The Role of DNA in Neural Development and Cognitive Function
Abstract
1. Introduction
2. Role of Genes in Brain Formation
2.1. Genetic Instructions for Neural Progenitor Identity
2.2. Genes and Neuronal Differentiation
2.3. Genetic Regulation of Neuronal Subtype Specification
2.4. Genes Involved in Synaptic Plasticity and Neurodegeneration
3. Epigenetic Regulation
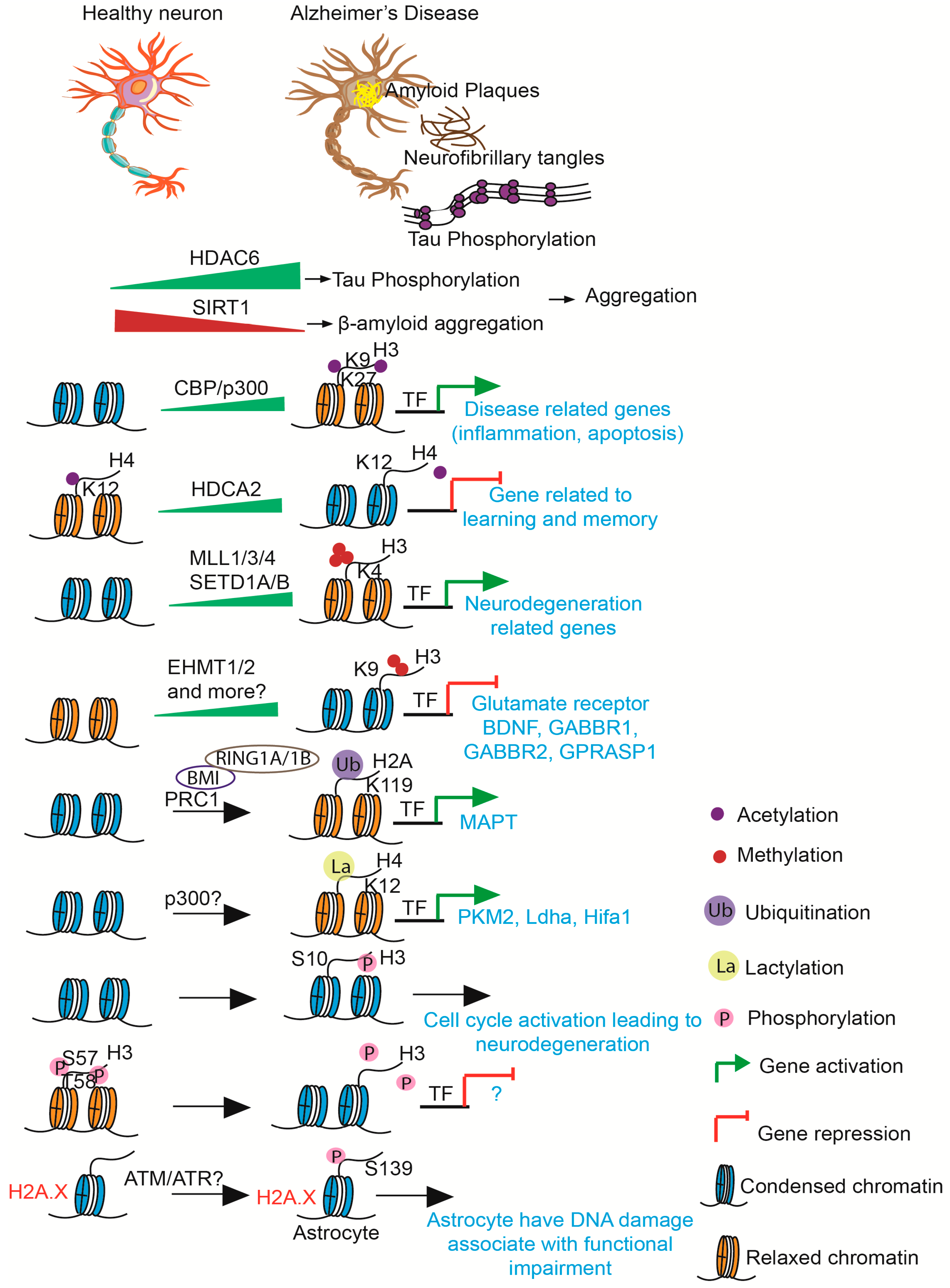
3.1. DNA Methylation and Histone Modifications
3.2. Role of Histone Modifications in Neural Lineage Differentiation and Synaptic Plasticity
3.3. Repetitive DNA Elements
3.4. Long Interspersed Nuclear Elements (L1) and Neural Progenitor Cells
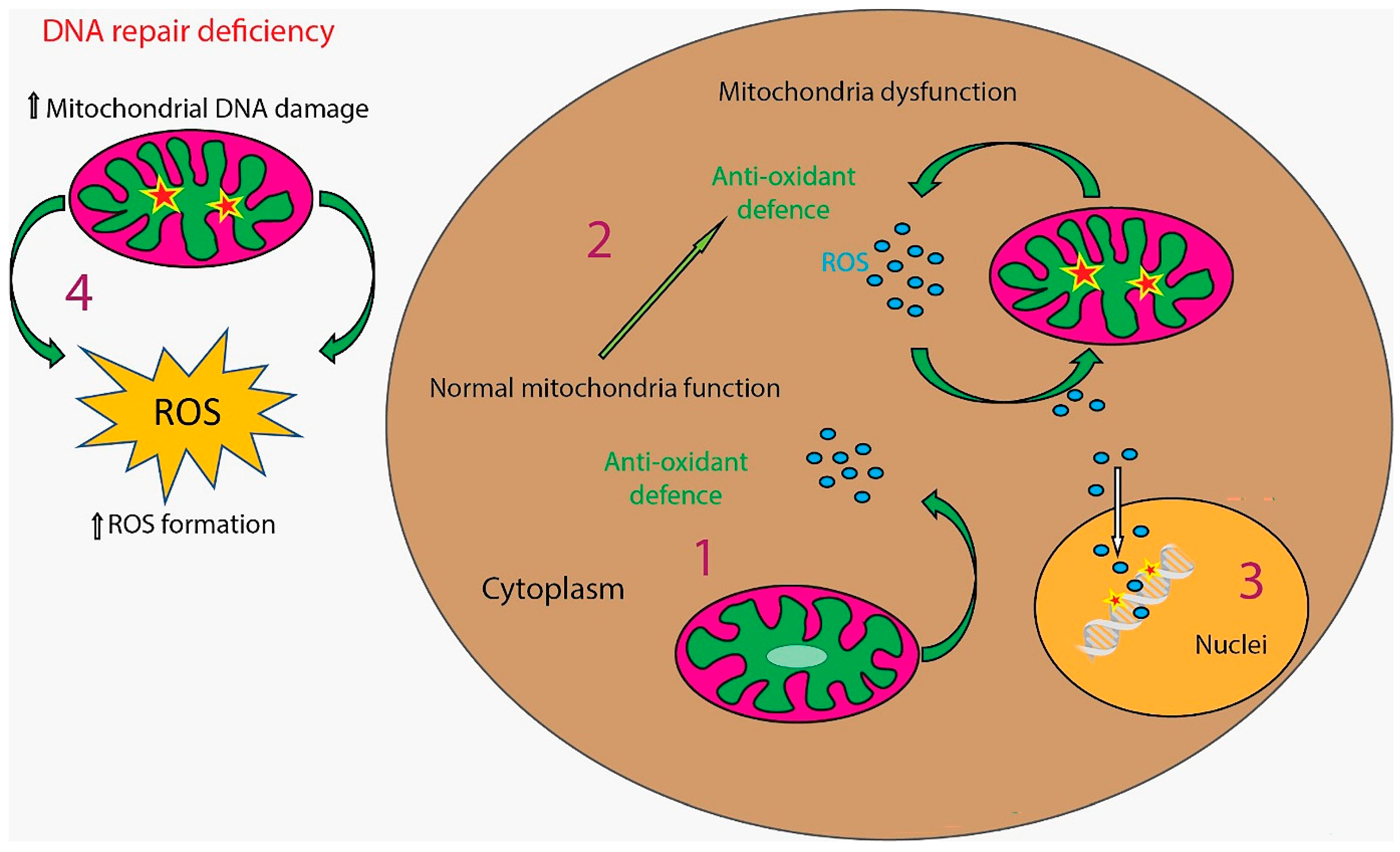
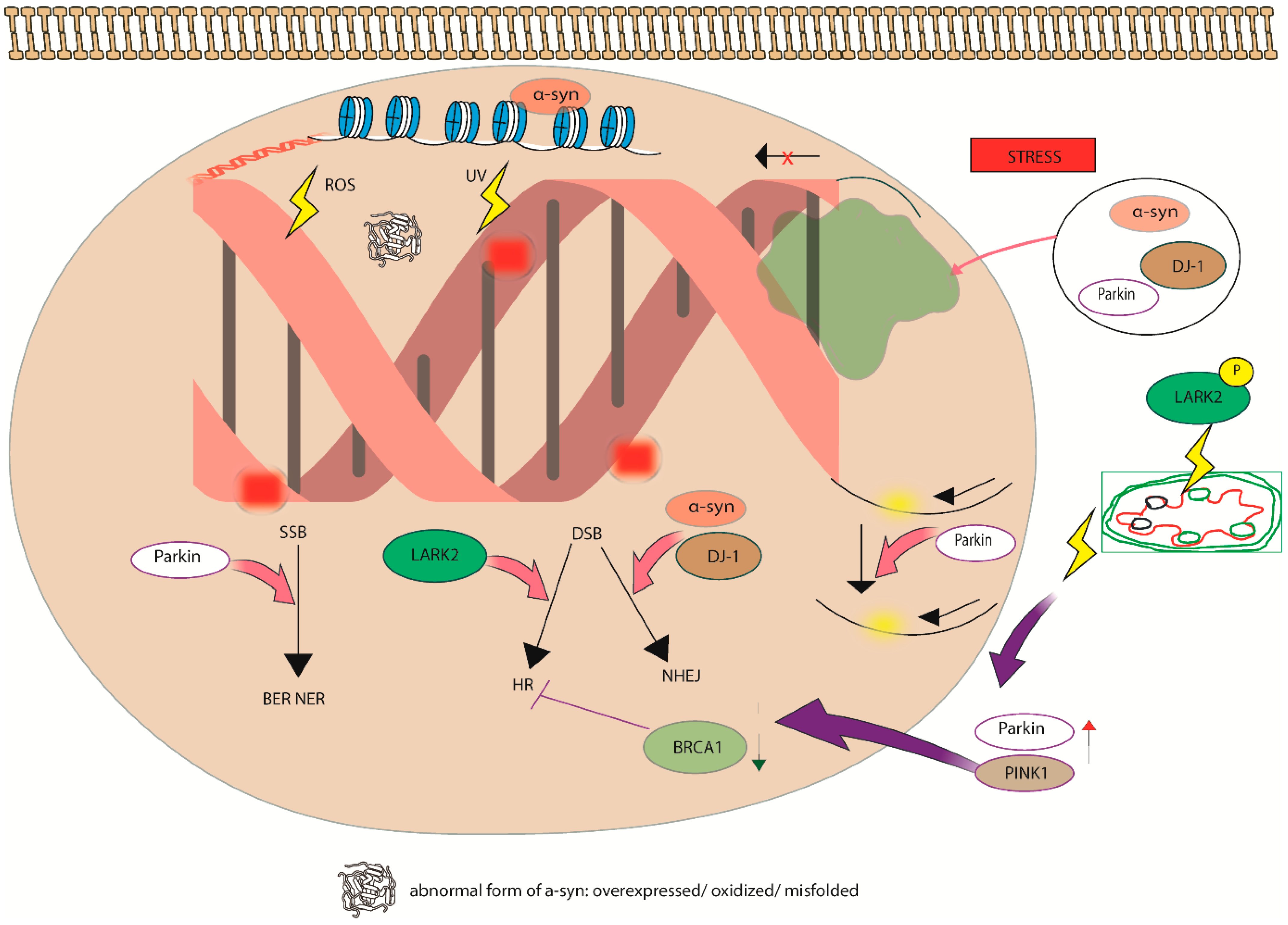
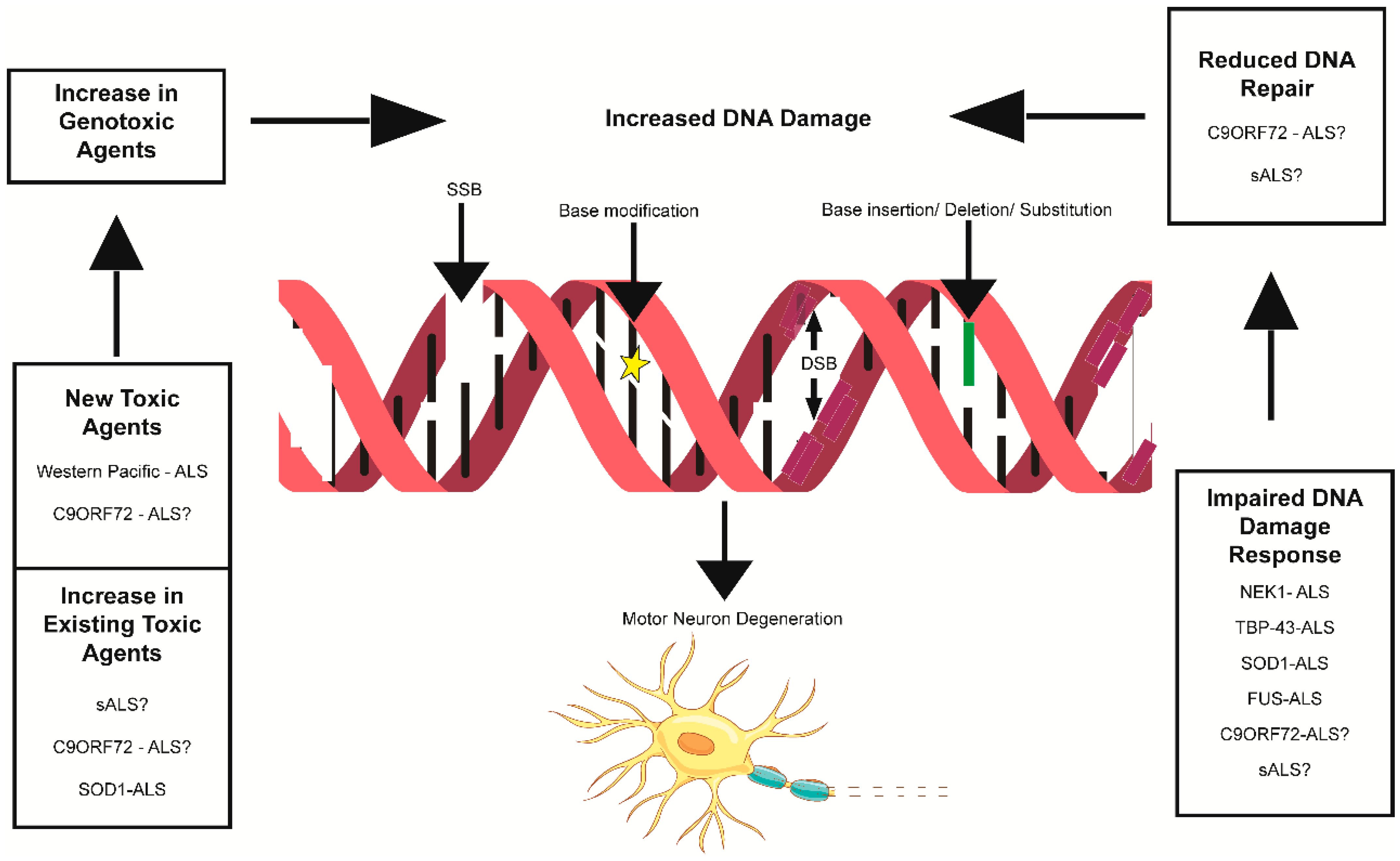
4. DNA Damage and Cellular Response
5. Cognitive Functions and Gene Expression
5.1. Influence of Genetic Factors on Cognition
5.2. Genetic Effects on Learning and Memory
5.3. Genetic and Environmental Contributions to Cognitive Functions
6. Pathophysiological Implications
7. Future Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| ALS | Amyotrophic Lateral Sclerosis |
| APOE | Apolipoprotein E |
| AT | axonal transport |
| BDNF | Brain-Derived Neurotrophic Factor |
| BER | base excision repair |
| bHLH | basic helix–loop–helix |
| CBP | CREB Binding Protein |
| CNS | central nervous system |
| COMT | Catechol O Methyltransferase |
| DNMTs | DNA methyltransferases |
| DSBs | double-strand breaks |
| fMRI | functional magnetic resonance imaging |
| GWAS | genome-wide association studies |
| HATs | histone acetyltransferases |
| HDACs | histone deacetylases |
| HR | homologous recombination |
| HMTs | histone methyltransferases |
| L1 | long interspersed nuclear elements |
| MeCP2 | methyl-CpG-binding protein 2 |
| MRN | MRE11 RAD50 NBS1 Complex |
| NDs | neurodegenerative diseases |
| Ngn | Neurogenin |
| NHEJ | non-homologous end joining |
| NPCs | neural progenitor cells |
| PFC | prefrontal cortex |
| Pol II | RNA polymerase II |
| PSEN1 | Presenilin 1 |
| QTL | quantitative trait locus |
| ROS | reactive oxygen species |
| SES | socioeconomic status |
| SSRs | simple sequence repeats |
| TEs | transposable elements |
| TSS | Transcription Start Site |
| mC | methylcytosine |
| hmC | hydroxymethylcytosine |
References
- Capella, J.; Telzer, E.H. A Framework for Integrating Neural Development and Social Networks in Adolescence. Dev. Cogn. Neurosci. 2024, 69, 101442. [Google Scholar] [CrossRef]
- Pan, Y.; Monje, M. Activity Shapes Neural Circuit Form and Function: A Historical Perspective. J. Neurosci. 2020, 40, 944–954. [Google Scholar] [CrossRef] [PubMed]
- Sporns, O.; Chialvo, D.R.; Kaiser, M.; Hilgetag, C.C. Organization, Development and Function of Complex Brain Networks. Trends Cogn. Sci. 2004, 8, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Cattane, N.; Richetto, J.; Cattaneo, A. Prenatal Exposure to Environmental Insults and Enhanced Risk of Developing Schizophrenia and Autism Spectrum Disorder: Focus on Biological Pathways and Epigenetic Mechanisms. Neurosci. Biobehav. Rev. 2020, 117, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Oldenburg, K.S.; O’Shea, T.M.; Fry, R.C. Genetic and Epigenetic Factors and Early Life Inflammation as Predictors of Neurodevelopmental Outcomes. Semin. Fetal Neonatal Med. 2020, 25, 101115. [Google Scholar] [CrossRef]
- Silbereis, J.C.; Pochareddy, S.; Zhu, Y.; Li, M.; Sestan, N. The Cellular and Molecular Landscapes of the Developing Human Central Nervous System. Neuron 2016, 89, 248–268. [Google Scholar] [CrossRef]
- Gilmore, J.H.; Knickmeyer, R.C.; Gao, W. Imaging Structural and Functional Brain Development in Early Childhood. Nat. Rev. Neurosci. 2018, 19, 123–137. [Google Scholar] [CrossRef]
- Bourgeron, T. From the Genetic Architecture to Synaptic Plasticity in Autism Spectrum Disorder. Nat. Rev. Neurosci. 2015, 16, 551–563. [Google Scholar] [CrossRef]
- Pescosolido, M.F.; Gamsiz, E.D.; Nagpal, S.; Morrow, E.M. Distribution of Disease-Associated Copy Number Variants Across Distinct Disorders of Cognitive Development. J. Am. Acad. Child Adolesc. Psychiatry 2013, 52, 414–430.e14. [Google Scholar] [CrossRef]
- Choi, G.B.; Yim, Y.S.; Wong, H.; Kim, S.; Kim, H.; Kim, S.V.; Hoeffer, C.A.; Littman, D.R.; Huh, J.R. The Maternal Interleukin-17a Pathway in Mice Promotes Autism-like Phenotypes in Offspring. Science 2016, 351, 933–939. [Google Scholar] [CrossRef]
- Schrott, R.; Song, A.; Ladd-Acosta, C. Epigenetics as a Biomarker for Early-Life Environmental Exposure. Curr. Environ. Health Rep. 2022, 9, 604–624. [Google Scholar] [CrossRef]
- Gartstein, M.A.; Skinner, M.K. Prenatal Influences on Temperament Development: The Role of Environmental Epigenetics. Dev. Psychopathol. 2018, 30, 1269–1303. [Google Scholar] [CrossRef]
- Xie, J.; Xie, L.; Wei, H.; Li, X.-J.; Lin, L. Dynamic Regulation of DNA Methylation and Brain Functions. Biology 2023, 12, 152. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Valnegri, P.; Yang, Y. Higher-Order Genome Organization in the Control of Neuronal Identity and Neural Circuit Plasticity. In Transcriptional Regulation by Neuronal Activity: To the Nucleus and Back; Springer Nature: Cham, Switzerland, 2024; pp. 251–274. [Google Scholar]
- Ali, H.H.; Fazil, H. Epigenetic Factors Affecting Children with Autism Spectrum Disorder: Perspectives of Therapeutic Staff. Remit. Rev. 2024, 9, 1678–1695. [Google Scholar]
- Acharjee, S.; Chauhan, S.; Pal, R.; Tomar, R.S. Mechanisms of DNA Methylation and Histone Modifications. Prog. Mol. Biol. Transl. Sci. 2023, 197, 51–92. [Google Scholar] [CrossRef]
- Bure, I.V.; Nemtsova, M.V.; Kuznetsova, E.B. Histone Modifications and Non-Coding RNAs: Mutual Epigenetic Regulation and Role in Pathogenesis. Int. J. Mol. Sci. 2022, 23, 5801. [Google Scholar] [CrossRef]
- Gagnidze, K.; Pfaff, D.W. Epigenetic Mechanisms: DNA Methylation and Histone Protein Modification. In Neuroscience in the 21st Century: From Basic to Clinical; Springer International Publishing: Cham, Switzerland, 2022; pp. 2677–2716. [Google Scholar]
- Lyko, F. The DNA Methyltransferase Family: A Versatile Toolkit for Epigenetic Regulation. Nat. Rev. Genet. 2018, 19, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Allison, J.; Kaliszewska, A.; Uceda, S.; Reiriz, M.; Arias, N. Targeting DNA Methylation in the Adult Brain through Diet. Nutrients 2021, 13, 3979. [Google Scholar] [CrossRef] [PubMed]
- Vaiserman, A.M. Epigenetic Programming by Early-Life Stress: Evidence from Human Populations. Dev. Dyn. 2015, 244, 254–265. [Google Scholar] [CrossRef]
- Leong, W.Y.; Lim, Z.H.; Korzh, V.; Pietri, T.; Goh, E.L.K. Methyl-CpG Binding Protein 2 (Mecp2) Regulates Sensory Function Through Sema5b and Robo2. Front. Cell. Neurosci. 2015, 9, 481. [Google Scholar] [CrossRef]
- Good, K.V.; Vincent, J.B.; Ausió, J. MeCP2: The Genetic Driver of Rett Syndrome Epigenetics. Front. Genet. 2021, 12, 620859. [Google Scholar] [CrossRef]
- Georgieff, M.K. Nutrition and the Developing Brain: Nutrient Priorities and Measurement. Am. J. Clin. Nutr. 2007, 85, 614S–620S. [Google Scholar] [CrossRef]
- Rao, R.; Tkac, I.; Townsend, E.L.; Gruetter, R.; Georgieff, M.K. Perinatal Iron Deficiency Alters the Neurochemical Profile of the Developing Rat Hippocampus. J. Nutr. 2003, 133, 3215–3221. [Google Scholar] [CrossRef]
- Beard, J.L.; Connor, J.R. Iron Status and Neural Functioning. Annu. Rev. Nutr. 2003, 23, 41–58. [Google Scholar] [CrossRef]
- Golub, M.; Takeuchi, P.; Keen, C.; Gershwin, M.; Hendrickx, A.; Lonnerdal, B. Modulation of Behavioral Performance of Prepubertal Monkeys by Moderate Dietary Zinc Deprivation. Am. J. Clin. Nutr. 1994, 60, 238–243. [Google Scholar] [CrossRef]
- Penland, J.G.; Prohaska, J.R. Abnormal Motor Function Persists Following Recovery from Perinatal Copper Deficiency in Rats. J. Nutr. 2004, 134, 1984–1988. [Google Scholar] [CrossRef]
- SanGiovanni, J.P.; Parra-Cabrera, S.; Colditz, G.A.; Berkey, C.S.; Dwyer, J.T. Meta-Analysis of Dietary Essential Fatty Acids and Long-Chain Polyunsaturated Fatty Acids as They Relate to Visual Resolution Acuity in Healthy Preterm Infants. Pediatrics 2000, 105, 1292–1298. [Google Scholar] [CrossRef]
- Park, J.; Lee, K.; Kim, K.; Yi, S.-J. The Role of Histone Modifications: From Neurodevelopment to Neurodiseases. Signal Transduct. Target. Ther. 2022, 7, 217. [Google Scholar] [CrossRef] [PubMed]
- Morelli, G.; Della Valle, F.; Orlando, V. Epigenetics and Brain Plasticity: Back to Function. In Neurobiological and Psychological Aspects of Brain Recovery; Springer International Publishing: Cham, Switzerland, 2023; pp. 237–252. [Google Scholar]
- Santana, D.A.; Smith, M.d.A.C.; Chen, E.S. Histone Modifications in Alzheimer’s Disease. Genes 2023, 14, 347. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Kundu, S.; Singh, A.; Singh, S. Understanding the Role of Histone Deacetylase and Their Inhibitors in Neurodegenerative Disorders: Current Targets and Future Perspective. Curr. Neuropharmacol. 2022, 20, 158–178. [Google Scholar] [CrossRef] [PubMed]
- Pappalardo, X.G.; Barra, V. Losing DNA Methylation at Repetitive Elements and Breaking Bad. Epigenetics Chromatin 2021, 14, 25. [Google Scholar] [CrossRef]
- Huda, A.; Mariño-Ramírez, L.; Landsman, D.; Jordan, I.K. Repetitive DNA Elements, Nucleosome Binding and Human Gene Expression. Gene 2009, 436, 12–22. [Google Scholar] [CrossRef]
- Reichard, J.; Zimmer-Bensch, G. The Epigenome in Neurodevelopmental Disorders. Front. Neurosci. 2021, 15, 776809. [Google Scholar] [CrossRef]
- Jiang, Y.; Sun, D.; Zhu, Y.; Peng, W.; Weng, J.; Dong, S.; Li, J.; Chen, Q.; Ge, C.; Liao, L.; et al. Epigenetic Modulation of Short Interspersed Nuclear Elements Activity Influences Neural Precursor Cell Proliferation. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Shadfar, S.; Brocardo, M.; Atkin, J.D. The Complex Mechanisms by Which Neurons Die Following DNA Damage in Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 2484. [Google Scholar] [CrossRef] [PubMed]
- Gohil, D.; Sarker, A.H.; Roy, R. Base Excision Repair: Mechanisms and Impact in Biology, Disease, and Medicine. Int. J. Mol. Sci. 2023, 24, 14186. [Google Scholar] [CrossRef] [PubMed]
- Bohr, V.A.; Dianov, G.L. Oxidative DNA Damage Processing in Nuclear and Mitochondrial DNA. Biochimie 1999, 81, 155–160. [Google Scholar] [CrossRef]
- Qiu, S.; Huang, J. MRN Complex Is an Essential Effector of DNA Damage Repair. J. Zhejiang Univ. Sci. B 2021, 22, 31–37. [Google Scholar] [CrossRef]
- Zhang, W.; Lukacsovich, D.; Young, J.I.; Gomez, L.; Schmidt, M.A.; Martin, E.R.; Kunkle, B.W.; Chen, X.; O’Shea, D.M.; Galvin, J.E.; et al. DNA Methylation Signature of a Lifestyle-Based Resilience Index for Cognitive Health. Res. Sq. 2024. [Google Scholar] [CrossRef]
- Shao, J.; Wang, J.; Li, B.; Liu, C. Potential Roles of Telomeres and Telomerase in Neurodegenerative Diseases. Ageing Neurodegener. Dis. 2024, 4, 1. [Google Scholar] [CrossRef]
- Cordeiro, A.; Gomes, C.; Bicker, J.; Fortuna, A. Aging and Cognitive Resilience: Molecular Mechanisms as New Potential Therapeutic Targets. Drug Discov. Today 2024, 29, 104093. [Google Scholar] [CrossRef] [PubMed]
- Hanawalt, P.C.; Spivak, G. Transcription-Coupled DNA Repair: Two Decades of Progress and Surprises. Nat. Rev. Mol. Cell Biol. 2008, 9, 958–970. [Google Scholar] [CrossRef]
- AlKhatib, M.I.; Maslat, A.O.; Ali, E.A.-H.; Al-Saqqar, T.; Khalil, R. A Cross-Sectional Study on the Association of KIBRA Genetic Polymorphism with Episodic Memory in North Jordanian Adults. Open Access Maced. J. Med. Sci. 2023, 11, 200–209. [Google Scholar] [CrossRef]
- Voineskos, A.N.; Hawco, C.; Neufeld, N.H.; Turner, J.A.; Ameis, S.H.; Anticevic, A.; Buchanan, R.W.; Cadenhead, K.; Dazzan, P.; Dickie, E.W.; et al. Functional Magnetic Resonance Imaging in Schizophrenia: Current Evidence, Methodological Advances, Limitations and Future Directions. World Psychiatry 2024, 23, 26–51. [Google Scholar] [CrossRef]
- Rosli, N.R.; Anuar, T.S.; Adenan, M.I.; Janor, R.M.; Ahmad, R.; Teh, L.K.; Salleh, M.Z.; Bakar, S.H.A.; James, R.J. Association of COMT Polymorphism and Academic Achievement among Female Undergraduate Students. Sci. Lett. 2021, 15, 90–101. [Google Scholar] [CrossRef]
- Goldberg, T.E.; Weinberger, D.R. Genes and the Parsing of Cognitive Processes. Trends Cogn. Sci. 2004, 8, 325–335. [Google Scholar] [CrossRef]
- Uffelmann, E.; Huang, Q.Q.; Munung, N.S.; De Vries, J.; Okada, Y.; Martin, A.R.; Martin, H.C.; Lappalainen, T.; Posthuma, D. Genome-Wide Association Studies. Nat. Rev. Methods Primers 2021, 1, 59. [Google Scholar] [CrossRef]
- Spengler, M.; Gottschling, J.; Hahn, E.; Tucker-Drob, E.M.; Harzer, C.; Spinath, F.M. Does the Heritability of Cognitive Abilities Vary as a Function of Parental Education? Evidence from a German Twin Sample. PLoS ONE 2018, 13, e0196597. [Google Scholar] [CrossRef]
- Ghirardi, G.; Gil-Hernández, C.J.; Bernardi, F.; Van Bergen, E.; Demange, P. Interaction of Family SES with Children’s Genetic Propensity for Cognitive and Noncognitive Skills: No Evidence of the Scarr-Rowe Hypothesis for Educational Outcomes. Res. Soc. Stratif. Mobil. 2024, 92, 100960. [Google Scholar] [CrossRef] [PubMed]
- Hart, E.R.; Bailey, D.H.; Luo, S.; Sengupta, P.; Watts, T.W. Fadeout and Persistence of Intervention Impacts on Social-Emotional and Cognitive Skills in Children and Adolescents: A Meta-Analytic Review of Randomized Controlled Trials. Psychol. Bull. 2024, 150, 1207–1236. [Google Scholar] [CrossRef] [PubMed]
- Nouspikel, T. DNA Repair in Mammalian Cells: Nucleotide Excision Repair: Variations on Versatility. Cell. Mol. Life Sci. 2009, 66, 994–1009. [Google Scholar] [CrossRef]
- Tesco, G.; Lomoio, S. Pathophysiology of Neurodegenerative Diseases: An Interplay among Axonal Transport Failure, Oxidative Stress, and Inflammation? Semin. Immunol. 2022, 59, 101628. [Google Scholar] [CrossRef]
- Knezevic, E.; Nenic, K.; Milanovic, V.; Knezevic, N.N. The Role of Cortisol in Chronic Stress, Neurodegenerative Diseases, and Psychological Disorders. Cells 2023, 12, 2726. [Google Scholar] [CrossRef] [PubMed]
- Kalia, L.V.; Lang, A.E. Parkinson’s Disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Hardiman, O.; Al-Chalabi, A.; Chio, A.; Corr, E.M.; Logroscino, G.; Robberecht, W.; Shaw, P.J.; Simmons, Z.; Van Den Berg, L.H. Amyotrophic Lateral Sclerosis. Nat. Rev. Dis. Primers 2017, 3, 17071. [Google Scholar] [CrossRef]
- Taylor, J.P.; Brown, R.H.; Cleveland, D.W. Decoding ALS: From Genes to Mechanism. Nature 2016, 539, 197–206. [Google Scholar] [CrossRef]
- Block, M.L.; Calderón-Garcidueñas, L. Air Pollution: Mechanisms of Neuroinflammation and CNS Disease. Trends Neurosci. 2009, 32, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Zar, H.J.; Ferkol, T.W. The Global Burden of Respiratory Disease-Impact on Child Health: The Global Burden of Respiratory Disease. Pediatr. Pulmonol. 2014, 49, 430–434. [Google Scholar] [CrossRef]
- Parrón, T.; Requena, M.; Hernández, A.F.; Alarcón, R. Association between Environmental Exposure to Pesticides and Neurodegenerative Diseases. Toxicol. Appl. Pharmacol. 2011, 256, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Chin-Chan, M.; Navarro-Yepes, J.; Quintanilla-Vega, B. Environmental Pollutants as Risk Factors for Neurodegenerative Disorders: Alzheimer and Parkinson Diseases. Front. Cell. Neurosci. 2015, 9, 124. [Google Scholar] [CrossRef]
- Gao, X.; Chen, H.; Fung, T.T.; Logroscino, G.; Schwarzschild, M.A.; Hu, F.B.; Ascherio, A. Prospective Study of Dietary Pattern and Risk of Parkinson Disease. Am. J. Clin. Nutr. 2007, 86, 1486–1494. [Google Scholar] [CrossRef] [PubMed]
- Nabi, M.; Tabassum, N. Role of Environmental Toxicants on Neurodegenerative Disorders. Front. Toxicol. 2022, 4, 837579. [Google Scholar] [CrossRef]
- Kaur, G.; Rathod, S.S.S.; Ghoneim, M.M.; Alshehri, S.; Ahmad, J.; Mishra, A.; Alhakamy, N.A. DNA Methylation: A Promising Approach in Management of Alzheimer’s Disease and Other Neurodegenerative Disorders. Biology 2022, 11, 90. [Google Scholar] [CrossRef]
- Zheng, K.; Lyu, Z.; Chen, J.; Chen, G. 5-Hydroxymethylcytosine: Far Beyond the Intermediate of DNA Demethylation. Int. J. Mol. Sci. 2024, 25, 11780. [Google Scholar] [CrossRef]
- Xu, C.; Fu, X.; Qin, H.; Yao, K. Traversing the Epigenetic Landscape: DNA Methylation from Retina to Brain in Development and Disease. Front. Cell. Neurosci. 2024, 18, 1499719. [Google Scholar] [CrossRef]
- Meng, M.; Guo, Y.; Kuang, Z.; Liu, L.; Cai, Y.; Ni, X. Risk of Stroke Among Different Metabolic Obesity Phenotypes: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2022, 9, 844550. [Google Scholar] [CrossRef]
- Jackson, R.J.; Hyman, B.T.; Serrano-Pozo, A. Multifaceted Roles of APOE in Alzheimer Disease. Nat. Rev. Neurol. 2024, 20, 457–474. [Google Scholar] [CrossRef]
- DeBroff, J.; Omer, N.; Cohen, B.; Giladi, N.; Kestenbaum, M.; Shirvan, J.C.; Cedarbaum, J.M.; Gana-Weisz, M.; Goldstein, O.; Orr-Urtreger, A.; et al. The Influence of GBA and LRRK2 on Mood Disorders in Parkinson’s Disease. Mov. Disord. Clin. Pract. 2023, 10, 606–616. [Google Scholar] [CrossRef]
- Brooks, A.C.; Henderson, B.J. Systematic Review of Nicotine Exposure’s Effects on Neural Stem and Progenitor Cells. Brain Sci. 2021, 11, 172. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Fontan, F.; Reeves, B.; Tuaño, K.; Colakoglu, S.; D’ Agostino, L.; Banegas, R. Tobacco Use and Neurogenesis: A Theoretical Review of Pathophysiological Mechanism Affecting the Outcome of Peripheral Nerve Regeneration. J. Orthop. 2020, 22, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Salman, A.; Kantor, A.; McClements, M.E.; Marfany, G.; Trigueros, S.; MacLaren, R.E. Non-Viral Delivery of CRISPR/Cas Cargo to the Retina Using Nanoparticles: Current Possibilities, Challenges, and Limitations. Pharmaceutics 2022, 14, 1842. [Google Scholar] [CrossRef] [PubMed]

| Aspects of Brain Development | Genes Involved | Functions | References |
|---|---|---|---|
| Neural Development and Regional Patterning | Otx1, Otx2 | Anterior neural structure induction, central nervous system patterning, and location of midbrain–hindbrain boundary. | [11,12] |
| Identification of Neural Progenitors | Neurogenin (Ngn1, Ngn2), Mash1 (Ascl1) | Define regional progenitor identification, start neural fate programs, and trigger neural commitment. | [13,14] |
| Differentiation of Neurons | NeuroD, Math3, Ngn1 | Encourage subtype specification, activate differentiation programs, and facilitate the development of sensory neurons. | [15] |
| Subtype Specification of Neurons | Ngn2 + Olig2, Mash1 (Ascl1) | Use region-specific gene synergy to generate motor and GABAergic neurons. | [16,17] |
| Genes That Affect Brain Development and Function | BDNF, COMT, APOE | COMT controls the metabolism of dopamine; BDNF enhances synaptic strength and survival; and lipid transport and the risk of Alzheimer’s disease: APOE. | [18,19,20] |
| Aspects of Brain Development | Genes Involved | Functions | References |
|---|---|---|---|
| The Impact of Genetics on Cognition | KIBRA | Connected to memory and Alzheimer’s disease risk; examined with imaging genetics and GWAS. | [43] |
| Imaging-Based Gene Identification | Genetic imaging and QTL research | Relationships between gene variations, brain anatomies, and cognitive characteristics based on genotyping and neuroimaging data. | [44] |
| Genetic Influences on Memory and Learning | COMT, BDNF | In the prefrontal cortex (PFC), COMT modulates dopamine, while BDNF affects memory function and hippocampus development. | [45,54] |
| Brain Activity Dependent on Genes | COMT, BDNF variations | Interact with brain circuits to influence learning and memory encoding; GWAS and imaging genetics are used to study this. | [46] |
| Environmental and Genetic Interactions | Genetic predispositions + SES (socioeconomic status) | SES influences genetic expression, which affects cognitive outcomes; 50–70% of cognition is heritable. | [47,48] |
| SES and Cognitive Heritability | Social and economic aspects | Low SES restricts cognitive growth through constrained surroundings, while high SES increases genetic potential. | [49] |
| Aspects | Key Points/Genes | Findings/Implications | References |
|---|---|---|---|
| ND General Pathophysiology | Axonal transport (AT), neuroinflammation, and oxidative stress | Disturb neuronal homeostasis; AT abnormalities contribute to neurodegeneration; early identification may facilitate therapies. | [50] |
| Changes in Epigenetics in NDs | Methylation of DNA (5 mC, 5 hmC) | Establish progenitor identity, initiate neural development, and ascertain neuronal lineage. | [51,52,53] |
| Subtype Specification of Neurons | PSEN1, APOE (particularly APOE4) | Variants affect the start and development of disease and increase susceptibility. | [55] |
| Parkinson’s Disease Genetic Risk | LRRK2 and GBA | Variants raise vulnerability and have an impact on the onset and progression of disease. | [56] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
William Raja, T.R.; Udaiyappan, J.P.; Pillay, M. The Role of DNA in Neural Development and Cognitive Function. DNA 2025, 5, 37. https://doi.org/10.3390/dna5030037
William Raja TR, Udaiyappan JP, Pillay M. The Role of DNA in Neural Development and Cognitive Function. DNA. 2025; 5(3):37. https://doi.org/10.3390/dna5030037
Chicago/Turabian StyleWilliam Raja, Tharsius Raja, Janakiraman Pillai Udaiyappan, and Michael Pillay. 2025. "The Role of DNA in Neural Development and Cognitive Function" DNA 5, no. 3: 37. https://doi.org/10.3390/dna5030037
APA StyleWilliam Raja, T. R., Udaiyappan, J. P., & Pillay, M. (2025). The Role of DNA in Neural Development and Cognitive Function. DNA, 5(3), 37. https://doi.org/10.3390/dna5030037







