1. Introduction
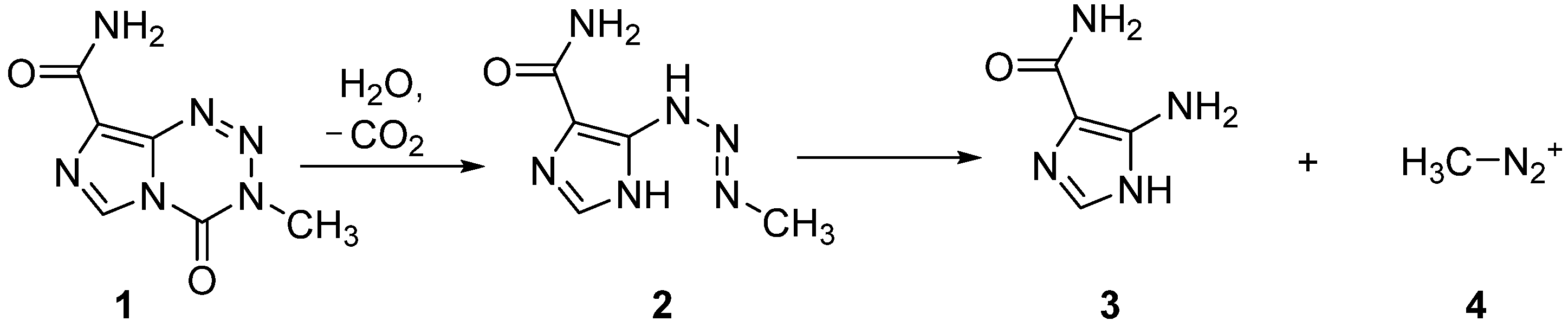
DNA alkylating agent temozolomide (
1) is an important drug used for the treatment of glioblastoma multiforme, the most common primary brain malignancy in adults [
1]. A dacarbazine derivative, temozolomide is a pro-drug [
2] that is converted by hydrolysis, under physiological conditions, to 5-(3-methyltriazen-1-yl)imidazole-4-carboxamide (
2) that is metabolised further to 5-aminoimidazole-4-carboxamide (
3) with concomitant release of electrophilic, methyl diazonium ion (
4) [
3] (
Figure 1). Temozolomide induces methylation at various sites within the DNA bases, particularly the middle guanine (G) in runs of three (GGG). Although O6-methylguanine is the least abundant of the resultant DNA adducts that are formed, it is this O6 methylation that is mainly responsible for the cytotoxic effect of temozolomide [
4].
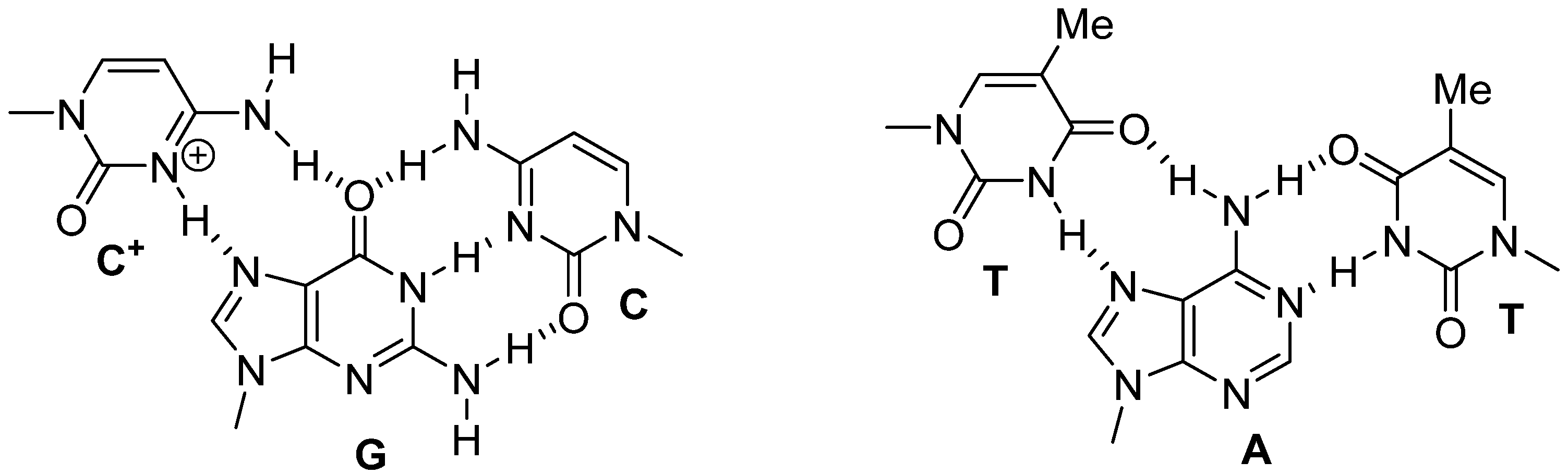
Covalent conjugation [
5] of temozolomide to triplex-forming oligonucleotides [
6] could facilitate better sequence discrimination when targeted to the middle guanine in runs of three, in DNA. Fewer off-target effects would potentially reduce the debilitating side-effects associated with conventional chemotherapy. Representative triplex structures are shown, whereby the Watson–Crick GC and AT DNA base pairs are targeted by protonated C
+ (
Figure 2, left) and T (
Figure 2, right), in the triplex-forming oligonucleotide.
The delivery of therapeutic oligonucleotides and other macromolecular entities to the brain presents challenges that have been addressed using various surgically non-invasive strategies including the covalent attachment to a blood–brain barrier-penetrating, ApoE-derived peptide [
7]. Efficient delivery strategies would therefore facilitate targeting of triplex-forming oligonucleotide conjugates of temozolomide to provide further treatment options for glioblastoma multiforme [
8].
Solid-supported synthesis persists as a preferred method for the preparation of therapeutic oligonucleotides [
9,
10]. Whilst robust under acidic conditions, temozolomide (
1) is hydrolytically unstable [
11] above physiological pH, thus rendering the drug incompatible with aqueous, basic cleavage conditions that are commonly used for solid-supported synthesis of oligonucleotides and their conjugates [
12].
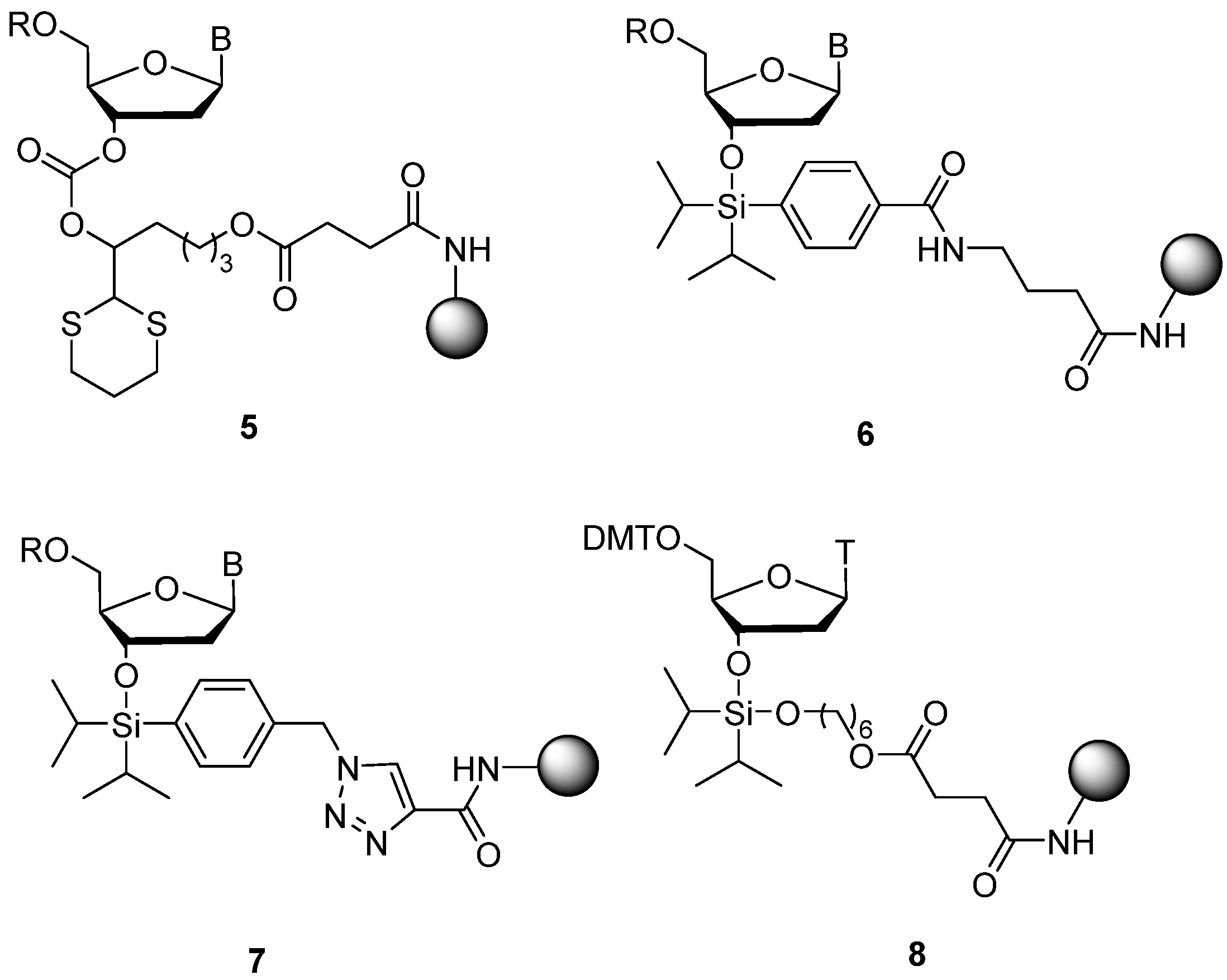
To facilitate solid-supported synthesis of base-sensitive oligodeoxynucleotides containing N4-acetyldeoxycytidine, N6-acetyladenosine N2-acetylguanosine, and N4-methyoxycarbonyldeoxycytidine, the 1,3-dithian-2-yl-methoxycarbonyl-linked solid support (
5) was prepared and shown to be cleavable under weakly basic, non-nucleophilic conditions [
13] (
Figure 3). Using sources of fluoride ion to facilitate cleavage, the phenyl di-
iso-propylsilyl linkers (
6) and (
7) have been successfully used to prepare base-sensitive oligonucleotides containing N4-acetyldeoxycytidine and other base-labile derivatives [
14,
15].
We reasoned that the presence of a second oxygen atom directly attached to silicon in the place of an aryl ring to give the di-iso-propylsilylene structure (
8) [
16] would allow cleavage under mild, acidic conditions [
17]. Here, we demonstrate the compatibility of long chain alkylamine controlled pore glass (LCAA-CPG) solid support (
8) with formation of a base-sensitive oligonucleotide conjugate of temozolomide (
26) and demonstrate its capability to engage in stable DNA triplex formation.
2. Materials and Methods
General. NMR spectra were recorded on a AC-250 spectrometer (Bruker, Rheinstetten, Germany) with
1H (250.1 MHz) spectra referenced to TMS,
13C spectra (62.9 MHz) referenced to CDCl
3 or (CD
3)
2SO, and
31P spectra (101.3 MHz) referenced to 85% H
3PO
4 (aq). Mass spectrometric analyses were carried out at NMSF (Swansea, UK) in EI
+ or CI
+ mode using a Quatro II (VG, Manchester, UK) or FAB
+ mode using a AutoSpec (VG, Manchester, UK) instrument or at Aston University in electrospray ionisation (ES) mode with a HP 5989B MS Engine (Hewlett-Packard, Palo Alto, CA, USA) apparatus using a HP 59987A API-electrospray LC/MS interface (Hewlett-Packard, CA, USA). Molecular weights of oligonucleotide sequences were determined by deconvolution of their multiple ion peaks. Infrared spectra were recorded using a Galaxy 2020 FT-IR Spectrophotometer (Mattson, Fremont, CA, USA). Ultraviolet spectra were recorded using a PU8730 Spectrophotometer (Unicam, Cambridge, UK). Melting points were measured on a Electrothermal Digital apparatus (Gallenkamp, Cambridge, UK) and are uncorrected. Flash column chromatography was performed using Sorbsil C60 silica (Fisher, Loughborough, UK) using the method described by Still, Kahn and Mitra [
18]. TLC was carried out on pre-coated 60 F
254 (Merck, Gillingham, UK) aluminium-backed plates and visualised using UV (254 and 366 nm) and vanillin reagent; vanillin (6.0 g), ethanol (250 mL), and conc. sulfuric acid (2 mL). Thermal UV analysis of oligonucleotides was conducted using a 1E UV-Visible Spectrophotometer (Varian Cary, Mulgrave, Australia) with a 12-sample heating block. Oligodeoxynucleotides were prepared on a Oligo 1000 DNA synthesizer (Beckman, Indianapolis, IN, USA) according to the manufacturer’s protocol using commercially available reagents (LINK Technologies, Bellshill, UK). Analysis and purification of oligonucleotides were performed by reversed-phase HPLC using gradient elution through C18 reversed-phase columns (250 mm × 4.6 mm) at a flow rate of 1 mL/min where solvent system A [1 M aqueous triethylammonium acetate (10%) and acetonitrile (2%) at pH 7.0] was mixed with solvent system B [1 M aqueous triethylammonium acetate (10%) and acetonitrile (80%) at pH 7.0]. The unmodified DNA sequences prepared for triplex studies were 20-mer 3′-dCCCCCTTTCTTTTCTCTCTT (
27); t
R 13.3 min; ESMS (MW 5885.7 calcd for C
191H
252N
49O
129P
19, found 5884.0); 20-mer 5′-dGGGGGAAAGAAAAGAGAGAA (
28) t
R 12.3 min, ESMS (MW calcd for C
200H
241N
100O
107P
19 6343.0, found 6345.1; 15-mer 5′-dTTTCTTTTCTCTCTT (
29) t
R 13.1 min, ESMS (MW calcd for C
146H
192N
34O
99P
14 4440.1, found 4438.7).
2.1. Preparation of 6-[bis(4-Methoxyphenyl)-phenyl-methoxy]hexan-1-ol (10)
To hexan-1,6-diol (6.20 g, 52.46 mmol), in dry pyridine (450 mL), under argon, a solution of DMT-Cl (15.98 g, 47.21 mmol) was added dropwise (4.5 h) with stirring overnight at room temperature. Methanol (100 mL) was added to the yellow product mixture, and the solvent evaporated under vacuum. To the residue, a mixture of hexane-ethyl acetate (1:1) (300 mL) containing triethylamine (5 mL) was added, and the resulting colourless precipitate was removed by filtration through Celite. The solvent was evaporated, and the residue purified by flash chromatography eluting with hexane-ethyl acetate (2:1) containing triethylamine (1%) to give the title compound (10) as a clear, yellow oil (11.40 g, 58%); TLC [hexane-ethyl acetate (2:1) containing triethylamine (1%)] Rf 0.31; IR (thin film): νmax 3388, 3060, 3027, 2953, 1606, 1096 cm−1; 1H NMR [(CD3)2SO]: δ 1.30–1.61 (m, 8H, 4 × CH2), 1.98 (s, 1H, OH), 3.03 (t, 2H, J 7.5 Hz, CH2O), 3.60 (t, 2H, J 7.5 Hz, CH2O), 3.78 (s, 6H, 2 × CH3O), 6.79 (d, 4H, J 8.3 Hz, 4 × CH (Ph)), 7.13–7.39 ppm (m, 9H, 9 × CH (Ar)); 13C NMR [(CD3)2SO]: δ 25.5, 26.1, 30.0, 32.6, 43.5, 55.1, 62.8, 63.2, 85.6, 112.9, 126.5, 127.6, 128.1, 129.9, 136.6, 145.3, 158.1 ppm; HRMS (calcd for [M+H] C27H33O4 421.230, found 421.339).
2.2. Preparation of 4-[6-[bis(4-Methoxyphenyl)-phenyl-methoxy]hexoxy]-4-oxo-butanoic Acid (11)
To alcohol (10), (672 mg, 1.60 mmol) succinic anhydride (176 mg, 1.76 mmol), DMAP (215 mg, 1.76 mmol), and 2,6-lutidine (8 mL) were added, and the mixture stirred (21 h) at room temperature. The product solution was diluted with dichloromethane (75 mL) and extracted with 3% (aq) citric acid (2 × 50 mL) followed by water (2 × 50 mL). The organic phase was dried (Mg2SO4), and the solvent evaporated. The residue was purified by flash column chromatography eluting sequentially with ethyl acetate-methanol 9:1, 4:1 and 2:1 to give product-containing fractions that were combined and concentrated to give the title product (11) as a hygroscopic, yellow viscous oil (564 mg, 63%); TLC [ethyl acetate-methanol (9:1)] Rf 0.54; IR (thin film): νmax 3395, 3030, 2950, 1715, 1605, 1450, 1089 cm−1; 1H NMR [(CD3)2SO]: δ 1.22–1.58 (m, 8H, 4 × CH2), 2.03 (s, 1H, OH), 2.57 (m, 4H, 2 × CH2CO), 3.01 (t, 2H, J 6.4 Hz, CH2O), 3.75 (s, 6H, 2 × CH3O), 4.03 (t, 2H, J 6.6 Hz, CH2O), 6.79 (d, 4H, J 8.8 Hz, 4 × CH (Ph)), 7.14–7.43 ppm (m, 9H, 9 × CH (Ar)); 13C NMR [(CD3)2SO]: δ 25.9, 26.3, 28.1, 30.0, 32.8, 55.0, 63.8, 65.0 80.9, 85.6, 111.9, 126.6, 127.7, 128.3, 129.8, 136.5, 145.4, 158.1, 158.4 ppm; HRMS (calcd for [M+H] C31H37O7 521.2461, found 521.2054).
2.3. Preparation of Di-iso-propylsilylene Linker (13)
To succinate (
11) (440 mg, 0.85 mmol) in DMF (10 mL), DhbtOH (207 mg, 1.27 mmol), DCC (201 mg, 1.27 mmol), DMAP (155 mg, 1.27 mmol), and LCAA-CPG (800 mg) were added, and the mixture was gently shaken (30 min) at room temperature. The mixture was filtered and washed successively with DMF (6 × 25 mL), methanol (3 × 25 mL), and diethyl ether (3 × 25 mL), and the solid dried to give the di-iso-propylsilylene linker (
12) with a loading of 68 µmol.g
−1 by trityl assay [
19]. Underivatised amino groups in linker (
12) were capped by acylation (30 min) using acetic anhydride (500 µL) and DMAP (125 mg, 1.02 mmol) in pyridine (10 mL) at room temperature. The linker (
12) was washed successively with methanol (3 × 25 mL) and diethyl ether (3 × 25 mL), after which it was subjected to detritylation (3 min) by gently shaking with dichloroacetic acid (3%) in dichloromethane (20 mL). The title product (
13) was filtered, washed successively with methanol (3 × 25 mL) and diethyl ether (3 × 25 mL), and dried to give the LCAA-CPG bound, di-iso-propylsilylene linker (
13) (999 mg).
2.4. Preparation of Di-iso-propylsilylene-linked Solid Support (8)
A solution of freshly sublimed imidazole (70 mg, 1.03 mmol) and bis-(trifluoromethanesulfonyl)diisopropylsilane (0.15 mL, 0.5 mmol) in mixture of anhydrous acetonitrile-DMF (1:1, 3 mL), was prepared (10 min) under argon atmosphere at −40 °C. To this solution, a solution 5′-O-DMT-2′-deoxythymidine (253 mg, 0.47 mmol) and imidazole (34 mg, 0.5 mmol) were added in anhydrous acetonitrile-DMF (1:1, 3 mL) in three portions (15 min). The mixture solution was allowed to warm to room temperature then added to a mixture of solid support (13) (450 mg, loading 55 µmol.g
−1), 2,6-lutidine (0.2 mL, 1.72 mmol), and DMAP (35 mg, 0.29 mmol) under argon. After gentle shaking (19 h), the solid support (8) was collected by filtration and washed successively with DMF (3 × 25 mL), methanol (3 × 25 mL), and diethyl ether (3 × 25 mL) to give the di-iso-propylsilylene-linked solid support (8) with a loading of 30 µmol.g
−1 based on trityl assay [
19].
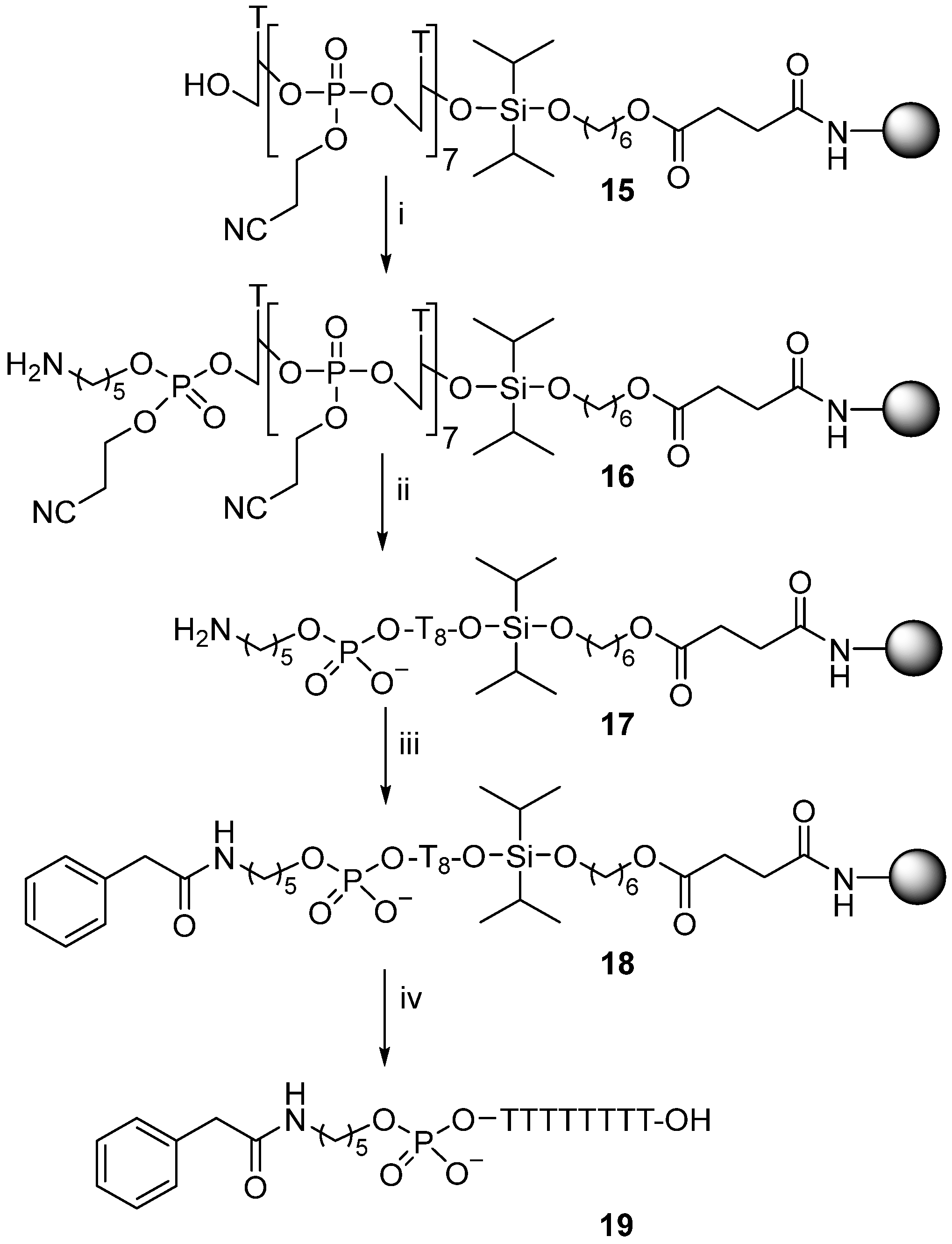
2.5. Preparation of Phenacetoylaminopentylphosphate-linked d[T8] Oligonucleotide Conjugate (19)
Support-bound oligonucleotide (15) was prepared (1 µmol scale) using 3-[(diisopropylamino)-[5-N-(4,4′demethoxytritylamino)pentoxy]phosphanyl]oxypropanenitrile (21) for attachment in the final coupling cycle with the synthesis concluded in “trityl-off” mode. This gave solid supported oligonucleotide conjugate (16) with appendant cyanoethyl-protecting groups that were removed (15.5 h) using tert-butylamine (1 mL) at room temperature. The solid support was filtered and washed with acetonitrile (3 × 1 mL) and diethyl ether (2 × 1 mL to give the support-bound conjugate (17). A solution of phenylacetic acid (16.3 mg, 120 µmol), PyBOP (0.62 mg, 120 µmol), and DIPEA (0.05 mL, 290 µmol) in DMF (0.30 mL) was prepared and allowed to stand (10 min) then added to the support-bound sequence (17), and the mixture was gently shaken (2 h). The solid support was filtered and washed successively with acetonitrile (2 × 1 mL), DMF (2 × 1 mL), acetonitrile (2 × 1 mL), and diethyl ether (2 × 1 mL), then treated (1 h) with TFA (2%) in dichloromethane (1 mL) to cleave oligonucleotide conjugate (18) from the solid support into solution. Product (19) was purified by reversed-phase HPLC; tR 16.6 min; ESMS (MW calcd for C93H124N17O58P8 2654.5, found 2654.0).
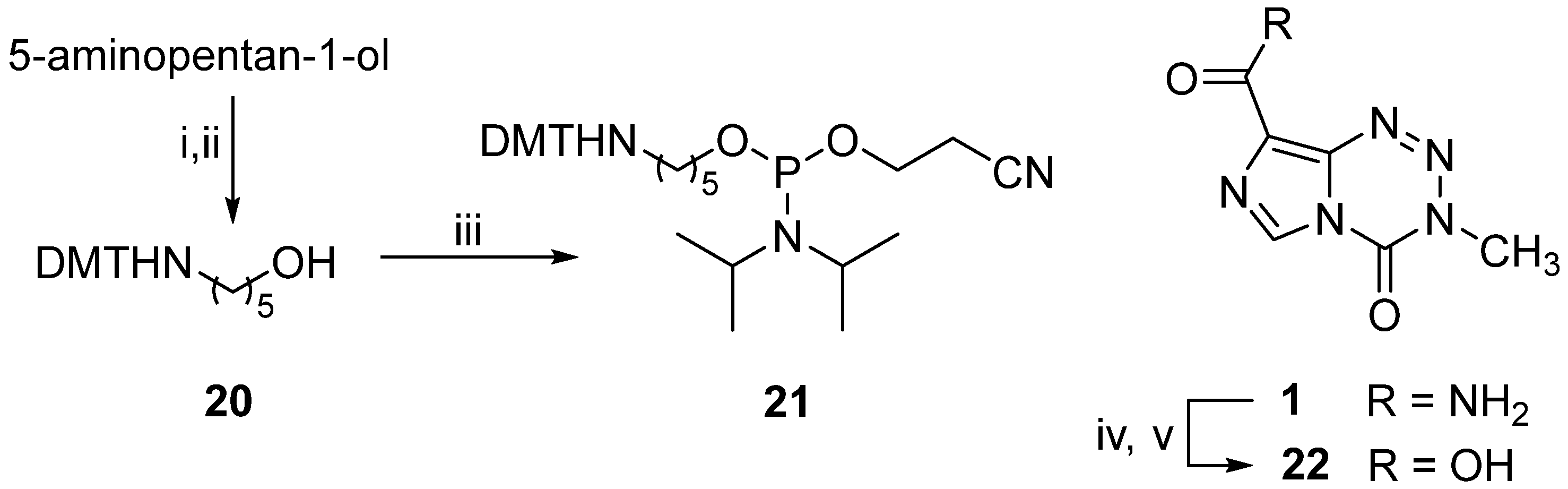
2.6. Preparation of 5-[[bis(4-Methoxyphenyl)-phenyl-methyl]amino]pentan-1-ol (20)
To 5-aminopentan-1-ol (0.5 mL, 4.60 mmol) in anhydrous pyridine (10 mL), trimethylsilyl chloride (1.75 mL, 13.79 mmol) was added at 0 °C under argon, and the mixture was allowed to warm (1 h) to room temperature. A solution of DMT chloride (1.55 g, 4.58 mmol) in anhydrous pyridine (5 mL) was then added, and the clear solution was stirred (12 h) at room temperature. Methanol (2 mL) was added, the solution was stirred (5 min), and the product mixture was concentrated by rotary evaporation. The residue was dissolved in ethyl acetate (40 mL) and washed with ice-cold, saturated (aq) sodium carbonate (50 mL), brine (50 mL), and water (50 mL), after which it was dried (Na2SO4). Evaporation of the solvent gave a yellow oil which was purified by flash chromatography, eluting with ethyl acetate-hexane-triethylamine (40:60:1) to give the title compound (20) as a viscous yellow oil (1.06 g, 57%); TLC [ethyl acetate-hexane-triethylamine (60:40:1)] Rf 0.56; IR (thin film): νmax 3600–3100, 3075, 2929, 1604, 1511 cm−1; 1H NMR [(CD3)2SO]: δ 1.16–1.40 (m, 6H, 3 × CH2), 1.94 (m, 2H, CH2NH), 2.37 (m, 1H, NH), 3.34 (t, 2H, J 6.3 Hz, CH2OH), 3.75 (s, 6H, 2 × CH3O), 4.32 (t, 1H, J 4.9 Hz, OH), 6.82 (d, 4H, J 8.3 Hz, 4 × CH (Ph)), 7.13–7.39 ppm (m, 9 H, 9 × CH (Ar)); 13C NMR [(CD3)2SO]: δ 23.5, 30.1, 32.6, 43.5, 55.0, 60.7, 69.4, 113.0, 125.8, 127.6, 128.2, 129.5, 138.6, 147.0, 157.3 ppm; HRMS (APCI+): Calcd for C26H31NO3: (M + H) 406.238. Found 406.238.
2.7. Preparation of 3-[(Diisopropylamino)-[5-N-(4,4′demethoxytritylamino)pentoxy]phosphanyl]oxypropanenitrile (21)
Alcohol (20) (0.28 g, 0.69 mmol) was dried by co-evaporation with anhydrous pyridine (3 × 3 mL) and dissolved in anhydrous tetrahydrofuran (4 mL) to which DIPEA (0.49 mL, 2.81 mmol) and 2-cyanoethyl-N,N-diisopropyl chlorophosphoramidite (0.23 mL, 1.03 mmol) were added with stirring under argon at room temperature. A precipitate formed (45 min), and the product mixture concentrated to an oil and was purified by flash chromatography eluting with ethyl acetate-hexane-triethylamine (25:75:1) to give the title compound (21) as a clear, yellow oil (0.37 g, 88%); TLC [ethyl acetate-hexane-triethylamine (25:75:1)]: Rf 0.27; IR (thin film): νmax 3054, 2962, 1604, 1506, 1459 cm−1; 1H NMR [(CD3)2SO]: δ 1.08–1.13 (m, 12H, 4 × CH3 (iso-Pr), 1.34–1.44 (m, 6H, 3 × CH2), 1.96 (m, 2H, CH2NH), 2.34 (t, 1H, J 7.8 Hz, NH), 2.72 (t, 2H, J 5.9 Hz, CH2CN), 3.49–3.70 (m, 12H, 2 × CH3O, 2 × NCH, POCH2CH2CN), 6.8–7.39 ppm (m, 13H, CH (Ar)); 13C NMR [(CD3)2SO]: δ 19.8, 23.4, 24.3, 24.4, 29.7, 30.7, 42.3, 42.5, 43.3, 54.9, 58.0, 63.0, 69.3, 112.9, 119.0, 125.7, 127.5, 128.1, 129.4, 138.5, 147.0, 157.2 ppm; 31P NMR ([(CD3)2SO]: δ 146.8 ppm; HRMS (FAB+): Calcd for C35H48N3O4P: (M + H) 606.346. Found 606.350.
2.8. Preparation of 3-Methyl-4-oxoimidazo [5,1-d]-1,2,3,5-tetrazine-8-carboxylic Acid (22)
Temozolomide (
1) (2.01 g, 0.01 mmol) was added slowly to concentrated sulfuric acid (50 mL) with stirring. Once dissolved, a solution of NaNO
2 (2.60 g, 0.04 mmol) in H
2O (4 mL) was added dropwise (50 min) with the temperature being kept below 60 °C. The resulting yellow solution was stirred (3 h) and poured onto ice to give a white precipitate that was filtered off and dried to give the title compound (
22) as a colourless solid (1.62 g, 80%); decomposed >170 °C (lit. [
20] dec. 177 °C);
1H NMR [(CD
3)
2SO]:
δ (s, 3H, NCH
3), 8.88 (s, 1 H, 6-CH), 13.5 ppm (br s, 1 H, CO
2H);
13C NMR [(CD
3)
2SO]:
δ 36.5, 127.8, 129.3, 136.7, 139.3, 162.0 ppm; MS (EI
+):
m/
z 195 (M
+, 2%), 138 (15%), 57 (100%).
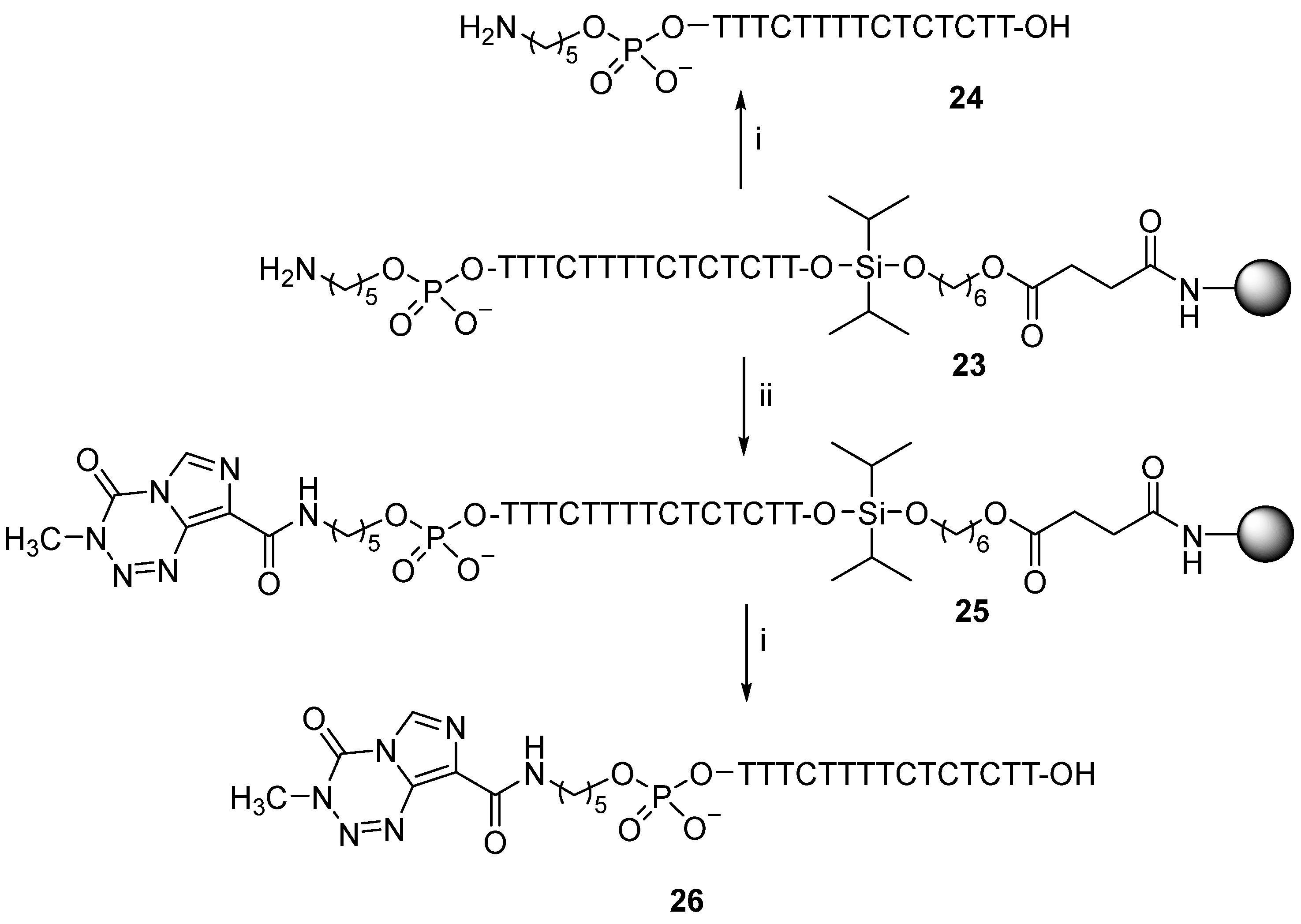
2.9. Preparation of the Aminopentylphosphate-linked d[TTTCTTTTCTCTCTT] Sequence (24)
Support-bound oligonucleotide (23) was prepared (1 µmol scale) using tert-BPA-protected C phosphoramidites and 3-[(diisopropylamino)-[5-N-(4,4′-dimethoxytritylamino)pentoxy]phosphanyl]oxypropanenitrile (21) for attachment in the final coupling cycle. This gave solid supported oligonucleotide conjugate (23) with appendant cyanoethyl and tert-BPA-protecting groups that were removed (15.5 h) using tert-butylamine (1 mL) at room temperature and with ethanolamine (1 mL) with vigorous shaking (30 min). The solid support (23) was washed with acetonitrile (2 × 1 mL), methanol (2 × 1 mL), and ethyl acetate, then treated (1 h) with TFA (2%) in dichloromethane (1 mL) to cleave oligonucleotide sequence (24) from the solid support into solution. Product (24) was purified by reversed-phase HPLC; tR 12.7 min; ESMS (MW calcd for C150H204N35O103P15 4607.8, found 4606.5).
2.10. Preparation of the Temozolomide Conjugate of Aminopentylphosphate-linked d[TTTCTTTTCTCTCTT] Sequence (26)
A solution of 3-methyl-4-oxoimidazo[5,1-d]-1,2,3,5-tetrazine-8-carboxylic acid (22) (0.76 mg, 3.9 µmol), PyBOP (2.03 mg, 3.9 µmol), DIPEA (1.7 µL, 9.7 µmol), and DMF (0.25 mL) was prepared and allowed to stand for 10 min then added to fully deprotected, solid supported oligonucleotide (23), and the mixture was gently shaken (2 h). The solid support (25) was filtered and washed successively with acetonitrile (2 × 1 mL), DMF (2 × 1 mL), acetonitrile (2 × 1 mL), and diethyl ether (2 × 1 mL), then treated (1 h) with TFA (2%) in dichloromethane (1 mL) to cleave oligonucleotide conjugate (25) from the solid support into solution. Product (26) was purified by reversed-phase HPLC; tR 16.5 min; ESMS (MW calcd for C156H206N40O105P15 4814.8, found 4812.7).
3. Results and Discussion
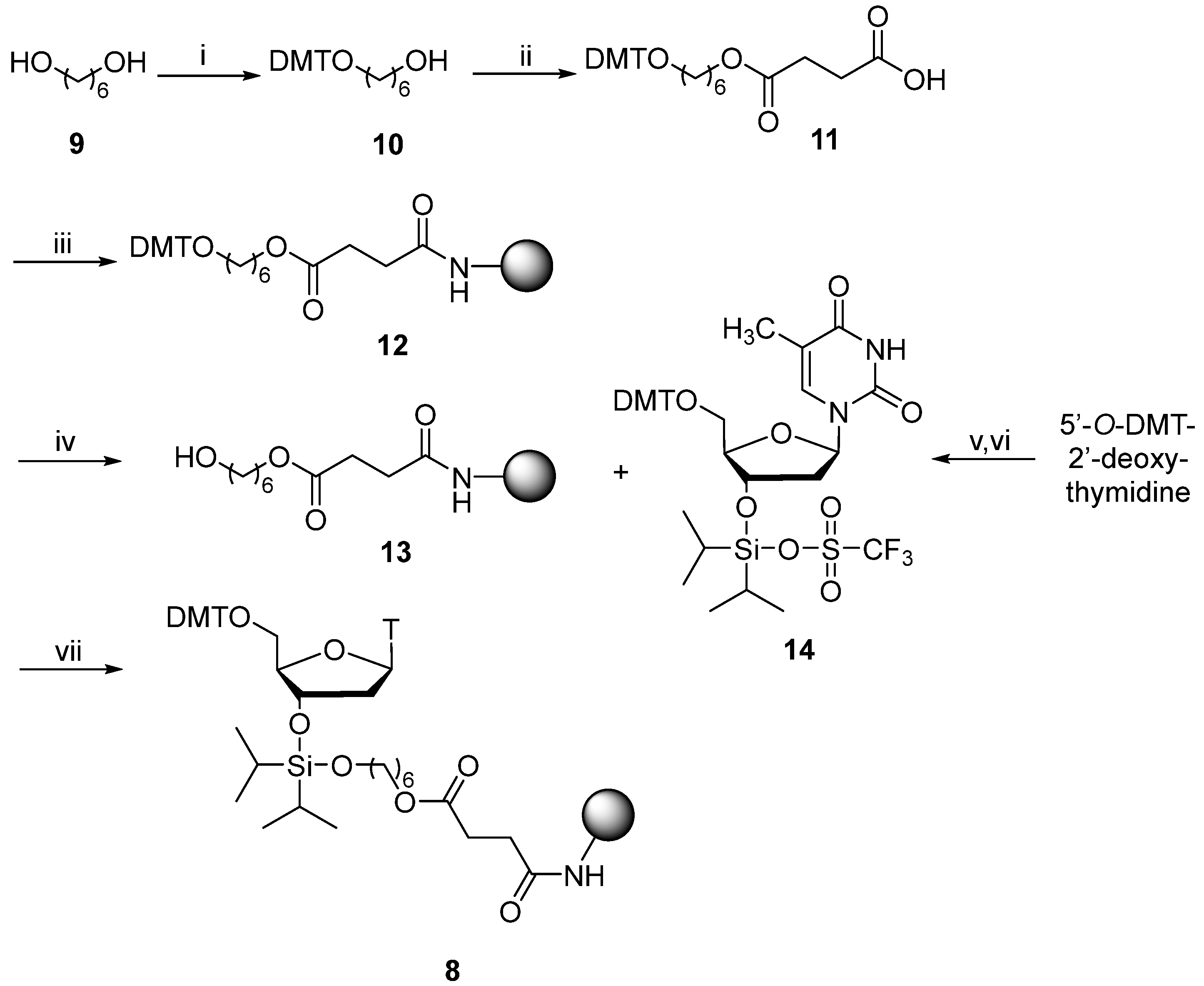
The di-iso-propylsilylene linker (
8) was prepared as shown in
Scheme 1. Firstly, 1,6-hexanediol (
9) was protected using DMT-Cl to give alcohol (
10) that was purified by flash column chromatography for subsequent conversion to the succinate (
11). Succinate (
11) was used to derivatise the primary amino groups of LCAA-CPG to give derivatised solid support (
12). Removal of the 5′-DMT-protecting group by brief (3 min) treatment with dichloroacetic acid (3%) in dichloromethane gave the primary alcohol (
13). This support-bound alcohol (
13) underwent derivatisation using triflate (
14) generated in situ by reacting 5′-
O-DMT-protected, 2′-deoxythymidine with
bis-(trifluoromethanesulfonyl)diisopropylsilane, to give the DMT-protected, di-iso-propylsilylene linker (
8) (
Scheme 1) with an acceptable nucleoside loading of 30 µmol.g
−1.
To establish suitable conditions to cleave di-iso-propylsilylene linker (
8), phenylacetic acid was used as a robust surrogate ligand for temozolomide to prepare and characterise conjugate (
19) (
Scheme 2).
Firstly, solid supported synthesis of the dT
8-containing oligomer (
15) was carried out. The final coupling cycle was concluded with attachment of the DMT-protected aminopentyl amidite (
21) (
Scheme 3) to give oligomer (
16) after removal of the 5′-DMT-protecting group. Care was needed to remove the cyanoethyl-protecting groups from phosphorus without inadvertent cleavage of the di-iso-propylsilylene linker. Thus, treatment of oligomer (
16) with
tert-butylamine gave the fully deprotected, support-bound oligonucleotide (
17) to which phenyl acetic acid, in the presence of PyBOP and DIPEA in DMF, was attached over 2 h, to form conjugate (
18).
Cleavage of the di-iso-propylsilylene linker in conjugate (18) was achieved within 1 h using 2% trifluoroacetic acid in dichloromethane to give the target conjugate (19), which was purified by reversed-phase HPLC (tR 16.6 min) and characterised by ESMS (MW calcd 2653.5, found 2654.0).
Having established reliable conditions for conjugate preparation, we carried out synthesis of the DNA triplex-forming oligonucleotide conjugate of temozolomide (
26) (
Scheme 4). Solid-phase synthesis of support-bound sequence (
23) was achieved whereby the necessary
tert-BPA-protecting groups on the cytosine bases were removed post-synthesis using ethanolamine after for removal of the cyanoethyl-protecting groups from the phosphate using
tert-butylamine. The di-iso-propylsilylene linker in solid-supported oligonucleotide (
25) was cleaved (2% TFA in CH
2Cl
2) to give C,T-containing, aminopentyl-linked oligonucleotide sequence (
24) that was purified by reversed-phase HPLC (t
R 12.7 min) and characterised by ESMS (MW calcd 4607.8, found 4606.5).
With conditions for the removal of the
tert-BPA-protecting groups from the C bases established, temozolomide oligonucleotide conjugate (
26) was prepared by reacting support-bound oligonucleotide (
23) with 21 molar equivalents of the carboxylic acid derivative of temozolomide (
22) (
Scheme 4) in the presence of PyBOP, DIPEA and DMF. The use of fewer than 21 molar equivalents of carboxylic acid (
22) failed to completely derivatise all the 5′-amino sites whereas use of more than 21 molar equivalents of carboxylic acid (
22) caused additional derivatisation of the amino group of the C nucleobases.
The oligonucleotide conjugate of temozolomide (26) was cleaved into solution on treatment (1 h) of solid-supported conjugate (25) with TFA (2%) in dichloromethane. Conjugate (26) which was purified by reversed-phase HPLC (tR 16.5 min) and characterised by ESMS (MW calcd 4814.8, found 4812.7).
DNA triplex-forming properties of the oligonucleotide conjugate of temozolomide (
26) and unconjugated oligonucleotide (
29) were determined by variable temperature UV analysis (
Table 1). The target DNA duplex was formed from equimolar amounts of polypyrimidine 20-mer sequence (
28) and polypurine 20-mer sequence (
29). The oligonucleotide conjugate of temozolomide (
26) formed a stable DNA triplex that was equal in stability to the triplex formed by the unconjugated sequence (
29). This demonstrated that drug conjugation was accommodated well and did not compromise DNA triplex-forming capability.