Abstract
Epilepsy remains a major therapeutic challenge, with approximately one-third of patients experiencing drug-resistant epilepsy (DRE) despite the availability of multiple antiseizure medications (ASMs). This review aims to evaluate emerging ASMs—cenobamate, fenfluramine, ganaxolone, ezogabine (retigabine), and perampanel—with a focus on their mechanisms of action, pharmacological profiles, and potential role in precision medicine. A comprehensive literature search was conducted using PubMed, Scopus, and Web of Science to identify preclinical and clinical studies evaluating the pharmacodynamics, pharmacokinetics, efficacy, and safety of the selected ASMs. Relevant trials, reviews, and mechanistic studies were reviewed to synthesize the current understanding of their application in DRE and specific epilepsy syndromes. Each ASM demonstrated unique mechanisms targeting hyperexcitability, including the modulation of γ-aminobutyric acid receptor A (GABA-A) receptors, sodium and potassium channels, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPA receptors), and serotonin systems. These mechanisms correspond with specific pathophysiological features in syndromes such as Dravet and Lennox–Gastaut. Evidence from clinical trials supports their use as adjunctive therapies with generally favorable tolerability, though adverse events and variable efficacy profiles were noted. The mechanistic diversity of these emerging ASMs supports their value in personalized epilepsy management, particularly in treatment-resistant cases. While the promise of precision medicine is evident, further studies are required to address challenges related to long-term safety, cost, and equitable access.
1. Introduction
Epilepsy ranks as one of the most common chronic diseases globally, impacting 50 million individuals, and this figure is often considered a significant underestimation. Seizures have a considerable effect on the health of 10% of the global population, whereas epilepsy impacts approximately 1% to 2% of individuals worldwide [1]. This condition significantly affects both quality of life and productivity. The Global Burden of Epilepsy Report indicates that epilepsy accounts for 13 million disability-adjusted life years each year [2]. There are more than 20 antiseizure drug treatments available for managing the symptoms associated with epileptic seizures; around one-third of individuals with epilepsy encounter seizures that do not respond to pharmacotherapy [3]. Epilepsy poses a significant challenge for physicians globally in their pursuit of effective treatments for symptomatic seizures. It is crucial to consider the patients facing structural cerebral damage, comorbidities such as osteoporosis, fractures, and cognitive impairment, as well as the heightened risk of mortality associated with suicide, vascular disease, sudden unexpected death in epilepsy, pneumonia, and accidents.
The current definition of epilepsy includes either two unprovoked seizures occurring at least 24 h apart, a single unprovoked seizure with a high risk of recurrence (greater than 60% over the next ten years), or a confirmed diagnosis of an epilepsy syndrome. Epileptogenesis denotes the process by which a non-epileptic brain evolves into one capable of generating spontaneous, recurring seizures. Seizures occur as a result of abnormal synchronized firing of neurons in a specific region of the brain or throughout the entire brain, often due to irregularly formed networks or structural abnormalities. The abnormalities and disruptions arise from a range of cellular mechanisms, including an imbalance between excitatory and inhibitory neurotransmission, changes in ion channels, synaptic plasticity, neuroinflammation, oxidative stress, and structural anomalies such as hippocampal sclerosis or cortical dysplasia [4]. The interplay of these factors plays a significant role in the pathophysiology of epilepsy and the onset of seizures [1]. Comprehending these cellular and molecular mechanisms is essential for formulating targeted therapies to manage epilepsy and enhance patient outcomes.
One of the major hypotheses explaining pharmacoresistance in epilepsy is the overexpression of drug efflux transporters, particularly P-glycoprotein (P-gp), which actively export antiseizure medications from the brain, reducing their therapeutic concentrations at the site of action [5]. While the primary compounds discussed in this review (cenobamate, fenfluramine, ezogabine, and perampanel) are generally not considered substrates for P-gp, emerging evidence suggests that some of their metabolites may interact with these transporters. For example, the N-acetyl metabolite of ezogabine (NAMR) has been shown to inhibit P-gp-mediated digoxin transport in vitro in a concentration-dependent manner, despite the parent compound not exhibiting such an effect [6]. However, little is known about the interaction of the other drugs with efflux transporters or cytochrome P450 enzymes, highlighting the need for further research in this area. Understanding whether these newer agents avoid transporter-mediated efflux could help explain their efficacy in drug-resistant epilepsy and may represent a crucial step toward overcoming this therapeutic challenge.
This review explores the distinctive mechanisms of action demonstrated by innovative epilepsy medications including cenobamate, fenfluramine, ezogabine, and perampanel. These medications present novel strategies for the management of neurological disorders. Importantly, they are not substrates for drug transporters, a characteristic that may help them overcome one of the most common mechanisms of drug resistance in epilepsy. Cenobamate functions as a positive allosteric modulator of the metabotropic glutamate receptor subtype 5 (mGluR5), fenfluramine influences serotonin signaling, ezogabine promotes neuronal inhibition through potassium channel activation, and perampanel diminishes glutamate-mediated excitatory neurotransmission by acting on AMPA receptors [6]. The recent advancements in pharmacological interventions exhibit potential efficacy in addressing particular forms of resistant epilepsy, where conventional treatment modalities have frequently proven inadequate. For example, perampanel has shown effectiveness in managing refractory focal-onset seizures, offering a significant alternative for patients who do not sufficiently respond to standard antiepileptic medications. Ezogabine is a novel antiepileptic drug that acts by opening neuronal KCNQ (Kv7) potassium channels, thereby enhancing membrane hyperpolarization and neuronal inhibition. This unique mechanism distinguishes it from traditional sodium channel blockers and may contribute to its effectiveness in treating drug-resistant focal seizures. Additionally, ezogabine is not a substrate for common drug efflux transporters, such as P-glycoprotein, which are often implicated in pharmacoresistance [5].
Fenfluramine has surfaced as a promising advancement for individuals afflicted with Dravet syndrome, a particularly severe variant of epilepsy that frequently proves resistant to conventional treatments, through its modulation of serotonin signaling. Cenobamate, through its positive allosteric modulation of the metabotropic glutamate receptor subtype 5 (mGluR5), offers a novel approach to tackling drug-resistant epilepsy, especially in instances where glutamatergic dysregulation is a critical factor. These medications signify significant progress in the treatment of resistant epilepsy, offering more precise and effective solutions for those with drug-resistant epilepsy [7,8,9,10,11,12]. This review focuses on the newly discovered antiepileptic medications in the era of precision medicine, how they work, and which types of resistant epilepsy they can effectively treat. It emphasizes how they can improve patient outcomes and how they advance epilepsy management.
2. Methods
We conducted a narrative review of the literature to explore the mechanisms of action, pharmacokinetics, clinical efficacy, and safety profiles of novel antiseizure medications. A comprehensive search was conducted using Pubmed, Scopus, and Web of Science, employing keywords related to cenobamate, fenfluramine, ganaxolone, ezogabine, and perampanel, as well as drug-resistant epilepsy and precision medicine. Inclusion criteria were as follows:
- Original studies, clinical trials, meta-analyses, or systematic reviews indexed in PubMed, Scopus, and Web of Science.
- Publications focused on cenobamate, fenfluramine, ganaxolone, ezogabine, or perampanel.
- Studies addressing pharmacokinetics, mechanism of action, clinical efficacy, or safety in drug-resistant epilepsy.
- Research conducted in pediatric, adolescent, or adult populations with genetic generalized epilepsy or specific epilepsy syndromes (e.g., Dravet syndrome, Lennox–Gastaut syndrome, CDKL5 deficiency disorder).
- Articles published in English between 2015 and 2025.
3. Cenobamate
Cenobamate is a compound designed for the treatment of focal-onset seizures in adults. Research has not yet been conducted to elucidate the precise mechanism of action; however, numerous animal models have demonstrated its efficacy as an antiepileptic treatment [4]. It has been demonstrated to diminish excitability in the CA3 region neurons of the hippocampus in rat models via the rapid and slow inactivation of sodium channels. Cenobamate has demonstrated selective inhibition of INaP as well as a potent ability to block neuronal hyperexcitability in CA3 hippocampal neurons, a structure that is highly implicated in limbic-onset seizures. This was supported by preclinical proof that this effect results in a reduced repetitive and burst firing with promising prospects for DRE due to hippocampal foci. While clinical trials have not formally stratified efficacy based on seizure focus, strong preclinical data in the mesial temporal model suggest that cenobamate may have focused benefit in mesial temporal epilepsy [13]. It has also shown effectiveness as a modulator of the high affinity of the GABA receptors in hippocampal rat tissue, acting through allosteric changes by binding to a non-benzodiazepine site [13]. Cenobamate demonstrates high oral bioavailability (88%) with a Tmax of 1–4 h. It exhibits a long half-life (37–57 h), enabling once-daily dosing. The drug is metabolized primarily via CYP3A4 and CYP2B6, necessitating dose adjustments when co-administered with CYP inducers or inhibitors. Consequently, it may interact with various medications, requiring possible dose modifications for drugs like lamotrigine, carbamazepine, and clobazam. Its bioavailability remains unaffected by other medications, with the exception of phenytoin, which requires vigilant monitoring and potential dose modifications when co-administered. Therefore, it is advised that the phenytoin dosage be progressively reduced by as much as 50%, and that phenobarbital dosages be diminished concurrently while adjusting the cenobamate dosage. The incorporation of cenobamate into the treatment protocol for adult patients with refractory focal-onset seizures is associated with a substantial reduction in seizure frequency [14]. Cenobamate reduces seizure frequency by up to 55% in drug-resistant focal epilepsy and achieves seizure freedom in up to 28% of patients. Its cost-effectiveness and manageable safety profile further enhance its utility as an adjunctive therapy. The clinical characteristics of cenobamate are outlined in Table 1.

Table 1.
Comparative summary of emerging antiseizure medications: mechanisms, metabolism, advantages, and disadvantages.
3.1. Novel Mechanism of Action
The exact mechanism of action of Cenobamate has yet to be described fully; however, insights have been described. It has been found to enhance the inactive state of voltage-gated sodium channels, known as VGSCs, and block persistent sodium current [13]. It is also mentioned that this drug improves the inhibition mediated by γ-aminobutyric acid (GABA) through positive allosteric modulation of the GABAA receptor through an effect on a binding site not sensitive to benzodiazepines (BZD) [14]. Research has shown that it functions as a positive allosteric modulator at synaptic and extrasynaptic GABAA receptors, modifying phasic (Iphasic) and tonic (Itonic) currents. Consequently, it enhances inhibitory neurotransmission within the brain. This unique GABA-modulating impact sets Cenobamate apart from other medications that target the GABAergic system solely [15]. Cenobamate’s dual action, sodium channel inactivation and GABA A receptor potentiation, targets key mechanisms of neuronal hyperexcitability. This mechanistic specificity supports precision medicine by aligning treatment with the underlying pathophysiology of drug-resistant epilepsy.
3.2. Pharmacokinetics
Cenobamate exhibits a remarkable solubility in aqueous solutions. The oral administration of cenobamate achieves an absorption rate of 88% within the gastrointestinal tract, exhibiting extensive distribution alongside minimal intra- and interindividual variability. The attainment of stationary plasmatic concentrations necessitates a regimen of daily dosage over a fortnight, subsequent to which both the areas under the curve (AUC) and the maximum plasma concentration (Cmax) exhibit a proportional increase within the therapeutic dose spectrum of 100–400 mg/day. The peak concentration is anticipated to occur within a timeframe of approximately 1 to 4 h. The distribution volume following oral administration is estimated to be approximately 40 to 50 L. Sixty percent of the union to plasmatic proteins occurs independently of the plasmatic concentration in vitro [16].
A study characterized by a double-blind, placebo-controlled, randomized design was executed, featuring a single ascending dose approach across four dosage levels: 50, 100, 200, and 400 mg. Subjects were administered either cenobamate or a placebo in a ratio of 6:2. The principal observations indicated that cenobamate demonstrated swift absorption, attaining its peak plasma concentration (Cmax) within a duration of 0.75 to 2.25 h, and exhibited elimination characterized by a mean half-life spanning from 37.0 to 57.7 h [17].
3.3. Advantages over Classic Antiepileptics
This study of patients with highly resistant focal epilepsy treated with cenobamate according to standard clinical protocols revealed significant reductions in seizure frequency and severity, along with a manageable safety profile, demonstrating greater efficacy than other antiepileptic medications. Validation via comparative trials remains essential. Nonetheless, reductions in monthly seizure frequency of up to 55% and seizure-free rates of up to 28% have been attained [18,19]. Cenobamate exhibited considerable efficacy in diminishing seizures, attaining an extraordinary seizure-free rate of up to 28%, a remarkable achievement. The negative consequences of hypersensitivity associated with the treatment seem to be mitigated by a properly calibrated titration schedule [16]. Cenobamate improved Quality-Adjusted Life Years (QALYs) and resulted in reduced costs relative to brivaracetam, eslicarbazepine, lacosamide, and perampanel. It is considered a cost-effective supplementary treatment option for individuals with drug-resistant focal seizures [20]. Cenobamate demonstrates efficacy as an antiseizure medication (ASM) for patients with drug-resistant genetic generalized epilepsy (GGE) and combined generalized and focal epilepsy [21].
3.4. Clinical Studies That Have Supported Its Use
In a multicenter, double-blind, placebo-controlled study targeting adults aged 18 to 65 with focal seizures, participants were randomized 1:1 into cenobamate and placebo groups following an 8-week baseline period. This study showed that Cenobamate exhibited a substantial median percent seizure reduction compared to the placebo (55.6% vs. 21.5%) and a notably higher responder rate (50.4% vs. 22.2%). The reduction in focal seizures, including those with a motor component, impaired awareness, and focal to bilateral tonic–clonic seizures, was significantly more pronounced with cenobamate. During the maintenance phase, 28.3% of cenobamate-treated patients achieved seizure freedom, contrasting with 8.8% in the placebo group. The safety profile indicated that cenobamate, at 200 mg/d, was well-tolerated, with somnolence and dizziness being the most commonly reported adverse events. In this regard, adjunctive treatment with cenobamate demonstrated significant efficacy in improving seizure control among adults with uncontrolled focal seizures while maintaining favorable tolerability [22].
4. Fenfluramine
Fenfluramine is a serotonin-reducing agent. This means it initially increases serotonin release but can lead to reduced serotonergic signaling over time through receptor downregulation. This modulation may help control seizures. It was initially prescribed as an appetite suppressant. Nonetheless, its utilization was identified as a contributing factor to heart valve disease, particularly associated with mitral valve dysfunction [23]. These effects are thought to be associated with serotonergic mechanisms. In 2020, this medication received approval for the treatment of epilepsy in individuals with Dravet syndrome. Dravet syndrome, or severe myoclonic epilepsy of infancy (SMEI), is a rare genetic epileptic encephalopathy that usually manifests in infancy, marked by severe seizures, developmental delays, and cognitive deficits. It has exhibited a strong response regarding magnitude, consistency, and durability in patients experiencing seizure activity due to this syndrome [23]. Despite the availability of over 30 distinct pharmacological interventions for seizure management, the percentage of patients receiving antiseizure medication (ASM) remains constant. Fenfluramine is proposed as a potential therapy for individuals experiencing seizures associated with Dravet syndrome and Lennox–Gastaut syndrome [24]. It is suggested that fenfluramine may be helpful in treating seizures for people with Dravet syndrome (DS) and Lennox–Gastaut syndrome (LGS) as a result of the drug’s effects on serotonin. Despite DS being mainly attributed to mutations in the SCN1A gene with implications for sodium channel function, fenfluramine’s property of modulating serotonin neurotransmission and its sigma-1 receptor activity implies that it impinges also on the serotonergic system and the sigma-1 receptor pathways to restore the balance between excitatory and inhibitory transmitters, and to reduce seizures. There is also evidence of the effectiveness of fenfluramine in LGS, which is due to different seizure types of different etiology, probably because fenfluramine affects both serotonin receptors and sigma-1 receptors in order to modulate the more complex mechanisms underpinning seizure susceptibility in these patients. This broad serotonergic and neuromodulatory mechanism accounts for fenfluramine’s beneficial effects that extend beyond direct sodium channel influence [25]. Lennox–Gastaut syndrome is a severe epilepsy variant that typically manifests in childhood, characterized by various seizure types, including tonic, atonic, atypical absence, and occasionally tonic–clonic seizures. A randomized controlled trial demonstrated that it is an effective, long-lasting, and well-tolerated treatment for individuals diagnosed with Sunflower syndrome, with patients exhibiting no signs of cardiac valvulopathy or hypertension [24,25,26,27,28]. Sunflower syndrome is an uncommon form of photosensitive epilepsy distinguished by highly stereotypical seizures. Individuals with Sunflower syndrome direct their gaze toward a light source, frequently the sun, and gesture with one hand, exhibiting abducted fingers in front of their face. Eye fluttering or blinking is frequently linked to these episodes. Furthermore, these handwaving episodes may intermittently progress into other seizure types, such as generalized tonic–clonic seizures [26]. Additionally, these handwaving episodes can occasionally evolve into other seizure types, including generalized tonic-clonic seizures [27].
4.1. Novel Mechanism of Action
Fenfluramine has a novel action mechanism described as acting in the sigma-1 receptor of double action with serotonergic activity. Still, evidence supports the notion that there are other mechanisms of action. It modulates noradrenergic neurotransmission, associated clinically with patients with concentration problems, learning disabilities, or attention deficit hyperactivity disorder (ADHD). Another direct effect studied is its effect on adrenergic target receptors. At high supratherapeutic concentrations in vitro (>10 µM), dextro-FFA (dexfenfluramine) can stimulate alpha 1-adrenergic receptors, resulting in a metabolic shift from glucose production (gluconeogenesis) to glucose breakdown (glycolysis). It is speculated that increased glycolysis could be related to decreased epileptic activity since decreased glycolysis impairs neuronal function, and glycolysis maintains normal synaptic function [25,28].
4.2. Pharmacokinetics
A pharmacokinetic understanding of the drug has been achieved through studies of its concentration dynamics, isomers of d-F hydrochloride (d-F) and flenfluramine (1-F). Stable concentrations of each isomer were achieved after eight days, and the d isomer concentrations increased faster. In total, 80% to 90% of subjects achieved the maximum concentration after four days, while the one isomer concentration was 70% in the same time frame. After 24 h, plasmatic concentrations were 34 ng/mL for d-F and 18 ng/mL for d-NF, with no difference between the d and dl forms administered. Concentrations of 1-f were higher than the d isomer after two days of administration. The elimination half-life of NorFengluoramine was longer than that of fenfluramine, with the d isomer being similar for d-F and dl-F, and the one isomer being significantly longer. Substantial differences were observed between subjects in the relative rates of clearance and, therefore, metabolic inactivation of F and NF, depending on the metabolite ratios of the parent drug [29].
A study focused on the pharmacokinetics of Dravet’s syndrome showcased the following results. After oral administration, it is absorbed completely and has a bio disponibility of 68% to 74%. The maximum concentration was found in three hours. It was found that food ingestion did not alter fenfluramine absorption. Protein union is on approximately 50%. The medium plasmatic life of fenfluramine was 20 h, and for Norfenfluramine 23, fenfluramine reached a stable state after approximately seven days. In a stable state, concentration lasts 4 to 5 h. Fenfluramine comprises two stereoisomers, dexfenfluramine, and levofenfluramine, which are N-desethylated in the liver to produce the active metabolites of norfenfluramine. Over 75% of fenfluramine undergoes a metabolization process to norfenfluramine before its elimination, catalyzed mainly by CYP1A2, CYP2B6, and CYP2D6. There is potential for further metabolism through CYP2C9, CYP2C19, and CYP3A4. Norfenfluramine subsequently undergoes deamination and oxidation, leading to the formation of inactive metabolites. It should be noted that norfenfluramine is not classified as a potent substrate of any enzyme belonging to the CYP450 system. Furthermore, both fenfluramine and norfenfluramine do not show the ability to inhibit or induce any of the enzymes of the CYP450 system at clinically significant concentrations, which lowers the risk of drug interactions and contributes to a better safety profile. Ultimately, fenfluramine is eliminated primarily through urine, accounting for more than 90% of total excretion, while less than 5% is found in feces [23]. The metabolism of its major metabolite, norfenfluramine, seems less influenced by CYP inhibitors. The interaction between FFA and the CYP system underscores the potential for drug interactions. It necessitates caution when co-administering FFA with potent inhibitors or inducers of multiple enzymes involved in its metabolism [30].
4.3. Advantages over Classic Antiepileptics
Fenfluramine exerts a notably beneficial impact on conditions such as Dravet syndrome and Lennox–Gastaut syndrome. A cohort of 12 adult and pediatric patients diagnosed with Dravet’s syndrome underwent treatment with fenfluramine as an adjunct therapy, revealing encouraging long-term outcomes. Two of these patients had discontinued fenfluramine before the last follow-up. After an average follow-up period of 11 years, the findings indicated that 70% of participants were seizure-free for a minimum of 1 year, while 60% exhibited no epileptic activity during a 4 h electroencephalogram (EEG) [30]. Subsequently, they published data on a 5-year prospective follow-up on the same group of patients and reported that 3 out of 10 (30%) patients remained seizure-free for all 5 years, and 4 out of 10 (40%) were seizure-free for at least 2 years. Remarkably, one patient classified as a non-responder in the retrospective case series experienced a seizure-free period of 2 years during the prospective follow-up period. Two out of ten patients noted cardiac valve thickening, deemed clinically insignificant. No clinically significant valvular malfunction or pulmonary hypertension was observed [24]. In recent years, a phase 3 study investigated the efficacy of Dravet’s syndrome patients. It showcased a significant reduction in seizures in the group ingesting fenfluramine in comparison to the placebo group, this being without showcasing significant adverse effects such as the previously related ones in pulmonary arterial hypertension or cardiac abnormalities. Fenfluramine has showcased promising results for the treatment of Lennox–Gastaut syndrome. In a recently published phase 2 study, fefluoramine was administrated to pediatric patients ranging from 3 to 18 years of age with Lennox–Gastaut (LGS) syndrome who did not respond effectively to traditional treatment. A dose of 0.8 mg/kg/day is the maximum dose of 30 mg per day for 20 weeks. Dose escalation was stopped if the patient achieved a 50% reduction in seizures compared to their baseline before treatment. A median reduction of 53% in seizures was found in the 13 enrolled patients and 60% in the 10 who completed the study. Of the 13 patients, 8 (62%) experienced a 50% or more significant reduction in seizure frequency. Nine patients participated in a long-term extension of the study, demonstrating a median reduction in seizure frequency of 58% at the 15-month mark. Among adverse events, a decrease in appetite and reduced alertness were reported. Cardiac function was monitored through Doppler echocardiography, and no indications of valvulopathy or pulmonary hypertension were observed [31,32].
Recently, fenfluramine has been recognized as an effective treatment option for Sunflower syndrome. The drug demonstrated a significant decrease in seizure frequency and handwaving episodes at low doses. A follow-up study concluded that fenfluramine is a viable, enduring, and well-tolerated medication option for patients experiencing Sunflower syndrome, demonstrating effectiveness over time. This study notably indicated the absence of cardiac valvulopathy or pulmonary hypertension [33]. Fenfluramine demonstrates encouraging efficacy in addressing both less familiar and well-known triggers of epileptic seizure onset.
5. Ztalmy (Ganaxolone)
Ganaloxone is a synthetic neuroactive steroid that functions as a positive allosteric modulator of the gamma-aminobutyric acid (GABA) receptor. Ganaloxone obtained its initial approval in March 2022 for the treatment of seizures associated with cyclin-dependent kinase type 5 deficiency disorder (CDKL5) in individuals aged two years and older. Cyclin-dependent kinase-like 5 (CDKL5) deficiency disorder (CDD) is caused by pathogenic variants in the CDKL5 gene, leading to developmental encephalopathy. This unique condition presents with early-infantile-onset refractory epilepsy, hypotonia, developmental intellectual and motor deficits, and cortical visual impairment. The endorsement was predicated on a phase 3 trial demonstrating that Ganaloxone diminished the incidence of seizures in pediatric and adolescent patients [34,35]. The current evidence regarding this treatment as a neuroprotector is examined, demonstrating a high safety profile when administered post-hypoxia associated with childbirth, alongside evidence suggesting it may decrease the occurrence of enduring disabilities linked to preterm birth [34].
5.1. Action Mechanism
Ganaxolone (GX) is a synthetic analog of allopregnanolone with a 3-beta-methylated structure. It is an allosteric modulator of GABA(A) receptors with an action site that differs from the benzodiazepine binding site [36]. Insights on the action mechanism have yet to be mapped and described to provide a complete understanding of how the drug works. However, the recent literature has described it as a selective allosteric modulator and direct activator of extrasynaptic δGABA-AR receptors (δGABA-ARs). Like other neurosteroids, GX augments the activity of both extrasynaptic and synaptic GABA-ARs by binding to specific “neurosteroid-binding” sites, which are separate from the sites reserved for GABA, benzodiazepines, and barbiturates. This has been mainly localized in native hippocampal dentate gyrus granule cell (DGGC) neurons. At extrasynaptic locations, GX enhances and activates tonic currents directly in neurons possessing δGABA-ARs within hippocampal slices, maintaining functional synapses and dendritic connections [37].
5.2. Pharmacokinetics
The pharmacokinetic properties of ganaloxone have been reported from a double-blind, placebo-controlled volunteer human trial as well as from in vivo GLC (gas–liquid chromatography) analysis of Ganaloxone plasma levels with concomitant therapeutic challenges. High single doses were well-tolerated as bolus injections of Ganaloxone 50, 150, 300, 450, and 600 mg in healthy men. Its absorption is rapid and bi-exponential decay is in line with linear, proportional pharmacokinetics; plasma concentrations peaked within 1–3 h after administration. This bi-exponential decline in drug levels led to a long terminal half-life of ~37–70 h. The plasma concentration–time profiles are similar to lower doses with peak plasma concentrations (Tmax 1.5–3 h) during the first 12 h, and then slowly decrease over time into a range of lower-dose cycles. Ganaxolone plasma levels show a modest increase with the rising dose across the overall range, although levels at high doses are somewhat less than expected from the earlier study. When increasing the dose, there is a nearly proportional increase in the AUC (67% increased dose led to an 80% higher AUC), and peak levels increase to a lesser extent than at the steady state with identical dose increases [37].
5.3. Clinical Studies That Have Supported Its Use
Clinical trials have been conducted to evaluate the efficacy of Ganaxolone in addressing diverse types of seizures. A randomized, double-blind, placebo-controlled phase 2 study of ganaxolone as an adjunct therapy in adults experiencing uncontrolled partial-onset seizures was conducted, involving participants aged 18 to 69 years who were resistant to established antiepileptic drugs. Over an eight-week period at a dosage of 1500 mg/day, the study yielded promising results, indicating a reduction in the frequency of partial-onset seizures. Furthermore, ganaxolone was generally well-tolerated and considered safe. The results support the continued advancement of ganaxolone for adult individuals suffering from refractory partial-onset seizures [38].
Ganaloxone has undergone extensive testing as a therapeutic intervention for individuals experiencing seizures linked to cyclin-dependent kinase-like 5 deficiency disorder, evaluated through a placebo-controlled, double-blind study. This condition represents a unique disorder and is classified as a developmental epileptic encephalopathy. The condition is marked by the emergence of seizures during early life, generally occurring within the initial two months, and these seizures frequently demonstrate resistance to treatment with various medications [39]. Ganaxolone exhibited a significant decrease in the frequency of seizures linked to CDD in comparison to the placebo, and it was largely well-tolerated [40]. A comprehensive longitudinal study investigated the drug’s efficacy in achieving a sustained decrease in the frequency of primary motor seizures, yielding results that align consistently with those obtained in the earlier double-blind phase [40].
Ganaloxone has shown efficacy in reducing PCDH19-clustering seizures compared to placebo, and it has demonstrated favorable tolerability overall [41]. PCDH19-clustering epilepsy (PCDH19-CE) is a type of X-linked epilepsy disorder characterized by intellectual disability (ID) and behavioral disturbances. It is caused by variants in the PCDH19 gene. Interestingly, this condition leads to epilepsy in heterozygous females but not in hemizygous males, making its inheritance pattern unusual. The hypothesis of cellular interference has been proposed as a critical pathogenic mechanism underlying this disorder [42,43,44,45].
Additionally, ganaloxone has shown excellent efficacy in the treatment of refractory status epilepticus (RSE). A phase 2 open-label study evaluated the safety, efficacy, and dosing of intravenous (IV) ganaxolone in patients aged 12 and older with RSE who had failed treatment with benzodiazepines and at least one second-line antiseizure medication. Seventeen patients received ganaxolone infusions at varying doses alongside standard care. The treatment rapidly controlled seizures with a median cessation time of 5 min, and none required escalation to intravenous anesthetics within 24 h. Ganaxolone was generally well-tolerated, with two serious adverse events related to sedation and no deaths attributed to the drug. These findings suggest that IV ganaxolone may provide a safe and effective option for controlling RSE without the risks associated with anesthesia escalation [44].
5.4. Advantages over Classic Antiepileptics
Ganaxolone, an epalone, differentiates itself by a unique mechanism of action from current antiepileptic drugs. It does this by binding with GABAA receptors and activates both synaptic (sites which are different from benzodjsonaepines) and extrasynaptic sites, delivering both phasic and tonic inhibition. Pharmacological evidence from in vivo studies demonstrates that GNX has potent protective efficacy across a wide range of animal models. The study released as GNX exerts a significant anticonvulsant activity, and non-toxic doses, in the acute seizure model (clonic convulsion induced by PTZ) to rats and mice. In addition, it had potent anticonvulsant activity against seizures induced by bicuculline, t-butylbicyclophosphorothionate, and aminophylline in mice. Anticonvulsant properties of ganaxolone, a selective, high-affinity agonist for the neurosteroid recognition site of the GABAA receptor [46]. Ganaxolone attenuated AD elongation in 25-day-old rats, and delayed it in 12-day-old rats, with no effect on motor function at moderate doses. In contrast to the aforementioned response of immature rats, ganaxolone at anticonvulsant doses does not impair motor performance in adult rats [47]. Ganaxolone has also been suggested as a potential therapy for patients with temporal lobe seizures. In mice, behavioral and electrographic seizures following amygdala kindling stimulations of rapid trains at 60 pulses/second) were significantly suppressed by ganaxolone in a dose-dependent manner with an ED50 = 0.1 mg/kg, s.c., which was similar to the effect produced by clonazepam. This study strengthens the rationale for the effectiveness of ganaxolone for patients with temporal lobe seizures based on its efficacy in ameliorating amygdala kindling, which is a model for mesial temporal lobe epilepsy [48].
It can be more likely to worsen certain seizure types, but this is less clear. Ganaxolone has been tested in low-dose pentylenetetrazol (PTZ) and gamma-hydroxybutyric acid (GHB) rat models of absence seizures. This finding merits consideration, as the role of GABAergic signaling is complex in the context of absence seizures. Although Ganaxolone primarily enhances GABA-A benzodiazepine receptor activity, increased GABAergic neurotransmission can indirectly influence thalamocortical networks, potentially facilitating the hypersynchronous oscillations characteristic of absence seizures. Therefore, careful monitoring and further evaluation in preclinical and clinical settings are essential to determine Ganaxolone’s overall risk–benefit profile for absence epilepsy. In both the PTZ and GHB models, there was a significant prolongation of absence seizures with pretreatment with ganaxolone. Furthermore, doses of ganaxolone greater than 20 mg/kg alone caused bisynchronous spike–wave discharges (SWDs) together with behavioral arrest. These results suggest that the activation of the GABA(A-BZD) receptor complex by neurosteroids can act as a pro-absence epileptogenic signal. Ganaxolone, a selective high-affinity steroid modulator of the gamma-aminobutyric acid-A receptor, exacerbates seizures in animal models of absence seizures [49]. The most significant aspect of this drug is the low risk of interactions and both oral and IV formulation offers a flexibility that could be beneficial in the management of acute as well as home environments. The highly lipophilic profile allows a high exposition on brain tissue. In terms of the safety profile, it is to be highlighted that Ganaxolone has not demonstrated any evidence of organ toxicity or teratogenicity occurring as well. It is possible that some of the CNS effects from ganaxolone could be beneficial in epilepsy with comorbid anxiety or depression, and its GABAergic interactions may have either positive or negative impacts based on different receptors and where they are found in the brain [50].
6. Potiva (Ezogabine or Retigabine)
Ezogabine, also known as Retigabine, represents a novel addition to the anticonvulsant pharmacotherapy landscape, characterized by its unique mechanism of action that involves the activation of low-threshold potassium-dependent channels, thereby facilitating the membrane’s transition to a hyperpolarization potential, attaining equilibrium of the resting membrane potential and inhibiting repetitive firing [38]. The phenomenon has been demonstrated in PC12 cells that have undergone differentiation through nerve growth factor, facilitating membrane repolarization. The effects are contingent upon the dosage; nevertheless, it is common for drowsiness, dizziness, vertigo, and confusion to manifest as well. It is noted that, owing to its distinctive properties, it stands out as an exceptional medication for the management of epileptic seizures [51].
6.1. Novel Mechanism of Action
Differences between retigabine and other anticonvulsant drugs include Retigabine having a novel action mechanism with characteristics not shared by any of the clinically available antiepileptic medications. Its main mechanism of action is as a positive allosteric modulator of KCNQ2-5 ion channels (K(v) 7.2–7.5), making it the first neuronal potassium (K(+)) channel opener designed to treat epilepsy. The KCNQ2-5 channels are neuronal, widely distributed voltage-gated potassium channels that play a critical role in controlling cellular excitability especially because human genetic mutations in these channels induce inherited disorders, such as benign familial neonatal seizure syndrome in KCNQ2/3. The compound Retigabine has shown a capacity to stabilize the open conformation of these KCNQ2-5 channels based on one study. In particular, the lack of KCNQ1 is responsible for the drug’s activity on potassium channels other than cardiac KCNQ. KCNQ channels operate near the normal resting membrane potential of cells and contribute to a permanent hyperpolarizing action, leading to increased cellular excitability stability. The mechanism of action of RTG/EZG is to increase the activation of KCNQ channels in their closed configuration, thus over-priming the cell for an enhanced more rapid and sustained response to membrane depolarization or increased neuronal excitation. Thus, due to this, RTG/EZG /RN enriches natural inhibitory power over the brain and works as a check to prevent sustained depolarization era (firing of action potentials), responsible for the era and growth of epileptic activity in CNS [52].
6.2. Pharmacokinetics
Retigabine is rapidly absorbed and the Tmax ranges from 0.5 to 2 h after administration. Following this, plasma levels decrease in a monoexponential manner with an average half-life of 6–8 h. Orally administered retigabine is about 60% bioavailable. Retigabine is mainly metabolized by N-acetylation and subsequent N-glucuronidation. Preclinical in vitro and in vivo data have shown a low drug–drug interaction potential of retigabine. Retigabine is linear in pharmacokinetics within the dose range of 200–400 mg tablets, with an intersubject variability of approximately 35–50%. Systemic exposure was unaffected by a high-fat meal, but the Cmax increased by ~14% and ~38% between the fed and fasted states with respect to 200 mg and 400 mg tablets, respectively. Renal elimination of retigabine-related material is the major route, with unchanged retigabine accounting for about 36% of toenails. The plasma clearance of retigabine decreased in parallel to the severity of renal and hepatic impairment. Retigabine does not demonstrate gender-specific systemic exposure after the body weight-normalized concentration. Systemic exposure to retigabine was greater and the half-life longer in elderly individuals relative to younger subjects [53].
6.3. Advantages over Classic Antiepileptics
Ezogabine has advantages over many available anticonvulsants because it is not metabolized through the cytochrome P450 system and is not highly protein-bound, limiting its potential for drug interactions. Exclusive to ezogabine in comparison with other anticonvulsants, its association with urinary retention stands out due to its effect on the potassium channels in the urothelium of the bladder.
The effect of ezogabine on urinary symptoms may be clinically advantageous for those with an overactive bladder. Ezogabine is used with a dose–response reduction in contractility and overall tone of isolated rat bladder tissue, regardless of the stimulation method [51,52,53,54].
6.4. Clinical Studies That Have Supported Its Use
In a multicenter, randomized, double-blind, placebo-controlled trial study of 538 patients, the erzogabazine-treated group at 600 mg and 900 mg doses saw statistically significant decreases in both median seizure percentage reduction and responder rates when compared with the placebo. However, erzogabazine was more commonly associated with treatment discontinuations related to adverse events, of which the most frequent included dizziness, drowsiness, headache, and fatigue. Erzogabazine was efficacious and generally well-tolerated in adults with refractory partial-onset seizures as adjunctive therapy. While no stratification analysis for ezogabine efficacy itself by seizure onset zone was made in the RESTORE 2 trial, it is valuable to note that the mode of action of ezogabine is via stabilization of Kv7.2–Kv7.5-mediated K(+) channels for decreasing neuronal excitability, which could potentially be used in limbic-onset seizures that frequently occur in temporal lobe epilepsy. Furthermore, the drug’s localized interaction with the potassium channels encoded by KCNQ genes, which are involved in familial epilepsies, is consistent with its place in a precision medicine approach—particularly in patients whose seizures may involve ion channel dysfunctions. Further studies are needed to delineate its utility across specific focal epilepsy subtypes, including limbic epilepsy [54].
7. Perampanel
Perampanel serves as a therapeutic agent, functioning as a non-competitive agonist of α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) [55]. AMPA receptors play a crucial role in the mediation of glutamate at the excitatory synapse. Preclinical studies have indicated that the blockade of AMPA receptors represents a promising avenue for antiepileptic therapy [38]. Research demonstrates that this treatment exhibits significant efficacy and safety in individuals with uncommon genetic anomalies. The list encompasses epilepsies characterized by a deficiency in γ-aminobutyric acid inhibition, such as those linked to SCN1A, alongside mutations in GNAO1, PIGA, PCDH19, SYNGAP1, CDKL5, NEU1, and POLG, indicating a direct influence on glutamate transmission [56].
7.1. Novel Action Mechanism
Perampanel serves as a selective non-competitive antagonist of the ionotropic alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor, with a particular focus on post-synaptic neurons. Nevertheless, the precise pathway through which this antiepileptic effect operates has yet to be thoroughly established. Perampanel has received approval from both the European Medicines Agency (EMA) and the Food and Drug Administration (FDA) for its use as an adjunctive treatment for partial-onset seizures, with or without secondarily generalized seizures, in a diverse age group of patients with epilepsy aged 12 years and older, in addition to its application for primary generalized tonic–clonic seizures [57].
7.2. Pharmakocinetics
Perampanel exhibits linear pharmacokinetics and is rapidly absorbed following oral administration, achieving a time to maximum plasma concentration (Tmax) within 0.5 to 2 h. It demonstrates nearly complete bioavailability (approximately 100%) and has a small volume of distribution (Vd) of 1.1 L/kg. Notably, perampanel is highly protein-bound in plasma, with 98% of the drug bound to plasma proteins.
Metabolism of perampanel occurs extensively in the liver, predominantly mediated by the cytochrome P450 enzyme CYP3A4, leading to the formation of multiple metabolites. In adults, the elimination half-life (t½) of perampanel is approximately 48 h.
The pharmacokinetic profile of perampanel is susceptible to drug–drug interactions, particularly with concomitant medications that induce or inhibit CYP3A4. Co-administration with enzyme-inducing antiepileptic drugs (AEDs) such as carbamazepine, oxcarbazepine, and phenytoin reduces the plasma concentrations of perampanel, whereas CYP3A4 inhibitors like ketoconazole increase its plasma levels.
In phase III clinical trials, the plasma concentrations of perampanel in responders ranged between 180 and 980 µg/L, highlighting interindividual variability and the need for careful therapeutic monitoring when managing drug interactions or optimizing treatment regimens [58,59].
7.3. Clinical Studies That Have Supported Its Use
An analysis of a large cohort (n = 2396) provides valuable insights into the effectiveness and safety profile of adjunctive perampanel in real-world clinical practice for epilepsy management. Among the participants at one center, 95% experienced focal seizures, underscoring the predominance of this seizure type in the study population. The cohort had a median epilepsy duration of 27 years, reflecting the chronic nature of epilepsy within this group. The average exposure to antiepileptic drugs (AEDs) was moderate, with patients using a median of two concomitant AEDs, but prior AED exposure was extensive, with a median history of six previously tried AEDs. A key finding was the 1-year retention rate of 48%, suggesting a notable degree of tolerance and long-term acceptance of perampanel as an adjunctive therapy.
Clinically meaningful benefits were observed in a subset of patients, with a 1-year seizure freedom rate of 9.2%, defined as more than six months without seizures. The median duration of perampanel treatment in this subgroup was 11.3 months. Treatment-emergent adverse events (TEAEs) were consistent with those documented in earlier clinical studies, with fewer events observed in this real-world setting compared to clinical trial data. Importantly, no new or unexpected TEAEs were reported.
Although observational studies are inherently subject to limitations, the findings suggest that adjunctive perampanel may provide significant therapeutic benefits in a subset of patients with epilepsy, contributing to improved seizure control and long-term treatment adherence [60].
7.4. Advantages over Classic Antiepileptics
Clinical studies have shown good tolerability for perampanel and will be critical with respect to long-term patient treatment adherence. Clinical data have supported its use for focal seizures with or without secondary generalization and primary generalized tonic–clonic seizures (PGTC) and other seizure types. Such extensive clinical experience in the real world confirms that perampanel is as efficacious in potentially more representative clinical populations beyond trials. Of added significance is that perampanel has not only proven efficacy across diverse seizure types, including those of absence or myoclonic seizure etiology, but also a positive safety profile in this real-world setting. Perampanel, the first antiepileptic drug of its class, works differently from some other AEDs as it is expected to provide added benefit for patients with refractory epilepsy. Given its novel mechanism, perampanel has the potential to be a broad-spectrum agent. In patients with treatment-resistant focal seizures, with or without secondary generalization, PHWT may represent a useful adjunctive therapy and PGTC is a potentially rational polytherapy option [60,61,62,63].
8. Discussion
Epilepsy remains a pervasive global health challenge, with nearly one-third of patients experiencing drug-resistant epilepsy (DRE) despite the availability of over 20 antiseizure medications (ASMs). This persistent unmet need underscores the necessity for innovative therapeutic approaches. The recent emergence of novel ASMs, including cenobamate, fenfluramine, ganaxolone, ezogabine, and perampanel, represents a transformative evolution in epilepsy management by targeting specific pathophysiological mechanisms (Table 1).
The novel mechanisms of action underlying these ASMs offer significant therapeutic advantages in addressing specific epilepsy subtypes, see Figure 1. Cenobamate, with its dual mechanism targeting GABAA receptors and voltage-gated sodium channels, has shown notable efficacy in refractory focal seizures, achieving complete seizure freedom in some patients. Fenfluramine’s modulation of serotonin and noradrenergic pathways provides substantial benefits in managing Dravet syndrome and Lennox–Gastaut syndrome, offering improved seizure control and mitigating comorbidities. Ganaxolone’s unique modulation of GABAA receptors offers a promising treatment avenue for rare genetic epileptic syndromes, such as CDKL5 deficiency disorder. Similarly, ezogabine, with its minimal interaction with the cytochrome P450 system, reduces the risk of drug–drug interactions, while perampanel’s AMPA receptor antagonism demonstrates broad-spectrum efficacy, making it a valuable option for polytherapy in refractory epilepsy.
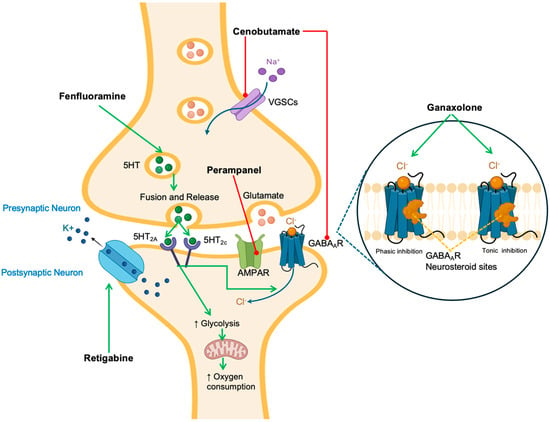
Figure 1.
Mechanism of action of novel antiepileptic medications. Green arrows indicate activation while red arrows indicate inhibition. Medications are highlighted in bold fonts. Abbreviations: K+: potassium; Na+: sodium; SHT: serotonin, AMPAR: α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; GABAAR: γ-aminobutyric acid receptor A; Cl−: chloride; VGSCs: voltage-gated sodium channels. The black arrow connects Perampanel to the AMPAR (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor) on the postsynaptic neuron.
Despite these advancements, challenges persist. The high cost and limited accessibility of molecular diagnostics, including genetic testing, pose barriers to the broader implementation of precision therapies. Variability in patient responses underscores the multifactorial nature of epilepsy, necessitating a more personalized approach to treatment. Furthermore, the limited availability of long-term safety and efficacy data, particularly for newer ASMs such as fenfluramine and ganaxolone, complicates their routine integration into clinical practice. Understanding the real-world impact of these therapies will require comprehensive post-marketing surveillance and longitudinal studies.
Economic barriers also hinder the adoption of innovative ASMs, particularly in low- and middle-income countries. Addressing these disparities is critical to ensuring equitable access to these advancements. The integration of advanced diagnostic technologies with therapeutic interventions holds promise for improving outcomes. Biomarkers for treatment response could optimize therapy selection and minimize trial-and-error approaches, while emerging innovations in gene therapy and machine learning offer the potential to revolutionize epilepsy management.
Collaboration among researchers, clinicians, and policymakers will be essential to overcome these challenges. Efforts should focus on enhancing the cost-effectiveness of precision therapies, expanding access to molecular diagnostics, and fostering international partnerships to ensure equitable treatment availability. By addressing these obstacles, the field can move closer to a future where tailored, effective treatments are accessible to all patients, improving both clinical outcomes and quality of life.
8.1. Age-Dependent Efficacy and Pharmacokinetics of Emerging ASMs
It is important to mention that this set of five novel ASMs have distinct profiles in efficacy when comparing pediatric and adult populations and it is necessary to take into consideration developmental pharmacokinetics and utility in specific syndromes. Cenobamate has demonstrated exceptional efficacy in adult populations with seizures with resistance characteristics. A recent meta-analysis highlighted Cenobamate as a third-generation ASM with the highest efficacy for focal epilepsy [64]. Experience with Cenobamate in pediatric populations is limited, yet still promising. A retrospective study performed in subjects at adolescent age, with a mean of 16 years of age, who were patients with refractory focal epilepsy, reported significant results of a median 93.7% seizure frequency reduction, 62.5% achieving ≥50% seizure reduction, and 19% seizure-free. This pediatric efficacy is mirrored in adult outcomes [65]. Pharmacodynamic potency is maintained through the different age groups when weight-adjusted dosing and slow titration in order to mitigate DRESS risk are utilized. Although age-related biological differences are correlated to differences in the response in specific epileptic syndromes, in a case series of six pediatric patients with Dravet syndrome, cenobamate was found ineffective. None of the subjects met the responder criteria and half had seizure worsening [66]. This contrasts with a small series of adult Dravet patients where cenobamate did reduce seizures >80% [67]. This contrast between these results underscores the importance of the pediatric developing brain and genetic context as primary influencers in an ASM’s efficacy. These showcase that cenobamate’s broad adult efficacy may not generalize to certain pediatric epilepsies [66].
Fenfluramine contrasts this by exhibiting an elevated efficacy in pediatric populations, specifically correlated to syndromes, and has a more circumscribed role in adult populations. In Dravet syndrome (a pediatric-onset DRE), fenfluramine recently established new efficacy benchmarks; across three pivotal trials, there was a 54–65% greater reduction in convulsive seizure frequency compared to the placebo. Notably, approximately half of patients with Dravet syndrome had a ≥75% reduction in seizures with fenfluramine (vs. ~2–4% on placebo) [68], a level of response not reported among this treatment-refractory population. This exceptional efficacy in children has resulted in regulatory approval for Dravet, and positive trials led to its use being expanded to LGS (2–35 years of age). In a landmark phase III trial in LGS, a high dose of fenfluramine (0.7 mg/kg/day) resulted in a median change in drop seizure frequency of 26.5% vs. 7.6% with the placebo [69]. Although the absolute reduction in seizures in LGS was less dramatic than in Dravet, fenfluramine was nonetheless clearly superior to the placebo and seemed especially effective against generalized tonic–clonic seizures in this relatively heterogeneous age range. There are limited data on adults as we do not use fenfluramine to the same extent in adult focal epilepsies but adults with lifelong genetic epilepsies (e.g., older Dravet/LGS patients) appear likely to benefit to a similar degree in percentage seizure reduction as children [69]. Pharmacokinetic variations by age are addressed through dosage based on weight (maximum 26 mg/day in trials), and no significant age-related metabolism concerns have been reported. Therefore, fenfluramine’s clinical importance is extremely age-specific and is a “game changer” for pediatric-onset EE, but its role in adult-onset epilepsy is likely to be limited.
Ganaxolone is a prototype treatment for children specifically and with a neuroactive steroid mechanism of action. As an analog of allopregnanolone, the mechanism of action of ganaxolone is through the modulation of GABAA receptors at sites distinct from those that interact with the GABA molecule, which results in an increase in inhibitory tone. That trial was the phase 3 Marigold study and it should be noted that the drug in question was recently approved as the first drug to treat CDKL5 deficiency disorder—a rare X-linked pediatric epilepsy. In that trial (patients 2 to 21 years of age, mostly female children), ganaxolone demonstrated a median reduction of 30.7% in 28-day major motor seizure frequency compared to 6.9% for the placebo [42].
Ganaxolone showed age-focused efficacy in pediatric patients with CDKL5 deficiency disorder (CDD), a rare, early-onset, and severe developmental and epileptic encephalopathy resulting from a mutation of the CDKL5 gene that normally presents in the first few months of life. In a double-blind, randomized, placebo-controlled phase 3 trial in 101 children with CDKL5 deficiency disorder aged 2–21 years (median age 6) and also on ≥2 antiepileptic drugs, adjunctive ganaxolone significantly reduced the rate of major motor seizures by a median of 30.7% over the 17-week treatment period, vs. 6.9% in those receiving the placebo (p = 0.0036), demonstrating a clinically significant benefit in such a severely refractory pediatric population. The safety profile was good, with the most frequent adverse events being somnolence, pyrexia, and upper respiratory tract infections, whereas serious adverse events were recorded in 12% of patients receiving ganaxolone compared with 10% on the placebo, with no deaths reported. The data support the positioning of ganaxolone as a targeted antiseizure therapy for children and adolescents with CDD, and emphasize its potential directive in a population with limited treatment options [42].
Importantly, the mechanism of action of ezogabine is well suited to some pediatric encephalopathies. In human infants with KCNQ2 developmental and epileptic encephalopathy (KCNQ2-DEE), heterozygous loss-of-function mutations in the K_v7.2 impact the development of drug-resistant early-onset seizures. A pediatric formulation of ezogabine (XEN496) has been formulated for the treatment of this condition. The rationale approach has substantial back-up, as ezogabine can activate mutant K_v7 channels and hyperpolarize neurons [70], perhaps treating the disease at its channelopathy source. Initial case series were promising (some infants had decreased seizures and improved development while taking ezogabine), which has resulted in an active phase 3 study in KCNQ2-DEE. Pending the results, this precision medicine approach is an illustrative example of an ASM with little use in adults that may be repurposed for a discrete peds subgroup. Outside of this specific niche, ezogabine is no longer available for common use; its effectiveness in adults (≈30–40% response rates in focal seizures) was similar to other ASMs but for safety reasons, it was withdrawn. If XEN496 is successful, it will support the age-specific indication of ezogabine in neonates/infants, taking advantage of developmental pharmacology (granular presentation, potential different dosing frequency) and the genetic basis that underpins this therapy to improve outcomes [70].
In the specific case of Perampanel, pediatric populations have higher clearance for Perampanel so dosing in kids tends to be in higher mg/kg (and it is now approved in many areas for ages 4–11 with focal seizures, and for generalized tonic–clonic seizures). Similar (if not higher) efficacy in children compared to adults has been shown in both clinical trials and real-world studies. In a single large pediatric series (n = 216; age 4–12), ~63–68% of patients had equal to more than 50% seizure frequency reduction at 3–12 months while taking adjunctive perampanel [71], which is a responder rate that is consistent with or higher than in adult trials. Demonstrated effectiveness was seen across seizure types at the focal and generalized, myoclonic, and atonic and absence seizures in line with perampanel’s numerous target mechanisms. Children also had a relatively high retention on therapy (65% at 1 year), emphasizing the clinical benefit. Adverse event profiles are similar between children and adults (dizziness, somnolence, irritability) with some behavioral adverse events (aggression, irritability) occurring somewhat more frequently in younger patients [71]. In the reported cohort focused on pediatric patients, 8.3% experienced irritability/aggression and ~6.5% somnolence, leading to ~5.6% discontinuation Considerations include dosing by weight and drug–drug interactions in pediatrics. In general, perampanel is consistently effective in all age groups, with dose increases to allow for the greater metabolism in kids. Its approval for both focal and generalized tonic–clonic seizures in both adult and adolescent patients and pediatric patients adds to its utility if side effects can be managed.
Age plays a significant role in determining the clinical function of every ASM. Some serve poorly met pediatric niches (e.g., fenfluramine in Dravet/LGS) that arise from the biology of childhood-onset epilepsies as the target. Others, such as cenobamate and perampanel, have demonstrative efficacy across a range of adults and, less so, adolescents, but warrant careful generation down to younger children. The cenobamate story in Dravet syndrome serves as a cautionary note that an ASM’s mechanism (e.g., sodium channel modulation) may in fact be counter-productive when interfering with the genetic disease etiology of a pediatric epilepsy. Even so, pediatric use of “adult” ASMs frequently requires extra proof and, when we can, a precision medicine approach (using drugs by a mechanism that fits the child’s epilepsy subtype). Table 1 compares these five adult and pediatric ASMs [66].
8.2. Sex Differences in Pharmacodynamics, Efficacy, and Safety
To date, antiseizure drug development and trials have not focused on sex-based differences; indeed, men and women have been found—consistent with this sample—to respond similarly to most ASMs in terms of efficacy in most studies. A systematic review reported few distinct sex-related differences in seizure control. Nevertheless, new information and clinical experience demonstrate significant sex effects on PK and AEs, as well as hormone-related changes in seizure patterns that interact with ASM treatment [72].
8.2.1. Pharmacokinetic and Dose Considerations
Women show different drug metabolism rates, as compared with men, and this can impact on the serum levels of some ASMs. A pooled analysis of the phase III studies of perampanel discovered that oral clearance was approximately 17% lower in females compared with males (in the absence of enzyme-inducing co-therapies). As a result, female patients had greater perampanel exposure at equivalent doses, and the reduction in seizure frequency and the responder rate was greater in females than males in studies [72]. This indicates that women may fare better in seizure control with any given perampanel dose, albeit also be prone to more adverse effects. Furthermore, the analysis demonstrated that common adverse events, including headache and dizziness, were more common in female patients. This means that, clinically, dosing may need to be titrated individually—females may need slower titration or lower target doses to compensate for the higher levels of the drug [72]. A further point is the interaction between drugs and hormonal contraceptives: perampanel (≥12 mg) can lead to the CYP3A4-mediated metabolism of oral contraceptives [73]. Women of potential childbearing status using perampanel should be advised to utilize high-dose or non-hormonal contraceptive methods to prevent inadvertent pregnancy. In contrast, enzyme-inducing ASMs (none of the five inputs, but, e.g., phenytoin, carbamazepine) are recognized to lower perampanel levels. Notably, such inducer use is more prevalent among men in some cohorts, which might partially offset the sex differences in perampanel exposure in the real world [72]. For the other two novel ASMs, the extent of sex-specific PK characteristics has not been extensively studied. Cenobamate and fenfluramine do not have documented significant sex-mediated differences in metabolism, but general physiology would indicate that higher body fat in women, and a differing CYP expression compared to men, may subtly influence the metabolism of lipophilic drugs like cenobamate. To date, the efficacy of these agents has not been found to differ between sexes in trials but one review suggested that women may be more susceptible to cutaneous side effects of ASMs overall [74]. Ezogabine and ganaxolone, too, do not have sex-specific pharmacokinetic data; however, due to the hepatic pathways that ganaxolone metabolizes, one would expect that pregnancy or oral contraceptive steroids (which affect the activity of liver enzymes) would affect levels—something that needs to be studied.
8.2.2. Hormonal Influences on Seizures
Sex hormones influence epilepsy, especially in females, which is a well-known concept. Estrogen is more likely to exert pro- rather than anticonvulsant effects and the other way round as regards progesterone, after its conversion to neurosteroids, including allopregnanolone. These mechanisms form the basis and are the underlying cause of a number of catamenial epilepsies, in which the seizure number increases disproportionately during a certain stratified period of the menstrual cycle, most commonly in the peri-menstrual period, when progesterone falls, or before ovulation when estrogen rises [75]. These hormonal imbalances may influence the responsiveness of ASMs or the need for individualized treatment interventions. Critically, the mode of action of ganaxolone directly leverages the progesterone–neurosteroid pathway. As an exogenous analog of allopregnenolone, ganaxolone might increase endogenous neurosteroids levels, which decrease prior to catamenial seizures. No published study has directly addressed the role of ganaxolone in catamenial epilepsy; however, the development of this compound was in part driven by this concept [75]. In a large phase 2 trial of ganaxolone in refractory focal epilepsy, a subgroup analysis indicated that women with catamenial patterns appeared to derive some benefit, which prompted the investigators to attribute a “perceived benefit” of ganaxolone in catamenial epilepsy. That trial (1500 mg/d ganaxolone vs. placebo) showed a substantial reduction in seizure frequency in women endorsing catamenial pattern, supporting ganaxolone as a potentially safe and well-tolerated adjunct to catamenial cases [42]. Although further data are required before prescriptive recommendations are made, this suggests that tailored sex-specific utilization of ASMs (e.g., neurosteroid replacement for catamenial seizures) can lead to improved outcomes [76]. In contrast, some seizure types have a different prevalence in men or women—for example, juvenile myoclonic epilepsy may be slightly more common in women, whereas males are more likely to pore posttraumatic epilepsy—but there is no evidence that the five ASMs under consideration here have differential efficacy in these situations. What has also been highlighted is that adverse effect profiles may be exclusive of each other; in addition to the example of perampanel, women with epilepsy, for instance, have reported higher rates of weight gain for some ASMs and increased susceptibility to ASM hypersensitivity syndrome [74]. Whether any of the novel ASMs have sex-differential adverse effects is still to be determined. Fenfluramine causes appetite suppression and weight loss, effects that might a priori impact adolescent female patients, which could be at risk of underweight or nutritional deficiencies. Differently than males, trials have not reported a sex difference in discontinuation rates. Likewise, aggression on perampanel, while idiosyncratic, may be noted more in male adolescents in some clinicians’ experience, but controlled studies have not confirmed a robust sex predisposition for this side effect.
Table 2 compares the five emerging ASMs across age and sex dimensions, summarizing their mechanisms, efficacy, and key safety considerations in adult vs. pediatric and male vs. female contexts.

Table 2.
Comparative efficacy and safety of five emerging ASMs by age and sex.
9. Conclusions
Emerging ASMs exemplify the shift toward precision medicine, with tailored approaches demonstrating the potential to improve seizure control and the overall quality of life in patients with DRE. Future research must prioritize integrating advanced diagnostics with therapeutic innovations, developing biomarkers for treatment response, and addressing systemic inequities in access to care. This multidisciplinary effort will be pivotal in translating these breakthroughs into meaningful real-world benefits, ultimately advancing epilepsy treatment and enhancing the lives of individuals affected by this debilitating condition.
Author Contributions
Conceptualization, G.d.J.A.-V., A.A.-P., and J.E.V.; methodology, G.d.J.A.-V., A.A.-P., and J.E.V.; formal analysis, G.d.J.A.-V. and M.P.F.G.; investigation, G.d.J.A.-V., L.M., M.F.T.-P., and M.P.F.G.; resources, E.L.-C.; data curation, G.d.J.A.-V., M.F.T.-P., and M.P.F.G.; writing—original draft preparation, G.d.J.A.-V., L.M., M.F.T.-P., and M.P.F.G.; writing—review and editing, G.d.J.A.-V., A.A.-P., M.P.F.G., and E.L.-C.; supervision, G.d.J.A.-V., A.A.-P., and J.E.V. All authors have read and agreed to the published version of the manuscript.
Funding
This manuscript was not funded by any external source.
Conflicts of Interest
All authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ASM | Antiseizure Medication |
| DRE | Drug-Resistant Epilepsy |
| GABA | Gamma-Aminobutyric Acid |
| GABAA | GABA type A receptor |
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor |
| CYP | Cytochrome P450 (enzyme system) |
| VGSC | Voltage-Gated Sodium Channels |
| NAMR | N-acetyl metabolite of retigabine (ezogabine) |
| P-gp | P-glycoprotein |
| BZD | Benzodiazepine |
| Iphasic | Phasic GABA current |
| Itonic | Tonic GABA current |
| Tmax | Time to maximum plasma concentration |
| AUC | Area Under the Curve (pharmacokinetics) |
| Cmax | Maximum plasma concentration |
| QALY | Quality-Adjusted Life Year |
| CDKL5 | Cyclin-Dependent Kinase-Like 5 (gene associated with deficiency disorder) |
| CDD | CDKL5 Deficiency Disorder |
| ADHD | Attention Deficit Hyperactivity Disorder |
| d-F | dexfenfluramine (stereoisomer of fenfluramine) |
| 1-F | levofenfluramine (stereoisomer of fenfluramine) |
| d-NF | dexnorfenfluramine (metabolite) |
| LGS | Lennox–Gastaut Syndrome |
| DS | Dravet Syndrome |
| SCN1A | Sodium Channel, Neuronal Type I Alpha Subunit gene |
| SHT | Serotonin (5-HT) |
| SWD | Spike–Wave Discharge |
| CNS | Central Nervous System |
| KCNQ | Potassium Voltage-Gated Channel Subfamily Q |
| Kv7 | Voltage-gated potassium channel 7 (same as KCNQ) |
| PC12 | Pheochromocytoma Cell Line (neuronal differentiation model) |
| PCDH19 | Protocadherin 19 gene |
| PCDH19-CE | PCDH19 Clustering Epilepsy |
| GLC | Gas–Liquid Chromatography |
| EMA | European Medicines Agency |
| FDA | Food and Drug Administration |
| AED | Antiepileptic Drug |
| TEAE | Treatment-Emergent Adverse Event |
| RSE | Refractory Status Epilepticus |
| DEE | Developmental and Epileptic Encephalopathy |
| Vd | Volume of distribution |
| t½ | Elimination half-life |
| BZD | Benzodiazepine |
| CYP3A4 | Cytochrome P450 3A4 (major enzyme for drug metabolism) |
References
- Beghi, E. The Epidemiology of Epilepsy. Neuroepidemiology 2020, 54, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Falco-Walter, J. Epilepsy-Definition, Classification, Pathophysiology, and Epidemiology. Semin. Neurol. 2020, 40, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Sander, J.W. The global burden of epilepsy report: Implications for low- and middle-income countries. Epilepsy Behav. 2020, 105, 106949. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W.; Potschka, H.; Sisodiya, S.M.; Vezzani, A. Drug Resistance in Epilepsy: Clinical Impact, Potential Mechanisms, and New Innovative Treatment Options. Pharmacol. Rev. 2020, 72, 606–638. [Google Scholar] [CrossRef]
- Leandro, K.; Bicker, J.; Alves, G.; Falcão, A.; Fortuna, A. ABC transporters in drug-resistant epilepsy: Mechanisms of upregulation and therapeutic approaches. Pharmacol. Res. 2019, 144, 357–376. [Google Scholar] [CrossRef]
- Tang, F.; Hartz, A.M.S.; Bauer, B. Drug-resistant epilepsy: Multiple hypotheses, few answers. Front. Neurol. 2017, 8, 301. [Google Scholar] [CrossRef]
- Lagogianni, C.; Gatzonis, S.; Patrikelis, P. Fatigue and cognitive functions in epilepsy: A review of the literature. Epilepsy Behav. 2021, 114, 107541. [Google Scholar] [CrossRef]
- Laxer, K.D.; Trinka, E.; Hirsch, L.J.; Cendes, F.; Langfitt, J.; Delanty, N.; Resnick, T.; Benbadis, S.R. The consequences of refractory epilepsy and its treatment. Epilepsy Behav. 2014, 37, 59–70. [Google Scholar] [CrossRef]
- Roberti, R.; De Caro, C.; Iannone, L.F.; Zaccara, G.; Lattanzi, S.; Russo, E. Pharmacology of Cenobamate: Mechanism of Action, Pharmacokinetics, Drug-Drug Interactions and Tolerability. CNS Drugs 2021, 35, 609–618. [Google Scholar] [CrossRef]
- Sullivan, J.; Simmons, R. Fenfluramine for treatment-resistant epilepsy in Dravet syndrome and other genetically mediated epilepsies. Drugs Today 2021, 57, 449–454. [Google Scholar] [CrossRef]
- Pong, A.W.; Xu, K.J.; Klein, P. Recent advances in pharmacotherapy for epilepsy. Curr. Opin. Neurol. 2023, 36, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Knight, D.; Mahida, S.; Kelly, M.; Poduri, A.; Olson, H.E. Ezogabine impacts seizures and development in patients with KCNQ2 developmental and epileptic encephalopathy. Epilepsia 2023, 64, e143–e147. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Cho, J.H.; Shin, H.; Jang, I.S. Effects of cenobamate (YKP3089), a newly developed anti-epileptic drug, on voltage-gated sodium channels in rat hippocampal CA3 neurons. Eur. J. Pharmacol. 2019, 855, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Nakamura, M.; Neupane, C.; Jeon, B.H.; Shin, H.; Melnick, S.M.; Glenn, K.J.; Jang, I.S.; Park, J.B. Positive allosteric modulation of GABAA receptors by a novel antiepileptic drug cenobamate. Eur. J. Pharmacol. 2020, 879, 173117. [Google Scholar] [CrossRef]
- Lattanzi, S.; Trinka, E.; Zaccara, G.; Striano, P.; Del Giovane, C.; Silvestrini, M.; Brigo, F. Adjunctive Cenobamate for Focal-Onset Seizures in Adults: A Systematic Review and Meta-Analysis. CNS Drugs 2020, 34, 1105–1120. [Google Scholar] [CrossRef]
- Serrano-Castro, P.J.; Rodríguez-Uranga, J.J.; Cabezudo-García, P.; García-Martín, G.; Romero-Godoy, J.; Estivill-Torrús, G.; Ciano-Petersen, N.L.; Oliver, B.; Ortega-Pinazo, J.; López-Moreno, Y.; et al. Cenobamate and Clobazam Combination as Personalized Medicine in Autoimmune-Associated Epilepsy With Anti-Gad65 Antibodies. Neurol. Neuroimmunol. Neuroinflamm. 2023, 10, e200151. [Google Scholar] [CrossRef]
- Specchio, N.; Pietrafusa, N.; Vigevano, F. Is Cenobamate the Breakthrough We Have Been Wishing for? Int. J. Mol. Sci. 2021, 22, 9339. [Google Scholar] [CrossRef]
- Yang, E.; Sunwoo, J.; Huh, K.Y.; Kim, Y.K.; Lee, S.; Jang, I.J.; Yu, K.S. Pharmacokinetics and safety of cenobamate, a novel antiseizure medication, in healthy Japanese, and an ethnic comparison with healthy non-Japanese. Clin. Transl. Sci. 2022, 15, 490–500. [Google Scholar] [CrossRef]
- Beltrán-Corbellini, Á.; Romeral-Jiménez, M.; Mayo, P.; Román, I.S.M.; Iruzubieta, P.; Chico-García, J.L.; Parra-Díaz, P.; García-Morales, I.; Toledano, R.; Aledo-Serrano, Á.; et al. Cenobamate in patients with highly refractory focal epilepsy: A retrospective real-world study. Seizure-Eur. J. Epilepsy 2023, 111, 71–77. [Google Scholar] [CrossRef]
- Wheless, J.W. Adjunctive cenobamate for the treatment of focal onset seizures in adults with epilepsy: A critical review. Expert. Rev. Neurother. 2020, 20, 1085–1098. [Google Scholar] [CrossRef]
- Buckley, C.T.; Waters, O.R.; DeMaagd, G. Cenobamate: A New Adjunctive Agent for Drug-Resistant Focal Onset Epilepsy. Ann. Pharmacother. 2021, 55, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.S.; French, J.A.; Kowalski, J.; Krauss, G.L.; Lee, S.K.; Maciejowski, M.; Rosenfeld, W.E.; Sperling, M.R.; Mizne, S.; Kamin, M. Randomized phase 2 study of adjunctive cenobamate in patients with uncontrolled focal seizures. Neurology 2020, 94, e2311–e2322. [Google Scholar] [CrossRef] [PubMed]
- Aman, M.G.; Kern, R.A. Review of Fenfluramine in the Treatment of the Developmental Disabilities. J. Am. Acad. Child. Adolesc. Psychiatry 1989, 28, 549–565. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, E.; Grau, J.B.; Fortier, J.H.; Salvati, E.; Levy, R.J.; Ferrari, G. Serotonin and catecholamines in the development and progression of heart valve diseases. Cardiovasc. Res. 2017, 113, 849–857. [Google Scholar] [CrossRef]
- Balagura, G.; Cacciatore, M.; Grasso, E.A.; Striano, P.; Verrotti, A. Fenfluramine for the Treatment of Dravet Syndrome and Lennox-Gastaut Syndrome. CNS Drugs 2020, 34, 1001–1007. [Google Scholar] [CrossRef]
- Samanta, D. Fenfluramine: A Review of Pharmacology, Clinical Efficacy, and Safety in Epilepsy. Children 2022, 9, 1159. [Google Scholar] [CrossRef]
- Patel, S.; Geenen, K.R.; Dowless, D.; Bruno, P.L.; Thiele, E.A. Follow-up to low-dose fenfluramine for Sunflower syndrome: A non-randomized controlled trial. Dev. Med. Child Neurol. 2023, 65, 961–967. [Google Scholar] [CrossRef]
- Geenen, K.R.; Patel, S.; Thiele, E.A. Sunflower syndrome: A poorly understood photosensitive epilepsy. Dev. Med. Child Neurol. 2021, 63, 259–262. [Google Scholar] [CrossRef]
- Sourbron, J.; Lagae, L. Fenfluramine: A plethora of mechanisms? Front. Pharmacol. 2023, 14, 1192022. [Google Scholar] [CrossRef]
- Caccia, S.; Conforti, I.; Duchier, J.; Garattini, S. Pharmacokinetics of fenfluramine and norfenfluramine in volunteers given d- and dl-fenfluramine for 15 days. Eur. J. Clin. Pharmacol. 1985, 29, 221–224. [Google Scholar] [CrossRef]
- Martin, P.; Czerwiński, M.; Limaye, P.B.; Muranjan, S.; Ogilvie, B.W.; Smith, S.; Boyd, B. In vitro evaluation of fenfluramine and norfenfluramine as victims of drug interactions. Pharmacol. Res. Perspect. 2022, 10, e00958. [Google Scholar] [CrossRef] [PubMed]
- Ceulemans, B.; Boel, M.; Leyssens, K.; Van Rossem, C.; Neels, P.; Jorens, P.G.; Lagae, L. Successful use of fenfluramine as an add-on treatment for Dravet syndrome. Epilepsia 2012, 53, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Ceulemans, B.; Schoonjans, A.S.; Marchau, F.; Paelinck, B.P.; Lagae, L. Five-year extended follow-up status of 10 patients with Dravet syndrome treated with fenfluramine. Epilepsia 2016, 57, e129–e134. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Giraldo, E.; Sullivan, J.E. Advances in the Treatment of Drug-Resistant Pediatric Epilepsy. Semin. Neurol. 2020, 40, 257–262. [Google Scholar] [CrossRef]
- Olson, H.E.; Demarest, S.T.; Pestana-Knight, E.M.; Swanson, L.C.; Iqbal, S.; Lal, D.; Leonard, H.; Cross, J.H.; Devinsky, O.; Benke, T.A. Cyclin-Dependent Kinase-Like 5 Deficiency Disorder: Clinical Review. Pediatr. Neurol. 2019, 97, 18–25. [Google Scholar] [CrossRef]
- Yawno, T.; Miller, S.L.; Bennet, L.; Wong, F.; Hirst, J.J.; Fahey, M.; Walker, D.W. Ganaxolone: A New Treatment for Neonatal Seizures. Front. Cell. Neurosci. 2017, 11, 246. Available online: https://www.frontiersin.org/journals/cellular-neuroscience/articles/10.3389/fncel.2017.00246/full (accessed on 6 July 2024). [CrossRef]
- Nohria, V.; Giller, E. Ganaxolone. Neurother 2007, 4, 102–105. [Google Scholar] [CrossRef]
- Reddy, D.S. Neurosteroids as Novel Anticonvulsants for Refractory Status Epilepticus and Medical Countermeasures for Nerve Agents: A 15-Year Journey to Bring Ganaxolone from Bench to Clinic. J. Pharmacol. Exp. Ther. 2024, 388, 273–300. [Google Scholar] [CrossRef]
- Monaghan, E.P.; Navalta, L.A.; Shum, L.; Ashbrook, D.W.; Lee, D.A. Initial Human Experience with Ganaxolone, a Neuroactive Steroid with Antiepileptic Activity. Epilepsia 1997, 38, 1026–1031. [Google Scholar] [CrossRef]
- Sperling, M.R.; Klein, P.; Tsai, J. Randomized, double-blind, placebo-controlled phase 2 study of ganaxolone as add-on therapy in adults with uncontrolled partial-onset seizures. Epilepsia 2017, 58, 558–564. [Google Scholar] [CrossRef]
- Leonard, H.; Downs, J.; Benke, T.A.; Swanson, L.; Olson, H.; Demarest, S. CDKL5 deficiency disorder: Clinical features, diagnosis, and management. Lancet Neurol. 2022, 21, 563–576. [Google Scholar] [CrossRef] [PubMed]
- Knight, E.M.P.; Amin, S.; Bahi-Buisson, N.; Benke, T.A.; Cross, J.H.; Demarest, S.T.; Olson, H.E.; Specchio, N.; Fleming, T.R.; Aimetti, A.A.; et al. Safety and efficacy of ganaxolone in patients with CDKL5 deficiency disorder: Results from the double-blind phase of a randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2022, 21, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Olson, H.E.; Amin, S.; Bahi-Buisson, N.; Devinsky, O.; Marsh, E.D.; Pestana-Knight, E.; Rajaraman, R.R.; Aimetti, A.A.; Rybak, E.; Kong, F.; et al. Long-term treatment with ganaxolone for seizures associated with cyclin-dependent kinase-like 5 deficiency disorder: Two-year open-label extension follow-up. Epilepsia 2024, 65, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Vaitkevicius, H.; Ramsay, R.E.; Swisher, C.B.; Husain, A.M.; Aimetti, A.; Gasior, M. Intravenous ganaxolone for the treatment of refractory status epilepticus: Results from an open-label, dose-finding, phase 2 trial. Epilepsia 2022, 63, 2381–2391. [Google Scholar] [CrossRef]
- Kowkabi, S.; Yavarian, M.; Kaboodkhani, R.; Mohammadi, M.; Badv, R.S. PCDH19-clustering epilepsy, pathophysiology and clinical significance. Epilepsy Behav. 2024, 154, 109730. Available online: https://www.epilepsybehavior.com/article/S1525-5050(24)00111-2/abstract (accessed on 6 July 2024). [CrossRef]
- Carter, R.B.; Wood, P.L.; Wieland, S.; Hawkinson, J.E.; Belelli, D.; Lambert, J.J.; White, H.S.; Wolf, H.H.; Mirsadeghi, S.; Tahir, S.H.; et al. Characterization of the anticonvulsant properties of ganaxolone (CCD 1042, 3alpha-hydroxy-3beta-methyl-5alpha-pregnan-20-one), a selective, high-affinity, steroid modulator of the gamma-aminobutyric acid (A) receptor. J. Pharmacol. Exp. Ther. 1997, 280, 1284–1295. [Google Scholar] [CrossRef]
- Mares, P.; Stehlíková, M. Anticonvulsant doses of ganaxolone do not compromise motor performance in immature rats. Neurosci. Lett. 2010, 469, 396–399. [Google Scholar] [CrossRef]
- Reddy, D.S.; Rogawski, M.A. Ganaxolone suppression of behavioral and electrographic seizures in the mouse amygdala kindling model. Epilepsy Res. 2010, 89, 254–260. [Google Scholar] [CrossRef]
- Snead, O.C., III. Ganaxolone, a selective, high-affinity steroid modulator of the γ-aminobutyric acid-A receptor, exacerbates seizures in animal models of absence. Ann. Neurol. 1998, 44, 688–691. [Google Scholar] [CrossRef]
- Lattanzi, S.; Riva, A.; Striano, P. Ganaxolone treatment for epilepsy patients: From pharmacology to place in therapy. Expert. Rev. Neurother. 2021, 21, 1317–1332. [Google Scholar] [CrossRef]
- Splinter, M.Y. Ezogabine (Retigabine) and Its Role in the Treatment of Partial-Onset Seizures: A Review. Clin. Ther. 2012, 34, 1845–1856. [Google Scholar] [CrossRef] [PubMed]
- Gunthorpe, M.J.; Large, C.H.; Sankar, R. The mechanism of action of retigabine (ezogabine), a first-in-class K+ channel opener for the treatment of epilepsy. Epilepsia 2012, 53, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Tompson, D.J.; Crean, C.S. Clinical pharmacokinetics of retigabine/ezogabine. Curr. Clin. Pharmacol. 2013, 8, 319–331. [Google Scholar] [CrossRef]
- Brodie, M.J.; Lerche, H.; Gil-Nagel, A.; Elger, C.; Hall, S.; Shin, P.; Nohria, V.; Mansbach, H. Efficacy and safety of adjunctive ezogabine (retigabine) in refractory partial epilepsy. Neurology 2010, 75, 1817–1824. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Gil-Nagel, A.; Wheless, J.W.; Kim, J.H.; Wechsler, R.T. Perampanel monotherapy for the treatment of epilepsy: Clinical trial and real-world evidence. Epilepsy Behav. 2022, 136, 108885. [Google Scholar] [CrossRef]
- Nissenkorn, A.; Kluger, G.; Schubert-Bast, S.; Bayat, A.; Bobylova, M.; Bonanni, P.; Ceulemans, B.; Coppola, A.; Di Bonaventura, C.; Feucht, M.; et al. Perampanel as precision therapy in rare genetic epilepsies. Epilepsia 2023, 64, 866–874. [Google Scholar] [CrossRef]
- Patsalos, P.N.; Spencer, E.P.; Berry, D.J. Therapeutic Drug Monitoring of Antiepileptic Drugs in Epilepsy: A 2018 Update. Ther. Drug Monit. 2018, 40, 526–548. [Google Scholar] [CrossRef]
- Strzelczyk, A.; Schubert-Bast, S. Expanding the Treatment Landscape for Lennox-Gastaut Syndrome: Current and Future Strategies. CNS Drugs 2021, 35, 61–83. [Google Scholar] [CrossRef]
- Rohracher, A.; Zimmermann, G.; Villanueva, V.; Garamendi, I.; Sander, J.W.; Wehner, T.; Shankar, R.; Ben-Menachem, E.; Brodie, M.J.; Pensel, M.C.; et al. Perampanel in routine clinical use across Europe: Pooled, multicenter, observational data. Epilepsia 2018, 59, 1727–1739. [Google Scholar] [CrossRef]
- Potschka, H.; Trinka, E. Perampanel: Does it have broad-spectrum potential? Epilepsia 2019, 60 (Suppl. 1), 22–36. [Google Scholar] [CrossRef]
- Mesraoua, B.; Brigo, F.; Lattanzi, S.; Abou-Khalil, B.; Hail, H.A.; Asadi-Pooya, A.A. Drug-resistant epilepsy: Definition, pathophysiology, and management. J. Neurol. Sci. 2023, 452, 120766. Available online: https://www.jns-journal.com/article/S0022-510X(23)00227-7/abstract (accessed on 28 July 2024). [CrossRef] [PubMed]
- Berg, A.T.; Coryell, J.; Saneto, R.P.; Grinspan, Z.M.; Alexander, J.J.; Kekis, M.; Sullivan, J.E.; Wirrell, E.C.; Shellhaas, R.A.; Mytinger, J.R.; et al. Early-Life Epilepsies and the Emerging Role of Genetic Testing. JAMA Pediatr. 2017, 171, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Balaji, A.; Mohanlal, S.; Pachat, D.; Babu, S.S.; EK, S.K.; Mamukoya, N.; Das, S. Genome-Based Therapeutics: Era of Precision Medicine in Genetic Epilepsies and Epileptic Encephalopathies. Ann. Indian. Acad. Neurol. 2023, 26, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Gjerulfsen, C.E.; Oudin, M.J.; Furia, F.; Gverdtsiteli, S.; Landmark, C.J.; Trivisano, M.; Balestrini, S.; Guerrini, R.; Aledo-Serrano, A.; Morcos, R.; et al. Cenobamate as add-on treatment for SCN8A developmental and epileptic encephalopathy. medRxiv 2024, 66, 1119–1128. [Google Scholar] [CrossRef]
- Varughese, R.T.; Shah, Y.D.; Karkare, S.; Kothare, S.V. Adjunctive use of cenobamate for pediatric refractory focal-onset epilepsy: A single-center retrospective study. Epilepsy Behav. 2022, 130, 108679. [Google Scholar] [CrossRef]
- Cagigal, R.; Romero-Del-Rincon, C.; Fernandez-Perrone, A.; Cruz, R.; Møller, R.S.; Aledo-Serrano, A. Lack of effectiveness and seizure worsening with cenobamate in pediatric patients with Dravet syndrome. Epilepsia 2025, 66, e83–e89. [Google Scholar] [CrossRef]
- Makridis, K.L.; Friedo, A.L.; Kellinghaus, C.; Losch, F.P.; Schmitz, B.; Boßelmann, C.; Kaindl, A.M. Successful treatment of adult Dravet syndrome patients with cenobamate. Epilepsia 2022, 63, e164–e171. [Google Scholar] [CrossRef]
- Sullivan, J.; Helen Cross, J. Raising the bar: Fenfluramine sets new treatment standards for Dravet syndrome. Epilepsy Behav. 2021, 121, 108061. [Google Scholar] [CrossRef]
- Knupp, K.G.; Scheffer, I.E.; Ceulemans, B.; Sullivan, J.E.; Nickels, K.C.; Lagae, L.; Guerrini, R.; Zuberi, S.M.; Nabbout, R.; Riney, K.; et al. Efficacy and Safety of Fenfluramine for the Treatment of Seizures Associated With Lennox-Gastaut Syndrome: A Randomized Clinical Trial. JAMA Neurol. 2022, 79, 554–564. [Google Scholar] [CrossRef]
- Perucca, E.; Taglialatela, M. Targeting Kv7 Potassium Channels for Epilepsy. CNS Drugs 2025, 39, 263–288. [Google Scholar] [CrossRef]
- Zeng, Q.; Xia, X.; Jiang, L.; Chen, J.; Liu, Y.; Hu, Y. Efficacy and safety of adjunctive perampanel treatment in pediatric patients with epilepsy aged 4-12 years: A real-world study. J. Neurol. 2024, 271, 4566–4576. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, B.; Yang, H.; Williams, B.; Zhou, S.; Laurenza, A. Perampanel efficacy and safety by gender: Subanalysis of phase III randomized clinical studies in subjects with partial seizures. Epilepsia 2015, 56, e90–e94. [Google Scholar] [CrossRef] [PubMed]
- Gidal, B.E.; Laurenza, A.; Hussein, Z.; Yang, H.; Fain, R.; Edelstein, J.; Kumar, D.; Ferry, J. Perampanel efficacy and tolerability with enzyme-inducing AEDs in patients with epilepsy. Neurology 2015, 84, 1972–1980. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, L.; Durante, V.; Battaglia, G.; Gasparini, S.; Zambrelli, E.; Ermio, C.; La Neve, A.; Mostacci, B.; Epilepsy, Gender Commission of the LICE (Italian chapter of the ILAE). Sex Differences in Adverse Effects of Antiseizure Medications in Adults with Epilepsy: A Systematic Review. CNS Drugs 2024, 38, 409–423. [Google Scholar] [CrossRef]
- Eunhae, Y.; Emily, S.; Natalie, R.; Nicholas, P.; Aaron, A.; Fadi, M.; Graham, R.H. Catamenial epilepsy. Pract. Neurol. 2022, 35–39, 44. Available online: https://practicalneurology.com/diseases-diagnoses/epilepsy-seizures/catamenial-epilepsy/31944/ (accessed on 31 May 2025).
- Girgis, M.M.F.; Farkasinszky, G.; Fekete, K.; Fekete, I.; Vecsernyés, M.; Bácskay, I.; Horváth, L. Sex differences in suspected adverse drug reactions of anti-seizure medications reported in Eudra Vigilance. Eur. J. Pharm. Sci. 2025, 210, 107119. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).