et al. Gemcitabine and capecitabine with or without telomerase peptide vaccine GV1001 in patients with locally advanced or metastatic pancreatic cancer (TeloVac): An open-label, randomised, phase 3 trial. Lancet Oncol. 2014, 15, 829–840. [Google Scholar] [CrossRef]
- Jo, J.H.; Kim, Y.-T.; Choi, H.S.; Kim, H.G.; Lee, H.S.; Choi, Y.W.; Kim, D.U.; Lee, K.H.; Kim, E.J.; Han, J.; et al. Efficacy of GV1001 with gemcitabine/capecitabine in previously untreated patients with advanced pancreatic ductal adenocarcinoma having high serum eotaxin levels (KG4/2015): An open-label, randomised, Phase 3 trial. Br. J. Cancer. 2024, 130, 43–52, Erratum in Br. J. Cancer 2024, 130, 163. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Wang-Gillam, A.; Picozzi, V.; Greten, T.F.; Crocenzi, T.; Springett, G.; Morse, M.; Zeh, H.; Cohen, D.; Fine, R.L.; et al. Safety and Survival with GVAX Pancreas Prime and Listeria Monocytogenes Expressing Mesothelin (CRS-207) Boost Vaccines for Metastatic Pancreatic Cancer. J. Clin. Oncol. 2015, 33, 1325–1333. [Google Scholar] [CrossRef]
- Lopez, J.; Powles, T.; Braiteh, F.; Siu, L.L.; LoRusso, P.; Friedman, C.F.; Balmanoukian, A.S.; Gordon, M.; Yachnin, J.; Rottey, S.; et al. Autogene cevumeran with or without atezolizumab in advanced solid tumors: A phase 1 trial. Nat. Med. 2025, 31, 152–164. [Google Scholar] [CrossRef]
- Rojas, L.A.; Sethna, Z.; Soares, K.C.; Olcese, C.; Pang, N.; Patterson, E.; Lihm, J.; Ceglia, N.; Guasp, P.; Chu, A.; et al. Personalized RNA neoantigen vaccines stimulate T cells in pancreatic cancer. Nature 2023, 618, 144–150. [Google Scholar] [CrossRef]
- Sethna, Z.; Guasp, P.; Reiche, C.; Milighetti, M.; Ceglia, N.; Patterson, E.; Lihm, J.; Payne, G.; Lyudovyk, O.; Rojas, L.A.; et al. RNA neoantigen vaccines prime long-lived CD8+ T cells in pancreatic cancer. Nature 2025, 639, 1042–1051. [Google Scholar] [CrossRef]
- Stallard, J. In Early-Phase Pancreatic Cancer Clinical Trial, Investigational mRNA Vaccine Induces Sustained Immune Activity in Small Patient Group. 2025. Available online: https://www.mskcc.org/news/can-mrna-vaccines-fight-pancreatic-cancer-msk-clinical-researchers-are-trying-find-out (accessed on 21 July 2025).
- Jia, W.; Zhang, T.; Huang, H.; Feng, H.; Wang, S.; Guo, Z.; Luo, Z.; Ji, X.; Cheng, X.; Zhao, R. Colorectal cancer vaccines: The current scenario and future prospects. Front Immunol. 2022, 13, 942235. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.; Castañón, E.; Perez-Gracia, J.L.; Rodriguez, I.; Viudez, A.; Alfaro, C.; Oñate, C.; Perez, G.; Rotellar, F.; Inogés, S.; et al. A randomized phase II clinical trial of dendritic cell vaccination following complete resection of colon cancer liver metastasis. J. Immunother. Cancer 2018, 6, 96. [Google Scholar] [CrossRef] [PubMed]
- Tada, F.; Abe, M.; Hirooka, M.; Ikeda, Y.; Hiasa, Y.; Lee, Y.; Jung, N.; Lee, W.; Lee, H.; Bae, Y.; et al. Phase I/II study of immunotherapy using tumor antigen-pulsed dendritic cells in patients with hepatocellular carcinoma. Int. J. Oncol. 2012, 41, 1601–1609. [Google Scholar] [CrossRef] [PubMed]
- Tojjari, A.; Saeed, A.; Singh, M.; Cavalcante, L.; Sahin, I.H.; Saeed, A. A Comprehensive Review on Cancer Vaccines and Vaccine Strategies in Hepatocellular Carcinoma. Vaccines 2023, 11, 1357. [Google Scholar] [CrossRef]
- Andersson, M.; Jalnefjord, O.; Montelius, M.; Rizell, M.; Sternby Eilard, M.; Ljungberg, M. Evaluation of response in patients with hepatocellular carcinoma treated with intratumoral dendritic cell vaccination using intravoxel incoherent motion (IVIM) MRI and histogram analysis. Acta Radiol. 2023, 64, 32–41. [Google Scholar] [CrossRef]
- Rizell, M.; Sternby Eilard, M.; Andersson, M.; Andersson, B.; Karlsson-Parra, A.; Suenaert, P. Phase 1 Trial with the Cell-Based Immune Primer Ilixadencel, Alone, and Combined with Sorafenib, in Advanced Hepatocellular Carcinoma. Front. Oncol. 2019, 9, 19. [Google Scholar] [CrossRef]
- Kaida, M.; Morita-Hoshi, Y.; Soeda, A.; Wakeda, T.; Yamaki, Y.; Kojima, Y.; Ueno, H.; Kondo, S.; Morizane, C.; Ikeda, M.; et al. Phase 1 Trial of Wilms Tumor 1 (WT1) Peptide Vaccine and Gemcitabine Combination Therapy in Patients with Advanced Pancreatic or Biliary Tract Cancer. J. Immunother. 2011, 34, 92–99. [Google Scholar] [CrossRef]
- Kalyan, A.; Khosla, H.; Kim, R.D. Immunotherapy in Biliary Tract Cancers: Where Are We? Curr. Oncol. Rep. 2022, 24, 1821–1828. [Google Scholar] [CrossRef]
- Aruga, A.; Takeshita, N.; Kotera, Y.; Okuyama, R.; Matsushita, N.; Ohta, T.; Takeda, K.; Yamamoto, M. Long-term Vaccination with Multiple Peptides Derived from Cancer-Testis Antigens Can Maintain a Specific T-cell Response and Achieve Disease Stability in Advanced Biliary Tract Cancer. Clin. Cancer Res. 2013, 19, 2224–2231. [Google Scholar] [CrossRef] [PubMed]
- Aruga, A.; Takeshita, N.; Kotera, Y.; Okuyama, R.; Matsushita, N.; Ohta, T.; Takeda, K.; Yamamoto, M. Phase I clinical trial of multiple-peptide vaccination for patients with advanced biliary tract cancer. J. Transl. Med. 2014, 12, 61. [Google Scholar] [CrossRef] [PubMed]
- Murahashi, M.; Tsuruta, T.; Yamada, K.; Hijikata, Y.; Ogata, H.; Kishimoto, J.; Yoshimura, S.; Hikichi, T.; Nakanishi, Y.; Tani, K. Clinical Trial of a Cancer Vaccine Targeting VEGF and KIF20A in Advanced Biliary Tract Cancer. Anticancer Res. 2021, 41, 1485–1496. [Google Scholar] [CrossRef]
- Shioyama, Y.; Yakeishi, Y.; Watanabe, T.; Nakanura, K.; Kunitake, N.; Kimura, M.; Sasaki, M.; Honda, H.; Terashima, H.; Masuda, K. Long-Term Control for a Retroperitoneal Metastasis of Malignant Gastrointestinal Stromal Tumor after Chemoradiotherapy and Immunotherapy. Acta Oncol. 2001, 40, 102–104. [Google Scholar] [CrossRef]
- Fröbom, R.; Berglund, E.; Berglund, D.; Nilsson, I.; Åhlén, J.; von Sivers, K.; Linder-Stragliotto, C.; Suenaert, P.; Karlsson-Parra, A.; Bränström, R. Phase I trial evaluating safety and efficacy of intratumorally administered inflammatory allogeneic dendritic cells (ilixadencel) in advanced gastrointestinal stromal tumors. Cancer Immunol. Immunother. 2020, 69, 2393–2401. [Google Scholar] [CrossRef]
- Karlsson-Parra, A.; Fröbom, R.; Berglund, E.; Nilsson, I.; Linder-Stragliotto, C.; Suenaert, P.; Bränström, R. Phase I trial evaluating safety and efficacy of intratumorally administered allogeneic monocyte-derived cells (ilixadencel) in advanced gastrointestinal stromal tumors. J. Clin. Oncol. 2020, 38, 15. [Google Scholar] [CrossRef]
- Conlon, K.C.; Miljkovic, M.D.; Waldmann, T.A. Cytokines in the Treatment of Cancer. J. Interferon Cytokine Res. 2019, 39, 6–21. [Google Scholar] [CrossRef]
- Partyka, S.; Dumas, P.; Ajani, J. Combination chemotherapy with granulocyte-macrophage-colony stimulating factor in patients with locoregional and metastatic gastric adenocarcinoma. Cancer 1999, 85, 2336–2339. [Google Scholar] [CrossRef]
- Badamgarav, E.; Lyman, G.H.; Pinto, L.; Bernal, M.; Dubois, R.W. Efficacy of GM-CSF vs G-CSF in reducing chemotherapy-induced complications (CIC): A systematic review of literature. J. Clin. Oncol. 2004, 22, 6140. [Google Scholar] [CrossRef]
- Mavroudis, D.; Kourousis, C.; Androulakis, N.; Kalbakis, K.; Agelaki, S.; Kakolyris, S.; Souglakos, J.; Sarra, E.; Vardakis, N.; Hatzidaki, D.; et al. Frontline treatment of advanced gastric cancer with docetaxel and granulocyte colony-stimulating factor (G-CSF): A phase II trial. Am. J. Clin. Oncol. 2000, 23, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Kornek, G.V.; Raderer, M.; Schüll, B.; Fiebiger, W.; Gedlicka, C.; Lanauer, A.; Depisch, D.; Schneeweiss, B.; Lang, F.; Scheithauer, W. Effective combination chemotherapy with paclitaxel and cisplatin with or without human granulocyte colony-stimulating factor and/or erythropoietin in patients with advanced gastric cancer. Br. J. Cancer 2002, 86, 1858–1863. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dadgar, N.; Sherry, C.; Zimmerman, J.; Park, H.; Lewis, C.; Donnenberg, A.; Zaidi, A.H.; Fan, Y.; Xiao, K.; Bartlett, D.; et al. Targeting interleukin-6 as a treatment approach for peritoneal carcinomatosis. J. Transl. Med. 2024, 22, 402. [Google Scholar] [CrossRef] [PubMed]
- Ham, I.-H.; Oh, H.J.; Jin, H.; Bae, C.A.; Jeon, S.; Choi, K.S.; Son, S.; Han, S.; Brekken, R.A.; Lee, D.; et al. Targeting interleukin-6 as a strategy to overcome stroma-induced resistance to chemotherapy in gastric cancer. Mol. Cancer 2019, 18, 68. [Google Scholar] [CrossRef]
- Ruzzo, A.; Catalano, V.; Canestrari, E.; Giacomini, E.; Santini, D.; Tonini, G.; Vincenzi, B.; Fiorentini, G.; Magnani, M.; Graziano, F. Genetic modulation of the interleukin 6 (IL-6) system in patients with advanced gastric cancer: A background for an alternative target therapy. BMC Cancer 2014, 14, 357. [Google Scholar] [CrossRef]
- Nukui, Y.; Picozzi, V.J.; Traverso, L.W. Interferon-based adjuvant chemoradiation therapy improves survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Am. J. Surg. 2000, 179, 367–371. [Google Scholar] [CrossRef]
- Schmidt, J.; Jäger, D.; Hoffmann, K.; Büchler, M.W.; Märten, A. Impact of interferon-alpha in combined chemoradioimmunotherapy for pancreatic adenocarcinoma (CapRI): First data from the immunomonitoring. J. Immunother. 2007, 30, 108–115. [Google Scholar] [CrossRef]
- Picozzi, V.J.; Abrams, R.A.; Decker, P.A.; Traverso, W.; O’Reilly, E.M.; Greeno, E.; Martin, R.C.; Wilfong, L.S.; Rothenberg, M.L.; Posner, M.C.; et al. Multicenter phase II trial of adjuvant therapy for resected pancreatic cancer using cisplatin, 5-fluorouracil, and interferon-alfa-2b–based chemoradiation: ACOSOG Trial Z05031. Ann. Oncol. 2011, 22, 348–354. [Google Scholar] [CrossRef]
- Blaauboer, A.; Van Koetsveld, P.; Mustafa, D.; Dumas, J.; Dogan, F.; Van Zwienen, S.; Van Ejijck, C.H.J.; Hofland, L.J. Immunomodulatory antitumor effect of interferon-beta combined with gemcitabine in pancreatic cancer. Int. J. Oncol. 2022, 61, 97. [Google Scholar] [CrossRef]
- Infante, J.R.; Naing, A.; Papadopoulos, K.P.; Autio, K.A.; Ott, P.A.; Wong, D.J.L.; Falchook, G.S.; Patel, M.R.; Pant, S.; Whiteside, M.; et al. A first-in-human dose escalation study of PEGylated recombinant human IL-10 (AM0010) in advanced solid tumors. J. Clin. Oncol. 2015, 33, 3017. [Google Scholar] [CrossRef]
- Chen, L.L.; Chen, X.; Choi, H.; Sang, H.; Chen, L.C.; Zhang, H.; Gouw, L.; Andtbacka, R.H.; Chan, B.K.; Rodesch, C.K.; et al. Exploiting antitumor immunity to overcome relapse and improve remission duration. Cancer Immunol. Immunother. 2012, 61, 1113–1124. [Google Scholar] [CrossRef]
- Tavartkiladze, A.A.; Khutsishvili, R.; Revazishvili, P.; Maisuradze, M.; Tavartkiladze, L.; Tavartkiladze, G. Treatment of refractory recurrent gastrointestinal stromal tumors with adoptive cellular immunotherapy (TILs) and personalized vaccine. Ann. Oncol. 2019, 30, xi13. [Google Scholar] [CrossRef]
- Green, B.L.; Davis, J.L. Gastric adenocarcinoma peritoneal carcinomatosis: A narrative review. Dig Med. Res. 2022, 5, 37. [Google Scholar] [CrossRef] [PubMed]
- Wagner, P.L.; Knotts, C.M.; Donnenberg, V.S.; Dadgar, N.; Pico, C.X.C.; Xiao, K.; Zaidi, A.; Schiffman, S.C.; Allen, C.J.; Donnenberg, A.D.; et al. Characterizing the Immune Environment in Peritoneal Carcinomatosis: Insights for Novel Immunotherapy Strategies. Ann. Surg. Oncol. 2024, 31, 2069–2077. [Google Scholar] [CrossRef] [PubMed]
- Riihimäki, M.; Hemminki, A.; Sundquist, K.; Sundquist, J.; Hemminki, K. Metastatic spread in patients with gastric cancer. Oncotarget. 2016, 7, 52307–52316. [Google Scholar] [CrossRef] [PubMed]
- Thadi, A.; Khalili, M.; Morano, W.; Richard, S.; Katz, S.; Bowne, W. Early Investigations and Recent Advances in Intraperitoneal Immunotherapy for Peritoneal Metastasis. Vaccines 2018, 6, 54. [Google Scholar] [CrossRef]
- Chelius, D.; Ruf, P.; Gruber, P.; Plöscher, M.; Liedtke, R.; Gansberger, E.; Hess, J.; Wasiliu, M.; Lindhofer, H. Structural and functional characterization of the trifunctional antibody catumaxomab. mAbs 2010, 2, 309–319. [Google Scholar] [CrossRef]
- Tocilizumab Delivered Via Pleural and Peritoneal Catheters in Patients with Advanced Metastatic Cancer. Available online: https://clinicaltrials.gov/study/NCT06016179 (accessed on 24 February 2024).
- Li, Y.; Duan, H.; Yang, K.; Ye, J. Advancements and challenges in oncolytic virus therapy for gastrointestinal tumors. Biomed. Pharmacother. 2023, 168, 115627. [Google Scholar] [CrossRef]
- Zhang, B.; Huang, J.; Tang, J.; Hu, S.; Luo, S.; Luo, Z.; Zhou, F.; Tan, S.; Ying, J.; Chang, Q.; et al. Intratumoral OH2, an oncolytic herpes simplex virus 2, in patients with advanced solid tumors: A multicenter, phase I/II clinical trial. J Immunother. Cancer 2021, 9, e002224. [Google Scholar] [CrossRef]
- Shirakawa, Y.; Tazawa, H.; Tanabe, S.; Kanaya, N.; Noma, K.; Koujima, T.; Kashima, H.; Kata, T.; Kuroda, S.; Kikuchi, S.; et al. Phase I dose-escalation study of endoscopic intratumoral injection of OBP-301 (Telomelysin) with radiotherapy in oesophageal cancer patients unfit for standard treatments. Eur. J. Cancer 2021, 153, 98–108. [Google Scholar] [CrossRef]
- Noonan, A.M.; Farren, M.R.; Geyer, S.M.; Huang, Y.; Tahiri, S.; Ahn, D.; Mikhail, S.; Ciombor, K.K.; Pant, S.; Apara, S.; et al. Randomized Phase 2 Trial of the Oncolytic Virus Pelareorep (Reolysin) in Upfront Treatment of Metastatic Pancreatic Adenocarcinoma. Mol. Ther. 2016, 24, 1150–1158. [Google Scholar] [CrossRef]
- Mahalingam, D.; Goel, S.; Aparo, S.; Arora, S.P.; Noronha, N.; Tran, H.; Chakrabarty, R.; Selvaggi, G.; Gutierrez, A.; Coffey, M.; et al. A Phase II Study of Pelareorep (REOLYSIN®) in Combination with Gemcitabine for Patients with Advanced Pancreatic Adenocarcinoma. Cancers 2018, 10, 160. [Google Scholar] [CrossRef]
- Mahalingam, D.; Wilkinson, G.A.; Eng, K.H.; Fields, P.; Raber, P.; Moseley, J.L.; Cheetham, K.; Coffey, M.; Nuovo, G.; Kalinski, P.; et al. Pembrolizumab in Combination with the Oncolytic Virus Pelareorep and Chemotherapy in Patients with Advanced Pancreatic Adenocarcinoma: A Phase Ib Study. Clin. Cancer Res. 2020, 26, 71–81. [Google Scholar] [CrossRef]
- Yoon, S.S.; Carroll, N.M.; Chiocca, E.A.; Tanabe, K.K. Cancer gene therapy using a replication-competent herpes simplex virus type 1 vector. Ann. Surg. 1998, 228, 366–374. [Google Scholar] [CrossRef]
- Park, S.H.; Breitbach, C.J.; Lee, J.; Park, J.O.; Lim, H.Y.; Kang, W.K.; Moon, A.; Mun, J.; Sommermann, E.M.; Avidal, L.M.; et al. Phase 1b Trial of Biweekly Intravenous Pexa-Vec (JX-594), an Oncolytic and Immunotherapeutic Vaccinia Virus in Colorectal Cancer. Mol. Ther. 2015, 23, 1532–1540. [Google Scholar] [CrossRef]
- Jonker, D.J.; Tang, P.A.; Kennecke, H.; Welch, S.A.; Cripps, M.C.; Asmis, T.; Chalchal, H.; Tomiak, A.; Lim, H.; Ko, Y.; et al. A Randomized Phase II Study of FOLFOX6/Bevacizumab with or without Pelareorep in Patients with Metastatic Colorectal Cancer: IND.210, a Canadian Cancer Trials Group Trial. Clin. Color. Cancer 2018, 17, 231–239.e7. [Google Scholar] [CrossRef] [PubMed]
- Moehler, M.; Heo, J.; Lee, H.C.; Tak, W.Y.; Chao, Y.; Paik, S.W.; Yim, H.J.; Byun, K.S.; Baron, A.; Ungerechts, G.; et al. Vaccinia-based oncolytic immunotherapy Pexastimogene Devacirepvec in patients with advanced hepatocellular carcinoma after sorafenib failure: A randomized multicenter Phase IIb trial (TRAVERSE). Oncoimmunology 2019, 8, 1615817. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.; Chao, Y.; Jonker, D.J.; Baron, A.D.; Habersetzer, F.; Burke, J.; Breitbach, C.; Patt, R.H.; Lencioni, R.; Homerin, M.; et al. Phase IIb randomized trial of Pexa-Vec (pexastimogene devacirepvec; JX-594), a targeted oncolytic vaccinia virus, plus best supportive care (BSC) versus BSC alone in patients with advanced hepatocellular carcinoma who have failed sorafenib treatment (TRAVERSE). J. Clin. Oncol. 2013, 31, TPS4161. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Galle, P.R.; Chao, Y.; Erinjeri, J.; Heo, J.; Borad, M.J.; Luca, A.; Burke, J.; Pelusio, A.; Agathon, D.; et al. PHOCUS: A Phase 3, Randomized, Open-Label Study of Sequential Treatment with Pexa-Vec (JX-594) and Sorafenib in Patients with Advanced Hepatocellular Carcinoma. Liver Cancer 2024, 13, 248–264. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Galle, P.R.; Chao, Y.; Brown, K.T.; Heo, J.; Borad, M.J.; Luca, A.; Pelusio, A.; Agathon, D.; Lusky, M.; et al. PHOCUS: A phase 3 randomized, open-label study comparing the oncolytic immunotherapy Pexa-Vec followed by sorafenib (SOR) vs SOR in patients with advanced hepatocellular carcinoma (HCC) without prior systemic therapy. J. Clin. Oncol. 2016, 34, TPS4146. [Google Scholar] [CrossRef]
- Miller, A.M.; Lemke-Miltner, C.D.; Blackwell, S.; Tomanek-Chalkley, A.; Gibson-Corely, K.N.; Coleman, K.L.; Weiner, G.J.; Chan, C.H.F. Intraperitoneal CMP-001: A Novel Immunotherapy for Treating Peritoneal Carcinomatosis of Gastrointestinal and Pancreaticobiliary Cancer. Ann. Surg. Oncol. 2021, 28, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Hu, W. Advances in adoptive cellular therapy for colorectal cancer: A narrative review. Ann. Transl. Med. 2022, 10, 1404. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, S.; Ikeda, H.; Miyahara, Y.; Imai, N.; Ishihara, M.; Saito, K.; Sugino, S.; Ueda, S.; Ishikawa, T.; Kokura, S.; et al. Adoptive Transfer of MAGE-A4 T-cell Receptor Gene-Transduced Lymphocytes in Patients with Recurrent Esophageal Cancer. Clin. Cancer Res. 2015, 21, 2268–2277. [Google Scholar] [CrossRef]
- Gu, Y.-M.; Zhuo, Y.; Chen, L.-Q.; Yuan, Y. The Clinical Application of Neoantigens in Esophageal Cancer. Front. Oncol. 2021, 11, 703517. [Google Scholar] [CrossRef]
- Shi, L.; Zhou, Q.; Wu, J.; Ji, M.; Li, G.; Jiang, J.; Wu, C. Efficacy of adjuvant immunotherapy with cytokine-induced killer cells in patients with locally advanced gastric cancer. Cancer Immunol. Immunother. 2012, 61, 2251–2259. [Google Scholar] [CrossRef]
- Kono, K.; Takahashi, A.; Ichihara, F.; Amemiya, H.; Iizuka, H.; Fujii, H.; Sekikawa, T.; Matsumoto, Y. Prognostic Significance of Adoptive Immunotherapy with Tumor-associated Lymphocytes in Patients with Advanced Gastric Cancer: A Randomized Trial. Clin. Cancer Res. 2002, 8, 1767–1771. [Google Scholar]
- Hadfield, M.J.; Safran, H.; Purbhoo, M.A.; Grossman, J.E.; Buell, J.S.; Carneiro, B.A. Overcoming resistance to programmed cell death protein 1 (PD-1) blockade with allogeneic invariant natural killer T-cells (iNKT). Oncogene 2024, 43, 758–762. [Google Scholar] [CrossRef]
- Carneiro, B.; Garmezy, B.; Hamm, J.T.; Sanborn, R.E.; Wise-Draper, T.; El-Khoueiry, A.; Wilky, B.; Hoon, D.S.B.; Buffa, A.; Michelet, X.; et al. Abstract CT275: Phase 1 clinical update of allogeneic invariant natural killer T cells (iNKTs), agenT-797, alone or in combination with pembrolizumab or nivolumab in patients with advanced solid tumors. Cancer Res. 2023, 83, CT275. [Google Scholar] [CrossRef]
- Leidner, R.; Sanjuan Silva, N.; Huang, H.; Sprott, D.; Zheng, C.; Shih, Y.; Leung, A.; Payne, R.; Sutcliffe, K.; Cramer, J.; et al. Neoantigen T-Cell Receptor Gene Therapy in Pancreatic Cancer. N. Engl. J. Med. 2022, 386, 2112–2119. [Google Scholar] [CrossRef]
- Reusch, U.; Sundaram, M.; Davol, P.A.; Olson, S.D.; Davis, J.B.; Demel, K.; Nissim, J.; Rathore, R.; Liu, P.Y.; Lum, L.G. Anti-CD3 x anti-epidermal growth factor receptor (EGFR) bispecific antibody redirects T-cell cytolytic activity to EGFR-positive cancers in vitro and in an animal model. Clin. Cancer Res. 2006, 12, 183–190. [Google Scholar] [CrossRef]
- Lum, L.G.; Thakur, A.; Choi, M.; Deol, A.; Kondadasula, V.; Schalk, D.; Fields, K.; Dufrense, M.; Philip, P.; Dyson, G.; et al. Clinical and immune responses to anti-CD3 x anti-EGFR bispecific antibody armed activated T cells (EGFR BATs) in pancreatic cancer patients. Oncoimmunology 2020, 9, 1773201. [Google Scholar] [CrossRef]
- Li, C.; Yang, N.; Li, H.; Wang, Z. Robo1-specific chimeric antigen receptor natural killer cell therapy for pancreatic ductal adenocarcinoma with liver metastasis. J. Cancer Res. Ther. 2020, 16, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, Z.; Yang, Z.; Wang, W.; Li, S.; Li, Y.; Zhang, R.; Xiong, Z.; Wei, Z.; Shen, J.; et al. Phase I Escalating-Dose Trial of CAR-T Therapy Targeting CEA+ Metastatic Colorectal Cancers. Mol. Ther. 2017, 25, 1248–1258. [Google Scholar] [CrossRef] [PubMed]
- Shaza, L.; Henlisz, A.; Awada, A.; Canon, J.; Carrasco, J.; Van Cutsem, E.; Dekervel, J.; Alcantar-Orozco, E.; Renard, F.; Cerf, E.; et al. Results from the completed dose-escalation phase I SHRINK study evaluating the autologous NKG2D-based CAR T-cell therapy CYAD-01 in metastatic colorectal cancer patients. Lung 2019, 3, 33. [Google Scholar]
- Prenen, H.; Dekervel, J.; Hendlisz, A.; Anguille, S.; Awada, A.; Cerf, E.; Lonez, C.; Breman, E.; Dheur, M.; Alcantar-Orozco, E.; et al. Updated data from alloSHRINK phase I first-in-human study evaluating CYAD-101, an innovative non-gene edited allogeneic CAR-T in mCRC. J. Clin. Oncol. 2021, 39, 74. [Google Scholar] [CrossRef]
- Tran, E.; Robbins, P.F.; Lu, Y.-C.; Prickett, T.D.; Gartner, J.J.; Jia, L.; Pasetto, A.; Zheng, Z.; Ray, S.; Groh, E.M.; et al. T-Cell Transfer Therapy Targeting Mutant KRAS in Cancer. N. Engl. J. Med. 2016, 375, 2255–2262. [Google Scholar] [CrossRef]
- National Cancer Institute: Immunotherapy Using Tumor Infiltrating Lymphocytes for Patients with Metastatic Cancer. Available online: https://clinicaltrials.gov/study/NCT01174121?cond=nci%2001174121&term=NCT01174121&rank=1#publications (accessed on 7 February 2025).
- A Study of TBio-4101 (TIL) and Pembrolizumab in Patients with Advanced Solid Tumors (STARLING). Available online: https://clinicaltrials.gov/study/NCT05576077?cond=nci%2001174121&term=NCT05576077&rank=1 (accessed on 12 September 2024).
- Zhu, Y.; Li, X.; Chen, T.; Wang, J.; Zhou, Y.; Mu, X.; Du, Y.; Wang, J.; Tang, J.; Liu, J. Personalised neoantigen-based therapy in colorectal cancer. Clin. Transl. Med. 2023, 13, e1461. [Google Scholar] [CrossRef]
- Qin, W.; Cao, Z.-Y.; Liu, S.-Y.; Xu, X.-D. Recent advances regarding tumor microenvironment and immunotherapy in hepatocellular carcinoma. Hepatoma Res. 2020, 6, 24. [Google Scholar] [CrossRef]
- Huang, Z.-M.; Li, W.; Li, S.; Gao, F.; Zhou, Q.; Wu, F.; He, N.; Pan, C.; Xia, J.; Wu, P.; et al. Cytokine-induced Killer Cells in Combination with Transcatheter Arterial Chemoembolization and Radiofrequency Ablation for Hepatocellular Carcinoma Patients. J. Immunother. 2013, 36, 287–293. [Google Scholar] [CrossRef]
- Yu, X.; Zhao, H.; Liu, L.; Cao, S.; Ren, B.; Zhang, N.; An, X.; Yu, J.; Li, H.; Ren, X. A randomized phase II study of autologous cytokine-induced killer cells in treatment of hepatocellular carcinoma. J. Clin. Immunol. 2014, 34, 194–203. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, J.-H.; Lim, Y.-S.; Yeon, J.E.; Song, T.; Yu, S.J.; Gwak, G.; Kim, K.M.; Kim, Y.J.; Lee, J.W.; et al. Adjuvant Immunotherapy with Autologous Cytokine-Induced Killer Cells for Hepatocellular Carcinoma. Gastroenterology 2015, 148, 1383–1391.e6. [Google Scholar] [CrossRef] [PubMed]
- Carisma Therapeutics: Carisma Therapeutics Announces Nomination of First In Vivo CAR-M Development Candidate for Hepatocellular Carcinoma Under Collaboration with Moderna. 2024. Available online: https://www.prnewswire.com/news-releases/carisma-therapeutics-announces-nomination-of-first-in-vivo-car-m-development-candidate-for-hepatocellular-carcinoma-under-collaboration-with-moderna-302185002.html (accessed on 12 September 2024).
- Liu, D.; Heij, L.R.; Czigany, Z.; Dahl, E.; Lang, S.A.; Ulmer, T.F.; Luedde, T.; Neumann, U.P.; Bednarsch, J. The role of tumor-infiltrating lymphocytes in cholangiocarcinoma. J. Exp. Clin. Cancer Res. 2022, 41, 127. [Google Scholar] [CrossRef] [PubMed]
- Sawasdee, N.; Thepmalee, C.; Sujjitjoon, J.; Yongpitakwattana, P.; Junking, M.; Poungvarin, N.; Yenchitsomanus, P.; Panya, A. Gemcitabine enhances cytotoxic activity of effector T-lymphocytes against chemo-resistant cholangiocarcinoma cells. Int. Immunopharmacol. 2020, 78, 106006. [Google Scholar] [CrossRef]
- Rogers, J.E.; Leung, M.; Johnson, B. Metastatic or Locally Recurrent Anal Squamous Cell Carcinoma (SCAC): Current Clinical Trial Landscape and Novel Approaches. Cancer Manag. Res. 2022, 14, 2065–2077. [Google Scholar] [CrossRef]
- Stevanović, S.; Helman, S.R.; Wunderlich, J.R.; Langhan, M.M.; Doran, S.L.; Kwong, M.L.M.; Somerville, R.P.T.; Klebanoff, C.A.; Kammula, U.S.; Sherry, R.M.; et al. A Phase II Study of Tumor-infiltrating Lymphocyte Therapy for Human Papillomavirus-associated Epithelial Cancers. Clin. Cancer Res. 2019, 25, 1486–1493. [Google Scholar] [CrossRef]
- Bustamante, L.; Frakes, J.; Hoffe, S.; Kim, R. Investigational drugs for treating anal cancer and future perspectives. Expert. Opin. Investig. Drugs. 2016, 25, 51–62. [Google Scholar] [CrossRef]
- Safran, H.; Leonard, K.L.; DiPetrillo, T.A.; Klipfel, A.; Schechter, S.; Oldenburg, N.; Vrees, M.; Roth, L.; Shah, N.; Mantripragada, K.C.; et al. ADXS11-001 Lm-LLO Immunotherapy, Mitomycin, 5-fluorouracil (5-FU) and Intensity-modulated radiation therapy (IMRT) for Anal Cancer. J. Clin. Oncol. 2017, 35, e15072. [Google Scholar] [CrossRef]
- Katz, S.C.; Point, G.R.; Cunetta, M.; Thorn, M.; Guha, P.; Espat, N.J.; Boutros, C.; Hanna, N.; Junghans, R.P. Regional CAR-T cell infusions for peritoneal carcinomatosis are superior to systemic delivery. Cancer Gene Ther. 2016, 23, 142–148. [Google Scholar] [CrossRef]
- Wang, D.; Lin, J.; Yang, X.; Long, J.; Bai, Y.; Yang, X.; Mao, Y.; Sang, X.; Seery, S.; Zhao, H. Combination regimens with PD-1/PD-L1 immune checkpoint inhibitors for gastrointestinal malignancies. J. Hematol. Oncol. 2019, 12, 42. [Google Scholar] [CrossRef]
- Abdul-Latif, M.; Townsend, K.; Dearman, C.; Shiu, K.-K.; Khan, K. Immunotherapy in gastrointestinal cancer: The current scenario and future perspectives. Cancer Treat. Rev. 2020, 88, 102030. [Google Scholar] [CrossRef]
- McGrath, K.; Dotti, G. Combining Oncolytic Viruses with Chimeric Antigen Receptor T Cell Therapy. Hum. Gene Ther. 2021, 32, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Lu, S.; Shi, M.; Yang, Z.; Liu, W.; Ni, Z.; Yao, X.; Hua, Z.; Feng, R.; Zheng, Y.; et al. Sintilimab combined neoadjuvant intraperitoneal and systemic chemotherapy in gastric cancer with peritoneal metastasis. Future Oncol. 2023, 19, 2517–2523. [Google Scholar] [CrossRef] [PubMed]
- Anhui Provincial Hospital: Postoperative Radiotherapy Followed by Immunotherapy for Locally Advanced Esophageal Carcinoma. Available online: https://www.clinicaltrials.gov/study/NCT05937438 (accessed on 7 February 2025).
- Huang, X.; Tang, H.; Jiao, H.; Yin, J.; Wang, H.; Xu, W.; Yin, H.; Yang, S.; Wang, Q.; Zeng, M.; et al. Neoadjuvant Immunotherapy combined with Chemoradiotherapy VS. Neoadjuvant Chemoradiotherapy for Locally Advanced Esophageal Squamous Cell Carcinoma(cStageII-III): Study Protocol For A Multi-center Prospective Randomized Clinical Trial. Res. Sq. 2024. [Google Scholar] [CrossRef]
- Chung, V.; Guthrie, K.A.; Pishvaian, M.J.; Reiss, K.A.; Lowy, A.M.; Sohal, D.; Colby, S.; Sharon, E.; Allegra, C.J.; O’Reilly, E.M.; et al. Randomized phase II trial of olaparib + pembrolizumab vs olaparib alone as maintenance therapy in metastatic pancreatic cancer patients with germline BRCA1 or BRCA2 (gBRCA1/2+) pathogenic variants: SWOG S2001. J. Clin. Oncol. 2023, 41, TPS4198. [Google Scholar] [CrossRef]
- Chung, V.; Guthrie, K.A.; Pishvaian, M.J.; Reiss, K.A.; Lowy, A.M.; Sohal, D.; Colby, S.; Sharon, E.; Allegra, C.J.; O’Reilly, E.M.; et al. Randomized phase II trial of olaparib + pembrolizumab vs olaparib alone as maintenance therapy in patients with metastatic pancreatic cancer with germline BRCA1 or BRCA2 mutations: SWOG S2001 NCT04548752. J. Clin. Oncol. 2025, 43, TPS793. [Google Scholar] [CrossRef]
- Peking Union Medical College Hospital: Pembrolizumab with or without Lenvatinib or Chemotherapy in First-Line Treatment of Advanced Biliary Tract Cancer. Available online: https://www.clinicaltrials.gov/study/NCT06230471 (accessed on 7 February 2025).
- Eastern Hepatobiliary Surgery Hospital: Envafolimab Combined with GEMOX in First-line Treatment of Advanced GBC. Available online: https://www.clinicaltrials.gov/study/NCT06013943 (accessed on 7 February 2025).
- Sinicrope, F.A.; Ou, F.-S.; Zemla, T.; Nixon, A.B.; Mody, K.; Levasseur, A.; Dueck, A.C.; Dhanarajan, A.R.; Lieu, C.H.; Cohen, D.J.; et al. Randomized trial of standard chemotherapy alone or combined with atezolizumab as adjuvant therapy for patients with stage III colon cancer and deficient mismatch repair (ATOMIC, Alliance A021502). J. Clin. Oncol. 2019, 37, e15169. [Google Scholar] [CrossRef]
- Yamazaki, K.; Satake, H.; Takashima, A.; Mizusawa, J.; Kataoka, T.; Fukuda, H.; Ishizuka, Y.; Suwa, Y.; Numata, K.; Shibata, N.; et al. 446TiP Randomized phase III study of bi-weekly trifluridine/tipiracil (FTD/TPI) plus bevacizumab (BEV) vs. FTD/TPI for chemorefractory metastatic colorectal cancer (mCRC): ROBiTS/JCOG2014. Ann. Oncol. 2022, 33, S738. [Google Scholar] [CrossRef]
- Tongji Hospital: Adjuvant PD-1 Inhibitor for Patients with Early-stage Hepatocellular Carcinoma Following Microwave Ablation. Available online: https://www.clinicaltrials.gov/study/NCT06248554 (accessed on 7 February 2025).
- Centre Leon Berard: A Prospective, Randomized, Multicenter, Comparative Study of the Efficacy of Imatinib Resumption Combined with Atezolizumab Versus Imatinib Resumption Alone in Patients with Unresectable Advanced Gastrointestinal Stromal Tumors (GIST) After Failure of Standard Treatments (ATEZOGIST). Available online: https://clinicaltrials.gov/study/NCT05152472 (accessed on 7 February 2025).
- Sun Yat-sen University. Surufatinib and Sintilimab in Combination with Capecitabine for Metastatic Adenocarcinoma of Small Intestine or Appendix Carcinoma. Available online: https://clinicaltrials.gov/study/NCT05472948?term=NCT05472948&rank=1 (accessed on 7 February 2025).
- Overman, M.J.; Guthrie, K.A.; Salem, M.E.; Pedersen, K.S.; Kalyan, A.; Bellasea, S.; Philip, P.A. Randomized phase II selection study of ramucirumab and paclitaxel versus FOLFIRI in refractory small bowel adenocarcinoma: SWOG S1922. J. Clin. Oncol. 2022, 40, TPS643. [Google Scholar] [CrossRef]
- Overman, M.J.; Guthrie, K.A.; Salem, M.E.; Pederson, K.S.; Kalyan, A.; Colby, S.; Fakih, M.; Gholami, S.; Gold, P.J.; Chiorean, E.G.; et al. Randomized, phase II selection study of ramucirumab and paclitaxel versus FOLFIRI in refractory small bowel adenocarcinoma: SWOG S1922. J. Clin. Oncol. 2023, 41, TPS784. [Google Scholar] [CrossRef]
- National Cancer Institute: Nivolumab After Combined Modality Therapy in Treating Patients with High Risk Stage II-IIIB Anal Cancer. Available online: https://www.clinicaltrials.gov/study/NCT03233711 (accessed on 7 February 2025).
- Martin, D.; Balermpas, P.; Gollrad, J.; Weiβ, C.; Valentini, C.; Stuschke, M.; Schafer, H.; Henckenberens, C.; Debus, J.; Krug, D.; et al. RADIANCE—Radiochemotherapy with or without Durvalumab in the treatment of anal squamous cell carcinoma: A randomized multicenter phase II trial. Clin. Transl. Radiat. Oncol. 2020, 23, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Jones, M.; Bowman, J.; Tian, C.; Spano, J.-P. POD1UM-303/InterAACT 2, A phase III, global, randomized, double-blind study of retifanlimab or placebo plus carboplatin–paclitaxel in patients with locally advanced or metastatic squamous cell anal carcinoma. Front Oncol. 2022, 12, 935383. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-T.; Chiu, C.-F.; Bai, H.-J.; Bai, L.-Y. Intraperitoneal nivolumab in a patient with pancreatic cancer and refractory malignant ascites. Eur. J. Cancer 2021, 148, 48–50. [Google Scholar] [CrossRef]
- Lewis, C.R.; Dadgar, N.; Yellin, S.A.; Donnenberg, V.S.; Donnenberg, A.D.; Bartlett, D.L.; Allen, C.J.; Wagner, P.L. Regional Immunotherapy for Peritoneal Carcinomatosis in Gastroesophageal Cancer: Emerging Strategies to Re-Condition a Maladaptive Tumor Environment. Cancers 2023, 15, 5107. [Google Scholar] [CrossRef]
- QI, C.; Wu, S.; Kim, I.-H.; Cai, S.; Wang, J.; Kim, S.T.; Wang, J.; Bai, L.; Lin, C.; Liang, Z.; et al. Global multi-center phase I trial of the intraperitoneal infusion of anti-EpCAM x anti-CD3 bispecific antibody catumaxomab for advanced gastric carcinoma with peritoneal metastasis. J. Clin. Oncol. 2022, 40, e16102. [Google Scholar] [CrossRef]
- Aldesleukin with Nivolumab and Standard Chemotherapy for Treatment of Gastric Cancer with Peritoneal Metastasis. Available online: https://clinicaltrials.gov/study/NCT05802056 (accessed on 25 February 2024).
- Wagner, P.L.; Park, H.Y.; Blodgett, R.J.; Donnenberg, V.; Lewis, C.R.; Dadgar, N.; Xiao, K.; Zaidi, A.; Bartlett, D.L.; Donnenberg, A. 658 Intra-cavitary tocilizumab immunotherapy for malignant pleural effusions and ascites: In progress update from the regional immuno-oncology trial (RIOT)-2. J. Immunother. Cancer 2024, 12, A755. [Google Scholar] [CrossRef]
- Park, H.; Lewis, C.; Dadgar, N.; Sherry, C.; Evans, S.; Ziobert, S.; Omstead, A.; Zaidi, A.; Xiao, K.; Ghosh, S.; et al. Intra-pleural and intra-peritoneal tocilizumab therapy for managing malignant pleural effusions and ascites: The Regional Immuno-Oncology Trial (RIOT)−2 study protocol. Surg. Oncol. Insight. 2024, 1, 100045. [Google Scholar] [CrossRef]
- Aznar Gomez, M.A.; Good, C.R.; Barber-Rotenberg, J.S.; Agarwal, S.; Wilson, W.; Gonzales, D.; Watts, A.C.; Hwang, W.; Jadlowsky, J.K.; Yong, R.M.; et al. 237 Enhancing mesothelin-directed CAR T cell therapy for unresectable or metastatic pancreatic adenocarcinoma. J. Immunother. Cancer 2024, 12, A272. [Google Scholar] [CrossRef]
- Chudasama, R.; Phung, Q.; Hsu, A.; Almhanna, K. Vaccines in Gastrointestinal Malignancies: From Prevention to Treatment. Vaccines 2021, 9, 647. [Google Scholar] [CrossRef]
- Guo, Z.; Yuan, Y.; Chen, C.; Lin, J.; Ma, Q.; Liu, G.; Gao, Y.; Huang, Y.; Chen, L.; Chen, L.; et al. Durable complete response to neoantigen-loaded dendritic-cell vaccine following anti-PD-1 therapy in metastatic gastric cancer. NPJ Precis Oncol. 2022, 6, 34. [Google Scholar] [CrossRef]
- Flatmark, K.; Torgunrud, A.; Fleten, K.G.; Davidson, B.; Juul, H.V.; Mensali, N.; Lund-Anderson, C.; Inderberg, E.M. Peptide vaccine targeting mutated GNAS: A potential novel treatment for pseudomyxoma peritonei. J Immunother Cancer 2021, 9, e003109. [Google Scholar] [CrossRef]
- Ramanathan, R.; Choudry, H.; Jones, H.; Girgis, M.; Gooding, W.; Kalinski, P.; Bartlett, D.L. Phase II Trial of Adjuvant Dendritic Cell Vaccine in Combination with Celecoxib, Interferon-α, and Rintatolimod in Patients Undergoing Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Peritoneal Metastases. Ann. Surg. Oncol. 2021, 28, 4637–4646. [Google Scholar] [CrossRef] [PubMed]
- Al-Batran, S.-E.; Mueller, D.W.; Rafiyan, M.-R.; Kiselicki, D.; Atmaca, A.; Habibzada, T.; Mueller, C.; Brignone, C.; Triebel, F.; Loose, M.; et al. A soluble LAG-3 protein (eftilagimod alpha) and an anti-PD-L1 antibody (avelumab) tested in a phase I trial: A new combination in immuno-oncology. ESMO Open 2023, 8, 101623. [Google Scholar] [CrossRef] [PubMed]
- Mueller, D.W.; Goetze, T.O.; Eickhoff, R.; Reichart, A.; Al-Batran, S.-E. The ‘INSIGHT’ trial: An explorative, open-labeled phase I study to evaluate the feasibility and safety of intra-tumoral, intra-peritoneal, and subcutaneous injections with IMP321 (LAG-3Ig fusion protein) for advanced stage solid tumor entities. J. Clin. Oncol. 2018, 36, TPS3129. [Google Scholar] [CrossRef]
- Lewis, C.; Dadgar, N.; Najafi, M.; Park, H.; Sherry, C.; Lucas, A.; Zaidi, A.; Xiao, K.; Omstead, A.; Donnenberg, A.; et al. Intra-Tumoral Immunomodulatory Therapy for Advanced Abdominal Cancers using Lipopolysaccharide: The Regional Immuno-Oncology Trial-1 (RIOT-1) Protocol. Surg. Oncol. Insight 2024, 1, 100042. [Google Scholar] [CrossRef]
- Park, H.Y.; Lewis, C.R.; Blodgett, R.J.; Dadgar, N.; Omstead, A.; Xiao, K.; Zaidi, A.; Donnenberg, A.; Donnenberg, V.; Bartlet, D.L.; et al. 637 Regional immuno-oncology trial 1: A phase I trial of laparoscopic intra-tumoral lipopolysaccharide injection in peritoneal tumors. J. Immunother. Cancer 2024, 12, A731. [Google Scholar] [CrossRef]
- Chen, J.J.; Vincent, M.Y.; Shepard, D.; Peereboom, D.; Mahalingam, D.; Battiste, J.; Patel, M.R.; Juric, D.; Wen, P.Y.; Bullock, A.; et al. Phase 1 dose expansion and biomarker study assessing first-in-class tumor microenvironment modulator VT1021 in patients with advanced solid tumors. Commun. Med. 2024, 4, 95. [Google Scholar] [CrossRef]
- Cartwright, E.; Turkes, F.; Saffery, C.; Kalaitzaki, E.; Powell, R.; Wotherspoon, A.; De Paepe, K.; von Loga, K.; Hubank, M.; Rao, S.; et al. EMERGE: Epigenetic modulation of the immune response in gastrointestinal cancers. Ann. Oncol. 2019, 30, v251–v252. [Google Scholar] [CrossRef]
- Cartwright, E.; Slater, S.; Saffery, C.; Tran, A.; Turkes, F.; Smith, G.; Aresu, M.; Kohoutova, D.; Terlizzo, M.; Zhitkov, O.; et al. Phase II trial of domatinostat (4SC-202) in combination with avelumab in patients with previously treated advanced mismatch repair proficient oesophagogastric and colorectal adenocarcinoma: EMERGE. ESMO Open. 2024, 9, 102971. [Google Scholar] [CrossRef]
- Christenson, E.; Lim, S.J.; Wang, H.; Ferguson, A.; Parkinson, R.; Cetasaan, Y.; Rodriguez, C.; Burkhart, R.; De Jesus-Acosta, A.; He, J.; et al. Nivolumab and a CCR2/CCR5 dual antagonist (BMS-813160) with or without GVAX for locally advanced pancreatic ductal adenocarcinomas: Results of phase I study. J. Clin. Oncol. 2023, 41, 730. [Google Scholar] [CrossRef]
- Choo, J.; Rha, S.Y.; Jung, M.; Tan, H.L.; Chan, G.; Ho, J.S.; Walsh, R.J.; Chee, C.E.; Raghav, S.; Yong, W.P. 1255P da VINci: Safety and efficacy of the OTSGC-A24 vaccine and nivolumab in metastatic gastric cancer. Ann. Oncol. 2022, 33, S1121. [Google Scholar] [CrossRef]
- Istituto Scientifico Romagnolo per lo Studio e la cura dei Tumori: Vaccination with Autologous Dendritic Cells Loaded with Autologous Tumour Homogenate After Curative Resection for Stage IV Colorectal Cancer (COREVAX-1). Available online: https://clinicaltrials.gov/study/NCT02919644 (accessed on 7 February 2025).
- Kirk, A.M.; Crawford, J.C.; Kamdar, Z.; Baretti, M.; Yarchoan, M.; Thomas, P.G. 897-B A DNAJB1-PRKACA fusion peptide vaccine combined with ipilimumab and nivolumab elicits polyclonal fusion-specific T cell responses in fibrolamellar carcinoma. J. Immunother. Cancer 2023, 11, A1820. [Google Scholar] [CrossRef]
- Baretti, M.; Ladle, B.; Nauroth, J.; Wang, H.; Durham, J.; Thoburn, C.; Ferguson, A.; Thompson, A.; Brancati, M.; Griffith, P.; et al. 641 A pilot study of a DNAJB1-PRKACA fusion kinase peptide vaccine combined with nivolumab and ipilimumab for patients with fibrolamellar hepatocellular carcinoma. J. Immunother. Cancer 2023, 11, A733–A734. [Google Scholar] [CrossRef]
- Khanniche, A.; Zhang, Y.; Wu, Z.; Wang, H.; Kong Xi Gang, J.; Hu, L. 1499 TCR clonotype features correlate with responses to personalized neoantigen cancer vaccine in pancreatic ductal adenocarcinoma. J. Immunother. Cancer 2024, 12, A1728. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, Y.; Kong, X.; Wu, Z.; Wang, H. Impact of personalized peptide neoantigen vaccines on immunologic responses in patients with pancreatic cancer. J. Clin. Oncol. 2024, 42, e16351. [Google Scholar] [CrossRef]
- A Study of the Efficacy and Safety of Adjuvant Autogene Cevumeran Plus Atezolizumab and mFOLFIRINOX Versus mFOLFIRINOX Alone in Participants with Resected PDAC (IMCODE003). Available online: https://clinicaltrials.gov/study/NCT05968326 (accessed on 21 July 2025).
- Hoster, H.A.; Zanes, R.P.; von Haam, E. Studies in Hodgkin’s Syndrome: IX. The Association of “Viral” Hepatitis and Hodgkin’s Disease (A Preliminary Report). Cancer Res. 1949, 9, 473–480. [Google Scholar]
- Zolaly, M.A.; Mahallawi, W.; Khawaji, Z.Y.; Alahmadi, M.A. The Clinical Advances of Oncolytic Viruses in Cancer Immunotherapy. Cureus 2023, 15, e40742. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, B.; Tang, J.; Chang, Q.; Zhang, R.; Geng, C.; Wu, D.; Qi, L.; Gu, X.; Liu, B. Safety and tolerability of intratumorally administered OH2, an oncolytic herpes simplex virus 2, in patients with advanced solid tumors: A phase I dose escalation clinical study. J. Clin. Oncol. 2020, 38, 3139. [Google Scholar] [CrossRef]
- Lenz, H.-J.; Somasundaram, A.; Lee, M.S.; Sohal, D.; Safyan, R.A.; Zhu, M.; Jones, J.C.; Bhansali, A.; Kalwar, R.; Cho, M.; et al. An open-label clinical trial of RP2 and RP3 oncolytic immunotherapy in combination with atezolizumab plus bevacizumab for the treatment of patients with advanced colorectal carcinoma. J. Clin. Oncol. 2024, 42, TPS228. [Google Scholar] [CrossRef]
- Élez, E.; Shepard, D.; Smolenschi, C.; Bekaii-Saab, T.; Jac, J. P-58 An ongoing open-label, phase 2 trial of RP2 Or RP3 oncolytic immunotherapy in combination with atezolizumab and bevacizumab for the treatment of patients with advanced colorectal carcinoma. Ann. Oncol. 2023, 34, S34–S35. [Google Scholar] [CrossRef]
- Uppsala University: Study of Recombinant Adenovirus AdVince in Patients with Neuroendocrine Tumors; Safety and Efficacy (RADNET). Available online: https://clinicaltrials.gov/study/NCT02749331 (accessed on 7 February 2025).
- Chan, A. FDA Grants Orphan Drug Designation to ELC-100 for Pancreatic NETs. 2025. Available online: https://www.onclive.com/view/fda-grants-orphan-drug-designation-to-elc-100-for-pancreatic-nets (accessed on 7 February 2025).
- Musher, B.L.; Smaglo, B.G.; Abidi, W.; Othman, M.; Patel, K.; Jawaid, S.; Jing, J.; Brisco, A.; Wenthe, J.; Eriksson, E.; et al. A phase I/II study of LOAd703, a TMZ-CD40L/4-1BBL-armed oncolytic adenovirus, combined with nab-paclitaxel and gemcitabine in advanced pancreatic cancer. J. Clin. Oncol. 2022, 40, 4138. [Google Scholar] [CrossRef]
- Musher, B.L.; Rowinsky, E.K.; Smaglo, B.G.; Abidi, W.; Othman, M.; Patel, K.; Jawaid, S.; Jing, J.; Brisco, A.; Wenthe, J.; et al. LOAd703, an oncolytic virus-based immunostimulatory gene therapy, combined with chemotherapy for unresectable or metastatic pancreatic cancer (LOKON001): Results from arm 1 of a non-randomised, single-centre, phase 1/2 study. Lancet Oncol. 2024, 25, 488–500. [Google Scholar] [CrossRef]
- Wang, D.; Porter, C.E.; Lim, B.; Shaw, A.R.; Robertson, C.S.; Woods, M.L.; Xu, Y.; Biegert, G.G.W.; Morita, D.; Wang, T.; et al. Ultralow-dose binary oncolytic/helper-dependent adenovirus promotes antitumor activity in preclinical and clinical studies. Sci Adv. 2023, 9, eade6790. [Google Scholar] [CrossRef]
- Makawita, S.; Gibbs, J.M.; McFadden, D.R.; Porter, C.; Shaw, A.R.; Robertson, C.; Woods, M.L.; Wang, T.; Grilley, B.J.; Heslop, H.E.; et al. Binary oncolytic adenovirus in combination with HER2-specific autologous CAR VST for treatment of advanced HER2-positive solid tumors (VISTA). J. Clin. Oncol. 2024, 42, TPS2679. [Google Scholar] [CrossRef]
- Wang, T.; Zang, C.; Zhang, Y.; Ren, Z.; Liu, Y.; Yang, X.; Wang, Y. Efficacy of oncolytic virus combined with HAIC of mFOLFOX for IMCC: A single-center, single-arm, prospective study. J. Clin. Oncol. 2024, 42, e16265. [Google Scholar] [CrossRef]
- Fudan University: Oncolytic Virotherapy Combined with Tislelizumab Plus Lenvatinib in Patients with Advanced Biliary Tract Cancer (OPTIONS-05). Available online: https://clinicaltrials.gov/study/NCT05823987 (accessed on 7 February 2025).
- Wenthe, J.; Eriksson, E.; Sandin, L.; Lövgren, T.; Jarblad, J.L.; Dahlstrand, H.; Olsson-Strömberg, U.; Schiza, A.; Sundin, A.; Irenaeus, S.; et al. Abstract PO-018: Inflaming advanced solid tumors including pancreatic cancer using LOAd703, a TMZ-CD40L/4-1BBL-armed oncolytic virus. Cancer Res. 2021, 81, PO-018. [Google Scholar] [CrossRef]
- Wenthe, J.; Eriksson, E.; Naseri, S.; Sandin, L.; Hahn, A.; Irenaeus, S.; Sundin, A.; Leja-Jarblad, J.; Ullenhag, G.; Lövgren, T.; et al. Abstract 6663: Immunostimulatory gene therapy targeting CD40/4-1BB in combination with chemotherapy induces an inflammatory gene profile in tumors from patients with advanced disease. Cancer Res. 2023, 83, 6663. [Google Scholar] [CrossRef]
- Hahn, A.; Irenaeus, S.; Wenthe, J.; Eriksson, E.; Schiza, A.; Dahlstrand, H.; Olsson-Strömberg, U.; Krause, J.; Sundin, A.; Sandin, L.; et al. Abstract CT235: A phase I/II clinical study of an oncolytic adenovirus expressing the immunostimulatory transgenes TMZ-CD40L and 4-1BBL in advanced solid malignancies. Cancer Res. 2023, 83, CT235. [Google Scholar] [CrossRef]
- Lenz, H.-J.; Prenen, H.; Van Cutsem, E.; Kössler, T.; Mayol, J.; Trapani, F.; Tihy, M.; Rubbia-Brandt, L.; Toso, C.; Bogenrieder, T.; et al. Abstract CT507: ATP128 vaccine with ezabenlimab promotes antigen-specific immune responses in stage IV colorectal cancer in the KISIMA-01 phase 1b trial. Cancer Res. 2022, 82, CT507. [Google Scholar] [CrossRef]
- Lenz, H.-J.; Koessler, T.; Oberstein, P.; Van Cutsem, E.; Kim, S.; Fritsch, R.; Prenen, H.; Morse, M.; Gani, D.; Derouazi, M.; et al. Abstract CT570: KISIMA-01: A first-in-human trial of the heterologous prime-boost vaccine ATP128/VSV-GP128 with ezabenlimab (BI 754091) in patients with stage IV colorectal cancer. Cancer Res. 2022, 82, CT570. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Ueno, M.; Tanaka, M.; Ikeda, M. Results from phase I study of the oncolytic viral immunotherapy agent canerpaturev (C-REV) in combination with gemcitabine plus nab-paclitaxel for unresectable pancreatic cancer. J. Clin. Oncol. 2019, 37, 325. [Google Scholar] [CrossRef]
- Bębnowska, D.; Grywalska, E.; Niedźwiedzka-Rystwej, P.; Sosnowska-Pasiarska, B.; Smok-Kalwat, J.; Pasiarski, M.; Góźdź, S.; Rolinski, J.; Polkowski, W. CAR-T Cell Therapy—An Overview of Targets in Gastric Cancer. J. Clin. Med. 2020, 9, 1894. [Google Scholar] [CrossRef]
- Qi, C.; Gong, J.; Li, J.; Liu, D.; Qin, Y.; Ge, S.; Zhang, M.; Peng, Z.; Zhou, J.; Cao, Y.; et al. Claudin18.2-specific CAR T cells in gastrointestinal cancers: Phase 1 trial interim results. Nat. Med. 2022, 28, 1189–1198. [Google Scholar] [CrossRef]
- QI, C.; Liu, C.; Gong, J.; Li, J.; Liu, D.; Wang, X.; Zhang, P.; Qin, Y.; Zhang, M.; Peng, Z.; et al. Claudin18.2-targeted chimeric antigen receptor T cell-therapy for patients with gastrointestinal cancers: Final results of CT041-CG4006 phase 1 trial. J. Clin. Oncol. 2024, 42, 2501. [Google Scholar] [CrossRef]
- Butler, M.O.; Majeed, H.; Nelles, M.; Saibil, S.; Bonilla, L.; Boross-Harmer, S.; Sotov, V.; Elston, S.; Ross, K.; van As, B.; et al. Study of TBI-1301 (NY-ESO-1 specific TCR gene transduced autologous T lymphocytes) in patients with solid tumors. Ann. Oncol. 2018, 29, viii441. [Google Scholar] [CrossRef]
- Butler, M.O.; Sotov, V.; Saibil, S.; Bonilla, L.; Boross-Harmer, S.; Fyrsta, M.; Gray, D.; Nelles, M.; Le, M.; Lemiashkova, D.; et al. Adoptive T cell therapy with TBI-1301 results in gene-engineered T cell persistence and anti-tumour responses in patients with NY-ESO-1 expressing solid tumours. Ann. Oncol. 2019, 30, v481. [Google Scholar] [CrossRef]
- Ma, Q.; He, X.; Zhang, B.; Guo, F.; Ou, X.; Yang, Q.; Shu, P.; Chen, Y.; Li, K.; Gao, G.; et al. A PD-L1-targeting chimeric switch receptor enhances efficacy of CAR-T cell for pleural and peritoneal metastasis. Signal Transduct. Target. Ther. 2022, 7, 380. [Google Scholar] [CrossRef] [PubMed]
- Pang, N.; Shi, J.; Qin, L.; Chen, A.; Tang, Y.; Yang, H.; Huang, Y.; Wu, Q.; Li, X.; He, B.; et al. IL-7 and CCL19-secreting CAR-T cell therapy for tumors with positive glypican-3 or mesothelin. J. Hematol. Oncol. 2021, 14, 118. [Google Scholar] [CrossRef] [PubMed]
- Study on TIL for the Treatment of Advanced Hepatobiliary-Pancreatic Cancers. Available online: https://clinicaltrials.gov/study/NCT05098197 (accessed on 1 March 2024).
- CIK in Treating Patients with Esophageal Cancer. Available online: https://clinicaltrials.gov/study/NCT02490735 (accessed on 1 March 2024).
- Shanghai Juncell Therapeutics: Study on TIL for the Treatment of r/r Gastrointestinal Tumors. Available online: https://clinicaltrials.gov/study/NCT04960072 (accessed on 1 March 2024).
- Wang, S. The Safety and Efficacy of Specific TIL-TCM Cells for Advanced Relapse-refractory or Metastatic Pancreatic Cancer. Available online: https://clinicaltrials.gov/study/NCT05438797 (accessed on 1 March 2024).
- Immunotherapy Using Tumor Infiltrating Lymphocytes for Patients with Metastatic Cancer. Available online: https://clinicaltrials.gov/study/NCT01174121 (accessed on 1 March 2024).
- Mukherjee, S.; Boland, P.; Gupta, M.; Grimm, M.; Attwood, K.; Kalinski, P. 334 Phase II study evaluating a chemokine-modulatory (CKM) regimen in patients with colorectal cancer (CRC) metastatic to the liver. J. Immunother. Cancer 2020, 8, A360. [Google Scholar] [CrossRef]
- Palmer, D.; Webber, B.; Patel, Y.; Johnson, M.; Kariya, C.; Lahr, W.; Parkhurst, M.; Gartner, J.; Prickett, T.; Lowery, F.; et al. 333 Targeting the apical intracellular checkpoint CISH unleashes T cell neoantigen reactivity and effector program. J. Immunother. Cancer 2020, 8, A360. [Google Scholar] [CrossRef]
- Cytryn, S.L.; Maron, S.B.; Janjigian, Y.Y. A phase II study of agenT-797 (invariant natural killer T-cells), botensilimab (Fc-enhanced CTLA-4 inhibitor) and balstilimab (anti-PD-1) in patients with advanced, refractory gastroesophageal adenocarcinoma. J. Clin. Oncol. 2025, 43, 4. [Google Scholar] [CrossRef]
| Clinical Trial | Researchers | Phase | Treatment Protocol | Patients (N) | Outcome Measures | Status |
|---|---|---|---|---|---|---|
| Gastric Cancer | ||||||
| NCT05204173 (DRAGON-09) | Yuan et al. [180] | Phase II | Sintilimab, IP and IV PTX, plus oral S-1 | 30 | One-year survival rate, R0 resection rate, three-year OS, three-year PFS | Unknown |
| Esophageal Cancer | ||||||
| NCT05937438 | Qian et al. [181] | Phase I/II | Postoperative radiotherapy followed by tislelizumab or camrelizumab | 70 | One-year DFS, three-year DFS, three-year OS | Recruiting |
| NCT04973306 (iCROSS) | Huang et al. [182] | Phase II/III | Neoadjuvant CRT + tislelizumab followed by surgery vs. CRT + surgery | 176 | Pathologic response rate, OS, PFS, R0 resection rate | Recruiting |
| Pancreatic Cancer | ||||||
| NCT04548752 | Chung et al. [183,184] | Phase II | Pembrolizumab + Olaparib vs. Olaparib alone | 88 | PFS, safety, OS, ORR, DoR | Recruiting |
| Biliary Tract Cancer | ||||||
| NCT06230471 | Zhao et al. [185] | Phase II | Pembrolizumab with or without chemotherapy or Lenvatinib | 60 | ObRR, DCR, PFS, OS, DoR | Recruiting |
| NCT06013943 | Yuan et al. [186] | Phase II | Envafolimab + Gemox | 30 | 6-month PFS, PFS, OS, DCR, ObRR | Recruiting |
| Colorectal Cancer | ||||||
| NCT02912559 (ATOMIC) | Sinicrope et al. [187] | Phase III | Systemic chemotherapy with or without atezolizumab | 700 | DFS, OS, AE | Active, not recruiting |
| NCT05382741 (VIVA) | Yamazaki et al. [188] | Phase II | Adjuvant durvalumab + regorafenib vs. observation | 182 | DFS, 18-month DFS, OS, AE | Recruiting |
| Hepatocellular carcinoma | ||||||
| NCT06248554 | Zhang et al. [189] | N/A | Post-operative adjuvant PD-1 inhibitors | 200 | DFS, OS, AE | Recruiting |
| Gastrointestinal stromal tumor | ||||||
| NCT05152472 (ATEZOGIST) | Brahmi et al. [190] | Phase II | Imatinib with atezolizumab versus imatinib alone | 110 | PFS, ORR, TTF, OS | Recruiting |
| Small bowel and appendix | ||||||
| NCT05472948 | Deng et al. [191] | Phase II | Surufatinib and sintilimab in combination with capecitabine | 36 | PFS, ORR, OS, DCR, safety | Recruiting |
| NCT04205968 | Overman et al. [192,193] | Phase 2 | Ramucirumab + paclitaxel versus FOLFIRI | 94 | PFS, OS, ORR, AE, CEA levels | Recruiting |
| Anal cancer | ||||||
| NCT03233711 | Rajdev et al. [194] | Phase III | Nivolumab versus observation | 344 | DFS, ObRR, OS, colostomy-free survival | Active, not recruiting |
| NCT04230759 (RADIANCE) | Martin et al. [195] | Phase II | CRT versus CRT + durvalumab | 180 | DFS, AE, OS, colostomy-free survival, cCR | Active, not recruiting |
| NCT04472429 (POD1UM-303/InterAACT 2) | Rao et al. [196] | Phase III | Carboplatin + paclitaxel + placebo versus carboplatin + paclitaxel + retifanlimab | 308 | PFS, ORR, DoR, DCR, AE | Active, not recruiting |
| Clinical Trial | Researchers | Phase | Treatment Protocol | Patients (N) | Outcome Measures | Status |
|---|---|---|---|---|---|---|
| NCT04222114 | Qi et al. [199] | Phase III | IP catumaxomab vs. localized supportive treatment | 282 | OS, PFS, progression-free interval of peritoneal metastatic lesions | Unknown |
| NCT05802056 (CONTROL) | Grotz et al. [200] | Phase I | IP aldesleukin, IV nivolumab and systemic Chemotherapy | 15 | Reduction in PCI, AE, PFS, OS, histological response | Recruiting |
| NCT06016179 (RIOT2) | Wagner et al. [201,202] | Phase I | Intracavitary injection of tocilizumab | 12 | Successful administration of tocilizumab, AE, pharmaco-kinetic analysis, biomarkers | Recruiting |
| NCT03323944 | Aznar Gomez et al. [203] | Phase I | huCART meso cells IV versus IP versus intrahepatic infusion | 18 | AE, PFS, OS, ObRR | Recruiting |
| NCT04889768 | Zhejiang Cancer Hospital | N/A | HIPEC, camrelizumab, chemotherapy, and surgery | 46 | R0 resection, ObRR, AE, DFS, OS | Not yet recruiting |
| Clinical Trial | Researchers | Phase | Treatment Protocol | Patients (N) | Outcome Measures | Status |
|---|---|---|---|---|---|---|
| Immune Modulation | ||||||
| NCT03252938 (INSIGHT) | Al-Batran et al. [208,209] | Phase I | Intra-tumoral injection of IMP321 alone or bi-weekly IP injection of IMP321 | 110 | Feasibility rate, AE, objective response rate, PFS, OS, immune response | Recruiting |
| NCT05751837 (RIOT1) | Lewis et al. [210,211] | Phase I | Intra-tumoral injection of LPS | 12 | Safety and tolerability | LPS was feasible and safe |
| NCT03364400 | Chen et al. [212] | Phase I | Escalating doses of VT1021 | 116 | Identify phase 2 dose, AE, efficacy | DCR of 18% in pancreatic cancer |
| NCT03812796 (EMERGE) | Cartwright et al. [213,214] | Phase II | Domatinostat plus Avelumab | 75 | Safe and tolerable dose, objective response rate | Recruiting |
| Vaccination | ||||||
| NCT03767582 | Christenson et al. [215] | Phase I/II | Neoadjuvant/adjuvant nivolumab and BMS-813160 with or without GVAX | 30 | Drug-related toxicities, immune response, OS, MFS, LPFS, resectability rate, pathological response rate | Closed to accrual |
| NCT03784040 (da VINci) | Choo et al. [216] | Phase I | OTSGC-A24 + nivolumab or OTSGC-A24 + nivolumab + ipilimumab | 40 | AE, response rate, PFS, OS | Unknown |
| NCT02919644 (COREVAX-1) | De Rosa et al. [217] | Phase II | Autologous DC vaccine + IL-2 | 19 | AE, immunological efficacy, RFS, OS | Recruiting |
| NCT04248569 | Kirk et al. [218,219] | Phase I | DNAJB1-PRKACA peptide vaccine + ipilimumab and nivolumab | 56 | Drug-related toxicities, change in DNAJB1-PRKACA CD8 and CD4 T cells, ORR, DoR, DCR, PFS, OS | Recruiting |
| NCT03558945 | Khanniche et al. [220,221] | Phase I | Personalized neoantigen vaccine | 30 | AE, RFS, OS | Recruiting |
| NCT05968326 (IMCODE 003) | Genentech [222] | Phase II | Autogene Cevumeran + atezolizumab = mFOLFIRINOX | 260 | DFS, OS, AE | Recruiting |
| Clinical Trial | Researchers | Phase | Treatment Protocol | Patients (N) | Outcome Measures | Status |
|---|---|---|---|---|---|---|
| NCT03866525 | Zhang et al. [134,225] | Phase I/II | OH2 injection with or without irinotecan or HX008 | 300 | DLT, MTD, biodistribution and biologic effect of OH2, anti- tumor activity and immunogenicity of OH2 | Recruiting |
| NCT05733611 | Lenz et al. [226,227] | Phase II | RP2 or RP3 + bevacizumab and atezolizumab | 4 | ObRR, AE, OS, PFS, DoR, CRR | Active, not recruiting |
| NCT02749331 (RADNET) | Crona et al. [228,229] | Phase I/II | Infusion of AdVince into intrahepatic artery | 35 | AE, change in tumor size, change in tumor metabolic activity, PFS | FDA orphan drug designation |
| NCT02705196 (LOKON001) | Musher et al. [230,231] | Phase I/II | Intra-tumoral LOAd703 + gemcitabine + nab-paclitaxel with or without atezolizumab | 55 | DLT, ORR, OS | Recruiting |
| NCT03740256 (VISTA) | Wang et al. [232,233] | Phase I | Intra-tumoral CAdVEC | 45 | DLT, ORR, DCR, OS, PFS, AE | Recruiting |
| NCT05124002 | Wang, et al. [234] | Phase IV | FOLFOX + H101 | 66 | PFS, one-year survival rate, objective response rate, DCR | Recruiting |
| NCT05823987 (OPTIONS-05) | Wang et al. [235] | Phase II | H101 + tislelizumab + Lenvatinib | 25 | Objective response rate, DoR, PFS, OS, DCR, AE | Recruiting |
| NCT03225989 (LOKON002) | Wenthe et al. [236,237,238] | Phase I/II | LOAd703 + SOC chemotherapy or gemcitabine immune conditioning | 46 | AE, tolerability, OS, TTP, PFS, systemic immune activation | Objective clinical activity in advanced PDAC |
| NCT04046445 (KISIMA-01) | Lenz et al. [239,240] | Phase I | SC injection of ATP128 + BI 754,091 or ATP128 + BI 754,091 + VSV-GP128 | 96 | AE, PFS, overall response, confirm phase 2 dose, DoR, BOR, RFS | Active, not recruiting |
| NCT03252808 | Hashimoto et al. [241] | Phase I | HF10 + gemcitabine + Nab-paclitaxel or HF10 + TS-1 | 36 | DLT, AE, objective response rate, PFS, OS, one-year survival rate | Active, not recruiting |
| Clinical Trial | Researchers | Phase | Treatment Protocol | Patients (N) | Outcome Measures | Status |
|---|---|---|---|---|---|---|
| NCT03874897 | Qi et al. [243,244] | Phase I | CAR-Claudin18.2 T cells + toripalimab + first-line chemotherapy | 123 | DLT, MTD | ORR of 57.4% in gastric cancer patients |
| NCT02869217 | Butler et al. [245,246] | Phase I | Cyclophosphamide + TBI-1301 + fludarabine as retreatment vs. double infusion | 22 | AE, RP2D | Active, not recruiting |
| NCT04684459 | Ma et al. [247] | Phase I | Dual-targeting HER2 and PD-L1 CAR T cells | 18 | ORR, DoR | Recruiting |
| NCT03198546 | Pang et al. [248] | Phase I | GPC3 and/or TGFβ targeting CAR T cells | 30 | DLT, best response, median CAR T cell persistence | Recruiting |
| NCT05098197 | Shanghai Juncell Therapeutics [249] | Phase I | Adoptive transfer of TIL in hepatobiliary and pancreatic cancer | 50 | AE, ORR, DCR, DoR, PFS, OS, QOL | Recruiting |
| NCT02490735 | First People’s Hospital of Changzhou [250] | Phase II | Cytokine-induced killer cells + chemotherapy versus chemotherapy alone | 2000 | PFS, OS | Not yet recruiting |
| NCT04960072 | Xu et al. [251] | Phase I | Adoptive transfer of TIL in advanced GI cancers | 50 | ORR, DCR, DoR, PFS, OS, QOL | Recruiting |
| NCT05438797 | Wang et al. [252] | Phase I | Adoptive TIL-TCM transfer therapy | 3 | DLT, MTD, AE, ORR, DoR, PFS | Unknown |
| NCT01174121 | Rosenberg et al. [253] | Phase II | CD8+ TIL vs. unselected TIL + aldesleukin + cyclophosphamide + fludarabine; unselected TIL + pembrolizumab | 332 | RR, safety of pembrolizumab + TIL, AE | Recruiting |
| NCT04426669 | Mukherjee et al. [254,255] | Phase I/II | CISH CRISPR TIL | 20 | MTD, efficacy, safety, PFS, OS, toxicity | Active, not recruiting |
| NCT06251973 | Cytryn et al. [256] | Phase II | agenT-797, Botensilimab, Balstilimab, Ramucirumab, Paclitaxel | 37 | Overall RR | Recruiting |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewis, C.R.; Samhouri, Y.; Sherry, C.; Dadgar, N.; Raj, M.S.; Wagner, P.L. Current and Future Directions in Immunotherapy for Gastrointestinal Malignancies. Int. J. Transl. Med. 2025, 5, 33. https://doi.org/10.3390/ijtm5030033
Lewis CR, Samhouri Y, Sherry C, Dadgar N, Raj MS, Wagner PL. Current and Future Directions in Immunotherapy for Gastrointestinal Malignancies. International Journal of Translational Medicine. 2025; 5(3):33. https://doi.org/10.3390/ijtm5030033
Chicago/Turabian StyleLewis, Catherine R., Yazan Samhouri, Christopher Sherry, Neda Dadgar, Moses S. Raj, and Patrick L. Wagner. 2025. "Current and Future Directions in Immunotherapy for Gastrointestinal Malignancies" International Journal of Translational Medicine 5, no. 3: 33. https://doi.org/10.3390/ijtm5030033
APA StyleLewis, C. R., Samhouri, Y., Sherry, C., Dadgar, N., Raj, M. S., & Wagner, P. L. (2025). Current and Future Directions in Immunotherapy for Gastrointestinal Malignancies. International Journal of Translational Medicine, 5(3), 33. https://doi.org/10.3390/ijtm5030033







