The Natural History of SARS-CoV-2-Incurred Disease: From Infection to Long COVID
Abstract
1. Background
2. The Natural History of SARS-CoV-2 and Human Interactions
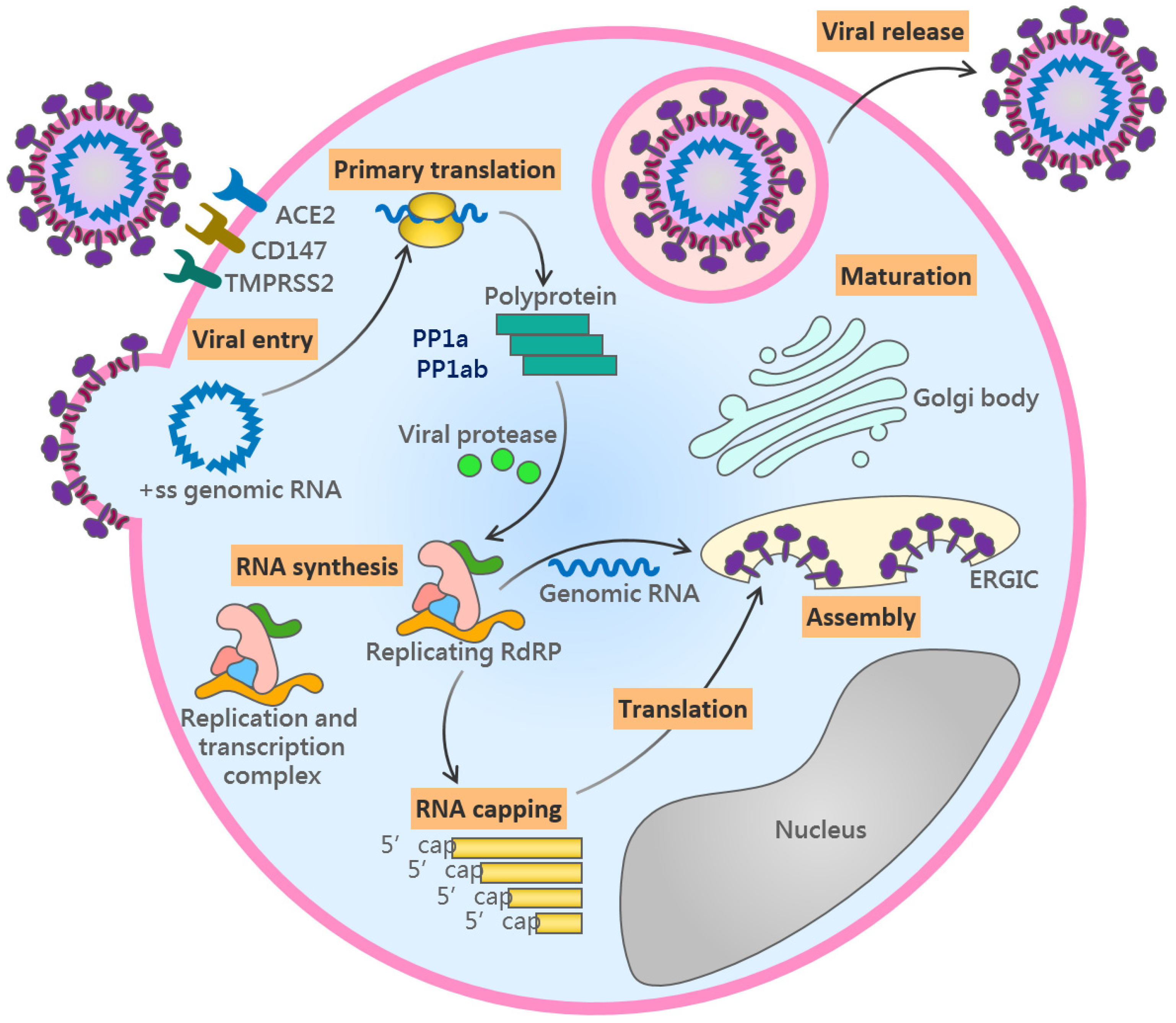
2.1. The Viral Life Cycle
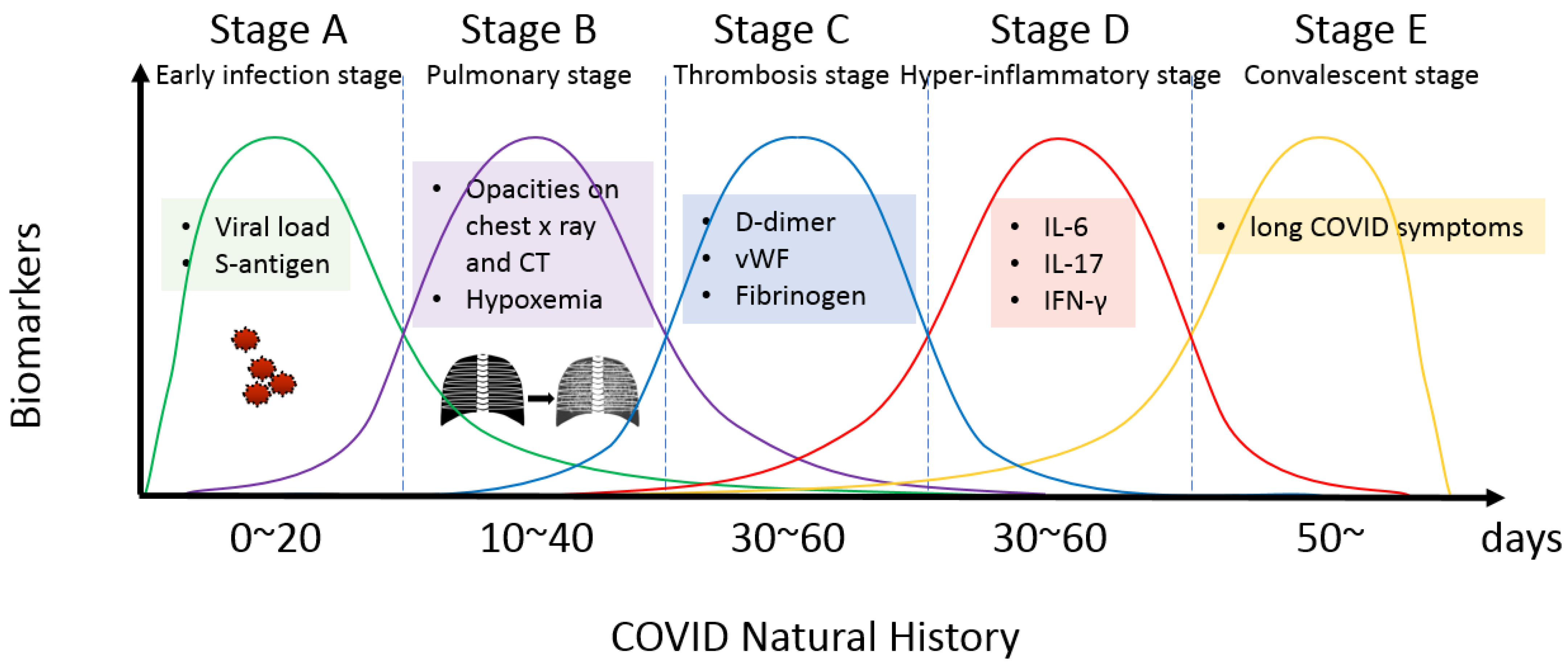
2.2. The Early Infection Stage
2.3. The Pulmonary Stage
2.4. Thrombosis Stage
2.5. The Hyper-Inflammatory Stage
2.6. The Convalescent Stage
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sohrabi, C.; Alsafi, Z.; O’Neill, N.; Khan, M.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, R. World health organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19). Int. J. Surg. 2020, 76, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Rao, Z. Structural biology of SARS-CoV-2 and implications for therapeutic development. Nat. Rev. Microbiol. 2021, 19, 685–700. [Google Scholar] [CrossRef] [PubMed]
- de Wit, E.; van Doremalen, N.; Falzarano, D.; Munster, V.J. Sars and mers: Recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 2016, 14, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Li, Y.; Zhang Annie, L.; Wang, Y.; Molina Mario, J. Identifying airborne transmission as the dominant route for the spread of COVID-19. Proc. Natl. Acad. Sci. USA 2020, 117, 14857–14863. [Google Scholar] [CrossRef] [PubMed]
- Malone, B.; Urakova, N.; Snijder, E.J.; Campbell, E.A. Structures and functions of coronavirus replication-transcription complexes and their relevance for SARS-CoV-2 drug design. Nat. Rev. Mol. Cell. Biol. 2022, 23, 21–39. [Google Scholar] [CrossRef] [PubMed]
- Giuntella, O.; Hyde, K.; Saccardo, S.; Sadoff, S. Lifestyle and mental health disruptions during COVID-19. Proc. Natl. Acad. Sci. USA 2021, 118, e2016632118. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Li, H.; Zhang, R. Effects of pandemic outbreak on economies: Evidence from business history context. Front. Public Health 2021, 9, 632043. [Google Scholar] [CrossRef]
- Duval, D.; Palmer, J.C.; Tudge, I.; Pearce-Smith, N.; O’Connell, E.; Bennett, A.; Clark, R. Long distance airborne transmission of SARS-CoV-2: Rapid systematic review. BMJ 2022, 377, e068743. [Google Scholar] [CrossRef]
- Cui, J.; Li, F.; Shi, Z.-L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2018, 17, 181–192. [Google Scholar] [CrossRef]
- Dhama, K.; Patel, S.K.; Sharun, K.; Pathak, M.; Tiwari, R.; Yatoo, M.I.; Malik, Y.S.; Sah, R.; Rabaan, A.A.; Panwar, P.K.; et al. SARS-CoV-2 jumping the species barrier: Zoonotic lessons from sars, mers and recent advances to combat this pandemic virus. Travel Med. Infect. Dis. 2020, 37, 101830. [Google Scholar] [CrossRef]
- Kadambari, S.; Goldacre, R.; Morris, E.; Goldacre, M.J.; Pollard, A.J. Indirect effects of the COVID-19 pandemic on childhood infection in England: Population based observational study. BMJ 2022, 376, e067519. [Google Scholar] [CrossRef] [PubMed]
- El Sahly, H.M.; Baden, L.R.; Essink, B.; Doblecki-Lewis, S.; Martin, J.M.; Anderson, E.J.; Campbell, T.B.; Clark, J.; Jackson, L.A.; Fichtenbaum, C.J.; et al. Efficacy of the mrna-1273 SARS-CoV-2 vaccine at completion of blinded phase. N. Engl. J. Med. 2021, 385, 1774–1785. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the bnt162b2 mrna COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.-H.; Hung, K.-F.; Wang, M.-L.; Chang, T.-J.; Cheng, Y.-F.; Chiang, S.-H.; Chen, M.-F.; Liao, Y.-T.; Chiou, S.-H.; Yang, D.-M. SARS-CoV-2 vaccines in children and adolescents: Can immunization prevent hospitalization? J. Chin. Med. Assoc. 2022, 85, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Shao, W.; Chen, X.; Zhang, B.; Wang, G.; Zhang, W. Real-world effectiveness of COVID-19 vaccines: A literature review and meta-analysis. Int. J. Infect. Dis. 2022, 114, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Mallapaty, S. Where did omicron come from? Three key theories. Nature 2022, 602, 26–28. [Google Scholar] [CrossRef] [PubMed]
- Kurhade, C.; Zou, J.; Xia, H.; Liu, M.; Chang, H.C.; Ren, P.; Xie, X.; Shi, P.Y. Low neutralization of SARS-CoV-2 omicron ba.2.75.2, bq.1.1, and xbb.1 by parental mrna vaccine or a ba.5-bivalent booster. Nat. Med. 2022, 29, 344–347. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, W.G. Natural history of COVID-19 and current knowledge on treatment therapeutic options. Biomed. Pharmacother. 2020, 129, 110493. [Google Scholar] [CrossRef]
- McCullough, P.A.; Alexander, P.E.; Armstrong, R.; Arvinte, C.; Bain, A.F.; Bartlett, R.P.; Berkowitz, R.L.; Berry, A.C.; Borody, T.J.; Brewer, J.H.; et al. Multifaceted highly targeted sequential multidrug treatment of early ambulatory high-risk SARS-CoV-2 infection (COVID-19). Rev. Cardiovasc. Med. 2020, 21, 517–530. [Google Scholar]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef]
- Soriano, J.B.; Murthy, S.; Marshall, J.C.; Relan, P.; Diaz, J.V. A clinical case definition of post-COVID-19 condition by a delphi consensus. Lancet Infect. Dis. 2022, 22, e102–e107. [Google Scholar] [CrossRef] [PubMed]
- Diamond, M.S.; Kanneganti, T.D. Innate immunity: The first line of defense against SARS-CoV-2. Nat. Immunol. 2022, 23, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Seth, S.; Batra, J.; Srinivasan, S. COVID-19: Targeting proteases in viral invasion and host immune response. Front. Mol. Biosci. 2020, 7, 215. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Cano, K.E.; Jia, L.; Drag, M.; Huang, T.T.; Olsen, S.K. Targeting SARS-CoV-2 proteases for COVID-19 antiviral development. Front. Chem. 2022, 9, 1221. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, Q.; Guo, D. Emerging coronaviruses: Genome structure, replication, and pathogenesis. J. Med. Virol. 2020, 92, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Hardenbrook, N.J.; Zhang, P. A structural view of the SARS-CoV-2 virus and its assembly. Curr. Opin. Virol. 2022, 52, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, S.G.; Sawicki, D.L.; Younker, D.; Meyer, Y.; Thiel, V.; Stokes, H.; Siddell, S.G. Functional and genetic analysis of coronavirus replicase-transcriptase proteins. PLoS Pathog. 2005, 1, e39. [Google Scholar] [CrossRef]
- Cubuk, J.; Alston, J.J.; Incicco, J.J.; Singh, S.; Stuchell-Brereton, M.D.; Ward, M.D.; Zimmerman, M.I.; Vithani, N.; Griffith, D.; Wagoner, J.A.; et al. The SARS-CoV-2 nucleocapsid protein is dynamic, disordered, and phase separates with rna. Nat. Commun. 2021, 12, 1936. [Google Scholar] [CrossRef]
- Huang, J.; Song, W.; Huang, H.; Sun, Q. Pharmacological therapeutics targeting rna-dependent rna polymerase, proteinase and spike protein: From mechanistic studies to clinical trials for COVID-19. J. Clin. Med. 2020, 9, 1131. [Google Scholar] [CrossRef]
- Bracquemond, D.; Muriaux, D. Betacoronavirus assembly: Clues and perspectives for elucidating SARS-CoV-2 particle formation and egress. mBio 2021, 12, e0237121. [Google Scholar] [CrossRef]
- Lauer, S.A.; Grantz, K.H.; Bi, Q.; Jones, F.K.; Zheng, Q.; Meredith, H.R.; Azman, A.S.; Reich, N.G.; Lessler, J. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: Estimation and application. Ann. Intern. Med. 2020, 172, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Looi, M.K. How are COVID-19 symptoms changing? BMJ 2023, 380, 3. [Google Scholar] [CrossRef] [PubMed]
- Kevadiya, B.D.; Machhi, J.; Herskovitz, J.; Oleynikov, M.D.; Blomberg, W.R.; Bajwa, N.; Soni, D.; Das, S.; Hasan, M.; Patel, M.; et al. Diagnostics for SARS-CoV-2 infections. Nat. Mater. 2021, 20, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Yuan, D.; Chen, D.G.; Ng, R.H.; Wang, K.; Choi, J.; Li, S.; Hong, S.; Zhang, R.; Xie, J.; et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell 2022, 185, 881–895.e820. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A.; Prattichizzo, F. Pharmacological management of COVID-19 in type 2 diabetes. J. Diabetes Complicat. 2021, 35, 107927. [Google Scholar] [CrossRef] [PubMed]
- Marconato, M.; Abela, I.A.; Hauser, A.; Schwarzmuller, M.; Katzensteiner, R.; Braun, D.L.; Epp, S.; Audige, A.; Weber, J.; Rusert, P.; et al. Antibodies from convalescent plasma promote SARS-CoV-2 clearance in individuals with and without endogenous antibody response. J. Clin. Investig. 2022, 132, e158190. [Google Scholar] [CrossRef] [PubMed]
- Stricker, R.B.; Fesler, M.C. Hydroxychloroquine pre-exposure prophylaxis for COVID-19 in healthcare workers from india: A meta-analysis. J. Infect. Public Health 2021, 14, 1161–1163. [Google Scholar] [CrossRef] [PubMed]
- Brouqui, P.; Million, M.; Parola, P.; McCullough, P.A.; Raoult, D. Outcomes after early treatment with hydroxychloroquine and azithromycin: An analysis of a database of 30,423 COVID-19 patients. New Microbes New Infect. 2023, 55, 101188. [Google Scholar] [CrossRef]
- Jolly, M.K.; Ward, C.; Eapen, M.S.; Myers, S.; Hallgren, O.; Levine, H.; Sohal, S.S. Epithelial-mesenchymal transition, a spectrum of states: Role in lung development, homeostasis, and disease. Dev. Dyn. 2018, 247, 346–358. [Google Scholar] [CrossRef]
- Cully, M. A tale of two antiviral targets—And the COVID-19 drugs that bind them. Nat. Rev. Drug Discov. 2021, 21, 3–5. [Google Scholar] [CrossRef]
- Sanders, J.M.; Monogue, M.L.; Jodlowski, T.Z.; Cutrell, J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19). JAMA 2020, 323, 1824–1836. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-ncov) in vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Samaddar, A.; Grover, M.; Nag, V.L. Pathophysiology and potential therapeutic candidates for COVID-19: A poorly understood arena. Front. Pharmacol. 2020, 11, 585888. [Google Scholar] [CrossRef] [PubMed]
- Hammond, J.; Leister-Tebbe, H.; Gardner, A.; Abreu, P.; Bao, W.; Wisemandle, W.; Baniecki, M.; Hendrick, V.M.; Damle, B.; Simón-Campos, A.; et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19. N. Engl. J. Med. 2022, 386, 1397–1408. [Google Scholar] [CrossRef] [PubMed]
- Najjar-Debbiny, R.; Gronich, N.; Weber, G.; Khoury, J.; Amar, M.; Stein, N.; Goldstein, L.H.; Saliba, W. Effectiveness of paxlovid in reducing severe COVID-19 and mortality in high risk patients. Clin. Infect. Dis. 2022, 76, e342–e349. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.M.; Joyce, B.; Plumb, I.D.; Sahakian, S.; Feldstein, L.R.; Barkley, E.; Paccione, M.; Deckert, J.; Sandmann, D.; Gerhart, J.L.; et al. Paxlovid associated with decreased hospitalization rate among adults with COVID-19—United States, April–September 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 1531–1537. [Google Scholar] [CrossRef] [PubMed]
- Jayk Bernal, A.; Gomes da Silva, M.M.; Musungaie, D.B.; Kovalchuk, E.; Gonzalez, A.; Delos Reyes, V.; Martin-Quiros, A.; Caraco, Y.; Williams-Diaz, A.; Brown, M.L.; et al. Molnupiravir for oral treatment of COVID-19 in nonhospitalized patients. N. Engl. J. Med. 2022, 386, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Radcliffe, C.; Palacios, C.F.; Azar, M.M.; Cohen, E.; Malinis, M. Real-world experience with available, outpatient COVID-19 therapies in solid organ transplant recipients during the omicron surge. Am. J. Transplant. 2022, 22, 2458–2463. [Google Scholar] [CrossRef]
- Luciani, F.; Cione, E.; Caroleo, M.C.; Colosimo, M.; Zanolini, A.; Barca, A.; Cosimo, S.; Pasqua, P.; Gallelli, L. SARS-CoV-2 translocate from nasopharyngeal to bronchoalveolar site: A case presentation. Reports 2020, 3, 23. [Google Scholar] [CrossRef]
- McGonagle, D.; Bridgewood, C.; Meaney, J.F.M. A tricompartmental model of lung oxygenation disruption to explain pulmonary and systemic pathology in severe COVID-19. Lancet Respir. Med. 2021, 9, 665–672. [Google Scholar] [CrossRef]
- Artifoni, M.; Danic, G.; Gautier, G.; Gicquel, P.; Boutoille, D.; Raffi, F.; Neel, A.; Lecomte, R. Systematic assessment of venous thromboembolism in COVID-19 patients receiving thromboprophylaxis: Incidence and role of d-dimer as predictive factors. J. Thromb. Thrombolysis 2020, 50, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Gavriilaki, E.; Anyfanti, P.; Gavriilaki, M.; Lazaridis, A.; Douma, S.; Gkaliagkousi, E. Endothelial dysfunction in COVID-19: Lessons learned from coronaviruses. Curr. Hypertens. Rep. 2020, 22, 63. [Google Scholar] [CrossRef]
- Vrints, C.J.M.; Krychtiuk, K.A.; Van Craenenbroeck, E.M.; Segers, V.F.; Price, S.; Heidbuchel, H. Endothelialitis plays a central role in the pathophysiology of severe COVID-19 and its cardiovascular complications. Acta Cardiol. 2021, 76, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, H.K.; Libby, P.; Ridker, P.M. COVID-19—A vascular disease. Trends Cardiovasc. Med. 2021, 31, 1–5. [Google Scholar] [CrossRef]
- Gando, S.; Wada, T. Thromboplasminflammation in COVID-19 coagulopathy: Three viewpoints for diagnostic and therapeutic strategies. Front. Immunol. 2021, 12, 649122. [Google Scholar] [CrossRef]
- Smadja, D.M.; Guerin, C.L.; Chocron, R.; Yatim, N.; Boussier, J.; Gendron, N.; Khider, L.; Hadjadj, J.; Goudot, G.; Debuc, B.; et al. Angiopoietin-2 as a marker of endothelial activation is a good predictor factor for intensive care unit admission of COVID-19 patients. Angiogenesis 2020, 23, 611–620. [Google Scholar] [CrossRef]
- Won, T.; Wood, M.K.; Hughes, D.M.; Talor, M.V.; Ma, Z.; Schneider, J.; Skinner, J.T.; Asady, B.; Goerlich, E.; Halushka, M.K.; et al. Endothelial thrombomodulin downregulation caused by hypoxia contributes to severe infiltration and coagulopathy in COVID-19 patient lungs. EBioMedicine 2022, 75, 103812. [Google Scholar] [CrossRef]
- Garnier, Y.; Claude, L.; Hermand, P.; Sachou, E.; Claes, A.; Desplan, K.; Chahim, B.; Roger, P.M.; Martino, F.; Colin, Y.; et al. Plasma microparticles of intubated COVID-19 patients cause endothelial cell death, neutrophil adhesion and netosis, in a phosphatidylserine-dependent manner. Br. J. Haematol. 2022, 196, 1159–1169. [Google Scholar] [CrossRef]
- Giordo, R.; Paliogiannis, P.; Mangoni, A.A.; Pintus, G. SARS-CoV-2 and endothelial cell interaction in COVID-19: Molecular perspectives. Vasc. Biol. 2021, 3, R15–R23. [Google Scholar] [CrossRef]
- Chow, J.H.; Khanna, A.K.; Kethireddy, S.; Yamane, D.; Levine, A.; Jackson, A.M.; McCurdy, M.T.; Tabatabai, A.; Kumar, G.; Park, P.; et al. Aspirin use is associated with decreased mechanical ventilation, intensive care unit admission, and in-hospital mortality in hospitalized patients with coronavirus disease 2019. Anesth. Analg. 2021, 132, 930–941. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Bai, H.; Chen, X.; Gong, J.; Li, D.; Sun, Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020, 18, 1094–1099. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, M.; Kuno, T.; Mikami, T.; Takagi, H.; Gronseth, G. Clinical characteristics of stroke with COVID-19: A systematic review and meta-analysis. J. Stroke Cerebrovasc. Dis. 2020, 29, 105288. [Google Scholar] [CrossRef] [PubMed]
- Machhi, J.; Herskovitz, J.; Senan, A.M.; Dutta, D.; Nath, B.; Oleynikov, M.D.; Blomberg, W.R.; Meigs, D.D.; Hasan, M.; Patel, M.; et al. The natural history, pathobiology, and clinical manifestations of SARS-CoV-2 infections. J. Neuroimmune Pharmacol. 2020, 15, 359–386. [Google Scholar] [CrossRef] [PubMed]
- Elezkurtaj, S.; Greuel, S.; Ihlow, J.; Michaelis, E.G.; Bischoff, P.; Kunze, C.A.; Sinn, B.V.; Gerhold, M.; Hauptmann, K.; Ingold-Heppner, B.; et al. Causes of death and comorbidities in hospitalized patients with COVID-19. Sci. Rep. 2021, 11, 4263. [Google Scholar] [CrossRef] [PubMed]
- Rabaan, A.A.; Al-Ahmed, S.H.; Muhammad, J.; Khan, A.; Sule, A.A.; Tirupathi, R.; Mutair, A.A.; Alhumaid, S.; Al-Omari, A.; Dhawan, M.; et al. Role of inflammatory cytokines in COVID-19 patients: A review on molecular mechanisms, immune functions, immunopathology and immunomodulatory drugs to counter cytokine storm. Vaccines 2021, 9, 436. [Google Scholar] [CrossRef] [PubMed]
- Ambrosino, P.; Calcaterra, I.L.; Mosella, M.; Formisano, R.; D’Anna, S.E.; Bachetti, T.; Marcuccio, G.; Galloway, B.; Mancini, F.P.; Papa, A.; et al. Endothelial dysfunction in COVID-19: A unifying mechanism and a potential therapeutic target. Biomedicines 2022, 10, 812. [Google Scholar] [CrossRef]
- Singh, V.; Obregon-Perko, V.; Lapp, S.A.; Horner, A.M.; Brooks, A.; Macoy, L.; Hussaini, L.; Lu, A.; Gibson, T.; Silvestri, G.; et al. Limited induction of SARS-CoV-2-specific t cell responses in children with multisystem inflammatory syndrome compared with COVID-19. JCI Insight 2022, 7, e155145. [Google Scholar] [CrossRef]
- Yu, M.; Charles, A.; Cagigi, A.; Christ, W.; Osterberg, B.; Falck-Jones, S.; Azizmohammadi, L.; Ahlberg, E.; Falck-Jones, R.; Svensson, J.; et al. Delayed generation of functional virus-specific circulating t follicular helper cells correlates with severe COVID-19. Nat. Commun. 2023, 14, 2164. [Google Scholar] [CrossRef]
- Group, R.C.; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in hospitalized patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar]
- Kocks, J.; Kerkhof, M.; Scherpenisse, J.; van de Maat, A.; van Geer-Postmus, I.; le Rütte, T.; Schaart, J.; Gans, R.O.B.; Kerstjens, H.A.M. A potential harmful effect of dexamethasone in non-severe COVID-19: Results from the copper-pilot study. ERJ Open Res. 2022, 8, 00129–02022. [Google Scholar] [CrossRef] [PubMed]
- Group, R.C. Tocilizumab in patients admitted to hospital with COVID-19 (recovery): A randomised, controlled, open-label, platform trial. Lancet 2021, 397, 1637–1645. [Google Scholar]
- Rosas, I.O.; Brau, N.; Waters, M.; Go, R.C.; Hunter, B.D.; Bhagani, S.; Skiest, D.; Aziz, M.S.; Cooper, N.; Douglas, I.S.; et al. Tocilizumab in hospitalized patients with severe COVID-19 pneumonia. N. Engl. J. Med. 2021, 384, 1503–1516. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re’em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 2021, 38, 101019. [Google Scholar] [CrossRef]
- Hastie, C.E.; Lowe, D.J.; McAuley, A.; Mills, N.L.; Winter, A.J.; Black, C.; Scott, J.T.; O’Donnell, C.A.; Blane, D.N.; Browne, S.; et al. Natural history of long-COVID in a nationwide, population cohort study. Nat. Commun. 2023, 14, 3504. [Google Scholar] [CrossRef]
- Ebinger, J.E.; Achamallah, N.; Ji, H.; Claggett, B.L.; Sun, N.; Botting, P.; Nguyen, T.T.; Luong, E.; Kim, E.H.; Park, E.; et al. Pre-existing traits associated with COVID-19 illness severity. PLoS ONE 2020, 15, e0236240. [Google Scholar] [CrossRef]
- Fernandez-de-Las-Penas, C.; Torres-Macho, J.; Velasco-Arribas, M.; Plaza-Canteli, S.; Arias-Navalon, J.A.; Hernandez-Barrera, V.; Guijarro, C. Preexisting hypertension is associated with a greater number of long-term post-COVID symptoms and poor sleep quality: A case-control study. J. Hum. Hypertens. 2022, 36, 582–584. [Google Scholar] [CrossRef]
- Yang, D.M.; Chang, T.J.; Hung, K.F.; Wang, M.L.; Cheng, Y.F.; Chiang, S.H.; Chen, M.F.; Liao, Y.T.; Lai, W.Q.; Liang, K.H. Smart healthcare: A prospective future medical approach for COVID-19. J. Chin. Med. Assoc JCMA 2023, 86, 138–146. [Google Scholar] [CrossRef]
- Caze, A.B.; Cerqueira-Silva, T.; Bomfim, A.P.; de Souza, G.L.; Azevedo, A.C.; Brasil, M.Q.; Santos, N.R.; Khouri, R.; Dan, J.; Bandeira, A.C.; et al. Prevalence and risk factors for long COVID after mild disease: A cohort study with a symptomatic control group. J. Glob. Health 2023, 13, 06015. [Google Scholar] [CrossRef]
- Subramanian, A.; Nirantharakumar, K.; Hughes, S.; Myles, P.; Williams, T.; Gokhale, K.M.; Taverner, T.; Chandan, J.S.; Brown, K.; Simms-Williams, N.; et al. Symptoms and risk factors for long COVID in non-hospitalized adults. Nat. Med. 2022, 28, 1706–1714. [Google Scholar] [CrossRef]
- Dagher, H.; Chaftari, A.-M.; Subbiah, I.M.; Malek, A.E.; Jiang, Y.; Lamie, P.; Granwehr, B.; John, T.; Yepez, E.; Borjan, J.; et al. Long COVID in cancer patients: Preponderance of symptoms in majority of patients over long time period. eLife 2023, 12, e81182. [Google Scholar] [CrossRef]
- Vasbinder, A.; Meloche, C.; Azam, T.U.; Anderson, E.; Catalan, T.; Shadid, H.; Berlin, H.; Pan, M.; O’Hayer, P.; Padalia, K.; et al. Relationship between preexisting cardiovascular disease and death and cardiovascular outcomes in critically ill patients with COVID-19. Circ. Cardiovasc. Qual. Outcomes 2022, 15, e008942. [Google Scholar] [CrossRef] [PubMed]
- Kerolos, M.M.; Ruge, M.; Gill, A.; Planek, M.I.; Volgman, A.S.; Du-Fay-De-Lavallaz, J.M.; Gomez, J.M.D.; Suboc, T.M.; Williams, K.A.; Abusin, S. Clinical outcomes of COVID-19 infection in patients with pre-existing cardiovascular disease. Am. Heart J. Plus 2022, 20, 100189. [Google Scholar] [CrossRef] [PubMed]
- Tana, C.; Bentivegna, E.; Cho, S.J.; Harriott, A.M.; Garcia-Azorin, D.; Labastida-Ramirez, A.; Ornello, R.; Raffaelli, B.; Beltran, E.R.; Ruscheweyh, R.; et al. Long COVID headache. J. Headache Pain 2022, 23, 93. [Google Scholar] [CrossRef] [PubMed]
- Waliszewska-Prosol, M.; Budrewicz, S. The unusual course of a migraine attack during COVID-19 infection—Case studies of three patients. J. Infect. Public Health 2021, 14, 903–905. [Google Scholar] [CrossRef] [PubMed]
- Straburzynski, M.; Kuca-Warnawin, E.; Waliszewska-Prosol, M. COVID-19-related headache and innate immune response—A narrative review. Neurol. Neurochir. Pol. 2023, 57, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Ayoubkhani, D.; Bermingham, C.; Pouwels, K.B.; Glickman, M.; Nafilyan, V.; Zaccardi, F.; Khunti, K.; Alwan, N.A.; Walker, A.S. Trajectory of long COVID symptoms after COVID-19 vaccination: Community based cohort study. BMJ 2022, 377, e069676. [Google Scholar] [CrossRef]
- Hulscher, N.; Procter, B.C.; Wynn, C.; McCullough, P.A. Clinical approach to post-acute sequelae after COVID-19 infection and vaccination. Cureus 2023, 15, e49204. [Google Scholar] [CrossRef]
- McCullough, P.A.; Procter, B.C.; Wynn, C. Clinical rationale for SARS-CoV-2 base spike protein detoxification in post COVID-19 and vaccine injury syndromes. J. Am. Physicians Surg. 2023, 28, 90–93. [Google Scholar]
- Kared, H.; Redd, A.D.; Bloch, E.M.; Bonny, T.S.; Sumatoh, H.; Kairi, F.; Carbajo, D.; Abel, B.; Newell, E.W.; Bettinotti, M.P.; et al. SARS-CoV-2-specific cd8+ t cell responses in convalescent COVID-19 individuals. J. Clin. Investig. 2021, 131, e145476. [Google Scholar] [CrossRef]
- Bull-Otterson, L.; Baca, S.; Saydah, S.; Boehmer, T.K.; Adjei, S.; Gray, S.; Harris, A.M. Post–COVID conditions among adult COVID-19 survivors aged 18–64 and ≥65 years—United States, March 2020–November 2021. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 713–717. [Google Scholar] [CrossRef]
- Thompson, R.C.; Simons, N.W.; Wilkins, L.; Cheng, E.; Del Valle, D.M.; Hoffman, G.E.; Cervia, C.; Fennessy, B.; Mouskas, K.; Francoeur, N.J.; et al. Molecular states during acute COVID-19 reveal distinct etiologies of long-term sequelae. Nat. Med. 2022, 29, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Loos, N.H.C.; Beijnen, J.H.; Schinkel, A.H. The mechanism-based inactivation of cyp3a4 by ritonavir: What mechanism? Int. J. Mol. Sci. 2022, 23, 9866. [Google Scholar] [CrossRef] [PubMed]
- Peter, L.; Wendering, D.J.; Schlickeiser, S.; Hoffmann, H.; Noster, R.; Wagner, D.L.; Zarrinrad, G.; Munch, S.; Picht, S.; Schulenberg, S.; et al. Tacrolimus-resistant SARS-CoV-2-specific t cell products to prevent and treat severe COVID-19 in immunosuppressed patients. Mol. Ther. Methods Clin. Dev. 2022, 25, 52–73. [Google Scholar] [CrossRef]
| Stage | Clinical Features | Biomarkers | Virus–Host Interactions/Immunology | Treatments |
|---|---|---|---|---|
| Early Infection Stage | Fever, cough, shortness of breath, muscle pain, fatigue, diarrhea, loss of taste or smell | Viral load by RT-PCR or viral antigens by antigenic assays from nasal swab or sputum samples | Viral entry through ACE2 receptors on host cells leading to replication and symptom development | Antiviral drugs and neutralizing antibodies to reduce disease progression |
| Pulmonary Stage | Breathing difficulties, lung inflammation | Chest X-ray or computed tomography showing lung inflammation | Immune response produces antibodies and activates immune cells (T cells, NK cells) to target and destroy the virus | Antiviral drugs targeting viral replication and assembly/release |
| Thrombosis Stage | Shortness of breath, chest pain, respiratory distress, oxygen desaturation | Elevated D-dimer and von Willebrand factor (vWF) levels | SARS-CoV-2 infection triggering endothelial injury and inflammation, leading to a prothrombotic state | Aspirin, low-molecular-weight heparin, novel oral anticoagulants |
| Hyper-Inflammatory Stage | Cytokine storm, widespread inflammation, severe illness, ARDS, sepsis, organ failure | Elevated levels of proinflammatory cytokines (e.g., IL-6, IL-17, IFN-γ) | Overproduction of proinflammatory cytokines and immune mediators causing organ damage and complications | Corticosteroids (e.g., dexamethasone) to reduce inflammation and improve oxygenation Supportive care (oxygen therapy, mechanical ventilation, ECMO) |
| Convalescent Stage | Gradual recovery after acute infection subsides Long COVID conditions with persisting symptoms | Supportive care to alleviate symptoms (pulmonary rehabilitation, cognitive-behavioral therapy) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, K.-H.; Teng, Y.-C.; Liao, Y.-T.; Yarmishyn, A.A.; Chiang, S.-H.; Hung, W.-C.; Hsiao, C.-Y.; Tsai, E.-T.; Chang, T.-J.; Yang, D.-M.; et al. The Natural History of SARS-CoV-2-Incurred Disease: From Infection to Long COVID. Int. J. Transl. Med. 2024, 4, 72-86. https://doi.org/10.3390/ijtm4010004
Liang K-H, Teng Y-C, Liao Y-T, Yarmishyn AA, Chiang S-H, Hung W-C, Hsiao C-Y, Tsai E-T, Chang T-J, Yang D-M, et al. The Natural History of SARS-CoV-2-Incurred Disease: From Infection to Long COVID. International Journal of Translational Medicine. 2024; 4(1):72-86. https://doi.org/10.3390/ijtm4010004
Chicago/Turabian StyleLiang, Kung-Hao, Yuan-Chi Teng, Yi-Ting Liao, Aliaksandr A. Yarmishyn, Su-Hua Chiang, Wei-Chun Hung, Chun-Yen Hsiao, En-Tung Tsai, Tai-Jay Chang, De-Ming Yang, and et al. 2024. "The Natural History of SARS-CoV-2-Incurred Disease: From Infection to Long COVID" International Journal of Translational Medicine 4, no. 1: 72-86. https://doi.org/10.3390/ijtm4010004
APA StyleLiang, K.-H., Teng, Y.-C., Liao, Y.-T., Yarmishyn, A. A., Chiang, S.-H., Hung, W.-C., Hsiao, C.-Y., Tsai, E.-T., Chang, T.-J., Yang, D.-M., & Wang, M.-L. (2024). The Natural History of SARS-CoV-2-Incurred Disease: From Infection to Long COVID. International Journal of Translational Medicine, 4(1), 72-86. https://doi.org/10.3390/ijtm4010004






