Loss of LsSOC1 Function Delays Bolting and Reprograms Transcriptional and Metabolic Responses in Lettuce
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
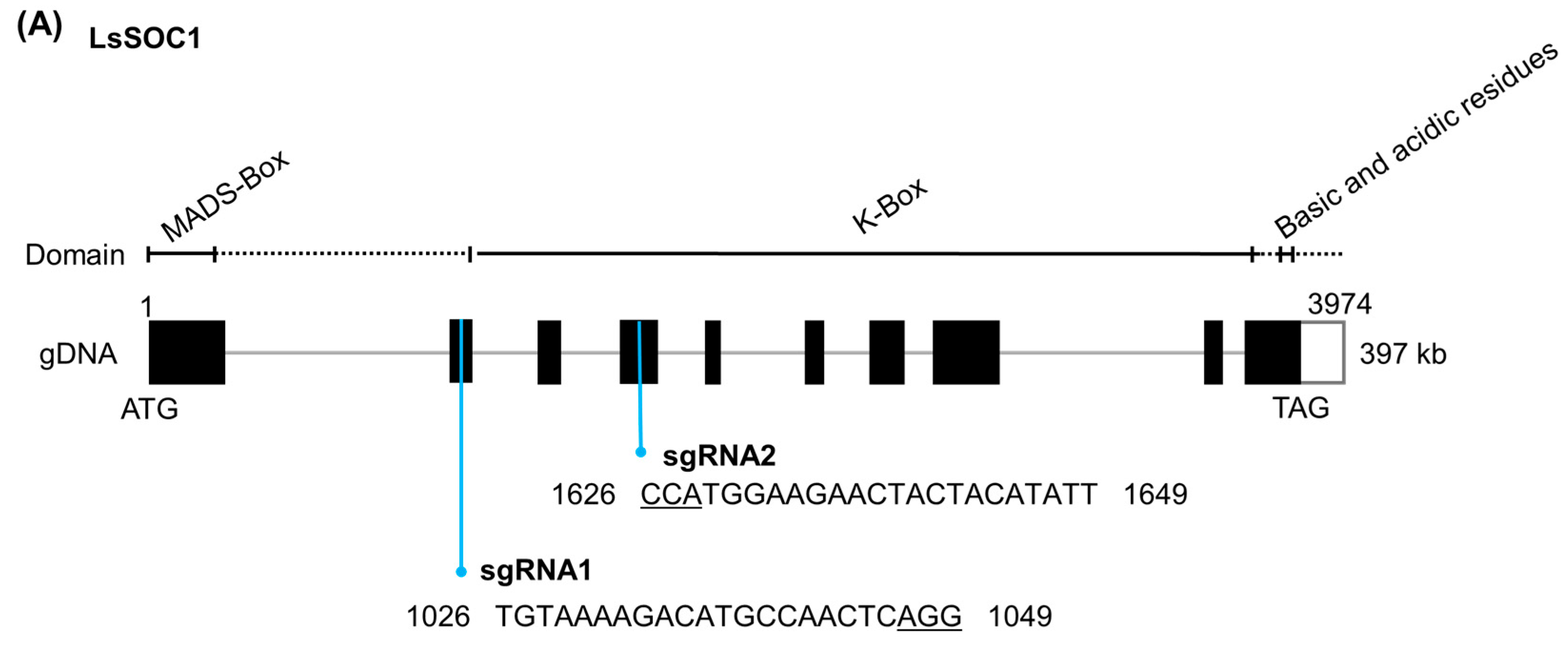
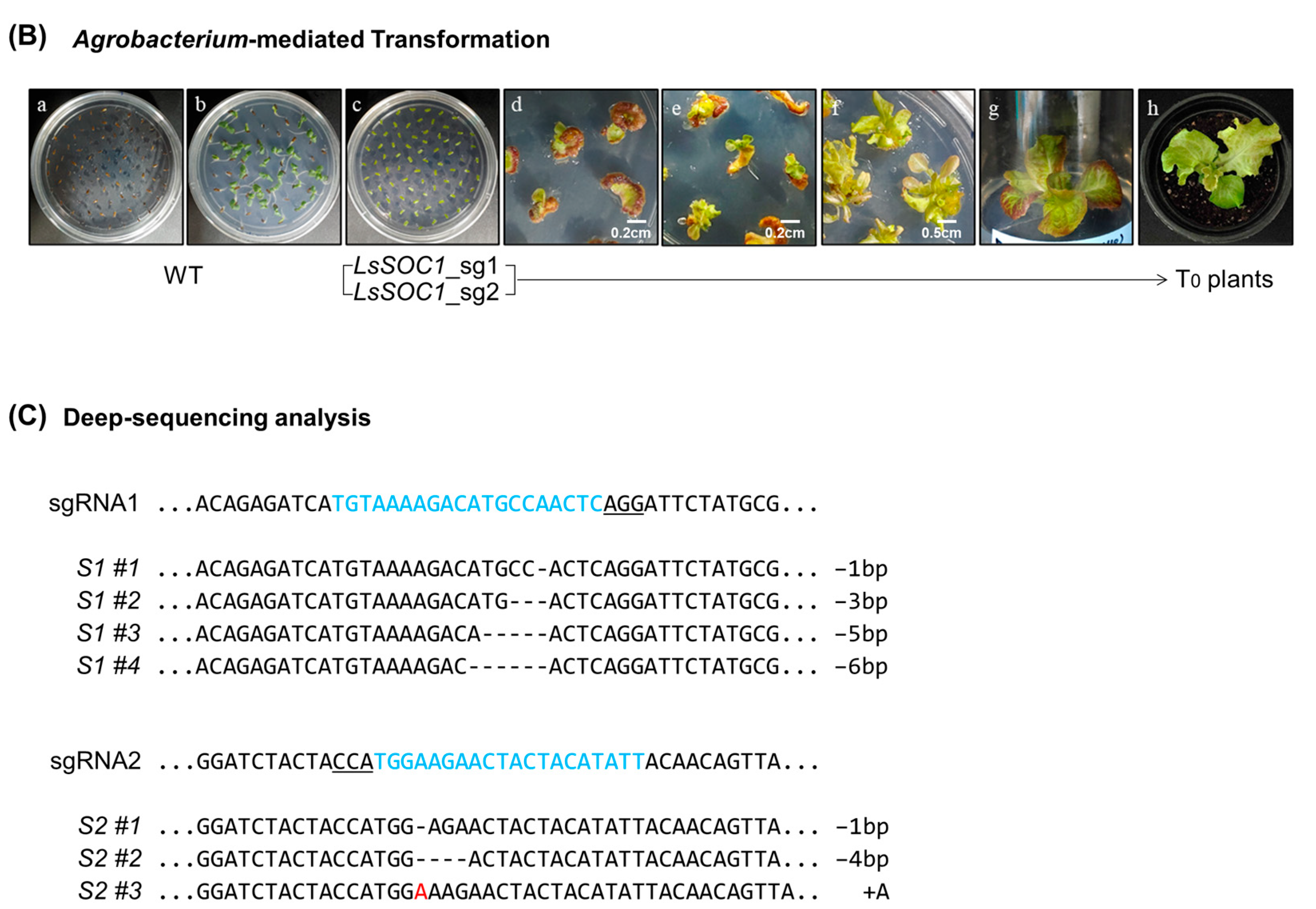
2.2. CRISPR/Cas9-Mediated Editing of LsSOC1
2.3. Selection of Transgenic Lines and Isolation of Null-Segments
2.4. Phenotypic Characterization and Bolting Assay
2.5. RNA-Sequencing
2.6. Anthocyanin Quantification
2.7. RNA Extraction and Quantitative Real-Time PCR (qRT-PCR)
2.8. Statistical Analysis
3. Results
3.1. Generation of LeSOC1 KO Lines via CRISPR/Cas9
3.2. Delayed Bolting and Reduced Stem Elongation in LsSOC1 KO Lines
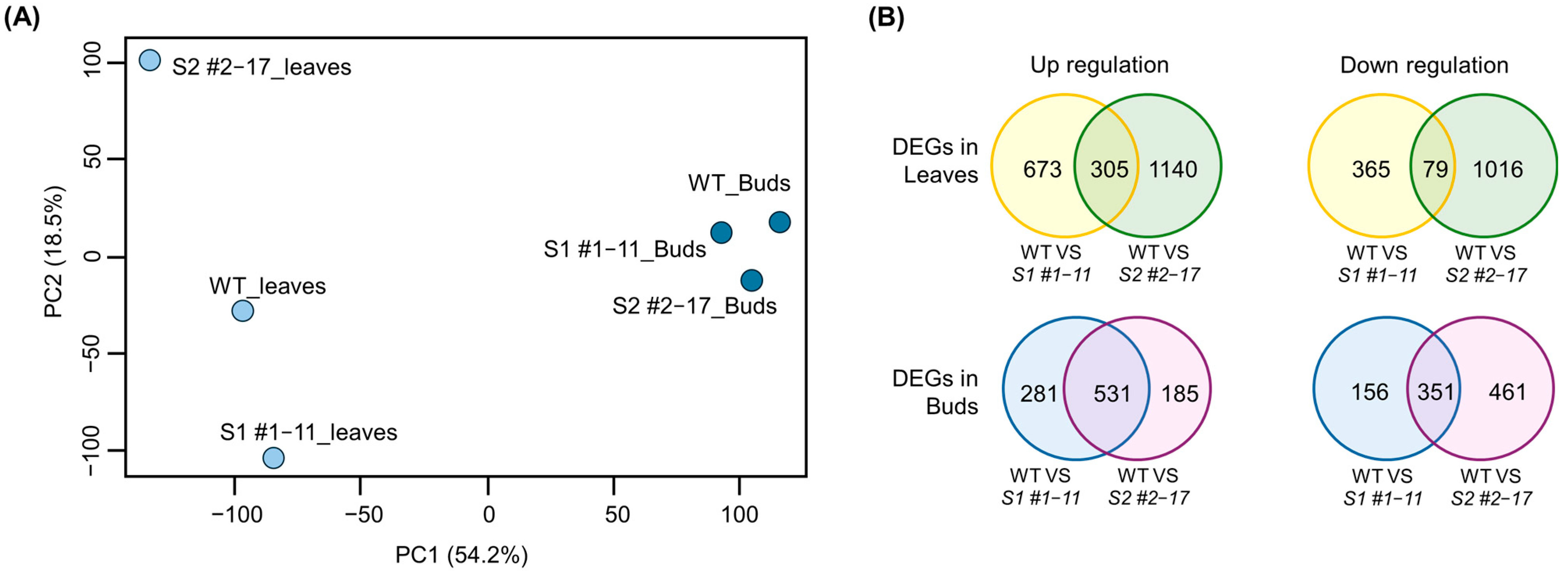
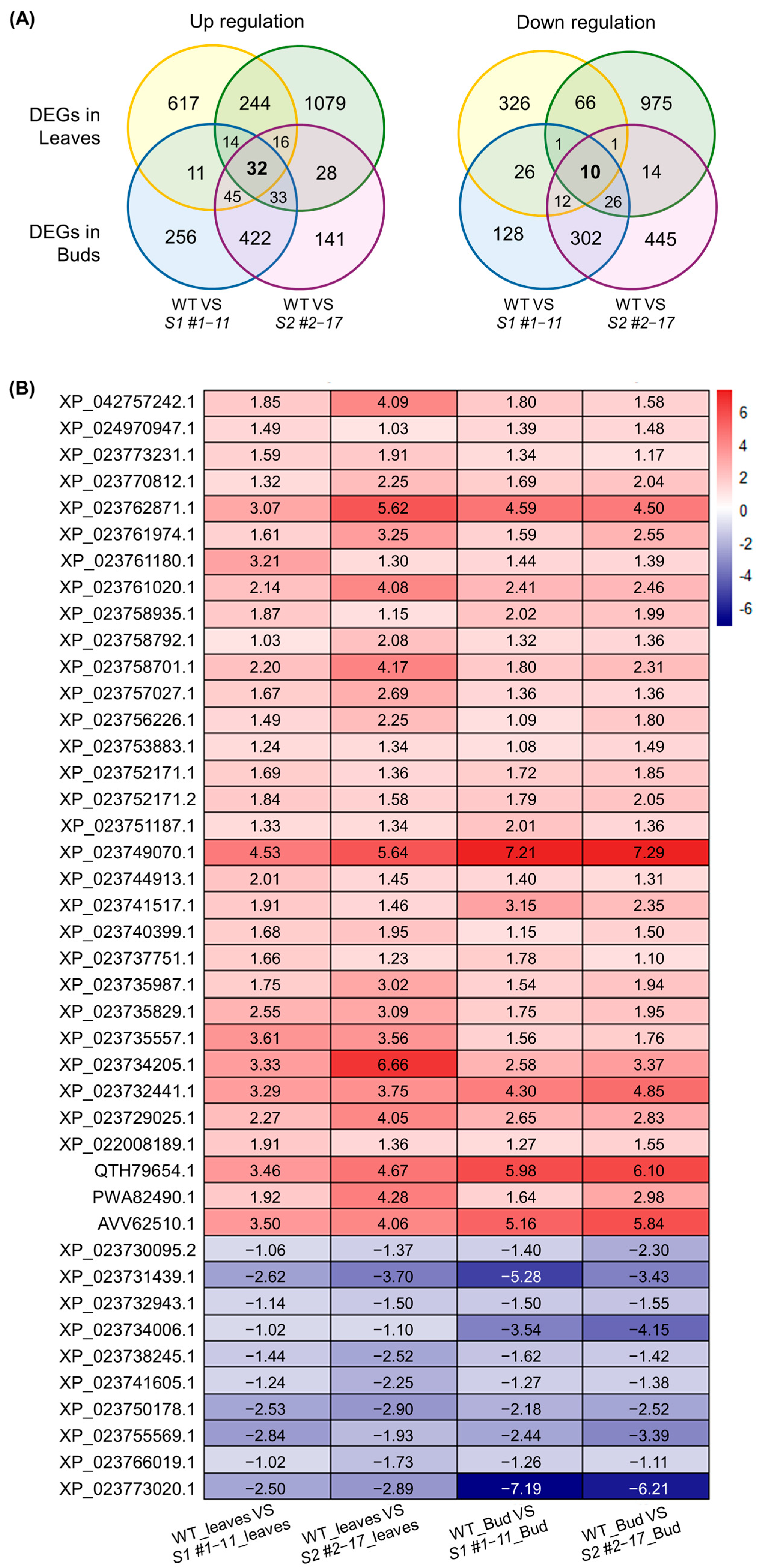
3.3. Transcriptome Analysis in LsSOC1 Knockout Lines
3.4. Consistent Transcriptomic Reprogramming in LsSOC1 Knockout Lettuce Reveals Enhanced Stress Responses and Repressed Floral Induction
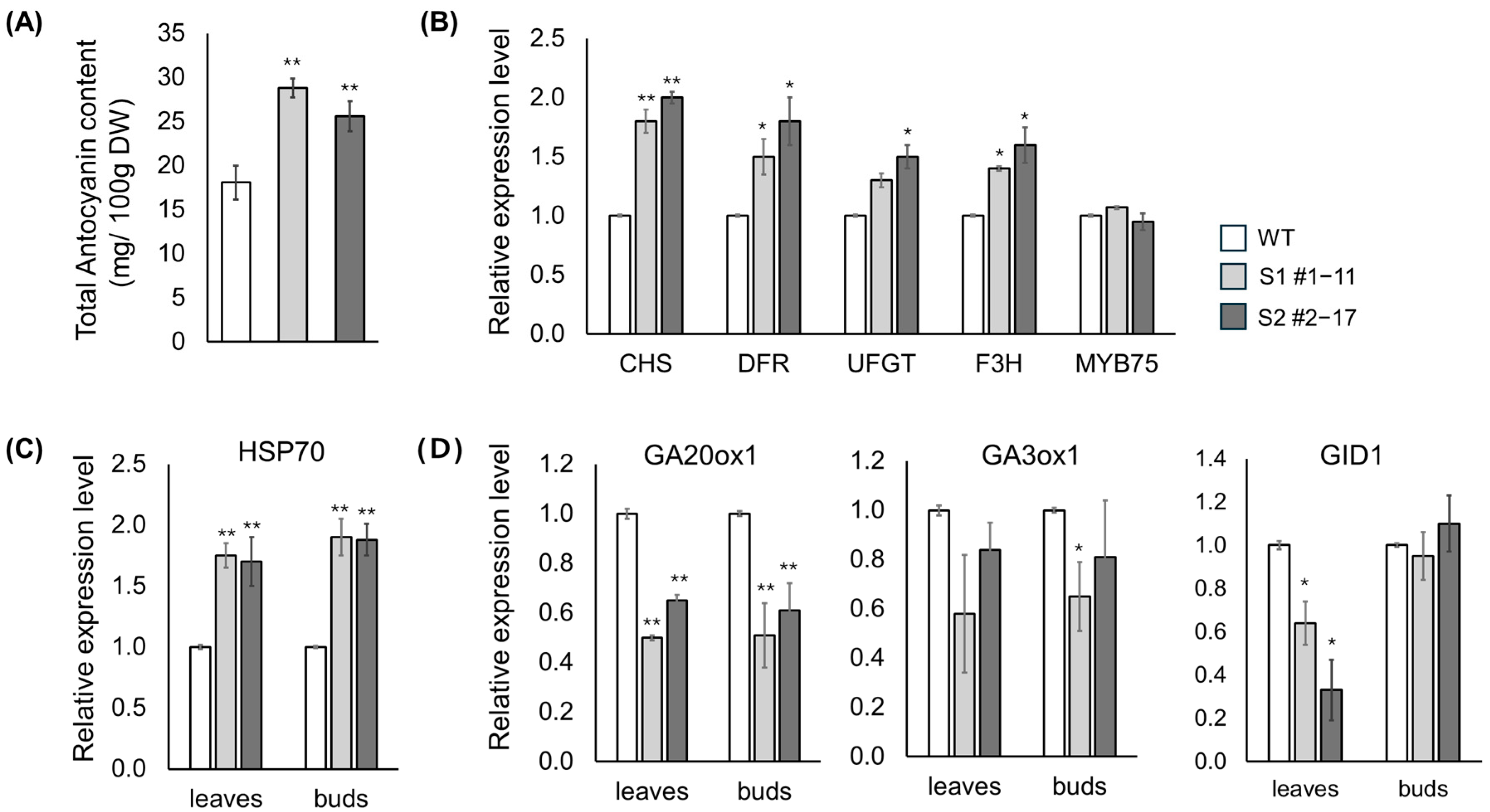
3.5. Validation of Anthocyanin Biosynthesis, GA Metabolism, and Heat-Responsive Gene Expression in LsSOC1 KO Lines
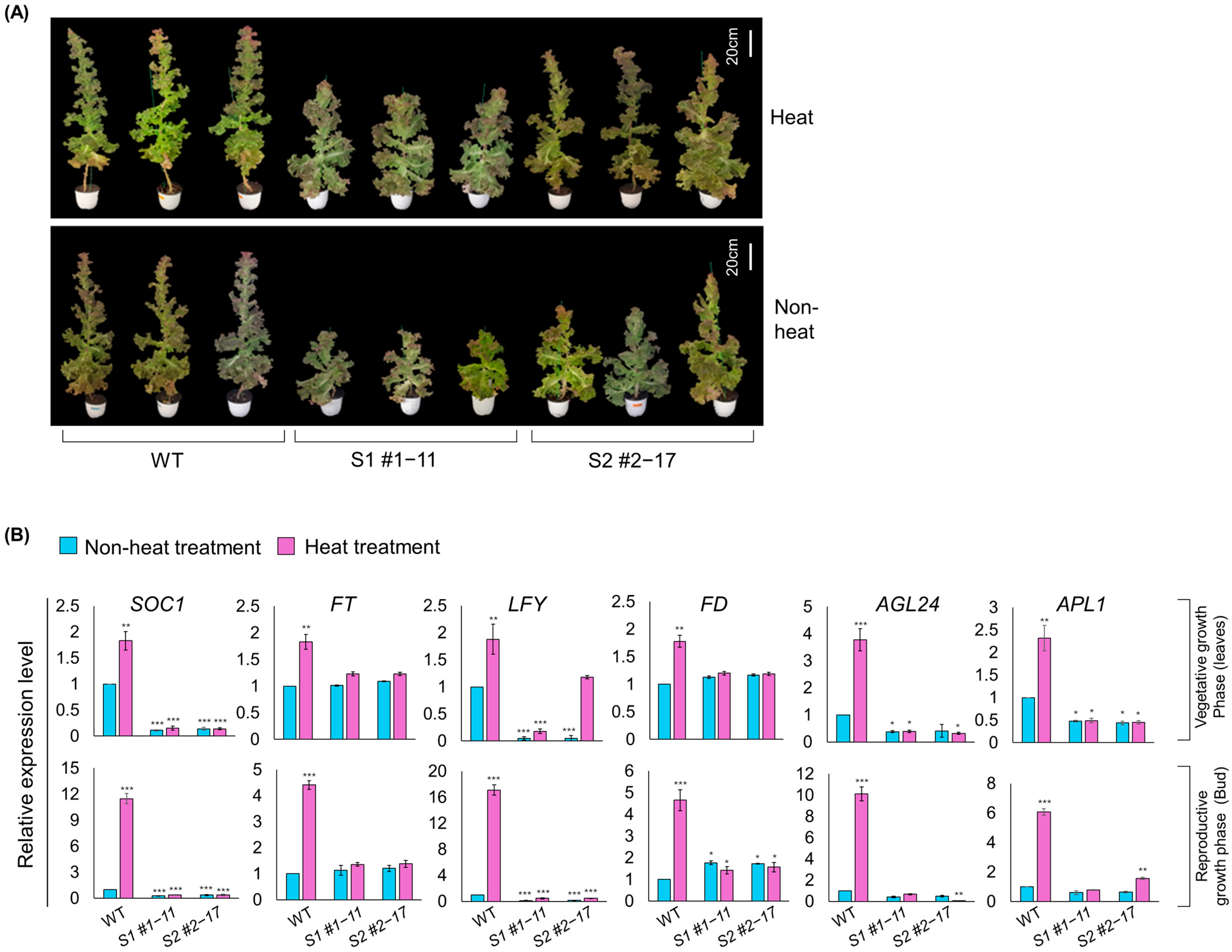
3.6. Functional Confirmation of Bolting Delay in LsSOC1 KO Lines Under High-Temperature Conditions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ryder, E.J. Lettuce: A Garden Heritage. HortScience 1999, 34, 715–719. [Google Scholar]
- Zhao, X.; Carey, E.E.; Wang, W. Influence of temperature and photoperiod on bolting in lettuce. J. Am. Soc. Hortic. Sci. 2007, 132, 492–496. [Google Scholar]
- Dufresne, C.J.; Wallace, D.H. Temperature-induced bolting in lettuce and spinach: A review. J. Am. Soc. Hortic. Sci. 1997, 122, 230–235. [Google Scholar]
- Zhu, Y.; Thakur, P.; Bai, Z.; Liang, Y.; Wang, H. Responses of plants to heat stress: Physiology, biochemical and molecular mechanisms. Plant Cell Rep. 2022, 41, 501–518. [Google Scholar] [CrossRef]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Han, Y.; Liu, X.; Benny, J.; Kim, H.J.; Park, E.J.; Kim, M.J.; Park, S.H.; Nou, I.S. High temperature accelerates lettuce bolting by inducing LsFT expression via LsELF3 degradation. Plant Physiol. 2021, 186, 1097–1110. [Google Scholar] [CrossRef]
- Bouché, F.; Lobet, G.; Tocquin, P.; Périlleux, C. Floral transition, gene networks and environmental interactions. Trends Plant Sci. 2016, 21, 378–388. [Google Scholar] [CrossRef]
- Moon, J.; Suh, S.S.; Lee, H.; Choi, K.; Hong, C.B.; Paek, N.-C.; Kim, S.-G.; Lee, I. The SOC1 MADS-box gene integrates vernalization and gibberellin signals for flowering in Arabidopsis. Plant J. 2003, 15, 635–646. [Google Scholar] [CrossRef]
- Lee, J.H.; Ryu, H.S.; Chung, K.S.; Pose, D.; Kim, S.; Schmid, M.; Ahn, J.H. Role of SVP in the control of flowering time by ambient temperature in Arabidopsis. Genes Dev. 2007, 21, 397–402. [Google Scholar] [CrossRef]
- Kim, D.H.; Doyle, M.R.; Sung, S.; Amasino, R.M. Vernalization: Winter and the timing of flowering in plants. Annu. Rev. Cell Dev. Biol. 2009, 25, 277–299. [Google Scholar] [CrossRef]
- Liu, C.; Teo, Z.W.N.; Bi, Y.; Song, S.; Xi, W.; Yang, X.; Yin, Z.; Yu, H. A conserved genetic pathway determines inflorescence architecture in Arabidopsis and rice. Dev. Cell 2013, 24, 612–622. [Google Scholar] [CrossRef]
- Joliffe, J.B.; Moser, C.; Pilati, S. The grapevine SOC1 homolog, VviMADS8/SOC1a, regulates floral organ specification in tomato. Fruit Res. 2024, 4, 703–715. [Google Scholar] [CrossRef]
- Tadege, M.; Sheldon, C.C.; Helliwell, C.A.; Stoutjesdijk, P.; Dennis, E.S.; Peacock, W.J. Control of flowering time by FLC orthologs in Brassica napus. Plant J. 2001, 28, 545–553. [Google Scholar] [CrossRef]
- Jung, C.; Müller, A.E. Flowering time control and applications in plant breeding. Trends Plant Sci. 2009, 14, 563–573. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, W.; Ge, D.; Han, Y.; Ning, K.; Luo, C.; Wang, S.; Lui, R.; Zhang, X.; Wang, Q. LCM-seq reveals the crucial role of LsSOC1 in heat-promoted bolting of lettuce (Lactuca sativa L.). Plant J. 2018, 95, 516–528. [Google Scholar] [CrossRef]
- Choi, S.H.; Ahn, W.S.; Jie, E.Y.; Cho, H.-S.; Kim, S.W. Development of late-bolting plants by CRISPR/Cas9-mediated genome editing from mesophyll protoplasts of lettuce. Plant Cell Rep. 2022, 41, 1627–1630. [Google Scholar] [CrossRef]
- Capovilla, G.; Pajoro, A.; Immink, R.G.H.; Angenent, G.C. The interplay of ambient temperature and flowering time. Trends Plant Sci. 2015, 20, 693–704. [Google Scholar] [CrossRef]
- Hedden, P.; Thomas, S.G. Gibberellin biosynthesis and its regulation. Biochem. J. 2012, 444, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Nakabayashi, R.; Yonekura-Sakakibara, K.; Urano, K.; Suzuki, M.; Yamada, Y.; Nishizawa, T.; Matsuda, F.; Kojima, M.; Sakakibara, H.; Shinozaki, K.; et al. Enhancement of oxidative and drought tolerance in Arabidopsis by overaccumulation of flavonoid biosynthesis products. Plant J. 2014, 77, 367–379. [Google Scholar] [CrossRef]
- Jung, Y.J.; Kang, K.K. Stable expression and characterization of brazzein, thaumatin and miraculin genes related to sweet protein in transgenic lettuce. J. Plant Biotechnol. 2018, 45, 257–265. [Google Scholar] [CrossRef]
- Jung, Y.J.; Lee, H.J.; Bae, S.; Kim, J.H.; Kim, D.H.; Kim, H.K.; Nam, K.H.; Nogoy, F.M.; Cho, Y.G.; Kang, K.K. Acquisition of seed dormancy breaking in rice (Oryza sativa L.) via CRISPR/Cas9-targeted mutagenesis of OsVP1 gene. Plant Biotechnol. Rep. 2019, 13, 511–520. [Google Scholar] [CrossRef]
- Park, J.; Lim, K.; Kim, J.S.; Bae, S. Cas-analyzer: An online tool for assessing genome editing results using NGS data. Bioinformatics 2016, 33, 286–288. [Google Scholar] [CrossRef] [PubMed]
- Ryder, E.J. Lettuce, Endive and Chicory; CABI Publishing: New York, NY, USA, 1999. [Google Scholar]
- Lafta, A.; Sandoya, G.; Mou, B. Genetic variation and genotype by environment interaction for heat tolerance in crisphead lettuce. HortScience 2021, 56, 126–135. [Google Scholar] [CrossRef]
- Lafta, A.; Sandoya, G.; Mou, B. Genetic variation and genotype by environment interaction for heat tolerance in crisphead lettuce. HortScience 2021, 56, 126–135. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization and Measurement of Anthocyanins by Uv-Visible Spectroscopy. In Current Protocols in Food Analytical Chemistry; Wiley: New York, NY, USA, 2001; pp. F1.2.1–F1.2.13. [Google Scholar] [CrossRef]
- Lee, J.; Lee, I. Regulation and function of SOC1, a flowering pathway integrator. J. Exp. Bot. 2010, 61, 2247–2254. [Google Scholar] [CrossRef]
- Moon, J.; Suh, S.S.; Lee, H.; Choi, K.R.; Hong, C.B.; Paek, N.C.; Kim, S.G.; Lee, I. The SOC1 MADS-box gene integrates vernalization and gibberellin signals for flowering in Arabidopsis. Plant J. 2003, 35, 613–623. [Google Scholar] [CrossRef]
- Ryu, C.H.; Lee, S.; Cho, L.H.; Kim, S.L.; Lee, Y.S.; Choi, S.C.; Jeong, H.J.; Yi, J.; Park, S.H.; Han, C.D.; et al. OsMADS50 and OsMADS56 function antagonistically in regulating long day (LD)-dependent flowering in rice. Plant Cell Environ. 2009, 32, 1412–1427. [Google Scholar] [CrossRef] [PubMed]
- Komiya, R.; Ikegami, A.; Tamaki, S.; Yokoi, S.; Shimamoto, K. Hd3a and RFT1 are essential for flowering in rice under long-day conditions. Plant Cell 2008, 20, 2960–2971. [Google Scholar] [CrossRef]
- Liu, X.; Lv, S.; Liu, R.; Fan, S.; Liu, C.; Liu, R.; Han, Y. Transcriptomic analysis reveals the roles of gibberellin-regulated genes and transcription factors in regulating bolting in lettuce (Lactuca sativa L.). PLoS ONE 2018, 13, e0191518. [Google Scholar] [CrossRef] [PubMed]
- Wigge, P.A.; Kim, M.C.; Jaeger, K.E.; Busch, W.; Schmid, M.; Lohmann, J.U.; Weigel, D. Integration of spatial and temporal information during floral induction in Arabidopsis. Science 2005, 309, 1056–1058. [Google Scholar] [CrossRef]
- Kotak, S.; Larkindale, J.; Lee, U.; von Koskull-Döring, P.; Vierling, E.; Scharf, K.D. Complexity of the heat stress response in plants. Curr. Opin. Plant Biol. 2007, 10, 310–316. [Google Scholar] [CrossRef]
- Dixon, D.P.; Lapthorn, A.; Edwards, R. Plant glutathione transferases. Genome Biol. 2002, 3, 3004.1. [Google Scholar] [CrossRef][Green Version]
- Winkel-Shirley, B. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 2001, 126, 485–493. [Google Scholar] [CrossRef]
- Nakashima, K.; Yamaguchi-Shinozaki, K. ABA signaling in stress-response and seed development. Plant Cell Rep. 2013, 32, 959–970. [Google Scholar] [CrossRef]
- Nakabayashi, R.; Saito, K. Integrated metabolomics for abiotic stress responses in plants. Curr. Opin. Plant Biol. 2015, 24, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Peer, W.A.; Bandyopadhyay, A.; Blakeslee, J.J.; Makam, S.N.; Chen, R.J.; Masson, P.H.; Murphy, A.S. Variation in expression and protein localization of the PIN family of auxin efflux facilitator proteins in flavonoid mutants with altered auxin transport in Arabidopsis thaliana. Plant Cell 2004, 16, 1898–1911. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, W.; Zhang, X.; Wang, L.; Jia, S.; Zhao, S.; Li, W.; Lu, R.; Ren, A.; Zhang, S. Transcriptomics and metabolomics analyses of Rosa hybrida to identify heat stress response genes and metabolite pathways. BMC Plant Biol. 2024, 24, 874. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.H.; Ito, S.; Imaizumi, T. Flowering time regulation: Photoperiod- and temperature-sensing in leaves. Trends Plant Sci. 2013, 18, 575–583. [Google Scholar] [CrossRef]
- Bouzroud, S.; Gouiaa, S.; Hu, N.; Bernadac, A.; Mila, I.; Bendaou, N.; Smouni, A.; Bouzayen., M.; Zouine, M. Auxin response factors (ARFs) are potential mediators of auxin action in tomato response to biotic and abiotic stress (Solanum lycopersicum). PLoS ONE 2018, 13, e0193517. [Google Scholar] [CrossRef]
- Ueguchi-Tanaka, M.; Ashikari, M.; Nakajima, M.; Itoh, H.; Katoh, E.; Kobayashi, M.; Chow, T.Y.; Hsing, Y.I.; Kitano, H.; Yamaguchi, I.; et al. Gibberellin Insensitive Dwarf1 encodes a soluble receptor for gibberellin. Nature 2005, 437, 693–698. [Google Scholar] [CrossRef]
- Tao, Z.; Shen, L.; Liu, C.; Liu, L.; Yan, Y.; Yu, H. Genome-wide identification of SOC1 and SVP targets during the floral transition in Arabidopsis. Plant J. 2012, 70, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Shen, L.; Gu, X.; Wang, Y.; Yu, H.; He, Y. Embryonic epigenetic reprogramming by a pioneer transcription factor in plants. Nature 2017, 551, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Long, H.; Qiu, F.; Wang, Y.; Zhang, M.; Chao, Y.; Chen, L. MADS-box protein MtSOC1c regulates flowering and seed development in Medicago truncatula. Ind. Crops Prod. 2023, 193, 116125. [Google Scholar] [CrossRef]
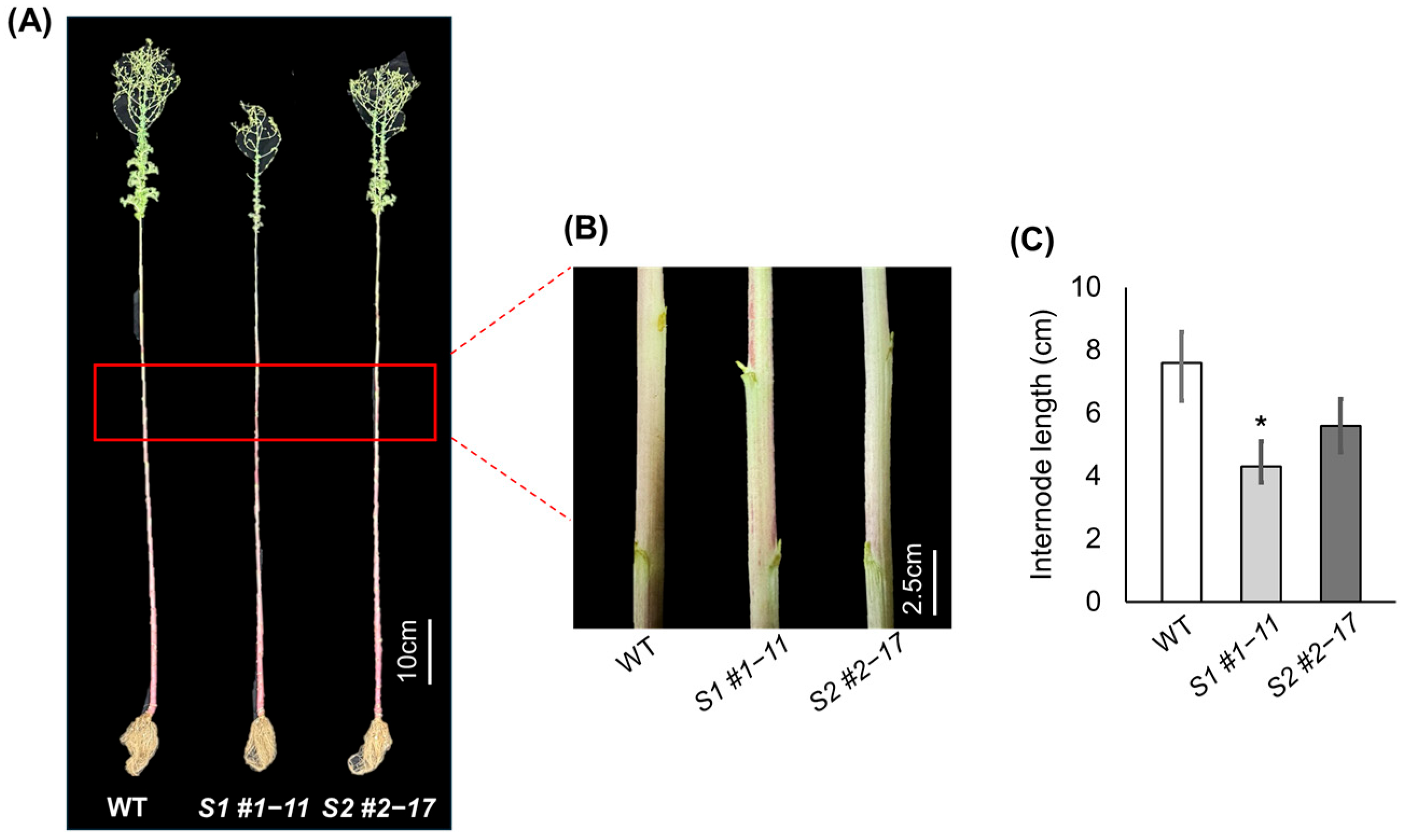
| Lines | Days to Bolting | Days to Flowering | Bolting Cumulative Temperature (°C) | Flowering Cumulative Temperature (°C) |
|---|---|---|---|---|
| WT | 106.67 ± 0.57 | 121.67 ± 1.53 | 1934.45 ± 8.96 | 2276.03 ± 90.93 |
| S1 #1–11 | 125.33 ± 1.57 *** | 139.67 ± 2.08 *** | 2332.41 ± 15.51 *** | 2675.12 ± 88.57 *** |
| S2 #2–17 | 115.67 ± 1.52 *** | 131.67 ± 1.53 ** | 2145.81 ± 8.01 *** | 2493.57 ± 20.77 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-Y.; Jang, Y.-H.; Kim, T.-S.; Jung, Y.-J.; Kang, K.-K. Loss of LsSOC1 Function Delays Bolting and Reprograms Transcriptional and Metabolic Responses in Lettuce. DNA 2025, 5, 40. https://doi.org/10.3390/dna5030040
Kim J-Y, Jang Y-H, Kim T-S, Jung Y-J, Kang K-K. Loss of LsSOC1 Function Delays Bolting and Reprograms Transcriptional and Metabolic Responses in Lettuce. DNA. 2025; 5(3):40. https://doi.org/10.3390/dna5030040
Chicago/Turabian StyleKim, Jin-Young, Young-Hee Jang, Tae-Sung Kim, Yu-Jin Jung, and Kwon-Kyoo Kang. 2025. "Loss of LsSOC1 Function Delays Bolting and Reprograms Transcriptional and Metabolic Responses in Lettuce" DNA 5, no. 3: 40. https://doi.org/10.3390/dna5030040
APA StyleKim, J.-Y., Jang, Y.-H., Kim, T.-S., Jung, Y.-J., & Kang, K.-K. (2025). Loss of LsSOC1 Function Delays Bolting and Reprograms Transcriptional and Metabolic Responses in Lettuce. DNA, 5(3), 40. https://doi.org/10.3390/dna5030040









