Bioluminescent ATP-Metry in Assessing the Impact of Various Microplastic Particles on Fungal, Bacterial, and Microalgal Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Microorganisms
2.3. Analytical Methods
2.4. Data Analysis
3. Results and Discussion
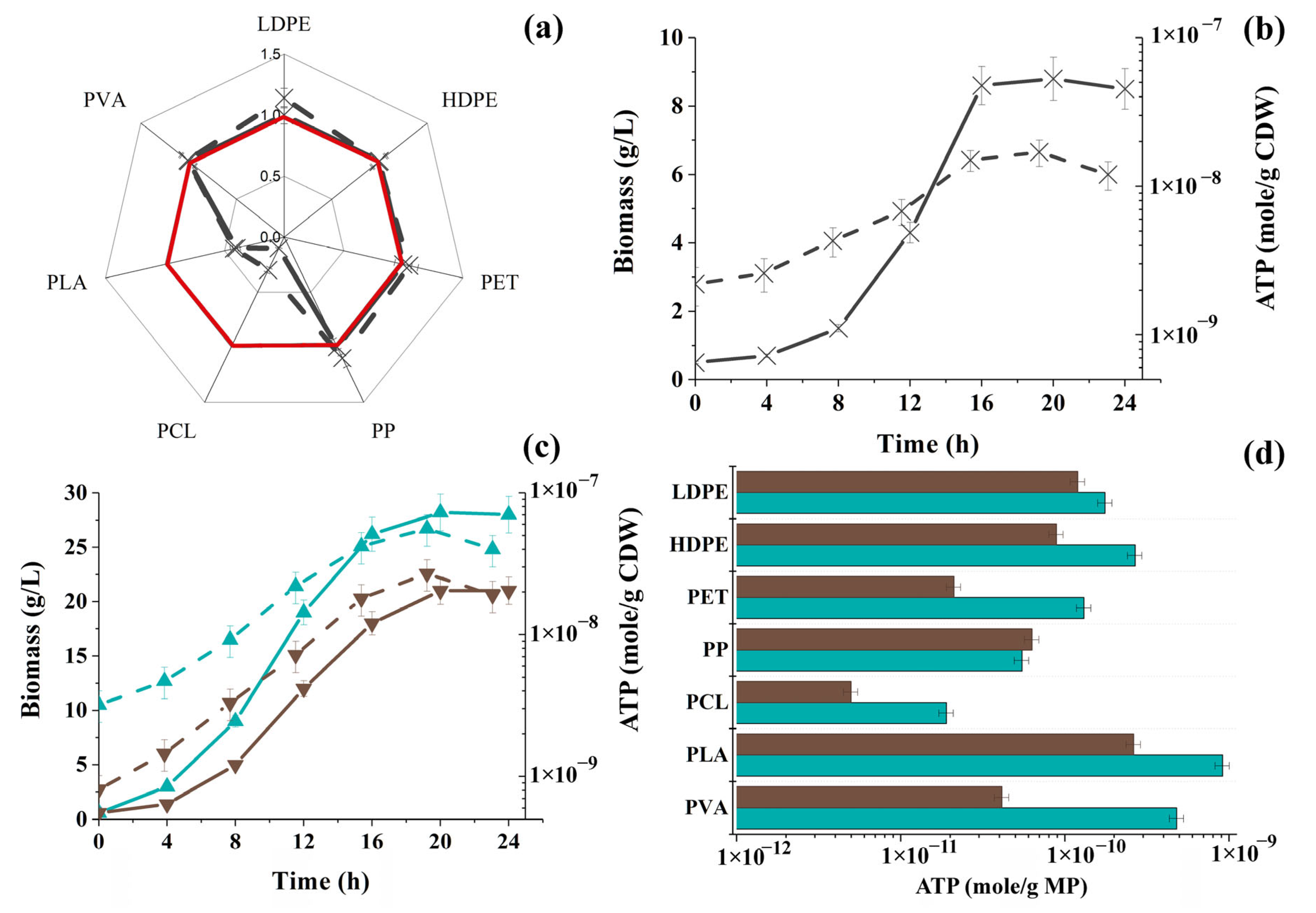
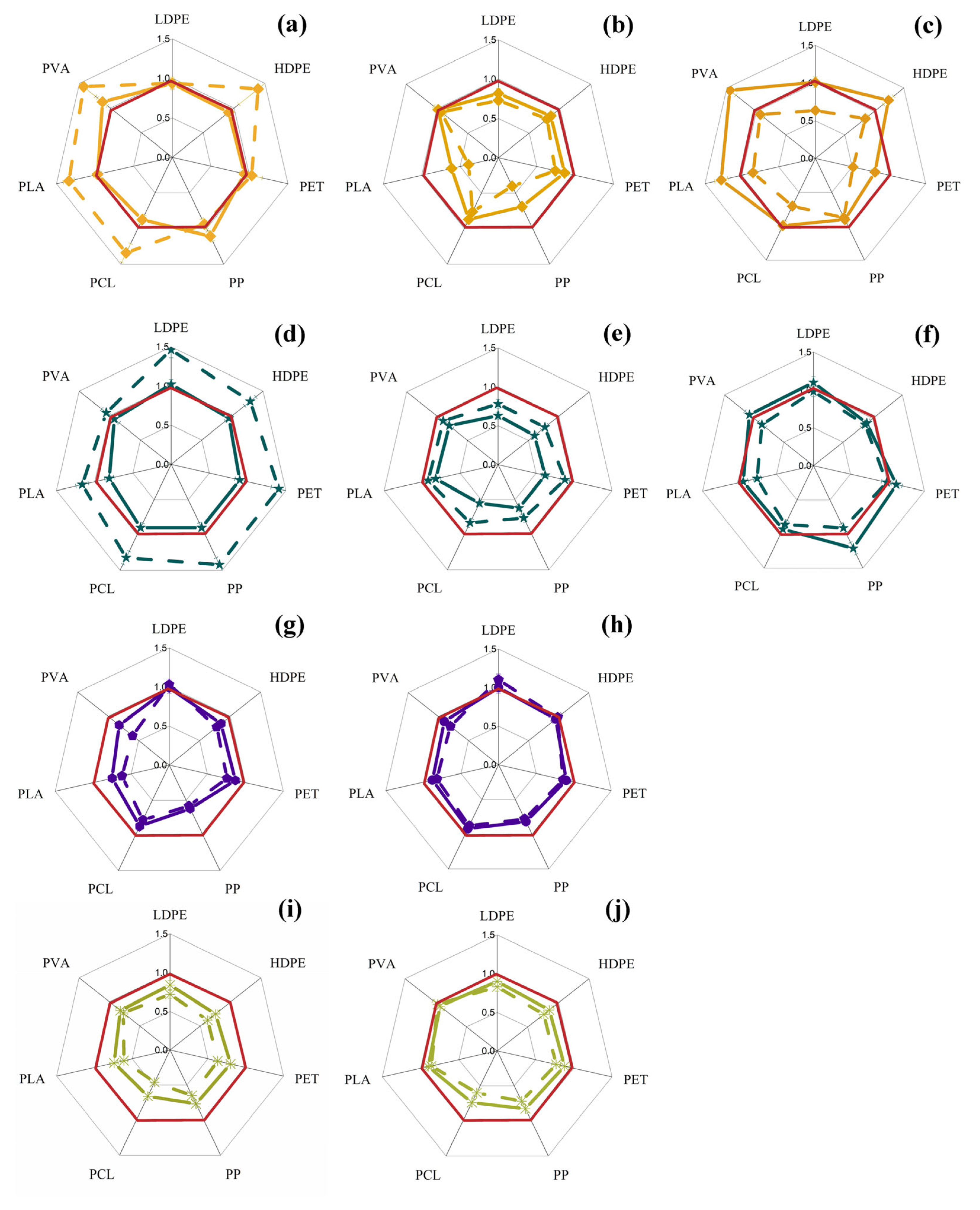
3.1. Changes in Intracellular ATP Concentration in Yeast and Filamentous Fungi in the Presence of MP Particles
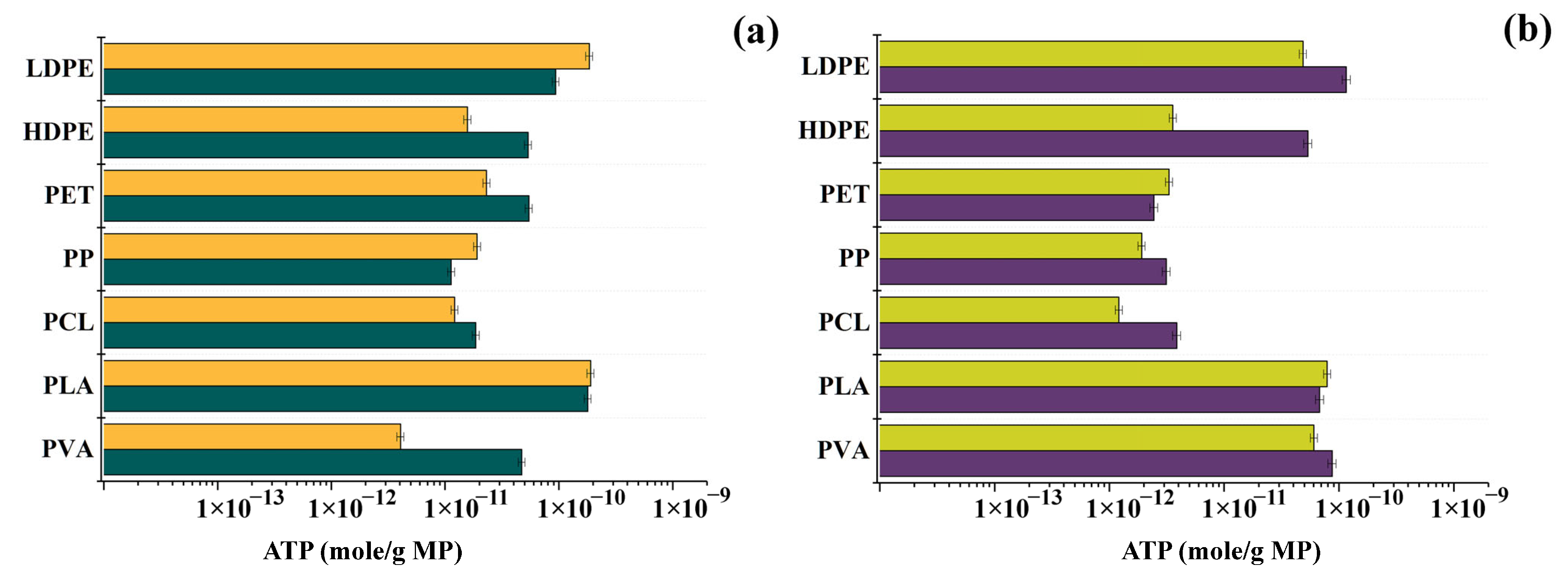
3.2. Changes in the Concentration of Intracellular ATP in Bacterial Cells in the Presence of MP Particles
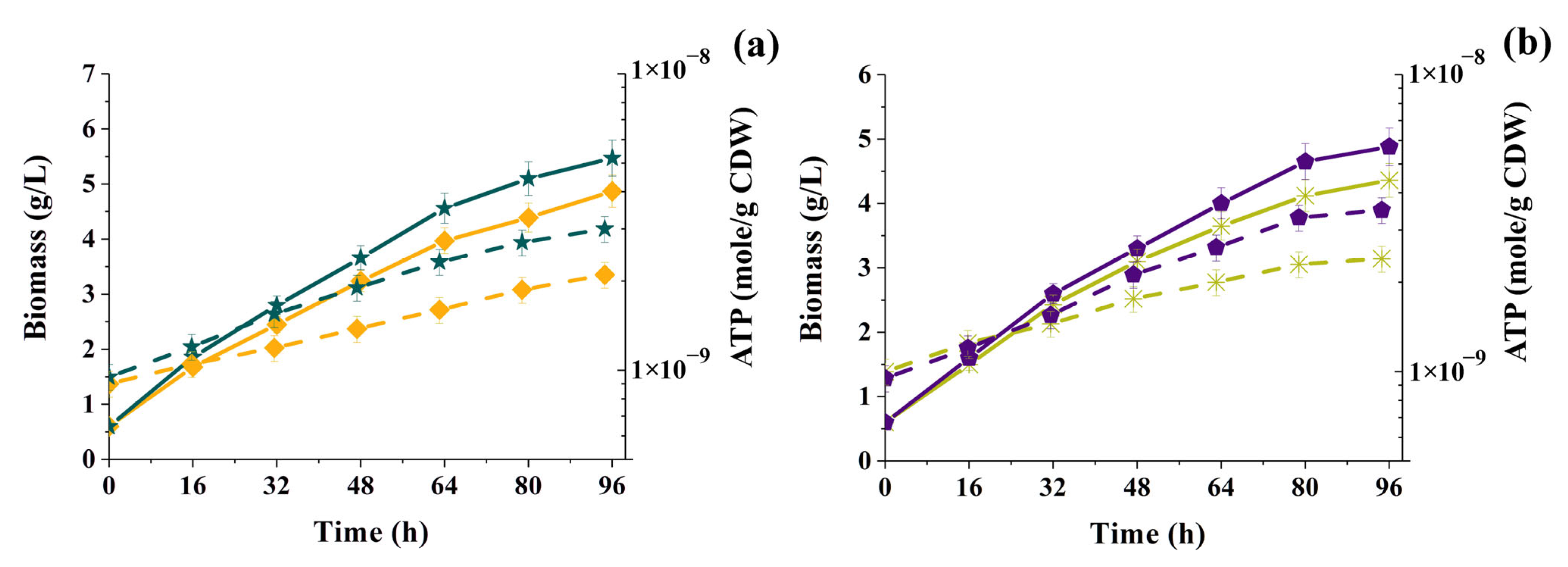
3.3. Changes in the Concentration of Intracellular ATP in the Cells of Phototrophic Microorganisms in the Presence of MP Particles
3.4. General Analysis of Obtained Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATP | Intracellular adenosine triphosphate |
| MPs | Microplastics |
| EPS | Exopolysaccharides |
| QS | Quorum Sensing |
| DCW | Dry cell weight |
| LDPE | Low-density polyethylene |
| HDPE | High-density polyethylene |
| PET | Polyethylene terephthalate |
| PP | Polypropylene |
| PVA | Polyvinyl alcohol |
| PCL | Polycaprolactone |
| PLA | Polylactic acid |
| DMSO | Dimethyl sulfoxide |
| PS | Polystyrene |
| PVC | Polyvinyl chloride |
| PE | Polyethylene |
References
- Martínez-Burgos, W.J.; Pozzan, R.; de Carvalho, J.C.; Cavali, M.; Mariano, A.B.; Vargas, J.V.; Ordonez, J.; Severo, I.A.; Soccol, C.R. The role of microalgae as bioindicators of aquatic contaminations. In Algae as a Natural Solution for Challenges in Water-Food-Energy Nexus; Kurniawan, T.A., Anouzla, A., Eds.; Springer Nature: Singapore, 2024; pp. 323–347. [Google Scholar] [CrossRef]
- Yadav, S.; Singh, A.K.; Verma, E. Cyanobacteria as bioindicator of water pollution. In Cyanobacterial Biotechnology in the 21st Century; Neilan, B., Passarini, M.R.Z., Singh, P.K., Kumar, A., Eds.; Springer Nature: Singapore, 2023; pp. 149–179. [Google Scholar] [CrossRef]
- Miglani, R.; Parveen, N.; Kumar, A.; Ansari, M.A.; Khanna, S.; Rawat, G.; Panda, A.K.; Bisht, S.S.; Upadhyay, J.; Ansari, M.N. Degradation of xenobiotic pollutants: An environmentally sustainable approach. Metabolites 2022, 12, 818. [Google Scholar] [CrossRef]
- Nicula, N.-O.; Lungulescu, E.-M.; Rîmbu, G.A.; Marinescu, V.; Corbu, V.M.; Csutak, O. Bioremediation of wastewater using yeast strains: An assessment of contaminant removal efficiency. Int. J. Environ. Res. Public Health 2023, 20, 4795. [Google Scholar] [CrossRef] [PubMed]
- Slaveykova, V.I.; Marelja, M. Progress in research on the bioavailability and toxicity of nanoplastics to freshwater plankton. Microplastics 2023, 2, 389–410. [Google Scholar] [CrossRef]
- Santonicola, S.; Volgare, M.; Cocca, M.; Colavita, G. Abundance and characteristics of fibrous microplastics and microfibers isolated in Mullus barbatus from the Adriatic Sea—Preliminary investigation. Microplastics 2023, 2, 411–421. [Google Scholar] [CrossRef]
- Hrovat, B.; Uurasjärvi, E.; Koistinen, A. Analysis of microplastics in industrial processes—Systematic analysis of digestion efficiency of samples from forestry, wastewater treatment plants and biogas industries. Microplastics 2024, 3, 634–652. [Google Scholar] [CrossRef]
- Efremenko, E.N.; Lyagin, I.V.; Maslova, O.V.; Senko, O.V.; Stepanov, N.A.; Aslanli, A.G. Catalytic degradation of microplastics. Russ. Chem. Rev. 2023, 92, 5069. [Google Scholar] [CrossRef]
- Ayush, P.T.; Ko, J.-H.; Oh, H.-S. Characteristics of initial attachment and biofilm formation of Pseudomonas aeruginosa on microplastic surfaces. Appl. Sci. 2022, 12, 5245. [Google Scholar] [CrossRef]
- Efremenko, E.; Lyagin, I.; Stepanov, N.; Senko, O.; Maslova, O.; Aslanli, A.; Ugarova, N. Luminescent bacteria as bioindicators in screening and selection of enzymes detoxifying various mycotoxins. Sensors 2024, 24, 763. [Google Scholar] [CrossRef]
- De Jesus, R.; Iqbal, S.; Mundra, S.; AlKendi, R. Heterogenous bioluminescence patterns, cell viability, and biofilm formation of Photobacterium leiognathi strains exposed to ground microplastics. Front. Toxicol. 2024, 6, 1479549. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, X.; Wang, J.; Tan, L. Toxic effects of microplastic on marine microalgae Skeletonema costatum: Interactions between microplastic and algae. Environ. Pollut. 2017, 220, 1282–1288. [Google Scholar] [CrossRef]
- Pencik, O.; Molnarova, K.; Durdakova, M.; Kolackova, M.; Klofac, D.; Kucsera, A.; Capal, P.; Svec, C.; Bytesnikova, Z.; Richtera, L.; et al. Not so dangerous? PET microplastics toxicity on freshwater microalgae and cyanobacteria. Environ. Pollut. 2023, 329, 121628. [Google Scholar] [CrossRef]
- Gopalakrishnan, K.K.; Kashian, D.R. Complex interactions among temperature, microplastics and cyanobacteria may facilitate cyanobacteria proliferation and microplastic deposition. Ecotoxicol. Environ. Saf. 2023, 263, 115259. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Lizon, F.; Gevaert, F.; Bialais, C.; Duong, G.; Ouddane, B.; Souissi, S. Assessing indicators of arsenic toxicity using variable fluorescence in a commercially valuable microalgae: Physiological and toxicological aspects. J. Hazard. Mater. 2023, 452, 131215. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Yin, G.; Gan, T.; Zhao, N.; Qi, P.; Chen, M.; Ding, Z.; Jia, R.; Liu, J.; Ma, M.; et al. Construction of characterization parameters of algal photosynthetic inhibition method for detection of comprehensive toxicity in water. Ecol. Indic. 2022, 136, 108651. [Google Scholar] [CrossRef]
- Efremenko, E.; Senko, O.; Stepanov, N.; Maslova, O.; Lomakina, G.Y.; Ugarova, N. Luminescent Analysis of ATP: Modern Objects and Processes for Sensing. Chemosensors 2022, 10, 493. [Google Scholar] [CrossRef]
- Bule Možar, K.; Miloloža, M.; Martinjak, V.; Cvetnić, M.; Ocelić Bulatović, V.; Mandić, V.; Bafti, A.; Ukić, Š.; Kučić Grgić, D.; Bolanča, T. Bacteria and yeasts isolated from the environment in biodegradation of PS and PVC microplastics: Screening and treatment optimization. Environments 2023, 10, 207. [Google Scholar] [CrossRef]
- Miloloža, M.; Bule, K.; Prevarić, V.; Cvetnić, M.; Ukić, Š.; Bolanča, T.; Kučić Grgić, D. Assessment of the influence of size and concentration on the ecotoxicity of microplastics to microalgae Scenedesmus sp., bacterium Pseudomonas putida and yeast Saccharomyces cerevisiae. Polymers 2022, 14, 1246. [Google Scholar] [CrossRef]
- Li, R.; Tao, J.; Huang, D.; Zhou, W.; Gao, L.; Wang, X.; Chen, H.; Huang, H. Investigating the effects of biodegradable microplastics and copper ions on probiotic (Bacillus amyloliquefaciens): Toxicity and application. J. Hazard. Mater. 2023, 443, 130081. [Google Scholar] [CrossRef]
- Yi, X.; Li, W.; Liu, Y.; Yang, K.; Wu, M.; Zhou, H. Effect of polystyrene microplastics of different sizes to Escherichia coli and Bacillus cereus. Bull. Environ. Contam. Toxicol. 2021, 107, 626–632. [Google Scholar] [CrossRef]
- Hossain, S.; Shukri, Z.N.A.; Waiho, K.; Ibrahim, Y.S.; Kamaruzzan, A.S.; Rahim, A.I.A.; Draham, A.S.; Wahab, W.; Khatoon, H.; Kasan, N.A. Biodegradation of polyethylene (PE), polypropylene (PP), and polystyrene (PS) microplastics by floc-forming bacteria, Bacillus cereus strain SHBF2, isolated from a commercial aquafarm. Environ. Sci. Pollut. Res. 2024, 31, 32225–32245. [Google Scholar] [CrossRef]
- Senko, O.; Stepanov, N.; Maslova, O.; Efremenko, E. Transformation of enzymatic hydrolysates of chlorella–fungus mixed biomass into poly(hydroxyalkanoates). Catalysts 2023, 13, 118. [Google Scholar] [CrossRef]
- Abomohra, A.; Hanelt, D. Recent advances in micro-/nanoplastic (MNPs) removal by microalgae and possible integrated routes of energy recovery. Microorganisms 2022, 10, 2400. [Google Scholar] [CrossRef]
- Abbasi, S.; Amiranipour, S.; Karimi, J.; Mohsenzadeh, S.; Turner, A. Impacts of polyethylene microplastics on the microalga, Spirulina (Arthrospira platensis). Environ. Pollut. 2023, 327, 121611. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, J.; Wang, W.; Bao, X.; Chang, D.; Hao, Z.; Qiu, M.; Liu, Y.; Teng, X.; Li, Z. Mechanisms of nanoplastic-induced energy metabolism reprogramming in juvenile Sepia esculenta: mRNA profile, miRNA/mRNA network, and ceRNA network. J. Environ. Chem. Ecotoxicol. 2025, 7, 1836–1849. [Google Scholar] [CrossRef]
- Simon-Sánchez, L.; Grelaud, M.; Lorenz, C.; Garcia-Orellana, J.; Vianello, A.; Liu, F.; Vollertsen, J.; Ziveri, P. Can a sediment core reveal the plastic age? Microplastic preservation in a coastal sedimentary record. Environ. Sci. Technol. 2022, 56, 16780–16788. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Chakma, S.; Alawa, B.; Kalyanasundaram, M.; Diwan, V. Microplastic pollution in terrestrial environment: Identification, characterization, and risk assessment in Indore, Central India. Soil Use Manag. 2024, 40, e13053. [Google Scholar] [CrossRef]
- Feng, S.; Wang, H.; Wang, Y.; Cheng, Q. A review of the occurrence and degradation of biodegradable microplastics in soil environments. Sci. Total Environ. 2023, 904, 166855. [Google Scholar] [CrossRef]
- Nigro, L.; Magni, S.; Ortenzi, M.A.; Gazzotti, S.; Della Torre, C.; Binelli, A. Are “liquid plastics” a new environmental threat? The case of polyvinyl alcohol. Aquat. Toxicol. 2022, 248, 106200. [Google Scholar] [CrossRef]
- Abbamondi, G.R.; Tommonaro, G. Research progress and hopeful strategies of application of quorum sensing in food, agriculture and nanomedicine. Microorganisms 2022, 10, 1192. [Google Scholar] [CrossRef]
- Senko, O.; Maslova, O.; Aslanli, A.; Efremenko, E. Impact of perfluorocarbons with gas transport function on growth of phototrophic microorganisms in a free and immobilized state and in consortia with bacteria. Appl. Sci. 2023, 13, 1868. [Google Scholar] [CrossRef]
- Efremenko, E.N.; Nikolskaya, A.B.; Lyagin, I.V.; Senko, O.V.; Makhlis, T.A.; Stepanov, N.A.; Maslova, O.V.; Mamedova, F.; Varfolomeev, S.D. Production of biofuels from pretreated microalgae biomass by anaerobic fermentation with immobilized Clostridium acetobutylicum cells. Bioresour. Technol. 2012, 114, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Algae. Consolidated Catalogue of Microbial Cultures Held in Russian Non-Medical Collections. Available online: https://vkm.ru/eCatalogue.htm (accessed on 4 July 2025).
- Ugarova, N.; Koksharov, M.; Lomakina, G. Reagent for Adenosine-5-Triphosphate Determination. Patent RU 2420594, 20 May 2009. [Google Scholar]
- Sánchez, C. Fungal potential for the degradation of petroleum-based polymers: An overview of macro-and microplastics biodegradation. Biotechnol. Adv. 2020, 40, 107501. [Google Scholar] [CrossRef] [PubMed]
- Baztan, J.; Carrasco, A.; Chouinard, O.; Cleaud, M.; Gabaldon, J.E.; Huck, T.; Jaffres, L.; Jorgensen, B.; Miquelez, A.; Paillard, C.; et al. Protected areas in the Atlantic facing the hazards of micro-plastic pollution: First diagnosis of three islands in the Canary current. Mar. Pollut. Bull. 2014, 80, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Meizoso-Regueira, T.; Fuentes, J.; Cusworth, S.J.; Rillig, M.C. Prediction of future microplastic accumulation in agricultural soils. Environ. Pollut. 2024, 359, 124587. [Google Scholar] [CrossRef]
- Mueller, R.-J. Biological degradation of synthetic polyesters—Enzymes as potential catalysts for polyester recycling. Process Biochem. 2006, 41, 2124–2128. [Google Scholar] [CrossRef]
- Hunková, Z.; Fencl, A. Toxic effects of fatty acids on yeast cells: Possible mechanisms of action. Biotechnol. Bioeng. 1978, 20, 1235–1247. [Google Scholar] [CrossRef]
- Fu, X.; Zhang, S.; Zhang, X.; Zhang, Y.; Li, B.; Jin, K.; Feng, X.; Hong, J.; Huang, X.; Cao, H.; et al. Sustainable microplastic remediation with record capacity unleashed via surface engineering of natural fungal mycelium framework. Adv. Funct. Mater. 2023, 33, 2212570. [Google Scholar] [CrossRef]
- Hadiyanto, H.; Joelyna, F.A.; Khoironi, A.; Sudarno, S.; Safaat, J.A.; Pratama, W.D.; Nur, M.M.A. Harnessing Chlorella vulgaris-Aspergilus niger Interactions for effective microplastic removal in aquatic ecosystems. Waste Biomass Valor. 2025, 1–17. [Google Scholar] [CrossRef]
- Krumov, N.; Atanasova, N.; Boyadzhieva, I.; Petrov, K.; Petrova, P. Biodegradation of Poly (ε-caprolactone): Microorganisms, Enzymes, and Mechanisms. Int. J. Mol. Sci. 2025, 26, 5826. [Google Scholar] [CrossRef]
- Báez-Flores, M.E.; Tiznado-Hernández, M.E.; Gracia-Valenzuela, M.H.; Troncoso-Rojas, R. Biosphere plastic contamination and microbial alternatives for a sustainable degradation of plastic waste. Microorganisms 2025, 13, 1246. [Google Scholar] [CrossRef]
- Fang, X.; Cai, Z.; Wang, X.; Liu, Z.; Lin, Y.; Li, M.; Gong, H.; Yan, M. Isolation and identification of four strains of bacteria with potential to biodegrade polyethylene and polypropylene from Mangrove. Microorganisms 2024, 12, 2005. [Google Scholar] [CrossRef]
- Boyandin, A.N.; Prudnikova, S.V.; Karpov, V.A.; Ivonin, V.N.; Dỗ, N.L.; Nguyễn, T.H.; Lê, T.M.H.; Filichev, N.L.; Levin, A.L.; Filipenko, M.L. Microbial degradation of polyhydroxyalkanoates in tropical soils. Int. Biodeterior. Biodegrad. 2013, 83, 77–84. [Google Scholar] [CrossRef]
- Cholewińska, P.; Moniuszko, H.; Wojnarowski, K.; Pokorny, P.; Szeligowska, N.; Dobicki, W.; Polechoński, R.; Górniak, W. The occurrence of microplastics and the formation of biofilms by pathogenic and opportunistic bacteria as threats in aquaculture. Int. J. Environ. Res. Public Health 2022, 19, 8137. [Google Scholar] [CrossRef]
- Aly, S.M.; ElBanna, N.I.; Fathi, M. Chlorella in aquaculture: Challenges, opportunities, and disease prevention for sustainable development. Aquac. Int. 2024, 32, 1559–1586. [Google Scholar] [CrossRef]
- Araujo, G.S.; Santiago, C.S.; Moreira, R.T.; Neto, M.P.D.; Fernandes, F.A. Nutrient removal by Arthrospira platensis cyanobacteria in cassava processing wastewater. J. Water Process Eng. 2021, 40, 101826. [Google Scholar] [CrossRef]
- Tabagari, I.; Kurashvili, M.; Varazi, T.; Adamia, G.; Gigolashvili, G.; Pruidze, M.; Chokheli, L.; Khatisashvili, G.; von Fragstein und Niemsdorff, P. Application of Arthrospira (Spirulina) platensis against chemical pollution of water. Water 2019, 11, 1759. [Google Scholar] [CrossRef]
- Gentscheva, G.; Nikolova, K.; Panayotova, V.; Peycheva, K.; Makedonski, L.; Slavov, P.; Radusheva, P.; Yotkovska, I. Application of Arthrospira platensis for medicinal purposes and the food industry: A review of the literature. Life 2023, 13, 845. [Google Scholar] [CrossRef] [PubMed]
- Park, B.S.; Choi, W.J.; Guo, R.; Kim, H.; Ki, J.S. Changes in free-living and particle-associated bacterial communities depending on the growth phases of marine green algae Tetraselmis suecica. J. Mar. Sci. Eng. 2021, 9, 171. [Google Scholar] [CrossRef]
- Marchi, A.; Benini, E.; Dondi, F.; Ferrari, M.G.; Scicchitano, D.; Palladino, G.; Candela, M.; Cerri, R.; Di Biase, F.; Vizcaino, A.J.; et al. The use of fishery and aquaculture by-products with Nannochloropsis sp. allows total dietary replacement of wild-caught fishmeal, fish oil and soy protein in European sea bass juveniles. Aquaculture 2024, 590, 741015. [Google Scholar] [CrossRef]
- Yue, Z.; Qian, J.; Li, W.; Liu, X.; Dai, H.; Liu, X.; Pi, F.; Wang, J. Spotlight on the long-term effects of micro/nanoplastics exposure on Spirulina platensis: Algal cells, extracellular polymeric substances, and phycocyanin. Food Chem. 2025, 472, 142940. [Google Scholar] [CrossRef]
- Xu, H.; Li, L.A.; Wang, Y.; Qiu, K.; Chen, S.; Zeng, J.; Liu, R.; Yang, Q.; Huang, W. Differential physiological response of marine and freshwater microalgae to polystyrene microplastics. J. Hazard. Mater. 2023, 448, 130814. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, I.; Cunha, C.; Kaufmann, M.; Faria, M.; Cordeiro, N. Microplastics reduce microalgal biomass by decreasing single-cell weight: The barrier towards implementation at scale. Sci. Total Environ. 2023, 877, 162950. [Google Scholar] [CrossRef] [PubMed]
- Cunha, C.; Faria, M.; Nogueira, N.; Ferreira, A.; Cordeiro, N. Marine vs freshwater microalgae exopolymers as biosolutions to microplastics pollution. Environ. Pollut. 2019, 249, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Qiu, S.; Xu, J.; Ge, S. Effect of undissociated n-caproic acid on methanogen activity and subsequent recovery: Methane anabolism and community structure. ACS ES&T Eng. 2024, 4, 706–716. [Google Scholar] [CrossRef]
- Oliveira, M.M.; Proenca, A.M.; Moreira-Silva, E.; Dos Santos, F.M.; Marconatto, L.; de Castro, A.M.; Medina-Silva, R. Biochemical features and early adhesion of marine Candida parapsilosis strains on high-density polyethylene. J. Appl. Microbiol. 2022, 132, 1954–1966. [Google Scholar] [CrossRef]
- Akinbobola, A.; Kean, R.; Quilliam, R.S. Plastic pollution as a novel reservoir for the environmental survival of the drug resistant fungal pathogen Candida auris. Mar. Pollut. Bull. 2024, 198, 115841. [Google Scholar] [CrossRef]
- Sponza, D.T.; Öztekin, R. Removals of some high-and low-density polyethylene (HDPE and LDPE), polypropylene (PP) and polyvinyl chloride (PVC) microplastics using some microalgae types, energy production and energy recovery. Mol. Sci. Appl. 2023, 3, 66–68. [Google Scholar] [CrossRef]
- Hadiyanto, H.; Khoironi, A.; Dianratri, I.; Suherman, S.; Muhammad, F.; Vaidyanathan, S. Interactions between polyethylene and polypropylene microplastics and Spirulina sp. microalgae in aquatic systems. Heliyon 2021, 7, e07676. [Google Scholar] [CrossRef]
- Alakomi, H.L.; Skytta, E.; Saarela, M.; Mattila-Sandholm, T.; Latva-Kala, K.; Helander, I.M. Lactic acid permeabilizes gram-negative bacteria by disrupting the outer membrane. Appl. Environ. Microbiol. 2000, 66, 2001–2005. [Google Scholar] [CrossRef]
- Su, Y.; Gao, L.; Xu, E.G.; Peng, L.; Diao, X.; Zhang, Y.; Bao, R. When microplastics meet microalgae: Unveiling the dynamic formation of aggregates and their impact on toxicity and environmental health. Water Res. 2025, 273, 123008. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, S.; Tan, H.; Wang, H.; Wang, J.; Song, K.; Xu, D.; Zhang, B.; Liu, Z.; Liu, X.; et al. Responses of different species of marine microalgae and their community to gear-derived microplastics. Water Res. 2025, 281, 123528. [Google Scholar] [CrossRef] [PubMed]
- Davarpanah, E.; Guilhermino, L. Single and combined effects of microplastics and copper on the population growth of the marine microalgae Tetraselmis chuii. Estuar. Coast. Shelf Sci. 2015, 167, 269–275. [Google Scholar] [CrossRef]
- Leonard, S.V.; Liddle, C.R.; Atherall, C.A.; Chapman, E.; Watkins, M.; Calaminus, S.D.; Rotchell, J.M. Microplastics in human blood: Polymer types, concentrations and characterization using mFTIR. Environ. Int. 2024, 188, 108751. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Jung, J.; Park, S.; Lee, Y.; Kim, J.; Han, C.; Kim, H.C.; Lee, J.H.; Hong, Y.C. Microplastic particles in human blood and their association with coagulation markers. Sci. Rep. 2024, 14, 30419. [Google Scholar] [CrossRef]
- Rajendran, D.; Chandrasekaran, N. Journey of micronanoplastics with blood components. RSC Adv. 2023, 13, 31435–31459. [Google Scholar] [CrossRef]
- da Costa, A.M.; de Oliveira Lopes, V.R.; Vidal, L.; Nicaud, J.M.; de Castro, A.M.; Coelho, M.A.Z. Poly (ethylene terephthalate)(PET) degradation by Yarrowia lipolytica: Investigations on cell growth, enzyme production and monomers consumption. Process Biochem. 2020, 95, 81–90. [Google Scholar] [CrossRef]
- Loll-Krippleber, R.; Sajtovich, V.A.; Ferguson, M.W.; Ho, B.; Burns, A.R.; Payliss, B.J.; Bellisssimo, J.; Peters, S.; Roy, P.G.; Wyatt, H.D.M.; et al. Development of a yeast whole-cell biocatalyst for MHET conversion into terephthalic acid and ethylene glycol. Microb. Cell Fact. 2022, 21, 280. [Google Scholar] [CrossRef]
- Giyahchi, M.; Moghimi, H. Acceleration a yeast-based biodegradation process of polyethylene terephthalate microplastics by Tween 20: Efficiency, by-product analysis, and metabolic pathway Prediction. Environ. Pollut. 2024, 351, 124106. [Google Scholar] [CrossRef]
- Araujo, J.; Monteiro, J.; Silva, D.; Alencar, A.; Silva, K.; Coelho, L.; Pacheco, W.; Silva, D.; Silva, M.; Silva, L.; et al. Surface-Active Compounds Produced by Microorganisms: Promising Molecules for the Development of Antimicrobial, Anti-Inflammatory, and Healing Agents. Antibiotics 2022, 11, 1106. [Google Scholar] [CrossRef]
- Senko, O.; Maslova, O.; Stepanov, N.; Aslanli, A.; Lyagin, I.; Efremenko, E. Role of Humic Substances in the (Bio)Degradation of Synthetic Polymers under Environmental Conditions. Microorganisms 2024, 12, 2024. [Google Scholar] [CrossRef]
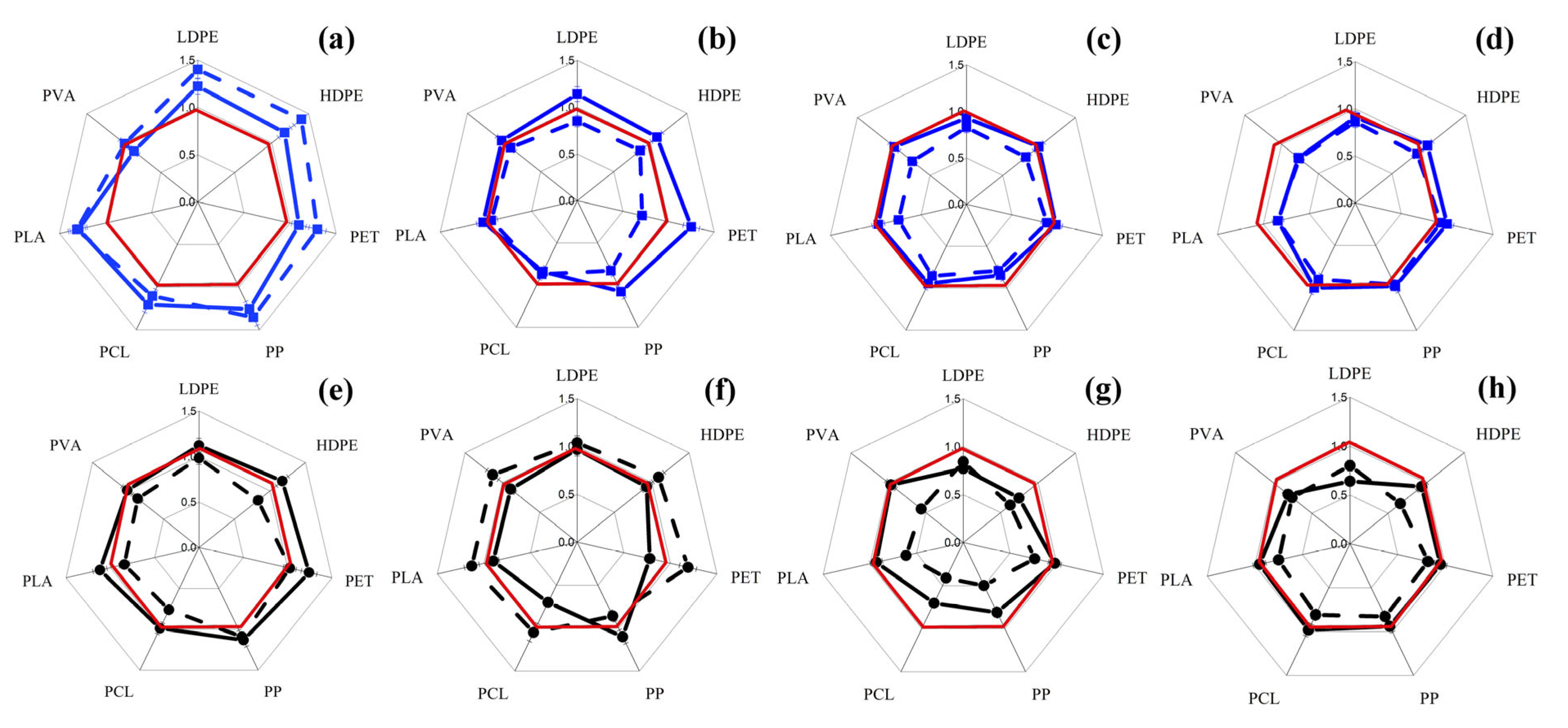
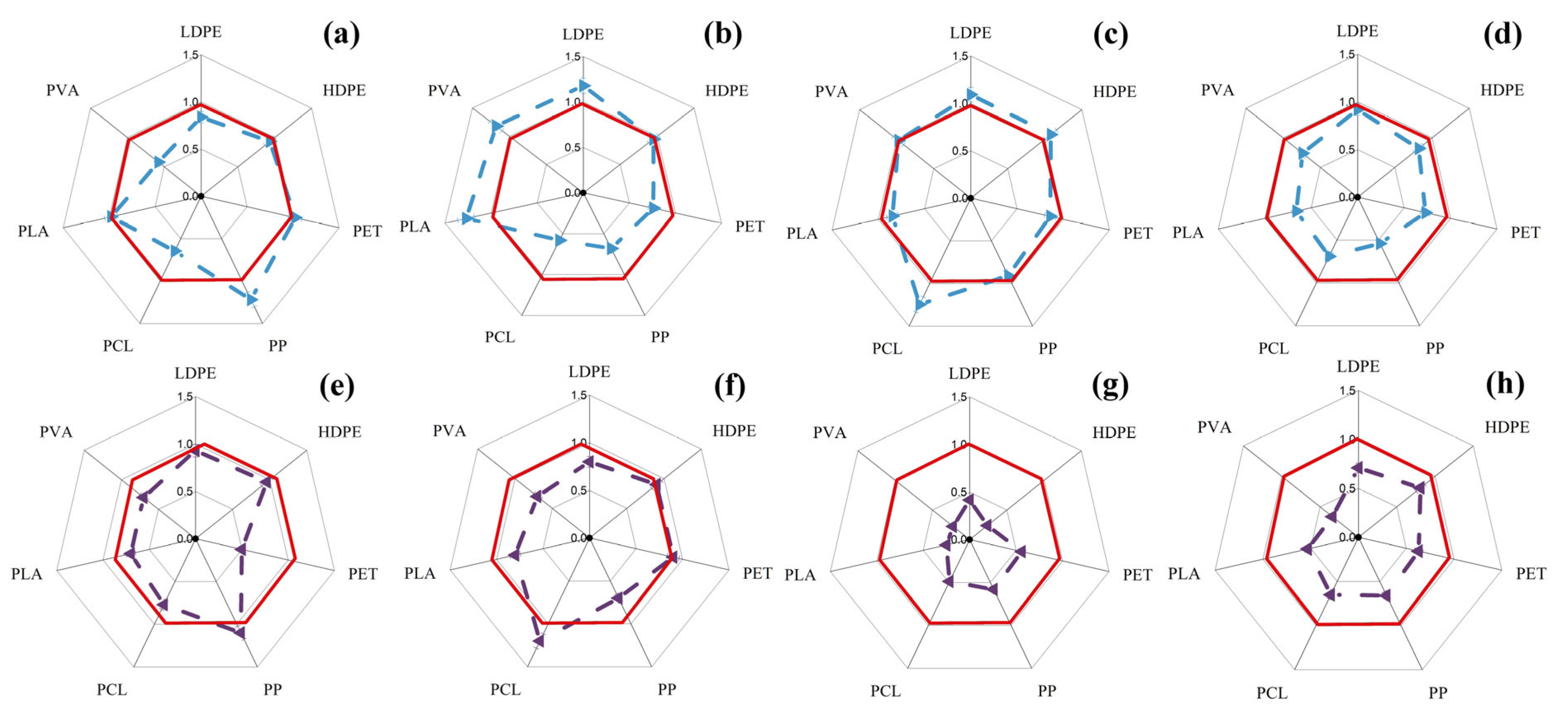

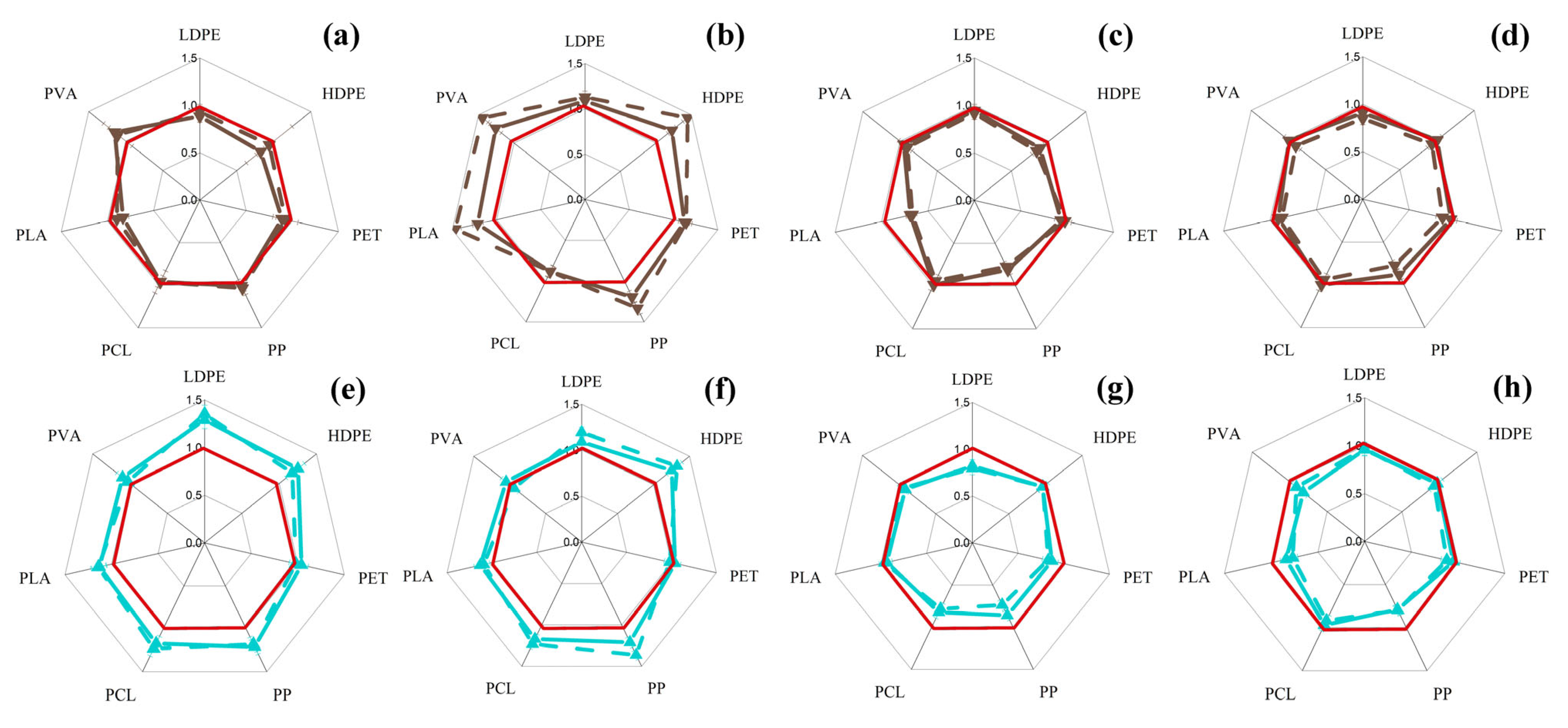
| MP | Sc | Cm | Ro | An | Bs | Pp | Php | Cv | Ap | Ns | Ts | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 g/L MP, Nutrient Medium, 104 Cells/mL | 2% NaCl | 5 g/L MP, MNM *, 104 Cells/mL | ||||||||||||||||||||
| LDPE | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B |
| HDPE | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B |
| PET | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B |
| PP | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B |
| PCL | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B |
| PLA | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B |
| PVA | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B |
| 5 g/L MP, nutrient medium, 107 cells/mL | 5 g/L MP, MNM, 107 cells/mL | |||||||||||||||||||||
| LDPE | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | ||
| HDPE | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | ||
| PET | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | ||
| PP | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | ||
| PCL | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | ||
| PLA | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | ||
| PVA | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | ||
| 5 g/L MP, saline, 104 cells/mL | 1 g/LMP, MNM, 107 cells/mL | |||||||||||||||||||||
| LDPE | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | ||||||
| HDPE | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | ||||||
| PET | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | ||||||
| PP | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | ||||||
| PCL | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | ||||||
| PLA | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | ||||||
| PVA | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | ||||||
| 5 g/L MP, saline, 107 cells/mL | ATP (A) and biomass (B) decreases in the parameters are highlighted in red, increases in green, and constant levels in yellow. Yeasts: Sc—S. cerevisiae and Cm—C. maltose; bacteria: Bs—B. subtillus, Pp—P. putida, and Php—P. phosphoreum; mycelial fungi: Ro—R. oryzae and An—A. niger; microalgae: Cv -C. vulgaris, Ns—Nannochloropsis sp., and Ts—T. suecica; cyanobacteria: Ap—A. platensis in the presence of MP under various experimental conditions. | |||||||||||||||||||||
| LDPE | A | B | A | B | A | B | A | B | A | B | A | B | ||||||||||
| HDPE | A | B | A | B | A | B | A | B | A | B | A | B | ||||||||||
| PET | A | B | A | B | A | B | A | B | A | B | A | B | ||||||||||
| PP | A | B | A | B | A | B | A | B | A | B | A | B | ||||||||||
| PCL | A | B | A | B | A | B | A | B | A | B | A | B | ||||||||||
| PLA | A | B | A | B | A | B | A | B | A | B | A | B | ||||||||||
| PVA | A | B | A | B | A | B | A | B | A | B | A | B | ||||||||||
 —stimulation of cell growth and metabolism;
—stimulation of cell growth and metabolism;  ,
,  —survival of cells due to the death of some of them, which means there is toxicity or lack of available nutrition;
—survival of cells due to the death of some of them, which means there is toxicity or lack of available nutrition;  —cells are tolerant to the presence of MPs, behaving as in the control in the absence of MPs;
—cells are tolerant to the presence of MPs, behaving as in the control in the absence of MPs;  —the culture cells are in the growth stage;
—the culture cells are in the growth stage;  —maintenance of metabolism occurs at the expense of the death of some cells, which indicates the presence of toxicity or lack of available nutrition;
—maintenance of metabolism occurs at the expense of the death of some cells, which indicates the presence of toxicity or lack of available nutrition;  —inhibition of cell growth and metabolism;
—inhibition of cell growth and metabolism;  —the culture is in the process of restructuring biochemical pathways to consume MPs;
—the culture is in the process of restructuring biochemical pathways to consume MPs;  —the culture is in the stage of division and restructuring of enzymatic systems for MP consumption.
—the culture is in the stage of division and restructuring of enzymatic systems for MP consumption.Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senko, O.; Stepanov, N.; Aslanli, A.; Efremenko, E. Bioluminescent ATP-Metry in Assessing the Impact of Various Microplastic Particles on Fungal, Bacterial, and Microalgal Cells. Microplastics 2025, 4, 72. https://doi.org/10.3390/microplastics4040072
Senko O, Stepanov N, Aslanli A, Efremenko E. Bioluminescent ATP-Metry in Assessing the Impact of Various Microplastic Particles on Fungal, Bacterial, and Microalgal Cells. Microplastics. 2025; 4(4):72. https://doi.org/10.3390/microplastics4040072
Chicago/Turabian StyleSenko, Olga, Nikolay Stepanov, Aysel Aslanli, and Elena Efremenko. 2025. "Bioluminescent ATP-Metry in Assessing the Impact of Various Microplastic Particles on Fungal, Bacterial, and Microalgal Cells" Microplastics 4, no. 4: 72. https://doi.org/10.3390/microplastics4040072
APA StyleSenko, O., Stepanov, N., Aslanli, A., & Efremenko, E. (2025). Bioluminescent ATP-Metry in Assessing the Impact of Various Microplastic Particles on Fungal, Bacterial, and Microalgal Cells. Microplastics, 4(4), 72. https://doi.org/10.3390/microplastics4040072






