Comparative Analysis of Virulence Genes and Antimicrobial Resistance in Escherichia coli from Poultry Meat and Poultry Farm Environments in Pakistan
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Preparation of Samples
2.2. Culturing, Isolation, and Biochemical Characterization
2.3. DNA Extraction and Molecular Detection of Virulence Genes
2.4. Antibiotic Susceptibility Test
3. Results
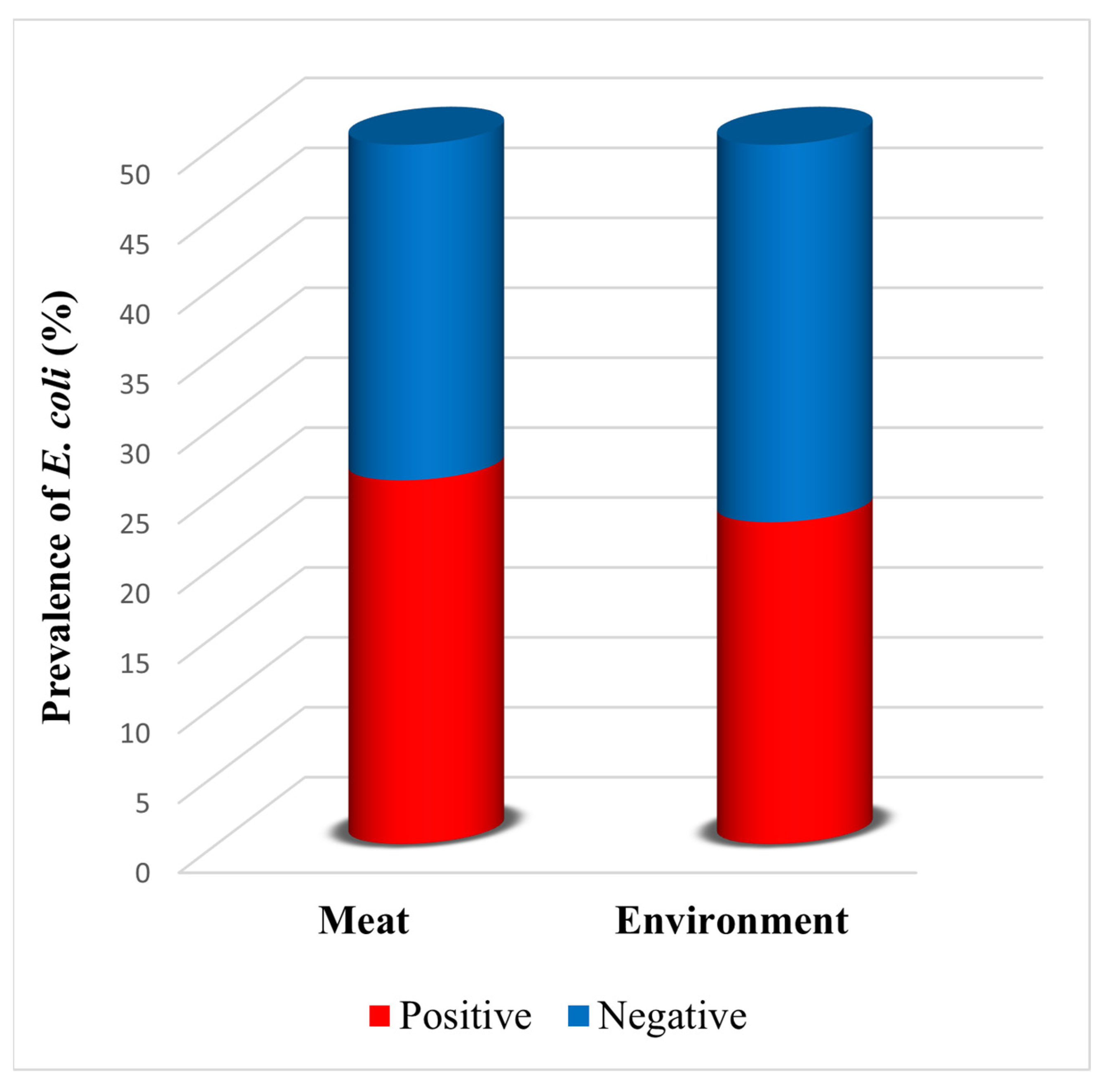
3.1. Prevalence of E. coli in Samples
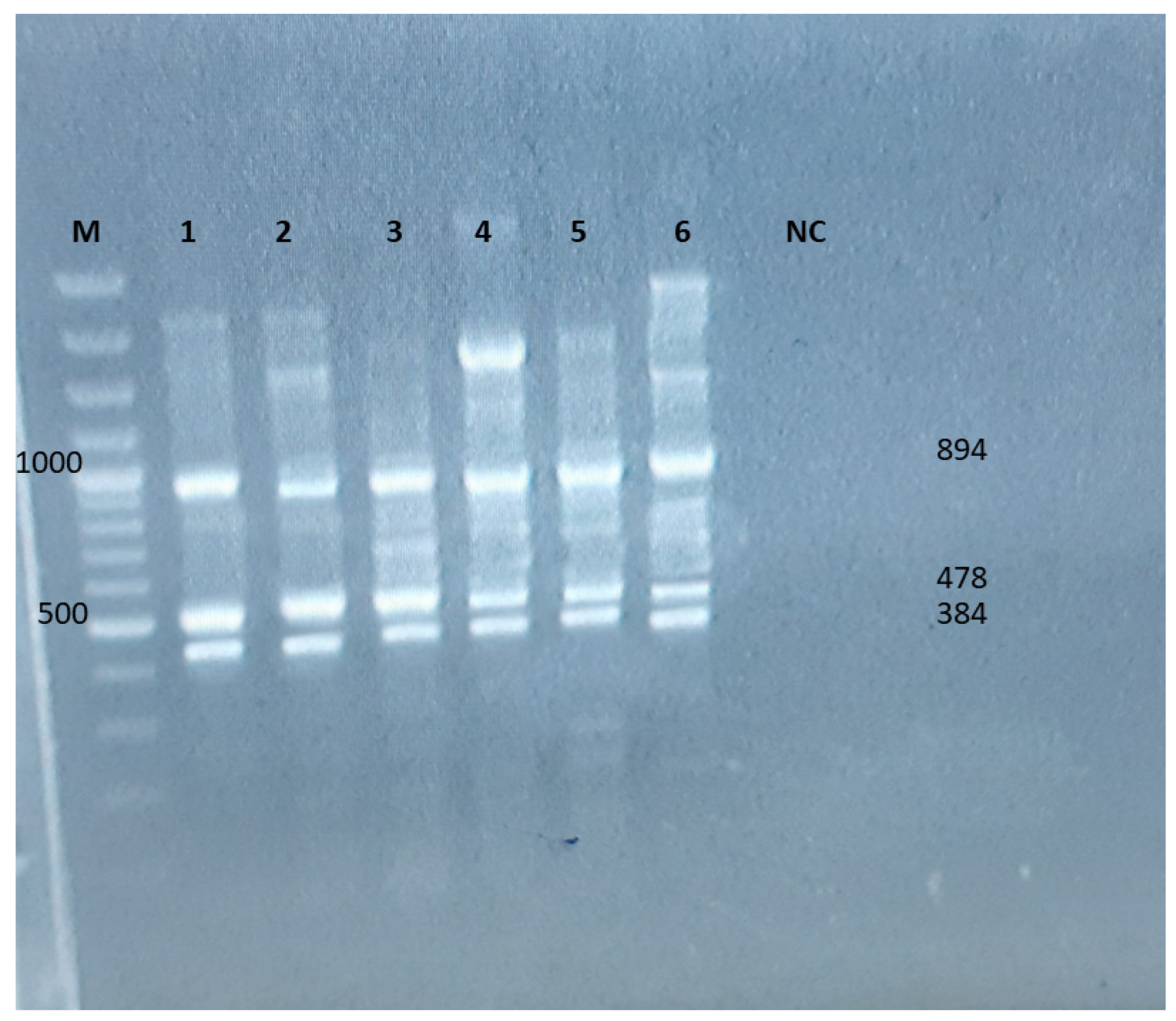
3.2. Molecular Detection of Virulence Genes
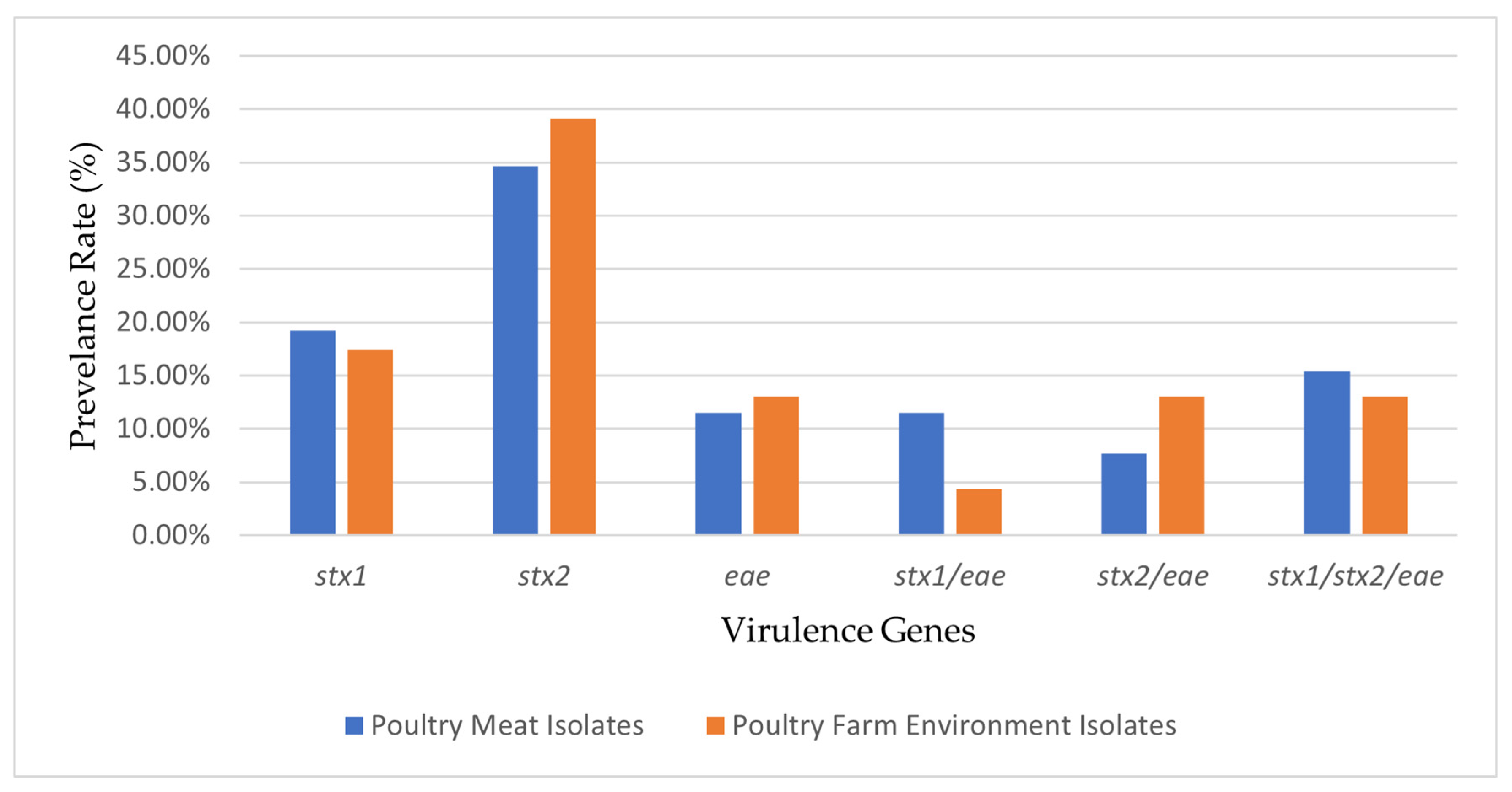
3.3. Comparative Analysis of Virulence Genes Detected in Poultry Meat and Poultry Farm Environment Isolates
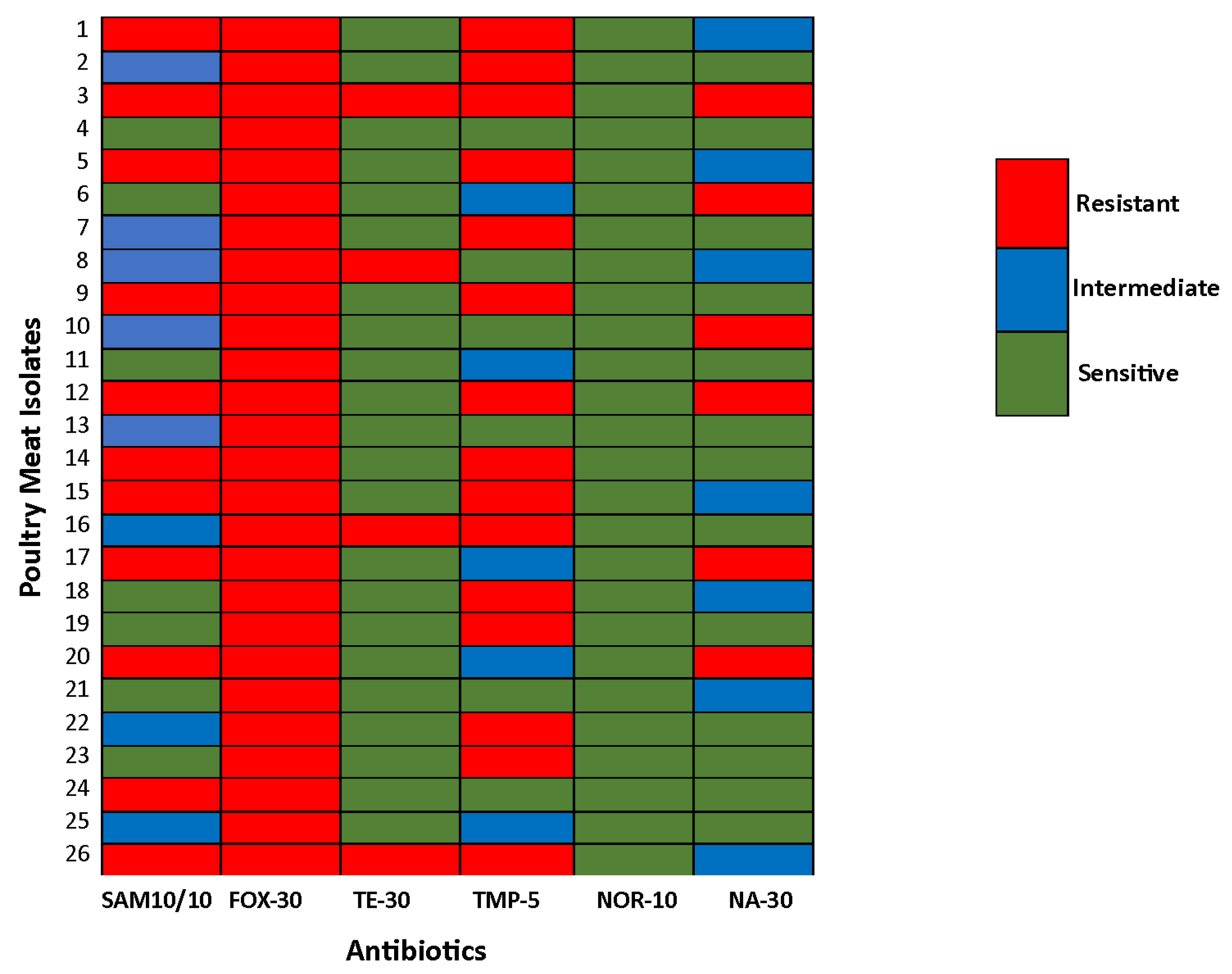
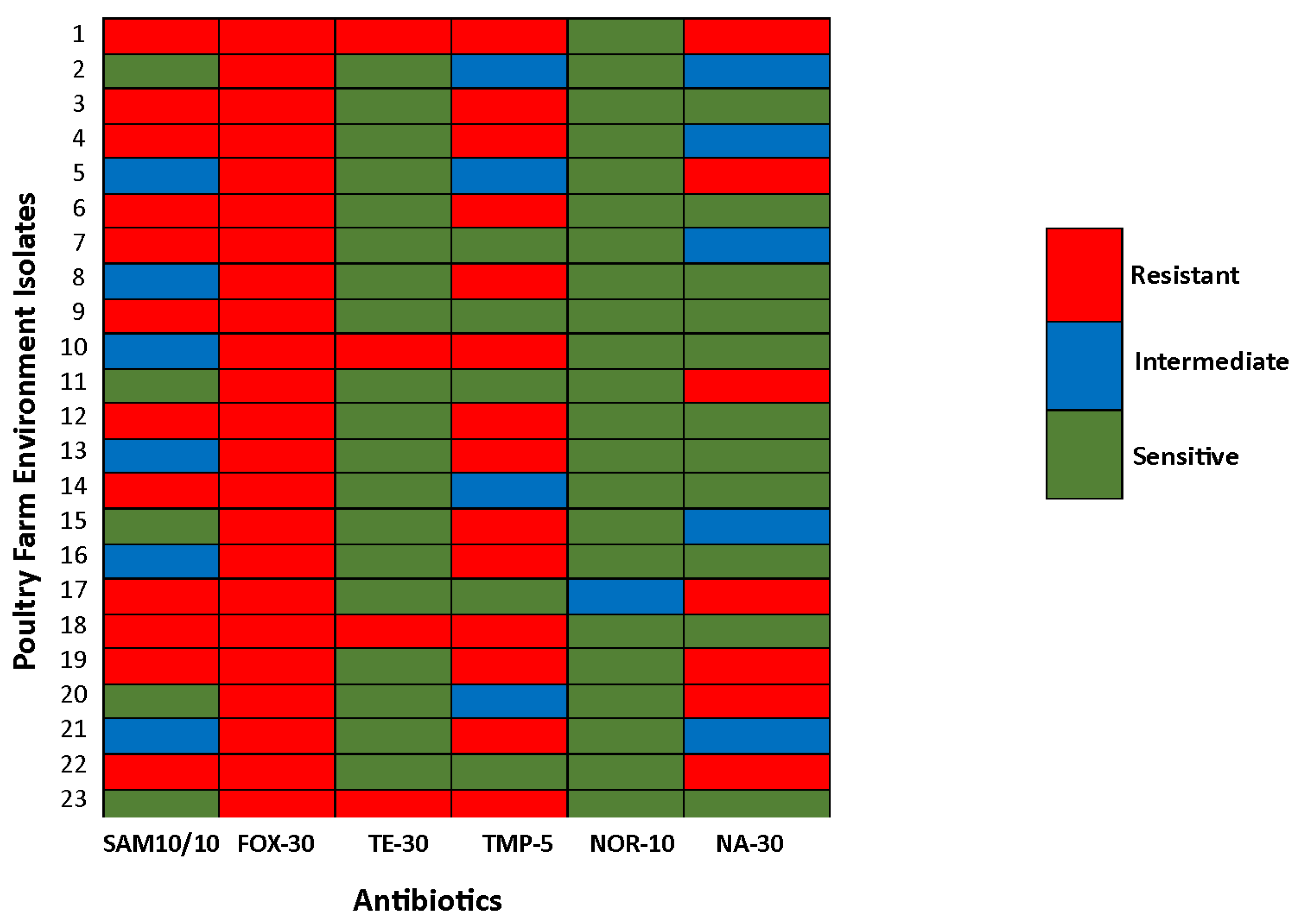
3.4. Antimicrobial Resistance Pattern
3.5. Comparative Analysis of AMR Patterns Between Poultry Meat and Poultry Farm Environment Isolates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raut, R.; Maharjan, P.; Fouladkhah, A.C. Practical Preventive Considerations for Reducing the Public Health Burden of Poultry-Related Salmonellosis. Int. J. Environ. Res. Public Health 2023, 20, 6654. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Hussain, J.; Usman, M.; Naseem, M.T.; Saleem, M.M.; Hashmi, S.G.M.D.; Latif, H.R.A.; Saleem, K.; Ahmad, S. Poultry Consumption and Perceptions in Tehsil Shakargarh, Punjab, Pakistan: Implications for Public Health during COVID-19. Heliyon 2024, 10, e29403. [Google Scholar] [CrossRef]
- Aslam, H.B.; Alarcon, P.; Yaqub, T.; Iqbal, M.; Häsler, B. A Value Chain Approach to Characterize the Chicken Sub-Sector in Pakistan. Front. Vet. Sci. 2020, 7, 361. [Google Scholar] [CrossRef]
- Khan, M.M.; Mushtaq, M.A.; Suleman, M.; Ahmed, U.; Ashraf, M.F.; Aslam, R.; Mohsin, M.; Rödiger, S.; Sarwar, Y.; Schierack, P.; et al. Fecal Microbiota Landscape of Commercial Poultry Farms in Faisalabad, Pakistan: A 16S rRNA Gene-Based Metagenomics Study. Poult. Sci. 2025, 104, 105089. [Google Scholar] [CrossRef]
- Feng, S.; Rao, A.; Pradhan, A.K. Chapter 3—Bacterial Contamination and Control in Food Products. In Antimicrobial Food Packaging, 2nd ed.; Barros-Velázquez, J., Ed.; Academic Press: San Diego, CA, USA, 2025; pp. 41–55. ISBN 978-0-323-90747-7. [Google Scholar]
- Ovuru, K.F.; Izah, S.C.; Ogidi, O.I.; Imarhiagbe, O.; Ogwu, M.C. Slaughterhouse Facilities in Developing Nations: Sanitation and Hygiene Practices, Microbial Contaminants and Sustainable Management System. Food Sci. Biotechnol. 2024, 33, 519–537. [Google Scholar] [CrossRef]
- Noorzai, A.Q.; Abro, S.H.; Abro, R.; Kalhoro, D.H.; Dasti, M.I.; Alhimaidi, A.R.; Ammari, A.A.; Abdel-Maksoud, M.A.; Kiani, B.H.; Jamali, F.H. Bacterial Assessment, Thermal Resilience and Antibiotic Resistance Profiles of Bacterial Contaminants in Retail Fish and Meat. LWT 2025, 217, 117462. [Google Scholar] [CrossRef]
- Kovačević, Z.; Čabarkapa, I.; Šarić, L.; Pajić, M.; Tomanić, D.; Kokić, B.; Božić, D.D. Natural Solutions to Antimicrobial Resistance: The Role of Essential Oils in Poultry Meat Preservation with Focus on Gram-Negative Bacteria. Foods 2024, 13, 3905. [Google Scholar] [CrossRef]
- Morshdy, A.; Mohamed, F.; El-tahlawy, A.; Mahmoud, A.; Basiony, S.; Mohamed, R.; Shosha, A.; Darwish, W.S.; Ali, E.-S. Prevalence of E. coli in The Retailed Chicken Meat Products with Protective Trials Using Carica Papaya and Moringa Oleifera Extracts. Egypt. J. Vet. Sci. 2025, 56, 1499–1506. [Google Scholar] [CrossRef]
- Duc, H.M.; Ha, C.T.T.; Hoa, T.T.K.; Hung, L.V.; Thang, N.V.; Son, H.M. Prevalence, Molecular Characterization, and Antimicrobial Resistance Profiles of Shiga Toxin-Producing Escherichia coli Isolated from Raw Beef, Pork, and Chicken Meat in Vietnam. Foods 2024, 13, 2059. [Google Scholar] [CrossRef]
- Shaikh, S.R.; Siddiqui, M.F.M.F.; MP, S. Importance of Colibacillosis in Poultry. Res. Perspect. Microbiol. Biotechnol. 2024, 1, 54–72. [Google Scholar] [CrossRef]
- Douwes, J.; Thorne, P.; Pearce, N.; Heederik, D. Bioaerosol Health Effects and Exposure Assessment: Progress and Prospects. Ann. Occup. Hyg. 2003, 47, 187–200. [Google Scholar] [CrossRef]
- Bruyand, M.; Mariani-Kurkdjian, P.; Gouali, M.; de Valk, H.; King, L.A.; Le Hello, S.; Bonacorsi, S.; Loirat, C. Hemolytic Uremic Syndrome Due to Shiga Toxin-Producing Escherichia coli Infection. Médecine Mal. Infect. 2018, 48, 167–174. [Google Scholar] [CrossRef]
- Proulx, F.; Turgeon, J.P.; Litalien, C.; Mariscalco, M.M.; Robitaille, P.; Seidman, E. Inflammatory Mediators in Escherichia coli O157:H7 Hemorrhagic Colitis and Hemolytic-Uremic Syndrome. Pediatr. Infect. Dis. J. 1998, 17, 899. [Google Scholar] [CrossRef]
- Farooq, M.; Smoglica, C.; Ruffini, F.; Soldati, L.; Marsilio, F.; Di Francesco, C.E. Antibiotic Resistance Genes Occurrence in Conventional and Antibiotic-Free Poultry Farming, Italy. Animals 2022, 12, 2310. [Google Scholar] [CrossRef]
- Vidovic, N.; Vidovic, S. Antimicrobial Resistance and Food Animals: Influence of Livestock Environment on the Emergence and Dissemination of Antimicrobial Resistance. Antibiotics 2020, 9, 52. [Google Scholar] [CrossRef]
- Osman, K.M.; Kappell, A.D.; Elhadidy, M.; ElMougy, F.; El-Ghany, W.A.A.; Orabi, A.; Mubarak, A.S.; Dawoud, T.M.; Hemeg, H.A.; Moussa, I.M.I.; et al. Poultry Hatcheries as Potential Reservoirs for Antimicrobial-Resistant Escherichia coli: A Risk to Public Health and Food Safety. Sci. Rep. 2018, 8, 5859. [Google Scholar] [CrossRef]
- Ali, N.H.; Farooqui, A.; Khan, A.; Khan, A.Y.; Kazmi, S.U. Microbial Contamination of Raw Meat and Its Environment in Retail Shops in Karachi, Pakistan. J. Infect. Dev. Ctries. 2010, 4, 382–388. [Google Scholar] [CrossRef]
- Soomro, A.H.; Khaskheli, M.; Bhutto, M.B.; Shah, G.; Memon, A.; Dewani, P. Prevalence and Antimicrobial Resistance of Salmonella Serovars Isolated from Poultry Meat in Hyderabad, Pakistan. Turk. J. Vet. Anim. Sci. 2010, 34, 455–460. [Google Scholar] [CrossRef]
- Miranda, J.M.; Vázquez, B.I.; Fente, C.A.; Barros-Velázquez, J.; Cepeda, A.; Franco Abuín, C.M. Antimicrobial Resistance in Escherichia coli Strains Isolated from Organic and Conventional Pork Meat: A Comparative Survey. Eur. Food Res. Technol. 2008, 226, 371–375. [Google Scholar] [CrossRef]
- Davis, G.S.; Waits, K.; Nordstrom, L.; Grande, H.; Weaver, B.; Papp, K.; Horwinski, J.; Koch, B.; Hungate, B.A.; Liu, C.M.; et al. Antibiotic-Resistant Escherichia coli from Retail Poultry Meat with Different Antibiotic Use Claims. BMC Microbiol. 2018, 18, 174. [Google Scholar] [CrossRef]
- Ugwu, I.C.; Wilson, F.; Ugwu, C.C. Multi-Drug Resistant Escherichia coli from Poultry: A Potential Source of Antimicrobial Resistance Spread in Poultry Farm Environment. Int. J. Enteric Pathog. 2022, 10, 8–12. [Google Scholar] [CrossRef]
- Dunislawska, A.; Pietrzak, E.; Bełdowska, A.; Siwek, M. Health in Poultry- Immunity and Microbiome with Regard to a Concept of One Health. Phys. Sci. Rev. 2024, 9, 477–495. [Google Scholar] [CrossRef]
- Elemuwa, C.O.; Raimi, M.O.; Geraldine, U.; Elemuwa, T.C.A. Decoding E. coli O157: H7: Insights from DNA Sequencing and Phylogenetic Analysis for Enhanced Public Health Strategies. JMIR Prepr. 2024, 26, 2024. [Google Scholar]
- Blanco, M.; Blanco, J.E.; Mora, A.; Rey, J.; Alonso, J.M.; Hermoso, M.; Hermoso, J.; Alonso, M.P.; Dahbi, G.; González, E.A.; et al. Serotypes, Virulence Genes, and Intimin Types of Shiga Toxin (Verotoxin)-Producing Escherichia coli Isolates from Healthy Sheep in Spain. J. Clin. Microbiol. 2003, 41, 1351–1356. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 30th ed.; CLSI Supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020. [Google Scholar]
- Abass, A.; Adzitey, F.; Huda, N. Escherichia coli of Ready-to-Eat (RTE) Meats Origin Showed Resistance to Antibiotics Used by Farmers. Antibiotics 2020, 9, 869. [Google Scholar] [CrossRef]
- Zhao, S.; Blickenstaff, K.; Bodeis-Jones, S.; Gaines, S.A.; Tong, E.; McDermott, P.F. Comparison of the Prevalences and Antimicrobial Resistances of Escherichia coli Isolates from Different Retail Meats in the United States, 2002 to 2008. Appl. Environ. Microbiol. 2012, 78, 1701–1707. [Google Scholar] [CrossRef]
- Muloi, D.; Ward, M.J.; Pedersen, A.B.; Fèvre, E.M.; Woolhouse, M.E.J.; van Bunnik, B.A.D. Are Food Animals Responsible for Transfer of Antimicrobial-Resistant Escherichia coli or Their Resistance Determinants to Human Populations? A Systematic Review. Foodborne Pathog. Dis. 2018, 15, 467–474. [Google Scholar] [CrossRef]
- Thongratsakul, S.; Amavisit, P.; Poolkhet, C. Antimicrobial Resistance in Poultry Farming: A Look Back at Environmental Escherichia coli Isolated from Poultry Farms during the Growing and Resting Periods. Vet. Med. Int. 2023, 2023, 8354235. [Google Scholar] [CrossRef]
- Pereira, A.; Sidjabat, H.E.; Davis, S.; Vong Da Silva, P.G.; Alves, A.; Dos Santos, C.; Jong, J.B.D.C.; Da Conceição, F.; Felipe, N.D.J.; Ximenes, A.; et al. Prevalence of Antimicrobial Resistance in Escherichia coli and Salmonella Species Isolates from Chickens in Live Bird Markets and Boot Swabs from Layer Farms in Timor-Leste. Antibiotics 2024, 13, 120. [Google Scholar] [CrossRef]
- Zhao, C.; Ge, B.; De Villena, J.; Sudler, R.; Yeh, E.; Zhao, S.; White, D.G.; Wagner, D.; Meng, J. Prevalence of Campylobacter Spp., Escherichia coli, and Salmonella Serovars in Retail Chicken, Turkey, Pork, and Beef from the Greater Washington, D.C., Area. Appl. Environ. Microbiol. 2001, 67, 5431–5436. [Google Scholar] [CrossRef]
- Mataseje, L.F.; Neumann, N.; Crago, B.; Baudry, P.; Zhanel, G.G.; Louie, M.; Mulvey, M.R. Characterization of Cefoxitin-Resistant Escherichia coli Isolates from Recreational Beaches and Private Drinking Water in Canada between 2004 and 2006. Antimicrob. Agents Chemother. 2009, 53, 3126–3130. [Google Scholar] [CrossRef]
- Amato, H.K.; Wong, N.M.; Pelc, C.; Taylor, K.; Price, L.B.; Altabet, M.; Jordan, T.E.; Graham, J.P. Effects of Concentrated Poultry Operations and Cropland Manure Application on Antibiotic Resistant Escherichia coli and Nutrient Pollution in Chesapeake Bay Watersheds. Sci. Total Environ. 2020, 735, 139401. [Google Scholar] [CrossRef]
- Similar Antimicrobial Resistance of Escherichia coli Strains Isolated from Retail Chickens and Poultry Farms|Foodborne Pathogens and Disease. Available online: https://www.liebertpub.com/doi/abs/10.1089/fpd.2021.0019?casa_token=dEBC39c-jDsAAAAA:_nz-r-8YdelEOoK7VhA8wknLhpv3kMA5khiJ58Uc-XX3VE0co-TQl1oAYOnrAl1EK25PipvzsBM (accessed on 8 May 2025).
- Akond, M.A.; Alam, S.; Hassan, S.M.R.; Shirin, M. Reza Antibiotic Resistance of Escherichia coli Isolated From Poultry and Poultry Environment of Bangladesh. Am. J. Environ. Sci. 2009, 5, 47–52. [Google Scholar] [CrossRef]
- Van Boeckel, T.P.; Gandra, S.; Ashok, A.; Caudron, Q.; Grenfell, B.T.; Levin, S.A.; Laxminarayan, R. Global Antibiotic Consumption 2000 to 2010: An Analysis of National Pharmaceutical Sales Data. Lancet Infect. Dis. 2014, 14, 742–750. [Google Scholar] [CrossRef]
- Foysal, M.; Imam, T.; Das, S.B.; Gibson, J.S.; Mahmud, R.; Gupta, S.D.; Fournié, G.; Hoque, M.A.; Henning, J. Association between Antimicrobial Usage and Resistance on Commercial Broiler and Layer Farms in Bangladesh. Front. Vet. Sci. 2024, 11, 1435111. [Google Scholar] [CrossRef]
- Agyare, C.; Etsiapa Boamah, V.; Ngofi Zumbi, C.; Boateng Osei, F. Antibiotic Use in Poultry Production and Its Effects on Bacterial Resistance. In Antimicrobial Resistance—A Global Threat; Kumar, Y., Ed.; IntechOpen: London, UK, 2019; ISBN 978-1-78985-783-2. [Google Scholar]
- Van Den Bogaard, A. Epidemiology of Resistance to Antibiotics Links between Animals and Humans. Int. J. Antimicrob. Agents 2000, 14, 327–335. [Google Scholar] [CrossRef]
- Parvin, M.S.; Talukder, S.; Ali, M.Y.; Chowdhury, E.H.; Rahman, M.T.; Islam, M.T. Antimicrobial Resistance Pattern of Escherichia coli Isolated from Frozen Chicken Meat in Bangladesh. Pathogens 2020, 9, 420. [Google Scholar] [CrossRef]
- Meena, P.R.; Priyanka, P.; Rana, A.; Raj, D.; Singh, A.P. Alarming Level of Single or Multidrug Resistance in Poultry Environments-associated Extraintestinal Pathogenic Escherichia coli Pathotypes with Potential to Affect the One Health. Environ. Microbiol. Rep. 2022, 14, 400–411. [Google Scholar] [CrossRef]
- Denamur, E.; Clermont, O.; Bonacorsi, S.; Gordon, D. The Population Genetics of Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2021, 19, 37–54. [Google Scholar] [CrossRef]
- Hughes, L.A.; Bennett, M.; Coffey, P.; Elliott, J.; Jones, T.R.; Jones, R.C.; Lahuerta-Marin, A.; McNIFFE, K.; Norman, D.; Williams, N.J.; et al. Risk Factors for the Occurrence of Escherichia Coli Virulence Genes Eae, Stx1 and Stx2 in Wild Bird Populations. Epidemiol. Infect. 2009, 137, 1574–1582. [Google Scholar] [CrossRef]
- Tayh, G.; Nsibi, F.; Chemli, K.; Daâloul-Jedidi, M.; Abbes, O.; Messadi, L. Occurrence, Antibiotic Resistance and Molecular Characterisation of Shiga Toxin-Producing Escherichia coli Isolated from Broiler Chickens in Tunisia. Br. Poult. Sci. 2024, 65, 751–761. [Google Scholar] [CrossRef]
- Hoang Minh, S.; Kimura, E.; Hoang Minh, D.; Honjoh, K.; Miyamoto, T. Virulence Characteristics of Shiga Toxin-producing Escherichia coli from Raw Meats and Clinical Samples. Microbiol. Immunol. 2015, 59, 114–122. [Google Scholar] [CrossRef]
- Escherichia coli from Commercial Broiler and Backyard Chickens Share Sequence Types, Antimicrobial Resistance Profiles, and Resistance Genes with Human Extraintestinal Pathogenic Escherichia coli|Foodborne Pathogens and Disease. Available online: https://www.liebertpub.com/doi/abs/10.1089/fpd.2019.2680?casa_token=Le6y0gcEVYcAAAAA:JbSmbRejQUlu_oBGqQn7349V5oqO3TEOhd2Naba7I3KgoSMoiEHSAKX7IK-hzbVqqzx8YPXTouE (accessed on 8 May 2025).
- Verweyen, H.M.; Karch, H.; Brandis, M.; Zimmerhackl, L.B. Enterohemorrhagic Escherichia coli Infections: Following Transmission Routes. Pediatr. Nephrol. 2000, 14, 73–83. [Google Scholar] [CrossRef]
- Sarowska, J.; Olszak, T.; Jama-Kmiecik, A.; Frej-Madrzak, M.; Futoma-Koloch, B.; Gawel, A.; Drulis-Kawa, Z.; Choroszy-Krol, I. Comparative Characteristics and Pathogenic Potential of Escherichia coli Isolates Originating from Poultry Farms, Retail Meat, and Human Urinary Tract Infection. Life 2022, 12, 845. [Google Scholar] [CrossRef]

| Biochemical Test | Reagents Added | E. coli | Original Control | Positive Control | Negative Control |
|---|---|---|---|---|---|
| Indole | Kovac’s reagent | + | Yellow | Red ring | Yellow |
| MR | MR indicator | + | Yellow | Red | Yellow |
| VP | Barritt’s reagent | − | Copper | Red | No color change |
| Citrate | Bromothymol indicator | + | Green | Blue | Green |
| Catalase | H2O2 | + | Clear | Bubble | No bubble |
| Lactose fermentation test | - | + | Red color with no gas production in the Durham tube | The yellow color along with gas production in the Durham tube | Red color, along with no gas production in the Durham tube |
| Primer | Oligonucleotide Sequence (5′-3′) | Amplicon Size (bp) | Reference |
|---|---|---|---|
| stx1 | F=CAGTTAATGTGGTGGCGAAG | 894 | [25] |
| R=CTGCTAATAGTTCTGCGCATC | |||
| stx2 | F=CTTCGGTATCCTATTCCCGG | 478 | [24] |
| R=GGATGCATCTCTGGTCATTG | |||
| eae | F=GACCCGGCACAAGCATAAGC | 384 | [25] |
| R=CCACCTGCAGCAACAAGAGG |
| Conditions | Temperature and Time |
|---|---|
| Initial Denaturation | 95 °C for 8 min |
| Denaturation | 94 °C for 1 min |
| Annealing | 55 °C for 45 s |
| Extension | 72 °C for 1 min |
| Final Extension | 72 °C for 10 min |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fatima, A.; Ali, S.; Raut, R. Comparative Analysis of Virulence Genes and Antimicrobial Resistance in Escherichia coli from Poultry Meat and Poultry Farm Environments in Pakistan. DNA 2025, 5, 42. https://doi.org/10.3390/dna5030042
Fatima A, Ali S, Raut R. Comparative Analysis of Virulence Genes and Antimicrobial Resistance in Escherichia coli from Poultry Meat and Poultry Farm Environments in Pakistan. DNA. 2025; 5(3):42. https://doi.org/10.3390/dna5030042
Chicago/Turabian StyleFatima, Arjmand, Sultan Ali, and Rabin Raut. 2025. "Comparative Analysis of Virulence Genes and Antimicrobial Resistance in Escherichia coli from Poultry Meat and Poultry Farm Environments in Pakistan" DNA 5, no. 3: 42. https://doi.org/10.3390/dna5030042
APA StyleFatima, A., Ali, S., & Raut, R. (2025). Comparative Analysis of Virulence Genes and Antimicrobial Resistance in Escherichia coli from Poultry Meat and Poultry Farm Environments in Pakistan. DNA, 5(3), 42. https://doi.org/10.3390/dna5030042








