Microalgae in Mitigating Industrial Pollution: Bioremediation Strategies and Biomagnification Potential
Abstract
1. Introduction
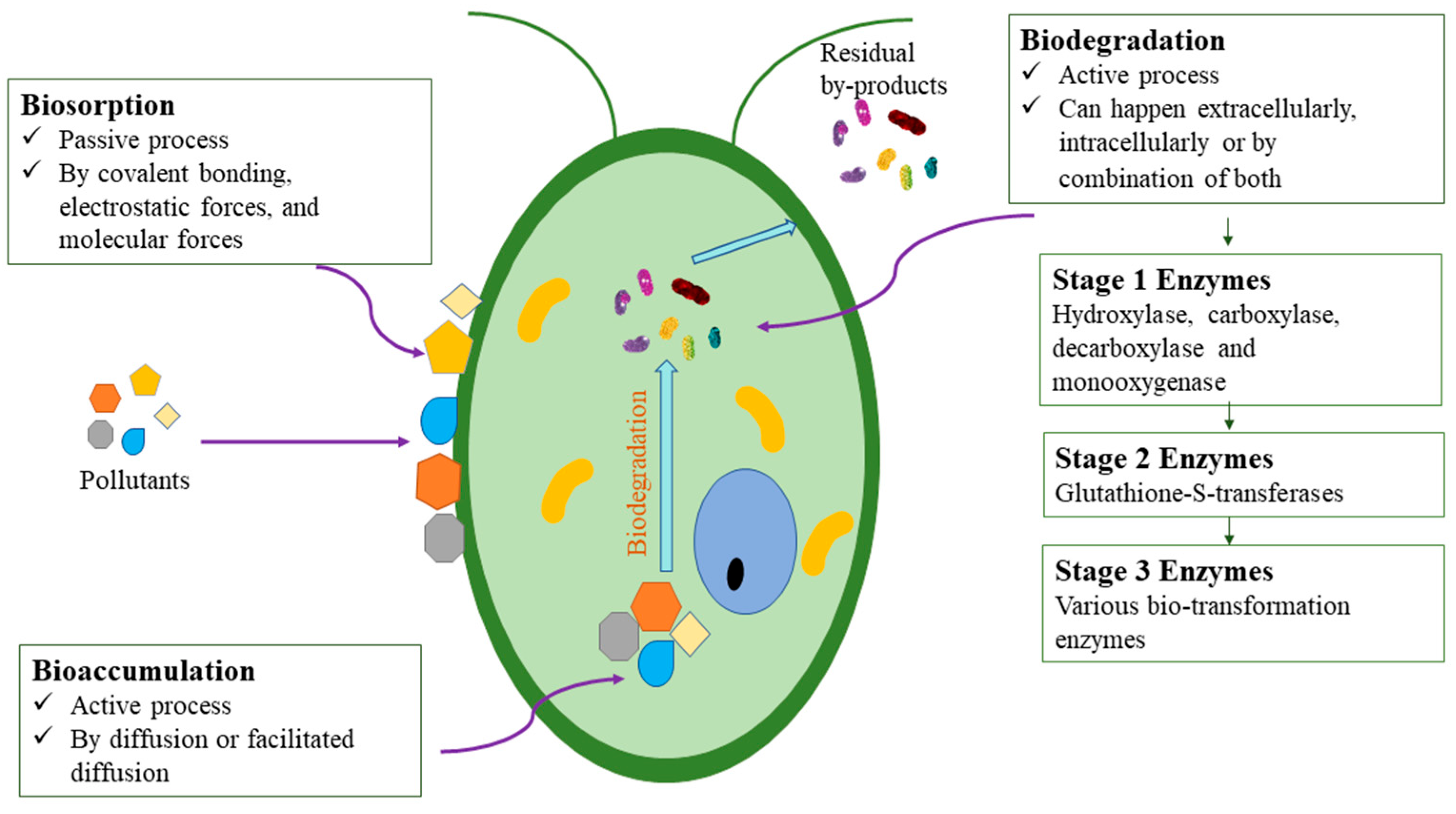
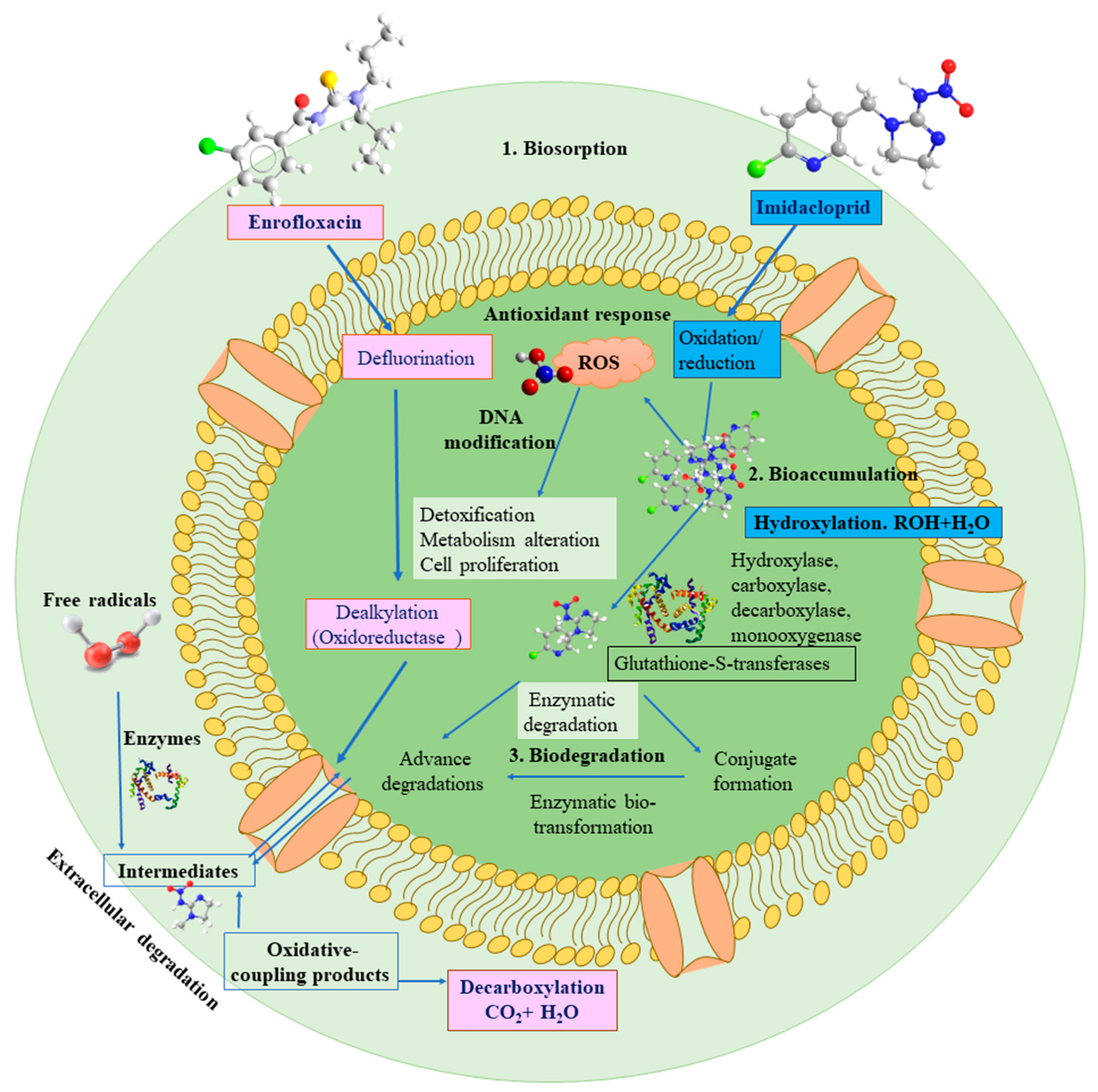
2. Bioremediation by Microalgae
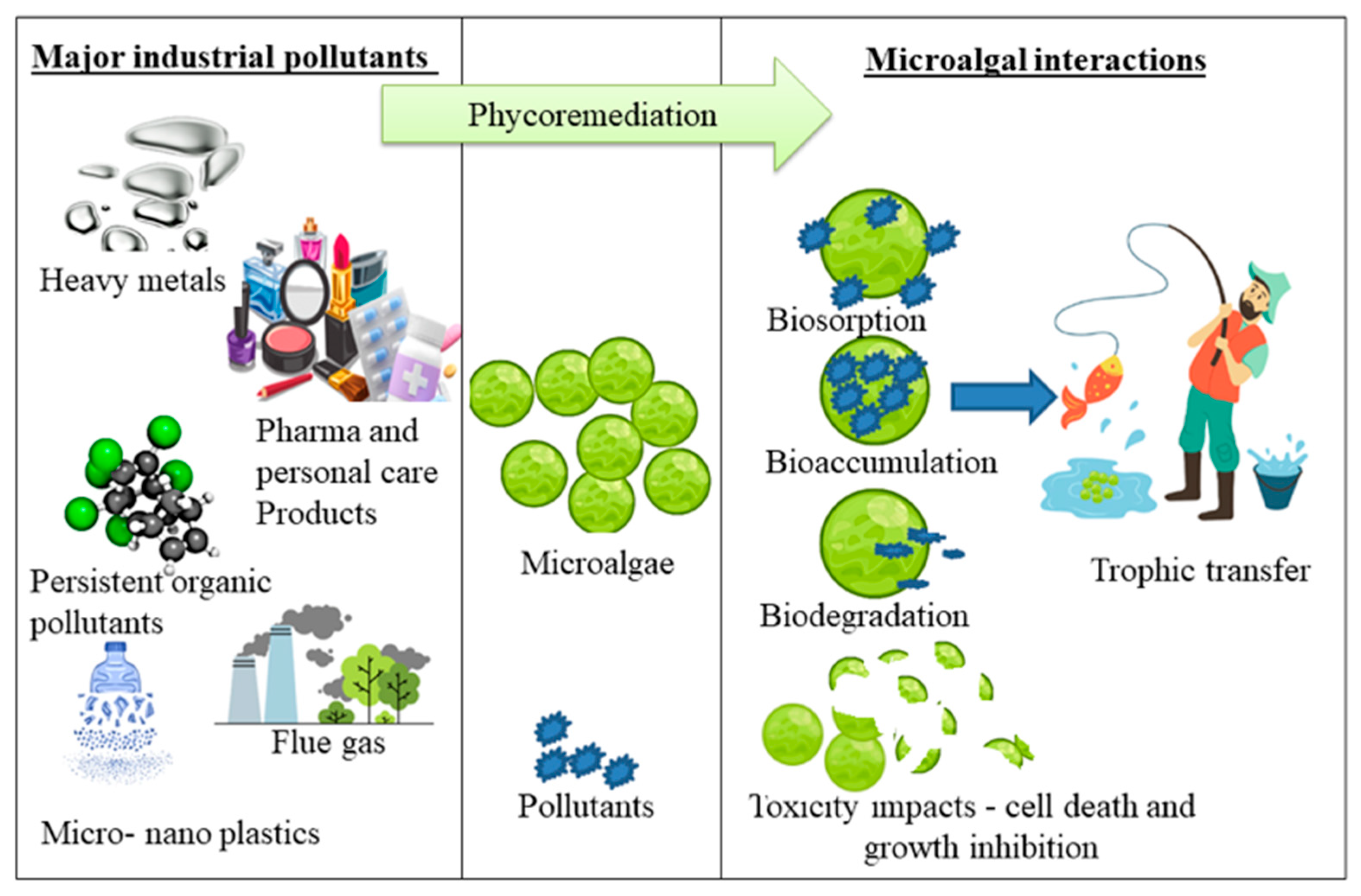
3. Industrial Pollutants
3.1. Heavy Metals
Heavy Metals—Microalgal Interactions and Bioremediation
3.2. Pharmaceuticals and Personal Care Products (PPCPs)
PPCPs—Microalgal Interactions and Bioremediation
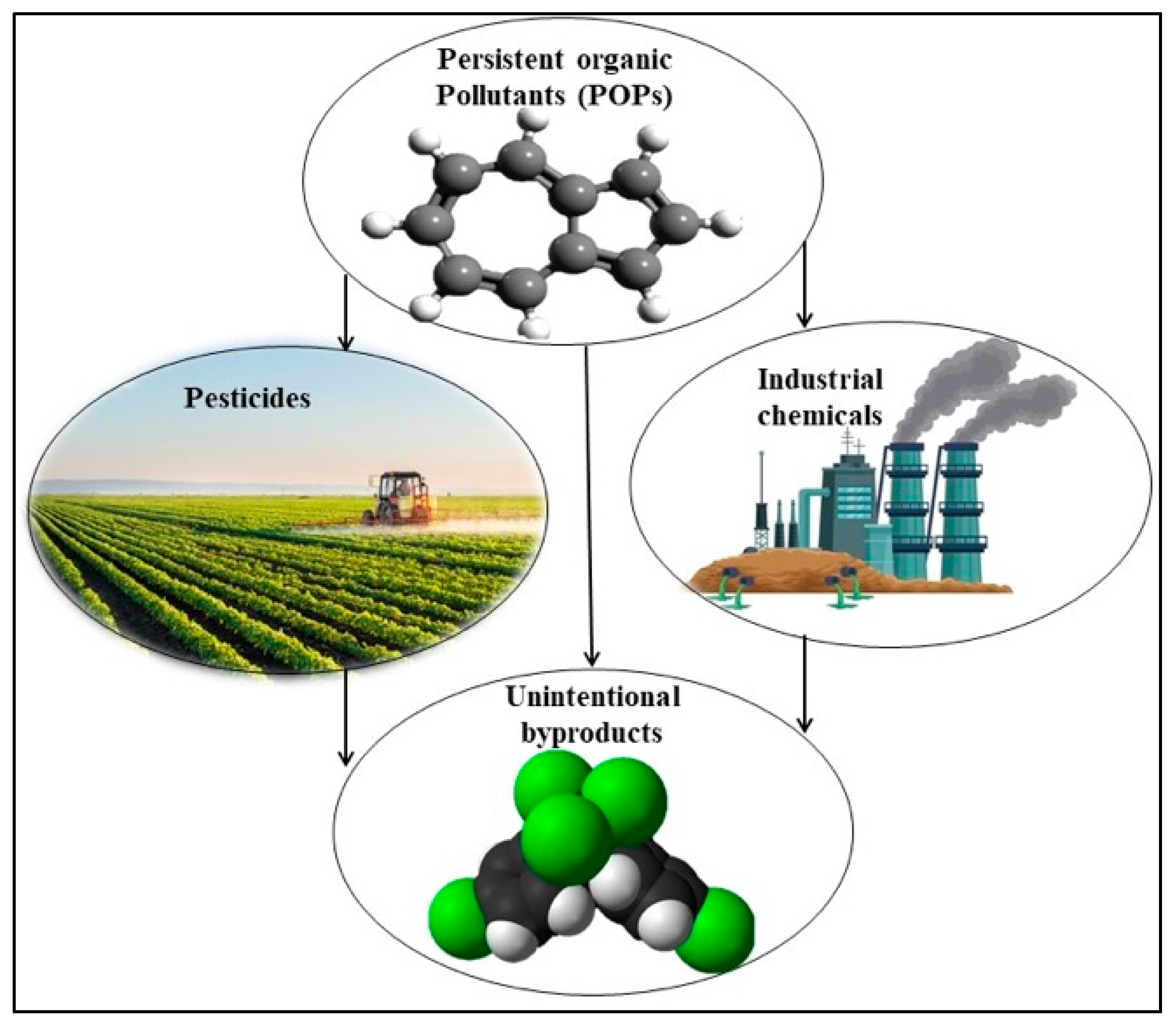
3.3. Persistent Organic Pollutants (POPs)
POPs—Microalgal Interactions and Bioremediation
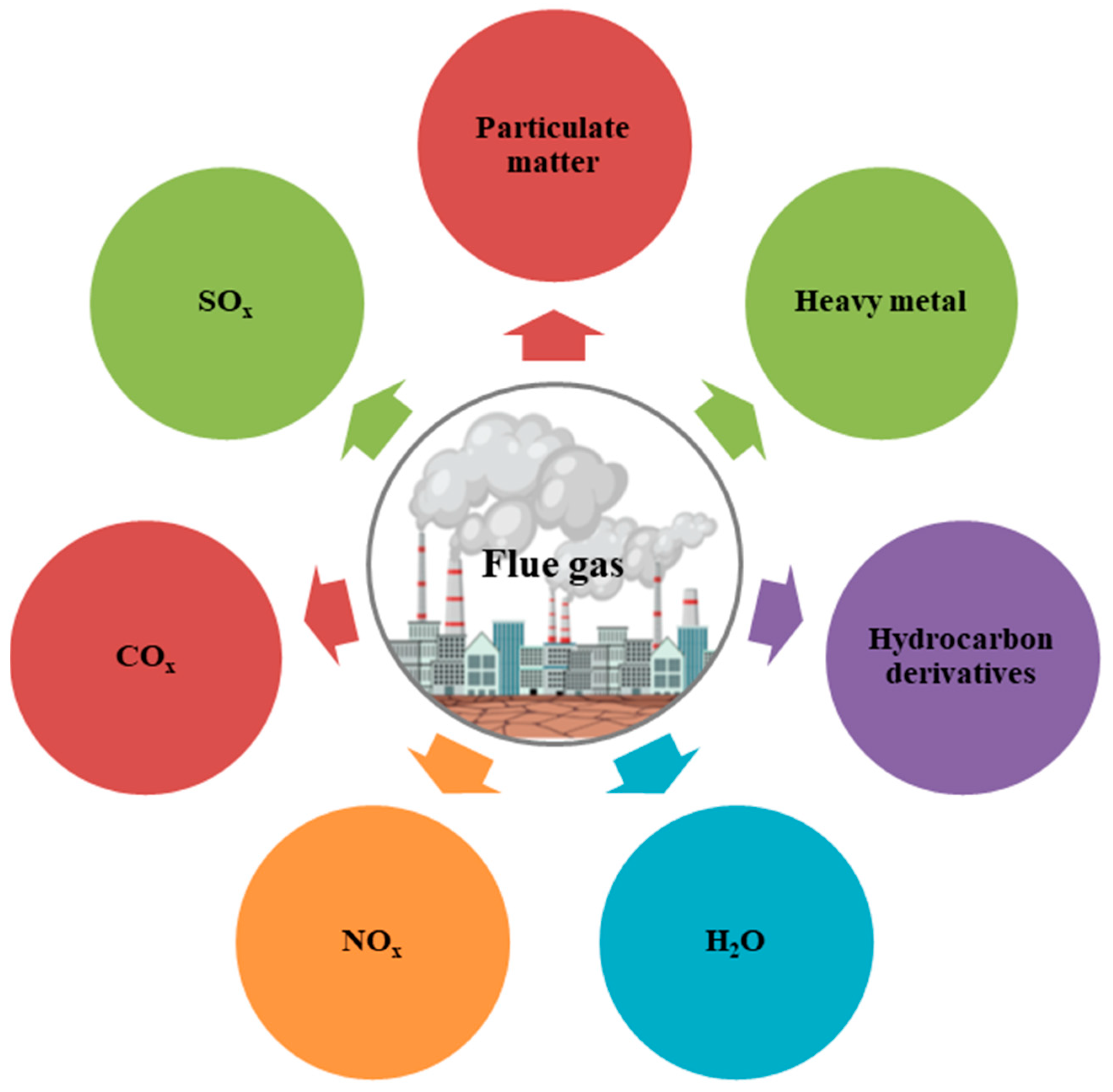
3.4. Flue Gas
Flue Gas—Microalgal Interactions and Bioremediation
3.5. Microplastics and Nanoplastics
Micro/Nanoplastics—Microalgal Interactions and Bioremediation
4. Biomagnification and Transfer of Toxins in Food Chain
5. Challenges and Future Prospects
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schlatter, C. Environmental pollution and human health. Sci. Total Environ. 1994, 143, 93–101. [Google Scholar] [CrossRef]
- Ajibade, F.O.; Adelodun, B.; Lasisi, K.H.; Fadare, O.O.; Ajibade, T.F.; Nwogwu, N.A.; Sulaymon, I.D.; Ugya, A.Y.; Wang, H.C.; Wang, A. Environmental pollution and their socioeconomic impacts. In Microbe Mediated Remediation of Environmental Contaminants; Elsevier: Amsterdam, The Netherlands, 2021; pp. 321–354. [Google Scholar]
- Xu, H.; Jia, Y.; Sun, Z.; Su, J.; Liu, Q.S.; Zhou, Q.; Jiang, G. Environmental pollution, a hidden culprit for health issues. Eco-Environ. Health 2022, 1, 31–45. [Google Scholar] [CrossRef]
- Ukaogo, P.O.; Ewuzie, U.; Onwuka, C.V. Environmental pollution: Causes, effects, and the remedies. In Microorganisms for Sustainable Environment and Health; Elsevier: Amsterdam, The Netherlands, 2020; pp. 419–429. [Google Scholar]
- Eskander, S.; Saleh, H. Biodegradation: Process mechanism. In Environmental Science and Engineering; Studium Press LLC: Houston, TX, USA, 2017; Volume 8, pp. 1–31. [Google Scholar]
- Fester, T.; Giebler, J.; Wick, L.Y.; Schlosser, D.; Kästner, M. Plant–microbe interactions as drivers of ecosystem functions relevant for the biodegradation of organic contaminants. Curr. Opin. Biotechnol. 2014, 27, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.; Ilyas, N.; Bibi, F.; Jayachandran, K.; Dattamudi, S.; Elgorban, A.M. Biodegradation of PAHs by Bacillus marsiflavi, genome analysis and its plant growth promoting potential. Environ. Pollut. 2022, 292, 118343. [Google Scholar] [CrossRef] [PubMed]
- Ceci, A.; Pinzari, F.; Russo, F.; Persiani, A.M.; Gadd, G.M. Roles of saprotrophic fungi in biodegradation or transformation of organic and inorganic pollutants in co-contaminated sites. Appl. Microbiol. Biotechnol. 2019, 103, 53–68. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Parakh, S.K.; Singh, S.P.; Parra-Saldívar, R.; Kim, S.-H.; Varjani, S.; Tong, Y.W. A critical review on microbes-based treatment strategies for mitigation of toxic pollutants. Sci. Total Environ. 2022, 834, 155444. [Google Scholar] [CrossRef]
- Martelli, F.; Cirlini, M.; Dellafiora, L.; Neviani, E.; Dall’Asta, C.; Bernini, V. Mitigation of marine toxins by interactions with bacteria: The case of okadaic acid and tetrodotoxin. Food Control 2022, 131, 108428. [Google Scholar] [CrossRef]
- Magnoli, K.; Carranza, C.; Aluffi, M.; Magnoli, C.; Barberis, C. Fungal biodegradation of chlorinated herbicides: An overview with an emphasis on 2, 4-D in Argentina. Biodegradation 2023, 34, 199–214. [Google Scholar] [CrossRef]
- Raj, R.; Manju, N.; Fazil, T.; Chatterjee, N.; Anandan, R.; Mathew, S. Seaweed and its Role in Bioremediation-A Review. Fish Technol. 2022, 59, 147–153. [Google Scholar]
- Touliabah, H.E.-S.; El-Sheekh, M.M.; Ismail, M.M.; El-Kassas, H. A review of microalgae-and cyanobacteria-based biodegradation of organic pollutants. Molecules 2022, 27, 1141. [Google Scholar] [CrossRef]
- Parial, D.; Dey, S. Potential Use of Algae and Bacteria for Bioremediation of Wastewater: A Critical Review. In Toxicity of Aquatic System and Remediation; CRC Press: Boca Raton, FL, USA, 2024; pp. 219–236. [Google Scholar]
- Chisti, Y. Society and microalgae: Understanding the past and present. In Microalgae in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2018; pp. 11–21. [Google Scholar]
- Bhola, V.; Swalaha, F.; Ranjith Kumar, R.; Singh, M.; Bux, F. Overview of the potential of microalgae for CO2 sequestration. Int. J. Environ. Sci. Technol. 2014, 11, 2103–2118. [Google Scholar] [CrossRef]
- Do Nascimento, M.; Rizza, L.S.; Di Palma, A.A.; de los Angeles Dublan, M.; Salerno, G.; Rubio, L.M.; Curatti, L. Cyanobacterial biological nitrogen fixation as a sustainable nitrogen fertilizer for the production of microalgal oil. Algal Res. 2015, 12, 142–148. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; El-Kassas, H.Y.; Ali, S.S. Microalgae-based bioremediation of refractory pollutants: An approach towards environmental sustainability. Microb. Cell Factories 2025, 24, 19. [Google Scholar] [CrossRef]
- Goswami, R.K.; Mehariya, S.; Verma, P. Advances in microalgae-based carbon sequestration: Current status and future perspectives. Environ. Res. 2024, 249, 118397. [Google Scholar] [CrossRef] [PubMed]
- Santos, B.; Freitas, F.; Sobral, A.J.; Encarnação, T. Microalgae and circular economy: Unlocking waste to resource pathways for sustainable development. Int. J. Sustain. Eng. 2025, 18, 2501488. [Google Scholar] [CrossRef]
- Wang, S.; Mukhambet, Y.; Esakkimuthu, S. Integrated microalgal biorefinery–Routes, energy, economic and environmental perspectives. J. Clean. Prod. 2022, 348, 131245. [Google Scholar] [CrossRef]
- Zhu, X.-G.; Long, S.P.; Ort, D.R. Improving photosynthetic efficiency for greater yield. Annu. Rev. Plant Biol. 2010, 61, 235–261. [Google Scholar] [CrossRef] [PubMed]
- Benner, P.; Meier, L.; Pfeffer, A.; Krüger, K.; Oropeza Vargas, J.E.; Weuster-Botz, D. Lab-scale photobioreactor systems: Principles, applications, and scalability. Bioprocess Biosyst. Eng. 2022, 45, 791–813. [Google Scholar] [CrossRef]
- Singh, U.B.; Ahluwalia, A. Microalgae: A promising tool for carbon sequestration. Mitig. Adapt. Strateg. Glob. Change 2013, 18, 73–95. [Google Scholar] [CrossRef]
- Wang, B.; Li, Y.; Wu, N.; Lan, C.Q. CO2 bio-mitigation using microalgae. Appl. Microbiol. Biotechnol. 2008, 79, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Fernández, F.G.A.; Reis, A.; Wijffels, R.H.; Barbosa, M.; Verdelho, V.; Llamas, B. The role of microalgae in the bioeconomy. New Biotechnol. 2021, 61, 99–107. [Google Scholar] [CrossRef]
- Bharti, R.K.; Singh, A.; Dhar, D.W.; Kaushik, A. Biological carbon dioxide sequestration by microalgae for biofuel and biomaterials production. In Biomass, Biofuels, Biochemicals; Elsevier: Amsterdam, The Netherlands, 2022; pp. 137–153. [Google Scholar]
- Barkia, I.; Saari, N.; Manning, S.R. Microalgae for high-value products towards human health and nutrition. Mar. Drugs 2019, 17, 304. [Google Scholar] [CrossRef] [PubMed]
- González-Pérez, B.K.; Rivas-Castillo, A.M.; Valdez-Calderón, A.; Gayosso-Morales, M.A. Microalgae as biostimulants: A new approach in agriculture. World J. Microbiol. Biotechnol. 2022, 38, 4. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, F.; Zhu, X.; Liao, Q.; Chang, J.-S.; Ho, S.-H. Biohydrogen production from microalgae for environmental sustainability. Chemosphere 2022, 291, 132717. [Google Scholar] [CrossRef]
- Rahman, A.; Miller, C. Microalgae as a source of bioplastics. In Algal Green Chemistry; Elsevier: Amsterdam, The Netherlands, 2017; pp. 121–138. [Google Scholar]
- Koller, M.; Muhr, A.; Braunegg, G. Microalgae as versatile cellular factories for valued products. Algal Res. 2014, 6, 52–63. [Google Scholar] [CrossRef]
- Leu, S.; Boussiba, S. Advances in the production of high-value products by microalgae. Ind. Biotechnol. 2014, 10, 169–183. [Google Scholar] [CrossRef]
- Bashir, I.; Lone, F.A.; Bhat, R.A.; Mir, S.A.; Dar, Z.A.; Dar, S.A. Concerns and threats of contamination on aquatic ecosystems. In Bioremediation and Biotechnology: Sustainable Approaches to Pollution Degradation; Springer: Cham, Switzerland, 2020; pp. 1–26. [Google Scholar]
- Glavan, M. Water Challenges of an Urbanizing World; BoD–Books on Demand: Norderstedt, Germany, 2018. [Google Scholar]
- Deletic, A.; Wang, H. Water pollution control for sustainable development. Engineering 2019, 5, 839–840. [Google Scholar] [CrossRef]
- Mantzorou, A.; Navakoudis, E.; Paschalidis, K.; Ververidis, F. Microalgae: A potential tool for remediating aquatic environments from toxic metals. Int. J. Environ. Sci. Technol. 2018, 15, 1815–1830. [Google Scholar] [CrossRef]
- Bhatnagar, S.; Kumari, R. Bioremediation: A sustainable tool for environmental management—A review. Annu. Res. Rev. Biol. 2013, 3, 974–993. [Google Scholar]
- Gani, P.; Sunar, N.M.; Matias-Peralta, H.; Parjo, U.K.; Razak, A.R.A. Phycoremediation of wastewaters and potential hydrocarbon from microalgae: A review. Adv. Environ. Biol. 2015, 9, 1–9. [Google Scholar]
- Diankristanti, P.A.; Ng, I.-S. Marine microalgae for bioremediation and waste-to-worth valorization: Recent progress and future prospects. Blue Biotechnol. 2024, 1, 10. [Google Scholar] [CrossRef]
- Daneshvar, E.; Zarrinmehr, M.J.; Hashtjin, A.M.; Farhadian, O.; Bhatnagar, A. Versatile applications of freshwater and marine water microalgae in dairy wastewater treatment, lipid extraction and tetracycline biosorption. Bioresour. Technol. 2018, 268, 523–530. [Google Scholar] [CrossRef]
- Torres Virviescas, M.J.; Henao-Castro, A.; Contreras-Vega, L. Bioremediation of Landfill Leachate Using Marine and Freshwater Microalgal Consortia Coupled with Fatty Acids Production for Biofuel. Available online: https://ssrn.com/abstract=4792684 (accessed on 21 June 2025). [CrossRef]
- Marella, T.K.; Saxena, A.; Tiwari, A. Diatom mediated heavy metal remediation: A review. Bioresour. Technol. 2020, 305, 123068. [Google Scholar] [CrossRef]
- Abdelfattah, A.; Ali, S.S.; Ramadan, H.; El-Aswar, E.I.; Eltawab, R.; Ho, S.-H.; Elsamahy, T.; Li, S.; El-Sheekh, M.M.; Schagerl, M. Microalgae-based wastewater treatment: Mechanisms, challenges, recent advances, and future prospects. Environ. Sci. Ecotechnol. 2023, 13, 100205. [Google Scholar] [CrossRef] [PubMed]
- Ansari, F.A.; Guldhe, A.; Gupta, S.K.; Rawat, I.; Bux, F. Improving the feasibility of aquaculture feed by using microalgae. Environ. Sci. Pollut. Res. 2021, 28, 43234–43257. [Google Scholar] [CrossRef]
- Ladakis, D.; Papapostolou, H.; Vlysidis, A.; Koutinas, A. Inventory of food processing side streams in European Union and prospects for biorefinery development. In Food Industry Wastes; Elsevier: Amsterdam, The Netherlands, 2020; pp. 181–199. [Google Scholar]
- Spennati, E.; Casazza, A.A.; Converti, A. Winery wastewater treatment by microalgae to produce low-cost biomass for energy production purposes. Energies 2020, 13, 2490. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. In Molecular, Clinical and Environmental Toxicology: Volume 3: Environmental Toxicology; Springer: Basel, Switzerland, 2012; pp. 133–164. [Google Scholar]
- Zamora-Ledezma, C.; Negrete-Bolagay, D.; Figueroa, F.; Zamora-Ledezma, E.; Ni, M.; Alexis, F.; Guerrero, V.H. Heavy metal water pollution: A fresh look about hazards, novel and conventional remediation methods. Environ. Technol. Innov. 2021, 22, 101504. [Google Scholar] [CrossRef]
- Boyd, C.E. Water Quality: An Introduction; Springer Nature: Cham, Switzerland, 2019. [Google Scholar]
- Malik, D.; Sharma, A.K.; Sharma, A.K.; Thakur, R.; Sharma, M. A review on impact of water pollution on freshwater fish species and their aquatic environment. In Advances in Environmental Pollution Management: Wastewater Impacts and Treatment Technologies; Agriculture and Environmental Science Academy: Haridwar, India, 2020; Volume 1, pp. 10–28. [Google Scholar] [CrossRef]
- Zeitoun, M.M.; Mehana, E. Impact of water pollution with heavy metals on fish health: Overview and updates. Glob. Vet. 2014, 12, 219–231. [Google Scholar]
- Malletzidou, L.; Kyratzopoulou, E.; Kyzaki, N.; Nerantzis, E.; Kazakis, N.A. Towards the sustainable removal of heavy metals from wastewater using Arthrospira platensis: A laboratory-scale approach in the context of a green circular economy. Appl. Sci. 2025, 15, 791. [Google Scholar] [CrossRef]
- Pinto, E.; Sigaud-kutner, T.C.; Leitao, M.A.; Okamoto, O.K.; Morse, D.; Colepicolo, P. Heavy metal–induced oxidative stress in algae 1. J. Phycol. 2003, 39, 1008–1018. [Google Scholar] [CrossRef]
- Danouche, M.; El Ghatchouli, N.; Arroussi, H. Overview of the management of heavy metals toxicity by microalgae. J. Appl. Phycol. 2022, 34, 475–488. [Google Scholar] [CrossRef]
- Banerjee, M.; Kalwani, P.; Chakravarty, D.; Pathak, P.; Agarwal, R.; Ballal, A. Modulation of oxidative stress machinery determines the contrasting ability of cyanobacteria to adapt to Se (VI) or Se (IV). Plant Physiol. Biochem. 2024, 211, 108673. [Google Scholar] [CrossRef]
- Zhao, D.-S.; Farooq, M.A.; Li, M.; Chen, Y.-T.; Xu, J.-M.; Liu, X.-L.; Zhang, A.; Yan, X.; Zou, H.-X.; Pang, Q. Acute toxicity of salicylic acid and its derivatives on the diatom Phaeodactylum tricornutum: Physico-Biochemical and transcriptomic insights. Aquat. Toxicol. 2024, 276, 107116. [Google Scholar] [CrossRef]
- Flori, S.; Dickenson, J.; Gaikwad, T.; Cole, I.; Smirnoff, N.; Helliwell, K.E.; Brownlee, C.; Wheeler, G.L. Diatoms exhibit dynamic chloroplast calcium signals in response to high light and oxidative stress. Plant Physiol. 2025, 197, kiae591. [Google Scholar] [CrossRef]
- Machado, M.D.; Soares, E.V. Integration of copper toxicity mechanisms in raphidocelis subcapitata: Advancing insights at environmentally relevant concentrations. Toxics 2024, 12, 905. [Google Scholar] [CrossRef]
- Zheng, H.; Zheng, Y.; Yuan, L.; Li, S.; Niu, J.; Dong, X.; Leong, Y.K.; Lee, D.-J.; Chang, J.-S. Oxidation effects on Microcystis aeruginosa inactivation through various reactive oxygen species: Degradation efficiency, mechanisms, and physiological properties. Bioresour. Technol. 2024, 402, 130806. [Google Scholar] [CrossRef]
- Pancheri, T.; Baur, T.; Roach, T. Singlet-Oxygen-Mediated Regulation of Photosynthesis-Specific Genes: A Role for Reactive Electrophiles in Signal Transduction. Int. J. Mol. Sci. 2024, 25, 8458. [Google Scholar] [CrossRef]
- Levin, G.; Yasmin, M.; Liran, O.; Hanna, R.; Kleifeld, O.; Horev, G.; Wollman, F.-A.; Schuster, G.; Nawrocki, W.J. Processes independent of nonphotochemical quenching protect a high-light-tolerant desert alga from oxidative stress. Plant Physiol. 2025, 197, e608. [Google Scholar] [CrossRef]
- Singh, R.P.; Yadav, P.; Sharma, H.; Kumar, A.; Hashem, A.; Abd_Allah, E.F.; Gupta, R.K. Unlocking the adaptation mechanisms of the oleaginous microalga Scenedesmus sp. BHU1 under elevated salt stress: A physiochemical, lipidomics and transcriptomics approach. Front. Microbiol. 2024, 15, 1475410. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, S.; Mochida, K.; Suzuki, K. Diverse biosynthetic pathways and protective functions against environmental stress of antioxidants in microalgae. Plants 2021, 10, 1250. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.M.; Castro, P.M.; Malcata, F.X. Metal uptake by microalgae: Underlying mechanisms and practical applications. Biotechnol. Prog. 2012, 28, 299–311. [Google Scholar] [CrossRef]
- Díaz, S.; De Francisco, P.; Olsson, S.; Aguilera, Á.; González-Toril, E.; Martín-González, A. Toxicity, physiological, and ultrastructural effects of arsenic and cadmium on the extremophilic microalga Chlamydomonas acidophila. Int. J. Environ. Res. Public Health 2020, 17, 1650. [Google Scholar] [CrossRef]
- Amunts, A.; Nelson, N. Plant photosystem I design in the light of evolution. Structure 2009, 17, 637–650. [Google Scholar] [CrossRef]
- Umena, Y.; Kawakami, K.; Shen, J.-R.; Kamiya, N. Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å. Nature 2011, 473, 55–60. [Google Scholar] [CrossRef]
- Wang, S.; Wufuer, R.; Duo, J.; Li, W.; Pan, X. Cadmium caused different toxicity to photosystem i and photosystem ii of freshwater unicellular algae Chlorella pyrenoidosa (Chlorophyta). Toxics 2022, 10, 352. [Google Scholar] [CrossRef] [PubMed]
- Dos Reis, L.L.; Alho, L.d.O.G.; de Abreu, C.B.; Melão, M.d.G.G. Using multiple endpoints to assess the toxicity of cadmium and cobalt for chlorophycean Raphidocelis subcapitata. Ecotoxicol. Environ. Saf. 2021, 208, 111628. [Google Scholar] [CrossRef]
- Bashir, K.M.I.; Lee, H.-J.; Mansoor, S.; Jahn, A.; Cho, M.-G. The effect of chromium on photosynthesis and lipid accumulation in two chlorophyte microalgae. Energies 2021, 14, 2260. [Google Scholar] [CrossRef]
- Pascual, G.; Sano, D.; Sakamaki, T.; Akiba, M.; Nishimura, O. The water temperature changes the effect of pH on copper toxicity to the green microalgae Raphidocelis subcapitata. Chemosphere 2022, 291, 133110. [Google Scholar] [CrossRef]
- Genevière, A.-M.; Derelle, E.; Escande, M.-L.; Grimsley, N.; Klopp, C.; Ménager, C.; Michel, A.; Moreau, H. Responses to iron oxide and zinc oxide nanoparticles in echinoderm embryos and microalgae: Uptake, growth, morphology, and transcriptomic analysis. Nanotoxicology 2020, 14, 1342–1361. [Google Scholar] [CrossRef] [PubMed]
- Zamani-Ahmadmahmoodi, R.; Malekabadi, M.B.; Rahimi, R.; Johari, S.A. Aquatic pollution caused by mercury, lead, and cadmium affects cell growth and pigment content of marine microalga, Nannochloropsis oculata. Environ. Monit. Assess. 2020, 192, 330. [Google Scholar] [CrossRef]
- Romero, N.; Visentini, F.F.; Marquez, V.E.; Santiago, L.G.; Castro, G.R.; Gagneten, A.M. Physiological and morphological responses of green microalgae Chlorella vulgaris to silver nanoparticles. Environ. Res. 2020, 189, 109857. [Google Scholar] [CrossRef]
- Romero-Freire, A.; Abdou, M.; Cobelo-García, A. Implications of kinetically-hindered metals in ecotoxicological studies: Effect of platinum spike aging on its toxicity to Dunaliella salina. Ecotoxicol. Environ. Saf. 2021, 227, 112924. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, D.; Song, B.; Li, Y. The potential of mercury methylation and demethylation by 15 species of marine microalgae. Water Res. 2022, 215, 118266. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Jacinto, V.; García-Barrera, T.; Gómez-Ariza, J.L.; Garbayo-Nores, I.; Vílchez-Lobato, C. Elucidation of the defence mechanism in microalgae Chlorella sorokiniana under mercury exposure. Identification of Hg–phytochelatins. Chem.-Biol. Interact. 2015, 238, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Tada, Y.; Marumoto, K. Uptake of methylmercury by marine microalgae and its bioaccumulation in them. J. Oceanogr. 2020, 76, 63–70. [Google Scholar] [CrossRef]
- Spain, O.; Plöhn, M.; Funk, C. The cell wall of green microalgae and its role in heavy metal removal. Physiol. Plant. 2021, 173, 526–535. [Google Scholar] [CrossRef]
- Priatni, S.; Ratnaningrum, D.; Warya, S.; Audina, E. Phycobiliproteins production and heavy metals reduction ability of Porphyridium sp. IOP Conf. Ser. Earth Environ. Sci. 2018, 160, 012006. [Google Scholar]
- Chugh, M.; Kumar, L.; Shah, M.P.; Bharadvaja, N. Algal Bioremediation of heavy metals: An insight into removal mechanisms, recovery of by-products, challenges, and future opportunities. Energy Nexus 2022, 7, 100129. [Google Scholar] [CrossRef]
- Mao, Q.; Xie, Z.; Irshad, S.; Zhong, Z.; Liu, T.; Pei, F.; Gao, B.; Li, L. Effect of arsenic accumulation on growth and antioxidant defense system of Chlorella thermophila SM01 and Leptolyngbya sp. XZMQ. Algal Res. 2022, 66, 102762. [Google Scholar] [CrossRef]
- Leong, Y.K.; Chang, J.-S. Bioremediation of heavy metals using microalgae: Recent advances and mechanisms. Bioresour. Technol. 2020, 303, 122886. [Google Scholar] [CrossRef]
- Goswami, R.; Agrawal, K.; Shah, M.; Verma, P. Bioremediation of heavy metals from wastewater: A current perspective on microalgae-based future. Lett. Appl. Microbiol. 2022, 75, 701–717. [Google Scholar] [CrossRef]
- Noor, I.; Sohail, H.; Akhtar, M.T.; Cui, J.; Lu, Z.; Mostafa, S.; Hasanuzzaman, M.; Hussain, S.; Guo, N.; Jin, B. From stress to resilience: Unraveling the molecular mechanisms of cadmium toxicity, detoxification and tolerance in plants. Sci. Total Environ. 2024, 954, 176462. [Google Scholar] [CrossRef]
- Ran, Y.; Sun, D.; Liu, X.; Zhang, L.; Niu, Z.; Chai, T.; Hu, Z.; Qiao, K. Chlorella pyrenoidosa as a potential bioremediator: Its tolerance and molecular responses to cadmium and lead. Sci. Total Environ. 2024, 912, 168712. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tian, Q.; Zhou, H.; Kang, J.; Yu, X.; Qiu, G.; Shen, L. Physiological regulation of microalgae under cadmium stress and response mechanisms of time-series analysis using metabolomics. Sci. Total Environ. 2024, 916, 170278. [Google Scholar] [CrossRef] [PubMed]
- Danouche, M.; El Ghachtouli, N.; El Arroussi, H. Phycoremediation mechanisms of heavy metals using living green microalgae: Physicochemical and molecular approaches for enhancing selectivity and removal capacity. Heliyon 2021, 7, e07609. [Google Scholar] [CrossRef]
- Geraei, H.; Shokrkar, H. Investigation of the biological removal of nickel and copper ions from aqueous solutions using mixed microalgae. Biofuels Bioprod. Biorefin. 2025, 19, 18–33. [Google Scholar] [CrossRef]
- Kumar, V.S.; Sarkar, D.J.; Das, B.K.; Samanta, S.; Tripathi, G.; Sarkar, S.D.; Nag, S.K. Utilizing microalgae biofilm for reducing arsenic toxicity on fish grown in contaminated waters: An innovative solution for safer and cleaner aquaculture in arsenic endemic areas. Aquaculture 2025, 599, 742121. [Google Scholar] [CrossRef]
- Urrutia, C.; Yañez-Mansilla, E.; Jeison, D. Bioremoval of heavy metals from metal mine tailings water using microalgae biomass. Algal Res. 2019, 43, 101659. [Google Scholar] [CrossRef]
- Abdel-Razek, M.A.; Abozeid, A.M.; Eltholth, M.M.; Abouelenien, F.A.; El-Midany, S.A.; Moustafa, N.Y.; Mohamed, R.A. Bioremediation of a pesticide and selected heavy metals in wastewater from various sources using a consortium of microalgae and cyanobacteria. Slov. Vet. 2019, 56, 61–73. [Google Scholar] [CrossRef]
- Sayadi, M.H.; Rashki, O.; Shahri, E. Application of modified Spirulina platensis and Chlorella vulgaris powder on the adsorption of heavy metals from aqueous solutions. J. Environ. Chem. Eng. 2019, 7, 103169. [Google Scholar] [CrossRef]
- Bauenova, M.O.; Sadvakasova, A.K.; Mustapayeva, Z.O.; Kokociński, M.; Zayadan, B.K.; Wojciechowicz, M.K.; Balouch, H.; Akmukhanova, N.R.; Alwasel, S.; Allakhverdiev, S.I. Potential of microalgae Parachlorella kessleri Bh-2 as bioremediation agent of heavy metals cadmium and chromium. Algal Res. 2021, 59, 102463. [Google Scholar] [CrossRef]
- Mubashar, M.; Naveed, M.; Mustafa, A.; Ashraf, S.; Shehzad Baig, K.; Alamri, S.; Siddiqui, M.H.; Zabochnicka-Świątek, M.; Szota, M.; Kalaji, H.M. Experimental investigation of Chlorella vulgaris and Enterobacter sp. MN17 for decolorization and removal of heavy metals from textile wastewater. Water 2020, 12, 3034. [Google Scholar] [CrossRef]
- Ibuot, A.; Webster, R.E.; Williams, L.E.; Pittman, J.K. Increased metal tolerance and bioaccumulation of zinc and cadmium in Chlamydomonas reinhardtii expressing a AtHMA4 C-terminal domain protein. Biotechnol. Bioeng. 2020, 117, 2996–3005. [Google Scholar] [CrossRef]
- Jiang, X.; Zhou, X.; Li, C.; Wan, Z.; Yao, L.; Gao, P. Adsorption of copper by flocculated Chlamydomonas microsphaera microalgae and polyaluminium chloride in heavy metal-contaminated water. J. Appl. Phycol. 2019, 31, 1143–1151. [Google Scholar] [CrossRef]
- Kang, J.-K.; Pham, B.; Lee, C.-G.; Park, S.-J. Biosorption of Cd2+, Cu2+, Ni2+, Pb2+ by four different macroalgae species (Costaria costata, Hizikia fusiformis, Gracilaria verrucosa, and Codium fragile). Int. J. Environ. Sci. Technol. 2023, 20, 10113–10122. [Google Scholar] [CrossRef]
- Sun, J.; Cheng, J.; Yang, Z.; Zhou, J. Effect of Hg2+ on the microphysical and chemical properties of oil-producing Nannochloropsis sp. Algal Res. 2021, 60, 102525. [Google Scholar] [CrossRef]
- Martínez-Macias, M.d.R.; Correa-Murrieta, M.A.; Villegas-Peralta, Y.; Dévora-Isiordia, G.E.; Álvarez-Sánchez, J.; Saldivar-Cabrales, J.; Sánchez-Duarte, R.G. Uptake of copper from acid mine drainage by the microalgae Nannochloropsis oculata. Environ. Sci. Pollut. Res. 2019, 26, 6311–6318. [Google Scholar] [CrossRef] [PubMed]
- Wijayanti, T.A.; Ansori, M. Application of modified green algae Nannochloropsis sp. as adsorbent in the simultaneous adsorption of Methylene Blue and Cu (II) cations in solution. Sustain. Environ. Res. 2021, 31, 17. [Google Scholar] [CrossRef]
- Mohy El-Din, S.M.; Abdel-Kareem, M.S. Effects of copper and cadmium on the protein profile and DNA pattern of marine microalgae Chlorella salina and Nannochloropsis salina. Environ. Process. 2020, 7, 189–205. [Google Scholar] [CrossRef]
- Dey, S.; Bano, F.; Malik, A. Pharmaceuticals and personal care product (PPCP) contamination—A global discharge inventory. In Pharmaceuticals and Personal care Products: Waste Management and Treatment Technology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–26. [Google Scholar]
- Ohoro, C.; Adeniji, A.; Okoh, A.; Okoh, O. Distribution and chemical analysis of pharmaceuticals and personal care products (PPCPs) in the environmental systems: A review. Int. J. Environ. Res. Public Health 2019, 16, 3026. [Google Scholar] [CrossRef]
- Bu, Q.; Wang, B.; Huang, J.; Deng, S.; Yu, G. Pharmaceuticals and personal care products in the aquatic environment in China: A review. J. Hazard. Mater. 2013, 262, 189–211. [Google Scholar] [CrossRef]
- Liu, N.; Jin, X.; Feng, C.; Wang, Z.; Wu, F.; Johnson, A.C.; Xiao, H.; Hollert, H.; Giesy, J.P. Ecological risk assessment of fifty pharmaceuticals and personal care products (PPCPs) in Chinese surface waters: A proposed multiple-level system. Environ. Int. 2020, 136, 105454. [Google Scholar] [CrossRef]
- Sharma, B.M.; Bečanová, J.; Scheringer, M.; Sharma, A.; Bharat, G.K.; Whitehead, P.G.; Klánová, J.; Nizzetto, L. Health and ecological risk assessment of emerging contaminants (pharmaceuticals, personal care products, and artificial sweeteners) in surface and groundwater (drinking water) in the Ganges River Basin, India. Sci. Total Environ. 2019, 646, 1459–1467. [Google Scholar] [CrossRef]
- George, S.E.; Baker, T.R.; Baker, B.B. Nonlethal detection of PFAS bioaccumulation and biomagnification within fishes in an urban-and wastewater-dominant Great Lakes watershed. Environ. Pollut. 2023, 321, 121123. [Google Scholar] [CrossRef]
- Fabbri, E.; Franzellitti, S. Human pharmaceuticals in the marine environment: Focus on exposure and biological effects in animal species. Environ. Toxicol. Chem. 2016, 35, 799–812. [Google Scholar] [CrossRef] [PubMed]
- Ebele, A.J.; Abdallah, M.A.-E.; Harrad, S. Pharmaceuticals and personal care products (PPCPs) in the freshwater aquatic environment. Emerg. Contam. 2017, 3, 1–16. [Google Scholar] [CrossRef]
- Jin, X.; Wang, Y.; Jin, W.; Rao, K.; Giesy, J.P.; Hollert, H.; Richardson, K.L.; Wang, Z. Ecological risk of nonylphenol in China surface waters based on reproductive fitness. Environ. Sci. Technol. 2014, 48, 1256–1262. [Google Scholar] [CrossRef]
- Escapa, C.; Coimbra, R.N.; Khan, M.A.; Neuparth, T.; Santos, M.M.; Otero, M. Assessing the Efficiency of Microalgae in the Removal of Salicylic Acid from Contaminated Water: Insights from Zebrafish Embryo Toxicity Tests. Water 2024, 16, 1874. [Google Scholar] [CrossRef]
- Mojiri, A.; Zhou, J.L.; Ratnaweera, H.; Rezania, S.; Nazari, M. Pharmaceuticals and personal care products in aquatic environments and their removal by algae-based systems. Chemosphere 2022, 288, 132580. [Google Scholar] [CrossRef]
- Fernández-Pinos, M.-C.; Vila-Costa, M.; Arrieta, J.M.; Morales, L.; González-Gaya, B.; Piña, B.; Dachs, J. Dysregulation of photosynthetic genes in oceanic Prochlorococcus populations exposed to organic pollutants. Sci. Rep. 2017, 7, 8029. [Google Scholar] [CrossRef]
- de Wilt, A.; Butkovskyi, A.; Tuantet, K.; Leal, L.H.; Fernandes, T.V.; Langenhoff, A.; Zeeman, G. Micropollutant removal in an algal treatment system fed with source separated wastewater streams. J. Hazard. Mater. 2016, 304, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.-Q.; Kurade, M.B.; Jeon, B.-H. Biodegradation of levofloxacin by an acclimated freshwater microalga, Chlorella vulgaris. Chem. Eng. J. 2017, 313, 1251–1257. [Google Scholar] [CrossRef]
- Hena, S.; Gutierrez, L.; Croué, J.-P. Removal of pharmaceutical and personal care products (PPCPs) from wastewater using microalgae: A review. J. Hazard. Mater. 2021, 403, 124041. [Google Scholar] [CrossRef]
- Zhou, T.; Zhang, Z.; Liu, H.; Dong, S.; Nghiem, L.; Gao, L.; Chaves, A.V.; Zamyadi, A.; Li, X.; Wang, Q. A review on microalgae-mediated biotechnology for removing pharmaceutical contaminants in aqueous environments: Occurrence, fate, and removal mechanism. J. Hazard. Mater. 2022, 443, 130213. [Google Scholar] [CrossRef]
- Fomina, M.; Gadd, G.M. Biosorption: Current perspectives on concept, definition and application. Bioresour. Technol. 2014, 160, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Hifney, A.F.; Zien-Elabdeen, A.; Adam, M.S.; Gomaa, M. Biosorption of ketoprofen and diclofenac by living cells of the green microalgae Chlorella sp. Environ. Sci. Pollut. Res. 2021, 28, 69242–69252. [Google Scholar] [CrossRef]
- Kiki, C.; Rashid, A.; Wang, Y.; Li, Y.; Zeng, Q.; Yu, C.-P.; Sun, Q. Dissipation of antibiotics by microalgae: Kinetics, identification of transformation products and pathways. J. Hazard. Mater. 2020, 387, 121985. [Google Scholar] [CrossRef]
- Bai, X.; Acharya, K. Removal of trimethoprim, sulfamethoxazole, and triclosan by the green alga Nannochloris sp. J. Hazard. Mater. 2016, 315, 70–75. [Google Scholar] [CrossRef]
- Xie, P.; Chen, C.; Zhang, C.; Su, G.; Ren, N.; Ho, S.-H. Revealing the role of adsorption in ciprofloxacin and sulfadiazine elimination routes in microalgae. Water Res. 2020, 172, 115475. [Google Scholar] [CrossRef]
- da Silva Rodrigues, D.A.; da Cunha, C.C.R.F.; do Espirito Santo, D.R.; de Barros, A.L.C.; Pereira, A.R.; de Queiroz Silva, S.; da Fonseca Santiago, A.; de Cássia Franco Afonso, R.J. Removal of cephalexin and erythromycin antibiotics, and their resistance genes, by microalgae-bacteria consortium from wastewater treatment plant secondary effluents. Environ. Sci. Pollut. Res. 2021, 28, 67822–67832. [Google Scholar] [CrossRef]
- Hom-Diaz, A.; Jaén-Gil, A.; Rodríguez-Mozaz, S.; Barceló, D.; Vicent, T.; Blánquez, P. Insights into removal of antibiotics by selected microalgae (Chlamydomonas reinhardtii, Chlorella sorokiniana, Dunaliella tertiolecta and Pseudokirchneriella subcapitata). Algal Res. 2022, 61, 102560. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, L.; Han, Y.; Fang, J.; Wang, H. Degradation and metabolic pathways of sulfamethazine and enrofloxacin in Chlorella vulgaris and Scenedesmus obliquus treatment systems. Environ. Sci. Pollut. Res. 2020, 27, 28198–28208. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Liu, Y.; Zhang, J. Transcriptomic mechanisms for the promotion of cyanobacterial growth against eukaryotic microalgae by a ternary antibiotic mixture. Environ. Sci. Pollut. Res. 2022, 29, 58881–58891. [Google Scholar] [CrossRef]
- Teoh, M.-L.; Sanusi, N.S.; Wong, C.-Y.; Beardall, J. Effects of the sunscreen ultraviolet filter, oxybenzone, on green microalgae. Adv. Polar Sci. 2020, 31, 112–123. [Google Scholar]
- Michelon, W.; Matthiensen, A.; Viancelli, A.; Fongaro, G.; Gressler, V.; Soares, H.M. Removal of veterinary antibiotics in swine wastewater using microalgae-based process. Environ. Res. 2022, 207, 112192. [Google Scholar] [CrossRef] [PubMed]
- Šunta, U.; Prosenc, F.; Žagar Soderžnik, K.; Griessler Bulc, T.; Bavcon Kralj, M. Impact of microalgal biomass and microplastics on the sorption behaviour of pesticides in soil: A comparative study. Environ. Sci. Eur. 2025, 37, 57. [Google Scholar] [CrossRef]
- Ding, T.; Hou, Z.; Zhou, H.; Liu, L. Microplastics Alter the Distribution and Toxic Potential of Typical Pharmaceuticals in Aqueous Solutions: Mechanisms and Theory Calculations. ACS EST Water 2025, 5, 5605–5613. [Google Scholar]
- Zhao, W.; You, J.; Yin, S.; Yang, H.; He, S.; Feng, L.; Li, J.; Zhao, Q.; Wei, L. Extracellular polymeric substances—Antibiotics interaction in activated sludge: A review. Environ. Sci. Ecotechnol. 2023, 13, 100212. [Google Scholar]
- Yehya, T.; Favier, L.; Audonnet, F.; Fayad, N.; Bahry, H.; Bahrim, G.E.; Vial, C. Towards a better understanding of the removal of carbamazepine by Ankistrodesmus braunii: Investigation of some key parameters. Appl. Sci. 2020, 10, 8034. [Google Scholar] [CrossRef]
- Kariyawasam, T.; Helvig, C.; Petkovich, M.; Vriens, B. Pharmaceutical removal from wastewater by introducing cytochrome P450s into microalgae. Microb. Biotechnol. 2024, 17, e14515. [Google Scholar] [CrossRef]
- Liang, L.; Bai, X.; Hua, Z. Enhancement of the immobilization on microalgae protective effects and carbamazepine removal by Chlorella vulgaris. Environ. Sci. Pollut. Res. 2022, 29, 79567–79578. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Sandoval, D.S.; González-Ortega, O.; Vazquez-Martínez, J.; García de la Cruz, R.F.; Soria-Guerra, R.E. Diclofenac removal by the microalgae species Chlorella vulgaris, Nannochloropsis oculata, Scenedesmus acutus, and Scenedesmus obliquus. 3 Biotech 2022, 12, 210. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, W.; Li, J.; Yuan, M.; Zhang, J.; Xu, F.; Xu, H.; Zheng, X.; Wang, L. Ecotoxicological effects of sulfonamides and fluoroquinolones and their removal by a green alga (Chlorella vulgaris) and a cyanobacterium (Chrysosporum ovalisporum). Environ. Pollut. 2020, 263, 114554. [Google Scholar] [CrossRef]
- Villar-Navarro, E.; Baena-Nogueras, R.M.; Paniw, M.; Perales, J.A.; Lara-Martín, P.A. Removal of pharmaceuticals in urban wastewater: High rate algae pond (HRAP) based technologies as an alternative to activated sludge based processes. Water Res. 2018, 139, 19–29. [Google Scholar] [CrossRef]
- Vassalle, L.; García-Galán, M.J.; Aquino, S.F.; Afonso, R.J.d.C.F.; Ferrer, I.; Passos, F.; Mota, C.R. Can high rate algal ponds be used as post-treatment of UASB reactors to remove micropollutants? Chemosphere 2020, 248, 125969. [Google Scholar] [CrossRef]
- Jiménez-Bambague, E.M.; Florez-Castillo, J.S.; Gómez-Angulo, R.D.; Morales-Acosta, P.A.; Peña-Salamanca, E.J.; Machuca-Martínez, F.; Madera-Parra, C.A. Cell growth and removal capacity of ibuprofen and diclofenac by Parachlorella kessleri at bench scale. J. Chem. Technol. Biotechnol. 2022, 97, 1416–1423. [Google Scholar] [CrossRef]
- Silva, A.; Coimbra, R.N.; Escapa, C.; Figueiredo, S.A.; Freitas, O.M.; Otero, M. Green microalgae Scenedesmus obliquus utilization for the adsorptive removal of nonsteroidal anti-inflammatory drugs (NSAIDs) from water samples. Int. J. Environ. Res. Public Health 2020, 17, 3707. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.-Q.; Govindwar, S.; Kurade, M.B.; Paeng, K.-J.; Roh, H.-S.; Khan, M.A.; Jeon, B.-H. Toxicity of sulfamethazine and sulfamethoxazole and their removal by a green microalga, Scenedesmus obliquus. Chemosphere 2019, 218, 551–558. [Google Scholar] [CrossRef]
- Bano, F.; Malik, A.; Ahammad, S.Z. Removal of estradiol, diclofenac, and triclosan by naturally occurring microalgal consortium obtained from wastewater. Sustainability 2021, 13, 7690. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, Y.; Li, R.; Yin, Q.; Song, C. Removal of cefradine by Chlorella sp. L166 and Scenedesmus quadricauda: Toxicity investigation, degradation mechanism and metabolic pathways. Process Saf. Environ. Prot. 2022, 160, 632–640. [Google Scholar] [CrossRef]
- Yang, L.; Ren, L.; Tan, X.; Chu, H.; Chen, J.; Zhang, Y.; Zhou, X. Removal of ofloxacin with biofuel production by oleaginous microalgae Scenedesmus obliquus. Bioresour. Technol. 2020, 315, 123738. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhou, Z.; Ou, L.; Yu, J.; Fu, H.; Yang, A. The Toxicological Effects and Removal Characterization of Carbamazepine Using Chlorella vulgaris in Aqueous Media. Water Air Soil Pollut. 2022, 233, 368. [Google Scholar] [CrossRef]
- Ouada, S.B.; Ali, R.B.; Cimetiere, N.; Leboulanger, C.; Ouada, H.B.; Sayadi, S. Biodegradation of diclofenac by two green microalgae: Picocystis sp. and Graesiella sp. Ecotoxicol. Environ. Saf. 2019, 186, 109769. [Google Scholar] [CrossRef]
- Kariyawasam, T.; Petkovich, M.; Vriens, B. Diclofenac degradation by immobilized Chlamydomonas reinhardtii and Scenedesmus obliquus. MicrobiologyOpen 2024, 13, e70013. [Google Scholar] [CrossRef]
- Poddar, B.; Mandal, S.; Das, S.; Mukherjee, A. Interactive toxicity effects of metronidazole, diclofenac, ibuprofen, and differently functionalized nanoplastics on marine algae Chlorella sp. Environ. Sci. Process. Impacts 2025, 27, 901–916. [Google Scholar]
- Filote, C.; Roșca, M.; Hlihor, R.M.; Cozma, P.; Simion, I.M.; Apostol, M.; Gavrilescu, M. Sustainable application of biosorption and bioaccumulation of persistent pollutants in wastewater treatment: Current practice. Processes 2021, 9, 1696. [Google Scholar] [CrossRef]
- Mondal, M.; Halder, G.; Oinam, G.; Indrama, T.; Tiwari, O.N. Bioremediation of organic and inorganic pollutants using microalgae. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 223–235. [Google Scholar]
- Akhtar, A.B.T.; Naseem, S.; Yasar, A.; Naseem, Z. Persistent organic pollutants (POPs): Sources, types, impacts, and their remediation. In Environmental Pollution and Remediation; Springer: Singapore, 2021; pp. 213–246. [Google Scholar]
- Kim, Y.A.; Park, J.B.; Woo, M.S.; Lee, S.Y.; Kim, H.Y.; Yoo, Y.H. Persistent organic pollutant-mediated insulin resistance. Int. J. Environ. Res. Public Health 2019, 16, 448. [Google Scholar] [CrossRef]
- Wang, H.; Guo, R.; Ki, J.-S. 6.0 K microarray reveals differential transcriptomic responses in the dinoflagellate Prorocentrum minimum exposed to polychlorinated biphenyl (PCB). Chemosphere 2018, 195, 398–409. [Google Scholar] [CrossRef]
- Othman, H.B.; Pick, F.R.; Hlaili, A.S.; Leboulanger, C. Effects of polycyclic aromatic hydrocarbons on marine and freshwater microalgae—A review. J. Hazard. Mater. 2022, 441, 129869. [Google Scholar] [CrossRef]
- Gardia-Parège, C.; Kim Tiam, S.; Budzinski, H.; Mazzella, N.; Devier, M.-H.; Morin, S. Pesticide toxicity towards microalgae increases with environmental mixture complexity. Environ. Sci. Pollut. Res. 2022, 29, 29368–29381. [Google Scholar] [CrossRef] [PubMed]
- Chalifour, A.; Tam, N.F.-Y. Tolerance of cyanobacteria to the toxicity of BDE-47 and their removal ability. Chemosphere 2016, 164, 451–461. [Google Scholar] [CrossRef]
- Liu, D.; Qv, M.; Dai, D.; Wang, X.; Zhu, L. Toxic responses of freshwater microalgae Chlorella sorokiniana due to exposure of flame retardants. Chemosphere 2023, 310, 136808. [Google Scholar] [CrossRef]
- Liu, D.; Yang, W.; Lv, Y.; Li, S.; Qv, M.; Dai, D.; Zhu, L. Pollutant removal and toxic response mechanisms of freshwater microalgae Chlorella sorokiniana under exposure of tetrabromobisphenol A and cadmium. Chem. Eng. J. 2023, 461, 142065. [Google Scholar] [CrossRef]
- Mustafa, S.; Bhatti, H.N.; Maqbool, M.; Iqbal, M. Microalgae biosorption, bioaccumulation and biodegradation efficiency for the remediation of wastewater and carbon dioxide mitigation: Prospects, challenges and opportunities. J. Water Process Eng. 2021, 41, 102009. [Google Scholar] [CrossRef]
- Tufail, M.A.; Iltaf, J.; Zaheer, T.; Tariq, L.; Amir, M.B.; Fatima, R.; Asbat, A.; Kabeer, T.; Fahad, M.; Naeem, H. Recent advances in bioremediation of heavy metals and persistent organic pollutants: A review. Sci. Total Environ. 2022, 850, 157961. [Google Scholar] [CrossRef]
- Chan, S.S.; Khoo, K.S.; Chew, K.W.; Ling, T.C.; Show, P.L. Recent advances biodegradation and biosorption of organic compounds from wastewater: Microalgae-bacteria consortium—A review. Bioresour. Technol. 2022, 344, 126159. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Singh, M.P.; Kumar, S.; Srivastava, S. Phycoremediation of persistent organic pollutants from wastewater: Retrospect and prospects. In Application of Microalgae in Wastewater Treatment: Volume 1: Domestic and Industrial Wastewater Treatment; Springer: Cham, Switzerland, 2019; pp. 207–235. [Google Scholar]
- Avila, R.; García-Vara, M.; López-García, E.; Postigo, C.; de Alda, M.L.; Vicent, T.; Blánquez, P. Evaluation of an outdoor pilot-scale tubular photobioreactor for removal of selected pesticides from water. Sci. Total Environ. 2022, 804, 150040. [Google Scholar] [CrossRef]
- Hu, N.; Xu, Y.; Sun, C.; Zhu, L.; Sun, S.; Zhao, Y.; Hu, C. Removal of atrazine in catalytic degradation solutions by microalgae Chlorella sp. and evaluation of toxicity of degradation products via algal growth and photosynthetic activity. Ecotoxicol. Environ. Saf. 2021, 207, 111546. [Google Scholar] [CrossRef]
- Nicodemus, T.J.; DiRusso, C.C.; Wilson, M.; Black, P.N. Reactive Oxygen Species (ROS) mediated degradation of organophosphate pesticides by the green microalgae Coccomyxa subellipsoidea. Bioresour. Technol. Rep. 2020, 11, 100461. [Google Scholar] [CrossRef]
- Deng, Z.; Zhu, J.; Yang, L.; Zhang, Z.; Li, B.; Xia, L.; Wu, L. Microalgae fuel cells enhanced biodegradation of imidacloprid by Chlorella sp. Biochem. Eng. J. 2022, 179, 108327. [Google Scholar]
- Lv, M.; Tang, X.; Zhao, Y.; Li, J.; Zhang, B.; Li, L.; Jiang, Y.; Zhao, Y. The toxicity, bioaccumulation and debromination of BDE-47 and BDE-209 in Chlorella sp. under multiple exposure modes. Sci. Total Environ. 2020, 723, 138086. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Ghosh, P.; Thakur, I.S. Development of artificial consortia of microalgae and bacteria for efficient biodegradation and detoxification of lindane. Bioresour. Technol. Rep. 2020, 10, 100415. [Google Scholar] [CrossRef]
- Zheng, S.; Qi, C.; Duan, S. Adsorption and degradation effects of three PAHs by Chlamydomonas reinhardtii. Int. J. Sci. 2019, 6, 203–212. [Google Scholar]
- Yang, W.; Gao, X.; Wu, Y.; Wan, L.; Tan, L.; Yuan, S.; Ding, H.; Zhang, W. The combined toxicity influence of microplastics and nonylphenol on microalgae Chlorella pyrenoidosa. Ecotoxicol. Environ. Saf. 2020, 195, 110484. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Zheng, X.; Yang, M.; Gao, X.; Huang, J.; Zhang, L.; Fan, Z. Toxic effects and mechanisms of PFOA and its substitute GenX on the photosynthesis of Chlorella pyrenoidosa. Sci. Total Environ. 2021, 765, 144431. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, X.; Tang, X.; Zhao, Y. Toxicity of 2, 2′, 4, 4′-tetrabromodiphenyl ether (BDE-47) on the green microalgae Chlorella sp. and the role of cellular oxidative stress. Mar. Pollut. Bull. 2022, 180, 113810. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Zhao, Y.; Li, D.; Zhang, B.; Li, L.; Liu, Z.; Tang, X.; Zhao, Y. The adsorption and absorption kinetics of BDE-47 by Chlorella sp. and the role of extracellular polymer substances influenced by environmental factors. Environ. Res. 2023, 216, 114698. [Google Scholar] [CrossRef] [PubMed]
- Esperanza, M.; Seoane, M.; Servia, M.J.; Cid, Á. Effects of Bisphenol A on the microalga Chlamydomonas reinhardtii and the clam Corbicula fluminea. Ecotoxicol. Environ. Saf. 2020, 197, 110609. [Google Scholar] [CrossRef]
- Volgusheva, A.A.; Todorenko, D.A.; Konyukhov, I.V.; Voronova, E.N.; Pogosyan, S.I.; Plyusnina, T.Y.; Khruschev, S.S.; Antal, T.K. Acclimation Response of Green Microalgae Chlorella sorokiniana to 2, 3′, 4, 4′, 6-Pentachlorobiphenyl. Photochem. Photobiol. 2023, 99, 1106–1114. [Google Scholar] [CrossRef]
- Yang, W.; Huang, X.; Wu, Q.; Shi, J.; Zhang, X.; Ouyang, L.; Crump, D.; Zhang, X.; Zhang, R. Acute toxicity of polychlorinated diphenyl ethers (PCDEs) in three model aquatic organisms (Scenedesmus obliquus, Daphnia magna, and Danio rerio) of different trophic levels. Sci. Total Environ. 2022, 805, 150366. [Google Scholar] [CrossRef]
- Wan, L.; Wu, Y.; Ding, H.; Zhang, W. Toxicity, biodegradation, and metabolic fate of organophosphorus pesticide trichlorfon on the freshwater algae Chlamydomonas reinhardtii. J. Agric. Food Chem. 2020, 68, 1645–1653. [Google Scholar] [CrossRef]
- Luo, J.; Deng, J.; Cui, L.; Chang, P.; Dai, X.; Yang, C.; Li, N.; Ren, Z.; Zhang, X. The potential assessment of green alga Chlamydomonas reinhardtii CC-503 in the biodegradation of benz (a) anthracene and the related mechanism analysis. Chemosphere 2020, 249, 126097. [Google Scholar] [CrossRef]
- Yien, C.Y. Ecotoxicolgical Effects of Atrazine and Endosulfan on the Growth and Biomarker Expression of Three Green Algal Species (Chlorophyta) Under Various Nutrient Conditions. Doctoral Dissertation, International Medical University, Kuala Lumpur, Malaysia, 2021. [Google Scholar]
- Volgusheva, A.A.; He, Y.; Maksimov, G.V.; Maksimov, E.G.; Kukarskikh, G.P.; Antal, T.K.; Rubin, A.B. The resistance of Chlamydomonas reinhardtii cells to the neonicotinoid clothianidin. Ecotoxicology 2025, 34, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Materazzi, M.; Lettieri, P. Fluidized beds for the thermochemical processing of waste. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Speight, J.G. Unconventional gas. In Natural Gas; Speight, J.G., Ed.; Gulf Professional Publishing: Boston, MA, USA, 2019; pp. 59–98. [Google Scholar]
- IPCC; Houghton, J. Revised 1996 IPCC Guidelines for National Greenhouse Gas Inventories: Greenhouse Gas Inventory Workbook; OECD: Paris, France, 1996. [Google Scholar]
- Tripathi, S.; Choudhary, S.; Meena, A.; Poluri, K.M. Carbon capture, storage, and usage with microalgae: A review. Environ. Chem. Lett. 2023, 21, 2085–2128. [Google Scholar] [CrossRef]
- Calatrava, V.; Ballester, D.G.; Dubini, A. Microalgae for bioremediation: Advances, challenges, and public perception on genetic engineering. BMC Plant Biol. 2024, 24, 1261. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Li, X.; Yuan, Y.; Yang, C.; Tang, T.; Zhao, Q.; Sun, Y. Adaptive evolution and carbon dioxide fixation of Chlorella sp. in simulated flue gas. Sci. Total Environ. 2019, 650, 2931–2938. [Google Scholar] [CrossRef]
- Vuppaladadiyam, A.K.; Yao, J.G.; Florin, N.; George, A.; Wang, X.; Labeeuw, L.; Jiang, Y.; Davis, R.W.; Abbas, A.; Ralph, P. Impact of flue gas compounds on microalgae and mechanisms for carbon assimilation and utilization. ChemSusChem 2018, 11, 334–355. [Google Scholar] [CrossRef]
- Morales-Pineda, M.; García-Gómez, M.E.; Bedera-García, R.; García-González, M.; Couso, I. CO2 levels modulate carbon utilization, energy levels and inositol polyphosphate profile in Chlorella. Plants 2022, 12, 129. [Google Scholar] [CrossRef]
- Peng, H.; Wei, D.; Chen, G.; Chen, F. Transcriptome analysis reveals global regulation in response to CO2 supplementation in oleaginous microalga Coccomyxa subellipsoidea C-169. Biotechnol. Biofuels 2016, 9, 151. [Google Scholar] [CrossRef]
- Ma, S.; Li, D.; Yu, Y.; Li, D.; Yadav, R.S.; Feng, Y. Application of a microalga, Scenedesmus obliquus PF3, for the biological removal of nitric oxide (NO) and carbon dioxide. Environ. Pollut. 2019, 252, 344–351. [Google Scholar] [CrossRef]
- Van Den Hende, S.; Vervaeren, H.; Boon, N. Flue gas compounds and microalgae:(Bio-) chemical interactions leading to biotechnological opportunities. Biotechnol. Adv. 2012, 30, 1405–1424. [Google Scholar] [CrossRef]
- Choi, H.I.; Hwang, S.-W.; Kim, J.; Park, B.; Jin, E.; Choi, I.-G.; Sim, S.J. Augmented CO2 tolerance by expressing a single H+-pump enables microalgal valorization of industrial flue gas. Nat. Commun. 2021, 12, 6049. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Xu, G.; Rong, J.; Chen, H.; He, C.; Giordano, M.; Wang, Q. The acclimation of Chlorella to high-level nitrite for potential application in biological NOx removal from industrial flue gases. J. Plant Physiol. 2016, 195, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Aslam, A.; Thomas-Hall, S.R.; Mughal, T.; Zaman, Q.-u.; Ehsan, N.; Javied, S.; Schenk, P.M. Heavy metal bioremediation of coal-fired flue gas using microalgae under different CO2 concentrations. J. Environ. Manag. 2019, 241, 243–250. [Google Scholar] [CrossRef]
- Yadav, G.; Dubey, B.K.; Sen, R. A comparative life cycle assessment of microalgae production by CO2 sequestration from flue gas in outdoor raceway ponds under batch and semi-continuous regime. J. Clean. Prod. 2020, 258, 120703. [Google Scholar] [CrossRef]
- Du, K.; Wen, X.; Wang, Z.; Liang, F.; Luo, L.; Peng, X.; Xu, Y.; Geng, Y.; Li, Y. Integrated lipid production, CO2 fixation, and removal of SO2 and NO from simulated flue gas by oleaginous Chlorella pyrenoidosa. Environ. Sci. Pollut. Res. 2019, 26, 16195–16209. [Google Scholar] [CrossRef]
- Naď, M.; Brummer, V.; Lošák, P.; Máša, V.; Sukačová, K.; Tatarová, D.; Pernica, M.; Procházková, M. Waste-to-energy plants flue gas CO2 mitigation using a novel tubular photobioreactor while producing Chlorella algae. J. Clean. Prod. 2023, 385, 135721. [Google Scholar] [CrossRef]
- Wang, Z.; Cheng, J.; Zhang, X.; Yang, W.; Park, J.-Y.; Kim, H.-T.; Xu, L.-H. Spermidine protects Chlorella sp. from oxidative damage caused by SO2 in flue gas from coal-fired power plants. ACS Sustain. Chem. Eng. 2020, 8, 15179–15188. [Google Scholar] [CrossRef]
- Wang, Z.; Cheng, J.; Zhang, X.; Chen, L.; Liu, J. Metabolic pathways of Chlorella sp. cells induced by exogenous spermidine against nitric oxide damage from coal-fired flue gas. Bioresour. Technol. 2021, 328, 124827. [Google Scholar] [CrossRef]
- Yu, Q.; Yin, M.; Chen, Y.; Liu, S.; Wang, S.; Li, Y.; Cui, H.; Yu, D.; Ge, B.; Huang, F. Simultaneous carbon dioxide sequestration and nitrate removal by Chlorella vulgaris and Pseudomonas sp. consortium. J. Environ. Manag. 2023, 333, 117389. [Google Scholar] [CrossRef]
- Cheng, D.; Li, X.; Yuan, Y.; Zhao, Q. Kinetic model for effects of simulated flue gas onto growth profiles of Chlorella sp. AE10 and Chlorella sp. Cv. Biotechnol. Appl. Biochem. 2020, 67, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Varshney, P.; Beardall, J.; Bhattacharya, S.; Wangikar, P.P. Effect of elevated carbon dioxide and nitric oxide on the physiological responses of two green algae, Asterarcys quadricellulare and Chlorella sorokiniana. J. Appl. Phycol. 2020, 32, 189–204. [Google Scholar] [CrossRef]
- Huang, G.; Fan, W.; Ren, Q.; He, H. Transcriptome Differential Analysis of Chlorella sp. Cultivated with Simulated Flue Gases. Environ. Prog. Sustain. Energy 2019, 38, 13217. [Google Scholar] [CrossRef]
- Ayatollahi, S.Z.; Esmaeilzadeh, F.; Mowla, D. Integrated CO2 capture, nutrients removal and biodiesel production using Chlorella vulgaris. J. Environ. Chem. Eng. 2021, 9, 104763. [Google Scholar] [CrossRef]
- Hariz, H.B.; Takriff, M.S.; Yasin, N.H.M.; Ba-Abbad, M.M.; Hakimi, N.I.N.M. Potential of the microalgae-based integrated wastewater treatment and CO2 fixation system to treat Palm Oil Mill Effluent (POME) by indigenous microalgae; Scenedesmus sp. and Chlorella sp. J. Water Process Eng. 2019, 32, 100907. [Google Scholar] [CrossRef]
- Kong, W.; Kong, J.; Ma, J.; Lyu, H.; Feng, S.; Wang, Z.; Yuan, P.; Shen, B. Chlorella vulgaris cultivation in simulated wastewater for the biomass production, nutrients removal and CO2 fixation simultaneously. J. Environ. Manag. 2021, 284, 112070. [Google Scholar] [CrossRef]
- Rodas-Zuluaga, L.I.; Castañeda-Hernández, L.; Castillo-Vacas, E.I.; Gradiz-Menjivar, A.; López-Pacheco, I.Y.; Castillo-Zacarías, C.; Boully, L.; Iqbal, H.M.; Parra-Saldívar, R. Bio-capture and influence of CO2 on the growth rate and biomass composition of the microalgae Botryococcus braunii and Scenedesmus sp. J. CO2 Util. 2021, 43, 101371. [Google Scholar] [CrossRef]
- Badgujar, N.R.; Di Capua, F.; Papirio, S.; Pirozzi, F.; Lens, P.N.; Esposito, G. CO2 Biofixation by Chlamydomonas reinhardtii Using Different CO2 Dosing Strategies. In Frontiers in Water-Energy-Nexus—Nature-Based Solutions, Advanced Technologies and Best Practices for Environmental Sustainability, Proceedings of the 2nd WaterEnergyNEXUS Conference, Salerno, Italy, 14-17 November 2018; Springer: Cham, Switzerland, 2020; pp. 321–324. [Google Scholar]
- Hu, X.; Wei, X.; Yang, Y.; Zhang, X.; Chen, X.; Tian, J.; Zhao, J.; Yu, X. Enhancement performance of CO2 on the organic toxicity removal of sludge by Scenedesmus obliquus with proteomics analysis. J. CO2 Util. 2022, 61, 102038. [Google Scholar] [CrossRef]
- Cruz, T.J.; Calixto, G.Q.; Câmara, F.R.d.A.; Teixeira, D.I.; Braga, R.M.; Pergher, S.B. Cultivation of Chlorella sp. in a Closed System Using Mining Wastewater and Simulated Flue Gas: Biomass Production and CO2 Fixation Potential. Sustain. Chem. 2025, 6, 11. [Google Scholar] [CrossRef]
- Alimi, O.S.; Farner Budarz, J.; Hernandez, L.M.; Tufenkji, N. Microplastics and nanoplastics in aquatic environments: Aggregation, deposition, and enhanced contaminant transport. Environ. Sci. Technol. 2018, 52, 1704–1724. [Google Scholar] [CrossRef]
- Rochman, C.M.; Hoh, E.; Hentschel, B.T.; Kaye, S. Long-term field measurement of sorption of organic contaminants to five types of plastic pellets: Implications for plastic marine debris. Environ. Sci. Technol. 2013, 47, 1646–1654. [Google Scholar] [CrossRef]
- Ivleva, N.P. Chemical analysis of microplastics and nanoplastics: Challenges, advanced methods, and perspectives. Chem. Rev. 2021, 121, 11886–11936. [Google Scholar] [CrossRef]
- Mendoza, L.M.R.; Karapanagioti, H.; Álvarez, N.R. Micro (nanoplastics) in the marine environment: Current knowledge and gaps. Curr. Opin. Environ. Sci. Health 2018, 1, 47–51. [Google Scholar] [CrossRef]
- Ma, H.; Pu, S.; Liu, S.; Bai, Y.; Mandal, S.; Xing, B. Microplastics in aquatic environments: Toxicity to trigger ecological consequences. Environ. Pollut. 2020, 261, 114089. [Google Scholar] [CrossRef]
- Gao, N.; Ning, R.; Deng, X. Feasibility, challenges, and future prospects of microalgae-based bioremediation technique for removing microplastics from wastewater. Front. Bioeng. Biotechnol. 2023, 11, 1288439. [Google Scholar] [CrossRef]
- Tripathi, M.; Singh, P.; Pathak, S.; Manimekalai, R.; Garg, D.; Dashora, K. Strategies for the remediation of micro-and Nanoplastics from contaminated food and water: Advancements and challenges. J. Xenobiotics 2025, 15, 30. [Google Scholar] [CrossRef]
- Xiao, Y.; Jiang, X.; Liao, Y.; Zhao, W.; Zhao, P.; Li, M. Adverse physiological and molecular level effects of polystyrene microplastics on freshwater microalgae. Chemosphere 2020, 255, 126914. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, X.; Wang, J.; Tan, L. Toxic effects of microplastic on marine microalgae Skeletonema costatum: Interactions between microplastic and algae. Environ. Pollut. 2017, 220, 1282–1288. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Liu, Z.; Wang, C.; Li, S.; Kitamura, Y. Different interaction performance between microplastics and microalgae: The bio-elimination potential of Chlorella sp. L38 and Phaeodactylum tricornutum MASCC-0025. Sci. Total Environ. 2020, 723, 138146. [Google Scholar] [CrossRef] [PubMed]
- Tamayo-Belda, M.; Vargas-Guerrero, J.J.; Martin-Betancor, K.; Pulido-Reyes, G.; González-Pleiter, M.; Leganés, F.; Rosal, R.; Fernandez-Piñas, F. Understanding nanoplastic toxicity and their interaction with engineered cationic nanopolymers in microalgae by physiological and proteomic approaches. Environ. Sci. Nano 2021, 8, 2277–2296. [Google Scholar] [CrossRef]
- Khoshnamvand, M.; Hanachi, P.; Ashtiani, S.; Walker, T.R. Toxic effects of polystyrene nanoplastics on microalgae Chlorella vulgaris: Changes in biomass, photosynthetic pigments and morphology. Chemosphere 2021, 280, 130725. [Google Scholar] [CrossRef]
- Li, X.; Qiu, H.; Zhang, P.; Song, L.; Romero-Freire, A.; He, E. Role of heteroaggregation and internalization in the toxicity of differently sized and charged plastic nanoparticles to freshwater microalgae. Environ. Pollut. 2023, 316, 120517. [Google Scholar] [CrossRef]
- Yang, W.; Gao, P.; Nie, Y.; Huang, J.; Wu, Y.; Wan, L.; Ding, H.; Zhang, W. Comparison of the effects of continuous and accumulative exposure to nanoplastics on microalga Chlorella pyrenoidosa during chronic toxicity. Sci. Total Environ. 2021, 788, 147934. [Google Scholar] [CrossRef]
- Yang, W.; Gao, P.; Ma, G.; Huang, J.; Wu, Y.; Wan, L.; Ding, H.; Zhang, W. Transcriptome analysis of the toxic mechanism of nanoplastics on growth, photosynthesis and oxidative stress of microalga Chlorella pyrenoidosa during chronic exposure. Environ. Pollut. 2021, 284, 117413. [Google Scholar] [CrossRef]
- Giri, S.; Mukherjee, A. Ageing with algal EPS reduces the toxic effects of polystyrene nanoplastics in freshwater microalgae Scenedesmus obliquus. J. Environ. Chem. Eng. 2021, 9, 105978. [Google Scholar] [CrossRef]
- Cunha, C.; Silva, L.; Paulo, J.; Faria, M.; Nogueira, N.; Cordeiro, N. Microalgal-based biopolymer for nano-and microplastic removal: A possible biosolution for wastewater treatment. Environ. Pollut. 2020, 263, 114385. [Google Scholar] [CrossRef]
- Lotfigolsefidi, F.; Davoudi, M.; Sarkhosh, M.; Bonyadi, Z. Removal of microplastics by algal biomass from aqueous solutions: Performance, optimization, and modeling. Sci. Rep. 2025, 15, 501. [Google Scholar] [CrossRef]
- Fu, D.; Zhang, Q.; Fan, Z.; Qi, H.; Wang, Z.; Peng, L. Aged microplastics polyvinyl chloride interact with copper and cause oxidative stress towards microalgae Chlorella vulgaris. Aquat. Toxicol. 2019, 216, 105319. [Google Scholar] [CrossRef]
- Li, Z.; Yi, X.; Zhou, H.; Chi, T.; Li, W.; Yang, K. Combined effect of polystyrene microplastics and dibutyl phthalate on the microalgae Chlorella pyrenoidosa. Environ. Pollut. 2020, 257, 113604. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-R.; Wang, H.-Y. Highly effective removal of microplastics by microalgae Scenedesmus abundans. Chem. Eng. J. 2022, 435, 135079. [Google Scholar] [CrossRef]
- Rummel, C.D.; Schäfer, H.; Jahnke, A.; Arp, H.P.H.; Schmitt-Jansen, M. Effects of leachates from UV-weathered microplastic on the microalgae Scenedesmus vacuolatus. Anal. Bioanal. Chem. 2022, 414, 1469–1479. [Google Scholar] [CrossRef]
- Wang, Q.; Wangjin, X.; Zhang, Y.; Wang, N.; Wang, Y.; Meng, G.; Chen, Y. The toxicity of virgin and UV-aged PVC microplastics on the growth of freshwater algae Chlamydomonas reinhardtii. Sci. Total Environ. 2020, 749, 141603. [Google Scholar] [CrossRef]
- Sun, A.; Xu, L.; Zhou, G.; Yin, E.; Chen, T.; Wang, Y.; Li, X. Roles of polystyrene micro/nano-plastics as carriers on the toxicity of Pb2+ to Chlamydomonas reinhardtii. Chemosphere 2022, 309, 136676. [Google Scholar] [CrossRef]
- Li, Z.; Dong, S.; Huang, F.; Lin, L.; Hu, Z.; Zheng, Y. Toxicological Effects of Microplastics and Sulfadiazine on the Microalgae Chlamydomonas reinhardtii. Front. Microbiol. 2022, 13, 865768. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Xiang, Y.; He, D.; Li, Y.; Zhao, Y.; Wang, S.; Pan, X. Leaching behavior of fluorescent additives from microplastics and the toxicity of leachate to Chlorella vulgaris. Sci. Total Environ. 2019, 678, 1–9. [Google Scholar] [CrossRef]
- Thiagarajan, V.; Iswarya, V.; Seenivasan, R.; Chandrasekaran, N.; Mukherjee, A. Influence of differently functionalized polystyrene microplastics on the toxic effects of P25 TiO2 NPs towards marine algae Chlorella sp. Aquat. Toxicol. 2019, 207, 208–216. [Google Scholar]
- Miloloža, M.; Bule, K.; Prevarić, V.; Cvetnić, M.; Ukić, Š.; Bolanča, T.; Kučić Grgić, D. Assessment of the Influence of Size and Concentration on the Ecotoxicity of Microplastics to Microalgae Scenedesmus sp., Bacterium Pseudomonas putida and Yeast Saccharomyces cerevisiae. Polymers 2022, 14, 1246. [Google Scholar] [CrossRef]
- Li, R.-R.; Wang, B.-L.; Nan, F.-R.; Lv, J.-P.; Liu, X.-D.; Liu, Q.; Feng, J.; Xie, S.-L. Effects of polystyrene nanoplastics on the physiological and biochemical characteristics of microalga Scenedesmus quadricauda. Environ. Pollut. 2023, 319, 120987. [Google Scholar] [CrossRef]
- Feng, L.-J.; Sun, X.-D.; Zhu, F.-P.; Feng, Y.; Duan, J.-L.; Xiao, F.; Li, X.-Y.; Shi, Y.; Wang, Q.; Sun, J.-W. Nanoplastics promote microcystin synthesis and release from cyanobacterial Microcystis aeruginosa. Environ. Sci. Technol. 2020, 54, 3386–3394. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Xu, L.; Zhang, W.; Yang, G.; Zhao, Z.; Wang, Y.; Li, X. Comparative toxic effects of microplastics and nanoplastics on Chlamydomonas reinhardtii: Growth inhibition, oxidative stress, and cell morphology. J. Water Process Eng. 2021, 43, 102291. [Google Scholar] [CrossRef]
- Hazeem, L.J.; Yesilay, G.; Bououdina, M.; Perna, S.; Cetin, D.; Suludere, Z.; Barras, A.; Boukherroub, R. Investigation of the toxic effects of different polystyrene micro-and nanoplastics on microalgae Chlorella vulgaris by analysis of cell viability, pigment content, oxidative stress and ultrastructural changes. Mar. Pollut. Bull. 2020, 156, 111278. [Google Scholar] [CrossRef]
- Natarajan, L.; Omer, S.; Jetly, N.; Jenifer, M.A.; Chandrasekaran, N.; Suraishkumar, G.; Mukherjee, A. Eco-corona formation lessens the toxic effects of polystyrene nanoplastics towards marine microalgae Chlorella sp. Environ. Res. 2020, 188, 109842. [Google Scholar]
- Nasrabadi, A.E.; Bonyadi, Z. Evaluation of the rate of Chlorella vulgaris biofilm on polyvinyl chloride microplastics in aqueous solutions. Results Eng. 2025, 26, 105189. [Google Scholar] [CrossRef]
- Mackay, D.; Celsie, A.K.; Arnot, J.A.; Powell, D.E. Processes influencing chemical biomagnification and trophic magnification factors in aquatic ecosystems: Implications for chemical hazard and risk assessment. Chemosphere 2016, 154, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, Y.; Kong, F.; Li, M.; Xi, M.; Yu, Z. How do trophic magnification factors (TMFs) and biomagnification factors (BMFs) perform on toxic pollutant bioaccumulation estimation in coastal and marine food webs. Reg. Stud. Mar. Sci. 2021, 44, 101797. [Google Scholar] [CrossRef]
- Verhaert, V.; Newmark, N.; D’Hollander, W.; Covaci, A.; Vlok, W.; Wepener, V.; Addo-Bediako, A.; Jooste, A.; Teuchies, J.; Blust, R. Persistent organic pollutants in the Olifants River Basin, South Africa: Bioaccumulation and trophic transfer through a subtropical aquatic food web. Sci. Total Environ. 2017, 586, 792–806. [Google Scholar] [CrossRef]
- Souza, I.C.; Morozesk, M.; Azevedo, V.C.; Mendes, V.A.; Duarte, I.D.; Rocha, L.D.; Matsumoto, S.T.; Elliott, M.; Baroni, M.V.; Wunderlin, D.A. Trophic transfer of emerging metallic contaminants in a neotropical mangrove ecosystem food web. J. Hazard. Mater. 2021, 408, 124424. [Google Scholar] [CrossRef]
- Zheng, R.; Liu, Y.; Zhang, Z. Trophic transfer of heavy metals through aquatic food web in the largest mangrove reserve of China. Sci. Total Environ. 2023, 899, 165655. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Fu, S.; Zou, H.; Su, Y.; Zhang, Y. Effects of nanoplastics on microalgae and their trophic transfer along the food chain: Recent advances and perspectives. Environ. Sci. Process. Impacts 2021, 23, 1873–1883. [Google Scholar] [CrossRef]
- Amorim, M.L.; Soares, J.; Coimbra, J.S.d.R.; Leite, M.d.O.; Albino, L.F.T.; Martins, M.A. Microalgae proteins: Production, separation, isolation, quantification, and application in food and feed. Crit. Rev. Food Sci. Nutr. 2021, 61, 1976–2002. [Google Scholar] [CrossRef]
- Ma, M.; Hu, Q. Microalgae as feed sources and feed additives for sustainable aquaculture: Prospects and challenges. Rev. Aquac. 2024, 16, 818–835. [Google Scholar] [CrossRef]
- Tacon, A.G.; Lemos, D.; Metian, M. Fish for health: Improved nutritional quality of cultured fish for human consumption. Rev. Fish. Sci. Aquac. 2020, 28, 449–458. [Google Scholar] [CrossRef]
- Guedes, A.C.; Sousa-Pinto, I.; Malcata, F.X. Application of microalgae protein to aquafeed. In Handbook of Marine Microalgae; Elsevier: Amsterdam, The Netherlands, 2015; pp. 93–125. [Google Scholar]
- Vasseur, P.; Masfaraud, J.-F.; Blaise, C. Ecotoxicology, revisiting its pioneers. Environ. Sci. Pollut. Res. 2021, 28, 3852–3857. [Google Scholar]
- Naz, S.; Habib, S.S.; Arshad, M.; Majeed, S.; Acar, Ü.; Kesbiç, O.S.; Mohany, M.; Aragona, F.; Fazio, F. Human health risk of heavy metal biomagnification: Trophic transfer patterns in aquatic ecosystems. J. Trace Elem. Med. Biol. 2025, 91, 127704. [Google Scholar] [CrossRef]
- Wang, Z.; Xia, B.; Chen, B.; Sun, X.; Zhu, L.; Zhao, J.; Du, P.; Xing, B. Trophic transfer of TiO2 nanoparticles from marine microalga (Nitzschia closterium) to scallop (Chlamys farreri) and related toxicity. Environ. Sci. Nano 2017, 4, 415–424. [Google Scholar] [CrossRef]
- Wei, S.; Xu, P.; Mao, Y.; Shi, Y.; Liu, W.; Li, S.; Tu, Z.; Chen, L.; Hu, M.; Wang, Y. Differential intestinal effects of water and foodborne exposures of nano-TiO2 in the mussel Mytilus coruscus under elevated temperature. Chemosphere 2024, 355, 141777. [Google Scholar] [CrossRef]
- Wang, C.; Yan, C.; Qiu, J.; Liu, C.; Yan, Y.; Ji, Y.; Wang, G.; Chen, H.; Li, Y.; Li, A. Food web biomagnification of the neurotoxin β-N-methylamino-L-alanine in a diatom-dominated marine ecosystem in China. J. Hazard. Mater. 2021, 404, 124217. [Google Scholar] [CrossRef]
- Sokołowski, A.; Mordec, M.; Caban, M.; Øverjordet, I.B.; Wielogórska, E.; Włodarska-Kowalczuk, M.; Balazy, P.; Chełchowski, M.; Lepoint, G. Bioaccumulation of pharmaceuticals and stimulants in macrobenthic food web in the European Arctic as determined using stable isotope approach. Sci. Total Environ. 2024, 909, 168557. [Google Scholar] [CrossRef] [PubMed]
- Babaei, M.; Tayemeh, M.B.; Jo, M.S.; Yu, I.J.; Johari, S.A. Trophic transfer and toxicity of silver nanoparticles along a phytoplankton-zooplankton-fish food chain. Sci. Total Environ. 2022, 842, 156807. [Google Scholar] [CrossRef]
- Hedgespeth, M.L.; Taylor, D.L.; Balint, S.; Schwartz, M.; Cantwell, M.G. Ecological characteristics impact PFAS concentrations in a US North Atlantic food web. Sci. Total Environ. 2023, 880, 163302. [Google Scholar] [CrossRef]
- Deprá, M.C.; Nörnberg, M.L.; Schneider, A.T.; Dias, R.R.; Zepka, L.Q.; Jacob-Lopes, E. Microalgae: Green cell biofactories in food and feed supply chains. In Advances in Sustainable Applications of Microalgae; Elsevier: Amsterdam, The Netherlands, 2025; pp. 301–315. [Google Scholar]
- Ayub, A.; Rahayu, F.; Khamidah, A.; Antarlina, S.S.; Iswari, K.; Supriyadi, K.; Mufidah, E.; Singh, A.; Chopra, C.; Wani, A.K. Harnessing microalgae as a bioresource for nutraceuticals: Advancing bioactive compound exploration and shaping the future of health and functional food innovation. Discov. Appl. Sci. 2025, 7, 389. [Google Scholar] [CrossRef]
- Mesa, A.P.; Grattz, P.A.C.; Vargas, J.J.V.; Ríos, L.A.; Echeverri, D.O.; Parra, A.M.M. Feasibility of nitrogen and phosphorus removal from treated wastewater using microalgae and potential microalgae use as biofertilizer. J. Water Process Eng. 2025, 70, 107023. [Google Scholar] [CrossRef]
- Faruque, M.O.; Uddin, S.; Mohammed, T.; Hossain, M.M.; Razzak, S.A. Bioremediation and Biofuel Production Potential of Chlorella sorokiniana Cultivated in Petroleum-Derived Produced Water. Arab. J. Sci. Eng. 2025, 1–21. [Google Scholar] [CrossRef]
- Kilic, L.; Liu, J.; Engel, B.; Jafvert, C.T.; Bhatt, P.; Brunnquell, J.; Simsek, H. Biological carbon capture from egg-washing wastewater using microalgae for sustainable biofuel production. Sci. Total Environ. 2025, 966, 178708. [Google Scholar] [CrossRef]
- Sridhar, N.; Manian, R. Advances in biochar production from microalgae: Techniques, challenges, and environmental benefits. Clean Technol. Environ. Policy 2025, 27, 2715–2739. [Google Scholar] [CrossRef]
- Ayilara, M.S.; Babalola, O.O. Bioremediation of environmental wastes: The role of microorganisms. Front. Agron. 2023, 5, 1183691. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, C.; Wang, Y.; Chen, G. A review of the current and emerging detection methods of marine harmful microalgae. Sci. Total Environ. 2022, 815, 152913. [Google Scholar] [CrossRef]
- Rao, N.; Beyer, V.; Thielemans, W.; Muylaert, K. Techno-economic comparison of flocculation combined with dissolved air flotation versus sedimentation for microalgae harvesting. Algal Res. 2024, 81, 103581. [Google Scholar] [CrossRef]
- Geng, Y.; Shaukat, A.; Azhar, W.; Raza, Q.-U.-A.; Tahir, A.; Abideen, M.Z.u.; Zia, M.A.B.; Bashir, M.A.; Rehim, A. Microalgal biorefineries: A systematic review of technological trade-offs and innovation pathways. Biotechnol. Biofuels Bioprod. 2025, 18, 93. [Google Scholar] [CrossRef]
- Kumar, N.; Shukla, P. Microalgal multiomics-based approaches in bioremediation of hazardous contaminants. Environ. Res. 2024, 247, 118135. [Google Scholar] [CrossRef]
- Zhao, W.; Zhu, J.; Yang, S.; Liu, J.; Sun, Z.; Sun, H. Microalgal metabolic engineering facilitates precision nutrition and dietary regulation. Sci. Total Environ. 2024, 951, 175460. [Google Scholar] [CrossRef]
- Pradhan, B.; Bhuyan, P.P.; Nayak, R.; Patra, S.; Behera, C.; Ki, J.-S.; Ragusa, A.; Lukatkin, A.S.; Jena, M. Microalgal phycoremediation: A glimpse into a sustainable environment. Toxics 2022, 10, 525. [Google Scholar] [CrossRef]
- Dell’Anno, F.; Rastelli, E.; Sansone, C.; Brunet, C.; Ianora, A.; Dell’Anno, A. Bacteria, fungi and microalgae for the bioremediation of marine sediments contaminated by petroleum hydrocarbons in the omics era. Microorganisms 2021, 9, 1695. [Google Scholar] [CrossRef]
- Omar, H.H. Algal decolorization and degradation of monoazo and diazo dyes. Pak. J. Biol. Sci. 2008, 11, 1310–1316. [Google Scholar] [CrossRef]
- Gowthami, A.; Marjuk, M.S.; Santhanam, P.; Thirumurugan, R.; Muralisankar, T.; Perumal, P. Marine microalgae–Mediated biodegradation of polystyrene microplastics: Insights from enzymatic and molecular docking studies. Chemosphere 2025, 370, 144024. [Google Scholar] [CrossRef] [PubMed]
- Dutra Molino, J.V.; Saucedo, B.; Kang, K.; Walsh, C.; Diaz, C.J.; Tessman, M.; Simkovsky, R.; Mayfield, S. Efficient secretion of a plastic degrading enzyme from the green algae Chlamydomonas reinhardtii. Sci. Rep. 2025, 15, 24690. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Ye, X.; Bi, H.; Shen, Z. Microalgae biofuels: Illuminating the path to a sustainable future amidst challenges and opportunities. Biotechnol. Biofuels Bioprod. 2024, 17, 10. [Google Scholar] [CrossRef] [PubMed]
- Webster, L.J.; Villa-Gomez, D.; Brown, R.; Clarke, W.; Schenk, P.M. A synthetic biology approach for the treatment of pollutants with microalgae. Front. Bioeng. Biotechnol. 2024, 12, 1379301. [Google Scholar] [CrossRef]
- Dasan, Y.K.; Lam, M.K.; Yusup, S.; Lim, J.W.; Lee, K.T. Life cycle evaluation of microalgae biofuels production: Effect of cultivation system on energy, carbon emission and cost balance analysis. Sci. Total Environ. 2019, 688, 112–128. [Google Scholar] [CrossRef]
- Li, R.; Guo, D.; Li, T.; Zhao, J.; Pan, J. Effect of C: N ratio on treatment of mixed industrial-domestic wastewater by microalgae-bacteria consortium. Biomass Convers. Biorefin. 2025, 15, 10829–10837. [Google Scholar] [CrossRef]

| Pollutant Name (Heavy Metals) | Microalgae | Observed Activity and Removal Efficiency | Analysis * Performed | References |
|---|---|---|---|---|
| Cu, Ni | Mixed culture (Spirogyra, Chlamydomonas, Eudorina, Spirulina) | Adsorption mechanism Removal efficiency: 90.20% Cu and 78.25% Ni. | Growth kinetics, adsorption efficiency, Dry weight analysis, SEM analysis and thermodynamic analysis. | [90] |
| As(III) | Biofilm (Chlorella sp., Cladophora sp., Microspora sp., Gomphonema sp., Nitzschia sp., Navicula sp., Scenedesmus spp.) | Biotransformation and bioaccumulation. 41–63% bioaccumulation. | Biofilm evaluation, biotransformation study, bioaccumulation evaluation if fish models, arsenic content analysis in serum and tissue, antioxidant gene expression analysis by PCR. | [91] |
| Cu, Mo | Chlorella vulgaris, Scenedesmus spinosus | Biosorption; C. vulgaris removal Cu 64.7%, Mo 99.9%, S. spinosus removal Cu (55%), Mo (80.3%). | Tolerance bioassays, bio removal assays, SEM-EDX, CLSM, ash content, lipid analysis, GCMS analysis. | [92] |
| Cd, Ni, Pb | Chlorella vulgaris, Scenedesmus quadricuda, Spirulina platensis | Bioremediation. The consortium removed Pb 89%, Cd 88% and Ni 95%. | Growth kinetics. | [93] |
| Cd, Pb, Cu | Spirulina platensis, Chlorella vulgaris | Biosorption. Removal efficiency: S. platensis Cd 47.84%, Pb 47.39% and Cu 45.04% C. vulgaris Cd 48.54%, Pb 48.30% and Cu 47.72%. | FTIR analysis, adsorption kinetics. | [94] |
| Cd, Cr. | Parachlorella kessleri Bh-2 | Bioaccumulation. Removal efficiency: 94.80% removal. | Genomic DNA extraction, PCR sequencing, phylogenetic analysis, AAS, TEM, polysaccharide analysis, toxicity analysis. | [95] |
| Cu, Cr, Pb, Cd | Chlorella vulgaris | Removal efficiency: 79% Cr, 93% Cd, 72% Cu, and 79% Pb. | Growth kinetics, dry weight analysis, decolorization assay. | [96] |
| Zn, Cd | Chlamydomonas reinhardtii | Biosorption; bioremediation. | Cellular tolerance analysis, cellular uptake analysis. | [97] |
| Cu2+, Na+, Ca2+, Fe3+ | Chlamydomonas microsphaera | Biosorption. | Absorption kinetics, SEM, EDAX, absorption isotherm, pH influence study. | [98] |
| Cd2+, Cu2+, Ni2+, Pb2+ | Costaria costata, Hizikia fusiformis, Gracilaria verrucosa, Codium fragile | Biosorption. Highest adsorption capacity by C. costata. | Solution chemistry effect, SEM, FTIR, and kinetic sorption analysis. | [99] |
| Hg2+ | Nannochloropsis sp. | Bioaccumulation; The maximum adsorption rate 6.96 μg/(g·day) at day 1. For 0.7 ppb concentration of Hg2+ 50% adsorption in 30 min. | Kinetics, morphology by SEM, adsorption isotherms, FTIR spectrometry, fatty acid analysis by GC-MS. | [100] |
| Cu | Nannochloropsis oculata | Cu remediation; adsorption. Removal efficiency: 99.92%. | Cell density, growth rate analysis, lipid and fatty acid profile analysis, FTIR spectrometry. | [101] |
| Cu | Nannochloropsis sp. | Absorption. The adsorption capacity of Cu(II) 5.32 × 10− 1 mmol/g. | FTIR, SEM, EDX, XRD, particle size, charge, adsorption kinetics, sequential desorption. | [102] |
| Cu, Cd | Chlorella salina, Nannochloropsis salina | Bioremediation. | Growth inhibition, protein pattern analysis, gene analysis (RAPD-PCR). | [103] |
| Pollutant Name (PPCPs) | Microalgae | Observed Activity and Removal Efficiency | Analysis Performed | References |
|---|---|---|---|---|
| Diclofenac | Chlorella vulgaris, Nannochloropsis oculata, Scenedesmus acutus, and Scenedesmus obliquus | Biosorption and bioremediation. | Growth kinetics, chlorophyll evaluation, removal efficiency comparison analysis. | [137] |
| Sulfonamides, fluoroquinolones | Chlorella vulgaris, cyanobacterium (Chrysosporum ovalisporum) | Biosorption/bioaccumulation. | Growth inhibition assay, growth kinetics, pigment fluorescence, antioxidant enzyme evaluation, LPO evaluation residual antibiotics. | [138] |
| Ibuprofen, salicylic acid, acetaminophen, diclofenac, tetracycline | Coelastrum sp. | Pharmaceutical removal (mechanism not mentioned). Ibuprofen, salicylic acid, and acetaminophen—89.4–99.8% removal, diclofenac 55%, and tetracycline 100%. | Evaluation of removal efficiency, conventional treatment line efficiency. | [139] |
| EE2—ethinylestradiol, E2—estradiol, ibuprofen, estrone, gemfibrozil, bisphenol A | Chlorella vulgaris, Scenedesmus sp., Westella botryoides, and diatoms species | Photodegradation, bioadsorption and biodegradation. EE2—25.12%, E2—84.91%, ibuprofen 64.8%, estrone 95%, gemfibrozil 39%, and bisphenol A 43%. | Sewage quality analyses, GC-MS analysis, behavior and fate of micropollutants. | [140] |
| Ibuprofen, diclofenac | Parachlorella kessleri | Bioaccumulation and adsorption, growth inhibition, photosynthetic imbalance, and chlorophyll variation. Ibuprofen 51.3% and diclofenac 55.7%. | Growth kinetics, chlorophyll content, adsorption rate/removal efficiency, photolysis analysis. | [141] |
| Salicylic acid, ibuprofen | Scenedesmus Obliquus | Biosorption. Adsorptive removal. Maximum adsorption capacities for salicylic acid 60 mg/g and ibuprofen 12 mg/g. | Adsorption kinetics, thermodynamic adsorption, FTIR, point of zero charge determination, SEM. | [142] |
| Sulfamethazine, sulfamethoxazole | Scenedesmus obliquus | Biosorption, bioaccumulation, and biodegradation as an adaptive mechanism to antibiotics. sulfamethazine 31.4–62.3%, sulfamethoxazole 27.7–46.8%. | Growth kinetics, analysis of biochemical content, elemental analysis, FTIR, HPLC. | [143] |
| Estradiol, Diclofenac, Triclosan | Microalgal consortium—Chlorella sp., Merismopedia sp., Closteriopsis sp., and Scenedesmus sp. | Biodegradation. Growth inhibition and variations in chlorophyll content. Estradiol 91.73%, diclofenac 74.68%, and triclosan 78.47%. | Growth kinetics, chlorophyll analysis IC50 assays, drug adsorption HPLC, SEM, and degradation studies. | [144] |
| Cefradine | Chlorella sp. L166 and Scenedesmus quadricauda | Biodegradation. Three degradation pathways—decarboxylation, hydroxylation, demethylation. 97.27% by Chlorella sp. and 98.50% by Scenedesmus quadricauda. | Growth kinetics, pigment changes, antioxidant enzymes evaluation, removal efficiency, metabolic identification. | [145] |
| Ofloxacin | Scenedesmus obliquus | Ofloxacin stress influenced microalgae photosynthetic system, leading to carbon redistribution, increased lipid accumulation. Ofloxacin 39.24% removal by Scenedesmus obliquus. | HPLC, biomass productivity, biomolecule analysis, pigment content analysis. | [146] |
| Carbamazepine | Chlorella vulgaris | Biodegradation; bioaccumulation; biosorption. Removal efficiency: 79.16%. | Toxicity analysis, photosynthesis influence, antioxidant enzymes activity, removal mechanism. | [147] |
| Diclofenac | Picocystis sp. and Graesiella sp. | Biodegradation; biotransformation. Removal efficiency: Picocystis and Graesiella 73% and 52% of 25 mg/L initial diclofenac concentration. | Growth inhibition, photosynthetic activity, diclofenac removal and degradation intermediates, LCMS analysis. | [148] |
| Diclofenac | Chlamydomonas reinhardtii and Scenedesmus obliquus | Biodegradation; bioaccumulation; biosorption processes. Removal efficiency: Chlamydomonas 78%, Scenedesmus 80.1% (3 days); mixed culture: 91.4–92.3% (3 days), 100% (6 days). | Growth kinetics, diclofenac removal mechanisms (biodegradation, bioaccumulation, abiotic degradation) analysis. | [149] |
| Diclofenac, ibuprofen, metronidazole | Chlorella variabilis | Bioaccumulation; growth inhibition and oxidative stress. | Growth inhibition analysis, ROS production, antioxidant activity, photosynthetic activity, zeta potential analysis, hydrodynamic size measurements. | [150] |
| Pollutant Name (POPs) | Microalgae | Observed Activity and Removal Efficiency | Analysis Performed | References |
|---|---|---|---|---|
| Atrazine | Chlorella sp. | Growth inhibition; Degradation created three by-products—desisopropyl-atrazine, desethyl-atrazine, and dsethyl-desisopropyl-atrazine. Removal efficiency: 64.3–83.0%. | Photocatalytic degradation evaluation, growth kinetics, atrazine removal, GCMS analysis, photosynthetic activity. | [166] |
| Paraoxon, malathion and diazinon | Coccomyxa subellipsoidea | Degradation; inhibition of photosystem II; mitochondrial ROS generation. | Degradation profile testing, GCMS analysis, singlet oxygen degradation assay, ROS-dependent degradation, photosynthesis efficiency. | [167] |
| Imidacloprid | Chlorella sp. | Degradation by hydroxylation and oxidation. Removal efficiency: 57.20–61.66%. | Cell growth kinetics. Assays (SOD, ROS and MDA), pigment evaluation. | [168] |
| BDE-47 or BDE-209 | Chlorella sp. | Debromination; bioaccumulation; degradation; cell growth inhibition. Removal efficiency:82.2–93.5%. | Toxicity profile, bioaccumulation analysis, debromination process, extraction from microalgae and analysis by GC. Evaluation of biomolecules. | [169] |
| Lindane | Scenedesmus sp. ISTGA1 | Bioremediation; synergistic effect on the degradation and detoxification. | Growth kinetics, evaluation of ions, GCMS analysis, detoxification analysis, cytotoxicity and EROD activity analysis by MTT and EROD assays. | [170] |
| Naphthalene, anthracene, benzo[a]pyrene | Chlamydomonas reinhardtii | Adsorption; degradation Removal efficiency: naphthalene 85.5%, anthracene 89.5%, and benzo[a]pyrene 16.9% by degradation, naphthalene 13.1%, anthracene 9.8%, and benzo[a]pyrene 82.0% by adsorption. | Growth kinetics, HPLC analysis, chlorophyll quantification. | [171] |
| Nonylphenol | Chlorella pyrenoidosa | Growth inhibition; synergistic toxicity. | Growth kinetics, chlorophyll fluorescence analysis, toxicity analysis, antioxidant assays (SOD, MDA, and CAT) assays. | [172] |
| PFOA and GenX | Chlorella pyrenoidosa | Growth inhibition; gene down regulation; photosynthesis variations; physical damage; metabolic disorders. | Growth kinetics, photosynthetic parameters evaluation, gene analysis. | [173] |
| BDE-47 | Chlorella sp. | Growth inhibition; photosynthetic damage; low antioxidant level; oxidative stress; programmed cell death. EC50 (96 h)—64.7 μg/L. | Growth kinetics, photosynthetic parameters evaluation, antioxidant evaluation, TEM analysis, ROS detection. | [174] |
| BDE-47 | Chlorella sp. | Extra cellular adsorption; intracellular absorption; bioaccumulation. Removal efficiency: 82.1–84.2%. | Adsorption and absorption kinetics, effects of EPS, light limitation, nitrogen starvation, FTIR analysis, GCMS analysis. | [175] |
| Bisphenol A | Chlamydomonas reinhardtii | Growth inhibition; cytotoxicity; oxidative stress. Bioaccumulation—0.16 pg BPA/cell. | Growth inhibition analysis, toxicity assays, ROS assays, FCM analysis biomarkers analysis. | [176] |
| PCB | Chlorella Sorokiniana | Cytotoxicity; chlorophyll degradation; growth inhibition; recovery of after stress. | Growth kinetics, fluorescent measurement, chlorophyll analysis, oxidation kinetics. | [177] |
| Polychlorinated diphenyl ethers | Scenedesmus obliquus | Low bioavailability; toxicity responses. | Concentration exposure studies, toxicity analysis, toxicity comparison with similar structure chemicals, oxidative stress evaluation. | [178] |
| Trichlorfon | Chlamydomonas reinhardtii | Biotransformation; biodegradation; toxic metabolite; chlorophyll level reduction. | Biodegradation analysis, growth kinetics, pigment analysis, antioxidant enzyme evaluation, HPLC analysis, GCMS analysis, chlorophyll fluorescence, and antioxidant enzymes. | [179] |
| Benz(a)anthracene | Chlamydomonas reinhardtii CC-503 | Sorption and biodegradation. Upregulation of 4 genes and 5 enzymes—degradation process. Removal efficiency: 80% degradation efficiency 10 mg/L. | Growth kinetics, biomolecules and intermediate metabolites analysis by GC, gene regulation evaluation by qRT-PCR, enzyme activity analysis. | [180] |
| Atrazine, endosulfan | Scenedesmus arcuatus, Chlorella sp., Pseudokirchneriella subcapitata | Cytotoxicity; oxidative stress; ROS production; lipid peroxidation. | Oxidative stress biomarkers, photosynthetic biomarkers and morphological biomarkers. | [181] |
| Clothianidin | Chlamydomonas reinhardtii | Biosorption; biodegradation. Cytotoxicity; decrease in photochemical activity; resistance to the insecticide. Removal efficiency: 50% in 10 days. | Growth kinetics, chlorophyll analysis, microscopic evaluation, and pigment analysis. | [182] |
| Pollutant Name (Flue Gas Elements) | Microalgae | Observed Activity and Removal Efficiency | Analysis Performed | References |
|---|---|---|---|---|
| CO2, SO2, and NO | Chlorella pyrenoidosa | Active CO2 biofixation. SO2 neutralization and NO detoxification. Improved growth and lipid production. Removal efficiency: CO2—95.9%, SO2—100% and NO—84.2%. | Growth kinetics, chlorophyll evaluation, lipid/biomolecule evaluation by GCMS and efficiency calculation. | [198] |
| CO2 | Chlorella pyrenoidosa Chick (IPPAS C2) | Biofixation of CO2, improved biomass production. CO2 bio-fixation 0.790 g/Ld (10% CO2). | Growth evaluation, CO2 sequestration, dry weight analysis. | [199] |
| SO2 | Chlorella sp. | Spermidine induced SO2 detoxification, cell protection, enhanced antioxidant activity, improved photosynthesis, and improved biomolecule production. | Growth analysis, chlorophyll fluorescence analysis, maximum photochemical efficiency evaluation, antioxidant assays, biomolecule e valuation by GC, effect of spermidine in presence of SO2. | [200] |
| NO | Chlorella sp. | Spermidine-induced NO assimilation: antioxidant enzymes prevented peroxidation damage, proteomic/metabolomic adjustments improved photosynthesis. | Growth kinetics, spermidine influence analysis, photosynthetic pigment concentration, enzyme assays, proteomic analysis, metabolomics analysis. | [201] |
| CO2, NO3 | Chlorella vulgaris | Bioaccumulation, fixation of CO2, nitrate removal and biomass improvement. | Growth kinetics, photosynthetic activity analysis and carbonic anhydrase activity. | [202] |
| CO2, NOx, SOx | Chlorella sp. AE10; Chlorella sp. Cv | CO2 fixation and tolerance evaluation of CO2, NOx, SOx. | Evaluation of growth kinetics, tolerance levels of CO2, NOx, SOx and photosynthetic activity. | [203] |
| CO2, NO | Asterarcys quadricellulare; Chlorella sorokiniana | Bio-fixation of CO2 and NO. | Non-photochemical quenching analysis, photosynthetic oxygen evolution measurement, fluorescence measurements. | [204] |
| CO2, SO2, NO | Chlorella sp. | Reduced biomass yield and the content of carbohydrate, down regulation of 3 enzymes, growth inhibition. | Growth kinetics, flue gas effect on biomass and biomolecules, gene expressions evaluation. | [205] |
| CO2 | Chlorella vulgaris | Biofixation. 5% CO2 showed best growth capability with enhanced biomass. Removal efficiency: 76.92% CO2. CO2 fixation rate: 0.318 ± 0.009 (g/Ld) for 5% (v/v) CO2 input. | Growth kinetics, morphology analysis, functional group analysis, CO2 removal efficiency, CO2 fixation rate, biodiesel properties. | [206] |
| CO2 | Scenedesmus sp. (UKM9); Chlorella sp. (UKM2) | Fixation of carbon, nitrogen and phosphate. Chlorella sp. recovered CO2 12.435 g/L after 15 days of CO2 fixation. | Growth rate analysis, biomass productivity evaluation, biochemical evaluation, CO2 fixation efficiency. | [207] |
| CO2, NO | Chlorella vulgaris | Carbon fixation. Improved biomass and enhanced biomolecule content CO2 fixation rate 47.51 mg/Ld. | Growth kinetics, biomass productivity, fixation of CO2 and nitrogen, biomolecule analysis with HPLC. | [208] |
| CO2 | Botryococcus braunii; Scenedesmus sp. | Carbon fixation. Optimum CO2 removal efficiency 10% for B. braunii and 20% for Scenedesmus sp. | Growth evaluation, CO2 sequestration analysis, biomolecule evaluation. | [209] |
| CO2 | Chlamydomonas reinhardtii | Carbon fixation. Maximum CO2 sequestration observed at 30% CO2. | Growth analysis, pigment evaluation, CO2 sequestration analysis. | [210] |
| CO2 | Scenedesmus obliquus | Carbon fixation. Improved protein kinase and ATPase activity and oxidative phosphorylation process. | Growth analysis, oxidative stress response evaluation, enzyme activity evaluation and proteomics analysis. | [211] |
| CO2, N2, O2 | Chlorella sp. | Carbon fixation. CO2 fixation rate 0.90 g/L·day. Improved lipids, proteins, and carbohydrates 20.95%, 26.48%, and 9.3%, respectively. | Growth kinetics, biomass productivity and biochemical composition evaluations. | [212] |
| Pollutant Name (Micro- and Nanoplastics) | Microalgae | Observed Activity and Removal Efficiency | Analysis Performed | References |
|---|---|---|---|---|
| Polyvinyl chloride | Chlorella vulgaris | Adsorption; aggregation; precipitation in cells. Dose dependent growth inhibition; high oxidative stress; improved enzymatic activity. | Toxicity analysis, growth kinetics, SEM, biomass production, antioxidant enzyme analysis. | [231] |
| Polystyrene, dibutyl phthalate | Chlorella pyrenoidosa | Growth inhibition; cytotoxicity. Synergistic toxicity effect lead to morphological variations and total pigment content changes. IC50 value (96 h) of dibutyl phthalate was 2.41 mg/L and for polystyrene 6.90–7.19 mg/L. | Growth inhibition analysis, morphology, cytotoxicity analysis. | [232] |
| Polystyrene, poly(methyl methacrylate), polylactide | Scenedesmus abundans | Adsorption; hetero-aggregation Removal efficiency > 84%. | Growth kinetics, flow cytometry, removal efficiency calculations, SEM, EPS quantification. | [233] |
| Polyethylene, polyethylene terephthalate, polypropylene, polystyrene | Scenedesmus vacuolatus | Growth inhibition, oxidative stress, toxicity. | Growth inhibition study, microplastic leachates/degradation products effects, cytotoxicity evaluation. | [234] |
| Virgin polyvinyl chloride, UV-aged polyvinyl chloride | Chlamydomonas reinhardtii | Aged polyvinyl chloride more toxic; growth inhibition; reduction in chlorophyll-a content’ oxidative stress; antioxidant enzyme activation. | Growth kinetics, chlorophyll content evaluation, antioxidant enzyme activity assays, FTIR. | [235] |
| Polystyrene (nano- and microsizes) | Chlamydomonas reinhardtii | Nanoplastics showed higher adsorption capacity and lower desorption rate to lead ions. Growth inhibition, oxidative stress and cytotoxicity. | Adsorption/desorption tests, particle charge evaluation, toxicity analysis, biochemical analysis, elemental analysis and morphological analysis. | [236] |
| Polystyrene (1, 5 µm) | Chlamydomonas reinhardtii | Growth inhibition; cytotoxicity; antioxidant enzyme activity; chlorophyll a content decrease; hetero-aggregation of microplastics. | Growth measurement, antioxidant enzyme analysis, toxicity analysis, Pigment quantification and photosynthetic activity measurement, SEM. | [237] |
| Polystyrene microplastics | Chlorella vulgaris | Leaching depends on time, pH, water sources. High microplastic concentration lead to inhibitory effects. | Growth kinetics, chlorophyll fluorescence, cell distribution analysis. | [238] |
| Polystyrene microplastics (6 μm)—plain polystyrene, aminated polystyrene, carboxylated polystyrene | Chlorella sp. | Toxicity; growth inhibition; oxidative stress; hetero aggregation. | Growth analysis, toxicity evaluation, SEM, oxidative stress determination. | [239] |
| Polyethylene, polypropylene, polystyrene, polyvinyl chloride, polyethylene terephthalate (200–600 µm) | Scenedesmus sp. | Growth inhibition; Smaller size particles induced higher inhibition. | Microplastics preparation, Characterization, growth kinetics, growth inhibition analysis. | [240] |
| Polystyrene | Scenedesmus quadricauda | Cell wall thickening; internalization; aggregation. Highest concentration 200 mg/L—enhanced growth, improved levels of chlorophyll, polysaccharide and soluble proteins. | Growth kinetics, chlorophyll content analysis antioxidant enzyme activity assays, biomolecule quantifications. | [241] |
| Amino-modified polystyrene nanoplastics (50 nm) | Microcystis aeruginosa FACHB 905 (single cells), FACHB 1327 (small colonies), and FACHB 1338 (large colonies) | Enhanced microcystin production, inhibition of photosystem II efficiency, oxidative stress, gene expression, protein up regulation and decreased cell membrane integrity. | Algal growth evaluation, chlorophyll a content analysis, evaluation of cell membrane integrity, antioxidant enzyme activity biomolecules, evaluation of gene expression and proteomic response. | [242] |
| Polystyrene microbeads (100 nm, 100 μm) | Chlamydomonas reinhardtii | Nanosized plastics showed higher growth inhibition via shading effect, oxidative stress and cell damage. Higher antioxidant enzyme activity and cell toxicity also observed. | Algal cultivation evaluation, chlorophyll content analysis, evaluation of biomolecules, cell morphology analysis by flow cytometry and SEM analysis, antioxidant enzyme activity and lipid peroxidation assays. | [243] |
| Polystyrene (20, 50 and 500 nm) | Chlorella vulgaris | Growth inhibition; ROS generation; antioxidant enzyme activity; membrane damage; stress conditions. | Growth kinetics, growth inhibition analysis, chlorophyll a content analysis, LDH assay, FTIR, TEM/SEM, ROS associated oxidative stress determination. | [244] |
| Polystyrene nanoplastics (200 nm) | Chlorella sp. | Cytotoxicity; cell viability reduced; loss of membrane integrity; reduced photosynthetic activity; enhanced ROS level; oxidative stress; EPS formation. | Growth kinetics, growth inhibition by cell viability, elevated intracellular ROS and specific radicals (hydroxyl and superoxide), membrane integrity analysis, percentage of maximum quantum yield of PSII evaluation. | [245] |
| polyvinyl chloride (≤85 µm) | Chlorella vulgaris | Growth inhibition; biofilm inhibition; chlorophyll reduction; EPS production. | Growth kinetics, biofilm formation analysis, FTIR, FESEM, chlorophyll concentration analysis, EPS analysis. | [246] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, R.G.; Chandrasekharan Nair, S.; Joller-Vahter, L.; Kikas, T. Microalgae in Mitigating Industrial Pollution: Bioremediation Strategies and Biomagnification Potential. Biomass 2025, 5, 61. https://doi.org/10.3390/biomass5040061
Bai RG, Chandrasekharan Nair S, Joller-Vahter L, Kikas T. Microalgae in Mitigating Industrial Pollution: Bioremediation Strategies and Biomagnification Potential. Biomass. 2025; 5(4):61. https://doi.org/10.3390/biomass5040061
Chicago/Turabian StyleBai, Renu Geetha, Salini Chandrasekharan Nair, Liina Joller-Vahter, and Timo Kikas. 2025. "Microalgae in Mitigating Industrial Pollution: Bioremediation Strategies and Biomagnification Potential" Biomass 5, no. 4: 61. https://doi.org/10.3390/biomass5040061
APA StyleBai, R. G., Chandrasekharan Nair, S., Joller-Vahter, L., & Kikas, T. (2025). Microalgae in Mitigating Industrial Pollution: Bioremediation Strategies and Biomagnification Potential. Biomass, 5(4), 61. https://doi.org/10.3390/biomass5040061








