Comparative Assessment of Protocols for Microplastic Quantification in Wastewater
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents & Equipment
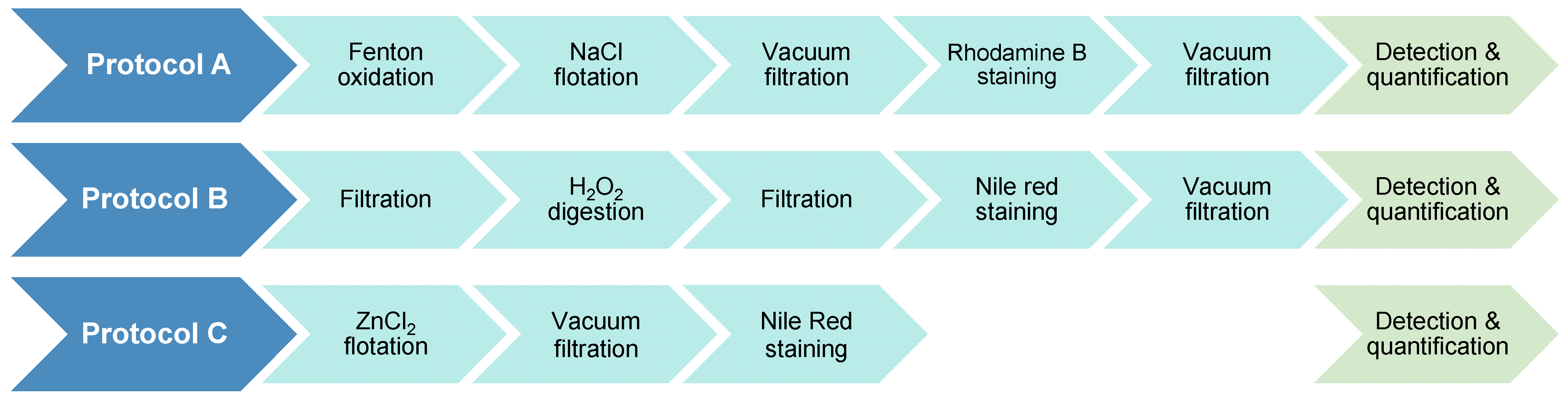
2.2. Description of Evaluated Protocols
2.2.1. Protocol A: Rhodamine B Staining Detection with NaCl Flotation and Fenton Oxidation
2.2.2. Protocol B: Nile Red Staining Detection with H2O2 Digestion
2.2.3. Protocol C: Nile Red Staining Detection with ZnCl2 Flotation
2.3. Statistical Analysis
3. Results and Discussion
3.1. Protocols Testing
3.2. Protocols Statistical Comparison
3.3. Implementation Cost Estimation
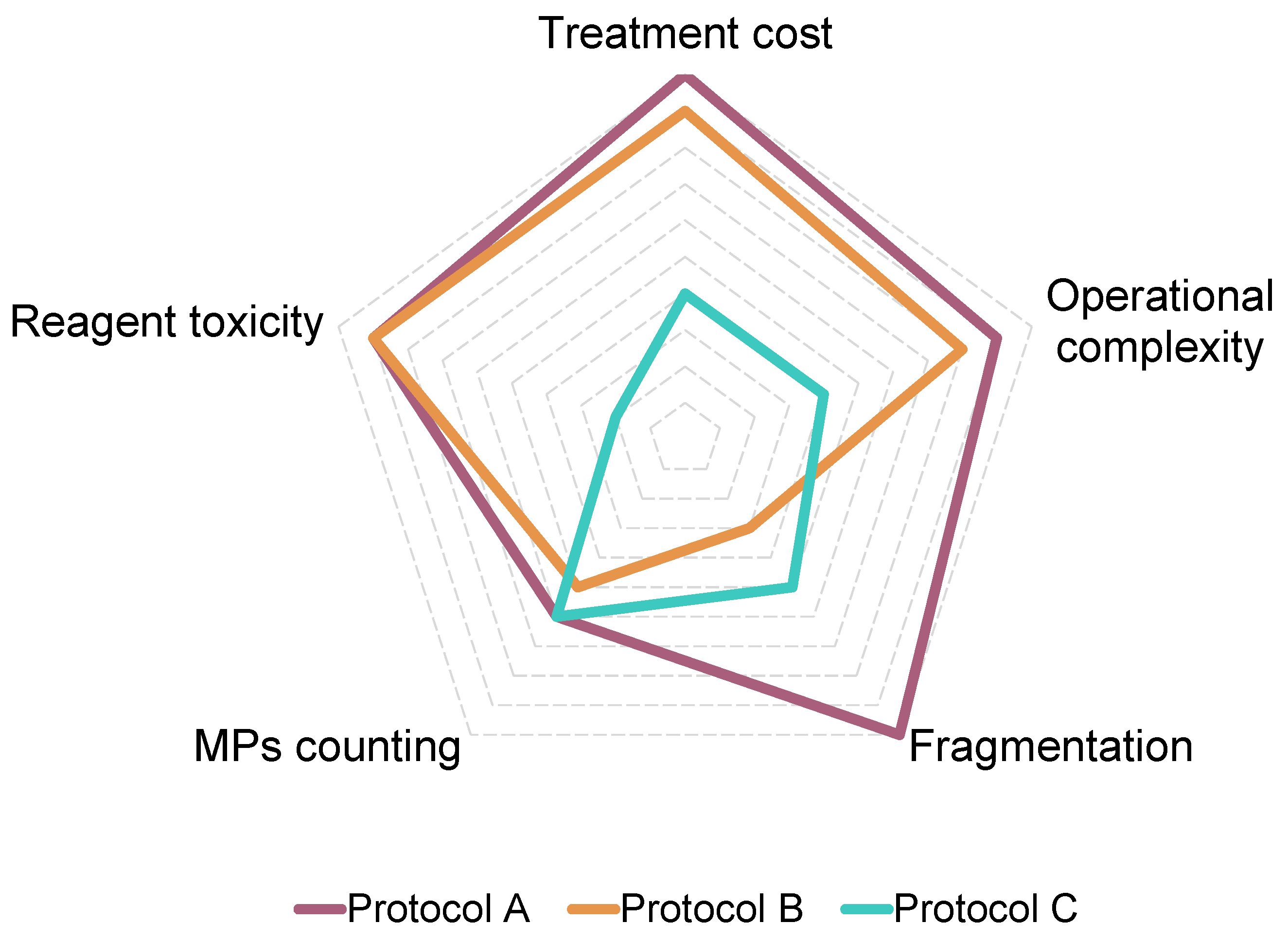
3.4. Integrated Evaluation of Protocol Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, H.; Zhu, L.; Ma, M.; Wu, H.; An, L.; Yang, Z. Occurrence of microplastics in commercially sold bottled water. Sci. Total Environ. 2023, 867, 61553. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, R.; Singh, S.; Kaur, G. Sorption of pharmaceuticals over microplastics’ surfaces: Interaction mechanisms and governing factors. Environ. Monit. Assess. 2022, 194, 803. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, V.; Chauhan, J.S. Microplastic in freshwater ecosystem: Bioaccumulation, trophic transfer, and biomagnification. Environ. Sci. Pollut. Res. 2022, 30, 9389–9400. [Google Scholar] [CrossRef] [PubMed]
- Almuhtaram, H.; Andrews, R.C. Sampling Microplastics in Water Matrices: A Need for Standardization. ACS EST Water 2022, 2, 1276–1278. [Google Scholar] [CrossRef]
- Prata, J.C.; Padrão, J.; Khan, M.T.; Walker, T.R. Do’s and don’ts of microplastic research: A comprehensive guide. Water Emerg. Contam. Nanoplast. 2024, 3, 8. [Google Scholar] [CrossRef]
- Quinn, B.; Murphy, F.; Ewins, C. Validation of density separation for the rapid recovery of microplastics from sediment. Anal. Methods 2017, 9, 1491–1498. [Google Scholar] [CrossRef]
- Rani, M.; Ducoli, S.; Depero, L.E.; Prica, M.; Tubić, A.; Ademovic, Z.; Morrison, L.; Federici, S. A Complete Guide to Extraction Methods of Microplastics from Complex Environmental Matrices. Molecules 2023, 28, 5710. [Google Scholar] [CrossRef]
- Pfeiffer, F.; Fischer, E.K. Various Digestion Protocols Within Microplastic Sample Processing—Evaluating the Resistance of Different Synthetic Polymers and the Efficiency of Biogenic Organic Matter Destruction. Front. Environ. Sci. 2020, 8, 572424. [Google Scholar] [CrossRef]
- Prasad, S.; Bennett, A.; Triantafyllou, M. Characterization of Nile Red-Stained Microplastics through Fluorescence Spectroscopy. J. Mar. Sci. Eng. 2024, 12, 1403. [Google Scholar] [CrossRef]
- Tong, H.; Jiang, Q.; Zhong, X.; Hu, X. Rhodamine B dye staining for visualizing microplastics in laboratory-based studies. Environ. Sci. Pollut. Res. 2021, 28, 4209–4215. [Google Scholar] [CrossRef]
- Aoki, H. Material-Specific Determination Based on Microscopic Observation of Single Microplastic Particles Stained with Fluorescent Dyes. Sensors 2022, 22, 3390. [Google Scholar] [CrossRef]
- Kang, H.; Park, S.; Lee, B.; Ahn, J.; Kim, S. Modification of a Nile Red Staining Method for Microplastics Analysis: A Nile Red Plate Method. Water 2020, 12, 3251. [Google Scholar] [CrossRef]
- Li, Y.; Chen, P.; Tang, Y.; Yang, Y.; Zhou, C.; Bu, J.; Zhong, S. Microplastics in Water: A Review of Characterization and Removal Methods. Sustainability 2024, 16, 4033. [Google Scholar] [CrossRef]
- Lv, L.; Yan, X.; Feng, L.; Jiang, S.; Lu, Z.; Xie, H.; Sun, S.; Chen, J.; Li, C. Challenge for the detection of microplastics in the environment. Water Environ. Res. 2021, 93, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Bakir, A.; McGoran, A.R.; Silburn, B.; Russell, J.; Nel, H.; Lusher, A.L.; Amos, R.; Shadrack, R.S.; Arnold, S.J.; Castillo, C.; et al. Creation of an international laboratory network towards global microplastics monitoring harmonization. Sci. Rep. 2024, 14, 12714. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.-C.; Ziajahromi, S.; Neale, P.A.; Leusch, F.D.L. A systematic review of freshwater microplastics in water and sediments: Recommendations for harmonisation to enhance future study comparisons. Sci. Total Environ. 2021, 781, 146693. [Google Scholar] [CrossRef]
- Rodríguez-Alegre, R.; Durán-Videra, S.; Megías, L.P.; Pérez-Moya, M.; García-Montaño, J.; Saiz, C.A.; You, X. Effect of Microfiltration Membrane Configuration in Microplastics Recovery from Wastewater Treatment Effluent. Membranes 2025, 15, 137. [Google Scholar] [CrossRef]
- Sturm, M.T.; Myers, E.; Schober, D.; Korzin, A.; Schuhen, K. Development of an inexpensive and comparable microplastic detection method using fluorescent staining with novel nile red derivatives. Analytica 2023, 4, 27–44. [Google Scholar] [CrossRef]
- Maes, T.; Jessop, R.; Wellner, N.; Haupt, K.; Mayes, A.G. A rapid-screening approach to detect and quantify microplastics based on fluorescent tagging with Nile Red. Sci. Rep. 2017, 7, 44501. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Upadhyay, V.K. Fenton’s reagent dose calculation with respect to COD value and the process requirement optimization for effective oxidation of Aqueous Mother Liquor Effluent of an API manufacturing industry at large scale. Int. J. Adv. Res. 2013, 1, 158–164. [Google Scholar]
- Munno, K.; Helm, P.A.; Jackson, D.A.; Rochman, C.; Sims, A. Impacts of temperature and selected chemical digestion methods on microplastic particles. Environ. Toxicol. Chem. 2017, 37, 91–98. [Google Scholar] [CrossRef]
- Ormaniec, P. Occurrence and analysis of microplastics in municipal wastewater, Poland. Environ. Sci. Pollut. Res. 2024, 31, 49646–49655. [Google Scholar] [CrossRef]
- Bodzek, M.; Pohl, A.; Rosik-Dulewska, C. Microplastics in Wastewater Treatment Plants: Characteristics, Occurrence and Removal Technologies. Water 2024, 16, 3574. [Google Scholar] [CrossRef]
- Miino, M.C.; Galafassi, S.; Zullo, R.; Torretta, V.; Rada, E.C. Microplastics removal in wastewater treatment plants: A review of the different approaches to limit their release in the environment. Sci. Total Environ. 2024, 930, 172675. [Google Scholar] [CrossRef]
- García-Sobrino, R.; Cortés, A.; Calderón-Villajos, R.; Díaz, J.G.; Muñoz, M. Novel and Accessible Physical Recycling for Expanded Polystyrene Waste with the Use of Acetone as a Solvent and Additive Manufacturing (Direct Ink-Write 3D Printing). Polymers 2023, 15, 3888. [Google Scholar] [CrossRef]
- Bianco, A.; Carena, L.; Peitsaro, N.; Sordello, F.; Vione, D.; Passananti, M. Rapid detection of nanoplastics and small microplastics by Nile-Red staining and flow cytometry. Environ. Chem. Lett. 2023, 21, 647–653. [Google Scholar] [CrossRef]
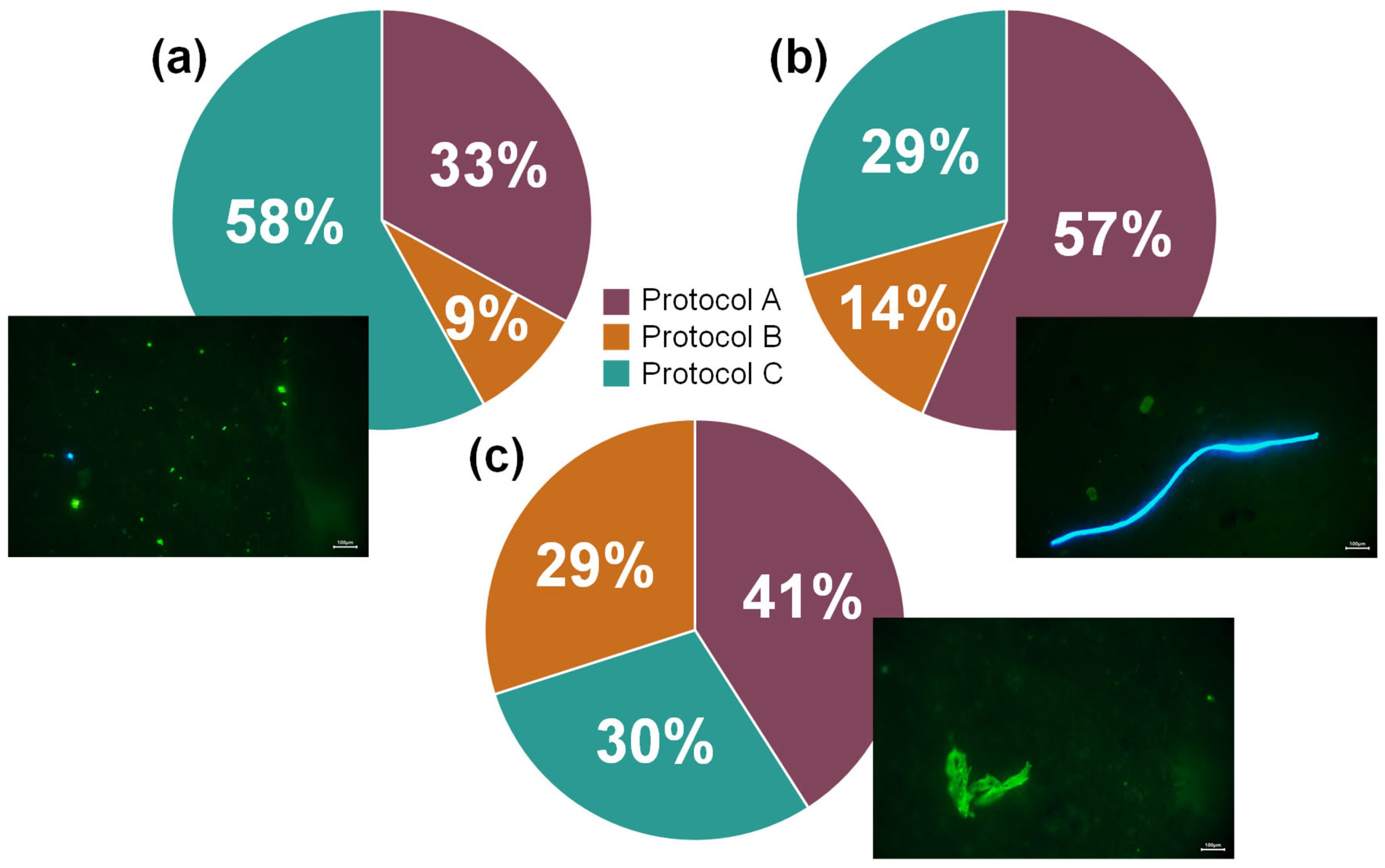

| Protocol | Unit | Fragments | Fibres | Films | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <50 µm | 50–100 µm | >100 µm | <100 µm | 100–500 µm | 500–1000 µm | >1000 µm | <100 µm | 100–500 µm | 500–1000 µm | >1000 µm | ||
| A | MP sample−1 | 1819 ± 1557 | 135 ± 57 | 25 ± 5 | 13 ± 2 | 89 ± 29 | 47 ± 28 | 43 ± 19 | 1 ± 1 | 15 ± 1 | 1 ± 2 | 00 ± 00 |
| % | 83.09 | 6.15 | 1.16 | 0.61 | 4.05 | 2.16 | 1.98 | 0.03 | 0.70 | 0.06 | 0.00 | |
| B | MP sample−1 | 499 ± 301 | 26 ± 28 | 13 ± 3 | 3 ± 1 | 36 ± 12 | 5 ± 1 | 4 ± 3 | 00 ± 00 | 11 ± 3 | 1 ± 1 | 1 ± 1 |
| % | 83.44 | 4.35 | 2.12 | 0.45 | 6.02 | 0.89 | 0.67 | 0.00 | 1.78 | 0.17 | 0.11 | |
| C | MP sample−1 | 3335 ± 1332 | 75 ± 15 | 73 ± 41 | 15 ± 9 | 58 ± 38 | 13 ± 6 | 13 ± 17 | 2 ± 2 | 9 ± 4 | 1 ± 1 | 1 ± 1 |
| % | 92.75 | 2.09 | 2.02 | 0.43 | 1.61 | 0.37 | 0.37 | 0.06 | 0.24 | 0.04 | 0.02 | |
| Reagent | Quantity | Unit | Price | Unit | Needed | Unit | Total | Unit |
|---|---|---|---|---|---|---|---|---|
| Protocol A | ||||||||
| NaCl (99%) | 1000 | g | 37.50 | € | 60.00 | g | 2.250 | € |
| Glass fibre filter | 100 | u | 20.46 | € | 1.00 | u | 0.205 | € |
| H2O2 (30%) | 1000 | mL | 24.59 | € | 3.00 | mL | 0.074 | € |
| Rhodamine B | 100,000 | mg | 80.68 | € | 1.00 | mg | 0.001 | € |
| Ethanol | 1000 | mL | 25.01 | € | 5.00 | mL | 0.125 | € |
| FeSO4·7H2O | 1000 | g | 45.50 | € | 0.27 | g | 0.012 | € |
| Total | 2.667 | € sample−1 | ||||||
| Protocol B | ||||||||
| Glass fibre filter | 100 | u | 20.46 | € | 1.00 | u | 0.205 | € |
| H2O2 (35%) | 1000 | mL | 60.90 | € | 20.00 | mL | 0.006 | € |
| Red Nile | 250 | mg | 164.63 | € | 0.50 | mg | 0.329 | € |
| Acetone | 1000 | mL | 34.77 | € | 5.00 | mL | 0.174 | € |
| FeSO4·7H2O | 1000 | g | 45.50 | € | 0.32 | g | 0.015 | € |
| Total | 0.728 | € sample−1 | ||||||
| Protocol C | ||||||||
| Glass fibre filter | 100 | u | 20.46 | € | 1.00 | u | 0.205 | € |
| Red Nile | 250 | mg | 164.63 | € | 5.00 | mg | 3.293 | € |
| Acetone | 1000 | mL | 34.77 | € | 5.00 | mL | 0.174 | € |
| ZnCl2 | 1000 | g | 62.48 | € | 185.00 | g | 11.559 | € |
| Total | 15.230 | € sample−1 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Alegre, R.; Durán-Videra, S.; Carmona-Fernández, D.; Pérez Megías, L.; Andecochea Saiz, C.; You, X. Comparative Assessment of Protocols for Microplastic Quantification in Wastewater. Microplastics 2025, 4, 49. https://doi.org/10.3390/microplastics4030049
Rodríguez-Alegre R, Durán-Videra S, Carmona-Fernández D, Pérez Megías L, Andecochea Saiz C, You X. Comparative Assessment of Protocols for Microplastic Quantification in Wastewater. Microplastics. 2025; 4(3):49. https://doi.org/10.3390/microplastics4030049
Chicago/Turabian StyleRodríguez-Alegre, Rubén, Sergi Durán-Videra, David Carmona-Fernández, Laura Pérez Megías, Carlos Andecochea Saiz, and Xialei You. 2025. "Comparative Assessment of Protocols for Microplastic Quantification in Wastewater" Microplastics 4, no. 3: 49. https://doi.org/10.3390/microplastics4030049
APA StyleRodríguez-Alegre, R., Durán-Videra, S., Carmona-Fernández, D., Pérez Megías, L., Andecochea Saiz, C., & You, X. (2025). Comparative Assessment of Protocols for Microplastic Quantification in Wastewater. Microplastics, 4(3), 49. https://doi.org/10.3390/microplastics4030049