Seasonal Comparative Monitoring of Plastic and Microplastic Pollution in Lake Garda (Italy) Using Seabin During Summer–Autumn 2024
Abstract
1. Introduction
2. Materials and Methods
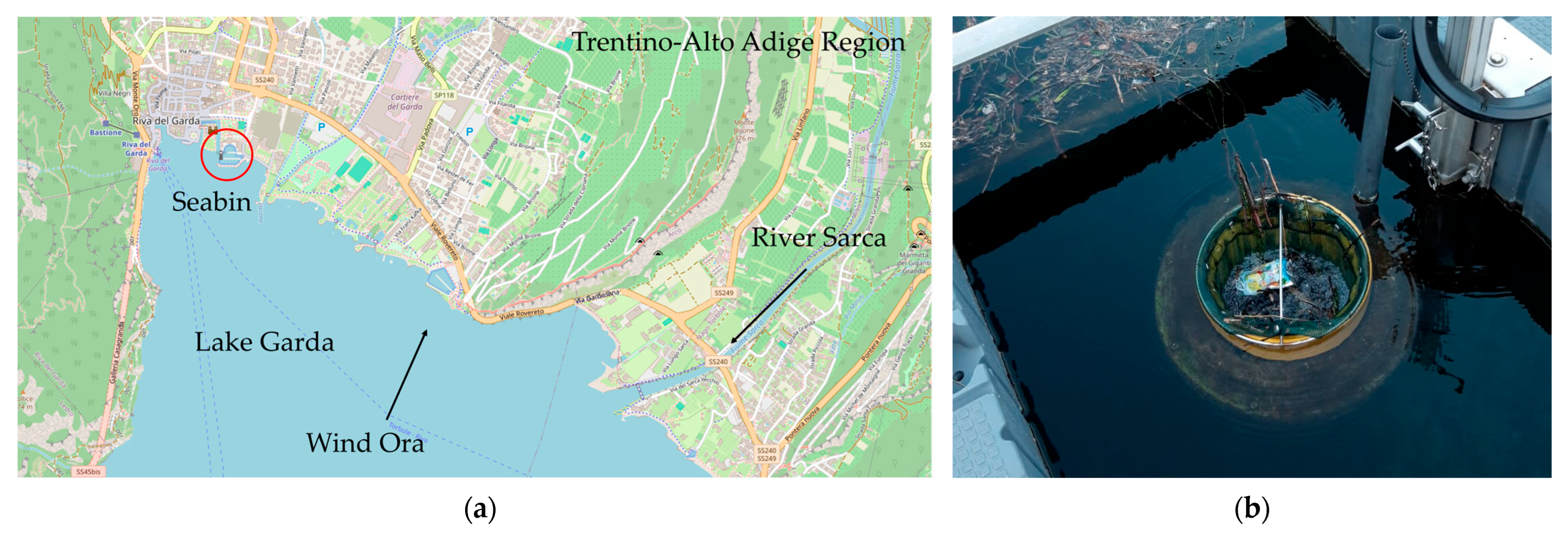
2.1. Study Area
2.2. Seabin
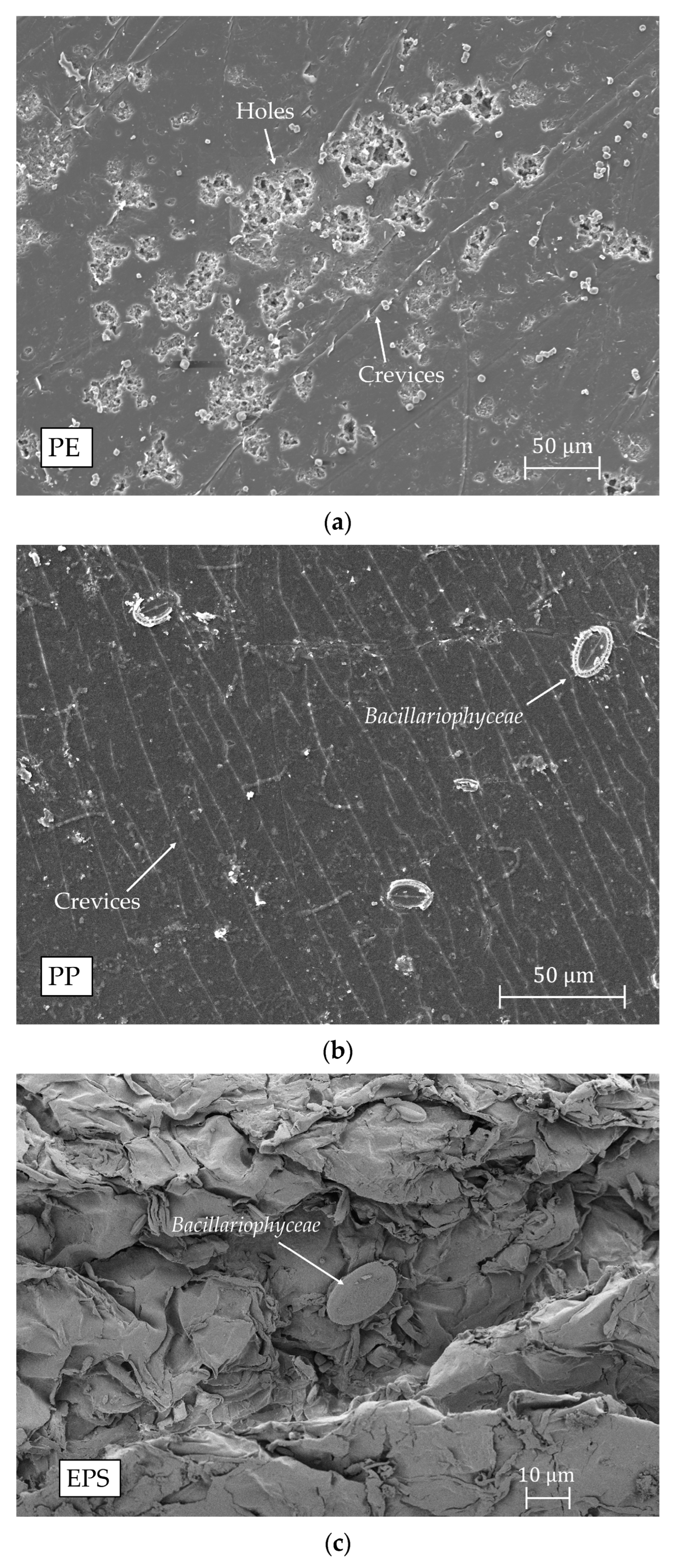
2.3. Scanning Electron Microscopy (SEM)
2.4. Fourier-Transform Infrared Spectroscopy (FTIR)
2.5. Differential Scanning Calorimetry (DSC)
2.6. Statistical Analysis of Data
3. Results and Discussion
3.1. Seabin
- Cups 15.0%;
- Bottles 41.1%;
- Ropes/wires/threads 18.3%;
- Packaging 24.4%;
- Cigarette filters 0.6%;
- Foams 0.3%.
3.2. Case Study
- 190 °C for aged PE;
- 218 °C for pristine PE;
- 206 °C for aged PP;
- 223 °C for pristine PP;
- 198 °C for aged EPS;
- 258 °C for pristine EPS.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATR | Attenuated Total Reflectance |
| DSC | Differential Scanning Calorimeter |
| EPS | Expanded Polystyrene |
| FTIR | Fourier-Transform InfraRed |
| HDPE | High-density polyethylene |
| LDPE | Low-density polyethylene |
| MP | Microplastic |
| OOT | Oxidation Onset Temperature |
| PA | Polyamide |
| PE | Polyethylene |
| PET | Polyethylene Terephthalate |
| PLA | Polylactic Acid |
| PP | Polypropylene |
| PP&A | Polyesters, polyamides, and acrylic fibers |
| PS | Polystyrene |
| PVC | Polyvinyl Chloride |
| PU | Polyurethane |
| P | Macroplastic |
| SEM | Scanning electron microscope |
Appendix A
Seabin Geometrical Aspects


Appendix B
Raw Data
| Sample | Date (Start) | Seabin Working Time (h) | Total Wet Mass (g) | P Item Count (n) | P Mass (g) |
|---|---|---|---|---|---|
| 1 | 28/08/24 | 48 | 3385 | 37 | 19.45 |
| 2 | 01/09/24 | 24 | 2455 | 30 | 45.32 |
| 3 | 03/09/24 | 48 | 2865 | 18 | 4.71 |
| Mean summer | 2902 ± 466 | 28.3 ± 9.6 | 23 ± 21 | ||
| 4 | 29/11/24 | 24 | 440 | 39 | 31.27 |
| 5 | 30/11/24 | 48 | 275 | 0 | 0 |
| 6 | 14/12/24 | 72 | 350 | 5 | 0.25 |
| Mean autumn | 355 ± 83 | 14.7 ± 21.2 | 11 ± 18 |
| Sample | Date (Start) | Seabin Working Time (h) | MP Item Count (n) | MP Mass (g) | Mean MP Mass (mg) | Ratio MP/ Total Wet Mass (ppm) |
|---|---|---|---|---|---|---|
| 1 | 28/08/24 | 48 | 111 | 0.7233 | 6.5 | 213.7 |
| 2 | 01/09/24 | 24 | 56 | 0.5912 | 10.6 | 240.8 |
| 3 | 03/09/24 | 48 | 57 | 0.3214 | 5.6 | 112.2 |
| Mean summer | 74.7 ± 31.5 | 0.55 ± 0.20 | 7.6 ± 2.6 | 188.9 ± 67.8 | ||
| 4 | 29/11/24 | 24 | 20 | 0.2672 | 13.4 | 607.3 |
| 5 | 30/11/24 | 48 | 25 | 0.0165 | 0.7 | 60.0 |
| 6 | 14/12/24 | 72 | 44 | 0.0411 | 0.9 | 117.4 |
| Mean autumn | 29.7 ± 12.7 | 0.11 ± 0.14 | 5.0 ± 7.3 | 261.6 ± 300.8 |
| Sample | Seabin Working Time (h) | PE (n) | PE (g) | PP (n) | PP (g) | EPS and PS * (n) | EPS and PS * (g) | Other (n) | Other (g) | Total (n) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 48 | 82 | 11.6262 | 38 | 6.5270 | 22 | 0.1239 | 6 | 1.8928 | 148 |
| 2 | 24 | 30 | 2.8394 | 28 | 13.9035 | 7 | 0.0258 | 21 | 29.1488 | 86 |
| 3 | 48 | 18 | 2.5894 | 45 | 1.9123 | 5 | 0.2288 | 7 | 0.3003 | 75 |
| 4 | 24 | 28 | 5.2957 | 24 | 26.0099 | 4 | 0.2065 | 3 | 0.0256 | 59 |
| 5 | 48 | 0 | 0 | 0 | 0 | 25 | 0.0165 | 0 | 0 | 25 |
| 6 | 72 | 10 | 0.0733 | 2 | 0.1532 | 26 | 0.0155 | 11 | 0.0497 | 49 |
| Total | 168 | 22.4240 | 137 | 48.5059 | 89 | 0.617 | 48 | 31.4172 | 442 |
| Sample | Seabin Working Time (h) | PE (n) | PE (mg) | PP (n) | PP (mg) | EPS (n) | EPS (mg) | Other (n) | Other (mg) | Total (n) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 48 | 69 | 609.5 | 20 | 69.9 | 21 | 40.0 | 1 | 3.9 | 111 |
| 2 | 24 | 20 | 240.5 | 11 | 31.6 | 7 | 25.8 | 18 | 293.3 | 56 |
| 3 | 48 | 15 | 70.0 | 36 | 248.0 | 1 | 1.6 | 5 | 1.8 | 57 |
| 4 | 24 | 13 | 215.7 | 1 | 24.2 | 3 | 1.7 | 3 | 25.6 | 20 |
| 5 | 48 | 0 | 0 | 0 | 0 | 25 | 16.5 | 0 | 0 | 25 |
| 6 | 72 | 9 | 14.6 | 1 | 0.6 | 26 | 15.5 | 8 | 10.4 | 44 |
| Total | 126 | 1150.3 | 69 | 374.3 | 83 | 101.1 | 35 | 334.2 | 313 | |
| Mean MP mass (mg) | 8.7 ± 5.9 | 7.6 ± 9.5 | 1.5 ± 1.2 | 6.1 ± 6.5 | ||||||
| Sample | Seabin Working Time (h) | Cups (PP) | Bottles (PET) | Ropes/ Wires/ Threads (mainly PE) | Packaging (mainly PE) | Cigarette Filters (Cellulose Acetate) | Foam (EPS) |
|---|---|---|---|---|---|---|---|
| 1 | 48 | 1 | - | 1 | 15 | - | 22 * |
| 2 | 24 | 1 | 1 | 1 | 15 | 3 | 7 |
| 3 | 48 | - | - | 5 | 20 | - | 1 |
| 4 | 24 | - | - | - | 16 | - | 3 |
| 5 | 48 | - | - | - | - | - | 25 |
| 6 | 72 | - | - | - | 2 | - | 26 |
| Total | 2 | 1 | 7 | 68 | 3 | 84 |
| Sample | Seabin Working Time (h) | Cups (g) (PP) | Bottles (g) (PET) | Ropes/ Wires/ Threads (g) (Mainly PE) | Packaging (g) (Mainly PE) | Cigarette Filters (g) (Cellulose Acetate) | Foams (g) (EPS) |
|---|---|---|---|---|---|---|---|
| 1 | 48 | 2.56 | - | 7.39 | 4.35 | - | 0.12 |
| 2 | 24 | 7.69 | 28.24 | 2.93 | 5.99 | 0.44 | 0.03 |
| 3 | 48 | - | - | 2.17 | 1.79 | - | 0.00 |
| 4 | 24 | - | - | - | 4.37 | - | 0.00 |
| 5 | 48 | - | - | - | - | - | 0.02 |
| 6 | 72 | - | - | - | 0.16 | - | 0.02 |
| Total | 10.25 | 28.24 | 12.49 | 16.66 | 0.44 | 0.19 |

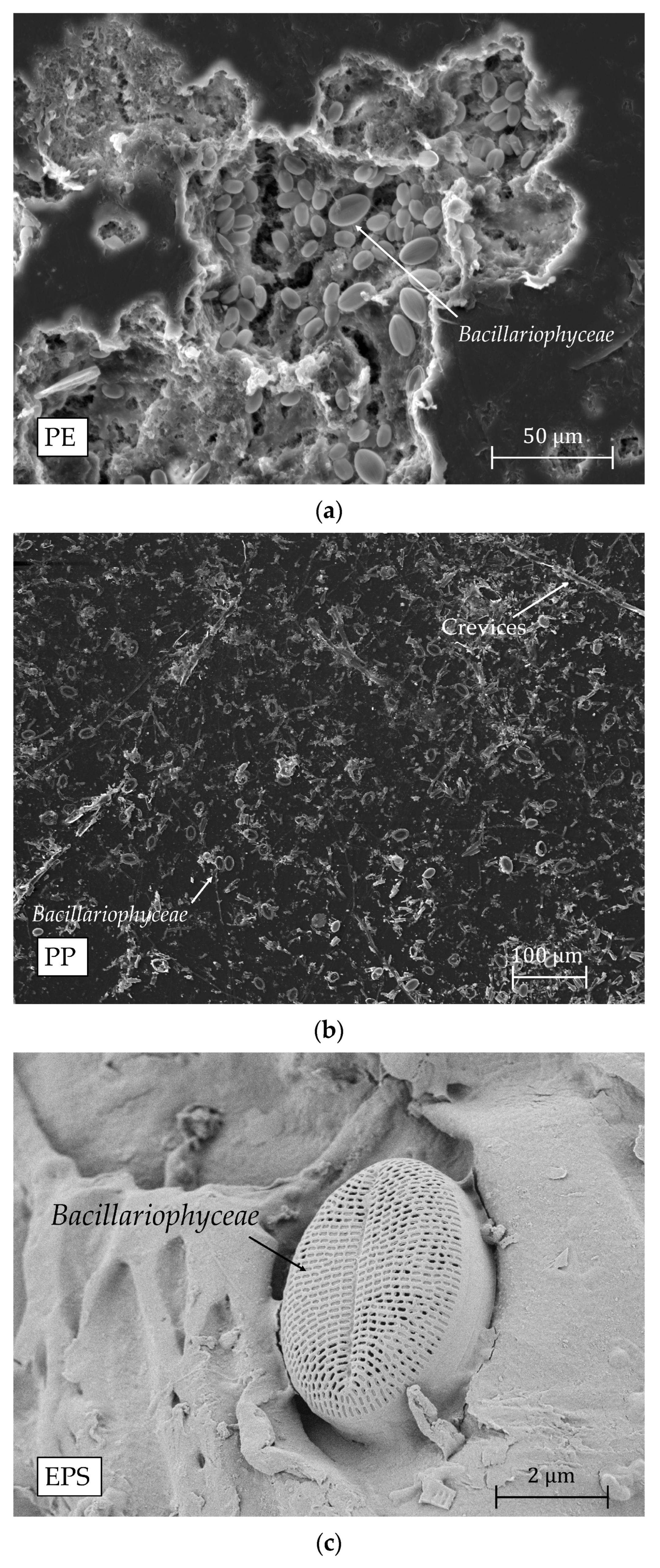
References
- Plastics Europe. Plastics—The Facts. 2022. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2022/ (accessed on 16 December 2024).
- Ellen MacArthur Foundation. The New Plastics Economy: Rethinking the Future of Plastics & Catalysing Action. ellenmacarthurfoundation.org. Available online: https://ellenmacarthurfoundation.org/the-new-plastics-economy-catalysing-action (accessed on 23 September 2023).
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.; Olsen, Y.; Mitchell, R.; Davis, A.; Rowland, S.; John, A.; McGonigle, D.F.; Russell, A. Lost at Sea: Where Is All the Plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef] [PubMed]
- ISO/TR 21960:2020; Plastics—Environmental Aspects—State of Knowledge and Methodologies. International Organization for Standardization (ISO): Geneva, Switzerland, 2020.
- Joint Research Centre: Institute for Environment Sustainability. Guidance on Monitoring of Marine Litter in European Seas; Publications Office of the European Union: Luxembourg, 2013. [Google Scholar]
- GESAMP. Guidelines for the Monitoring and Assessment of Plastic Litter and Microplastics in the Ocean; Kershaw, P.J., Turra, A., Galgani, F., Eds.; GESAMP Reports and Studies, No. 99; GESAMP Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection: London, UK, 2019; 130p. [Google Scholar]
- Wright, S.L.; Thompson, R.C.; Galloway, T.S. The physical impacts of microplastics on marine organisms: A review. Environ. Pollut. 2013, 178, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental exposure to microplastics: An overview on possible human health effects. Sci. Total Environ. 2020, 702, 134455. [Google Scholar] [CrossRef]
- Sutherland, W.J.; Clout, M.; Côté, I.M.; Daszak, P.; Depledge, M.H.; Fellman, L.; Fleishman, E.; Garthwaite, R.; Gibbons, D.W.; De Lurio, J.; et al. A horizon scan of global conservation issues for 2010. Trends. Ecol. Evol. 2010, 25, 1–7. [Google Scholar] [CrossRef]
- Eerkes-Medrano, D.; Thompson, R.C.; Aldridge, D.C. Microplastics in freshwater systems: A review of the emerging threats, identification of knowledge gaps and prioritisation of research needs. Water Res. 2015, 75, 63–82. [Google Scholar] [CrossRef]
- Free, C.M.; Jensen, O.P.; Mason, S.A.; Eriksen, M.; Williamson, N.J.; Boldgiv, B. High-levels of microplastic pollution in a large, remote, mountain lake. Mar. Pollut. Bull. 2014, 85, 156–163. [Google Scholar] [CrossRef]
- Sighicelli, M.; Pietrelli, L.; Lecce, F.; Iannilli, V.; Falconieri, M.; Coscia, L.; Di Vito, S.; Nuglio, S.; Zampetti, G. Microplastic pollution in the surface waters of Italian Subalpine Lakes. Environ. Pollut. 2018, 236, 645–651. [Google Scholar] [CrossRef]
- Binelli, A.; Pietrelli, L.; Di Vito, S.; Coscia, L.; Sighicelli, M.; Torre, C.D.; Parenti, C.C.; Magni, S. Hazard evaluation of plastic mixtures from four Italian subalpine great lakes on the basis of laboratory exposures of zebra mussels. Sci. Total Environ. 2020, 699, 134366. [Google Scholar] [CrossRef]
- Salikova, N.S.; Rodrigo-Ilarri, J.; Makeyeva, L.A.; Rodrigo-Clavero, M.-E.; Tleuova, Z.O.; Makhmutova, A.D. Monitoring of Microplastics in Water and Sediment Samples of Lakes and Rivers of the Akmola Region (Kazakhstan). Water 2024, 16, 1051. [Google Scholar] [CrossRef]
- Cozzarini, L.; Buoninsegni, J.; Corbau, C.; Lughi, V. Characterization of Large Microplastic Debris in Beach Sediments in the Po Delta Area. Microplastics 2023, 2, 147–157. [Google Scholar] [CrossRef]
- Mabry, L.; Urban-Rich, J. Seasonal and Distributional Changes in the Composition and Flux of Anthropogenic Microparticles in the Surface Waters of the Charles River, Massachusetts, United States. Microplastics 2024, 3, 539–558. [Google Scholar] [CrossRef]
- Seabin. Available online: https://seabin.io/home (accessed on 12 December 2024).
- Seabin V5. Available online: https://www.maris.com.tr/en/products/seabin-v5 (accessed on 22 May 2025).
- Paris, A.; Kwaoga, A.; Hewavitharane, C. An assessment of floating marine debris within the breakwaters of the University of the South Pacific, Marine Studies Campus at Laucala Bay. Mar. Pollut. Bull. 2022, 174, 113290. [Google Scholar] [CrossRef]
- Maglić, L.; Maglić, L.; Grbčić, A.; Gulić, M. Composition of Floating Marine Litter in Port Areas of the Island of Mallorca. J. Mar. Sci. Eng. 2022, 10, 1079. [Google Scholar] [CrossRef]
- Parker-Jurd, F.N.F.; Smith, N.S.; Gibson, L.; Nuojua, S.; Thompson, R.C. Evaluating the performance of the ‘Seabin’—A fixed point mechanical litter removal device for sheltered waters. Mar. Pollut. Bull. 2022, 184, 114199. [Google Scholar] [CrossRef]
- Nikiema, J.; Asiedu, Z. A review of the cost and effectiveness of solutions to address plastic pollution. Environ. Sci. Pollut. Res. 2022, 29, 24547–24573. [Google Scholar] [CrossRef]
- Sherlock, C.; Gutierrez, R.F.; David, M.; Rochman, C.M. A methodology for quantifying and characterizing litter from trash capture devices (TCDs) to measure impact and inform upstream solutions. Facets 2023, 8, 1–12. [Google Scholar] [CrossRef]
- DeYoung, M.; Fisher, M.; Hilkene, C. The Great Lakes Plastic Cleanup: An Effective Approach to Addressing Plastic Pollution in the Great Lakes. In Plastic Pollution: Nature Based Solutions and Effective Governance, 1st ed.; Krantzberg, G., Jetoo, S., Grover, V.I., Babel, S., Eds.; CRC Press: Boca Raton, FL, USA, 2023; pp. 1–16. [Google Scholar]
- Imhof, H.K.; Ivleva, N.P.; Schmid, J.; Niessner, R.; Laforsch, C. Contamination of beach sediments of a subalpine lake with microplastic particles. Curr. Biol. 2013, 23, R867–R868. [Google Scholar] [CrossRef] [PubMed]
- Fambri, L.; Bombardelli, G.; Gavazza, C.; Casagranda, A.; Battocchi, P.; Tomasi, R. Study of Plastics Debris Collected on the North Beaches of the Garda Lake After the Severe Storm Vaia in Autumn 2018. In Proceedings of the 2nd International Conference on Microplastic Pollution in the Mediterranean Sea, Naples, Italy, 15–18 September 2019; Cocca, M., Di Pace, E., Errico, M.E., Gentile, G., Montarsolo, A., Mossotti, R., Avella, M., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 212–225. [Google Scholar]
- Battistin, G.; Latella, L.; Iannilli, V. Microplastic pollution in the food web: Observation of ingestion by the talitrid amphipod Cryptorchestia garbinii on the shores of Lake Garda. Eur. Zool. J. 2023, 90, 73–82. [Google Scholar] [CrossRef]
- Zettler, E.R.; Mincer, T.J.; Amaral-Zettler, L.A. Life in the “Plastisphere”: Microbial Communities on Plastic Marine Debris. Environ. Sci. Technol. 2013, 47, 7137–7146. [Google Scholar] [CrossRef]
- Mato, Y.; Isobe, T.; Takada, H.; Kanehiro, H.; Ohtake, C.; Kaminuma, T. Plastic resin pellets as a transport medium for toxic chemicals in the marine environment. Environ. Sci. Technol. 2001, 35, 318–324. [Google Scholar] [CrossRef]
- LifeGate-PlasticLess Project. Available online: https://www.lifegate.it/nasce-lifegate-plasticless (accessed on 12 December 2024).
- Giovannini, L.; Laiti, L.; De Franceschi, M.; Zardi, D. Climatological characterisation of the Ora del Garda wind in the Alps. Int. J. Climatol. 2015, 2015, 4103–4115. [Google Scholar] [CrossRef]
- ISTAT. Available online: https://demo.istat.it/app/?a=2024&i=D7B (accessed on 12 December 2024).
- La Destinazione Garda Trentino. Available online: https://data.gardatrentino.it/it/data-garda-trentino.html (accessed on 12 December 2024).
- Campanale, C.; Savino, I.; Massarelli, C.; Uricchio, V.F. Fourier Transform Infrared Spectroscopy to Assess the Degree of Alteration of Artificially Aged and Environmentally Weathered Microplastics. Polymers 2023, 15, 911. [Google Scholar] [CrossRef]
- Gopanna, A.; Mandapati, R.N.; Thomas, S.P.; Rajan, K.; Chavali, M. Fourier transform infrared spectroscopy (FTIR), Raman spectroscopy and wide-angle X-ray scattering (WAXS) of polypropylene (PP)/cyclic olefin copolymer (COC) blends for qualitative and quantitative analysis. Polym. Bull. 2019, 76, 4259–4274. [Google Scholar] [CrossRef]
- Fang, J.; Xuan, Y.; Li, Q. Preparation of polystyrene spheres in different particle sizes and assembly of the PS colloidal crystals. Sci. China Technol. Sci. 2010, 53, 3088–3093. [Google Scholar] [CrossRef]
- Mecozzi, M.; Nisini, L. The differentiation of biodegradable and non-biodegradable polyethylene terephthalate (PET) samples by FTIR spectroscopy: A potential support for the structural differentiation of PET in environmental analysis. Infrared Phys. Technol. 2019, 101, 119–126. [Google Scholar] [CrossRef]
- Djebara, M.; Stoquert, J.P.; Abdesselam, M.; Muller, D.; Chami, A.C. FTIR analysis of polyethylene terephthalate irradiated by MeV He+. Nucl. Instrum. Methods Phys. Res. B 2012, 274, 70–77. [Google Scholar] [CrossRef]
- ASTM E2009-08; Standard Test Method for Oxidation Onset Temperature of Hydrocarbons by Differential Scanning Calorimetry. ASTM International: West Conshohocken, PA, USA, 2014.
- Maes, T.; Van der Meulen, M.D.; Devriese, L.I.; Leslie, H.A.; Huvet, A.; Frère, L.; Robbens, J.; Vethaak, A.D. Microplastics Baseline Surveys at the Water Surface and in Sediments of the North-East Atlantic. Front. Mar. Sci. 2017, 4, 135. (In English) [Google Scholar] [CrossRef]
- Pasquier, G.; Doyen, P.; Kazour, M.; Dehaut, A.; Diop, M.; Duflos, G.; Amara, R. Manta Net: The Golden Method for Sampling Surface Water Microplastics in Aquatic Environments. Front. Environ. Sci. 2022, 10, 811112. [Google Scholar] [CrossRef]
- Montoto-Martínez, T.; Meléndez-Díez, C.; Melián-Ramírez, A.; Hernández-Brito, J.J.; Gelado-Caballero, M.D. Comparison between the traditional Manta net and an innovative device for microplastic sampling in surface marine waters. Mar. Pollut. Bull. 2022, 185, 114237. [Google Scholar] [CrossRef]
- Bergmann, M.; Arp, H.P.H.; Carney Almroth, B.; Cowger, W.; Eriksen, M.; Dey, T.; Gündoğdu, S.; Helm, R.R.; Krieger, A.; Syberg, K.; et al. Moving from symptom management to upstream plastics prevention: The fallacy of plastic cleanup technology. One Earth 2023, 6, 1439–1442. [Google Scholar] [CrossRef]
- Gulmine, J.; Janissek, P.; Heise, H.; Akcelrud, L. Polyethylene characterization by FTIR. Polym. Test. 2002, 21, 557–563. [Google Scholar] [CrossRef]
- Karlsson, T.M.; Kärrman, A.; Rotander, A.; Hassellöv, M. Comparison between manta trawl and in situ pump filtration methods, and guidance for visual identification of microplastics in surface waters. Environ. Sci. Pollut. Res. 2020, 27, 5559–5571. [Google Scholar] [CrossRef] [PubMed]
- Rytelewska, S.; Dąbrowska, A. The Raman Spectroscopy Approach to Different Freshwater Microplastics and Quantitative Characterization of Polyethylene Aged in the Environment. Microplastics 2022, 1, 263–281. [Google Scholar] [CrossRef]
- Lim, Y.K.; Lee, K.-W.; Hong, S.H.; Park, J.G.; Baek, S.H. Differential impact of planktonic and periphytic diatoms on aggregation and sinking of microplastics in a simulated marine environment. Mar. Pollut. Bull. 2024, 199, 115961. [Google Scholar] [CrossRef]
- Microplastics a Macro Problem. Available online: https://diinews.unitn.it/en/microplastics-a-macro-problem/ (accessed on 16 April 2025).



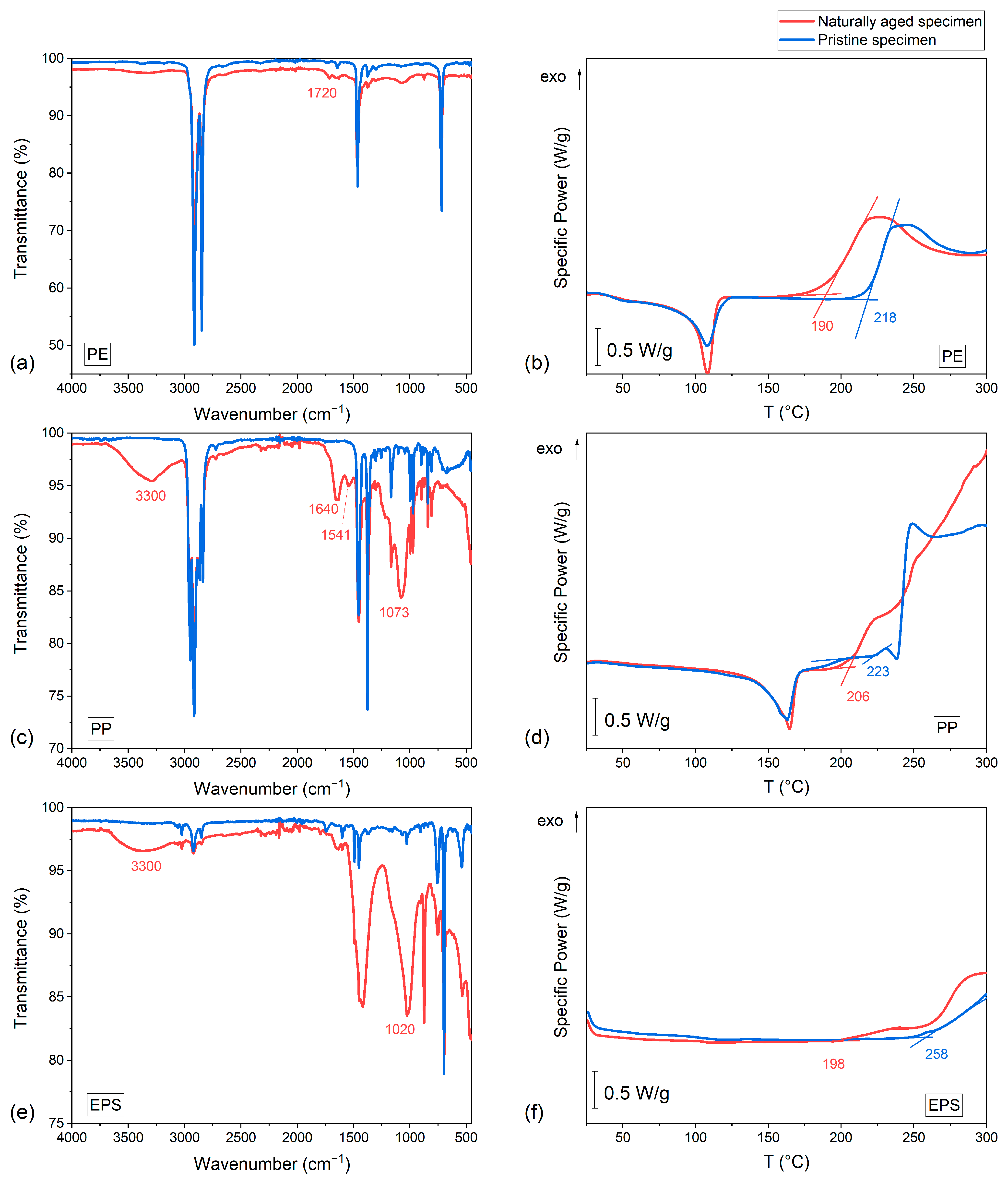
| Material | Wavenumber (cm−1) | Peak Intensity * | Peak Assignment |
|---|---|---|---|
| PE [35] | 2915 | vs | –CH2 stretching |
| 2847 | vs | –CH2 stretching | |
| 1462 | m | CH=CH stretching | |
| 719 | m | –CH2 rocking | |
| PP [35,36] | 2952 | s | –CH3 stretching |
| 2917 | s | –CH2 stretching | |
| 2839 | m | –CH2 stretching | |
| 1455 | m | –CH2 bending | |
| 1375 | s | –CH3 bending | |
| 997 | w | –CH3 rocking | |
| 972 | w | –CH3 rocking | |
| PS [35,37] | 3060 | w | C–H aromatic stretch |
| 3026 | w | C–H aromatic stretch | |
| 1600 | w | C=C aromatic stretch | |
| 1492 | m | C=C aromatic stretch | |
| 1452 | m | C=C aromatic stretch | |
| 756 | m | C–H out-of-plane bending | |
| 698 | s | C–H out-of-plane bending | |
| PET [38,39] | 1715 | vs | C=O stretching |
| 1470 | w | –CH2 bending | |
| 1370 | w | –CH2 gauche wagging | |
| 1340 | m | –CH2 trans wagging | |
| 1250 | vs | C–C–O asymmetric stretching | |
| 1100 | vs | C–O–C stretching | |
| 973 | m | Oxy-methylene bending | |
| 898 | w | Oxy-methylene bending | |
| 724 | vs | Aromatic ring C–H out-of plane and C–C bending |
| Summer | Autumn | |
|---|---|---|
| Mass of P/water filtered (g/m3) | 0.0319 ± 0.0383 | 0.0174 ± 0.0300 |
| Mass of MP/water filtered (g/m3) | 0.0006 ± 0.0004 | 0.0002 ± 0.0002 |
| Mass of P+ MP/water filtered (g/m3) | 0.0325 ± 0.0386 | 0.0176 ± 0.0303 |
| Number of P particles/water filtered (1/m3) | 0.0319 ± 0.0175 | 0.0226 ± 0.0368 |
| Number of MP particles/water filtered (1/m3) | 0.0778 ± 0.0262 | 0.0262 ± 0.0064 |
| Number of P+ MP particles/water filtered (1/m3) | 0.1097 ± 0.0421 | 0.0488 ± 0.0430 |
| Mass of vegetation and animal residues/water filtered (g/m3) | 0.45 ± 0.30 | 0.15 ± 0.23 |
| Ratio MP/total wet mass (ppm) | 189 ± 68 | 262 ± 301 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papparotto, M.; Gavazza, C.; Matteotti, P.; Fambri, L. Seasonal Comparative Monitoring of Plastic and Microplastic Pollution in Lake Garda (Italy) Using Seabin During Summer–Autumn 2024. Microplastics 2025, 4, 44. https://doi.org/10.3390/microplastics4030044
Papparotto M, Gavazza C, Matteotti P, Fambri L. Seasonal Comparative Monitoring of Plastic and Microplastic Pollution in Lake Garda (Italy) Using Seabin During Summer–Autumn 2024. Microplastics. 2025; 4(3):44. https://doi.org/10.3390/microplastics4030044
Chicago/Turabian StylePapparotto, Marco, Claudia Gavazza, Paolo Matteotti, and Luca Fambri. 2025. "Seasonal Comparative Monitoring of Plastic and Microplastic Pollution in Lake Garda (Italy) Using Seabin During Summer–Autumn 2024" Microplastics 4, no. 3: 44. https://doi.org/10.3390/microplastics4030044
APA StylePapparotto, M., Gavazza, C., Matteotti, P., & Fambri, L. (2025). Seasonal Comparative Monitoring of Plastic and Microplastic Pollution in Lake Garda (Italy) Using Seabin During Summer–Autumn 2024. Microplastics, 4(3), 44. https://doi.org/10.3390/microplastics4030044






