Direct Chromosome Preparation Method in Avian Embryos for Cytogenetic Studies: Quick, Easy and Cheap
Abstract
:1. Introduction
2. Materials and Methods
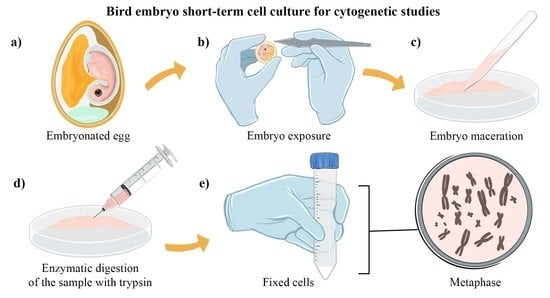
2.1. Bird Embryo Cell Culture Preparation
2.2. Fluorescent In Situ Hybridization (FISH)
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Degrandi, T.M.; Barcellos, S.A.; Costa, A.L.; Garnero, A.D.V.; Hass, I.; Gunski, R.J. Introducing the Bird Chromosome Database: An Overview of cytogenetic studies in birds. Cytogenet. Genome Res. 2020, 160, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Schartl, M.; Schmid, M.; Nanda, I. Dynamics of vertebrate sex chromosome evolution: From equal size to giants and dwarfs. Chromosoma 2016, 125, 553–571. [Google Scholar] [CrossRef] [PubMed]
- Schmid, M.; Nanda, I.; Guttenbach, M.; Steinlein, C.; Hoehn, M.; Schartl, M.; Haaf, T.; Weigend, S.; Fries, R.; Buerstedde, J.M.; et al. First report on chicken genes and chromosomes 2000. Cytogenet. Genome Res. 2000, 90, 169–218. [Google Scholar] [CrossRef] [PubMed]
- Romero, L.M.; Romero, R.C. Corticosterone responses in wild birds: The importance of rapid initial sampling. Condor 2002, 104, 129–135. [Google Scholar] [CrossRef]
- Brown, M.B.; Brown, C.R. Blood sampling reduces annual survival in cliff swallows (Petrochelidon pyrrhonota). Auk 2009, 126, 853–861. [Google Scholar] [CrossRef]
- Kretschmer, R.; Ferguson-Smith, M.A.; de Oliveira, E.H.C. Karyotype evolution in birds: From conventional staining to chromosome painting. Genes 2018, 9, 181. [Google Scholar] [CrossRef] [Green Version]
- Habermann, F.A.; Cremer, M.; Walter, J.; Kreth, G.; von Hase, J.; Bauer, K.; Wienberg, J.; Cremer, C.; Cremer, T.; Solovei, I. Arrangements of macro-and microchromosomes in chicken cells. Chromosome Res. 2001, 9, 569–584. [Google Scholar] [CrossRef]
- Nanda, I.; Schrama, D.; Feichtinger, W.; Haaf, T.; Schartl, M.; Schmid, M. Distribution of telomeric (TTAGGG) in sequences in avian chromosomes. Chromosoma 2002, 111, 215–227. [Google Scholar] [CrossRef]
- Itoh, Y.; Arnold, A.P. Chromosomal polymorphism and comparative painting analysis in the zebra finch. Chromosome Res. 2005, 13, 47–56. [Google Scholar] [CrossRef]
- Tsuda, Y.; Nishida-Umehara, C.; Ishijima, J.; Yamada, K.; Matsuda, Y. Comparison of the Z and W sex chromosomal architectures in elegant crested tinamou (Eudromia elegans) and ostrich (Struthio camelus) and the process of sex chromosome differentiation in palaeognathous birds. Chromosoma 2007, 116, 159–173. [Google Scholar] [CrossRef] [Green Version]
- Degrandi, T.M.; de Furo, I.O.; de Oliveira, E.H.C.; Costa, A.L.; Ferguson-Smith, M.A.; O’Brien, P.C.M.; Pereira, J.C.; Garnero, A.D.V.; Gunski, R.J.; Artoni, R.F. Comparative chromosome painting in hummingbirds (Trochilidae). Genet. Mol. Biol. 2020, 43, e20200162. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, B.S.; Kretschmer, R.; Gunski, R.J.; Garnero, A.D.V.; O’Brien, P.C.M.; Ferguson-Smith, M.; de Oliveira, E.H.C. Chromosome painting in Tyrant Flycatchers confirms a set of inversions shared by Oscines and Suboscines (Aves, Passeriformes). Cytogenet. Genome Res. 2017, 153, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Pigozzi, M.I.; Solari, A.J. Germ cell restriction and regular transmission of an accessory chromosome that mimics a sex body in the zebra finch, Taeniopygia guttata. Chromosome Res. 1998, 6, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Degrandi, T.M.; Gunski, R.J.; Garnero, A.D.V.; de Oliveira, E.H.C.; Kretschmer, R.; Santos, M.S.; Barcellos, S.A.; Hass, I. The distribution of 45S rDNA sites in bird chromosomes suggests multiple evolutionary histories. Genet. Mol. Biol. 2020, 43, e20180331. [Google Scholar] [CrossRef]
- Poignet, M.; Pokorná, M.J.; Altmanová, M.; Majtánová, Z.; Dedukh, D.; Albrecht, T.; Reif, J.; Osiejuk, T.S.; Reifová, R. Comparison of karyotypes in two hybridizing passerine species: Conserved chromosomal structure but divergence in centromeric repeats. Front. Genet. 2021, 12, 768987. [Google Scholar] [CrossRef]
- Pinheiro, M.L.S.; Nagamachi, C.Y.; Ribas, T.F.A.; Diniz, C.G.; O’Brien, P.C.M.; Ferguson-Smith, M.; Yang, F.; Pieczarka, J.C. Chromosomal painting of the sandpiper (Actitis macularius) detects several fissions for the Scolopacidae family (Charadriiformes). BMC Ecol. Evol. 2021, 21, 8. [Google Scholar] [CrossRef]
- Sasaki, M.; Ikeuchi, T.; Makino, S. A feather pulp culture technique for avian chromosomes, with notes on the chromosomes of the peafowl and the ostrich. Experientia 1968, 24, 1292–1293. [Google Scholar] [CrossRef]
- Garnero, A.D.V.; Gunski, R.J. Comparative analysis of the karyotype of Nothura maculosa and Rhynchotus rufescens (Aves: Tinamidae). A case of chromosomal polymorphism. Nucleus 2000, 43, 64–70. [Google Scholar]
- Bloom, S.E.; Buss, E.G. Triploid-diploid mosaic chicken embryo. Science 1966, 153, 759–760. [Google Scholar] [CrossRef]
- Bloom, S.E. Chromosome abnormalities in early chicken (Gallus domesticus) embryos. Chromosome 1969, 28, 357–369. [Google Scholar] [CrossRef]
- O’Connor, R.E.; Kiazim, L.; Skinner, B.; Fonseka, G.; Joseph, S.; Jennings, R.; Larkin, D.M.; Griffin, D.K. Patterns of microchromosome organization remain highly conserved throughout avian evolution. Chromosoma 2019, 128, 21–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christidis, L. Chromosomal evolution within the family Estrildidae (Aves) III. The Estrildae (waxbill finches). Genetica 1987, 72, 93–100. [Google Scholar] [CrossRef]
- De Lucca, E.J. Cariótipo de oito espécies de Aves. Rev. Bras. Biol. 1974, 34, 387–392. (In Portuguese) [Google Scholar] [PubMed]
- Carvalho, M.W. Estudos Citogenéticos na Família Fringillidae (Passeriformes: Aves). Master’s Thesis, Universidade Federal de Rio Grande do Sul, Porto Alegre, Brazil, 1989. (In Portuguese). [Google Scholar]
- Christidis, L. Animal Cytogenetics 4: Chordata 3 B: Aves; Gebrüder Borntraeger: Berlin, Germany, 1990; pp. 20–59. [Google Scholar]
- Vielmo, P.G. Bandeamentos Cromossômicos em Butorides Striata (Aves: Pelecaniformes) Revelam um Padrão Distinto Para o Cromossomo W; Universidade Federal do Pampa: São Gabriel, Brazil, 2019. (In Portuguese) [Google Scholar]
- Griffin, D.K.; Haberman, F.; Masabanda, J.; O’Brien, P.; Bagga, M.; Sazanov, A.; Smith, J.; Burt, D.W.; Ferguson-Smith, M.; Wienberg, J. Micro- and macrochromosome paints generated by flow cytometry and microdissection: Tools for mapping the chicken genome. Cytogenet. Genome Res. 1999, 87, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Ahlroth, M.K.; Kola, E.H.; Ewald, D.; Masabanda, J.; Sazanov, A.; Fries, R.; Kulomaa, M.S. Characterization and chromosomal localization of the chicken avidin gene family. Anim. Genet. 2000, 31, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Cockburn, A. Prevalence of different modes of parental care in birds. Proc. R. Soc. B 2006, 273, 1375–1383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bloom, S.E. Haploid chicken embryos: Evidence for diploid and triploid cell populations. Heredity 1970, 61, 147–150. [Google Scholar] [CrossRef]
- Bloom, S.E. Chromosome abnormalities in the chicken (Gallus domesticus) embryos: Types, frequencies and phenotypic effects. Chromosome 1972, 37, 309–326. [Google Scholar] [CrossRef] [PubMed]
- Goymann, W.; Safari, I.; Muck, C.; Schwabl, I. Sex roles, parental care and offspring growth in two contrasting coucal species. R. Soc. Open Sci. 2016, 167, 413–425. [Google Scholar] [CrossRef] [Green Version]
- Martin, T.E. Avian life history evolution in relation to nest sites, nest predation and food. Ecol. Monogr. 1995, 65, 101–127. [Google Scholar] [CrossRef]
- Cox, W.A.; Thompson, R.F.; Cox, A.S.; Faaborg, J. Post fledging survival in passerine birds and the value of post fledging studies to conservation. J. Wildl. Manag. 2014, 78, 183–193. [Google Scholar] [CrossRef]
- Ibáñez-Álamo, J.D.; Magrath, R.D.; Oteyza, J.C.; Chalfoun, A.D.; Haff, T.M.; Schmidt, K.A.; Thomson, R.L.; Martin, T.E. Nest predation research: Recent findings and future perspectives. J. Ornithol. 2015, 156, 247–262. [Google Scholar] [CrossRef] [Green Version]
- Burger, L.W.; Ryan, M.R.; Dailey, T.V.; Kurzejeski, E.W. Reproductive strategies, success, and mating systems of northern bobwhite in Missouri. J. Wildl. Manag. 1995, 59, 417–426. [Google Scholar] [CrossRef]
- Thompson, B.C.; Knadle, G.E.; Brubaker, D.L.; Brubaker, K.S. Nest Success Is Not an Adequate Comparative Estimate of Avian Reproduction (El Éxito al Anidar no es un Estimado Comparativo Adecuado de la Reproduccion en Aves). J. Field Ornithol. 2001, 72, 527–536. [Google Scholar] [CrossRef]
- Feng, S.; Stiller, J.; Deng, Y.; Armstrong, J.; Fang, Q.; Reeve, A.H.; Xie, D.; Chen, G.; Guo, C.; Faircloth, B.C.; et al. Dense sampling of bird diversity increases power of comparative genomics. Nature 2020, 587, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Rhie, A.; McCarthy, S.A.; Fedrigo, O.; Damas, J.; Formenti, G.; Koren, S.; Uliano-Silva, M.; Chow, W.; Fungtammasan, A.; Kim, J.; et al. Towards complete and error-free genome assemblies of all vertebrate species. Nature 2021, 592, 737–746. [Google Scholar] [CrossRef] [PubMed]



| Techniques | Advantages | Disadvantages |
|---|---|---|
| Colchicinized embryo technique developed by Bloom and Buss [19] |
|
|
| Fibroblast cell culture developed by Sasaki, Ikeuchi and Makino [17] |
|
|
| Colchicinized embryo technique developed by Bloom [20] |
|
|
| Bone marrow developed by Garnero and Gunski [18] |
|
|
| Fibroblast (skin and liver) developed by Itoh and Arnold [9] |
|
|
| Cell culture developed by Tsuda and Umehara [10] |
|
|
| Method described in this paper |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barcellos, S.A.; de Souza, M.S.; Tura, V.; Pereira, L.R.; Kretschmer, R.; Gunski, R.J.; Garnero, A.D.V. Direct Chromosome Preparation Method in Avian Embryos for Cytogenetic Studies: Quick, Easy and Cheap. DNA 2022, 2, 22-29. https://doi.org/10.3390/dna2010002
Barcellos SA, de Souza MS, Tura V, Pereira LR, Kretschmer R, Gunski RJ, Garnero ADV. Direct Chromosome Preparation Method in Avian Embryos for Cytogenetic Studies: Quick, Easy and Cheap. DNA. 2022; 2(1):22-29. https://doi.org/10.3390/dna2010002
Chicago/Turabian StyleBarcellos, Suziane Alves, Marcelo Santos de Souza, Victoria Tura, Larissa Rodrigues Pereira, Rafael Kretschmer, Ricardo José Gunski, and Analía Del Valle Garnero. 2022. "Direct Chromosome Preparation Method in Avian Embryos for Cytogenetic Studies: Quick, Easy and Cheap" DNA 2, no. 1: 22-29. https://doi.org/10.3390/dna2010002
APA StyleBarcellos, S. A., de Souza, M. S., Tura, V., Pereira, L. R., Kretschmer, R., Gunski, R. J., & Garnero, A. D. V. (2022). Direct Chromosome Preparation Method in Avian Embryos for Cytogenetic Studies: Quick, Easy and Cheap. DNA, 2(1), 22-29. https://doi.org/10.3390/dna2010002