Abstract
Objectives: Parental exposure to tobacco smoke is a significant public health concern, with over 1.1 billion smokers worldwide. The aim of this systematic review was to evaluate the impact of maternal, paternal, and dual-parental cigarette smoke exposure on offspring reproductive health. Methods: Original human clinical and animal research studies were included; titles and abstracts were manually scanned for relevance to the effect of parental smoking on offspring reproductive outcomes (Date of search:18/03/2025). Results: This systematic review incorporates 30 studies identified from three databases (PubMed, Web of Science, and Scopus). The results indicate that male offspring exhibit reduced spermatogenic capacity, characterized by decreased testicular size, lower sperm count, and impaired hormonal biosynthesis, with reductions of 30–40% in sperm production. Dual-parental smoking exacerbates these effects, with sperm counts averaging 85 million per ml in human male offspring from dual-smoking households, compared to 111 million per ml in single-smoking households. Animal studies provide mechanistic insights, revealing reduced testis weight in nicotine-exposed male rats and increased oxidative stress in offspring. Conclusions: This review highlights the dose-dependent and sex-specific effects of smoking on the fertility of offspring and underscores the need for standardized protocols to enhance the consistency and comparability of future research in both human and animal studies.
1. Introduction
Parental exposure to tobacco smoke is a significant public health concern, with over 1.1 billion smokers worldwide [1]. According to the World Health Organization (WHO), tobacco use is responsible for more than 8 million deaths annually, including 1.2 million attributed to second-hand smoke exposure.
Cigarette smoke contains over 7000 chemical compounds, including nicotine, polycyclic aromatic hydrocarbons (PAHs), and heavy metals, such as lead and cadmium, many of which are toxic to reproductive organs [2,3]. The Developmental Origins of Health and Disease (DOHaD) hypothesis has advanced considerably over the last two decades. It states that fetal adaptations to maternal and paternal environmental conditions during development significantly influence the development and functionality of their offspring’s organs and, ultimately, their long-term health. Epidemiological studies indicate that maternal smoking during pregnancy significantly increases the risk of low birth weight and perturbs placental function [4]. Low birth weight is linked to a range of adult-onset conditions [5]. Smoking during pregnancy is now linked to impairments in offspring fertility—giving the potential for there to be impacts across multiple generations, especially if the changes become germline, such as reduced body mass, body size, and tibia length of Drosophila melanogaster [6]. In vitro animal studies have also demonstrated that exposure to chemical compounds derived from cigarettes can damage the developing fetal ovary during the critical period of follicle formation [7].
Paternal smoking also affects the health of offspring, mediated through perturbed sperm quality, DNA fragmentation, and epigenetic changes [8]. Studies suggest that fathers who smoke have a 30% higher likelihood of sperm DNA fragmentation, which is linked to poor embryonic development and adverse reproductive outcomes [9].
The combined effects of maternal and paternal smoking further amplify these risks, but these are fewer in number than those examining the effect of just one parent. Research indicates that offspring born to smoking parents face compounded reproductive health challenges [10]. While animal studies are useful, especially from a mechanistic and ethical perspective (as human studies tend to be retrospective or cohort in nature), it is a common challenge to draw definitive conclusions and direct comparisons to humans. Variability in experimental protocols, including differences in dosage, duration, and timing of exposure, further complicates the interpretation and clinical relevance of results.
This systematic review provides the first known comprehensive analysis of studies reporting the effects of parental smoking on offspring reproductive health. With a focus on maternal and paternal influences, their combined impact, and dose–response relationships, it assesses the reliability of animal models, explores mechanisms, such as genetic and epigenetic damage, and identifies critical exposure thresholds.
2. Materials and Methods
2.1. Eligibility Criteria
This systematic review utilized comprehensive database searches, PRISMA-guided screening, and data extraction to ensure transparency and minimize bias in analyzing eligible studies.
2.2. Search Strategy
Articles were sourced from three databases: PubMed, Web of Science, and Scopus. Each database was filtered to include only articles published in English and to exclude reviews. The key terms used were as follows: smoking terms: “smoke,” “nicotine,” “tobacco,” “cigarette,” “e-cigarette,” and “JUUL”; time of exposure: “prenatal,” “maternal,” “during pregnancy,” “perinatal,” “lactation,” and “fetal exposure”; effect on offspring: “offspring,” “infant,” “boy,” “girl,” “male,” “female,” “age groups,” “son,” “newborn,” “in vitro,” and “children”; reproductive health: “reproductive outcome,” “reproductive hormone,” “semen quality,” “secondary sex,” “reproductive parameters,” “genital development,” “testis development,” “sperm count,” “oogonia,” “sexual behavior,” “genital anogenital,” “reproductive disorders,” “long-term fertility,” and “ovarian reserve.” See Supplement Table S1 for detailed search terms.
The search was conducted on 18 March 2025, yielding a total of 2552 articles: PubMed (683), Web of Science (479), and Scopus (1390). A total of 949 duplicates were then removed using EndNote software. The remaining titles and abstracts (1603) were manually scanned for relevance to the effect of parental smoking on offspring reproductive outcomes. This scanning process was validated by an independent reviewer. The remaining articles then had their full texts screened and were evaluated for inclusion in the study using the inclusion/exclusion criteria outlined in Table 1. Thirty studies met these eligibility criteria and so were included in this systematic review. A PRISMA 2020 flow diagram detailing the process of this systematic review is shown in Figure 1.

Table 1.
The inclusion and exclusion criteria used to determine eligibility.
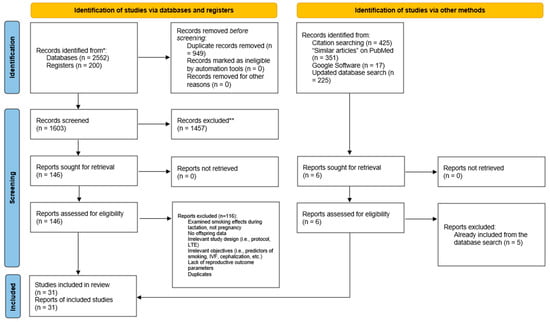
Figure 1.
Prisma flow diagram showing the detailed process of screening and study inclusion. * Databases included are PubMed, Web of Science, and Scopus. ** Excluded as per inclusion/exclusion criteria.
2.3. Data Extraction
Data extraction was conducted using a structured Excel spreadsheet. The data were exported into pre-defined tabular formats and categorized based on research characteristics, sources of smoking exposure, and outcomes. Coded data included the year of publication, study type, country of origin, number of participants, and detailed information on smoking exposure, such as the source (paternal, maternal, or both), material type, and dosage. Additional variables included study quality factors, reproductive function indicators (e.g., hormone levels, fertility indices, organ development), and offspring sex (male and/or female). The extracted reproductive endpoints encompassed hormonal assessments, semen quality, and other measures of human reproductive fitness. Measured outcomes such as sexual behavior and in vitro fertilization (IVF) were excluded from this review. Sexual behavior is generally not considered a direct reproductive outcome, as it primarily reflects behavioral patterns that are often studied as separate behavioral or neuroendocrine endpoints—such as mating attempts, libido, and courtship [11]. Moreover, sexual behavior is subject to high variability and presents interpretational challenges due to influencing factors such as stress, environmental differences, and social dynamics. IVF can be considered a reproductive outcome; however, it is often assessed in a clinical context. Individuals undergoing IVF may already have underlying fertility issues, which introduces additional variables and bias if, for instance, a birth outcome from IVF is used as a reproductive outcome. This structured rating system enabled a consistent comparison of study findings and their applicability. By consolidating the data, this approach facilitated the identification of common patterns and research gaps related to parental smoking and its impact on offspring reproductive health.
2.4. Quality Assessment
A customized scoring system was employed to evaluate the robustness of studies based on 16 parameters, such as sample size, methodological clarity, and outcome measurement (Table 2). Four aspects of the publications were assessed, namely the model system used, the sample quality (including sample size and controls), the methodology of the study, and the outcomes measured. The overall score was calculated by dividing the sum of the given scores by the total number of highest possible score.

Table 2.
Scoring system to assess the robustness of included papers.
3. Results
Thirty articles were included in the final analysis of the systematic review based on their inclusion criteria. Most of these studies examined the impact of maternal smoking (19 studies), followed by paternal smoking (4 studies) and dual-parental smoking (3 studies). Moreover, environmental tobacco smoke and other indirect exposures (referred to as passive source) were also the topic of interest in 4 studies. These articles comprised human observational studies, animal experiments, and a few studies involving both humans and animals.
3.1. Impact of Maternal Cigarette Smoke Exposure on Offspring Reproductive Health
There were 19 studies examining the effect of maternal smoking on offspring reproductive health (Table 3), with several studies highlighting an association between smoking while pregnant and adverse effects on the reproductive health of offspring. Numerous studies identified low sperm concentration and motility and alteration in the levels of testosterone. For instance, Ramlau-Hansen et al. (2007) and Ravnborg et al. (2011) observed that male babies born to smoking mothers had poor sperm quality and precocious puberty [12,13]. The baseline characteristics of included studies focusing on maternal smoking impact on offspring are summarized in Table 3.
Maternal smoking was mainly associated with adverse reproductive outcomes in male offspring. Testis size, a critical indicator of reproductive capacity, was consistently reduced in male offspring of smokers. For instance, Jensen (2004) observed that the mean testis volume in the smoking group was 19.1 mL (SD: 4.4), compared to 21.3 mL (SD: 5.0) in the non-smoking group [14]. This difference was statistically significant (p < 0.01), reinforcing the link between prenatal exposure and impaired testicular development. Similarly, semen volume was slightly reduced in the smoking group, with a mean of 3.0 mL (SD: 1.5), compared to 3.1 mL (SD: 1.5) in the non-smoking group, suggesting early developmental insults to reproductive structures. Sperm quality metrics, including testis volume at birth, concentration, and motility, were also negatively impacted by maternal smoking. Whether mothers smoked only during pregnancy or continued smoking after birth was not explicitly stated in studies. Ramlau-Hansen (2007) examined maternal smoking of more than 19 cigarettes per day, resulting in a mean sperm concentration of 2.6 million/mL (SD: 1.7), which was significantly lower than the 3.3 million/mL (SD: 2.5) observed in the non-smoking cohort [12]. Total sperm count followed a similar trend, with smoking groups reporting counts as low as 69 million, compared to 98 million in non-smoking groups. This substantial reduction revealed the dose-dependent nature of the impact of smoking on spermatogenesis. Puberty timing was not clarified in this study.
Adamcová (2017) reported maternal smoking also significantly influenced hormonal profiles in male offspring, with testosterone and estradiol levels showing notable deviations [15]. Ramlau-Hansen (2008) reported lower free testosterone levels in male offspring exposed to maternal smoking, with a mean of 16.4 nmol/L (95% CI: 13.3–19.4), compared to 16.7 nmol/L (95% CI: 14.1–20.3) in non-smoking groups [16]. Estradiol levels were similarly affected, with smoking groups exhibiting reduced concentrations (mean: 25.2 pg/mL, SD: 19.9) compared to non-smokers (mean: 27.2 pg/mL, SD: 21.8). Elevated sex hormone-binding globulin (SHBG) levels in smoking-exposed offspring suggest disruptions in androgen bioavailability and hormonal balance, with SHBG levels significantly higher in smokers (mean: 9.2 nmol/L, SD: 5.5) versus non-smokers (mean: 6.6 nmol/L, SD: 4.0).
Studies on female offspring revealed notable effects on pubertal timing and hormonal development. Zhang (2015) reported delayed menarche in daughters of smokers, with a mean age of 13.02 years (95% CI: 12.66–13.39), compared to 13.17 years (95% CI: 12.77–13.58) in non-smokers [17]. In animal study by Erdem Guzel (2020) maternal tobacco smoke advanced the onset of puberty in female offspring [18]. Brix (2019) found significant reductions in Tanner breast and pubic hair staging times, with breast stage 4 occurring approximately 2.8 months earlier in smoking-exposed daughters compared to non-exposed peers (p < 0.001) [19]. These findings revealed the sex-specific nature of smoking’s impact, with maternal smoking disproportionately affecting male reproductive metrics and paternal smoking influencing female hormonal profiles.
Anogenital distance (AGD) was another key metric affected by maternal smoking. Typically, male offspring exhibit a significantly longer AGD than females due to the higher levels of testosterone during a critical window of fetal development. Because of this consistent sex difference, AGD is commonly used in both animal and human studies to assess developmental effects related to sex. Fowler (2011) reported shorter AGD measurements in male fetuses exposed to maternal smoking (mean: 17.6 mm, SD: 2.5) compared to non-smoking groups (mean:13.9 mm, SD: 2.3) [20]. By contrast, female offspring exhibited longer AGD in smoking groups, as reported by Kızılay (2021) (mean: 14 mm, SD: 2.5) compared to non-smoking groups (mean: 13.1 mm, SD: 2.2) [21]. In the cohort study by Cirillo (2011), maternal smoking during pregnancy was associated with a higher risk of cryptorchidism (aHR = 1.18, 95% CI: 1.12, 1.24) in male offspring [22]. These findings indicate that maternal smoking exposure disrupts sexually dimorphic development, with implications for reproductive health and disease risk.
Dose–response analyses highlighted the proportional relationship between cigarette consumption and reproductive outcomes. Higher maternal smoking doses (>19 cigarettes/day) consistently resulted in more significant reductions in sperm concentration, testis size, and hormonal levels compared to lighter smoking (<10 cigarettes/day). Lighter smoking (<10 cigarettes/day) also showed significant reductions in sperm concentration and hormonal levels compared to non-smoking. For example, Ramlau-Hansen (2007) reported that heavy maternal smoking reduced total sperm count by 30–40% compared to non-smokers and 17% lower sperm concentration (p = 0.47) in lighter smoking compared with non-smokers [12].

Table 3.
A descriptive summary of included studies focusing on the impact of maternal smoking on offspring.
Table 3.
A descriptive summary of included studies focusing on the impact of maternal smoking on offspring.
| First Author (Year) [Citation Number] | Study Design | Model | Method | Sample Size | Smoking Material | Source of Smoking | Smoking Dose | Offspring Sex |
|---|---|---|---|---|---|---|---|---|
| Adamcová (2017) [15] | Case-control study | Human | Changes in production of steroid hormones in pregnant smokers | 88 healthy women (17 active smokers and 71 non-smokers) | Cigarette | Maternal | 6–25 day | Both (Male and Female |
| Brix (2019) [19] | Population-based study | Human | 15,819 children (7696 male offspring and 8123 daughters) who were part of the Puberty Cohort, a sub cohort of the Danish National Birth Cohort | 15,819 children participated in the study | Cigarette tobacco | Maternal during pregnancy | Non-smoker, light-smoker (1–10 daily cigarettes), heavy-smoker (>10 daily cigarettes) | Both (Male and Female) |
| Brix (2019) [23] | Population-based study | Human | 42,849 of 56,641 eligible boys and girls from the Danish National Birth Cohort born between 2000 and 2003 | 42,849 children after exclusions | Cigarette tobacco | Maternal smoking during the first trimester of pregnancy | Non-smoker, stopped smoking, 1–9 cigarettes/day, 10–14 cigarettes/day, 15+ cigarettes/day | Both (Male and Female) |
| Cirillo (2011) [22] | Retrospective cohort | Human | Girls aged 6–11 years from the Third National Health and Nutrition Examination Survey (NHANES III) | 705 girls with complete LH hormone measurements; of these, 689 had complete inhibin B analysis. | Cigarette | Maternal during pregnancy | Mean (SD) = 15.5 (9.7) | Male |
| Erdem Guzel (2020) [18] | Experimental study | Rats | Female rat offspring | 28 Sprague-Dawley female rats | Tobacco | Maternal | 20 g of tobacco per/day | Female |
| Fowler (2009) [24] | Observational study | Human | In total, 46 fetuses were used to determine circulating hormones and cotinine levels from cardiac blood | 46 mothers and fetuses | Tobacco | Maternal during pregnancy | Mean (SD) = 12 (1). 12/day = heavy smoking | Male |
| Fowler (2011) [20] | Retrospective cohort | Human | 83 electively terminated, normally progressing, second-trimester fetuses (11 to 20 weeks’ gestation) | 83 fetuses | Cigarette tobacco | Maternal during pregnancy | Unknown | Male |
| Gollenberg (2015) [25] | Retrospective study | Human | Girls aged 6–11 years from the Third National Health and Nutrition Examination Survey (NHANES III) | 705 female offspring (girls 6–11 years) | Cigarette smoke | Maternal during pregnancy and current environmental tobacco smoke (ETS) exposure | Unknown | Female |
| Gordon, 2022 [26] | Prospective cohort | Human | >18 years old with a singleton pregnancy with female fetus | 64 female infants | Self-reported smoking | Maternal | Active smoker (≥1/2 pack/day) | Female |
| Hærvig (2022) [27] | Retrospective study | Human | Pregnant women | 984 male offspring | Cigarette smoke | Maternal | Light (≤10 cigarettes/day); Heavy smokers (>10 cigarettes/day) | Male |
| Jensen (2004) [14] | Follow-up study | Human | Young men from general populations undergoing military examination | 889 (Denmark), 221 (Norway), 313 (Finland), 157 (Lithuania), 190 (Estonia) | Tobacco | Maternal, during fetal life | Light smoking (1–9 cigarettes/day); Medium smoking (10–19 cigarettes/day); Heavy smoking (≥20 cigarettes/day) | Male |
| Kızılay (2020) [21] | Prospective case-control study | Human | 120 infants (56 female and 64 male) from mothers who smoked during pregnancy and a control group of 120 infants (56 female, 64 male) whose mothers had no active or passive smoke exposure | 240 infants evaluated, 120 in the study group (56 female and 64 male), and 120 in the control group (56 female, 64 male) included in the study after exclusions | Cigarette tobacco | Maternal during pregnancy | Unknown | Both (Male and Female) |
| Lindbo (2022) [28] | Register-based, sibling-matched cohort | Human | 823,670 live-born, singleton boys born in Denmark between 1st January 1991, and 31st December 2016 | 823,670 singleton boys | Cigarette tobacco | Maternal during pregnancy | (≤5, 6–10, 11–20, and ≥21 cigarettes/day) | Male |
| Lutterodt (2009) [29] | Prospective study | Human | First-trimester women >18 years of age | 28 Fetuses | Cigarette smoke | 1–5, 6–10, 11–15, 16–20 cigarettes/day | Both (Male and Female) | |
| Mamsen (2010) [30] | Prospective study | Human | First-trimester women >18 years of age | 24 Fetuses (embryonic testes) | Cigarette smoke | Ranging from 1 to 25 | Both (Male and Female) | |
| Ramlau-Hansen (2007) [12] | Follow-up study | Human | Male offspring of mothers in the Healthy Habits for Two cohort | 347 men | Tobacco | Unknown | Male | |
| Ramlau-Hansen (2008) [16] | Population-based follow-up study | Human | Male offspring of mothers in Healthy Habits for Two cohort | 347 men | Tobacco | Light smoking (1–9 cigarettes/day); Medium smoking (10–19 cigarettes/day); Heavy smoking (≥20 cigarettes/day) | Male | |
| Ravnborg (2011) [13] | Semen-quality study | Human | 4862 Danish men from the Copenhagen area | 3486 men | Tobacco | Light smoking (1–10 cigarettes/day); Heavy smoking (>10 cigarettes/day) | Male | |
| Zhang (2015) [17] | Retrospective study | Human | 751 female students aged 8 to 20 years from a suburban district in Shanghai | 751 girls included for data analysis after exclusions | Tobacco smoke | Unknown | Female |
In summary, of the 19 studies examining maternal smoking, 14 studies examined the effect of maternal smoking in male offspring, and this was linked to adverse outcomes such as reduced sperm quality, disrupted gonadal development, hormonal imbalances, and higher risk of cryptorchidism in male offspring [12]. Female offspring were studied less, with only 5 studies, showing disrupted ovarian reserve and hormonal regulation [18]. There was no justification in studies provided why female offspring were less studied compared to male offspring. Active maternal smoking during pregnancy was associated with lower testosterone levels, reduced anogenital distance (AGD), and delayed pubertal development in male offspring [24]. Environmental tobacco smoke (ETS) was also linked to hormonal disruptions in both sexes [21]. Hormonal changes included alterations in testosterone and estradiol levels. Fowler (2009) and Fowler (2011) demonstrated that maternal smoking during pregnancy reduced testosterone levels in male fetuses, impacting anogenital distance (AGD) and pubertal onset [20,24]. Gordon (2022) observed increased maternal testosterone levels associated with active smoking, suggesting potential androgenic effects [26]. By contrast, Cirillo (2011) observed no effect on male offspring semen quality associated with maternal smoking [22]. Studies on female offspring, including Zhang (2015) and Gordon (2022), linked maternal smoking to delayed pubertal onset and reduced ovarian reserve [17,26]. An animal study by Erdem Guzel (2020) found that maternal tobacco smoke advanced onset of puberty in female offspring, an increase of addition apoptotic cell markers in the rat ovary [18]. The variability in findings was compounded by inconsistent definitions and classifications of smoking exposure, tobacco content of products used, exact amount of nicotine smoked in milligrams, and differences between cigarette brands. While most studies outlined specific smoking dose ranges, five studies lacked precise categorization, obscuring the dose–response relationship.
A customized scoring system was then employed to evaluate the robustness of studies based on 16 parameters, such as sample size, methodological quality, and outcome measurement. Of the 19 studies examining the effect of maternal smoking on offspring health, scores were relatively high, ranging from 0.438 to 0.75 (Table 4). Human studies consistently scored higher, with Ravnborg (2011) achieving a score of 0.750 due to its large sample size, detailed exposure classifications, and comprehensive reproductive outcomes [13]. Fowler (2009) and Hærvig (2022) scored 0.688, reflecting their strengths in longitudinal design and standardized methodologies (Table 4) [24,31].

Table 4.
A summary of the sample and methodological quality of each maternal smoking study included in this systematic review. The overall score was calculated by dividing the sum of the given scores by the total number of highest possible scores.
3.2. Impact of Paternal Cigarette Smoke Exposure on Offspring Reproductive Health
Four studies investigated paternal smoking on reproductive health and revealed effects on offspring health comparable to those observed with maternal smoking. Table 5 summarizes the baseline characteristics of these studies assessing the impact of paternal smoking on offspring.
The primary mechanisms through which paternal smoking affects offspring appear to be genetic and epigenetic, particularly through alterations occurring during spermatogenesis. Multiple studies have found that paternal smoking is associated with reduced sperm quality, shortened reproductive lifespan, and altered testosterone levels. Specifically, Haervig (2025) reported that male offspring of pre-conceptional smokers had lower semen quality [31]. For example, the percentage of progressive spermatozoa was slightly lower in the smoking group (63%, SD: 29) compared to the non-smoking group (64%, SD: 30). More notably, total sperm count showed a substantial difference, with smoker offspring having a mean count of 130 million (SD: 111) versus 230 million (SD: 225) in non-smoker offspring. Hormonal indicators also revealed detrimental effects. Inhibin B, a marker of spermatogenic activity, was also lower among sons of smokers (mean: 191 pg/mL, SD: 88) versus non-smokers (mean: 204 pg/mL, SD: 98). While individual studies have shown negative impacts, an updated and republished metanalysis of 867 young adult men found no overall association between paternal preconception smoking and semen parameters or testicular size [32]. In females, data from Fukuda (2011) suggest that smoking reduces the reproductive lifespan by leading to earlier menopause (p = 0.059) [33].

Table 5.
Baseline characteristics of studies examining the impact of paternal smoking on offspring.
Table 5.
Baseline characteristics of studies examining the impact of paternal smoking on offspring.
| First Author (Year) [Citation Number] | Investigation Period | Study Design | Model | Method | Sample Size | Smoking Material | Smoking Dose | Source of Smoking | Offspring Sex |
|---|---|---|---|---|---|---|---|---|---|
| Fukuda (2011) [33] | June 2007 to December 2009 | Observational study | Human | 1093 postmenopausal women attending clinics for gynecological assessment | 1093 daughters out of 1164 approached | Cigarette tobacco | Unknown | Paternal smoking around the time of conception | Female |
| Haervig (2020) [34] | 1996–2003 | Population-based follow-up study within the Danish National Birth Cohort | Human | Adult male offspring born to mothers included in the DNBC (Danish National Birth Cohort) | 772 participated, 751 included for analysis after exclusions | Cigarette tobacco | Unknown | Paternal during and potentially before pregnancy | Male |
| Hærvig (2025) [32] | 2017–2019 | Retrospective cohort (reran analysis from 2023) | Human | 867 young adult men from the DNBC | 867 male offspring after exclusions | Self-reported smoking | Unknown | Paternal during gestational week 16 | Male |
| Pabarja (2021) [35] | Unknown | Observational study | Mice | 25 adult NMRI Naval Medical Research Institute mice (19 females and 6 male) aged 8–10 weeks | 25 adult mice | Injection | Control, saline solution | Paternal during and potentially before pregnancy | Male |
In summary, of the 4 studies examining paternal smoking, only one study examined the effect of paternal smoking in female offspring, and four of the studies examined male offspring, with effects appearing to be genetic, epigenetic, and hormone related.
As above, a customized scoring system was employed to evaluate the robustness of studies based on 16 parameters, such as sample size and methodological clarity (Table 6). Again, human studies consistently scored higher, with Fukuda (2011) achieving a score of 0.5363 due to its large sample size, detailed exposure classifications, and comprehensive reproductive outcomes [33]. Multiple references scored 0.438, reflecting their strengths in longitudinal design and standardized methodologies (Table 6). Most of studies focusing on parental smoking had mid-level overall quality score, ranging from 0.438 to 0.563.

Table 6.
A summary of the sample and methodological quality of each paternal smoking study included in this systematic review. The overall score was calculated by dividing the sum of the given scores by the total number of highest possible scores.
3.3. Impact of Dual-Parental Smoking on Offspring
Three studies examined dual-parental smoking, together suggesting an additive or even synergistic effect on offspring outcomes. The baseline characteristics of these studies are summarized in Table 7.

Table 7.
A descriptive summary of included studies focusing on the impact of dual-parental smoking on offspring.
Ernst (2012) and Axelsson (2013) reported compounded reductions in sperm quality, heightened hormonal disruptions, and increased developmental abnormalities in offspring when both parents smoked [32,36]. These findings underscore the distinct and severe impact of dual-parental smoking, emphasizing the need for targeted public health interventions. The combined genetic, epigenetic, and environmental exposures from both parents further amplify these risks. Axelsson (2013) and Axelsson (2018) linked dual-parental smoking to disrupted reproductive hormone levels and altered gonadal morphology [36,37]. Addressing dual-parental smoking is crucial for mitigating cumulative reproductive health risks and improving offspring outcomes.
Axelsson (2013) also observed reductions in semen quality, with total sperm count significantly lower in dual-smoking households (mean: 85 million, SD: 27) compared to households where neither parent smoked (111 million, SD: 40) [36]. Sperm motility was also impaired, with motile sperm percentages averaging 50% (SD: 17) in dual-smoking households versus 54% (SD: 19) in non-smoking households. Hormonal disruptions were more significant in offspring exposed to dual-parental smoking. Elevated SHBG levels and altered free testosterone-to-estradiol ratios suggest compounded effects on androgen and estrogen balance. For instance, SHBG levels were significantly higher in dual-smoking groups (mean: 10.1 nmol/L, SD: 5.7) compared to single-parent smoking households (mean: 7.6 nmol/L, SD: 4.9), the sex was not specified.
In summary, Ernst et al. (2012), Axelsson et al. (2013), and Axelsson et al. (2018) highlighted that the combined smoking of both parents intensified cuts in sperm quality and hormonal imbalance [32,36,37]. Comparison across studies was again confounded due to the variability of smoking doses for each study.
Again, a customized scoring system was employed to evaluate the robustness of the studies based on 16 parameters. Axelsson (2018) achieved lowest score (0.5), while Ernst (2012) and Axelsson (2013) achieved moderate scores (0.625) due to their use of representative samples (Table 8) [32,36,37].

Table 8.
A summary of the sample and methodological quality, and measured outcomes, of each dual paternal smoking study included in this systematic review. The overall score was calculated by dividing the sum of the given scores by the total number of highest possible scores.
3.4. Impact of Passive Smoke Exposure on Offspring Reproductive Health
The final section of this systematic review focuses on passive smoking, where only animal models were used. Table 9 summarizes the baseline characteristics of these studies, along with the types of offspring affected. Camlin (2016) examined only female offspring and observed reduced oocyte quality, abnormal levels of cellular proliferation, and decreased interaction with wild-type sperm [38]. Al-Sawalha (2021) found elevated oxidative stress markers and reduced testosterone levels in rats exposed to waterpipe tobacco smoke, underscoring the generalizability of smoking’s reproductive effects across species [39]. Another animal study found that nicotine exposure reduced AGD at birth of both male and female offspring; however, by 34 days of age, this change was no longer apparent [40]. Lastly, Oyeyipo (2018) demonstrated that male rat offspring exposed to maternal nicotine exhibited significantly reduced fetal testis weight (mean: 21 mg, SD: 1) compared to controls (mean: 26 mg, SD: 4) [41].

Table 9.
Baseline summary of studies examining the impact of passive smoking (animal models) on offspring.
Animal studies generally scored lower due to smaller sample sizes and inconsistent exposure definitions. In fact, in four studies, the methods were either not clearly described or involved the use of alternative to cigarette smoke (waterpipe tobacco). Camlin (2016) and Oyeyipo (2018) scored 0.563 and 0.438, respectively, indicating limitations in replicability and statistical power (Table 10) [38,41]. In studies reporting passive smoking in animal models, scores were relatively high, ranging from 0.375 to 0.563.

Table 10.
The overall score was calculated by dividing the sum of the given scores by the total number of highest possible scores.
4. Discussion
The systematic review revealed the significant adverse effects of parental cigarette smoke exposure on offspring reproductive health, as evidenced by 30 studies. Maternal smoking was the most extensively studied, consistently linked to reduced sperm density, motility, hormonal imbalances, and impaired gonadal development in male offspring. Paternal smoking, though less frequently investigated, demonstrated similar negative effects mediated through sperm genetic and epigenetic pathways. Dual-parental smoking exhibited a compounded effect, amplifying reductions in sperm quality and endocrine disruptions in offspring.
With regard to maternal smoking, the placenta can act as a facilitator of toxicant transfer, as nicotine can cross the blood–placental barrier, with nicotine also detectable in fetal amniotic fluid. Maternal smoking induces increased oxidative stress and morphological changes in the placenta, which may contribute to adverse fetal health outcomes. However, the precise mechanism by which nicotine crosses the placenta remains unclear [42].
Overall, female offspring outcomes were underrepresented, leaving a critical void in understanding sex-specific reproductive risks. Future research should also prioritize standardizing smoking dose definitions, conducting longitudinal studies with consistent exposure characterisation, and investigating both male and female offspring outcomes. Refinement of animal models with larger sample sizes and consistent dosing protocols is also essential to enhance translational relevance.
Emerging evidence in other areas points to a synergistic effect of smoking, where dual-parental smoking leads to significantly higher risks to offspring health, such as increased risks of obesity and cardiovascular issues in adolescence and early adulthood [43]. Furthermore, there is an increasing likelihood that offspring will transition to daily smoking during adolescence and early adulthood when both parents smoke [44], suggesting a potential generational synergistic effect. Similar to the effects of smoking, other environmental perturbations have also shown enhanced outcomes when both parents are equally exposed. Finger (2015) reported that parental obesity negatively impacts pre-implantation mouse embryo development, including altered kinetics, morphology, and metabolism [45]. McPherson (2015) similarly emphasized that having two obese parents resulted in more detrimental impairments in embryo and fetal development when compared to either parent alone [46]. Ornellas (2015) further demonstrated that diet-induced obesity in both parents contributes more to the programming of obesity and related comorbidities in the offspring than either parent individually [47]. There is a potential for compounding effects of poor lifestyle choices, as individuals who smoke often have other adverse environmental exposures, such as poor diet.
Although the impact of smoking on sperm DNA fragmentation in the parent is established, whether smoking affects offspring gametes in the same way remains to be elucidated. In the E13.5 cultured mouse ovary, a smoking-related component (benzo[a]pyrene) caused DNA damage in mammalian germ cells and disrupted germ cell progression through early stages of prophase I in the first meiotic division [7].
Interestingly, studies have also shown how the effects of smoking can also persist across generations. A recent study by Watkins et al. (2022) suggested that the effects of grandmaternal smoking on offspring DNA methylation (DNAm) are unlikely to result solely from inherited DNA damage or stable epigenetic marks [48]. Rather, they proposed a mechanism of transcription factor-binding events shielding DNAm from being modified in early development or inducing DNAm changes consistent with ancestral smoking, as DNAm status can be restored by transcriptional factors during germline and embryonic development following erasure [48]. Furthermore, other societal and parental factors may be involved when looking at transgenerational influences. The observed enrichment of smoking-associated DNAm sites in males via the paternal line points to more complex mechanisms, such as sex-specific epigenetic reprogramming.
Mechanistically, cigarette smoke has been shown to modulate DNA methyltransferase 1 (DNMT1) expression and activity, potentially altering methylation patterns during critical windows of development. It can also induce double-stranded DNA breaks, recruiting DNMT1 to repair sites, and activate DNA-binding proteins like Sp1, which protect CpG sites from de novo methylation. During prenatal exposure, smoke-induced hypoxia may further influence DNAm by altering methyl group availability. These pathways highlight that DNAm is one part of a broader landscape of molecular responses (see our comment on transcription factor binding and parental influences), suggesting that multiple mechanisms, beyond direct epigenetic inheritance, may underlie the observed effects.
To highlight the potential persistent effect across generations, a recent study examining infertility rates in human adult offspring showed no clear link between prenatal exposure to maternal, paternal, or parental smoking and infertility in adult offspring. However, the limitations of this study were that it only focused on assisted reproductive technology (ART) treatments and non-ART treatments [49]. This study was not included in the current review, as it was published after the March 18th search date and included IVF outcomes.
Although cancer-specific outcomes were outside the scope of this review, a recent systematic review in the field did not provide evidence of an association between maternal exposure to cigarette smoke and risk of testicular cancer in offspring [50].
With the rise of e-cigarettes and vaping, future research should also address these emerging exposures. For example, in a recent study in cultured 5-week-old rat ovaries, nicotine had limited impact on the ovary, while in e-flavored e-cigarettes caused significant ovarian morphological damage, disruption of oxidative balance, and promotion of apoptosis [51].
There is a lack of mechanistic studies investigating how smoking affects the development of the testis and ovary, and how these effects manifest later in adulthood. Future studies should examine the combined influence of both parents, as dual-parental exposure may have a more significant impact on offspring reproductive and overall health across generations, along with the mechanisms involved.
A key strength of this review is its rigorous inclusion criteria, which ensured high-quality studies with clear exposure and outcome data. However, notable limitations include variability in smoking doses, reliance on self-reported data, and the exclusion of non-English studies and those published before 2000. These factors hindered the ability to perform robust meta-analyses and may have limited the breadth of findings. Additionally, small sample sizes and methodological inconsistencies in animal studies reduced their generalizability to human populations. Furthermore, while animal models provide mechanistic insights, challenges such as dose standardization and interspecies variability limit their translational relevance. Another limitation is the marked difference in reproductive and developmental physiologies between rodents and humans, particularly in hormonal regulation and placental development during pregnancy.
5. Conclusions
In conclusion, this systematic review shows the adverse effects of parental cigarette smoke exposure on offspring reproductive health as influenced by sex and dose of exposure. Maternal smoking affects impaired semen quality, hormonal imbalance, and disruption of gonadal development in male offspring, and paternal smoking affects both male and female fertility, genetically and epigenetically. Second-hand smoke from both parents escalated these risks, showing an adverse interaction effect of parental smoking. From this perspective, it can be agreed that although the results of the given studies are conclusive, there was appreciable heterogeneity concerning study designs and smoking dose definitions, providing the basis for standardized research and specific, evidence-based public health initiatives. The following topics and issues that should be further examined include the use of new tobacco products (i.e., e-cigarettes), the methods of assessing exposure to tobacco products, and the effects of tobacco use on the multigenerational span.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijtm5030034/s1. Supplement Tables.
Author Contributions
L.C.F., A.W., V.J., A.T. and Y.A. planned the systematic review design and reviewed the manuscript. Y.A. undertook the systematic review, created the scoring system, and wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The Ministry of Science and Education of Republic of Azerbaijan; Implementation Unit for “State Programme 2019-2023” Cohort.
Data Availability Statement
The original contributions presented in the study are included in the article, and further inquiries can be directed to the corresponding author.
Acknowledgments
The University of Nottingham provided access to online libraries and services. We also wish to thank Abdelaziz Abdelaal who validated the screening of publications.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| Abbreviation | Full Form |
| WHO | World Health Organization |
| ART | Assisted Reproductive Technology |
| PAHs | Polycyclic Aromatic Hydrocarbons |
| DOHaD | Developmental Origins of Health and Disease |
| AGD | Anogenital Distance |
| ETS | Environmental Tobacco Smoke |
| SHBG | Sex Hormone-Binding Globulin |
| SD | Standard Deviation |
| IVF | In Vitro Fertilization |
References
- Rosen, L.J.; Lev, E.; Guttman, N.; Tillinger, E.; Rosenblat, S.; Zucker, D.M.; Myers, V. Parental Perceptions and Misconceptions of Child Tobacco Smoke Exposure. Nicotine Tob. Res. 2018, 20, 1369–1377. [Google Scholar] [CrossRef]
- Bruin, J.E.; Gerstein, H.C.; Holloway, A.C. Long-term consequences of fetal and neonatal nicotine exposure: A critical review. Toxicol. Sci. 2010, 116, 364–374. [Google Scholar] [CrossRef]
- Deierlein, A.L.; Sun, Y.; Prado, G.; Stein, C.R. Socioeconomic Characteristics, Lifestyle Behaviors, and Health Conditions Among Males of Reproductive Age With and Without Disabilities, NHANES 2013–2018. Am. J. Mens. Health 2023, 17, 15579883221138190. [Google Scholar] [CrossRef]
- Falkner, B. Maternal and gestational influences on childhood blood pressure. Pediatr. Nephrol. 2020, 35, 1409–1418. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y. Adult-Onset Diseases in Low Birth Weight Infants: Association with Adipose Tissue Maldevelopment. J. Atheroscler. Thromb. 2020, 27, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Giannopoulos, A.S.; Giannakou, L.; Gourgoulianni, N.; Pitaraki, E.; Jagirdar, R.; Marnas, P.; Tzamalas, P.I.; Rouka, E.; Livanou, E.; Hatzoglou, C.; et al. The effect of cigarette smoke extract exposure on the size and sexual behaviour of Drosophila melanogaster. Environ. Toxicol. Pharmacol. 2023, 104, 104325. [Google Scholar] [CrossRef]
- Stefansdottir, A.; Marečková, M.; Matkovic, M.; Allen, C.M.; Spears, N. In vitro exposure to benzo[a]pyrene damages the developing mouse ovary. Reprod. Fertil. 2023, 4, e220071. [Google Scholar] [CrossRef] [PubMed]
- Gutvirtz, G.; Sheiner, E. Airway pollution and smoking in reproductive health. Best Pract. Res. Clin. Obstet. Gynaecol. 2022, 85, 81–93. [Google Scholar] [CrossRef]
- Kilic, S.; Yuksel, B.; Lortlar, N.; Sertyel, S.; Aksu, T.; Batioglu, S. Environmental tobacco smoke exposure during intrauterine period promotes granulosa cell apoptosis: A prospective, randomized study. J. Matern. Fetal Neonatal Med. 2012, 25, 1904–1908. [Google Scholar] [CrossRef]
- Kurti, A.N.; Bunn, J.Y.; Nighbor, T.; Cohen, A.H.; Bolívar, H.; Tang, K.J.; Dallery, J.; Higgins, S.T. Leveraging technology to address the problem of cigarette smoking among women of reproductive age. Prev. Med. 2019, 118, 238–242. [Google Scholar] [CrossRef]
- Jennings, K.J.; de Lecea, L. Neural and Hormonal Control of Sexual Behavior. Endocrinology 2020, 161, bqaa150. [Google Scholar] [CrossRef]
- Ramlau-Hansen, C.H.; Thulstrup, A.M.; Storgaard, L.; Toft, G.; Olsen, J.; Bonde, J.P. Is Prenatal Exposure to Tobacco Smoking a Cause of Poor Semen Quality? A Follow-up Study. Am. J. Epidemiol. 2007, 165, 1372–1379. [Google Scholar] [CrossRef]
- Ravnborg, T.L.; Jensen, T.K.; Andersson, A.-M.; Toppari, J.; Skakkebæk, N.E.; Jørgensen, N. Prenatal and adult exposures to smoking are associated with adverse effects on reproductive hormones, semen quality, final height and body mass index. Hum. Reprod. 2011, 26, 1000–1011. [Google Scholar] [CrossRef]
- Jensen, T.K.; Jørgensen, N.; Punab, M.; Haugen, T.B.; Suominen, J.; Zilaitiene, B.; Horte, A.; Andersen, A.-G.; Carlsen, E.; Magnus, Ø.; et al. Association of In Utero Exposure to Maternal Smoking with Reduced Semen Quality and Testis Size in Adulthood: A Cross-Sectional Study of 1,770 Young Men from the General Population in Five European Countries. Am. J. Epidemiol. 2004, 159, 49–58. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Adamcová, K.; Kolátorová, L.; Chlupáčová, T.; Šimková, M.; Jandíková, H.; Pařízek, A.; Stárka, L.; Dušková, M. Changes to fetal steroidogenesis caused by maternal smoking. Physiol. Res. 2017, 66, S375–S386. [Google Scholar] [CrossRef]
- Ramlau-Hansen, C.H.; Thulstrup, A.M.; Olsen, J.; Ernst, E.; Andersen, C.Y.; Bonde, J.P. Maternal smoking in pregnancy and reproductive hormones in adult sons. Int. J. Androl. 2008, 31, 565–572. [Google Scholar] [CrossRef]
- Zhang, B.; Shi, H.; Wang, Q.; Zhang, Z.; Li, M. Maternal Passive Smoking During Pregnancy and Age of Menarche in Daughters:A Study of Elementary and Middle School Students in Shanghai. Asia Pac. J. Public Health 2015, 27, 14S–20S. [Google Scholar] [CrossRef]
- Erdem Guzel, E.; Kaya, N.; Tektemur, A.; Ulker, N.; Yardimci, A.; Akkoc, R.F.; Canpolat, S.; Ozan, I.E. Chronic effects of maternal tobacco-smoke exposure and/or α-lipoic acid treatment on reproductive parameters in female rat offspring. Syst. Biol. Reprod. Med. 2020, 66, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Brix, N.; Ernst, A.; Lauridsen, L.L.B.; Parner, E.T.; Olsen, J.; Henriksen, T.B.; Ramlau-Hansen, C.H. Maternal Smoking During Pregnancy and Timing of Puberty in Sons and Daughters: A Population-Based Cohort Study. Am. J. Epidemiol. 2019, 188, 47–56. [Google Scholar] [CrossRef]
- Fowler, P.A.; Bhattacharya, S.; Flannigan, S.; Drake, A.J.; O’Shaughnessy, P.J. Maternal cigarette smoking and effects on androgen action in male offspring: Unexpected effects on second-trimester anogenital distance. J. Clin. Endocrinol. Metab. 2011, 96, E1502–E1506. [Google Scholar] [CrossRef] [PubMed]
- Kızılay, D.Ö.; Aydın, C.; Aygün, A.P.; Tuhan, H.Ü.; Olukman, Ö. Prenatal smoke exposure is associated with increased anogenital distance in female infants: A prospective case–control study. J. Pediatr. Endocrinol. Metab. 2021, 34, 79–88. [Google Scholar] [CrossRef]
- Cirillo, P.M.; Cohn, B.A.; Krigbaum, N.Y.; Lee, M.; Brazil, C.; Factor-Litvak, P. Effect of maternal coffee, smoking and drinking behavior on adult son’s semen quality: Prospective evidence from the Child Health and Development Studies. J. Dev. Orig. Health Dis. 2011, 2, 375–386. [Google Scholar] [CrossRef]
- Brix, N.; Ernst, A.; Lauridsen, L.L.B.; Parner, E.T.; Arah, O.A.; Olsen, J.; Henriksen, T.B.; Ramlau-Hansen, C.H. Maternal pre-pregnancy body mass index, smoking in pregnancy, and alcohol intake in pregnancy in relation to pubertal timing in the children. BMC Pediatr. 2019, 19, 338. [Google Scholar] [CrossRef] [PubMed]
- Fowler, P.A.; Bhattacharya, S.; Gromoll, J.; Monteiro, A.; O’Shaughnessy, P.J. Maternal smoking and developmental changes in luteinizing hormone (LH) and the LH receptor in the fetal testis. J. Clin. Endocrinol. Metab. 2009, 94, 4688–4695. [Google Scholar] [CrossRef]
- Gollenberg, A.L.; Addo, O.Y.; Zhang, Z.; Hediger, M.L.; Himes, J.H.; Lee, P.A. In utero exposure to cigarette smoking, environmental tobacco smoke and reproductive hormones in US girls approaching puberty. Horm. Res. Paediatr. 2015, 83, 36–44. [Google Scholar] [CrossRef]
- Gordon, S.S.; Dhanraj, D.N.; Ganga Devaiah, C.; Lambers, D.S. A Pilot Study of Exposure to Nicotine in Human Pregnancy and Maternal and Fetal Testosterone Levels at Birth. Reprod. Sci. 2022, 29, 3254–3259. [Google Scholar] [CrossRef]
- Hærvig, K.K.; Petersen, K.U.; Giwercman, A.; Hougaard, K.S.; Høyer, B.B.; Lindh, C.; Ramlau-Hansen, C.H.; Nybo Andersen, A.-M.; Toft, G.; Bonde, J.P.; et al. Fetal exposure to maternal cigarette smoking and male reproductive function in young adulthood. Eur. J. Epidemiol. 2022, 37, 525–538. [Google Scholar] [CrossRef] [PubMed]
- Lindbo, D.; Arendt, L.H.; Ernst, A.; Lunddorf, L.L.H.; Brix, N.; Ramlau-Hansen, C.H. Maternal Cigarette Smoking During Pregnancy and Genital Anomalies in Boys: A Register-Based Cohort and Sibling-Matched Design Study. Clin. Epidemiol. 2022, 14, 901–910. [Google Scholar] [CrossRef]
- Lutterodt, M.C.; Sørensen, K.P.; Larsen, K.B.; Skouby, S.O.; Andersen, C.Y.; Byskov, A.G. The number of oogonia and somatic cells in the human female embryo and fetus in relation to whether or not exposed to maternal cigarette smoking. Hum. Reprod. 2009, 24, 2558–2566. [Google Scholar] [CrossRef] [PubMed]
- Mamsen, L.S.; Lutterodt, M.C.; Andersen, E.W.; Skouby, S.O.; Sørensen, K.P.; Andersen, C.Y.; Byskov, A.G. Cigarette smoking during early pregnancy reduces the number of embryonic germ and somatic cells. Hum. Reprod. 2010, 25, 2755–2761. [Google Scholar] [CrossRef]
- Hærvig, K.K.; Petersen, K.U.; Dornfeldt, M.M.; Bonde, J.P.; Hougaard, K.S.; Ramlau-Hansen, C.H.; Toft, G.; Lindh, C.; Giwercman, A.; Tøttenborg, S.S. Paternal pre-conceptional smoking and semen quality in the adult son. Andrology 2025, 13, 82–88. [Google Scholar] [CrossRef]
- Ernst, A.; Kristensen, S.L.; Toft, G.; Thulstrup, A.M.; Håkonsen, L.B.; Olsen, S.F.; Ramlau-Hansen, C.H. Maternal smoking during pregnancy and reproductive health of daughters: A follow-up study spanning two decades. Hum. Reprod. 2012, 27, 3593–3600. [Google Scholar] [CrossRef]
- Fukuda, M.; Fukuda, K.; Shimizu, T.; Nobunaga, M.; Andersen, E.W.; Byskov, A.G.; Yding Andersen, C. Paternal smoking habits affect the reproductive life span of daughters. Fertil. Steril. 2011, 95, 2542–2544. [Google Scholar] [CrossRef] [PubMed]
- Hærvig, K.K.; Høyer, B.B.; Giwercman, A.; Hougaard, K.S.; Ramlau-Hansen, C.H.; Specht, I.O.; Toft, G.; Bonde, J.P.; Søgaard Tøttenborg, S. Fetal exposure to paternal smoking and semen quality in the adult son. Andrology 2020, 8, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- Pabarja, A.; Ganjalikhan Hakemi, S.; Musanejad, E.; Ezzatabadipour, M.; Nematollahi-Mahani, S.N.; Afgar, A.; Afarinesh, M.R.; Haghpanah, T. Genetic and epigenetic modifications of F1 offspring’s sperm cells following in utero and lactational combined exposure to nicotine and ethanol. Sci. Rep. 2021, 11, 12311. [Google Scholar] [CrossRef]
- Axelsson, J.; Rylander, L.; Rignell-Hydbom, A.; Silfver, K.Å.; Stenqvist, A.; Giwercman, A. The Impact of Paternal and Maternal Smoking on Semen Quality of Adolescent Men. PLoS ONE 2013, 8, e66766. [Google Scholar] [CrossRef]
- Axelsson, J.; Sabra, S.; Rylander, L.; Rignell-Hydbom, A.; Lindh, C.H.; Giwercman, A. Association between paternal smoking at the time of pregnancy and the semen quality in sons. PLoS ONE 2018, 13, e0207221. [Google Scholar] [CrossRef]
- Camlin, N.J.; Sobinoff, A.P.; Sutherland, J.M.; Beckett, E.L.; Jarnicki, A.G.; Vanders, R.L.; Hansbro, P.M.; McLaughlin, E.A.; Holt, J.E. Maternal Smoke Exposure Impairs the Long-Term Fertility of Female Offspring in a Murine Model1. Biol. Reprod. 2016, 94, 39. [Google Scholar] [CrossRef]
- Al-Sawalha, N.A.; Pokkunuri, I.D.; Alzoubi, K.H.; Khabour, O.F.; Almomani, B.N. Waterpipe Tobacco Smoke Exposure during Lactation—Susceptibility of Reproductive Hormones and Oxidative Stress Parameters in Male Progeny Rats. Reprod. Sci. 2021, 28, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Gyekis, J.; Anthony, K.; Foreman, J.E.; Klein, L.C.; Vandenbergh, D.J. Perinatal nicotine exposure delays genital development in mice. Reprod. Toxicol. 2010, 29, 378–380. [Google Scholar] [CrossRef]
- Oyeyipo, I.P.; Adeyemi, D.H.; Abe, T.R. Testosterone and testicular changes in F1 offspring of Wistar rats maternally exposed to nicotine during gestation. JBRA Assist. Reprod. 2018, 22, 162–166. [Google Scholar] [CrossRef]
- Lin, I.H.; Yang, L.; Dalley, J.W.; Tsai, T.-H. Trans-placental transfer of nicotine: Modulation by organic cation transporters. Biomed. Pharmacother. 2022, 145, 112489. [Google Scholar] [CrossRef]
- Dior, U.P.; Lawrence, G.M.; Sitlani, C.; Enquobahrie, D.; Manor, O.; Siscovick, D.S.; Friedlander, Y.; Hochner, H. Parental smoking during pregnancy and offspring cardio-metabolic risk factors at ages 17 and 32. Atherosclerosis 2014, 235, 430–437. [Google Scholar] [CrossRef]
- Alves, J.; Perelman, J.; Ramos, E.; Kunst, A.E. Intergenerational transmission of parental smoking: When are offspring most vulnerable? Eur. J. Public Health 2022, 32, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Finger, B.J.; Harvey, A.J.; Green, M.P.; Gardner, D.K. Combined parental obesity negatively impacts preimplantation mouse embryo development, kinetics, morphology and metabolism. Hum. Reprod. 2015, 30, 2084–2096. [Google Scholar] [CrossRef] [PubMed]
- McPherson, N.O.; Bell, V.G.; Zander-Fox, D.L.; Fullston, T.; Wu, L.L.; Robker, R.L.; Lane, M. When two obese parents are worse than one! Impacts on embryo and fetal development. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E568–E581. [Google Scholar] [CrossRef]
- Ornellas, F.; Souza-Mello, V.; Mandarim-de-Lacerda, C.A.; Aguila, M.B. Programming of Obesity and Comorbidities in the Progeny: Lessons from a Model of Diet-Induced Obese Parents. PLoS ONE 2015, 10, e0124737. [Google Scholar] [CrossRef] [PubMed]
- Watkins, S.H.; Iles-Caven, Y.; Pembrey, M.; Golding, J.; Suderman, M. Grandmaternal smoking during pregnancy is associated with differential DNA methylation in peripheral blood of their grandchildren. Eur. J. Hum. Genet. 2022, 30, 1373–1379. [Google Scholar] [CrossRef]
- Jørgensen Langergaard, M.; Ernst, A.; Håberg, S.E.; Ramlau-Hansen, C. O-035 Prenatal exposure to parental smoking and infertility in sons and daughters: A cohort study. Hum. Reprod. 2025, 40 (Suppl. S1), deaf097.035. [Google Scholar] [CrossRef]
- Beck, A.L.; Bräuner, E.V.; Hauser, R.; Lim, Y.H.; Uldbjerg, C.S.; Juul, A. Maternal Exposure to Cigarette Smoke during Pregnancy and Testicular Cancer in Offspring: A Systematic Review and Meta-Analysis. Life 2023, 13, 618. [Google Scholar] [CrossRef]
- Chen, T.; Wu, M.; Dong, Y.; Ren, H.; Wang, M.; Kong, B.; Cai, Y.; Hei, C.; Wu, K.; Zhao, C.; et al. Ovarian toxicity of e-cigarette liquids: Effects of components and high and low nicotine concentration e-cigarette liquid in vitro. Tob. Induc. Dis. 2023, 21, 128. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).