Hyperuricemia and Insulin Resistance: Interplay and Potential for Targeted Therapies
Abstract
1. Introduction
- How has research on hyperuricemia and metabolic diseases evolved over the past two decades?
- What are the most frequently studied themes and emerging trends in this field?
- What are the gaps in current knowledge regarding the role of SUA-lowering interventions in improving metabolic outcomes?
2. Evolution of Hyperuricemia, Insulin Resistance, and Metabolic Syndrome
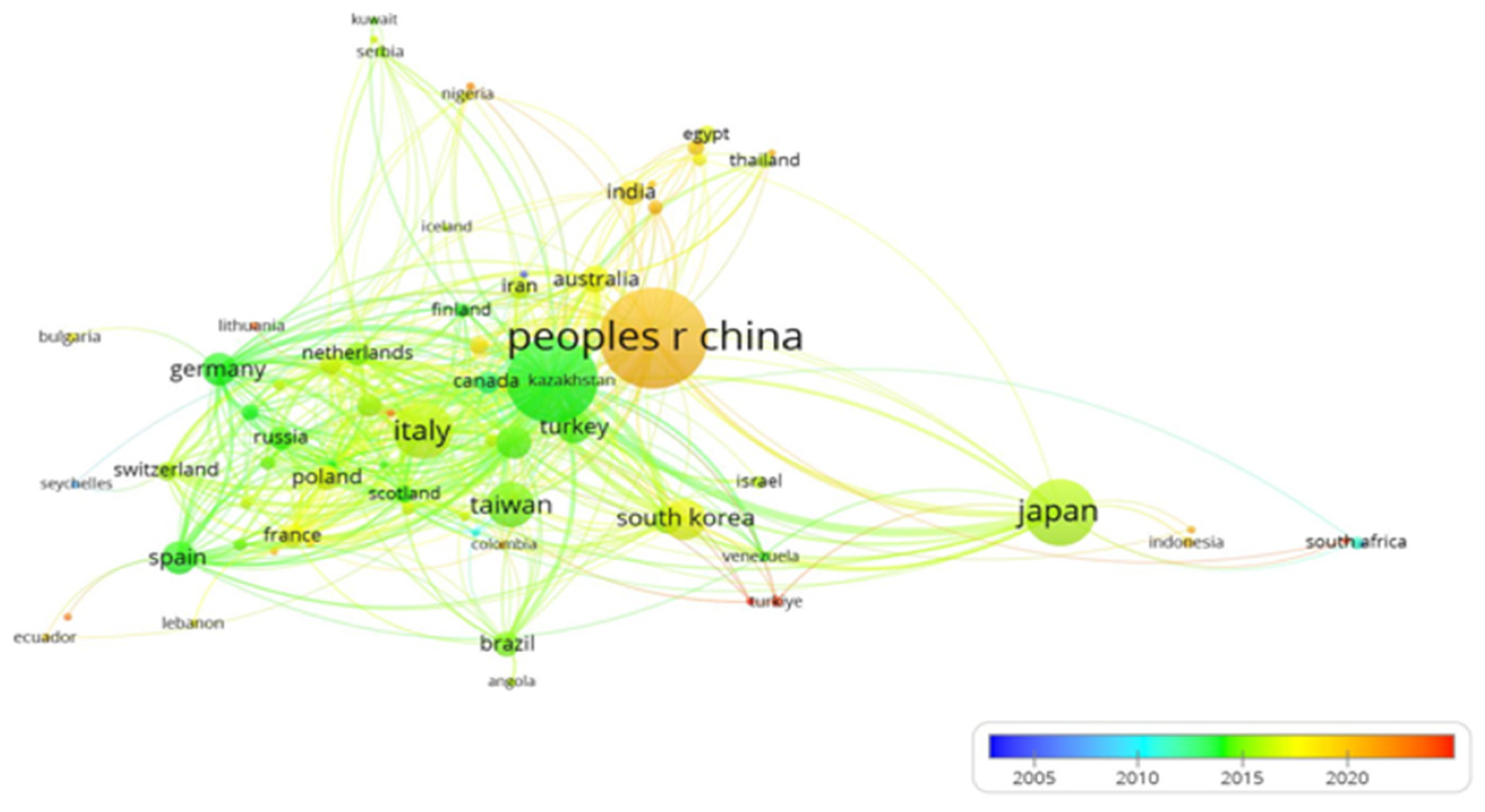
Research Trends over Time
3. The Complex Interplay Between Hyperuricemia, Insulin Resistance, and Type 2 Diabetes
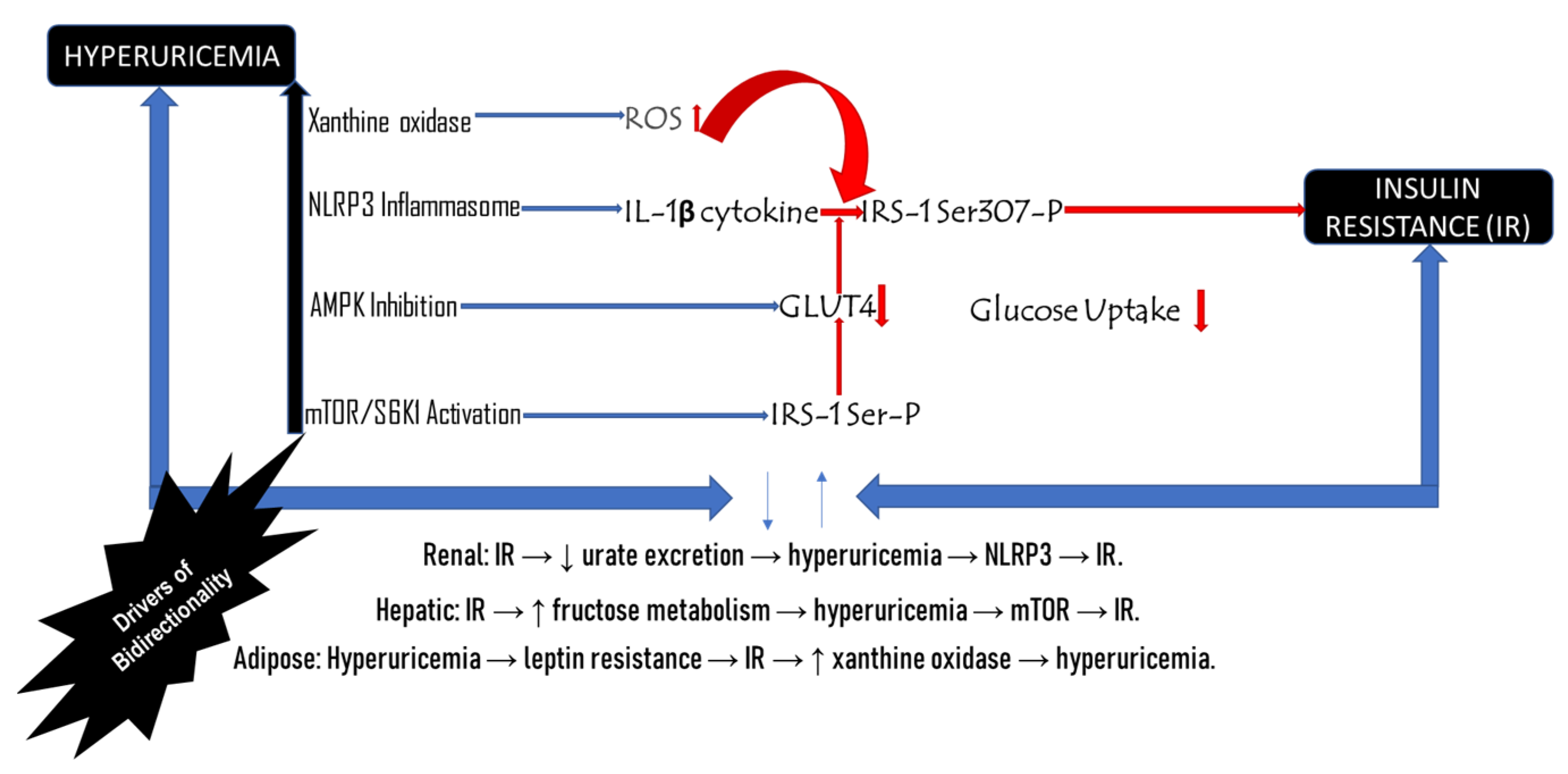
3.1. Bidirectional Relationship Between Hyperuricemia and Insulin Resistance
3.2. Gender Stratification in Hyperuricemia and Insulin Resistance
4. Molecular Processes Linking Hyperuricemia and Insulin Resistance
4.1. Oxidative Stress and Inflammation
4.2. Endothelial Dysfunction
4.3. Adipocyte Dysfunction
4.4. Activation of the Renin–Angiotensin System (RAS)
5. Clinical Evidence Supporting the Role of Hyperuricemia in Insulin Resistance
Clinical Trials and Interventions
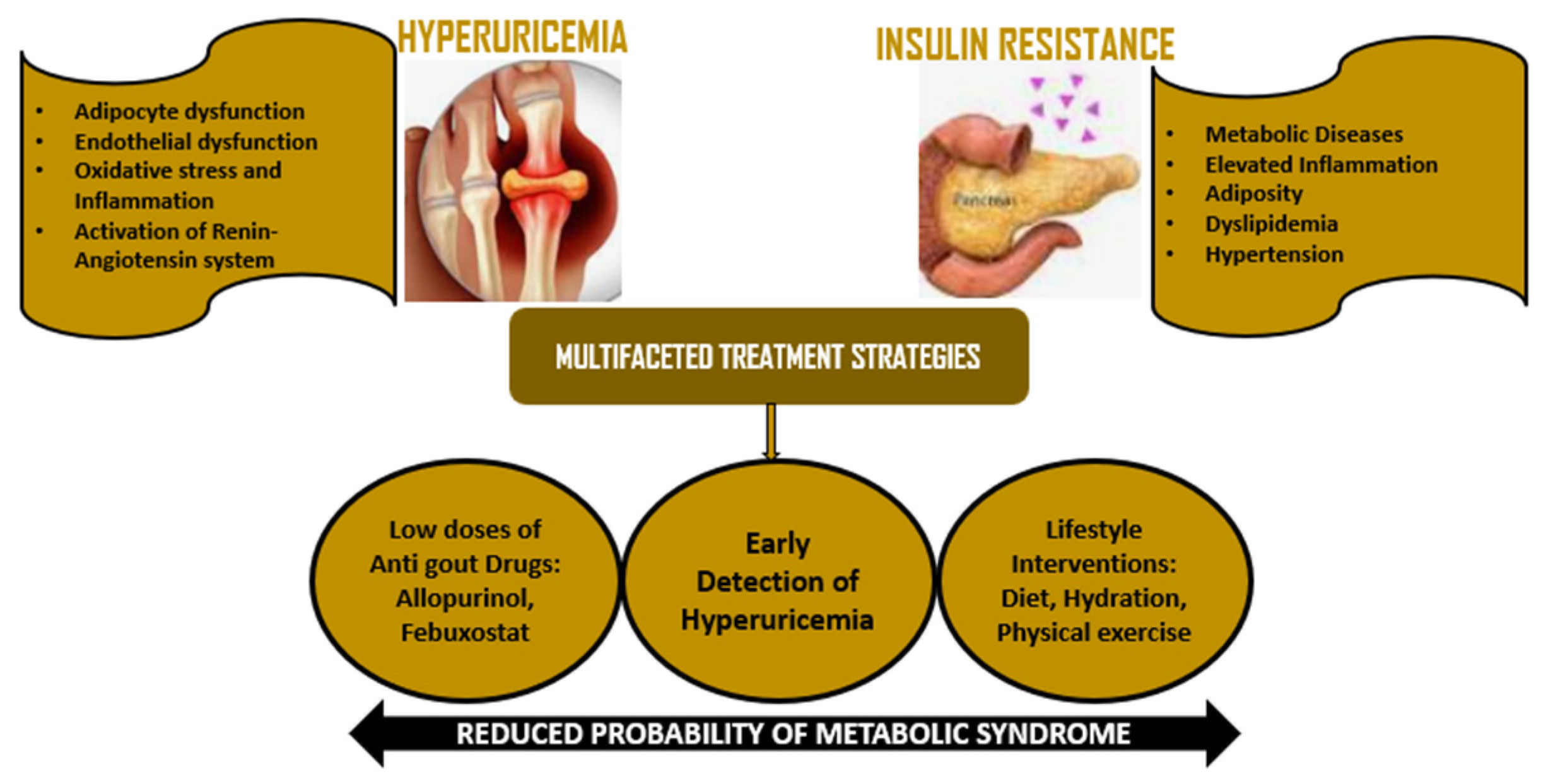
6. Implications for Management and Treatment
6.1. Drugs of Antigout and Antihyperuricemic Treatment
6.2. Lifestyle Interventions
6.3. Monitoring and Prevention Strategies
| Agent | Mechanism | Effect on IR | Key Evidence | Limitations | Reference |
|---|---|---|---|---|---|
| Allopurinol | Xanthine oxidase inhibitor lowers uric acid | Improves HOMA-IR (~15%) | RCT in metabolic syndrome | Weak effect in advanced T2D | Malorbeti et al. [1] |
| Febuxostat | Xanthine oxidase inhibitor (more potent) | Improves hepatic IR | Reduced mTORC1 activation in NAFLD | Cardiovascular safety debates | Yu et al. [58] |
| SGLT2 Inhibitors (e.g., Empagliflozin) | Increases Urinary urate excretion and reduces inflammation | Improves IR with reduced SUA | RCT in T2D (HOMA-IR reduced by 18%) | Genital infections, volume depletion | Wang et al. [92] |
| Metformin | AMPK activation leads to activation of GLUT4 | Indirectly counters uric acid’s AMPK blockade | Reversed leptin resistance in adipocytes | GI side effects | Agius et al. [93] |
| IL-1β Antagonists (e.g., Canakinumab) | Blocks NLRP3 inflammasome, resulting in reduced IL-1β | Preserves β-cell function | Restored GSIS in hyperuricemic mice | High cost, infection risk | Malorbeti et al. [1] |
| SGLT2i + Allopurinol | Dual urate-lowering + insulin-sensitizing | Synergistic HOMA-IR reduction | Clinical trials are ongoing (e.g., NCT04881110), University of Campania, Italy | Limited long-term data | Caruso et al. [94] |
| Diet/Lifestyle | Reduced fructose, an increase in fiber, and aerobic exercise | Reduced SUA with increased AMPK/GLUT4 | Ketogenic diet improved IR despite increase in uric acid | Adherence challenges | Yu et al. [58] |
7. Current Research and Future Trends
7.1. Insights from Large Cohort and Mendelian Randomization Studies
7.2. Genetic Factors
7.3. Novel Therapeutic Targets and Longitudinal Studies
8. Harnessing Explainable AI for Early Prediction and Personalized Treatment of Hyperuricemia
9. Study Limitations, Recommendations, and Future Outlook
9.1. Study Limitations
9.2. Recommendations
9.3. Conclusion and Future Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Maloberti, A.; Vanoli, J.; Finotto, A.; Bombelli, M.; Facchetti, R.; Redon, P.; Mancia, G.; Grassi, G. Uric acid relationships with lipid profile and adiposity indices: Impact of different hyperuricemic thresholds. J. Clin. Hypertens. 2022, 25, 78–85. [Google Scholar] [CrossRef]
- Fritz, J.; Brozek, W.; Concin, H.; Nagel, G.; Kerschbaum, J.; Lhotta, K.; Ulmer, H.; Zitt, E. The Association of Excess Body Weight with Risk of ESKD Is Mediated Through Insulin Resistance, Hypertension, and Hyperuricemia. J. Am. Soc. Nephrol. 2022, 33, 1377–1389. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Satoh, N.; Horita, S.; Nangaku, M. Insulin-induced mTOR signaling and gluconeogenesis in renal proximal tubules: A mini-review of current evidence and therapeutic potential. Front. Pharmacol. 2022, 13, 1015204. [Google Scholar] [CrossRef]
- Zhu, Y.; Hu, Y.; Huang, T.; Zhang, Y.; Li, Z.; Luo, C.; Luo, Y.; Yuan, H.; Hisatome, I.; Yamamoto, T.; et al. High uric acid directly inhibits insulin signalling and induces insulin resistance. Biochem. Biophys. Res. Commun. 2014, 447, 707–714. [Google Scholar] [CrossRef]
- Du, L.; Zong, Y.; Li, H.; Wang, Q.; Xie, L.; Yang, B.; Pang, Y.; Zhang, C.; Zhong, Z.; Gao, J. Hyperuricemia and its related diseases: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024, 9, 212. [Google Scholar] [CrossRef]
- Chen, J.; Ge, J.; Zha, M.; Miao, J.-J.; Sun, Z.-L.; Yu, J.-Y. Effects of Uric Acid-Lowering Treatment on Glycemia: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2020, 11, 507731. [Google Scholar] [CrossRef]
- Sun, H.; Chang, X.; Bian, N.; An, Y.; Liu, J.; Leng, S.; Wang, G. Adipose Tissue Insulin Resistance Is Positively Associated with Serum Uric Acid Levels and Hyperuricemia in Northern Chinese Adults. Front. Endocrinol. 2022, 13, 835154. [Google Scholar] [CrossRef]
- Bose, B.; Badve, S.V.; Hiremath, S.S.; Boudville, N.; Brown, F.G.; Cass, A.; de Zoysa, J.R.; Fassett, R.G.; Faull, R.; Harris, D.C.; et al. Effects of uric acid-lowering therapy on renal outcomes: A systematic review and meta-analysis. Nephrol. Dial. Transplant. 2014, 29, 406–413. [Google Scholar] [CrossRef]
- Hu, X.; Rong, S.; Wang, Q.; Sun, T.; Bao, W.; Chen, L.; Liu, L. Association between plasma uric acid and insulin resistance in type 2 diabetes: A Mendelian randomization analysis. Diabetes Res. Clin. Pr. 2020, 171, 108542. [Google Scholar] [CrossRef]
- Zehra, F. Association of serum uric acid with diabetes mellitus type-2: A narrative review. Pak. J. Physiol. 2024, 20, 52–57. [Google Scholar]
- Shu, J.; Zhao, R.; Xu, H.; Liu, X.; Guo, H.; Lu, C. Hyperuricemia is associated with metabolic syndrome: A cross-sectional analysis of the National Health and Nutrition Examination Survey (NHANES). Prev. Med. Rep. 2023, 36, 102520. [Google Scholar] [CrossRef] [PubMed]
- Luis-Rodríguez, D.; Donate-Correa, J.; Martín-Núñez, E.; Ferri, C.; Tagua, V.G.; Castro, A.P.; Mora-Fernández, C.; Navarro-González, J.F. Serum urate is related to subclinical inflammation in asymptomatic hyperuricaemia. Rheumatology 2021, 60, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Hua, R.; Hu, K.; Wang, Z. Carbohydrates deteriorate fatty liver by activating the inflammatory response. Nutr. Res. Rev. 2022, 35, 252–267. [Google Scholar] [CrossRef]
- Cuttone, A.; Cannavò, V.; Abdullah, R.M.S.; Fugazzotto, P.; Arena, G.; Brancati, S.; Muscarà, A.; Morace, C.; Quartarone, C.; Ruggeri, D.; et al. Expanding the Use of SGLT2 Inhibitors in T2D Patients Across Clinical Settings. Cells 2025, 14, 668. [Google Scholar] [CrossRef] [PubMed]
- Timsans, J.; Kauppi, J.; Rantalaiho, V.; Kerola, A.; Hakkarainen, K.; Lehto, T.; Kautiainen, H.; Kauppi, M. Serum Uric Acid Is Associated with Insulin Resistance in Non-Diabetic Subjects. J. Clin. Med. 2025, 14, 2621. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, N.; Zhang, W.; Yue, W.; Qu, X.; Li, Z.; Xu, G. The impact of uric acid on musculoskeletal diseases: Clinical associations and underlying mechanisms. Front. Endocrinol. 2025, 16, 1515176. [Google Scholar] [CrossRef]
- Badii, M.; Klück, V.; Gaal, O.; Cabău, G.; Hotea, I.; Nica, V.; Mirea, A.M.; Bojan, A.; Zdrenghea, M.; Novakovic, B.; et al. Regulation of SOCS3-STAT3 in urate-induced cytokine production in human myeloid cells. Jt. Bone Spine 2024, 91, 105698. [Google Scholar] [CrossRef]
- Nasser, S.; Solé, T.; Vega, N.; Thomas, T.; Balcerczyk, A.; Strigini, M.; Pirola, L. Ketogenic diet administration to mice after a high-fat-diet regimen promotes weight loss, glycemic normalization and induces adaptations of ketogenic pathways in liver and kidney. Mol. Metab. 2022, 65, 101578. [Google Scholar] [CrossRef]
- Rodriguez-Iturbe, B.; Johnson, R.J.; Lanaspa, M.A.; Nakagawa, T.; Garcia-Arroyo, F.E.; Sánchez-Lozada, L.G. Sirtuin deficiency and the adverse effects of fructose and uric acid synthesis. Am. J. Physiol. Integr. Comp. Physiol. 2022, 322, R347–R359. [Google Scholar] [CrossRef]
- Jing, P.; Shi, M.; Ma, L.; Fu, P. Mechanistic insights of soluble uric acid-related kidney disease. Curr. Med. Chem. 2020, 27, 5056–5066. [Google Scholar]
- Sridharan, S.; Basu, A. Distinct Roles of mTOR Targets S6K1 and S6K2 in Breast Cancer. Int. J. Mol. Sci. 2020, 21, 1199. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Mirmiran, P.; Kashfi, K.; Ghasemi, A. Hyperuricemia-induced endothelial insulin resistance: The nitric oxide connection. Pflüger Arch. Eur. J. Physiol. 2021, 474, 83–98. [Google Scholar] [CrossRef]
- Adnan, E.; Rahman, I.A.; Faridin, H.P. Relationship between insulin resistance, metabolic syndrome components and serum uric acid. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 2158–2162. [Google Scholar]
- Wan, X.; Xu, C.; Lin, Y.; Lu, C.; Li, D.; Sang, J.; He, H.; Liu, X.; Li, Y.; Yu, C. Uric acid regulates hepatic steatosis and insulin resistance through the NLRP3 inflammasome-dependent mechanism. J. Hepatol. 2016, 64, 925–932. [Google Scholar] [CrossRef]
- Lanaspa, M.A.; Sanchez-Lozada, L.G.; Choi, Y.J.; Cicerchi, C.; Kanbay, M.; Roncal-Jimenez, C.A.; Johnson, R.J. Uric acid induces hepatic steatosis by generation of mitochondrial oxidative stress: Potential role in fructose-dependent and-independent fatty liver. J. Biol. Chem. 2012, 287, 40732–40744. [Google Scholar]
- Baharuddin, B. The impact of fructose consumption on human health: Effects on obesity, hyperglycemia, diabetes, uric acid, and oxidative stress with a focus on the liver. Cureus 2024, 16, e70095. [Google Scholar]
- Gong, M.; Wen, S.; Nguyen, T.; Wang, C.; Jin, J.; Zhou, L. Converging Relationships of Obesity and Hyperuricemia with Special Reference to Metabolic Disorders and Plausible Therapeutic Implications. Diabetes Metab. Syndr. Obesity Targets Ther. 2020, 13, 943–962. [Google Scholar] [CrossRef]
- McCormick, N.; O’cOnnor, M.J.; Yokose, C.; Merriman, T.R.; Mount, D.B.; Leong, A.; Choi, H.K. Assessing the Causal Relationships Between Insulin Resistance and Hyperuricemia and Gout Using Bidirectional Mendelian Randomization. Arthritis Rheumatol. 2021, 73, 2096–2104. [Google Scholar] [CrossRef]
- Dong, M.; Chen, H.; Wen, S.; Yuan, Y.; Yang, L.; Xu, D.; Zhou, L. The Mechanism of Sodium-Glucose Cotransporter-2 Inhibitors in Reducing Uric Acid in Type 2 Diabetes Mellitus. Diabetes Metab. Syndr. Obesity Targets Ther. 2023, 16, 437–445. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, T.-T.; Xie, H.-A.; Hu, P.P.; Li, P. Experimental cell models of insulin resistance: Overview and appraisal. Front. Endocrinol. 2024, 15, 1469565. [Google Scholar] [CrossRef]
- Redon, P.; Maloberti, A.; Facchetti, R.; Redon, J.; Lurbe, E.; Bombelli, M.; Mancia, G.; Grassi, G. Gender-related differences in serum uric acid in treated hypertensive patients from central and east European countries: Findings from the Blood Pressure control rate and CArdiovascular Risk profilE study. J. Hypertens. 2019, 37, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Yamase, Y.; Horibe, H.; Kato, K.; Oguri, M.; Fujimaki, T.; Hibino, T.; Kondo, T.; Sakuma, J.; Takeuchi, I.; Murohara, T.; et al. P4470Identification of four genes as novel susceptibility loci for early-onset type 2 diabetes mellitus, metabolic syndrome, or hyperuricemia in Japanese. Eur. Hear. J. 2019, 40, 867. [Google Scholar] [CrossRef]
- Alem, M.M. Effect of low dose allopurinol on glycemic control and glycemic variability in patients with type 2 diabetes mellitus: A cross-sectional study. Heliyon 2022, 8, e11549. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Lan, L.; Qu, R.; Xu, Q.; Jiang, R.; Na, L.; Sun, C. Temporal Relationship Between Hyperuricemia and Insulin Resistance and Its Impact on Future Risk of Hypertension. Hypertension 2017, 70, 703–711. [Google Scholar] [CrossRef]
- Sakr, H.F.; Sirasanagandla, S.R.; Das, S.; Bima, A.I.; Elsamanoudy, A.Z. Insulin Resistance and Hypertension: Mechanisms Involved and Modifying Factors for Effective Glucose Control. Biomedicines 2023, 11, 2271. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Zeng, C. Update on the epidemiology, genetics, and therapeutic options of hyperuricemia. Am. J. Transl. Res. 2020, 12, 3167. [Google Scholar]
- Jung, J.H.; Song, G.G.; Lee, Y.H.; Kim, J.-H.; Hyun, M.H.; Choi, S.J. Serum uric acid levels and hormone therapy type: A retrospective cohort study of postmenopausal women. Menopause 2018, 25, 77–81. [Google Scholar] [CrossRef]
- Meloni, A.; Cadeddu, C.; Cugusi, L.; Donataccio, M.P.; Deidda, M.; Sciomer, S.; Gallina, S.; Vassalle, C.; Moscucci, F.; Mercuro, G.; et al. Gender Differences and Cardiometabolic Risk: The Importance of the Risk Factors. Int. J. Mol. Sci. 2023, 24, 1588. [Google Scholar] [CrossRef]
- Joosten, L.A.B.; Crişan, T.O.; Bjornstad, P.; Johnson, R.J. Asymptomatic hyperuricaemia: A silent activator of the innate immune system. Nat. Rev. Rheumatol. 2019, 16, 75–86. [Google Scholar] [CrossRef]
- Tan, H.; Zhang, S.; Liao, J.; Qiu, X.; Zhang, Z.; Wang, Z.; Geng, H.; Zhang, J.; Jia, E. Mechanism of macrophages in gout: Recent progress and perspective. Heliyon 2024, 10, e38288. [Google Scholar] [CrossRef]
- Kim, S.-K. The mechanism of the NLRP3 inflammasome activation and pathogenic implication in the pathogenesis of gout. J. Rheum. Dis. 2022, 29, 140–153. [Google Scholar] [CrossRef] [PubMed]
- Baratta, F.; Moscucci, F.; Ettorre, E.; Bocale, R.; Cicero, A.F.G.; Cirillo, P.; Fogacci, F.; Lospinuso, I.; Savoia, C.; Mengozzi, A.; et al. Influence of Uric Acid on Vascular and Cognitive Functions: Evidence for an Ambivalent Relationship. Metabolites 2024, 14, 642. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-κB in biology and targeted therapy: New insights and translational implications. Signal Transduct. Target. Ther. 2024, 9, 1–37. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, D.; Yang, L. Targeting AMPK Signaling in the Liver: Implications for Obesity and Type 2 Diabetes Mellitus. Curr. Drug Targets 2022, 23, 1057–1071. [Google Scholar] [CrossRef]
- Goel, S.; Singh, R.; Singh, V.; Singh, H.; Kumari, P.; Chopra, H.; Sharma, R.; Nepovimova, E.; Valis, M.; Kuca, K.; et al. Metformin: Activation of 5′ AMP-activated protein kinase and its emerging potential beyond anti-hyperglycemic action. Front. Genet. 2022, 13, 1022739. [Google Scholar] [CrossRef]
- Liu, N.; Xu, H.; Sun, Q.; Yu, X.; Chen, W.; Wei, H.; Jiang, J.; Xu, Y.; Lu, W.; Tocchetti, C.G. The Role of Oxidative Stress in Hyperuricemia and Xanthine Oxidoreductase (XOR) Inhibitors. Oxidative Med. Cell. Longev. 2021, 2021, 380. [Google Scholar] [CrossRef]
- Li, J.; Guo, C.; Yang, X.; Xie, W.; Mi, W.; Hua, C.; Tang, C.; Wang, H. Effects of natural products on macrophage immunometabolism: A new frontier in the treatment of metabolic diseases. Pharmacol. Res. 2025, 213, 107634. [Google Scholar] [CrossRef]
- Ndrepepa, G. Uric acid and cardiovascular disease. Clin. Chim. Acta 2018, 484, 150–163. [Google Scholar] [CrossRef]
- Kumar, A.N.; Aruna, P.; Naidu, J.N.; Kumar, R.; Srivastava, A.K. Review of concepts and controversies of uric acid as antioxidant and pro-oxidant. Arşiv Kaynak Tarama Derg. 2015, 24, 19–40. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, M.; Huang, S.; Lan, X.; Zheng, J.; Luo, H.; He, Y.; Lei, W. Hyperuricemia: A key contributor to endothelial dysfunction in cardiovascular diseases. FASEB J. 2023, 37, e23012. [Google Scholar] [CrossRef]
- Rhea, E.M.; Banks, W.A.; Raber, J. Insulin Resistance in Peripheral Tissues and the Brain: A Tale of Two Sites. Biomedicines 2022, 10, 1582. [Google Scholar] [CrossRef] [PubMed]
- Dilworth, L.; Facey, A.; Omoruyi, F. Diabetes Mellitus and Its Metabolic Complications: The Role of Adipose Tissues. Int. J. Mol. Sci. 2020, 22, 7644. [Google Scholar] [CrossRef]
- Kosmas, C.E.; Bousvarou, M.D.; Kostara, C.E.; Papakonstantinou, E.J.; Salamou, E.; Guzman, E. Insulin resistance and cardiovascular disease. J. Int. Med Res. 2023, 51, 30006. [Google Scholar] [CrossRef]
- Manrique, C.; Lastra, G.; Sowers, J.R. New insights into insulin action and resistance in the vasculature. Ann. N. Y. Acad. Sci. 2014, 1311, 138–150. [Google Scholar] [CrossRef]
- La Russa, D.; Cerra, M.C.; Pellegrino, D.; Montesanto, A. Chronic kidney disease as an age-related disease: New study perspectives fron animal models to hospitalized patents. Dissertation 2019. [Google Scholar] [CrossRef]
- Russo, E.; Bertolotto, M.; Zanetti, V.; Picciotto, D.; Esposito, P.; Carbone, F.; Montecucco, F.; Pontremoli, R.; Garibotto, G.; Viazzi, F.; et al. Role of Uric Acid in Vascular Remodeling: Cytoskeleton Changes and Migration in VSMCs. Int. J. Mol. Sci. 2023, 24, 2960. [Google Scholar]
- Zhang, Z.; Jiang, S.-M.; Ma, Y.-P.; Dai, P.-L.; Wang, Y.-N.; Zou, G.-M.; Gao, H.-M.; Yang, Y.; Li, W.-G. Expression of the intrarenal angiotensin receptor and the role of renin-angiotensin system inhibitors in IgA nephropathy. Mol. Cell. Biochem. 2019, 453, 103–110. [Google Scholar] [CrossRef]
- Yu, W.; Xie, D.; Yamamoto, T.; Koyama, H.; Cheng, J. Mechanistic insights of soluble uric acid-induced insulin resistance: Insulin signaling and beyond. Rev. Endocr. Metab. Disord. 2023, 24, 327–343. [Google Scholar] [CrossRef]
- McMullen, M.K. Fructose increases uric acid contributing to metabolic syndrome-herbal, nutritional and dietary strategies to reduce uric acid. OBM Integr. Complement. Med. 2018, 3, 1–49. [Google Scholar]
- Rong, J.; Fang, C.; Chen, X.; Hong, C.; Huang, L. Association of serum uric acid with prognosis in patients with myocardial infarction: An update systematic review and meta-analysis. BMC Cardiovasc. Disord. 2023, 23, 512. [Google Scholar]
- Sakalli, A.S.A.; Küçükerdem, H.S.S.; Aygün, O.S. What is the relationship between serum uric acid level and insulin resistance? A case-control study. Medicine 2023, 102, e36732. [Google Scholar] [CrossRef]
- Liu, J.; Tao, L.; Zhao, Z.; Mu, Y.; Zou, D.; Zhang, J.; Guo, X. Two-Year Changes in Hyperuricemia and Risk of Diabetes: A Five-Year Prospective Cohort Study. J. Diabetes Res. 2018, 2018, 6905720. [Google Scholar] [CrossRef]
- Billing, A.M.; Kim, Y.C.; Gullaksen, S.; Schrage, B.; Raabe, J.; Hutzfeldt, A.; Demir, F.; Kovalenko, E.; Lassé, M.; Dugourd, A.; et al. Metabolic communication by SGLT2 inhibition. Circulation 2024, 149, 860–884. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.-Y.; Liu, J.-C.; Chen, H.-Y.; Chen, J.-J.; Hao, W.-R.; Cheng, T.-H. Hyperuricemia and epiretinal pathologies: A review of pathophysiological links and clinical implications. Explor. Med. 2024, 5, 732–749. [Google Scholar] [CrossRef]
- Dhungana, A.; Pandeya, A.; Pant, S.; Pokharel, B. Evaluation Of Serum Uric Acid, Glucose And Other Glycemic Parameter In Type II Diabetic Individuals. J. Chitwan Med. Coll. 2022, 12, 68–73. [Google Scholar]
- Krishnan, E.; Pandya, B.J.; Chung, L.; Hariri, A.; Dabbous, O. Hyperuricemia in Young Adults and Risk of Insulin Resistance, Prediabetes, and Diabetes: A 15-Year Follow-up Study. Am. J. Epidemiol. 2012, 176, 108–116. [Google Scholar] [CrossRef]
- Kanbay, M.; Xhaard, C.; Le Floch, E.; Dandine-Roulland, C.; Girerd, N.; Ferreira, J.P.; Boivin, J.-M.; Wagner, S.; Bacq-Daian, D.; Deleuze, J.-F.; et al. Weak association between genetic markers of hyperuricemia and cardiorenal outcomes: Insights from the STANISLAS study cohort with a 20-year follow-up. J. Am. Heart Assoc. 2022, 11, e023301. [Google Scholar] [CrossRef]
- Bashir, A.A.; Bathija, D.; Chandrakar, S. A Study Of Prevalence Of Metabolic Syndrome And Hyperuricemia In Type 2 Diabetes Mellitus. Int. J. Acad Med. Pharm. 2024, 6, 1153–1158. [Google Scholar] [CrossRef]
- Han, R.; Zhang, Y.; Jiang, X. Relationship Between Four Non-Insulin-Based Indexes of Insulin Resistance and Serum Uric Acid in Patients with Type 2 Diabetes: A Cross-Sectional Study. Diabetes Metab. Syndr. Obes. Targets Ther. 2022, 15, 1461–1471. [Google Scholar] [CrossRef]
- Maloberti, A.; Giannattasio, C.; Bombelli, M.; Desideri, G.; Cicero, A.F.G.; Muiesan, M.L.; Rosei, E.A.; Salvetti, M.; Ungar, A.; Rivasi, G.; et al. Working Group on Uric Acid and Cardiovascular Risk of the Italian Society of Hypertension (SIIA). Hyperuricemia and Risk of Cardiovascular Outcomes: The Experience of the URRAH (Uric Acid Right for Heart Health) Project. High Blood Press. Cardiovasc. Prev. 2020, 27, 121–128. [Google Scholar] [CrossRef]
- Fu, Y.; Yu, Y.; Wang, Y.; Sun, Y.; Zhang, K.; Xu, F.; Wang, N.; Wang, B.; Lu, Y. Nonlinear relationship between body mass index and serum uric acid: An observational and Mendelian randomization study among Chinese adults. Nutr. Metab. Cardiovasc. Dis. 2024, 34, 998–1007. [Google Scholar] [CrossRef] [PubMed]
- Pinz, M.P.; Medeiros, I.; Carvalho, L.A.d.C.; Meotti, F.C. Is uric acid a true antioxidant? Identification of uric acid oxidation products and their biological effects. Redox Rep. 2025, 30, 2498105. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Wang, H.; Han, L.; Lyu, G.; Li, S. Effect of uric acid on lipid metabolism assessed via restricted cubic splines: A new insight. Heliyon 2024, 10, e37408. [Google Scholar] [CrossRef] [PubMed]
- Chien, H.-T.; Lin, Y.-W.; Shen, L.-J.; Hsieh, S.-C.; Lin, L.-Y.; Chen, Y.-A.; Lin, F.-J. Sex-specific associations between prolonged serum uric acid levels and risk of major adverse cardiovascular events. Int. J. Cardiol. Cardiovasc. Risk Prev. 2024, 22, 200302. [Google Scholar] [CrossRef]
- Gonzalez-Martin, G.; Cano, J.; Carriazo, S.; Kanbay, M.; Perez-Gomez, M.V.; Fernandez-Prado, R.; Ortiz, A. The dirty little secret of urate-lowering therapy: Useless to stop chronic kidney disease progression and may increase mortality. Clin. Kidney J. 2020, 13, 936–947. [Google Scholar] [CrossRef]
- Zhang, W. Hyperuricemia: Current State and Prospects. Explor. Res. Hypothesis Med. 2025, 10, 49–55. [Google Scholar]
- Kumaresan, S.; Palanisamy, S. Serum uric acid levels in type 2 diabetes patients: Exploring the association with cardiovascular disease-a systematic. Int. J. Acad. Med. Pharm. 2024, 6, 415–419. [Google Scholar] [CrossRef]
- Ruoff, G.; Edwards, N.L. Overview of Serum Uric Acid Treatment Targets in Gout: Why Less than 6 mg/dL? Postgrad. Med. 2016, 128, 706–715. [Google Scholar] [CrossRef]
- Latourte, A.; Pascart, T.; Flipo, R.-M.; Chalès, G.; Coblentz-Baumann, L.; Cohen-Solal, A.; Ea, H.-K.; Grichy, J.; Letavernier, E.; Lioté, F.; et al. 2020 Recommendations from the French Society of Rheumatology for the management of gout: Management of acute flares. Jt. Bone Spine 2020, 87, 387–393. [Google Scholar] [CrossRef]
- Yadav, S.; Khandelwal, N.; Nath, S.K.; Rai, S. A Hospital-Based Cross-Sectional Study of Patients with Plantar Fasciitis: Is Hyperuricemia Screening Needed? Cureus 2023, 15, e37088. [Google Scholar] [CrossRef]
- Richette, P.; Doherty, M.; Pascual, E.; Bardin, T. SUA levels should not be maintained <3 mg/dL for several years. Response to ‘EULAR gout treatment guidelines by Richette et al: Uric acid and neurocognition by Singh et al’. Ann. Rheum. Dis. 2018, 77, e21. [Google Scholar] [CrossRef] [PubMed]
- Tsunoda, S.; Kamide, K.; Minami, J.; Kawano, Y. Decreases in serum uric acid by amelioration of insulin resistance in overweight hypertensive patients: Effect of a low-energy diet and an insulin-sensitizing agent. Am. J. Hypertens. 2002, 15, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Takir, M.; Kostek, O.; Ozkok, A.; Elcioglu, O.C.; Bakan, A.; Erek, A.; Mutlu, H.H.; Telci, O.; Semerci, A.; Odabas, A.R.; et al. Lowering Uric Acid with Allopurinol Improves Insulin Resistance and Systemic Inflammation in Asymptomatic Hyperuricemia. J. Investig. Med. 2015, 63, 924–929. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.A.; MacDonald, P.A.; Hunt, B.J.; Jackson, R.L. Diabetes and gout: Efficacy and safety of febuxostat and allopurinol. Diabetes Obes. Metab. 2013, 15, 1049–1055. [Google Scholar] [CrossRef]
- Spatola, L.; Ferraro, P.M.; Gambaro, G.; Badalamenti, S.; Dauriz, M. Metabolic syndrome and uric acid nephrolithiasis: Insulin resistance in focus. Metabolism 2018, 83, 225–233. [Google Scholar] [CrossRef]
- Bai, Y.; Wu, B.; Gou, L.; Fang, Z.; Xu, T.; Zhang, T.; Li, Y. Cardiovascular Safety Evaluation of Febuxostat and Allopurinol: Findings from the FDA Adverse Event Reporting System. J. Clin. Med. 2022, 12, 6089. [Google Scholar] [CrossRef]
- Caliceti, C.; Calabria, D.; Roda, A.; Cicero, A.F.G. Fructose Intake, Serum Uric Acid, and Cardiometabolic Disorders: A Critical Review. Nutrients 2017, 9, 395. [Google Scholar] [CrossRef]
- Suijk, D.L.; van Baar, M.J.; van Bommel, E.J.; Iqbal, Z.; Krebber, M.M.; Vallon, V.; Touw, D.; Hoorn, E.J.; Nieuwdorp, M.; Kramer, M.M.; et al. SGLT2 Inhibition and Uric Acid Excretion in Patients with Type 2 Diabetes and Normal Kidney Function. Clin. J. Am. Soc. Nephrol. 2022, 17, 663–671. [Google Scholar] [CrossRef]
- Hegazi, O.; Alalalmeh, S.; Shahwan, M.; Jairoun, A.A.; Alourfi, M.M.; Bokhari, G.A.; Alkhattabi, A.; Alsharif, S.; Aljehani, M.A.; Alsabban, A.M.; et al. Exploring Promising Therapies for Non-Alcoholic Fatty Liver Disease: A ClinicalTrials.gov Analysis. Diabetes Metab. Syndr. Obesity Targets Ther. 2024, 17, 545–561. [Google Scholar] [CrossRef]
- Kakutani-Hatayama, M.; Kadoya, M.; Okazaki, H.; Kurajoh, M.; Shoji, T.; Koyama, H.; Tsutsumi, Z.; Moriwaki, Y.; Namba, M.; Yamamoto, T. Nonpharmacological Management of Gout and Hyperuricemia: Hints for Better Lifestyle. Am. J. Lifestyle Med. 2015, 11, 321–329. [Google Scholar] [CrossRef]
- Cleveland, C. Hyperuricemia (High Uric Acid Level): Symptoms, Causes & Treatment. Available online: https://my.clevelandclinic.org/health/diseases/17808-hyperuricemia-high-uric-acid-level (accessed on 19 December 2024).
- Wang, Z.; Li, Y.; Liao, W.; Huang, J.; Liu, Y.; Li, Z.; Tang, J. Gut microbiota remodeling: A promising therapeutic strategy to confront hyperuricemia and gout. Front. Cell Infect Microbiol. 2022, 12, 935723. [Google Scholar] [CrossRef]
- Agius, L.; Ford, B.E.; Chachra, S.S. The Metformin Mechanism on Gluconeogenesis and AMPK Activation: The Metabolite Perspective. Int. J. Mol. Sci. 2020, 21, 3240. [Google Scholar] [CrossRef]
- Caruso, P.; Maiorino, M.I.; Longo, M.; Porcellini, C.; Matrone, R.; Selvaggio, L.D.; Gicchino, M.; Carbone, C.; Scappaticcio, L.; Bellastella, G.; et al. Liraglutide for lower limb perfusion in people with type 2 diabetes and peripheral artery disease: The STARDUST randomized clinical trial. JAMA Netw. Open 2024, 7, e241545. [Google Scholar]
- Sullivan, V.K.; Kim, H.; Caulfield, L.E.; Steffen, L.M.; Selvin, E.; Rebholz, C.M. Plant-Based Dietary Patterns and Incident Diabetes in the Atherosclerosis Risk in Communities (ARIC) Study. Diabetes Care 2024, 47, 803–809. [Google Scholar] [CrossRef]
- Mulugeta, A.; Hyppönen, E.; Ala-Korpela, M.; Mäkinen, V.-P. Cross-sectional metabolic subgroups and 10-year follow-up of cardiometabolic multimorbidity in the UK Biobank. Sci. Rep. 2022, 12, 8905. [Google Scholar] [CrossRef]
- Cho, C.; Kim, B.; Kim, D.S.; Hwang, M.Y.; Shim, I.; Song, M.; Lee, Y.C.; Jung, S.-H.; Cho, S.K.; Park, W.-Y.; et al. Large-scale cross-ancestry genome-wide meta-analysis of serum urate. Nat. Commun. 2024, 15, 3411. [Google Scholar] [CrossRef]
- Vaskimo, L.M.; Gomon, G.; Naamane, N.; Cordell, H.J.; Pratt, A.; Knevel, R. The Application of Genetic Risk Scores in Rheumatic Diseases: A Perspective. Genes 2023, 14, 2167. [Google Scholar] [CrossRef]
- Yang, B.; Xin, M.; Liang, S.; Xu, X.; Cai, T.; Dong, L.; Wang, C.; Wang, M.; Cui, Y.; Song, X.; et al. New insight into the management of renal excretion and hyperuricemia: Potential therapeutic strategies with natural bioactive compounds. Front. Pharmacol. 2022, 13, 1026246. [Google Scholar] [CrossRef]
- Armand, T.P.T.; Mozumder, A.I.; Carole, K.S.; Deji-Oloruntoba, O.; Kim, H.-C.; Ajakwe, S.O. ELIPF: Explicit Learning Framework for Pre-Emptive Forecasting, Early Detection and Curtailment of Idiopathic Pulmonary Fibrosis Disease. BioMedInformatics 2024, 4, 1807–1821. [Google Scholar] [CrossRef]
- Metta, C.; Beretta, A.; Pellungrini, R.; Rinzivillo, S.; Giannotti, F. Towards Transparent Healthcare: Advancing Local Explanation Methods in Explainable Artificial Intelligence. Bioengineering 2024, 11, 369. [Google Scholar] [CrossRef]
- Ajakwe, S.O.; Okoloegbo, C.A.; Lee, J.M.; Kim, D.S. Machine Learning Models for Drone Security: Cognitive Versus Cyber Intelligence for Safety Operations. In Machine Learning for Drone-Enabled IoT Networks; Springer: Cham, Switzerland, 2025; pp. 121–139. [Google Scholar]
- Alkhanbouli, R.; Almadhaani, H.M.A.; Alhosani, F.; Simsekler, M.C.E. The role of explainable artificial intelligence in disease prediction: A systematic literature review and future research directions. BMC Med. Inform. Decis. Mak. 2025, 25, 110. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Lv, H.; Zhang, G. Ensemble machine learning prediction of hyperuricemia based on a prospective health checkup population. Front. Physiol. 2024, 15, 1357404. [Google Scholar] [CrossRef]
- Ajakwe, S.O.; Saviour, I.I.; Ihekoronye, V.U.; Nwankwo, O.U.; Dini, M.A.; Uchechi, I.U.; Kim, D.-S.; Lee, J.M. Medical IoT Record Security and Blockchain: Systematic Review of Milieu, Milestones, and Momentum. Big Data Cogn. Comput. 2024, 8, 121. [Google Scholar] [CrossRef]
- Ajakwe, S.O.; Ihekoronye, V.U.; Ajakwe, I.U.; Jun, T.; Kim, D.S.; Lee, J.M. Connected Intelligence for Smart Water Quality Monitoring System in IIoT. In Proceedings of the 2022 13th International Conference on Information and Communication Technology Convergence (ICTC), Jeju, Republic of Korea, 9–21 October 2022; pp. 2386–2391. [Google Scholar]

| Study | Study Type | Key Findings | Mechanistic Insights | Therapeutic Implications |
|---|---|---|---|---|
| Gao et al. [13] | Human studies | Uric acid activates mTORC1-S6K1 in hepatocytes, worsening hepatic insulin resistance. | Uric acid disrupts IRS-1/Akt signaling via oxidative stress. | Febuxostat improves insulin sensitivity in NAFLD patients. |
| Cuttone et al. [14] | Human studies on SGLT2 inhibitors | SGLT2 inhibitors (empagliflozin) lower uric acid and improve insulin sensitivity in T2D. | Reduced renal urate reabsorption (URAT1 inhibition) with anti-inflammatory effects. Other insights are increased urinary UA excretion and anti-inflammatory and AMPK activation effects. | SGLT2 may be dual-purpose for hyperuricemia and diabetes. |
| Hu et al. [15] | Human studies | Mendelian randomization confirms a causal link between uric acid and insulin resistance, and Variants in SLC2A9 (urate transporter) are linked to higher T2D risk. | Genetic variants in SLC2A9/ABCG2 affect both urate and glucose metabolism. | Supports early urate-lowering therapy (ULT) in prediabetes. |
| Zhang et al. [16] | Human studies on xanthine oxidase inhibitors | Allopurinol reduces fasting insulin in hyperuricemic patients with metabolic syndrome. Additionally, Alopurinol with metformin reduces HOMA-IR more than alone. | Xanthine oxidase inhibition lowers TNF-α and IL-6, improving insulin signaling. Also, Xanthine oxidase inhibition reduces oxidative stress but may not fully reverse IR pathways. | Lowers HOMA-IR by ~15% in 6 months. |
| Zhang et al. [16] | Human Studies on Lifestyle Intervention | Low-purine diet and exercise reduce SUA and improve IR; also, Mediterranean diet lowers UA and IR. | Reduced fructose intake decreases UA synthesis, and exercise enhances insulin sensitivity. | First-line strategy for HU and metabolic syndrome. |
| Badii et al. [17] | Human studies | Leptin resistance mediates hyperuricemia-induced insulin resistance in adipose tissue. | Uric acid upregulates SOCS3, blocking leptin/insulin receptor crosstalk. | Potential for leptin sensitizers (e.g., metformin adjunct). |
| Nasser et al. [18] | Animal studies | Ketogenic diets raise uric acid but may paradoxically improve insulin sensitivity via β-hydroxybutyrate. This suggests a complex context-dependent effect. | Confirms context-dependent effects of uric acid (antioxidant vs. pro-oxidant). | Cautions against high-purine diets in susceptible individuals. |
| Rodriguez-Iturbe et al. [19] | Human studies | NLRP3 inflammasome activation by uric acid crystals drives pancreatic β-cell dysfunction. | Uric acid reduces GSIS (glucose-stimulated insulin secretion). | Anakinra (IL-1 antagonist) trials show promise in T2D. |
| Yu et al. [20] | Human studies | Uric acid-induced inflammasome activation promotes hepatic insulin resistance. | NLRP3 inflammasome drives hepatic inflammation. | Anti-inflammatory agents (e.g., IL-1β antagonists) may improve hepatic insulin sensitivity. |
| Sridharan, S. and Basu, A. [21] | Human studies | Uric acid activates mTOR/S6K1 pathway, inducing insulin receptor substrate-1 (IRS-1) serine phosphorylation. | mTOR/S6K1 pathway mediates insulin resistance. | mTOR inhibitors (e.g., rapamycin) may have adjunct benefits. |
| Bahadoran et al. [22] | Human Studies | Uric acid impairs insulin signaling in endothelial cells. | ROS generation via NADPH oxidase activation. | Antioxidant therapies (e.g., vitamin C, allopurinol) may restore insulin sensitivity. |
| Adnan et al. [23] | Human studies | Elevated serum uric acid (SUA) is associated with a higher incidence of metabolic syndrome and insulin resistance. | Uric acid impairs endothelial function and reduces nitric oxide (NO) bioavailability. | Xanthine oxidase inhibitors (e.g., allopurinol) may improve insulin sensitivity. |
| Wan et al. [24] | Animal studies | Hyperuricemia independently predicts insulin resistance and type 2 diabetes (T2D) development. | Uric acid activates inflammatory pathways (e.g., NLRP3 inflammasome). | Urate-lowering therapy (ULT) may delay T2D onset. |
| Lanaspa et al. [25], Baharudin [26] | Animal studies (uric acid-induced IR models) | Fructose metabolism increases uric acid, leading to mitochondrial oxidative stress and insulin resistance. | Fructose-induced uric acid production inhibits AMPK. | Reducing fructose intake or blocking uric acid synthesis may improve metabolic health. |
| Gong et al. [27] | Review | Hyperuricemia induces adipocyte dysfunction and systemic inflammation. | Uric acid stimulates leptin resistance and adipokine dysregulation. | Targeting adipocyte-uric acid interaction may mitigate insulin resistance. |
| Gong et al. [27] | Animal studies on uricosurics | Probenecid improves IR in obese mice. | Enhances UA excretion, and reduces renal lipotoxicity. | URAT1 inhibitors may be promising. |
| Yu et al. [10] | Animal studies (anti-inflammatory approach) | IL-1β knockout mice resist fructose-induced IR | UA triggers NLRP3 inflammasome leading to IL-1β and then IR. | IL-1 blocker may help gout and metabolic syndrome. |
| Zhang et al. [16] | Animal studies on xanthine oxidase inhibitors | Allopurinol/febuxostat reverse IR in fructose-fed rats. | Reduces oxidative stress and improves endothelial function. | Stronger IR benefits in animals than humans. |
| Gao et al. [13] | Animal study on gene therapy (uricase) | PEGylated uricase reverses IR in KO mice | Degrades UA reduces oxidative stress and inflammation. | Potential for severe HU, but human trials are needed. |
| Aspect | Men | Women (Premenopausal) | Women (Postmenopausal) | Ref. |
|---|---|---|---|---|
| Uric Acid Levels | Higher (androgen-driven reabsorption) | Lower (estrogen promotes excretion) | Rises (loss of estrogen protection) | Li et al. [36] |
| Insulin Resistance (IR) Risk | Earlier onset (visceral fat dominance) | Lower risk (estrogen protective) | Sharply increases (visceral fat shift) | Redon et al. [31] |
| Hyperuricemia–IR Link | Stronger association (oxidative stress, endothelial dysfunction) | Weaker (estrogen-mediated protection) | Strengthens (resembles male pattern) | Meloni et al. [38] |
| Key Influences | Testosterone, muscle mass, and diet | Estrogen, subcutaneous fat | Declining estrogen, rising androgens | Meloni et al. [38] |
| Clinical Implications | Early urate-lowering therapy may benefit metabolic health | Monitor postmenopausal transition | Hormone replacement therapy may modulate risk and screen for metabolic syndrome. | Jung et al. [37] |
| Issue/Dimension | Traditional Binary Approach | Limitations of the Binary Approach | Proposed Continuous/Stratified Approach | References |
|---|---|---|---|---|
| Classification of SUA and IR | Hyperuricemia vs. Normouricemia; Insulin Resistant vs. Insulin Sensitive | Oversimplifies dynamic biological relationships; ignores gradient risk | Model SUA and IR as continuous variables to capture the full physiological range and subtle trends. | Han et al. [69] |
| Threshold Effects | Fixed cutoffs (e.g., SUA > 6.8 mg/dL) | Misses early metabolic risks; may delay intervention | Use data-driven, sex-specific thresholds (e.g., 5.5 mg/dL in men, 4.6 mg/dL in women). | Malorbeti et al. [1] |
| Nonlinear Associations | Assumes linear or stepwise risk | Ignores U- or J-shaped patterns; overlooks potential harm at low SUA levels | Employ nonlinear modeling (e.g., splines) to detect risk inflection points across the SUA spectrum. | Fu et al. [71] Pinz et al. [72] |
| Gender Differences | Uniform cutoffs across sexes | Fails to account for hormonal and physiological variability; menopause alters SUA-IR linkage | Conduct sex-stratified analyses; adjust for menopausal status. | - |
| Biological Interpretation | Uric acid as an isolated metabolic marker | Misrepresents uric acid’s dual role as an antioxidant and pro-oxidant based on context and concentration | View SUA as a context-sensitive biomarker requiring nuanced interpretation. | Pinz et al. [72] |
| Statistical Modeling | Logistic regression or categorical analysis | Reduces statistical power and granularity | Apply flexible modeling techniques: restricted cubic splines and quantile regression. | Xiao et al. [73] |
| Clinical Implications | One-size-fits-all diagnostic and therapeutic thresholds | Poor risk stratification may overlook at-risk patients with “normal” SUA | Enable early detection, individualized risk scoring, and targeted interventions | Hu et al. [9] |
| Research Design | Cross-sectional studies with single-time-point measurements | Cannot capture temporal dynamics or causality | Promote longitudinal studies with repeated SUA/IR assessments; use Mendelian randomization. | Chien et al. [74] |
| Therapeutic Targeting | Universal urate-lowering approach | Risk of overcorrection in low-SUA individuals; unintended oxidative stress | Test SUA modulation across stratified levels to identify safe and effective intervention windows. | Gonzalez-Martin et al. [75] |
| Guideline Development | Static cutoffs dominate clinical protocols | Limit precision medicine applications | Advocate for dynamic sex- and age-sensitive clinical guidelines. | Zhang, [76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deji-Oloruntoba, O.O.; Balogun, J.O.; Elufioye, T.O.; Ajakwe, S.O. Hyperuricemia and Insulin Resistance: Interplay and Potential for Targeted Therapies. Int. J. Transl. Med. 2025, 5, 30. https://doi.org/10.3390/ijtm5030030
Deji-Oloruntoba OO, Balogun JO, Elufioye TO, Ajakwe SO. Hyperuricemia and Insulin Resistance: Interplay and Potential for Targeted Therapies. International Journal of Translational Medicine. 2025; 5(3):30. https://doi.org/10.3390/ijtm5030030
Chicago/Turabian StyleDeji-Oloruntoba, Opeyemi. O., James Onoruoiza Balogun, Taiwo. O. Elufioye, and Simeon Okechukwu Ajakwe. 2025. "Hyperuricemia and Insulin Resistance: Interplay and Potential for Targeted Therapies" International Journal of Translational Medicine 5, no. 3: 30. https://doi.org/10.3390/ijtm5030030
APA StyleDeji-Oloruntoba, O. O., Balogun, J. O., Elufioye, T. O., & Ajakwe, S. O. (2025). Hyperuricemia and Insulin Resistance: Interplay and Potential for Targeted Therapies. International Journal of Translational Medicine, 5(3), 30. https://doi.org/10.3390/ijtm5030030







