Abstract
Gastrointestinal (GI) malignancies are diverse and particularly challenging in terms of current immunotherapy but hold great opportunity for impact given that they constitute the highest cancer incidence and mortality rates worldwide. Traditional treatment options for solid GI malignancies include surgical intervention, chemotherapy, radiation, or a combination of these treatments. Emerging modalities within immunotherapy are anticipated to extend the results with conventional therapy by stimulating the patient’s own intrinsic potential for tumor-specific immunologic rejection. Combination regimens of chemotherapy and tumor-infiltrating lymphocyte (TIL) therapy in advanced colorectal cancer and pancreatic cancer, autologous monocyte therapy in advanced gastric cancer, and CAR-T therapy trained against GI-selective tumor antigens such as carcinoembryonic antigen are currently being studied. Clinical trials are underway to study the combination of various chemotherapeutic agents along with immunotherapy in the management of cholangiocarcinoma, hepatocellular carcinoma, and esophageal cancer. Alternative therapies are needed based on the tumor immune microenvironment, which can lead to a personalized approach to treatment. In this review, we discuss the current status of various modalities of immunotherapy in common GI malignancies, along with their mechanisms of immune activation and cancer suppression. We will also discuss the use of immunotherapy in less common solid GI malignancies and touch on recent advancements and clinical trials.
1. Introduction
The concept of cancer immune surveillance was first introduced in 1967 by Burnet [1], based on the hypothesis that the immune system can suppress progression or development of spontaneous malignancies. Naturally occurring T lymphocytes that infiltrate tumor tissue are, under the right circumstances, capable of recognition and destruction of cancer cells [2,3,4]. Cancer cells can evade immune destruction by inhibiting antigen presentation, cytokine production, and cytotoxicity by antigen-presenting cells and CD4+ and CD8+ T lymphocytes [2,4]. As such, an increased number of intra-tumoral T cells is associated with better clinical outcomes and improved survival [3]. This led to great interest in the development of drugs designed to enhance the intrinsic potential of a patient’s immune system to mount tumor-specific rejection [5].
Immunotherapy is broadly defined as any biologic or targeted agent that increases or restores the immune system’s ability to detect and destroy cancer cells [6]. Immunotherapy includes agents with a broad range of mechanisms of action. These include but are not limited to vaccination with tumor antigens, cytokine therapy, stimulation of antigen presentation, and enhancement of antitumor T cell function [5,7,8,9]. Adoptive cell transfer (ACT) is a cell-based therapy that uses reinfused natural killer (NK) cells, TILs, activated peripheral blood mononuclear cells, chimeric antigen receptor (CAR) T cells, or cytotoxic T-lymphocytes (CTLs) to target tumor cells [7,10]. Tumor-infiltrating lymphocyte (TIL) therapy achieved success in the management of several metastatic malignancies, specifically melanoma, where the FDA approved the first TIL product in February 2024 [11]. CAR-T therapy achieved dramatic success in B-cell malignancies, but its utility in solid tumors remains elusive due to the extensive risk of off-target toxicity [12].
The advancements in immunotherapy have, to date, been most dramatic in immunogenic tumors such as melanoma, or in liquid tumors such as B-cell leukemia where lineage-specific antigenic targets are well-defined. In GI cancers, only modest efficacy has been achieved with ICI therapy, both in upper GI malignancies, such as gastroesophageal cancers, and in microsatellite-unstable colorectal cancers. Overall, however, most GI malignancies are not highly immunogenic or lack tumor-specific antigenic targets, rendering the development of rational immunotherapies to be extremely challenging.
In this review, we will discuss the current use of existing immunotherapeutic approaches in GI malignancies, their targets, and their use in combination therapy. We will also discuss emerging technologies and clinical trials involving immunotherapy in GI malignancies, focusing on ACT regimens. Figure 1 describes the immunotherapy agents mentioned in this manuscript.
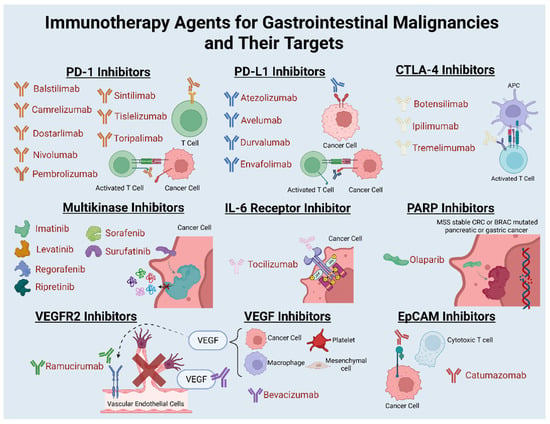
Figure 1.
Immunotherapy agents for gastrointestinal malignancies and their targets. Abbreviations: PD-1: programmed cell death protein 1; PD-L1: programmed death-ligand 1; CTLA: cytotoxic T-lymphocyte-associated protein; IL: interleukin; PARP: poly(ADP-ribose) polymerase; VEGF: vascular endothelial growth factor; VEGFR: vascular endothelial growth factor receptor; and EpCAM: epithelial cell adhesion molecule.
2. Immune Checkpoint Inhibition in Gastrointestinal Malignancies
2.1. Esophageal Cancer
Esophageal cancer has a poor prognosis, with an overall five-year survival rate of 20% [6,13]. Current treatment regimens are anchored by surgery and perioperative chemotherapy based on data from clinical trials, including the MAGIC and FLOT-4 trials. The MAGIC trial demonstrated decreased tumor size, decreased stage, and improved progression-free survival and overall survival in patients with operable gastric or lower esophageal adenocarcinoma treated with perioperative chemotherapy [14,15]. The FLOT4 randomized phase 2/3 trial demonstrated that perioperative 5-fluorouracil (5-FU), leucovorin, oxaliplatin (FOLFOX), and docetaxel improved overall survival as compared to the perioperative regimen used in the MAGIC trial [15]. However, responses to standard therapies are poor, and there remains intense interest in utilizing immunotherapy as a potential treatment option in advanced or recurrent cases.
Immune checkpoint inhibition has been extensively studied in esophageal cancer. The CHECKMATE 577 phase 3 clinical trial included patients with esophageal squamous cell carcinomas or adenocarcinomas who were treated with nivolumab, a monoclonal PD-1 antibody, as adjuvant therapy. Patients with R0 stage II or stage III esophageal or gastroesophageal junction cancer who received neoadjuvant chemoradiation and had residual pathological disease were included. Compared to the placebo group, patients who received adjuvant nivolumab had longer disease-free survival (DFS) [16]. Janjigian et al. demonstrated in the randomized phase 3 CheckMate 649 clinical trial that nivolumab plus chemotherapy versus chemotherapy alone led to prolonged overall survival and progression-free survival [17]. In the KEYNOTE-590 trial, patients with previously untreated, locally advanced, or metastatic esophageal cancer were randomized to receive pembrolizumab with or without chemotherapy. Pembrolizumab with chemotherapy was superior to placebo for progression-free survival [18]. With these studies, nivolumab or pembrolizumab plus chemotherapy were granted FDA approval for specific subsets of esophageal cancer patients and are recommended as first-line treatment in untreated and unresectable patients with esophageal adenocarcinoma [17,19]. This further demonstrates that the cytotoxic effects of chemotherapy may sensitize tumors to ICIs, thereby illustrating a benefit of combination therapy [20,21].
2.2. Gastric Cancer
Curative resection with or without chemotherapy is the gold standard for treatment of gastric cancer, and many recommendations for immunotherapy are extrapolated from trials focusing on esophageal or GE junction cancer. In cohort 1 of the KEYNOTE-059 study, patients with advanced gastric cancer treated with pembrolizumab alone had an overall response rate of 11.6% [22,23,24]. In cohort 2, patients received pembrolizumab, cisplatin, and 5-fluorouracil. The overall response rate was 60% with a complete response in one patient [25].
The ATTRACTION-2 randomized phase 3 trial studied nivolumab versus placebo in patients with advanced gastric cancer who had previously been treated with at least two chemotherapy regimens. The primary endpoint was overall survival. Median overall survival was 5.26 months in the nivolumab group and 4.14 months in the placebo group [26]. When combined with ipilimumab, objective response and 12-month progression-free survival rates were 24% and 35%, respectively, in patients with metastatic and chemotherapy-resistant gastric cancer [27]. Ongoing studies are in progress to further delineate the role of ICIs in the treatment of gastric cancer.
2.3. Pancreatic Cancer
Immune checkpoint inhibition has been largely disappointing in pancreatic ductal adenocarcinoma (PDAC) due to its immunosuppressive microenvironment, scant T cell infiltration, and limited neoantigen load [20,28]. These factors rendered ICI therapy ineffective, particularly when used as monotherapy [20,29]. In preclinical studies, however, immunotherapy has been associated with improved overall survival and tumor regression in PDAC when used in combination with other treatment regimens, raising hope that rationally selected combination regimens may be beneficial in patients. For example, neoadjuvant immunotherapy may increase the presence of TILs in the pancreatic tumor microenvironment, thus reducing the immunosuppressive effects of surgery with systemic release of glucocorticoids that induce apoptosis of naïve T cells and suppression of T-cell proliferation [30].
PDAC tumors with high microsatellite instability (MSI) due to mismatch repair enzyme (MMR) deficiency respond well to immunotherapy [28,31]. Le et al., hypothesized that ICIs induce peripheral expansion of tumor-specific T cells and that MMR-deficient tumors have functional mutation-associated neoantigen (MANA)-specific T cells. They studied the efficacy of PD-1 blockade with pembrolizumab in MMR-deficient solid tumors. Out of eight patients with PDAC, objective response to treatment was seen in five patients, with complete response in two of these individuals [31]. This led to FDA approval of pembrolizumab for high MSI and MMR-deficient tumors [28,29,31]. However, only a small percentage of patients with PDAC [20,28,29] exhibit MSI phenotype, limiting the efficacy of ICI in this disease.
2.4. Colorectal Cancer
Lymphocytic infiltration in CRC has been associated with improved prognosis [32]. MMR-deficient tumors have elevated levels of DNA microsatellite instability, which leads to overexpression of genes specific to CTLs. The high mutational burden in these tumors generate antigens that can be recognized as foreign, and therefore mount an immune response [2,9]. High MSI tumors make up approximately 6% of advanced-stage CRCs, and these tumors demonstrate upregulation of immune checkpoint proteins such as PD-1 and programmed death ligand (PD-L1) that permit immune evasion by TILs [33].
The benefit of immunotherapy in MMR-deficient and high-MSI tumors has been established in several clinical trials, for example, in a phase 2 clinical trial including stage IV CRC patients with or without MMR deficiency by Le et al. Patients treated with pembrolizumab every 14 days showed an objective response rate of 40% compared to 0% in MMR-proficient patients [31,34]. The results of these studies led to FDA approval of pembrolizumab for patients with advanced CRC who progressed through standard chemotherapy. CheckMate 142 was a multicenter, phase 2 study that included MMR-deficient and MMR-proficient patients with CRC. Patients received nivolumab monotherapy until disease progression, death, or unacceptable toxicity. Immunotherapy was more beneficial in those with MMR-deficiency or high MSI tumors, with an objective response rate of 31%, and 68% disease control at 12 weeks [35] leading to FDA approval in 2017 for nivolumab in these patients. The combination of nivolumab plus ipilimumab was subsequently evaluated in 119 patients. The overall response rate was 55% at 13.4 months with a disease control rate greater than 80% at 12 weeks [36]. The combination regimen was granted FDA approval in 2018 as first-line therapy in MSI-high and MMR-deficient metastatic CRC patients [9,33].
Single-agent immunotherapy has also been studied in rectal cancer. In a prospective phase 2 study, dostarlimab, an anti-PD-1 monoclonal antibody, was given every 3 weeks for 6 months to patients with MMR-deficient stage II or stage III rectal cancer. Standard chemoradiation and surgery were then performed. Patients with complete clinical response after completing dostarlimab continued without chemoradiation or surgery. Twelve patients completed treatment with dostarlimab, with 100% demonstrating a complete clinical response after at least six months of follow-up. Although longer follow-up is needed, these results indicate that MMR-deficient locally advanced rectal cancer is sensitive to single-agent dostarlimab [37].
2.5. Hepatocellular Carcinoma
Hepatocellular carcinoma (HCC) is the most common liver cancer, and the first-generation tyrosine-kinase inhibitor (TKI) sorafenib remains standard therapy in patients with advanced disease and acceptable hepatic function [38]. HCC is a biologically complex malignancy, with multiple candidate pathways for immunotherapy. The CheckMate 040 study was a phase 1/2 trial of nivolumab in adults with advanced HCC with or without hepatitis B or C. Patients previously treated with agents targeting T-cell costimulation or checkpoint pathways were excluded, but prior treatment with sorafenib was acceptable. Patients received intravenous nivolumab 0.1–10 mg/kg every 2 weeks in a dose-escalation design. In the dose-expansion phase, patients received nivolumab 3 mg/kg every 2 weeks in four cohorts: patients without hepatitis who were intolerant or not previously treated with sorafenib, patients who progressed on sorafenib without hepatitis, those with hepatitis B infection, and those with hepatitis C infection. The objective response rate was 20% in the dose-expansion cohort and 15% in the dose-escalation phase [39]. These findings led to FDA approval of nivolumab for HCC in patients with disease progression or treatment-limiting sides effects on sorafenib [40].
Yau et al., in the phase 3 CheckMate 459 randomized, multicenter trial, compared nivolumab versus sorafenib in advanced HCC. They observed a favorable safety profile for nivolumab. However, nivolumab did not improve overall survival as compared to sorafenib [41]. As a result of this pivotal study, nivolumab as a single agent may be considered as first-line therapy in patients with Child-Pugh A or B liver disease who have contraindications to sorafenib [40,41].
Finn et al., in the phase 3 IMbrave150 randomized study, compared combination therapy with the PDL-1 inhibitor atezolizumab and the vascular endothelial growth factor (VEGF) inhibitor bevacizumab and observed improved overall survival (OS) and progression-free survival (PFS) in patients with unresectable HCC compared to sorafenib [42]. Sangro et al., in the phase 3 HIMALAYA study showed that in unresectable HCC, STRIDE (Single Tremelimumab Regular Interval Durvalumab) significantly improved OS as compared to sorafenib [43]. These two trials confirm that dual immunotherapy with biological agents has positive and beneficial outcomes in unresectable HCC.
2.6. Biliary Tract Cancers
Cisplatin plus gemcitabine is standard first-line therapy for metastatic cholangiocarcinoma, while radical surgery is the mainstay of treatment for resectable cholangiocarcinoma. Despite recent advances in surgical and systemic therapy, recurrence rates are high, leading investigators to explore the use of immunotherapy in biliary tract cancers [44]. The global TOPAZ-1 study was a double-blind phase 3 study in which patients who had not been treated for unresectable locally advanced, recurrent, or metastatic biliary tract cancer were randomized to receive 1500 mg durvalumab, an anti-PD-L1 antibody, every 3 weeks or placebo plus gemcitabine/cisplatin on days 1 and 8 every 3 weeks for up to 8 cycles, followed by durvalumab or placebo until disease progression or unacceptable toxicity. The combination of durvalumab plus gemcitabine/cisplatin significantly improved overall survival versus placebo plus gemcitabine/cisplatin. PFS was also significantly improved with durvalumab versus placebo. The objective response rate was 26.7% with durvalumab and 18.7% with placebo [45]. In September 2022, durvalumab gained FDA approval when combined with chemotherapy as first-line treatment in advanced biliary tract cancer and is now included in NCCN guidelines [13,46]. The recently completed DurGAP trial (NCT05640791) demonstrated promising overall response and disease control rates with neoadjuvant durvalumab combined with gemcitabine/cisplatin and Nab-paclitaxel [47].
Treatment with pembrolizumab as monotherapy has been used to successfully treat high-MSI cholangiocarcinoma in several case reports. All patients had disease recurrence after standard chemotherapy and/or surgery [48,49,50,51]. In the KEYNOTE-028 and KEYNOTE-158 basket studies, patients with incurable biliary tract cancer that progressed after prior standard treatment regimens (without immunotherapy) were given pembrolizumab 10 mg/kg every two weeks or 200 mg every three weeks, respectively. The primary endpoint was the overall response rate in both studies. All patients in KEYNOTE-028 had PD-L1-positive tumors whereas only 61 had PD-L1-positive tumors in KEYNOTE-158. The 12-month overall survival rate was 32.7% in KEYNOTE-158 and 27.6% in KEYNOTE-028. These results emphasize that pembrolizumab provided durable antitumor activity regardless of PD-L1 expression. In KEYNOTE-158, no patients had high-MSI tumors [52]. However, MMR deficiency or high-MSI tumors have been shown to be associated with durable responses to ICIs, and pembrolizumab is approved for previously treated patients with MMR-deficient or high-MSI tumors regardless of histology [53,54,55].
Additional clinical trials included other ICIs in combination with chemotherapy. In the KEYNOTE-966 phase 3 clinical trial, patients with metastatic or unresectable biliary tract cancer without prior systemic therapy were randomized to receive pembrolizumab or placebo added to gemcitabine and cisplatin. The primary endpoint was overall survival. The 24-month overall survival was 24.9% for the pembrolizumab group versus 18.1% for the placebo group, demonstrating a statistically significant and clinically meaningful improvement [56]. There have also been case reports of pembrolizumab, combined with chemotherapy and surgery, used to successfully treat a patient with gallbladder cancer [57].
The IMbrave 151 phase 2 randomized clinical trial was the first to evaluate the combination of immunotherapy, VEGF blockade, and chemotherapy as first-line treatment in advanced biliary tract cancer [58]. Patients were randomized to Arm A (atezolizumab + bevacizumab + gemcitabine/cisplatin) or Arm B (atezolizumab + placebo + gemcitabine/cisplatin). Both combinations had a manageable safety profile [59]. Results indicate only a modest benefit to combining atezolizumab with bevacizumab and chemotherapy [60]. In contrast, Shi et al., combined toripalimab, lenvatinib, and gemcitabine plus oxaliplatin in patients with advanced intrahepatic cholangiocarcinoma. Toripalimab is an anti-PD-1 monoclonal antibody and lenvatinib is a multikinase inhibitor. Thirty patients received gemcitabine and oxaliplatin every three weeks for six cycles along with intravenous toripalimab and oral lenvatinib for one year. The median follow-up time was 23.5 months with an objective response rate of 80%. The median overall survival was 22.5 months with a disease control rate of 93.3%. The results of this trial indicate that the combination of toripalimab, lenvatinib, and gemcitabine/oxaliplatin is a promising first-line treatment option for advanced intrahepatic cholangiocarcinoma [61].
2.7. Gastrointestinal Stromal Tumor
Gastrointestinal stromal tumor (GIST) is the most common digestive mesenchymal tumor and can occur throughout the entire GI tract. Surgical resection and TKIs are the primary management for localized and recurrent or metastatic GISTs, respectively. Imatinib, a KIT- and platelet-derived growth factor receptor α (PDGFRA)-directed TKI, is FDA approved as first-line treatment of GIST. It acts by inhibiting KIT signaling pathways that affect cell proliferation and survival. The efficacy of imatinib is potentiated by its effect on the immune system, partially mediated by its activation and proliferation of CD8+T cells which induce apoptosis of Treg cells and stimulate the production of IFN-γ by NK cells [62,63]. Resistance to treatment is a challenge for patients with advanced GIST and sunitinib and regorafenib have been approved as second- and third-line treatment for GIST, respectively. More recently, ripretinib, a switch-control TKI that inhibits both KIT and PDGFRA kinase through a dual mechanism of action, was FDA approved in patients with advanced GIST who failed previous treatment with three or more TKIs [64].
Due to the high prevalence of resistance to treatment with TKIs, PD-1/PD-L1 blockade has been studied as a promising strategy to improve the effects of TKIs. In a GIST mouse model and in tissue specimens from GIST patients who underwent surgery, Siefert et al., demonstrated that PD-1 was expressed at an increased frequency in intratumoral T cells. In tumor cells treated with either anti-PD-1 or anti-PD-L1 and imatinib, PD-1/PD-L1 blockade enhanced the effects of imatinib by increasing CD8+ T cell function leading to tumor cell apoptosis [65]. These data emphasize the importance of combination immunotherapy in improving the effects of targeted therapy for GIST [62,65].
2.8. Small Bowel Adenocarcinoma
Small bowel adenocarcinomas are typically treated as other GI malignancies with fluoropyrimidine-based chemotherapy [40]. Immunotherapy in small bowel adenocarcinoma targeted microsatellite status and tumor mutational burden [13]. In the phase 2 KEYNOTE-158 study, 233 patients with advanced, pretreated MSI-H, or MMR-deficient GI tumors received pembrolizumab as monotherapy. Of these patients, only nineteen patients had small bowel adenocarcinoma. The objective response rate was 42.1% in those with small bowel cancer versus 34.3% in all patients. Median PFS was 9.1 months in those with small bowel cancer and 4.1 months in all patients, although the study was not powered to evaluate between subgroups [66]. Because small bowel adenocarcinoma has a high rate of microsatellite instability and T-lymphocyte infiltration as compared to other GI cancers, the ZEBRA study sought to determine if pembrolizumab, a PD-1 inhibitor, could induce an antitumor response. Pembrolizumab did not induce the hypothesized response rate, with the observed rate a disappointing 8% [67,68]. Continued research is warranted in this area, as there is currently no FDA-approved ICI for small bowel cancer [40].
2.9. Neuroendocrine Tumors
Neuroendocrine neoplasms (NETs) have a low tumor mutation burden and have a poor response rate to ICIs [40]. However, Klein et al., in the CA209-538 study, demonstrated a response to ipilimumab and nivolumab in patients with high-grade pancreatic NETs and NECs of the gastroesophageal junction and pancreas [69]. A multicenter phase II study combining ipilimumab with nivolumab demonstrated a 26% overall response rate in patients with NECs [70]. In another retrospective study of patients with NECs without a specific location who received at least two previous lines of treatment, the objective response rate was 14.7% [71]. This suggests that a regimen of ipilimumab and nivolumab in NECs that have progressed despite previous chemotherapy may be beneficial [40,71]. The recently completed AveNEC and CABOAveNEC trials (NCT03352934 and NCT05289856) demonstrate that the combination of avelumab and cabozantinib may be an effective option for second-line treatment in these aggressive tumors [72,73].
2.10. Anal Cancer
Anal cancer is a less prevalent GI malignancy that is primarily of squamous cell origin and is commonly associated with human papillomavirus (HPV) [13,40]. Most patients are cured with chemoradiation. However, there is no consensus treatment for those with recurrent metastatic disease [74]. HPV viral proteins E6 and E7 are immunogenic and can activate an anti-tumor host immune response by recruiting TILs [74,75]. Tumor cells express PD-L1, and upon binding the inhibitory receptor on T cells, T cell activation is diminished and hence the anti-tumor immune response is also decreased. In a multicenter phase 2 study in the United States, patients with refractory anal carcinoma were given 3 mg/kg nivolumab, a monoclonal antibody against PD-1, every 2 weeks. Encouragingly, 9 of 37 patients achieved a response, with an overall response rate of 24% and a manageable safety profile [74]. In the KEYNOTE-028 phase 1b trial, 25 patients with advanced anal carcinoma were treated with pembrolizumab, with an overall response rate of 17% [76]. The CORINTH phase 1b/2 trial studied patients with locally advanced anal cancer who received pembrolizumab concomitantly with standard chemoradiation. NCCN guidelines now recommend pembrolizumab or nivolumab as preferred treatment options for patients with metastatic anal cancer who progressed on first-line chemotherapy [77].
2.11. Appendiceal Cancer
There are no current indications for immunotherapy in the management of appendiceal cancer. Standard treatment follows protocols for CRC or are based on retrospective data [78]. However, recent studies indicated that appendiceal cancer has a distinct genomic landscape from CRC, leading to development of preclinical models to study appendiceal cancer [79]. The successful use of pembrolizumab in appendiceal cancer has only been demonstrated in a case report in which the patient was PD-L1 negative [80]. Forsythe et al. used patient tumor organoids (PTO) derived from 26 patients with appendiceal cancer with peritoneal metastasis (PM). The organoids were then treated with either pembrolizumab, ipilimumab, or nivolumab. An immunotherapy response was demonstrated in 21.1% of those treated with pembrolizumab and 10.5% of those treated with nivolumab. There was no response to ipilimumab [81]. In the future, information derived from testing in PTOs may be applied and used in clinical trials for those with appendiceal cancer [81]. A phase II study of nivolumab and ipilimumab in patients with mucinous appendiceal tumors was terminated due to slow enrollment and lack of efficacy (NCT 03693846) [82]. However, an open-label phase IIa clinical trial in which patients with unresectable metastatic appendiceal adenocarcinoma were treated with atezolizumab and bevacizumab demonstrated a 100% disease control rate with a progression-free survival of 18.3 months. Although the overall response rate was not achieved, and the data could not be generalized to patients with poorly differentiated appendiceal adenocarcinoma, and further study of combined PD-L1 and VEGF inhibition is warranted in appendiceal cancer [83].
3. Vaccines as Cancer Therapy
3.1. Esophageal Cancer
Immunotherapy modalities beyond ICI have been explored in esophageal cancer. For instance, an innovative Japanese study assessed vaccines in esophageal cancer patients who were also undergoing radiation and surgery. Fifteen patients with esophageal squamous cell carcinoma were enrolled, and patients received the S-588410 cancer peptide vaccine. Vaccination was shown to increase expression of PD-L1 and CD8+ and CD4+ TILs, leading to antitumor activity and a possible synergistic effect of the vaccine in combination with anti-PD-L1 antibodies in esophageal cancer [84]. However, in a follow-up phase 3, randomized, double-blind, multicenter trial in which patients with lymph node-positive esophageal cancer were treated with S-588410 peptide vaccine alone, there was no difference in relapse-free survival as compared to placebo [85]. This further emphasizes that peptide vaccines may be more beneficial when combined with other types of immunotherapies.
Dendritic cells have been used to create vaccines to treat cancer. Wang et al. conducted an observational cohort study with 40 patients with esophageal cancer. Patients were randomized 2:1, with the experimental group receiving a dendritic cell vaccine combined with adjuvant radiotherapy while the control group received conventional adjuvant radiotherapy. The vaccine was well-tolerated, and the 1- and 2-year survival was improved in patients treated with the vaccine. The 2-year survival in the vaccine group was 67.8% compared to 33.3% in the control group [86].
3.2. Gastric Cancer
Several cancer-testis antigens have been shown to be overexpressed in gastric cancer, such as FoxM1, which has been associated with chemoresistance. Patients with unresectable or recurrent gastric adenocarcinoma who failed standard therapy were treated with a vaccine consisting of five different peptides. No severe adverse events were observed. Patients with a CTL induction tended to have a better prognosis [87]. When the same vaccine was used in combination with S-1 in stage III gastric cancer patients in the adjuvant setting, the combination therapy was feasible and safe. However, further studies are necessary to determine its efficacy [88].
Sundar et al. conducted a phase I/Ib study using a peptide vaccine in patients with advanced gastric cancer. The OTSGC-A24 combined peptide cancer vaccine was administered to patients with inoperable or metastatic gastric cancer with the HLA-A*24:02 haplotype who were refractory to standard therapy. The vaccine was administered in three cohorts of patients with 1 mg of OTSGC-A24 being administered at 3-weekly, 2-weekly, and weekly intervals. A total of 24 patients were enrolled. The most common drug-related toxicity was injection site reactions, and 29% of patients experienced decreased appetite. Positive CTL induction with an antitumor effect was observed in 15 patients. Median progression-free survival and median overall survival was 1.7 months and 5.7 months, respectively [89]. This study demonstrated the safety of the OTSGC-A24 peptide vaccine consistent with other vaccines used in the treatment of solid tumors.
3.3. Pancreatic Cancer
Vaccines are the most commonly studied immunotherapy in PDAC. However, similar to ICIs, they have an insignificant effect when used as monotherapy [90]. In the TeloVac trial, patients with locally advanced or stage IV pancreatic cancer were randomized to receive chemotherapy including capecitabine and gemcitabine, subsequent chemoimmunotherapy, or concurrent chemoimmunotherapy using a human telomerase reverse transcriptase catalytic subunit peptide vaccine (GV1001). The findings are negative, as the addition of GV1001 did not improve overall survival [91]. A follow-up phase 3 trial by Jo et al., demonstrated that GV1001 plus gemcitabine/capecitabine improved overall survival and time to progression as compared to those treated with chemotherapy alone [92].
Vaccine effectiveness has been boosted in several trials by using attenuated or killed bacteria as immune modulators. GVAX is composed of two irradiated, granulocyte-macrophage colony-stimulating factor (GM-CSF)-secreting allogeneic PDAC cell lines given to inhibit regulatory T cells by inducing T cells against PDAC antigens. CRS-207 is a live-attenuated, double-deleted Listeria monocytogenes that has been engineered to secrete mesothelin, a tumor-associated antigen overexpressed in PDAC, into the cytosol of infected antigen-presenting cells. Stage IV PDAC patients who received at least one prior therapy were enrolled in a multicenter, randomized, phase 2 trial with two treatment arms. One arm received two doses of cyclophosphamide/GVAX and four doses of CRS-207 as an immunomodulator. The second treatment arm received six doses of cyclophosphamide/GVAX only. The primary objective was overall survival [93]. Patients treated with the immunomodulator CRS-207 had longer overall survival with minimal toxicity [90,93].
PDACs contain neoantigens that have the potential to stimulate T cells, and long-term PDAC survivors have been shown to mount T cell responses against tumor-specific neoantigens. Rojas et al. tested whether vaccines could stimulate neoantigen-specific T cells in patients with advanced PDAC. In a phase I clinical trial, patients with surgically resectable PDAC received adjuvant atezolizumab, autogene cevumeran (an individualized mRNA neoantigen vaccine), and modified FOLFIRINOX. Cevumeran demonstrated substantial expansion of neoantigen-specific, functional, and durable CD8+ T cells. Vaccine-expanded T cells persisted for up to 2 years post-vaccination [94,95]. In an extended follow-up study, autogene cevumeran-induced CD8+ T cell clones persisted up to 3.6 years post-vaccination [96]. The success of this mRNA neoantigen vaccine led to a global randomized trial (NCT05968326) in a larger group of patients [97].
3.4. Colorectal Cancer
OncoVax is the most widely studied vaccine used in CRC with clinical trials dating back to the 1980s. In this vaccine, autologous cancer cells are combined with the Bacille Calmette–Guérin (BCG) vaccine. The Eastern Cooperative Oncology Group conducted a phase III randomized trial in which 412 patients with CRC were treated with surgery alone or in combination with vaccination. Despite a follow-up period of more than seven years, there was no significant difference in disease-free or overall survival. However, subgroup analysis of patients with stage II CRC demonstrated improved overall and disease-free survival [98].
Ring finger protein 43 (RNF43) and 34-kDa translocase of the outer mitochondrial membrane (TOMM34) are two TAAs that were found to be overexpressed in 80% of CRC tissues. As such, HLA-A24-restricted epitope peptides from RNF43 and TOMM34 were identified. Okuno et al. conducted a phase I clinical trial using a peptide vaccination with RNF43 and TOMM34 in combination with a prodrug of 5-FU and uracil, an inhibitor of 5-FU degradation. Twenty-three patients with unresectable metastatic CRC were enrolled, and clinical response was detected via follow-up computed tomography (CT) scans and the induction of CTLs against the two peptides. There was stable disease in 16 patients. The median progression-free survival was 7.2 months and mean survival time was 24.4 months. Eight patients had CTL responses against both antigens and this group had the most long-term survivors. Patients with no CTL responses had the lowest survival [99].
Rodriguez et al. conducted a phase II clinical trial (NCT01348256) of patients with liver metastasis of CRC randomized 1:1 to receive two courses of dendritic cell intradermal vaccination four times daily versus observation. Although this study ended early due to budget restrictions, there were fewer and later relapses in patients in the vaccine arm, with a median disease-free survival of 25.3 months versus 9.5 months in the observation group [99]. Despite evidence of clinical benefit and induction of an immune response, the use of vaccines in CRC has not come to fruition due to lack of significant benefits in larger phase III clinical trials. It is speculated that the antitumor immunity induced may be insufficiently durable to make a meaningful impact on survival [98].
3.5. Hepatocellular Carcinoma
The feasibility of dendritic cell vaccines in patients with advanced HCC was tested in a small phase I/II trial of five patients using a multiple tumor-associated antigen (TAA)-pulsed dendritic cell vaccine. The vaccine consisted of pulsed dendritic cells with cytoplasmic transduction peptide-attached α-fetoprotein, glypcan-3, and MAGE-1 recombinant fusion proteins. At least six vaccinations were tolerated in all patients, and all patients showed T cell responses against TAAs [100].
Alpha-fetoprotein (AFP) is frequently overexpressed in HCC. A phase I trial (NCT01974661) evaluated COMBIG-C/ilixadencel, a dendritic cell-based vaccine primed with AFP. The vaccine was administered intratumorally in 18 patients. A total of three vaccinations were given. The trial demonstrated positive immunogenicity and a tolerable side effect profile [101]. Another study was conducted using the same vaccine in 17 patients on three separate occasions 2–5 weeks apart. Diffusion-weighted magnetic resonance imaging (MRI) was conducted before each injection and three months after the first injection. MRI was able to detect a significant reduction in diffusion early after treatment and decreased tumor growth at three months [102]. Rizell et al. demonstrated that HCC patients with ilixadencel had a median overall survival of 10.9 months [103]. These studies further suggest the effectiveness of dendritic cell vaccines in HCC, though further studies are necessary to determine the specific subset of patients in which a particular therapy is effective.
3.6. Biliary Tract Cancers
The Wilms’ tumor 1 (WT1) peptide vaccine has been tested as a potential therapeutic in a number of malignancies, including patients with biliary tract cancers. A phase I trial was conducted to determine the toxicity, safety, and immunologic dose of the WT1 vaccine in patients with advanced biliary tract cancer. HLA-A 0201, HLA-A 0206, and HLA-A 2402-positive patients with inoperable advanced biliary tract or pancreatic cancer who had not previously received gemcitabine were enrolled. Four doses of the WT1 peptide vaccine and six doses of gemcitabine were given over two months. Median survival of patients with biliary tract cancer was 288 days. However, the disease control rate was only 50% at two months [104,105].
Further trials were conducted using multi-antigen vaccines due to the modest effect of single-antigen vaccines. Aruga et al. conducted a phase I trial in nine patients with advanced biliary tract cancer who were refractory to standard chemotherapy. They were vaccinated subcutaneously weekly with doses of 0.5, 1, or 2 mg of a vaccine containing four peptides derived from cancer-testis antigens. Vaccination continued until disease progression. There were no grade 3 or 4 adverse events, and the median progression-free survival and overall survival were 156 and 380 days, respectively [106,107]. A three-peptide vaccine targeting VEGFR1, VEGFR2, and KIF20A also had positive effects on survival, further emphasizing the importance of more clinical trials regarding cancer vaccines [108].
3.7. Gastrointestinal Stromal Tumor
The first use of a vaccine in GIST was in 2001 in a case reported by Shioyama et al. [109]. In this case, a patient with retroperitoneal metastasis of GIST was treated with carboplatin, epirubicin, and radiotherapy, followed by four intratumoral injections of OK432 given in one-week intervals. The original tumor size was 11 cm in diameter, but decreased to 20 mm in diameter six years after treatment was completed.
Many GIST patients ultimately develop resistance to TKIs, making immunotherapy an appealing potential alternative for treatment. A phase I trial conducted in patients with advanced or metastatic GIST who failed at least second-line therapy with a TKI was carried out in seven patients, who received two intratumoral injections of ilixadencel while maintaining treatment with TKIs. Three patients developed donor-specific antibodies indicative of alloimmunization. Two patients had a partial response. However, disease progression was noted in five patients [110,111]. Further clinical trials are needed to optimize the use of vaccines in GIST.
4. Cytokine Therapy
4.1. Gastric Cancer
Granulocyte-macrophage colony-stimulating factor (GM-CSF) is a multi-lineage cytokine that stimulates antigen presentation by directly stimulating dendritic cells and macrophages [112]. Early studies demonstrated that the common regimen of etoposide, 5-FU, and folinic acid (ELF) had a response rate of 39% in patients with locally advanced gastric cancer. Partyka et al. added GM-CSF to the ELF treatment regimen to determine if the response rate would be improved. Thirty patients were treated with ELF for three consecutive days. GM-CSF was started on day 4 if patients developed an absolute granulocyte count < 500 cells/µL. Nineteen patients had progressive disease, and grade 4 neutropenia occurred in sixteen patients [113]. Granulocyte colony-stimulating factor (G-CSF) regulates neutrophil production only and is therefore thought to have less toxicity as compared to GM-CSF [114]. A phase II study was conducted in advanced gastric cancer patients who were treated with docetaxel and given prophylactic G-CSF. The overall response rate was 20%, and grade III or IV toxicity occurred in 36% of patients [115]. Kornek et al. also conducted a phase II clinical trial combining paclitaxel, cisplatin, and G-CSF in advanced gastric cancer patients. This combination was shown to have a decreased incidence of severe neutropenia and other adverse effects as compared to other trials [116]. Although there do not appear to be any studies regarding GM-CSF or G-CSF solo therapy in gastric cancer, its use in combination with standard therapy may allow for increased tolerability and decreased adverse effects.
IL-6 is a master cytokine that plays a role in immune evasion by tumors [117]. In in vitro and in vivo studies, Ham et al. demonstrated that upregulation of IL-6 in gastric cancer tissues correlated with a poor response to chemotherapy [118]. Ruzzo et al. studied patients with locally advanced metastatic gastric cancer who were undergoing palliative chemotherapy and had genetic variants of the IL-6 promoter and the IL-6 receptor that led to their upregulation. Patients with these variants had worse overall survival, supporting the hypothesis that targeting IL-6 is a feasible therapeutic option in gastric cancer [119]. Further clinical trials are necessary to access the feasibility of anti-IL-6 agents in the management of gastric and other malignancies.
4.2. Pancreatic Cancer
Interferons are secreted in response to immune stimulation and have been proven to upregulate MHC class I molecules, promote survival of CTLs, promote dendritic cell maturation, and have antiangiogenic effects on various tumors [112]. A phase II trial of patients with PDAC who underwent pancreaticoduodenectomy was performed by Nukui et al. In this trial, surgical patients were treated with standard adjuvant chemoradiation or standard therapy plus IFNα given every other day during the five weeks of radiation. The two-year overall survival was 84% in the IFNα group versus 54% in the control group [120]. In a phase III trial, patients receiving a low-dose IFNα injection prior to chemoradiation had a significant increase in cytotoxicity and activation of antigen-presenting cells and NK cells [121]. In the ACOSOG Z05031 multicenter phase II trial, PDAC patients were treated with adjuvant IFNa-2b. There was no long-term toxicity or toxicity-related deaths. Median disease-free survival and overall survival were 14.1 months and 25.4 months, respectively [122]. In a PDAC mouse model, IFNβ combined with gemcitabine led to smaller tumor volumes [123]. More robust clinical trials are necessary to determine the toxicity profile and effectiveness of interferons in combination with standard chemoradiation for PDAC.
4.3. Colorectal Cancer
Studies regarding cytokine therapy in CRC are limited. The first human dose escalation study of PEGylated recombinant human IL-10 (AM0010) was completed in patients with solid tumors in 2015. A total of 33 patients were enrolled and AM0010 was administered daily at doses of 1 to 40 µg/kg. Increasing doses of AM0010 had increased exposure, and IL-18 was increased in a dose-dependent fashion in all patients. At doses > 20 µg/kg, IFNγ, IL-4, GM-CSF, and IL-7 were increased. Of the six patients with CRC, four patients had stable disease at 8 weeks with one for greater than 40 weeks [124]. Further clinical studies evaluating the use of cytokine therapy in combination with other treatment modalities are imperative for CRC.
4.4. Gastrointestinal Stromal Tumor
Multiple therapies have been tested in an attempt to increase the efficacy of imantinib. IFNα and IFNβ are produced by dendritic cells, neutrophils, and macrophages in the tumor microenvironment. Peginterferon α-2b (PegIFNα2b) is a long-acting IFN that has been tested to treat GIST [63]. In a pre-clinical study, eight patients with stage III/IV GIST were administered PegIFNα2b along with imatinib. A partial or complete response rate was achieved in 100% of patients, compared to only 54% of patients treated with imantinib alone. These promising results suggest that cytokine therapy may help to eradicate drug-resistant clones in patients who no longer respond to imantinib [125].
Cytokines in combination with other types of immunotherapy have also been used to treat GIST. Eight patients with recurrent GIST refractory to standard therapy were treated with intravenous allogenous phytohemagglutinin-activated T cells in combination with IL-2 and a personalized vaccine adopted from donor B-cells and patient circulating tumor cells. Five patients achieved complete remission after 14 months of follow-up [126]. Combination immunotherapy continues to show promise when standard therapy has failed.
4.5. Peritoneal Carcinomatosis
Historically, PM has been treated following guidelines as per the primary tumor, with cytoreductive surgery (CRS) and heated intraperitoneal chemotherapy (HIPEC) being indicated for certain histological types. However, some studies demonstrated that peritoneal carcinomatosis may have a distinct biologic subtype as compared to the primary tumor [127,128].
PM is more common in signet ring adenocarcinomas of gastric origin and mucus produced by adenocarcinomas may help the tumor to invade the stroma and facilitate spread to the peritoneal fluid, resulting in malignant ascites [129]. Catumaxomab is a trifunctional antibody with a long-term vaccination effect that targets the innate and adaptive immune systems, leading to phagocytosis of tumor cells and cell death by initiating pro-apoptotic cytokines [130]. Catumaxomab was approved for the treatment of malignant ascites in Europe in 2009 [130,131]. We are currently conducting a phase 1 clinical trial to study the safety of IP administration of tocilizumab, an IL-6 receptor antagonist, in the treatment of malignant ascites (NCT06016179) [132].
5. Oncolytic Viral Therapy
5.1. Esophageal Cancer
Oncolytic virotherapy involves using natural or genetically engineered viruses to infect and replicate in tumor cells, causing direct tumor cell lysis that triggers the antitumor immune response [133,134]. OH2 is an oncolytic virus derived from herpes simplex virus (HSV) strain HG52. Preclinical studies demonstrated its safety and potent oncolytic activity. Zhang et al. conducted a multicenter phase I/II clinical trial in which patients with advanced solid tumors were treated with OH2 and HX008, an anti-PD-1 antibody. Of the fourteen patients with esophageal cancer, only one patient had stable disease [134]. Another phase I dose-escalation study evaluated OBP-301 (Telomelysin), an attenuated type-5 adenovirus containing human telomerase reverse transcriptase promoter. Thirteen patients who were unfit for surgery or chemotherapy received endoscopic injection of OBP-301 and radiation. Eight patients had a complete local response, and the objective response rate was 91.7% [135]. This study offers oncolytic viral therapy as an alternative for those who are unable to tolerate standard therapy.
5.2. Pancreatic Cancer
Taxane and platinum chemotherapy has been thought to induce antitumor immune activity, and the synergistic potential of reovirus in combination with carboplatin or paclitaxel has been studied previously. In a randomized phase II trial, patients with treatment-naïve metastatic PDAC were treated with paclitaxel/carboplatin or paclitaxel/carboplatin combined with the oncolytic reovirus pelareorep (Reolysin®). Although palereorep was shown to be safe, it did not improve progression-free survival when combined with paclitaxel/carboplatin [136]. When combined with gemcitabine, pelareorep was shown to complement gemcitabine with a median overall survival of 10.2 months [137]. Another phase Ib study evaluated patients with PDAC who had progressed after first-line treatment. Patients received chemotherapy (5-FU, gemcitabine, or irinotecan), pembrolizumab, and pelareorep until disease progression or unacceptable toxicity. Of the 11 patients enrolled, three patients had a partial response for up to 17.4 months [138]. Further studies are necessary to determine the optimal combination of therapy.
5.3. Colorectal Cancer
The effectiveness of oncolytic viral therapy in colon cancer has long been a subject of interest, going back to the studies by Yoon et al., who demonstrated that the HSV-1-derived vector hrR3 destroyed colon cancer cells both in vitro and in vivo [133,139]. JX-594 (Pexa-Vec) is a thymidine kinase gene-inactivated oncolytic vaccinia (cowpox) virus and was evaluated in 15 patients with treatment-resistant CRC in a phase Ib study. There were no dose-limiting toxicities, and 67% of patients had radiographically stable disease [140]. The oncolytic reovirus pelareorep was combined with FOLFOX and bevacizumab in 103 patients with metastatic CRC. Objective response rate was 53% in patients treated with pelareorep versus 35% in patients treated with chemotherapy alone. However, progression-free survival was inferior in the pelareorep group, suggesting that treatment intensity may have been decreased by standard therapy [141].
5.4. Hepatocellular Carcinoma
JX-594 has been used extensively in clinical trials in HCC [133]. In the TRAVERSE phase IIb clinical trial (NCT01387555), patients with advanced HCC who failed sorafenib treatment were randomized to Pexa-Vec plus best supportive care versus best supportive care alone [142,143]. Pexa-Vec was well-tolerated. However, there was no difference in overall response rate or time-to-progression in the two treatment arms [142]. In a phase III study of sequential treatment with Pexa-Vec and sorafenib in patients with no prior systemic treatment, there was again no clinical benefit in patients treated with Pexa-Vec plus sorafenib [144,145]. It is hoped that combining oncolytic viruses with other systemic therapies, such as ICIs, may yield benefit in future studies.
5.5. Peritoneal Carcinomatosis
Immunostimulatory virus-like particles are being studied as a means to modify the immunosuppressive peritoneal immune microenvironment. Miller et al. used the virus-like particle CMP-001 in an ex-vivo model of peritoneal cells isolated from patients with PM from GI and pancreaticobiliary cancers. CMP-001 is composed of a bacteriophage capsid protein that encapsulates a CpG-A oligodeoxynucleotide that activates plasmacytoid dendritic cells (pDCs) and activates IFNα release, leading to anti-tumor immune effects. In mice injected with either pancreatic or colon cancer cell lines, there was an increase in survival in those that received CMP-001 as compared to controls. There was also reduction in total tumor weight and volume of ascites, suggesting that CMP-001 may be an effective treatment for patients with PM [146].
6. Cellular Therapy
6.1. Esophageal Cancer
The use of ACT therapy has shown promising results in hematologic malignancies. However, their promise remains largely untapped in treating solid tumors [6]. Challenges result from low tumor tissue infiltration of CAR-T cells in solid tumors, an immunosuppressive tumor microenvironment, and toxic side effects [147]. CAR-T cells specific to the MAGE-A4 antigen were evaluated in ten patients with recurrent esophageal cancer in a phase 1 clinical trial. The patients were given T cell receptor lymphocytes followed by sequential MAGE-A4 peptide vaccinations on days 14 and 28. Only three patients demonstrated a durable response with a progression-free survival of 27 months, but they had a lower tumor burden as compared to the other patients [148]. These findings suggest that ACT may be more beneficial in patients with early-stage tumors [149].
6.2. Gastric Cancer
The normal immune response is known to be impaired in patients with advanced stages of cancer. Therefore, it is probable that cell therapy can improve the host’s immune function. Shi et al. evaluated the efficacy of immunotherapy with autologous cytokine-induced killer (CIK) cells in patients with gastric cancer. Patients with stage III or IV gastric cancer who had undergone gastrectomy were enrolled after receiving six cycles of adjuvant 5-FU-based chemotherapy. Patients in the immunotherapy group were treated with at least three cycles of CIK-cell therapy, while the control group did not receive immunotherapy. Patients in the immunotherapy group had significantly better disease-free survival (DFS). The 3- and 5-year DFS rates were 36.4% and 10.4% in the control group versus 47.3% and 28.3% in the immunotherapy group. These findings indicate that CIK-cell therapy has the potential to improve the host’s immune function after chemotherapy [150].
In a randomized Japanese study, Kono et al. investigated the use of adoptive immunotherapy (AIT) with tumor-associated lymphocytes in combination with chemotherapy in patients with stage IV gastric cancer. Patients in the AIT group were treated with AIT plus intravenous cisplatin/5-FU and oral doxifluridine, while those in the control group were treated with chemotherapy alone. All patients underwent surgery with adjuvant treatment beginning five weeks after surgery. Overall survival was significantly improved in the AIT group, with 50% survival rates of 11.5 and 8.3 months in the AIT group and control group, respectively [151].
Invariant NK T cells (iNKT) couple innate and adaptive immune responses by generating cytokine release in response to lipid antigens. AgenT-797 is a type of cell therapy that consists of allogenic human unmodified iNKT cells isolated from a healthy donor and expanded ex vivo. Hadfield et al. present a case of a patient with high-MSI advanced gastric adenocarcinoma who developed resistance to nivolumab treated with agenT-797. The patient received one infusion of agenT-797 while continuing maintenance nivolumab every two weeks. Gene expression analysis of tissue biopsies demonstrated increased tumor immune infiltration of TH-1 lymphocytes. The patient had a partial response as per RECIST 1.1 criteria [152]. A phase I clinical trial evaluating agenT-797 in combination with pembrolizumab or nivolumab demonstrated an overall response rate of 20% in patients with advanced solid tumors [153]. These data, along with the successful response of this patient to agenT-797, supports the development of clinical trials using iNKT in gastric cancer.
6.3. Pancreatic Cancer
ACT has also been explored in PDAC. Leidner et al. published a case of a patient with stage IIB PDAC who presented with bilateral lung metastases one year after completing chemoradiotherapy. The patient was refractory to standard therapy and TIL therapy. The patient was then subsequently treated with a single infusion of autologous T cells expressing T cell receptors targeting mutant KRAS G12D expressed by the tumor. The patient was noted to have an overall partial response of 72% with a clinical response of at least 6 months [154].
It has been proven that arming anti-CD3-activated T cells with anti-CD3, and anti-EGFR bispecific antibodies (EGFRBi) change activated T cells into specific CTLs [155]. Lum et al. then demonstrated in preclinical studies that EGFRBi-armed activated tumor cells killed pancreatic cancer cells in an in vitro model. This led to their phase 1/2 trial in which patients were infused intravenously with EGFRBi-armed activated tumor cells three weeks following chemotherapy with FOLFOX. Not only was the treatment deemed to be safe, but all patients also survived for more than one year with a median overall survival of 31 months [156].
NK cells serve as a safe and effective alternative to T cells. However, there are few clinical studies regarding CAR-NK cells as compared to CAR-T cells, and most studies involving GI malignancies are in preclinical trials or animal models. CAR-NK cells mainly kill cells with high expressions of specific antigens. Li et al. report use of Robo-1-specific CAR-NK cells in a patient with PDAC and liver metastasis. Robo1 has been shown to modulate T cell chemotaxis and plays a role in tumor angiogenesis. Its expression is increased in pancreatic cancer. The patient received intravenous and percutaneous injection of Robo1-CAR-NK cells weekly for five months. Imaging demonstrated resolution of the pancreatic and liver lesions at five months. Overall survival of the patient was eight months [157]. This case highlights the importance of the need for further clinical studies using alternative cellular therapies.
6.4. Colorectal Cancer
Because most targets of CAR-T cells in CRC are also expressed on normal cells, there is a substantial risk of off-tumor toxicity when using this approach in patients [147]. Carcinoembryonic antigen (CEA) is a biomarker widely expressed in CRC and was used as a target in a phase I clinical trial. CAR-T cell therapy directed against CEA-positive CRC patients with metastases was performed with five dose-escalating levels in ten patients. There were no severe adverse events, and seven patients had stable disease after CAR-T therapy. Serum CEA levels also decreased in these patients. After a second CAR-T therapy, CAR-T cell proliferation was also observed [158].
Other targets for CAR-T therapy have been explored in CRC. Ligands for the activating immunoreceptor NKG2D, which is highly expressed on cytotoxic immune cells, are expressed on malignant cells and are a potential target of CAR-T cells. The SHRINK trial was a dose-escalation trial of NKG2D CAR-T cells administered in combination with FOLFOX in patients with colon cancer liver metastasis in which there were no dose-limiting toxicities [159]. This was followed by the alloSHRINK trial in which dose-escalating doses of the NKG2D-based CAR-T cells were infused following pretreatment with FOLFOX. There were two partial responses and two patients who achieved stable disease. The median progression-free survival was 3.9 months [160].
Tran et al. reported the successful use of TILs in a patient with CRC [161]. A phase 2 clinical trial (NCT01174121) was designed to determine whether adoptive transfer of ex vivo TILs consisting of T cells against cancer neoepitopes would lead to regression of metastatic solid tumors [161,162]. In one patient with a history of CRC with only lung progression, regression of all lung lesions occurred 40 days after cell therapy. There was a 9-month partial response [161]. A phase I clinical trial (NCT05576077) is currently recruiting patients with CRC to investigate TBio-4101, a type of autologous TIL therapy that will utilize tumor specific antigens to expand patient-specific tumor-reactive T cells that will be reinfused back into the patient [163,164]. These clinical trials are important in the ongoing efforts to bring ACT to fruition.
6.5. Hepatocellular Carcinoma
Preclinical studies demonstrated that CIK cells have increased activity against HCC cells in vitro [165]. When CIK cell transfusions were combined with transcatheter arterial chemoembolization (TACE) and radiofrequency ablation (RFA), the disease control rate was higher in patients treated with CIK cells as opposed to TACE and RFA alone. However, the difference was not significant, and the two groups were not randomized [166]. A randomized phase II study, HCC patients without prior treatment were assigned to CIK plus standard treatment versus standard treatment alone. Median overall survival and progression-free survival was significantly higher in those treated with CIK [167]. Similarly, in a phase III trial of patients treated with adjuvant CIK, the median recurrence-free survival was 44.0 months in the CIK-treated group versus 30.0 months in the control group [168]. Larger randomized controlled studies are needed to further elucidate the role of CIK cells in the treatment of HCC.
Chimeric antigen receptor monocytes and macrophages (CAR-M), a type of ACT, are being developed to treat solid tumors. An in vivo CAR-M is being engineered by Carisma Therapeutics and will be used to target Glypican-3 (GPC3), a tumor antigen that is highly expressed in HCC. With the use of mRNA and a lipid nanoparticle, this novel in vivo cell therapy will aid in directing myeloid cells to kill cancer cells, further advancing the field of ACT [169].
6.6. Biliary Tract Cancers
Cholangiocarcinoma is categorized depending on the presence of immune cell infiltration into cancers that have been infiltrated by lymphocytes and those that have not been infiltrated. Tumors that have been infiltrated are immunologically responsive, with TILs being the most important determinant of the host immune response against tumor cells [170]. Systemic chemotherapy in cholangiocarcinoma can lead to resistance to available drugs, resulting in a low five-year survival rate. However, the immunomodulating properties of chemotherapy have been shown to enhance immune cell function, particularly with gemcitabine, which can sensitize cancer cells to be susceptible to CTLs. This led to the hypothesis that gemcitabine combined with CTLs would be an effective treatment against cholangiocarcinoma. Sawasdee et al., conducted an in vitro study where gemcitabine was combined with CTLs to treat gemcitabine-resistant cholangiocarcinoma cells. Cells pretreated with gemcitabine were killed by CTLs activated by self-differentiated dendritic cells more efficiently as opposed to CTLs activated with cancer cell lysate. These findings support the use of gemcitabine in combination with immunotherapy to treat chemo-resistant cholangiocarcinoma [171].
6.7. Anal Cancer
Due to its strong association with HPV, the majority of clinical trials addressing anal cancer are combined with other HPV-associated cancers such as cervical, vulvar, vaginal, penile, and oropharyngeal cancers [172]. A phase 2 study sponsored by the National Cancer Institute (NCI) studied two cohorts: cervical cancers and non-cervical cancers. Patients with metastatic HPV-associated cancers who received prior platinum-based chemotherapy or chemoradiotherapy were eligible. Patients were treated with a nonmyeloablative chemotherapy regimen, followed by a single intravenous infusion of HPV-TILs. Aldesleukin was then administered as an intravenous bolus every eight hours to tolerance or a maximum of fifteen doses. Five of the included patients had anal cancer, of which only one had a partial response of a 4-month duration [173]. Although the response rate was not very favorable for the patients with anal cancer, the feasibility of this approach and further clinical trials are warranted. Another approach was performed using ADXS11-001, a live attenuated Listeria monocytogenes bioengineered to secrete an HPV-16-E7 fusion protein targeting HPV-transformed cells. The tumor-associated antigen HPV E7 is present in anal cancer cells infected with HPV. ADXS11-001 causes stimulation of antigen-presenting cells to facilitate attachment of immune cells to cancer cells [174]. Patients with anal squamous cell cancer without metastasis were treated with two cycles of mitomycin and 5-FU, standard radiation, and one dose of ADXS11-001 prior to radiation followed by monthly doses after each session of radiation. Eight of nine patients had a complete response at a median follow-up of 34 months [175].
6.8. Peritoneal Carcinomatosis
CAR-T cell immunotherapy has been a focus of study in PM [130]. In a murine model of CRC metastasis to the peritoneal cavity, Katz et al., studied the intraperitoneal (IP) delivery of CAR-T cells, finding that direct IP infusion was more effective at reducing tumor burden as compared to systemic infusion, suggesting that combinatorial use of IP and systemic therapy is a promising therapeutic option [176].
7. Clinical Trials Involving Immunotherapy in Gastrointestinal Malignancies
Many clinical trials are underway to establish the efficacy of various immunotherapeutic agents in the treatment of GI malignancies. ICIs are the most studied and promising strategy for intervention as they have shown the potential for durable responses with manageable adverse effects. Some studies suggested that combining immunotherapy with chemotherapy and/or radiation may have an increased benefit. It has since been hypothesized that antitumor drugs that increase T cell immunity combined with PD-1 inhibition may have a synergistic effect [30,177]. The combination of endogenous T cell activation, vaccines, or ACT has also been studied synergistically with ICIs and standard chemoradiation in order to activate the host’s immune system [178]. Several preclinical studies demonstrated promising results when combining CAR T cells with oncolytic viruses, as the combination can increase anti-tumor activity [179]. In this section, we provide an overview of ongoing active clinical trials involving multiple modalities of immunotherapy in GI malignancies.
7.1. Immune Checkpoint Inhibition
Immune checkpoint inhibition has been the predominant focus of immunotherapy for GI malignancies since 2017 [20]. These promising immunotherapy drugs include anti-PD1 antibodies, anti-CTLA-4 antibodies, and anti-PD-L1 antibodies. Most studies involve the combination of immunotherapy with systemic chemotherapy, highlighting synergistic results with improved overall survival and prognosis. Table 1 summarizes select ongoing and active clinical trials focusing on the use of immune checkpoint inhibition in the management of GI malignancy.

Table 1.
Summary of ongoing clinical trials evaluating immune checkpoint inhibition in gastrointestinal malignancy.
7.2. Intraperitoneal Use of Immunotherapy
ICIs have the potential to enhance the immune response to tumor cells [8], with the same being true of intraperitoneal immunotherapy with ICIs [197]. ICIs may have an advantage in the peritoneal cavity, as the sequestered environment may allow for dosing regimens that may not be tolerated when given systemically [198]. Wang et al. presented the first case of a patient with pancreatic cancer and refractory ascites who was successfully treated with intraperitoneal nivolumab [197]. Table 2 summarizes upcoming and ongoing clinical trials focusing on intraperitoneal immunotherapy in the management of GI malignancies.

Table 2.
Summary of ongoing clinical trials evaluating intraperitoneal immunotherapy in gastrointestinal malignancies.
7.3. Vaccination and Immune Modulation
Therapeutic cancer vaccines are given to patients who have already been diagnosed with cancer, unlike prophylactic vaccines that are given to healthy subjects [204]. Cancer vaccines target antigens associated with tumor cells that are recognized as foreign by the host immune system and ideally target antigens only on cancer cells, those that are necessary for tumor survival, and are highly immunogenic [204]. Guo et al. reported successful treatment of a patient with advanced metastatic gastric cancer with a personalized neoantigen-loaded monocyte-derived dendritic cell vaccine [205]. Flatmark et al., introduced the concept of a peptide vaccine in patients with pseudomyxoma peritonei (PMP) and GNAS mutations, [206] while Ramanathan et al., demonstrated that use of an adjuvant dendritic cell vaccine was not efficacious in patients with PM undergoing CRS and HIPEC [207]. Table 3 reports both completed and ongoing clinical trials that use immune modulation or vaccination for potential treatment in GI malignancies.

Table 3.
Summary of ongoing clinical trials involving vaccination or immune modulation in gastrointestinal malignancy.
7.4. Oncolytic Viral Therapy
In addition to direct tumor cell lysis, oncolytic viruses have the potential to indirectly increase the effectiveness of T cells by stimulating the release of tumor-associated antigens, inflammatory cytokines, and chemokines [2]. The first clinical trial of viruses in malignancies was conducted in 1949 using hepatitis A virus [223]. Adenovirus, vaccinia virus, herpes simplex virus (HSV), and reovirus are four oncolytic virus families that are currently being studied in clinical trials, and in the modern era, the ideal virus is strategically selected for a given tumor type [2]. The route of administration is also important, and a majority of studies focused on intra-tumoral injection [224]. Table 4 describes current clinical trials utilizing oncolytic viral therapy in the management of GI malignancies.

Table 4.
Summary of ongoing clinical trials involving oncolytic virotherapy in gastrointestinal malignancy.
7.5. Adoptive Cell Therapy
Current targets of ACT include CAR-T cells that focus on carcinoembryonic antigen (CEA), HER-2, EpCAM, claudin 18.2, or mesothelin [242]. Kageyama et al. performed the first human clinical trial using ACT of MAGE-A4 T cell receptor gene-transduced lymphocytes in patients with MAGE-A4-expressing esophageal cancer. Their results indicate that the engineered T cells could be detected in peripheral blood of patients for up to five months [148]. TIL therapy is also under development as a treatment strategy for GI malignancy, although its efficacy has only been successfully demonstrated in melanoma and ovarian cancer to date [149]. Ongoing clinical trials are primarily investigating the combination of ACT with standard chemotherapy or immunotherapy regimens. Table 5 presents current clinical trials utilizing the technique of ACT in treating GI malignancies.

Table 5.
Summary of ongoing clinical trials involving adoptive cell therapy in gastrointestinal malignancy.
8. Conclusions
Despite advances in traditional treatment options for GI malignancies, prognosis remains poor, particularly in advanced or metastatic disease. Immunotherapy has become the standard of care in various skin, breast, and hematologic malignancies, and has become a promising approach for treating GI malignancies. However, the promise of immunotherapy beyond ICIs remains to be fully realized in GI malignancies.
A large array of combinations of systemic chemotherapy, radiation, ICIs, cancer vaccines, and other T cell-based therapies has also shown encouraging early results in GI malignancies (Figure 2). Despite these advancements, several challenges still exist. Different tumor characteristics and tumor microenvironment make certain ICIs ineffective in some tumors. For example, ICIs are more effective in colorectal cancer patients with MSI or MMR deficiencies. This requires use of ICIs and other immunotherapy techniques in combination. Although the combination may synergistically increase tumor cell death, the number of adverse events and the impact on quality of life is not well studied.
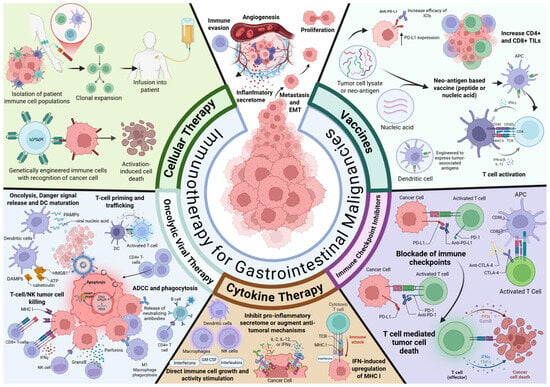
Figure 2.
Immunotherapy for gastrointestinal malignancies. Immunotherapy for gastrointestinal malignancies includes agents with a broad range of mechanisms of action. These include immune checkpoint inhibition, cytokine therapy, vaccines, oncolytic viral therapy, and cellular therapy.
The tumor microenvironment can diminish or inhibit T cells and other cells involved in cell-based therapy. Processes for large-scale development of CAR-T or CAR-NK cells are lacking, and future studies must be performed to determine methods to optimize the production of these cell products. Additionally, clinical trials comparing the different immunotherapy techniques are needed to determine the ideal treatment for different GI malignancies. The development of these and other therapies aimed at modulating the tumor immune microenvironment could eventually lead to a personalized approach to cancer treatment and ultimately better outcomes in patients with advanced GI malignancies.
Author Contributions
Conceptualization, C.R.L. and P.L.W.; writing—original draft preparation, C.R.L., Y.S., M.S.R. and P.L.W.; writing—review and editing, C.R.L., Y.S., C.S., N.D., M.S.R. and P.L.W.; Graphics and visualization—C.S. and N.D.; supervision, P.L.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Burnet, F.M. Immunological aspects of malignant disease. Lancet 1967, 289, 1171–1174. [Google Scholar] [CrossRef]
- Kazemi, M.H.; Sadri, M.; Najafi, A.; Rahimi, A.; Baghernejadan, Z.; Khorramdelazad, H.; Falak, R. Tumor-infiltrating lymphocytes for treatment of solid tumors: It takes two to tango? Front. Immunol. 2022, 13, 1018962. [Google Scholar] [CrossRef]
- McCarthy, P.M.; Valdera, F.A.; Smolinsky, T.R.; Adams, A.M.; O’Shea, A.E.; Thomas, K.K.; Van Decar, S.; Carpenter, E.L.; Tiwari, A.; Myers, J.W.; et al. Tumor infiltrating lymphocytes as an endpoint in cancer vaccine trials. Front. Immunol. 2023, 14, 1090533. [Google Scholar] [CrossRef] [PubMed]
- Qayoom, H.; Sofi, S.; Mir, M.A. Targeting tumor microenvironment using tumor-infiltrating lymphocytes as therapeutics against tumorigenesis. Immunol. Res. 2023, 71, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Wagner, K.; Wolchok, J.D.; Allison, J.P. Novel cancer immunotherapy agents with survival benefit: Recent successes and next steps. Nat. Rev. Cancer 2011, 11, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Teixeira Farinha, H.; Digklia, A.; Schizas, D.; Demartines, N.; Schäfer, M.; Mantziari, S. Immunotherapy for Esophageal Cancer: State-of-the Art in 2021. Cancers 2022, 14, 554. [Google Scholar] [CrossRef]
- Bashash, D.; Zandi, Z.; Kashani, B.; Pourbagheri-Sigaroodi, A.; Salari, S.; Ghaffari, S.H. Resistance to immunotherapy in human malignancies: Mechanisms, research progresses, challenges, and opportunities. J. Cell. Physiol. 2022, 237, 346–372. [Google Scholar] [CrossRef]
- Sathyanarayanan, V.; Neelapu, S.S. Cancer immunotherapy: Strategies for personalization and combinatorial approaches. Mol. Oncol. 2015, 9, 2043–2053. [Google Scholar] [CrossRef]
- Koustas, E.; Trifylli, E.-M.; Sarantis, P.; Papadopoulos, N.; Karapedi, E.; Aloizos, G.; Damaskos, C.; Garmpis, N.; Garmpi, A.; Papavassiliou, K.A.; et al. Immunotherapy as a Therapeutic Strategy for Gastrointestinal Cancer—Current Treatment Options and Future Perspectives. Int. J. Mol. Sci. 2022, 23, 6664. [Google Scholar] [CrossRef]
- Park, R.; Williamson, S.; Kasi, A.; Saeed, A. Immune Therapeutics in the Treatment of Advanced Gastric and Esophageal Cancer. Anticancer. Res. 2018, 38, 5569–5580. [Google Scholar] [CrossRef]
- Phillips, C. First Cancer TIL Therapy Gets FDA Approval for Advanced Melanoma. 2024. Available online: https://www.cancer.gov/news-events/cancer-currents-blog/2024/fda-amtagvi-til-therapy-melanoma (accessed on 11 September 2024).
- Wagner, J.; Wickman, E.; DeRenzo, C.; Gottschalk, S. CAR T Cell Therapy for Solid Tumors: Bright Future or Dark Reality? Mol. Ther. 2020, 28, 2320–2339. [Google Scholar] [CrossRef]
- Kelly, R.J.; Bever, K.; Chao, J.; Ciombor, K.K.; Eng, C.; Fakih, M.; Goyal, L.; Hubbard, J.; Iyer, R.; Kemberling, H.T.; et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immunotherapy for the treatment of gastrointestinal cancer. J. Immunother. Cancer 2023, 11, e006658. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, D.; Allum, W.H.; Stenning, S.P.; Thompson, J.N.; Van de Velde, C.J.H.; Nicolson, M.; Scarffe, J.H.; Lofts, F.J.; Falk, S.J.; Iveson, T.J.; et al. Perioperative Chemotherapy versus Surgery Alone for Resectable Gastroesophageal Cancer. N. Eng. J. Med. 2006, 355, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Al-Batran, S.E.; Homann, N.; Pauligk, C.; Goetze, T.O.; Meiler, J.; Kasper, S.; Kopp, H.; Mayer, F.; Haag, G.M.; Luley, K.; et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): A randomised, phase 2/3 trial. Lancet 2019, 393, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.J.; Ajani, J.A.; Kuzdzal, J.; Zander, T.; Van Cutsem, E.; Piessen, G.; Mendez, G.; Feliciano, J.; Motoyama, S.; Lièvre, A.; et al. Adjuvant Nivolumab in Resected Esophageal or Gastroesophageal Junction Cancer. N. Engl. J. Med. 2021, 384, 1191–1203, Erratum in N. Engl. J. Med. 2023, 388, 672. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Shitara, K.; Moehler, M.; Garrido, M.; Salman, P.; Shen, L.; Wyrwicz, L.; Yamaguchi, K.; Skoczylas, T.; Bragagnoli, A.C.; et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): A randomised, open-label, phase 3 trial. Lancet 2021, 398, 27–40. [Google Scholar] [CrossRef]
- Sun, J.-M.; Shen, L.; Shah, M.A.; Enzinger, P.; Adenis, A.; Doi, T.; Kojima, T.; Metges, J.; Li, Z.; Kim, S.; et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): A randomised, placebo-controlled, phase 3 study. Lancet 2021, 398, 759–771, Erratum in Lancet 2021, 398, 1874. [Google Scholar] [CrossRef]
- Ajani, J.A.; D’Amico, T.A.; Bentrem, D.J.; Cooke, D.; Corvera, C.; Das, P.; Enzinger, P.C.; Enzler, T.; Farjah, F.; Gerdes, H.; et al. Esophageal and Esophagogastric Junction Cancers, Version 2.2023, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2023, 21, 393–422. [Google Scholar] [CrossRef]
- Tieniber, A.D.; Perez, J.E.; Hanna, A.N.; DeMatteo, R.P. Immunotherapy for GI Malignancies, Ready for Prime Time? Ann. Surg. Oncol. 2023, 30, 1787–1793. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, P.; Pang, Q. Immune checkpoint inhibitors for esophageal squamous cell carcinoma: A narrative review. Ann. Transl. Med. 2020, 8, 1193. [Google Scholar] [CrossRef]
- Kono, K.; Nakajima, S.; Mimura, K. Current status of immune checkpoint inhibitors for gastric cancer. Gastric Cancer 2020, 23, 565–578. [Google Scholar] [CrossRef] [PubMed]
- Fashoyin-Aje, L.; Donoghue, M.; Chen, H.; He, K.; Veeraraghavan, J.; Goldberg, K.B.; Keegan, P.; McKee, A.E.; Pazdur, R. FDA Approval Summary: Pembrolizumab for Recurrent Locally Advanced or Metastatic Gastric or Gastroesophageal Junction Adenocarcinoma Expressing PD-L1. Oncologist 2019, 24, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, C.S.; Doi, T.; Jang, R.W.; Muro, K.; Satoh, T.; Machado, M.; Sun, W.; Jalal, S.I.; Shah, M.A.; Metges, J.; et al. Safety and Efficacy of Pembrolizumab Monotherapy in Patients with Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer. JAMA Oncol. 2018, 4, e180013, Erratum in JAMA Oncol. 2019, 5, 579. [Google Scholar] [CrossRef] [PubMed]
- Bang, Y.-J.; Kang, Y.-K.; Catenacci, D.V.; Muro, K.; Fuchs, C.S.; Geva, R.; Hara, H.; Golan, T.; Garrido, M.; Jalal, S.I.; et al. Pembrolizumab alone or in combination with chemotherapy as first-line therapy for patients with advanced gastric or gastroesophageal junction adenocarcinoma: Results from the phase II nonrandomized KEYNOTE-059 study. Gastric Cancer 2019, 22, 828–837. [Google Scholar] [CrossRef]
- Kang, Y.-K.; Boku, N.; Satoh, T.; Ryu, M.; Chao, Y.; Kato, K.; Chung, H.C.; Chen, J.; Muro, K.; Kang, W.K.; et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 390, 2461–2471. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Bendell, J.; Calvo, E.; Kim, J.W.; Ascierto, P.A.; Sharma, P.; Ott, P.A.; Peltola, K.; Jaeger, D.; Evans, J.; et al. CheckMate-032 Study: Efficacy and Safety of Nivolumab and Nivolumab Plus Ipilimumab in Patients with Metastatic Esophagogastric Cancer. J. Clin. Oncol. 2018, 36, 2836–2844, Erratum in J. Clin. Oncol. 2019, 37, 443. [Google Scholar] [CrossRef]
- Hilmi, M.; Bartholin, L.; Neuzillet, C. Immune therapies in pancreatic ductal adenocarcinoma: Where are we now? World J. Gastroenterol. 2018, 24, 2137–2151. [Google Scholar] [CrossRef]
- Dahiya, D.S.; Kichloo, A.; Singh, J.; Albosta, M.; Lekkala, M. Current immunotherapy in gastrointestinal malignancies A Review. J. Investig. Med. 2021, 69, 689–696. [Google Scholar] [CrossRef]
- Kole, C.; Charalampakis, N.; Tsakatikas, S.; Frountzas, M.; Apostolou, K.; Schizas, D. Immunotherapy in Combination with Well-Established Treatment Strategies in Pancreatic Cancer: Current Insights. Cancer Manag. Res. 2022, 14, 1043–1061. [Google Scholar] [CrossRef]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef]
- Ogino, S.; Nosho, K.; Irahara, N.; Meyerhardt, J.A.; Baba, Y.; Shima, K.; Glickman, J.N.; Ferrone, C.R.; Mino-Kenudson, M.; Tanaka, N.; et al. Lymphocytic reaction to colorectal cancer is associated with longer survival, independent of lymph node count, microsatellite instability, and CpG island methylator phenotype. Clin. Cancer Res. 2009, 15, 6412–6420. [Google Scholar] [CrossRef]
- Golshani, G.; Zhang, Y. Advances in immunotherapy for colorectal cancer: A review. Ther. Adv. Gastroenterol. 2020, 13, 1756284820917527. [Google Scholar] [CrossRef]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef]
- Overman, M.J.; McDermott, R.; Leach, J.L.; Lonardi, S.; Lenz, H.; Morse, M.A.; Desai, J.; Hill, A.; Axelson, M.; Moss, R.A.; et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): An open-label, multicentre, phase 2 study. Lancet Oncol. 2017, 18, 1182–1191, Erratum in Lancet Oncol. 2017, 18, 510. [Google Scholar] [CrossRef] [PubMed]
- Andre, T.; Lonardi, S.; Wong, M.; Lenz, H.; Gelsomino, F.; Aglietta, M.; Morse, M.; Van Cutsem, E.; McDermott, R.S.; Hill, A.G.; et al. Nivolumab + ipilimumab combination in patients with DNA mismatch repair-deficient/microsatellite instability-high (dMMR/MSI-H) metastatic colorectal cancer (mCRC): First report of the full cohort from CheckMate-142. J. Clin. Oncol. 2018, 36, 553. [Google Scholar] [CrossRef]
- Cercek, A.; Lumish, M.; Sinopoli, J.; Weiss, J.; Shia, J.; Lamendola-Essel, M.; El Dika, I.H.; Segal, N.; Shcherba, M.; Sugarman, R.; et al. PD-1 Blockade in Mismatch Repair–Deficient, Locally Advanced Rectal Cancer. N. Engl. J. Med. 2022, 386, 2363–2376. [Google Scholar] [CrossRef] [PubMed]
- Ascierto, P.A.; Addeo, R.; Cartenì, G.; Daniele, B.; De Laurentis, M.; Ianniello, G.P.; Morabito, A.; Palmieri, G.; Pepe, S.; Perrone, F.; et al. The role of immunotherapy in solid tumors: Report from the Campania Society of Oncology Immunotherapy (SCITO) meeting, Naples 2014. J. Transl. Med. 2014, 12, 291. [Google Scholar] [CrossRef]
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.; Choo, S.; Trojan, J.; Welling, T.H., 3rd; et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017, 389, 2492–2502. [Google Scholar] [CrossRef]
- DeCarli, K.; Strosberg, J.; Almhanna, K. Immune Checkpoint Inhibitors for Gastrointestinal Malignancies: An Update. Cancers 2022, 14, 4201. [Google Scholar] [CrossRef]
- Yau, T.; Park, J.-W.; Finn, R.S.; Cheng, A.; Mathurin, P.; Edeline, J.; Kudo, M.; Harding, J.J.; Merle, P.; Rosmorduc, O.; et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): A randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022, 23, 77–90. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Sangro, B.; Chan, S.L.; Kelley, R.K.; Lau, G.; Kudo, M.; Sukeepaisarnjaroen, W.; Yarchoan, M.; De Toni, E.N.; Furuse, J.; Kang, Y.K.; et al. Four-year overall survival update from the phase III HIMALAYA study of tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. Ann. Oncol. 2024, 35, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Ricci, A.D.; Brandi, G. Pemigatinib: Hot topics behind the first approval of a targeted therapy in cholangiocarcinoma. Cancer Treat. Res. Commun. 2021, 27, 100337. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.-Y.; He, A.R.; Qin, S.; Chen, L.; Okusaka, T.; Vogel, A.; Kim, J.W.; Suksombooncharoen, T.; Lee, M.A.; Kitano, M.; et al. A phase 3 randomized, double-blind, placebo-controlled study of durvalumab in combination with gemcitabine plus cisplatin (GemCis) in patients (pts) with advanced biliary tract cancer (BTC): TOPAZ-1. J. Clin. Oncol. 2022, 40, 378. [Google Scholar] [CrossRef]
- Speckart, J.; Rasmusen, V.; Talib, Z.; GnanaDev, D.A.; Rahnemai-Azar, A.A. Emerging Therapies in Management of Cholangiocarcinoma. Cancers 2024, 16, 613. [Google Scholar] [CrossRef]
- Li, R.; Liu, Y.; Liu, Y.; Fu, L.; Qi, Q.; Zhang, X.; Cui, Y.; Lu, W.; Li, H. Durvalumab plus gemcitabine/cisplatin/nab-paclitaxel in resectable biliary tract cancer: A phase II, single-arm, open-label study (DurGAP). J. Clin. Oncol. 2024, 42, e16260. [Google Scholar] [CrossRef]
- Naganuma, A.; Sakuda, T.; Murakami, T.; Aihara, K.; Watanuki, Y.; Suzuki, Y.; Shibasaki, E.; Masuda, T.; Uehara, S.; Yasuoka, H.; et al. Microsatellite Instability-high Intrahepatic Cholangiocarcinoma with Portal Vein Tumor Thrombosis Successfully Treated with Pembrolizumab. Int. Med. 2020, 59, 2261–2267. [Google Scholar] [CrossRef]
- Nakamura, M.; Ueno, M.; Hayami, S.; Kawai, M.; Miyamoto, A.; Suzaki, N.; Hironi, S.; Okada, K.; Miyazawa, M.; Kitahata, Y.; et al. Effective Response of Intrahepatic Cholangiocarcinoma to Pembrolizumab: A Case Report. Anticancer. Res. 2020, 40, 4123–4129. [Google Scholar] [CrossRef]
- Czink, E.; Kloor, M.; Goeppert, B.; Fröhling, S.; Uhrig, S.; Weber, T.F.; Meinel, J.; Sutter, C.; Weiss, K.H.; Schirmacher, P.; et al. Successful immune checkpoint blockade in a patient with advanced stage microsatellite-unstable biliary tract cancer. Mol. Case Stud. 2017, 3, a001974. [Google Scholar] [CrossRef]
- Ikeda, Y.; Ono, M.; Ohmori, G.; Ameda, S.; Yamada, M.; Abe, T.; Fujii, S.; Fujita, M.; Maeda, M. Successful pembrolizumab treatment of microsatellite instability-high intrahepatic cholangiocarcinoma: A case report. Clin. Case Rep. 2021, 9, 2259–2263. [Google Scholar] [CrossRef]
- Bang, Y.-J.; Ueno, M.; Malka, D.; Chung, H.C.; Nagrial, A.; Kelley, R.K.; Piha-Paul, S.A.; Ros, W.; Italiano, A.; Nakagawa, K.; et al. Pembrolizumab (pembro) for advanced biliary adenocarcinoma: Results from the KEYNOTE-028 (KN028) and KEYNOTE-158 (KN158) basket studies. J. Clin. Oncol. 2019, 37, 4079. [Google Scholar] [CrossRef]
- Saeed, A.; Park, R.; Al-Jumayli, M.; Al-Rajabi, R.; Sun, W. Biologics, Immunotherapy, and Future Directions in the Treatment of Advanced Cholangiocarcinoma. Clin. Color. Cancer 2019, 18, 81–90. [Google Scholar] [CrossRef]
- LaPelusa, M.; Heumann, T.; Goff, L.; Agarwal, R. Targeted therapies in advanced biliary tract cancers—A narrative review. Chin. Clin. Oncol. 2023, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Lo, J.H.; Agarwal, R.; Goff, L.W.; Heumann, T.R. Immunotherapy in Biliary Tract Cancers: Current Standard-of-Care and Emerging Strategies. Cancers 2023, 15, 3312. [Google Scholar] [CrossRef] [PubMed]
- Kelley, R.K.; Yoo, C.; Finn, R.S.; Furuse, J.; Ren, Z.; Yau, T.; Klümpen, H.; Chan, S.L.; Vogel, A.; Ozaka, M.; et al. Abstract CT008: Pembrolizumab (pembro) in combination with gemcitabine and cisplatin (gem/cis) for advanced biliary tract cancer (BTC): Phase 3 KEYNOTE-966 study. Cancer Res. 2023, 83, CT008. [Google Scholar] [CrossRef]
- Kawamoto, M.; Wada, Y.; Koya, N.; Takama, Y.; Saitsu, H.; Ishizaki, N.; Tabata, M.; Onishi, H.; Nakamura, M.; Morisaki, T. Long-term survival of a patient with recurrent gallbladder carcinoma, treated with chemotherapy, immunotherapy, and surgery: A case report. Surg. Case Rep. 2018, 4, 115. [Google Scholar] [CrossRef]
- Hack, S.P.; Verret, W.; Mulla, S.; Liu, B.; Wang, Y.; Macarulla, T.; Ren, Z.; El-Khoueiry, A.B.; Zhu, A.X. IMbrave 151: A randomized phase II trial of atezolizumab combined with bevacizumab and chemotherapy in patients with advanced biliary tract cancer. Ther. Adv. Med. Oncol. 2021, 13, 175883592110365. [Google Scholar] [CrossRef]
- El-Khoueiry, A.B.; Ren, Z.; Chon, H.; Park, J.O.; Kim, J.W.; Pressiani, T.; Li, D.; Zhukova, L.; Chen, M.; Hack, S.P.; et al. IMbrave151: A phase 2, randomized, double-blind, placebo-controlled study of atezolizumab with or without bevacizumab in combination with cisplatin plus gemcitabine in patients with untreated, advanced biliary tract cancer. J. Clin. Oncol. 2023, 41, 491. [Google Scholar] [CrossRef]
- El-Khoueiry, A.B.; Ren, Z.; Chon, H.J.; Park, J.O.; Kim, J.W.; Pressiani, T.; Li, D.; Zhukova, L.; Zhu, A.X.; Chen, M.; et al. Atezolizumab plus chemotherapy with or without bevacizumab in advanced biliary tract cancer: Results from a randomized proof-of-concept phase II trial (IMbrave151). J. Clin. Oncol. 2024, 42, 435. [Google Scholar] [CrossRef]
- Shi, G.-M.; Huang, X.-Y.; Wu, D.; Sun, H.; Liang, F.; Ji, Y.; Chen, Y.; Yang, G.; Lu, J.; Meng, X.; et al. Toripalimab combined with lenvatinib and GEMOX is a promising regimen as first-line treatment for advanced intrahepatic cholangiocarcinoma: A single-center, single-arm, phase 2 study. Signal Transduct. Target. Ther. 2023, 8, 106. [Google Scholar] [CrossRef]
- Tan, Y.; Trent, J.C.; Wilky, B.A.; Kerr, D.A.; Rosenberg, A.E. Current status of immunotherapy for gastrointestinal stromal tumor. Cancer Gene Ther. 2017, 24, 130–133. [Google Scholar] [CrossRef]
- Li, B.; Chen, H.; Yang, S.; Chen, F.; Xu, L.; Li, Y.; Li, M.; Zhu, C.; Shao, F.; Zhang, X.; et al. Advances in immunology and immunotherapy for mesenchymal gastrointestinal cancers. Mol. Cancer. 2023, 22, 71. [Google Scholar] [CrossRef]
- Blay, J.-Y.; Serrano, C.; Heinrich, M.C.; Zalcberg, J.; Bauer, S.; Gelderblom, H.; Schöffski, P.; Jones, R.L.; Attia, S.; D’Amato, G.; et al. Ripretinib in patients with advanced gastrointestinal stromal tumours (INVICTUS): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2020, 21, 923–934, Erratum in Lancet Oncol. 2020, 21, e341. [Google Scholar] [CrossRef] [PubMed]
- Seifert, A.M.; Zeng, S.; Zhang, J.Q.; Kim, T.S.; Cohen, N.A.; Beckman, M.J.; Medina, B.D.; Maltbaek, J.H.; Loo, J.K.; Crawley, M.H.; et al. PD-1/PD-L1 Blockade Enhances T-cell Activity and Antitumor Efficacy of Imatinib in Gastrointestinal Stromal Tumors. Clin. Cancer Res. 2017, 23, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.; Geva, R.; Gottfried, M.; et al. Efficacy of Pembrolizumab in Patients with Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef]
- Pedersen, K.S.; Foster, N.R.; Overman, M.J.; Boland, P.M.; Kim, S.S.; Arrambide, K.A.; Jaszewski, B.L.; Bekaii-Saab, T.; Graham, R.P.; Welch, J.; et al. ZEBRA: A Multicenter Phase II Study of Pembrolizumab in Patients with Advanced Small-Bowel Adenocarcinoma. Clin. Cancer Res. 2021, 27, 3641–3648. [Google Scholar] [CrossRef]
- Chae, Y.K.; Othus, M.; Patel, S.P.; Zalupski, M.; Kasi, A.; Khalil, M.; Kalyan, A.; Polite, B.; Fenton, S.; Gurung, S.; et al. Abstract 3417: A phase II basket trial of dual anti-CTLA-4 and anti-PD-1 blockade in rare tumors (DART) SWOG S1609: The small bowel tumor cohort. Cancer Res. 2020, 80, 3417. [Google Scholar] [CrossRef]
- Klein, O.; Kee, D.; Markman, B.; Michael, M.; Underhill, C.; Carlino, M.S.; Jackett, L.; Lum, C.; Scott, C.; Nagrial, A.; et al. Immunotherapy of Ipilimumab and Nivolumab in Patients with Advanced Neuroendocrine Tumors: A Subgroup Analysis of the CA209-538 Clinical Trial for Rare Cancers. Clin. Cancer Res. 2020, 26, 4454–4459. [Google Scholar] [CrossRef]
- Patel, S.P.; Mayerson, E.; Chae, Y.K.; Strosberg, J.; Wang, J.; Konda, B.; Hayward, J.; McLeod, C.M.; Chen, H.X.; Sharon, E.; et al. A phase II basket trial of Dual Anti-CTLA-4 and Anti-PD-1 Blockade in Rare Tumors (DART) SWOG S1609: High-grade neuroendocrine neoplasm cohort. Cancer 2021, 127, 3194–3201. [Google Scholar] [CrossRef]
- Al-Toubah, T.; Halfdanarson, T.; Gile, J.; Morse, B.; Sommerer, K.; Strosberg, J. Efficacy of ipilimumab and nivolumab in patients with high-grade neuroendocrine neoplasms. ESMO Open. 2022, 7, 100364. [Google Scholar] [CrossRef]
- Weber, M.M.M.; Apostolidis, L.; Krug, S.; Rinke, A.; Gruen, B.; Michl, P.; Gress, T.M.; Wagner, D.; Roth, W.; Mettler, E.; et al. 1185MO Activity and safety of avelumab alone or in combination with cabozantinib in patients with advanced high grade neuroendocrine neoplasias (NEN G3) progressing after chemotherapy. The phase II, open-label, multicenter AVENEC and CABOAVENEC trials. Ann. Oncol. 2023, 34, S702. [Google Scholar] [CrossRef]
- Fottner, C.; Apostolidis, L.; Krug, S.; Rinke, A.; Grün, B.; Michl, P.; Gress, T.M.; Wagner, D.; Roth, W.; Mettler, E.; et al. Activity and safety of avelumab in high-grade neuroendocrine tumors and poorly differentiated neuroendocrine carcinomas progressive after chemotherapy (AveNEC trial). Clin. Cancer Res. 2025, 31, 860–867. [Google Scholar] [CrossRef]
- Morris, V.K.; Salem, M.E.; Nimeiri, H.; Iqbal, S.; Singh, P.; Ciombor, K.; Polite, B.; Deming, D.; Chan, E.; Wade, J.L.; et al. Nivolumab for previously treated unresectable metastatic anal cancer (NCI9673): A multicentre, single-arm, phase 2 study. Lancet Oncol. 2017, 18, 446–453. [Google Scholar] [CrossRef]
- Lum, C.; Prenen, H.; Body, A.; Lam, M.; Segelov, E. A 2020 update of anal cancer: The increasing problem in women and expanding treatment landscape. Expert. Rev. Gastroenterol. Hepatol. 2020, 14, 665–680. [Google Scholar] [CrossRef]
- Ott, P.A.; Piha-Paul, S.A.; Munster, P.; Pishvaian, M.J.; van Brummelen, E.M.J.; Cohen, R.B.; Gomez-Roca, C.; Ejadi, S.; Stein, M.; Chan, E.; et al. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with recurrent carcinoma of the anal canal. Ann. Oncol. 2017, 28, 1036–1041. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Azad, N.; Chen, Y.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Garrido-Laguna, I.; et al. Anal Carcinoma, Version 2.2023, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2023, 21, 653–677. [Google Scholar] [CrossRef] [PubMed]
- Jayakrishnan, T.; Kamath, S.; Laderian, B.; Krishnamurthi, S. Recent Clinical Advances in Rare Gastrointestinal Tumors. Adv. Oncol. 2023, 3, 179–189. [Google Scholar] [CrossRef]
- Weitz, J.; de Mendoza, T.H.; Tiriac, H.; Lee, J.; Sun, S.; Garg, B.; Patel, J.; Li, K.; Baumgartner, J.; Kelly, K.J.; et al. An Ex Vivo Organotypic Culture Platform for Functional Interrogation of Human Appendiceal Cancer Reveals a Prominent and Heterogenous Immunological Landscape. Clin. Cancer Res. 2022, 28, 4793–4806. [Google Scholar] [CrossRef]
- Al Attar, L.; Truong, P. The Effect of Pembrolizumab in Absence of Programmed Death 1 Receptor. Cureus 2018, 10, e2896. [Google Scholar] [CrossRef]
- Forsythe, S.D.; Erali, R.A.; Sasikumar, S.; Laney, P.; Shelkey, E.; D’Agostino, R., Jr.; Miller, L.D.; Shen, P.; Levine, E.A.; Soker, S.; et al. Organoid Platform in Preclinical Investigation of Personalized Immunotherapy Efficacy in Appendiceal Cancer: Feasibility Study. Clin. Cancer Res. 2021, 27, 5141–5150. [Google Scholar] [CrossRef]
- Nivolumab and Ipilimumab in Mucinous Colorectal and Appendiceal Tumors. Available online: https://clinicaltrials.gov/study/NCT03693846 (accessed on 24 February 2024).
- Hornstein, N.J.; Zeineddine, M.A.; Gunes, B.B.; Pellatt, A.J.; Knafl, M.; Zhu, H.; Willett, A.F.; Yousef, A.; Liu, S.; Sun, R.; et al. Efficacy and Safety of Atezolizumab and Bevacizumab in Appendiceal Adenocarcinoma. Cancer Res. Commun. 2024, 4, 1363–1368. [Google Scholar] [CrossRef] [PubMed]
- Daiko, H.; Marafioti, T.; Fujiwara, T.; Shirakawa, Y.; Nakatsura, T.; Kato, K.; Puccio, I.; Hikichi, T.; Yoshimura, S.; Nakagawa, T.; et al. Exploratory open-label clinical study to determine the S-588410 cancer peptide vaccine-induced tumor-infiltrating lymphocytes and changes in the tumor microenvironment in esophageal cancer patients. Cancer Immunol. Immunother. 2020, 69, 2247–2257. [Google Scholar] [CrossRef] [PubMed]
- Makino, T.; Miyata, H.; Yasuda, T.; Kitagawa, Y.; Muro, K.; Park, J.; Hikichi, T.; Hasegawa, T.; Igarashi, K.; Iguchi, M.; et al. A phase 3, randomized, double-blind, multicenter, placebo-controlled study of S-588410, a five-peptide cancer vaccine as an adjuvant therapy after curative resection in patients with esophageal squamous cell carcinoma. Esophagus 2024, 21, 447–455. [Google Scholar] [CrossRef]
- Wang, C.; Pu, J.; Yu, H.; Liu, Y.; Yan, H.; He, Z.; Feng, X. A Dendritic Cell Vaccine Combined with Radiotherapy Activates the Specific Immune Response in Patients with Esophageal Cancer. J. Immunother. 2017, 40, 71–76. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Okada, K.; Omori, T.; Sugimura, K.; Miyata, H.; Ohue, M.; Kobayashi, S.; Takahashi, H.; Nakano, H.; Mochizuki, C.; et al. Multiple therapeutic peptide vaccines for patients with advanced gastric cancer. Int. J. Oncol. 2017, 50, 1655–1662. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Sugimura, K.; Miyata, H.; Omori, T.; Nakano, H.; Mochizuki, C.; Shimizu, K.; Saito, H.; Ashida, K.; Honjyo, S.; et al. A Pilot Study of Post-Operative Adjuvant Vaccine for Advanced Gastric Cancer. Yonago Acta Med. 2017, 60, 101–105. [Google Scholar] [CrossRef]
- Sundar, R.; Rha, S.Y.; Yamaue, H.; Katsuda, M.; Kono, K.; Kim, H.S.; Kim, C.; Mimura, K.; Kua, L.; Yong, W.P. A phase I/Ib study of OTSGC-A24 combined peptide vaccine in advanced gastric cancer. BMC Cancer 2018, 18, 332. [Google Scholar] [CrossRef]
- de Jesus, V.H.F.; Felismino, T.C.; de Barros e Silva, M.J.; de Souza e Silva, V.; Riechelmann, R.P. Current approaches to immunotherapy in noncolorectal gastrointestinal malignancies. Clinics 2018, 73, e510s. [Google Scholar] [CrossRef]
- Middleton, G.; Silcocks, P.; Cox, T.; Valle, J.; Wadsley, J.; Propper, D.; Coxon, F.; Ross, P.; Madhusudan, S.; Roques, T.; et al. Gemcitabine and capecitabine with or without telomerase peptide vaccine GV1001 in patients with locally advanced or metastatic pancreatic cancer (TeloVac): An open-label, randomised, phase 3 trial. Lancet Oncol. 2014, 15, 829–840. [Google Scholar] [CrossRef]
- Jo, J.H.; Kim, Y.-T.; Choi, H.S.; Kim, H.G.; Lee, H.S.; Choi, Y.W.; Kim, D.U.; Lee, K.H.; Kim, E.J.; Han, J.; et al. Efficacy of GV1001 with gemcitabine/capecitabine in previously untreated patients with advanced pancreatic ductal adenocarcinoma having high serum eotaxin levels (KG4/2015): An open-label, randomised, Phase 3 trial. Br. J. Cancer. 2024, 130, 43–52, Erratum in Br. J. Cancer 2024, 130, 163. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Wang-Gillam, A.; Picozzi, V.; Greten, T.F.; Crocenzi, T.; Springett, G.; Morse, M.; Zeh, H.; Cohen, D.; Fine, R.L.; et al. Safety and Survival with GVAX Pancreas Prime and Listeria Monocytogenes Expressing Mesothelin (CRS-207) Boost Vaccines for Metastatic Pancreatic Cancer. J. Clin. Oncol. 2015, 33, 1325–1333. [Google Scholar] [CrossRef]
- Lopez, J.; Powles, T.; Braiteh, F.; Siu, L.L.; LoRusso, P.; Friedman, C.F.; Balmanoukian, A.S.; Gordon, M.; Yachnin, J.; Rottey, S.; et al. Autogene cevumeran with or without atezolizumab in advanced solid tumors: A phase 1 trial. Nat. Med. 2025, 31, 152–164. [Google Scholar] [CrossRef]
- Rojas, L.A.; Sethna, Z.; Soares, K.C.; Olcese, C.; Pang, N.; Patterson, E.; Lihm, J.; Ceglia, N.; Guasp, P.; Chu, A.; et al. Personalized RNA neoantigen vaccines stimulate T cells in pancreatic cancer. Nature 2023, 618, 144–150. [Google Scholar] [CrossRef]
- Sethna, Z.; Guasp, P.; Reiche, C.; Milighetti, M.; Ceglia, N.; Patterson, E.; Lihm, J.; Payne, G.; Lyudovyk, O.; Rojas, L.A.; et al. RNA neoantigen vaccines prime long-lived CD8+ T cells in pancreatic cancer. Nature 2025, 639, 1042–1051. [Google Scholar] [CrossRef]
- Stallard, J. In Early-Phase Pancreatic Cancer Clinical Trial, Investigational mRNA Vaccine Induces Sustained Immune Activity in Small Patient Group. 2025. Available online: https://www.mskcc.org/news/can-mrna-vaccines-fight-pancreatic-cancer-msk-clinical-researchers-are-trying-find-out (accessed on 21 July 2025).
- Jia, W.; Zhang, T.; Huang, H.; Feng, H.; Wang, S.; Guo, Z.; Luo, Z.; Ji, X.; Cheng, X.; Zhao, R. Colorectal cancer vaccines: The current scenario and future prospects. Front Immunol. 2022, 13, 942235. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.; Castañón, E.; Perez-Gracia, J.L.; Rodriguez, I.; Viudez, A.; Alfaro, C.; Oñate, C.; Perez, G.; Rotellar, F.; Inogés, S.; et al. A randomized phase II clinical trial of dendritic cell vaccination following complete resection of colon cancer liver metastasis. J. Immunother. Cancer 2018, 6, 96. [Google Scholar] [CrossRef] [PubMed]
- Tada, F.; Abe, M.; Hirooka, M.; Ikeda, Y.; Hiasa, Y.; Lee, Y.; Jung, N.; Lee, W.; Lee, H.; Bae, Y.; et al. Phase I/II study of immunotherapy using tumor antigen-pulsed dendritic cells in patients with hepatocellular carcinoma. Int. J. Oncol. 2012, 41, 1601–1609. [Google Scholar] [CrossRef] [PubMed]
- Tojjari, A.; Saeed, A.; Singh, M.; Cavalcante, L.; Sahin, I.H.; Saeed, A. A Comprehensive Review on Cancer Vaccines and Vaccine Strategies in Hepatocellular Carcinoma. Vaccines 2023, 11, 1357. [Google Scholar] [CrossRef]
- Andersson, M.; Jalnefjord, O.; Montelius, M.; Rizell, M.; Sternby Eilard, M.; Ljungberg, M. Evaluation of response in patients with hepatocellular carcinoma treated with intratumoral dendritic cell vaccination using intravoxel incoherent motion (IVIM) MRI and histogram analysis. Acta Radiol. 2023, 64, 32–41. [Google Scholar] [CrossRef]
- Rizell, M.; Sternby Eilard, M.; Andersson, M.; Andersson, B.; Karlsson-Parra, A.; Suenaert, P. Phase 1 Trial with the Cell-Based Immune Primer Ilixadencel, Alone, and Combined with Sorafenib, in Advanced Hepatocellular Carcinoma. Front. Oncol. 2019, 9, 19. [Google Scholar] [CrossRef]
- Kaida, M.; Morita-Hoshi, Y.; Soeda, A.; Wakeda, T.; Yamaki, Y.; Kojima, Y.; Ueno, H.; Kondo, S.; Morizane, C.; Ikeda, M.; et al. Phase 1 Trial of Wilms Tumor 1 (WT1) Peptide Vaccine and Gemcitabine Combination Therapy in Patients with Advanced Pancreatic or Biliary Tract Cancer. J. Immunother. 2011, 34, 92–99. [Google Scholar] [CrossRef]
- Kalyan, A.; Khosla, H.; Kim, R.D. Immunotherapy in Biliary Tract Cancers: Where Are We? Curr. Oncol. Rep. 2022, 24, 1821–1828. [Google Scholar] [CrossRef]
- Aruga, A.; Takeshita, N.; Kotera, Y.; Okuyama, R.; Matsushita, N.; Ohta, T.; Takeda, K.; Yamamoto, M. Long-term Vaccination with Multiple Peptides Derived from Cancer-Testis Antigens Can Maintain a Specific T-cell Response and Achieve Disease Stability in Advanced Biliary Tract Cancer. Clin. Cancer Res. 2013, 19, 2224–2231. [Google Scholar] [CrossRef] [PubMed]
- Aruga, A.; Takeshita, N.; Kotera, Y.; Okuyama, R.; Matsushita, N.; Ohta, T.; Takeda, K.; Yamamoto, M. Phase I clinical trial of multiple-peptide vaccination for patients with advanced biliary tract cancer. J. Transl. Med. 2014, 12, 61. [Google Scholar] [CrossRef] [PubMed]
- Murahashi, M.; Tsuruta, T.; Yamada, K.; Hijikata, Y.; Ogata, H.; Kishimoto, J.; Yoshimura, S.; Hikichi, T.; Nakanishi, Y.; Tani, K. Clinical Trial of a Cancer Vaccine Targeting VEGF and KIF20A in Advanced Biliary Tract Cancer. Anticancer Res. 2021, 41, 1485–1496. [Google Scholar] [CrossRef]
- Shioyama, Y.; Yakeishi, Y.; Watanabe, T.; Nakanura, K.; Kunitake, N.; Kimura, M.; Sasaki, M.; Honda, H.; Terashima, H.; Masuda, K. Long-Term Control for a Retroperitoneal Metastasis of Malignant Gastrointestinal Stromal Tumor after Chemoradiotherapy and Immunotherapy. Acta Oncol. 2001, 40, 102–104. [Google Scholar] [CrossRef]
- Fröbom, R.; Berglund, E.; Berglund, D.; Nilsson, I.; Åhlén, J.; von Sivers, K.; Linder-Stragliotto, C.; Suenaert, P.; Karlsson-Parra, A.; Bränström, R. Phase I trial evaluating safety and efficacy of intratumorally administered inflammatory allogeneic dendritic cells (ilixadencel) in advanced gastrointestinal stromal tumors. Cancer Immunol. Immunother. 2020, 69, 2393–2401. [Google Scholar] [CrossRef]
- Karlsson-Parra, A.; Fröbom, R.; Berglund, E.; Nilsson, I.; Linder-Stragliotto, C.; Suenaert, P.; Bränström, R. Phase I trial evaluating safety and efficacy of intratumorally administered allogeneic monocyte-derived cells (ilixadencel) in advanced gastrointestinal stromal tumors. J. Clin. Oncol. 2020, 38, 15. [Google Scholar] [CrossRef]
- Conlon, K.C.; Miljkovic, M.D.; Waldmann, T.A. Cytokines in the Treatment of Cancer. J. Interferon Cytokine Res. 2019, 39, 6–21. [Google Scholar] [CrossRef]
- Partyka, S.; Dumas, P.; Ajani, J. Combination chemotherapy with granulocyte-macrophage-colony stimulating factor in patients with locoregional and metastatic gastric adenocarcinoma. Cancer 1999, 85, 2336–2339. [Google Scholar] [CrossRef]
- Badamgarav, E.; Lyman, G.H.; Pinto, L.; Bernal, M.; Dubois, R.W. Efficacy of GM-CSF vs G-CSF in reducing chemotherapy-induced complications (CIC): A systematic review of literature. J. Clin. Oncol. 2004, 22, 6140. [Google Scholar] [CrossRef]
- Mavroudis, D.; Kourousis, C.; Androulakis, N.; Kalbakis, K.; Agelaki, S.; Kakolyris, S.; Souglakos, J.; Sarra, E.; Vardakis, N.; Hatzidaki, D.; et al. Frontline treatment of advanced gastric cancer with docetaxel and granulocyte colony-stimulating factor (G-CSF): A phase II trial. Am. J. Clin. Oncol. 2000, 23, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Kornek, G.V.; Raderer, M.; Schüll, B.; Fiebiger, W.; Gedlicka, C.; Lanauer, A.; Depisch, D.; Schneeweiss, B.; Lang, F.; Scheithauer, W. Effective combination chemotherapy with paclitaxel and cisplatin with or without human granulocyte colony-stimulating factor and/or erythropoietin in patients with advanced gastric cancer. Br. J. Cancer 2002, 86, 1858–1863. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dadgar, N.; Sherry, C.; Zimmerman, J.; Park, H.; Lewis, C.; Donnenberg, A.; Zaidi, A.H.; Fan, Y.; Xiao, K.; Bartlett, D.; et al. Targeting interleukin-6 as a treatment approach for peritoneal carcinomatosis. J. Transl. Med. 2024, 22, 402. [Google Scholar] [CrossRef] [PubMed]
- Ham, I.-H.; Oh, H.J.; Jin, H.; Bae, C.A.; Jeon, S.; Choi, K.S.; Son, S.; Han, S.; Brekken, R.A.; Lee, D.; et al. Targeting interleukin-6 as a strategy to overcome stroma-induced resistance to chemotherapy in gastric cancer. Mol. Cancer 2019, 18, 68. [Google Scholar] [CrossRef]
- Ruzzo, A.; Catalano, V.; Canestrari, E.; Giacomini, E.; Santini, D.; Tonini, G.; Vincenzi, B.; Fiorentini, G.; Magnani, M.; Graziano, F. Genetic modulation of the interleukin 6 (IL-6) system in patients with advanced gastric cancer: A background for an alternative target therapy. BMC Cancer 2014, 14, 357. [Google Scholar] [CrossRef]
- Nukui, Y.; Picozzi, V.J.; Traverso, L.W. Interferon-based adjuvant chemoradiation therapy improves survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Am. J. Surg. 2000, 179, 367–371. [Google Scholar] [CrossRef]
- Schmidt, J.; Jäger, D.; Hoffmann, K.; Büchler, M.W.; Märten, A. Impact of interferon-alpha in combined chemoradioimmunotherapy for pancreatic adenocarcinoma (CapRI): First data from the immunomonitoring. J. Immunother. 2007, 30, 108–115. [Google Scholar] [CrossRef]
- Picozzi, V.J.; Abrams, R.A.; Decker, P.A.; Traverso, W.; O’Reilly, E.M.; Greeno, E.; Martin, R.C.; Wilfong, L.S.; Rothenberg, M.L.; Posner, M.C.; et al. Multicenter phase II trial of adjuvant therapy for resected pancreatic cancer using cisplatin, 5-fluorouracil, and interferon-alfa-2b–based chemoradiation: ACOSOG Trial Z05031. Ann. Oncol. 2011, 22, 348–354. [Google Scholar] [CrossRef]
- Blaauboer, A.; Van Koetsveld, P.; Mustafa, D.; Dumas, J.; Dogan, F.; Van Zwienen, S.; Van Ejijck, C.H.J.; Hofland, L.J. Immunomodulatory antitumor effect of interferon-beta combined with gemcitabine in pancreatic cancer. Int. J. Oncol. 2022, 61, 97. [Google Scholar] [CrossRef]
- Infante, J.R.; Naing, A.; Papadopoulos, K.P.; Autio, K.A.; Ott, P.A.; Wong, D.J.L.; Falchook, G.S.; Patel, M.R.; Pant, S.; Whiteside, M.; et al. A first-in-human dose escalation study of PEGylated recombinant human IL-10 (AM0010) in advanced solid tumors. J. Clin. Oncol. 2015, 33, 3017. [Google Scholar] [CrossRef]
- Chen, L.L.; Chen, X.; Choi, H.; Sang, H.; Chen, L.C.; Zhang, H.; Gouw, L.; Andtbacka, R.H.; Chan, B.K.; Rodesch, C.K.; et al. Exploiting antitumor immunity to overcome relapse and improve remission duration. Cancer Immunol. Immunother. 2012, 61, 1113–1124. [Google Scholar] [CrossRef]
- Tavartkiladze, A.A.; Khutsishvili, R.; Revazishvili, P.; Maisuradze, M.; Tavartkiladze, L.; Tavartkiladze, G. Treatment of refractory recurrent gastrointestinal stromal tumors with adoptive cellular immunotherapy (TILs) and personalized vaccine. Ann. Oncol. 2019, 30, xi13. [Google Scholar] [CrossRef]
- Green, B.L.; Davis, J.L. Gastric adenocarcinoma peritoneal carcinomatosis: A narrative review. Dig Med. Res. 2022, 5, 37. [Google Scholar] [CrossRef] [PubMed]
- Wagner, P.L.; Knotts, C.M.; Donnenberg, V.S.; Dadgar, N.; Pico, C.X.C.; Xiao, K.; Zaidi, A.; Schiffman, S.C.; Allen, C.J.; Donnenberg, A.D.; et al. Characterizing the Immune Environment in Peritoneal Carcinomatosis: Insights for Novel Immunotherapy Strategies. Ann. Surg. Oncol. 2024, 31, 2069–2077. [Google Scholar] [CrossRef] [PubMed]
- Riihimäki, M.; Hemminki, A.; Sundquist, K.; Sundquist, J.; Hemminki, K. Metastatic spread in patients with gastric cancer. Oncotarget. 2016, 7, 52307–52316. [Google Scholar] [CrossRef] [PubMed]
- Thadi, A.; Khalili, M.; Morano, W.; Richard, S.; Katz, S.; Bowne, W. Early Investigations and Recent Advances in Intraperitoneal Immunotherapy for Peritoneal Metastasis. Vaccines 2018, 6, 54. [Google Scholar] [CrossRef]
- Chelius, D.; Ruf, P.; Gruber, P.; Plöscher, M.; Liedtke, R.; Gansberger, E.; Hess, J.; Wasiliu, M.; Lindhofer, H. Structural and functional characterization of the trifunctional antibody catumaxomab. mAbs 2010, 2, 309–319. [Google Scholar] [CrossRef]
- Tocilizumab Delivered Via Pleural and Peritoneal Catheters in Patients with Advanced Metastatic Cancer. Available online: https://clinicaltrials.gov/study/NCT06016179 (accessed on 24 February 2024).
- Li, Y.; Duan, H.; Yang, K.; Ye, J. Advancements and challenges in oncolytic virus therapy for gastrointestinal tumors. Biomed. Pharmacother. 2023, 168, 115627. [Google Scholar] [CrossRef]
- Zhang, B.; Huang, J.; Tang, J.; Hu, S.; Luo, S.; Luo, Z.; Zhou, F.; Tan, S.; Ying, J.; Chang, Q.; et al. Intratumoral OH2, an oncolytic herpes simplex virus 2, in patients with advanced solid tumors: A multicenter, phase I/II clinical trial. J Immunother. Cancer 2021, 9, e002224. [Google Scholar] [CrossRef]
- Shirakawa, Y.; Tazawa, H.; Tanabe, S.; Kanaya, N.; Noma, K.; Koujima, T.; Kashima, H.; Kata, T.; Kuroda, S.; Kikuchi, S.; et al. Phase I dose-escalation study of endoscopic intratumoral injection of OBP-301 (Telomelysin) with radiotherapy in oesophageal cancer patients unfit for standard treatments. Eur. J. Cancer 2021, 153, 98–108. [Google Scholar] [CrossRef]
- Noonan, A.M.; Farren, M.R.; Geyer, S.M.; Huang, Y.; Tahiri, S.; Ahn, D.; Mikhail, S.; Ciombor, K.K.; Pant, S.; Apara, S.; et al. Randomized Phase 2 Trial of the Oncolytic Virus Pelareorep (Reolysin) in Upfront Treatment of Metastatic Pancreatic Adenocarcinoma. Mol. Ther. 2016, 24, 1150–1158. [Google Scholar] [CrossRef]
- Mahalingam, D.; Goel, S.; Aparo, S.; Arora, S.P.; Noronha, N.; Tran, H.; Chakrabarty, R.; Selvaggi, G.; Gutierrez, A.; Coffey, M.; et al. A Phase II Study of Pelareorep (REOLYSIN®) in Combination with Gemcitabine for Patients with Advanced Pancreatic Adenocarcinoma. Cancers 2018, 10, 160. [Google Scholar] [CrossRef]
- Mahalingam, D.; Wilkinson, G.A.; Eng, K.H.; Fields, P.; Raber, P.; Moseley, J.L.; Cheetham, K.; Coffey, M.; Nuovo, G.; Kalinski, P.; et al. Pembrolizumab in Combination with the Oncolytic Virus Pelareorep and Chemotherapy in Patients with Advanced Pancreatic Adenocarcinoma: A Phase Ib Study. Clin. Cancer Res. 2020, 26, 71–81. [Google Scholar] [CrossRef]
- Yoon, S.S.; Carroll, N.M.; Chiocca, E.A.; Tanabe, K.K. Cancer gene therapy using a replication-competent herpes simplex virus type 1 vector. Ann. Surg. 1998, 228, 366–374. [Google Scholar] [CrossRef]
- Park, S.H.; Breitbach, C.J.; Lee, J.; Park, J.O.; Lim, H.Y.; Kang, W.K.; Moon, A.; Mun, J.; Sommermann, E.M.; Avidal, L.M.; et al. Phase 1b Trial of Biweekly Intravenous Pexa-Vec (JX-594), an Oncolytic and Immunotherapeutic Vaccinia Virus in Colorectal Cancer. Mol. Ther. 2015, 23, 1532–1540. [Google Scholar] [CrossRef]
- Jonker, D.J.; Tang, P.A.; Kennecke, H.; Welch, S.A.; Cripps, M.C.; Asmis, T.; Chalchal, H.; Tomiak, A.; Lim, H.; Ko, Y.; et al. A Randomized Phase II Study of FOLFOX6/Bevacizumab with or without Pelareorep in Patients with Metastatic Colorectal Cancer: IND.210, a Canadian Cancer Trials Group Trial. Clin. Color. Cancer 2018, 17, 231–239.e7. [Google Scholar] [CrossRef] [PubMed]
- Moehler, M.; Heo, J.; Lee, H.C.; Tak, W.Y.; Chao, Y.; Paik, S.W.; Yim, H.J.; Byun, K.S.; Baron, A.; Ungerechts, G.; et al. Vaccinia-based oncolytic immunotherapy Pexastimogene Devacirepvec in patients with advanced hepatocellular carcinoma after sorafenib failure: A randomized multicenter Phase IIb trial (TRAVERSE). Oncoimmunology 2019, 8, 1615817. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.; Chao, Y.; Jonker, D.J.; Baron, A.D.; Habersetzer, F.; Burke, J.; Breitbach, C.; Patt, R.H.; Lencioni, R.; Homerin, M.; et al. Phase IIb randomized trial of Pexa-Vec (pexastimogene devacirepvec; JX-594), a targeted oncolytic vaccinia virus, plus best supportive care (BSC) versus BSC alone in patients with advanced hepatocellular carcinoma who have failed sorafenib treatment (TRAVERSE). J. Clin. Oncol. 2013, 31, TPS4161. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Galle, P.R.; Chao, Y.; Erinjeri, J.; Heo, J.; Borad, M.J.; Luca, A.; Burke, J.; Pelusio, A.; Agathon, D.; et al. PHOCUS: A Phase 3, Randomized, Open-Label Study of Sequential Treatment with Pexa-Vec (JX-594) and Sorafenib in Patients with Advanced Hepatocellular Carcinoma. Liver Cancer 2024, 13, 248–264. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Galle, P.R.; Chao, Y.; Brown, K.T.; Heo, J.; Borad, M.J.; Luca, A.; Pelusio, A.; Agathon, D.; Lusky, M.; et al. PHOCUS: A phase 3 randomized, open-label study comparing the oncolytic immunotherapy Pexa-Vec followed by sorafenib (SOR) vs SOR in patients with advanced hepatocellular carcinoma (HCC) without prior systemic therapy. J. Clin. Oncol. 2016, 34, TPS4146. [Google Scholar] [CrossRef]
- Miller, A.M.; Lemke-Miltner, C.D.; Blackwell, S.; Tomanek-Chalkley, A.; Gibson-Corely, K.N.; Coleman, K.L.; Weiner, G.J.; Chan, C.H.F. Intraperitoneal CMP-001: A Novel Immunotherapy for Treating Peritoneal Carcinomatosis of Gastrointestinal and Pancreaticobiliary Cancer. Ann. Surg. Oncol. 2021, 28, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Hu, W. Advances in adoptive cellular therapy for colorectal cancer: A narrative review. Ann. Transl. Med. 2022, 10, 1404. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, S.; Ikeda, H.; Miyahara, Y.; Imai, N.; Ishihara, M.; Saito, K.; Sugino, S.; Ueda, S.; Ishikawa, T.; Kokura, S.; et al. Adoptive Transfer of MAGE-A4 T-cell Receptor Gene-Transduced Lymphocytes in Patients with Recurrent Esophageal Cancer. Clin. Cancer Res. 2015, 21, 2268–2277. [Google Scholar] [CrossRef]
- Gu, Y.-M.; Zhuo, Y.; Chen, L.-Q.; Yuan, Y. The Clinical Application of Neoantigens in Esophageal Cancer. Front. Oncol. 2021, 11, 703517. [Google Scholar] [CrossRef]
- Shi, L.; Zhou, Q.; Wu, J.; Ji, M.; Li, G.; Jiang, J.; Wu, C. Efficacy of adjuvant immunotherapy with cytokine-induced killer cells in patients with locally advanced gastric cancer. Cancer Immunol. Immunother. 2012, 61, 2251–2259. [Google Scholar] [CrossRef]
- Kono, K.; Takahashi, A.; Ichihara, F.; Amemiya, H.; Iizuka, H.; Fujii, H.; Sekikawa, T.; Matsumoto, Y. Prognostic Significance of Adoptive Immunotherapy with Tumor-associated Lymphocytes in Patients with Advanced Gastric Cancer: A Randomized Trial. Clin. Cancer Res. 2002, 8, 1767–1771. [Google Scholar]
- Hadfield, M.J.; Safran, H.; Purbhoo, M.A.; Grossman, J.E.; Buell, J.S.; Carneiro, B.A. Overcoming resistance to programmed cell death protein 1 (PD-1) blockade with allogeneic invariant natural killer T-cells (iNKT). Oncogene 2024, 43, 758–762. [Google Scholar] [CrossRef]
- Carneiro, B.; Garmezy, B.; Hamm, J.T.; Sanborn, R.E.; Wise-Draper, T.; El-Khoueiry, A.; Wilky, B.; Hoon, D.S.B.; Buffa, A.; Michelet, X.; et al. Abstract CT275: Phase 1 clinical update of allogeneic invariant natural killer T cells (iNKTs), agenT-797, alone or in combination with pembrolizumab or nivolumab in patients with advanced solid tumors. Cancer Res. 2023, 83, CT275. [Google Scholar] [CrossRef]
- Leidner, R.; Sanjuan Silva, N.; Huang, H.; Sprott, D.; Zheng, C.; Shih, Y.; Leung, A.; Payne, R.; Sutcliffe, K.; Cramer, J.; et al. Neoantigen T-Cell Receptor Gene Therapy in Pancreatic Cancer. N. Engl. J. Med. 2022, 386, 2112–2119. [Google Scholar] [CrossRef]
- Reusch, U.; Sundaram, M.; Davol, P.A.; Olson, S.D.; Davis, J.B.; Demel, K.; Nissim, J.; Rathore, R.; Liu, P.Y.; Lum, L.G. Anti-CD3 x anti-epidermal growth factor receptor (EGFR) bispecific antibody redirects T-cell cytolytic activity to EGFR-positive cancers in vitro and in an animal model. Clin. Cancer Res. 2006, 12, 183–190. [Google Scholar] [CrossRef]
- Lum, L.G.; Thakur, A.; Choi, M.; Deol, A.; Kondadasula, V.; Schalk, D.; Fields, K.; Dufrense, M.; Philip, P.; Dyson, G.; et al. Clinical and immune responses to anti-CD3 x anti-EGFR bispecific antibody armed activated T cells (EGFR BATs) in pancreatic cancer patients. Oncoimmunology 2020, 9, 1773201. [Google Scholar] [CrossRef]
- Li, C.; Yang, N.; Li, H.; Wang, Z. Robo1-specific chimeric antigen receptor natural killer cell therapy for pancreatic ductal adenocarcinoma with liver metastasis. J. Cancer Res. Ther. 2020, 16, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, Z.; Yang, Z.; Wang, W.; Li, S.; Li, Y.; Zhang, R.; Xiong, Z.; Wei, Z.; Shen, J.; et al. Phase I Escalating-Dose Trial of CAR-T Therapy Targeting CEA+ Metastatic Colorectal Cancers. Mol. Ther. 2017, 25, 1248–1258. [Google Scholar] [CrossRef] [PubMed]
- Shaza, L.; Henlisz, A.; Awada, A.; Canon, J.; Carrasco, J.; Van Cutsem, E.; Dekervel, J.; Alcantar-Orozco, E.; Renard, F.; Cerf, E.; et al. Results from the completed dose-escalation phase I SHRINK study evaluating the autologous NKG2D-based CAR T-cell therapy CYAD-01 in metastatic colorectal cancer patients. Lung 2019, 3, 33. [Google Scholar]
- Prenen, H.; Dekervel, J.; Hendlisz, A.; Anguille, S.; Awada, A.; Cerf, E.; Lonez, C.; Breman, E.; Dheur, M.; Alcantar-Orozco, E.; et al. Updated data from alloSHRINK phase I first-in-human study evaluating CYAD-101, an innovative non-gene edited allogeneic CAR-T in mCRC. J. Clin. Oncol. 2021, 39, 74. [Google Scholar] [CrossRef]
- Tran, E.; Robbins, P.F.; Lu, Y.-C.; Prickett, T.D.; Gartner, J.J.; Jia, L.; Pasetto, A.; Zheng, Z.; Ray, S.; Groh, E.M.; et al. T-Cell Transfer Therapy Targeting Mutant KRAS in Cancer. N. Engl. J. Med. 2016, 375, 2255–2262. [Google Scholar] [CrossRef]
- National Cancer Institute: Immunotherapy Using Tumor Infiltrating Lymphocytes for Patients with Metastatic Cancer. Available online: https://clinicaltrials.gov/study/NCT01174121?cond=nci%2001174121&term=NCT01174121&rank=1#publications (accessed on 7 February 2025).
- A Study of TBio-4101 (TIL) and Pembrolizumab in Patients with Advanced Solid Tumors (STARLING). Available online: https://clinicaltrials.gov/study/NCT05576077?cond=nci%2001174121&term=NCT05576077&rank=1 (accessed on 12 September 2024).
- Zhu, Y.; Li, X.; Chen, T.; Wang, J.; Zhou, Y.; Mu, X.; Du, Y.; Wang, J.; Tang, J.; Liu, J. Personalised neoantigen-based therapy in colorectal cancer. Clin. Transl. Med. 2023, 13, e1461. [Google Scholar] [CrossRef]
- Qin, W.; Cao, Z.-Y.; Liu, S.-Y.; Xu, X.-D. Recent advances regarding tumor microenvironment and immunotherapy in hepatocellular carcinoma. Hepatoma Res. 2020, 6, 24. [Google Scholar] [CrossRef]
- Huang, Z.-M.; Li, W.; Li, S.; Gao, F.; Zhou, Q.; Wu, F.; He, N.; Pan, C.; Xia, J.; Wu, P.; et al. Cytokine-induced Killer Cells in Combination with Transcatheter Arterial Chemoembolization and Radiofrequency Ablation for Hepatocellular Carcinoma Patients. J. Immunother. 2013, 36, 287–293. [Google Scholar] [CrossRef]
- Yu, X.; Zhao, H.; Liu, L.; Cao, S.; Ren, B.; Zhang, N.; An, X.; Yu, J.; Li, H.; Ren, X. A randomized phase II study of autologous cytokine-induced killer cells in treatment of hepatocellular carcinoma. J. Clin. Immunol. 2014, 34, 194–203. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, J.-H.; Lim, Y.-S.; Yeon, J.E.; Song, T.; Yu, S.J.; Gwak, G.; Kim, K.M.; Kim, Y.J.; Lee, J.W.; et al. Adjuvant Immunotherapy with Autologous Cytokine-Induced Killer Cells for Hepatocellular Carcinoma. Gastroenterology 2015, 148, 1383–1391.e6. [Google Scholar] [CrossRef] [PubMed]
- Carisma Therapeutics: Carisma Therapeutics Announces Nomination of First In Vivo CAR-M Development Candidate for Hepatocellular Carcinoma Under Collaboration with Moderna. 2024. Available online: https://www.prnewswire.com/news-releases/carisma-therapeutics-announces-nomination-of-first-in-vivo-car-m-development-candidate-for-hepatocellular-carcinoma-under-collaboration-with-moderna-302185002.html (accessed on 12 September 2024).
- Liu, D.; Heij, L.R.; Czigany, Z.; Dahl, E.; Lang, S.A.; Ulmer, T.F.; Luedde, T.; Neumann, U.P.; Bednarsch, J. The role of tumor-infiltrating lymphocytes in cholangiocarcinoma. J. Exp. Clin. Cancer Res. 2022, 41, 127. [Google Scholar] [CrossRef] [PubMed]
- Sawasdee, N.; Thepmalee, C.; Sujjitjoon, J.; Yongpitakwattana, P.; Junking, M.; Poungvarin, N.; Yenchitsomanus, P.; Panya, A. Gemcitabine enhances cytotoxic activity of effector T-lymphocytes against chemo-resistant cholangiocarcinoma cells. Int. Immunopharmacol. 2020, 78, 106006. [Google Scholar] [CrossRef]
- Rogers, J.E.; Leung, M.; Johnson, B. Metastatic or Locally Recurrent Anal Squamous Cell Carcinoma (SCAC): Current Clinical Trial Landscape and Novel Approaches. Cancer Manag. Res. 2022, 14, 2065–2077. [Google Scholar] [CrossRef]
- Stevanović, S.; Helman, S.R.; Wunderlich, J.R.; Langhan, M.M.; Doran, S.L.; Kwong, M.L.M.; Somerville, R.P.T.; Klebanoff, C.A.; Kammula, U.S.; Sherry, R.M.; et al. A Phase II Study of Tumor-infiltrating Lymphocyte Therapy for Human Papillomavirus-associated Epithelial Cancers. Clin. Cancer Res. 2019, 25, 1486–1493. [Google Scholar] [CrossRef]
- Bustamante, L.; Frakes, J.; Hoffe, S.; Kim, R. Investigational drugs for treating anal cancer and future perspectives. Expert. Opin. Investig. Drugs. 2016, 25, 51–62. [Google Scholar] [CrossRef]
- Safran, H.; Leonard, K.L.; DiPetrillo, T.A.; Klipfel, A.; Schechter, S.; Oldenburg, N.; Vrees, M.; Roth, L.; Shah, N.; Mantripragada, K.C.; et al. ADXS11-001 Lm-LLO Immunotherapy, Mitomycin, 5-fluorouracil (5-FU) and Intensity-modulated radiation therapy (IMRT) for Anal Cancer. J. Clin. Oncol. 2017, 35, e15072. [Google Scholar] [CrossRef]
- Katz, S.C.; Point, G.R.; Cunetta, M.; Thorn, M.; Guha, P.; Espat, N.J.; Boutros, C.; Hanna, N.; Junghans, R.P. Regional CAR-T cell infusions for peritoneal carcinomatosis are superior to systemic delivery. Cancer Gene Ther. 2016, 23, 142–148. [Google Scholar] [CrossRef]
- Wang, D.; Lin, J.; Yang, X.; Long, J.; Bai, Y.; Yang, X.; Mao, Y.; Sang, X.; Seery, S.; Zhao, H. Combination regimens with PD-1/PD-L1 immune checkpoint inhibitors for gastrointestinal malignancies. J. Hematol. Oncol. 2019, 12, 42. [Google Scholar] [CrossRef]
- Abdul-Latif, M.; Townsend, K.; Dearman, C.; Shiu, K.-K.; Khan, K. Immunotherapy in gastrointestinal cancer: The current scenario and future perspectives. Cancer Treat. Rev. 2020, 88, 102030. [Google Scholar] [CrossRef]
- McGrath, K.; Dotti, G. Combining Oncolytic Viruses with Chimeric Antigen Receptor T Cell Therapy. Hum. Gene Ther. 2021, 32, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Lu, S.; Shi, M.; Yang, Z.; Liu, W.; Ni, Z.; Yao, X.; Hua, Z.; Feng, R.; Zheng, Y.; et al. Sintilimab combined neoadjuvant intraperitoneal and systemic chemotherapy in gastric cancer with peritoneal metastasis. Future Oncol. 2023, 19, 2517–2523. [Google Scholar] [CrossRef] [PubMed]
- Anhui Provincial Hospital: Postoperative Radiotherapy Followed by Immunotherapy for Locally Advanced Esophageal Carcinoma. Available online: https://www.clinicaltrials.gov/study/NCT05937438 (accessed on 7 February 2025).
- Huang, X.; Tang, H.; Jiao, H.; Yin, J.; Wang, H.; Xu, W.; Yin, H.; Yang, S.; Wang, Q.; Zeng, M.; et al. Neoadjuvant Immunotherapy combined with Chemoradiotherapy VS. Neoadjuvant Chemoradiotherapy for Locally Advanced Esophageal Squamous Cell Carcinoma(cStageII-III): Study Protocol For A Multi-center Prospective Randomized Clinical Trial. Res. Sq. 2024. [Google Scholar] [CrossRef]
- Chung, V.; Guthrie, K.A.; Pishvaian, M.J.; Reiss, K.A.; Lowy, A.M.; Sohal, D.; Colby, S.; Sharon, E.; Allegra, C.J.; O’Reilly, E.M.; et al. Randomized phase II trial of olaparib + pembrolizumab vs olaparib alone as maintenance therapy in metastatic pancreatic cancer patients with germline BRCA1 or BRCA2 (gBRCA1/2+) pathogenic variants: SWOG S2001. J. Clin. Oncol. 2023, 41, TPS4198. [Google Scholar] [CrossRef]
- Chung, V.; Guthrie, K.A.; Pishvaian, M.J.; Reiss, K.A.; Lowy, A.M.; Sohal, D.; Colby, S.; Sharon, E.; Allegra, C.J.; O’Reilly, E.M.; et al. Randomized phase II trial of olaparib + pembrolizumab vs olaparib alone as maintenance therapy in patients with metastatic pancreatic cancer with germline BRCA1 or BRCA2 mutations: SWOG S2001 NCT04548752. J. Clin. Oncol. 2025, 43, TPS793. [Google Scholar] [CrossRef]
- Peking Union Medical College Hospital: Pembrolizumab with or without Lenvatinib or Chemotherapy in First-Line Treatment of Advanced Biliary Tract Cancer. Available online: https://www.clinicaltrials.gov/study/NCT06230471 (accessed on 7 February 2025).
- Eastern Hepatobiliary Surgery Hospital: Envafolimab Combined with GEMOX in First-line Treatment of Advanced GBC. Available online: https://www.clinicaltrials.gov/study/NCT06013943 (accessed on 7 February 2025).
- Sinicrope, F.A.; Ou, F.-S.; Zemla, T.; Nixon, A.B.; Mody, K.; Levasseur, A.; Dueck, A.C.; Dhanarajan, A.R.; Lieu, C.H.; Cohen, D.J.; et al. Randomized trial of standard chemotherapy alone or combined with atezolizumab as adjuvant therapy for patients with stage III colon cancer and deficient mismatch repair (ATOMIC, Alliance A021502). J. Clin. Oncol. 2019, 37, e15169. [Google Scholar] [CrossRef]
- Yamazaki, K.; Satake, H.; Takashima, A.; Mizusawa, J.; Kataoka, T.; Fukuda, H.; Ishizuka, Y.; Suwa, Y.; Numata, K.; Shibata, N.; et al. 446TiP Randomized phase III study of bi-weekly trifluridine/tipiracil (FTD/TPI) plus bevacizumab (BEV) vs. FTD/TPI for chemorefractory metastatic colorectal cancer (mCRC): ROBiTS/JCOG2014. Ann. Oncol. 2022, 33, S738. [Google Scholar] [CrossRef]
- Tongji Hospital: Adjuvant PD-1 Inhibitor for Patients with Early-stage Hepatocellular Carcinoma Following Microwave Ablation. Available online: https://www.clinicaltrials.gov/study/NCT06248554 (accessed on 7 February 2025).
- Centre Leon Berard: A Prospective, Randomized, Multicenter, Comparative Study of the Efficacy of Imatinib Resumption Combined with Atezolizumab Versus Imatinib Resumption Alone in Patients with Unresectable Advanced Gastrointestinal Stromal Tumors (GIST) After Failure of Standard Treatments (ATEZOGIST). Available online: https://clinicaltrials.gov/study/NCT05152472 (accessed on 7 February 2025).
- Sun Yat-sen University. Surufatinib and Sintilimab in Combination with Capecitabine for Metastatic Adenocarcinoma of Small Intestine or Appendix Carcinoma. Available online: https://clinicaltrials.gov/study/NCT05472948?term=NCT05472948&rank=1 (accessed on 7 February 2025).
- Overman, M.J.; Guthrie, K.A.; Salem, M.E.; Pedersen, K.S.; Kalyan, A.; Bellasea, S.; Philip, P.A. Randomized phase II selection study of ramucirumab and paclitaxel versus FOLFIRI in refractory small bowel adenocarcinoma: SWOG S1922. J. Clin. Oncol. 2022, 40, TPS643. [Google Scholar] [CrossRef]
- Overman, M.J.; Guthrie, K.A.; Salem, M.E.; Pederson, K.S.; Kalyan, A.; Colby, S.; Fakih, M.; Gholami, S.; Gold, P.J.; Chiorean, E.G.; et al. Randomized, phase II selection study of ramucirumab and paclitaxel versus FOLFIRI in refractory small bowel adenocarcinoma: SWOG S1922. J. Clin. Oncol. 2023, 41, TPS784. [Google Scholar] [CrossRef]
- National Cancer Institute: Nivolumab After Combined Modality Therapy in Treating Patients with High Risk Stage II-IIIB Anal Cancer. Available online: https://www.clinicaltrials.gov/study/NCT03233711 (accessed on 7 February 2025).
- Martin, D.; Balermpas, P.; Gollrad, J.; Weiβ, C.; Valentini, C.; Stuschke, M.; Schafer, H.; Henckenberens, C.; Debus, J.; Krug, D.; et al. RADIANCE—Radiochemotherapy with or without Durvalumab in the treatment of anal squamous cell carcinoma: A randomized multicenter phase II trial. Clin. Transl. Radiat. Oncol. 2020, 23, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Jones, M.; Bowman, J.; Tian, C.; Spano, J.-P. POD1UM-303/InterAACT 2, A phase III, global, randomized, double-blind study of retifanlimab or placebo plus carboplatin–paclitaxel in patients with locally advanced or metastatic squamous cell anal carcinoma. Front Oncol. 2022, 12, 935383. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-T.; Chiu, C.-F.; Bai, H.-J.; Bai, L.-Y. Intraperitoneal nivolumab in a patient with pancreatic cancer and refractory malignant ascites. Eur. J. Cancer 2021, 148, 48–50. [Google Scholar] [CrossRef]
- Lewis, C.R.; Dadgar, N.; Yellin, S.A.; Donnenberg, V.S.; Donnenberg, A.D.; Bartlett, D.L.; Allen, C.J.; Wagner, P.L. Regional Immunotherapy for Peritoneal Carcinomatosis in Gastroesophageal Cancer: Emerging Strategies to Re-Condition a Maladaptive Tumor Environment. Cancers 2023, 15, 5107. [Google Scholar] [CrossRef]
- QI, C.; Wu, S.; Kim, I.-H.; Cai, S.; Wang, J.; Kim, S.T.; Wang, J.; Bai, L.; Lin, C.; Liang, Z.; et al. Global multi-center phase I trial of the intraperitoneal infusion of anti-EpCAM x anti-CD3 bispecific antibody catumaxomab for advanced gastric carcinoma with peritoneal metastasis. J. Clin. Oncol. 2022, 40, e16102. [Google Scholar] [CrossRef]
- Aldesleukin with Nivolumab and Standard Chemotherapy for Treatment of Gastric Cancer with Peritoneal Metastasis. Available online: https://clinicaltrials.gov/study/NCT05802056 (accessed on 25 February 2024).
- Wagner, P.L.; Park, H.Y.; Blodgett, R.J.; Donnenberg, V.; Lewis, C.R.; Dadgar, N.; Xiao, K.; Zaidi, A.; Bartlett, D.L.; Donnenberg, A. 658 Intra-cavitary tocilizumab immunotherapy for malignant pleural effusions and ascites: In progress update from the regional immuno-oncology trial (RIOT)-2. J. Immunother. Cancer 2024, 12, A755. [Google Scholar] [CrossRef]
- Park, H.; Lewis, C.; Dadgar, N.; Sherry, C.; Evans, S.; Ziobert, S.; Omstead, A.; Zaidi, A.; Xiao, K.; Ghosh, S.; et al. Intra-pleural and intra-peritoneal tocilizumab therapy for managing malignant pleural effusions and ascites: The Regional Immuno-Oncology Trial (RIOT)−2 study protocol. Surg. Oncol. Insight. 2024, 1, 100045. [Google Scholar] [CrossRef]
- Aznar Gomez, M.A.; Good, C.R.; Barber-Rotenberg, J.S.; Agarwal, S.; Wilson, W.; Gonzales, D.; Watts, A.C.; Hwang, W.; Jadlowsky, J.K.; Yong, R.M.; et al. 237 Enhancing mesothelin-directed CAR T cell therapy for unresectable or metastatic pancreatic adenocarcinoma. J. Immunother. Cancer 2024, 12, A272. [Google Scholar] [CrossRef]
- Chudasama, R.; Phung, Q.; Hsu, A.; Almhanna, K. Vaccines in Gastrointestinal Malignancies: From Prevention to Treatment. Vaccines 2021, 9, 647. [Google Scholar] [CrossRef]
- Guo, Z.; Yuan, Y.; Chen, C.; Lin, J.; Ma, Q.; Liu, G.; Gao, Y.; Huang, Y.; Chen, L.; Chen, L.; et al. Durable complete response to neoantigen-loaded dendritic-cell vaccine following anti-PD-1 therapy in metastatic gastric cancer. NPJ Precis Oncol. 2022, 6, 34. [Google Scholar] [CrossRef]
- Flatmark, K.; Torgunrud, A.; Fleten, K.G.; Davidson, B.; Juul, H.V.; Mensali, N.; Lund-Anderson, C.; Inderberg, E.M. Peptide vaccine targeting mutated GNAS: A potential novel treatment for pseudomyxoma peritonei. J Immunother Cancer 2021, 9, e003109. [Google Scholar] [CrossRef]
- Ramanathan, R.; Choudry, H.; Jones, H.; Girgis, M.; Gooding, W.; Kalinski, P.; Bartlett, D.L. Phase II Trial of Adjuvant Dendritic Cell Vaccine in Combination with Celecoxib, Interferon-α, and Rintatolimod in Patients Undergoing Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Peritoneal Metastases. Ann. Surg. Oncol. 2021, 28, 4637–4646. [Google Scholar] [CrossRef] [PubMed]
- Al-Batran, S.-E.; Mueller, D.W.; Rafiyan, M.-R.; Kiselicki, D.; Atmaca, A.; Habibzada, T.; Mueller, C.; Brignone, C.; Triebel, F.; Loose, M.; et al. A soluble LAG-3 protein (eftilagimod alpha) and an anti-PD-L1 antibody (avelumab) tested in a phase I trial: A new combination in immuno-oncology. ESMO Open 2023, 8, 101623. [Google Scholar] [CrossRef] [PubMed]
- Mueller, D.W.; Goetze, T.O.; Eickhoff, R.; Reichart, A.; Al-Batran, S.-E. The ‘INSIGHT’ trial: An explorative, open-labeled phase I study to evaluate the feasibility and safety of intra-tumoral, intra-peritoneal, and subcutaneous injections with IMP321 (LAG-3Ig fusion protein) for advanced stage solid tumor entities. J. Clin. Oncol. 2018, 36, TPS3129. [Google Scholar] [CrossRef]
- Lewis, C.; Dadgar, N.; Najafi, M.; Park, H.; Sherry, C.; Lucas, A.; Zaidi, A.; Xiao, K.; Omstead, A.; Donnenberg, A.; et al. Intra-Tumoral Immunomodulatory Therapy for Advanced Abdominal Cancers using Lipopolysaccharide: The Regional Immuno-Oncology Trial-1 (RIOT-1) Protocol. Surg. Oncol. Insight 2024, 1, 100042. [Google Scholar] [CrossRef]
- Park, H.Y.; Lewis, C.R.; Blodgett, R.J.; Dadgar, N.; Omstead, A.; Xiao, K.; Zaidi, A.; Donnenberg, A.; Donnenberg, V.; Bartlet, D.L.; et al. 637 Regional immuno-oncology trial 1: A phase I trial of laparoscopic intra-tumoral lipopolysaccharide injection in peritoneal tumors. J. Immunother. Cancer 2024, 12, A731. [Google Scholar] [CrossRef]
- Chen, J.J.; Vincent, M.Y.; Shepard, D.; Peereboom, D.; Mahalingam, D.; Battiste, J.; Patel, M.R.; Juric, D.; Wen, P.Y.; Bullock, A.; et al. Phase 1 dose expansion and biomarker study assessing first-in-class tumor microenvironment modulator VT1021 in patients with advanced solid tumors. Commun. Med. 2024, 4, 95. [Google Scholar] [CrossRef]
- Cartwright, E.; Turkes, F.; Saffery, C.; Kalaitzaki, E.; Powell, R.; Wotherspoon, A.; De Paepe, K.; von Loga, K.; Hubank, M.; Rao, S.; et al. EMERGE: Epigenetic modulation of the immune response in gastrointestinal cancers. Ann. Oncol. 2019, 30, v251–v252. [Google Scholar] [CrossRef]
- Cartwright, E.; Slater, S.; Saffery, C.; Tran, A.; Turkes, F.; Smith, G.; Aresu, M.; Kohoutova, D.; Terlizzo, M.; Zhitkov, O.; et al. Phase II trial of domatinostat (4SC-202) in combination with avelumab in patients with previously treated advanced mismatch repair proficient oesophagogastric and colorectal adenocarcinoma: EMERGE. ESMO Open. 2024, 9, 102971. [Google Scholar] [CrossRef]
- Christenson, E.; Lim, S.J.; Wang, H.; Ferguson, A.; Parkinson, R.; Cetasaan, Y.; Rodriguez, C.; Burkhart, R.; De Jesus-Acosta, A.; He, J.; et al. Nivolumab and a CCR2/CCR5 dual antagonist (BMS-813160) with or without GVAX for locally advanced pancreatic ductal adenocarcinomas: Results of phase I study. J. Clin. Oncol. 2023, 41, 730. [Google Scholar] [CrossRef]
- Choo, J.; Rha, S.Y.; Jung, M.; Tan, H.L.; Chan, G.; Ho, J.S.; Walsh, R.J.; Chee, C.E.; Raghav, S.; Yong, W.P. 1255P da VINci: Safety and efficacy of the OTSGC-A24 vaccine and nivolumab in metastatic gastric cancer. Ann. Oncol. 2022, 33, S1121. [Google Scholar] [CrossRef]
- Istituto Scientifico Romagnolo per lo Studio e la cura dei Tumori: Vaccination with Autologous Dendritic Cells Loaded with Autologous Tumour Homogenate After Curative Resection for Stage IV Colorectal Cancer (COREVAX-1). Available online: https://clinicaltrials.gov/study/NCT02919644 (accessed on 7 February 2025).
- Kirk, A.M.; Crawford, J.C.; Kamdar, Z.; Baretti, M.; Yarchoan, M.; Thomas, P.G. 897-B A DNAJB1-PRKACA fusion peptide vaccine combined with ipilimumab and nivolumab elicits polyclonal fusion-specific T cell responses in fibrolamellar carcinoma. J. Immunother. Cancer 2023, 11, A1820. [Google Scholar] [CrossRef]
- Baretti, M.; Ladle, B.; Nauroth, J.; Wang, H.; Durham, J.; Thoburn, C.; Ferguson, A.; Thompson, A.; Brancati, M.; Griffith, P.; et al. 641 A pilot study of a DNAJB1-PRKACA fusion kinase peptide vaccine combined with nivolumab and ipilimumab for patients with fibrolamellar hepatocellular carcinoma. J. Immunother. Cancer 2023, 11, A733–A734. [Google Scholar] [CrossRef]
- Khanniche, A.; Zhang, Y.; Wu, Z.; Wang, H.; Kong Xi Gang, J.; Hu, L. 1499 TCR clonotype features correlate with responses to personalized neoantigen cancer vaccine in pancreatic ductal adenocarcinoma. J. Immunother. Cancer 2024, 12, A1728. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, Y.; Kong, X.; Wu, Z.; Wang, H. Impact of personalized peptide neoantigen vaccines on immunologic responses in patients with pancreatic cancer. J. Clin. Oncol. 2024, 42, e16351. [Google Scholar] [CrossRef]
- A Study of the Efficacy and Safety of Adjuvant Autogene Cevumeran Plus Atezolizumab and mFOLFIRINOX Versus mFOLFIRINOX Alone in Participants with Resected PDAC (IMCODE003). Available online: https://clinicaltrials.gov/study/NCT05968326 (accessed on 21 July 2025).
- Hoster, H.A.; Zanes, R.P.; von Haam, E. Studies in Hodgkin’s Syndrome: IX. The Association of “Viral” Hepatitis and Hodgkin’s Disease (A Preliminary Report). Cancer Res. 1949, 9, 473–480. [Google Scholar]
- Zolaly, M.A.; Mahallawi, W.; Khawaji, Z.Y.; Alahmadi, M.A. The Clinical Advances of Oncolytic Viruses in Cancer Immunotherapy. Cureus 2023, 15, e40742. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, B.; Tang, J.; Chang, Q.; Zhang, R.; Geng, C.; Wu, D.; Qi, L.; Gu, X.; Liu, B. Safety and tolerability of intratumorally administered OH2, an oncolytic herpes simplex virus 2, in patients with advanced solid tumors: A phase I dose escalation clinical study. J. Clin. Oncol. 2020, 38, 3139. [Google Scholar] [CrossRef]
- Lenz, H.-J.; Somasundaram, A.; Lee, M.S.; Sohal, D.; Safyan, R.A.; Zhu, M.; Jones, J.C.; Bhansali, A.; Kalwar, R.; Cho, M.; et al. An open-label clinical trial of RP2 and RP3 oncolytic immunotherapy in combination with atezolizumab plus bevacizumab for the treatment of patients with advanced colorectal carcinoma. J. Clin. Oncol. 2024, 42, TPS228. [Google Scholar] [CrossRef]
- Élez, E.; Shepard, D.; Smolenschi, C.; Bekaii-Saab, T.; Jac, J. P-58 An ongoing open-label, phase 2 trial of RP2 Or RP3 oncolytic immunotherapy in combination with atezolizumab and bevacizumab for the treatment of patients with advanced colorectal carcinoma. Ann. Oncol. 2023, 34, S34–S35. [Google Scholar] [CrossRef]
- Uppsala University: Study of Recombinant Adenovirus AdVince in Patients with Neuroendocrine Tumors; Safety and Efficacy (RADNET). Available online: https://clinicaltrials.gov/study/NCT02749331 (accessed on 7 February 2025).
- Chan, A. FDA Grants Orphan Drug Designation to ELC-100 for Pancreatic NETs. 2025. Available online: https://www.onclive.com/view/fda-grants-orphan-drug-designation-to-elc-100-for-pancreatic-nets (accessed on 7 February 2025).
- Musher, B.L.; Smaglo, B.G.; Abidi, W.; Othman, M.; Patel, K.; Jawaid, S.; Jing, J.; Brisco, A.; Wenthe, J.; Eriksson, E.; et al. A phase I/II study of LOAd703, a TMZ-CD40L/4-1BBL-armed oncolytic adenovirus, combined with nab-paclitaxel and gemcitabine in advanced pancreatic cancer. J. Clin. Oncol. 2022, 40, 4138. [Google Scholar] [CrossRef]
- Musher, B.L.; Rowinsky, E.K.; Smaglo, B.G.; Abidi, W.; Othman, M.; Patel, K.; Jawaid, S.; Jing, J.; Brisco, A.; Wenthe, J.; et al. LOAd703, an oncolytic virus-based immunostimulatory gene therapy, combined with chemotherapy for unresectable or metastatic pancreatic cancer (LOKON001): Results from arm 1 of a non-randomised, single-centre, phase 1/2 study. Lancet Oncol. 2024, 25, 488–500. [Google Scholar] [CrossRef]
- Wang, D.; Porter, C.E.; Lim, B.; Shaw, A.R.; Robertson, C.S.; Woods, M.L.; Xu, Y.; Biegert, G.G.W.; Morita, D.; Wang, T.; et al. Ultralow-dose binary oncolytic/helper-dependent adenovirus promotes antitumor activity in preclinical and clinical studies. Sci Adv. 2023, 9, eade6790. [Google Scholar] [CrossRef]
- Makawita, S.; Gibbs, J.M.; McFadden, D.R.; Porter, C.; Shaw, A.R.; Robertson, C.; Woods, M.L.; Wang, T.; Grilley, B.J.; Heslop, H.E.; et al. Binary oncolytic adenovirus in combination with HER2-specific autologous CAR VST for treatment of advanced HER2-positive solid tumors (VISTA). J. Clin. Oncol. 2024, 42, TPS2679. [Google Scholar] [CrossRef]
- Wang, T.; Zang, C.; Zhang, Y.; Ren, Z.; Liu, Y.; Yang, X.; Wang, Y. Efficacy of oncolytic virus combined with HAIC of mFOLFOX for IMCC: A single-center, single-arm, prospective study. J. Clin. Oncol. 2024, 42, e16265. [Google Scholar] [CrossRef]
- Fudan University: Oncolytic Virotherapy Combined with Tislelizumab Plus Lenvatinib in Patients with Advanced Biliary Tract Cancer (OPTIONS-05). Available online: https://clinicaltrials.gov/study/NCT05823987 (accessed on 7 February 2025).
- Wenthe, J.; Eriksson, E.; Sandin, L.; Lövgren, T.; Jarblad, J.L.; Dahlstrand, H.; Olsson-Strömberg, U.; Schiza, A.; Sundin, A.; Irenaeus, S.; et al. Abstract PO-018: Inflaming advanced solid tumors including pancreatic cancer using LOAd703, a TMZ-CD40L/4-1BBL-armed oncolytic virus. Cancer Res. 2021, 81, PO-018. [Google Scholar] [CrossRef]
- Wenthe, J.; Eriksson, E.; Naseri, S.; Sandin, L.; Hahn, A.; Irenaeus, S.; Sundin, A.; Leja-Jarblad, J.; Ullenhag, G.; Lövgren, T.; et al. Abstract 6663: Immunostimulatory gene therapy targeting CD40/4-1BB in combination with chemotherapy induces an inflammatory gene profile in tumors from patients with advanced disease. Cancer Res. 2023, 83, 6663. [Google Scholar] [CrossRef]
- Hahn, A.; Irenaeus, S.; Wenthe, J.; Eriksson, E.; Schiza, A.; Dahlstrand, H.; Olsson-Strömberg, U.; Krause, J.; Sundin, A.; Sandin, L.; et al. Abstract CT235: A phase I/II clinical study of an oncolytic adenovirus expressing the immunostimulatory transgenes TMZ-CD40L and 4-1BBL in advanced solid malignancies. Cancer Res. 2023, 83, CT235. [Google Scholar] [CrossRef]
- Lenz, H.-J.; Prenen, H.; Van Cutsem, E.; Kössler, T.; Mayol, J.; Trapani, F.; Tihy, M.; Rubbia-Brandt, L.; Toso, C.; Bogenrieder, T.; et al. Abstract CT507: ATP128 vaccine with ezabenlimab promotes antigen-specific immune responses in stage IV colorectal cancer in the KISIMA-01 phase 1b trial. Cancer Res. 2022, 82, CT507. [Google Scholar] [CrossRef]
- Lenz, H.-J.; Koessler, T.; Oberstein, P.; Van Cutsem, E.; Kim, S.; Fritsch, R.; Prenen, H.; Morse, M.; Gani, D.; Derouazi, M.; et al. Abstract CT570: KISIMA-01: A first-in-human trial of the heterologous prime-boost vaccine ATP128/VSV-GP128 with ezabenlimab (BI 754091) in patients with stage IV colorectal cancer. Cancer Res. 2022, 82, CT570. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Ueno, M.; Tanaka, M.; Ikeda, M. Results from phase I study of the oncolytic viral immunotherapy agent canerpaturev (C-REV) in combination with gemcitabine plus nab-paclitaxel for unresectable pancreatic cancer. J. Clin. Oncol. 2019, 37, 325. [Google Scholar] [CrossRef]
- Bębnowska, D.; Grywalska, E.; Niedźwiedzka-Rystwej, P.; Sosnowska-Pasiarska, B.; Smok-Kalwat, J.; Pasiarski, M.; Góźdź, S.; Rolinski, J.; Polkowski, W. CAR-T Cell Therapy—An Overview of Targets in Gastric Cancer. J. Clin. Med. 2020, 9, 1894. [Google Scholar] [CrossRef]
- Qi, C.; Gong, J.; Li, J.; Liu, D.; Qin, Y.; Ge, S.; Zhang, M.; Peng, Z.; Zhou, J.; Cao, Y.; et al. Claudin18.2-specific CAR T cells in gastrointestinal cancers: Phase 1 trial interim results. Nat. Med. 2022, 28, 1189–1198. [Google Scholar] [CrossRef]
- QI, C.; Liu, C.; Gong, J.; Li, J.; Liu, D.; Wang, X.; Zhang, P.; Qin, Y.; Zhang, M.; Peng, Z.; et al. Claudin18.2-targeted chimeric antigen receptor T cell-therapy for patients with gastrointestinal cancers: Final results of CT041-CG4006 phase 1 trial. J. Clin. Oncol. 2024, 42, 2501. [Google Scholar] [CrossRef]
- Butler, M.O.; Majeed, H.; Nelles, M.; Saibil, S.; Bonilla, L.; Boross-Harmer, S.; Sotov, V.; Elston, S.; Ross, K.; van As, B.; et al. Study of TBI-1301 (NY-ESO-1 specific TCR gene transduced autologous T lymphocytes) in patients with solid tumors. Ann. Oncol. 2018, 29, viii441. [Google Scholar] [CrossRef]
- Butler, M.O.; Sotov, V.; Saibil, S.; Bonilla, L.; Boross-Harmer, S.; Fyrsta, M.; Gray, D.; Nelles, M.; Le, M.; Lemiashkova, D.; et al. Adoptive T cell therapy with TBI-1301 results in gene-engineered T cell persistence and anti-tumour responses in patients with NY-ESO-1 expressing solid tumours. Ann. Oncol. 2019, 30, v481. [Google Scholar] [CrossRef]
- Ma, Q.; He, X.; Zhang, B.; Guo, F.; Ou, X.; Yang, Q.; Shu, P.; Chen, Y.; Li, K.; Gao, G.; et al. A PD-L1-targeting chimeric switch receptor enhances efficacy of CAR-T cell for pleural and peritoneal metastasis. Signal Transduct. Target. Ther. 2022, 7, 380. [Google Scholar] [CrossRef] [PubMed]
- Pang, N.; Shi, J.; Qin, L.; Chen, A.; Tang, Y.; Yang, H.; Huang, Y.; Wu, Q.; Li, X.; He, B.; et al. IL-7 and CCL19-secreting CAR-T cell therapy for tumors with positive glypican-3 or mesothelin. J. Hematol. Oncol. 2021, 14, 118. [Google Scholar] [CrossRef] [PubMed]
- Study on TIL for the Treatment of Advanced Hepatobiliary-Pancreatic Cancers. Available online: https://clinicaltrials.gov/study/NCT05098197 (accessed on 1 March 2024).
- CIK in Treating Patients with Esophageal Cancer. Available online: https://clinicaltrials.gov/study/NCT02490735 (accessed on 1 March 2024).
- Shanghai Juncell Therapeutics: Study on TIL for the Treatment of r/r Gastrointestinal Tumors. Available online: https://clinicaltrials.gov/study/NCT04960072 (accessed on 1 March 2024).
- Wang, S. The Safety and Efficacy of Specific TIL-TCM Cells for Advanced Relapse-refractory or Metastatic Pancreatic Cancer. Available online: https://clinicaltrials.gov/study/NCT05438797 (accessed on 1 March 2024).
- Immunotherapy Using Tumor Infiltrating Lymphocytes for Patients with Metastatic Cancer. Available online: https://clinicaltrials.gov/study/NCT01174121 (accessed on 1 March 2024).
- Mukherjee, S.; Boland, P.; Gupta, M.; Grimm, M.; Attwood, K.; Kalinski, P. 334 Phase II study evaluating a chemokine-modulatory (CKM) regimen in patients with colorectal cancer (CRC) metastatic to the liver. J. Immunother. Cancer 2020, 8, A360. [Google Scholar] [CrossRef]
- Palmer, D.; Webber, B.; Patel, Y.; Johnson, M.; Kariya, C.; Lahr, W.; Parkhurst, M.; Gartner, J.; Prickett, T.; Lowery, F.; et al. 333 Targeting the apical intracellular checkpoint CISH unleashes T cell neoantigen reactivity and effector program. J. Immunother. Cancer 2020, 8, A360. [Google Scholar] [CrossRef]
- Cytryn, S.L.; Maron, S.B.; Janjigian, Y.Y. A phase II study of agenT-797 (invariant natural killer T-cells), botensilimab (Fc-enhanced CTLA-4 inhibitor) and balstilimab (anti-PD-1) in patients with advanced, refractory gastroesophageal adenocarcinoma. J. Clin. Oncol. 2025, 43, 4. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).