Valorization of Amazonian Fruit Biomass for Biosurfactant Production and Nutritional Applications
Abstract
1. Introduction
2. Material and Methods
2.1. Amazon Fruit Residues
2.2. Nutritional Composition of Fruit Residues
2.3. Isolation of Biosurfactant-Producing Bacteria
2.4. Molecular Identification of Biosurfactant-Producing Bacteria
2.5. Biosynthesis and Extraction of Biosurfactants
2.6. Characterization of Biosurfactants
2.7. Statistical Analysis
3. Results
3.1. Characterization of the Biosurfactant-Producing Bacterium
3.2. Production and Physicochemical Characterization of Biosurfactants
3.3. Nutritional Profiles of Fruit Residues
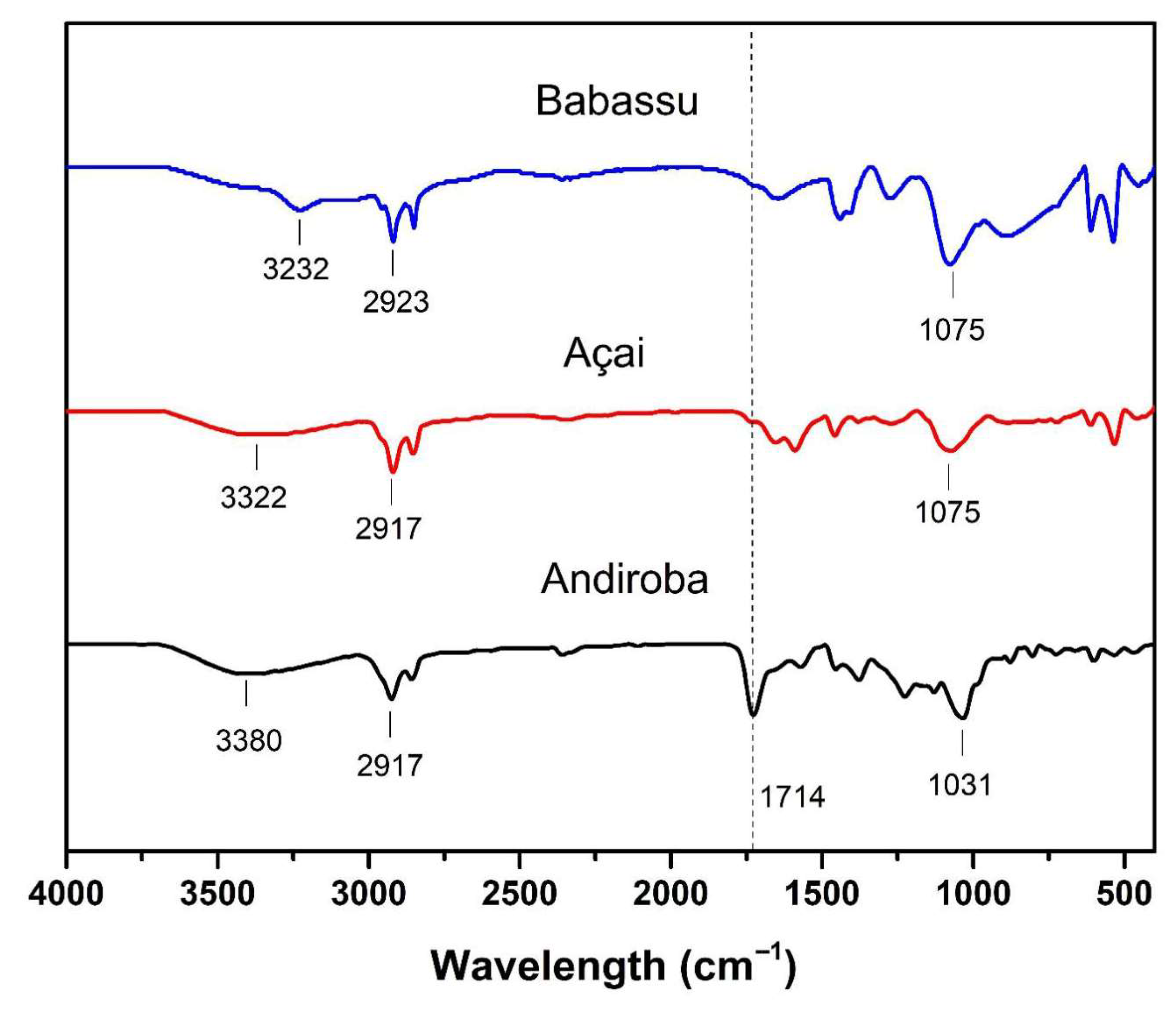
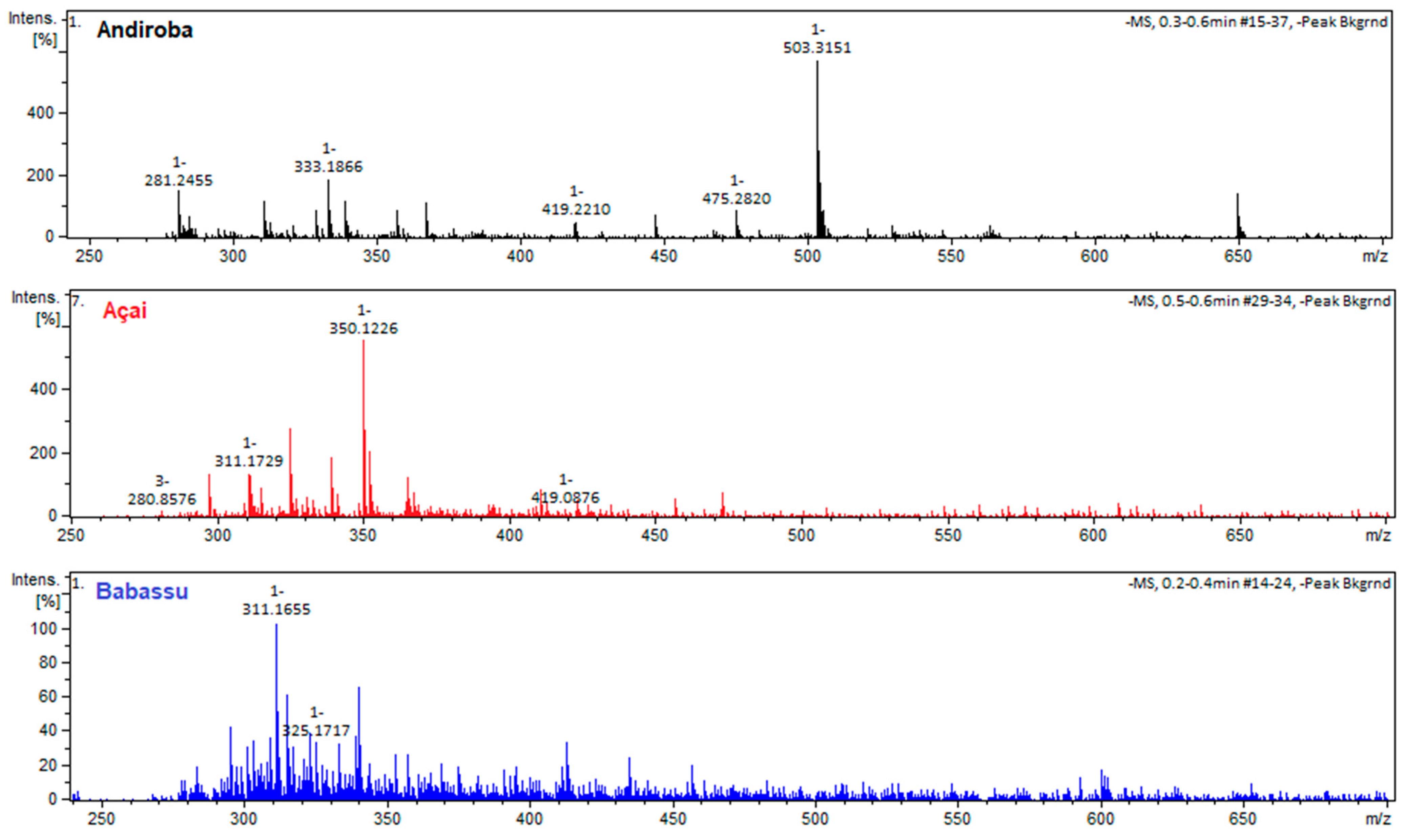
3.4. Biosurfactant Structural Characterization
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statements
Conflicts of Interest
References
- ter Steege, H.; Prado, P.I.; Lima, R.A.F.; Pos, E.; Coelho, L.S.; Filho, D.A.L.; Salomão, R.P.; Amaral, I.L.; Matos, F.D.A.; Castilho, C.V.; et al. Biased-corrected richness estimates for the Amazonian tree flora. Sci. Rep. 2020, 10, e10130. [Google Scholar] [CrossRef] [PubMed]
- Silveira, J.T.; Rosa, A.P.C.; Morais, M.G.; Victoria, F.N.; Costa, J.A.V. An integrative review of Açaí (Euterpe oleracea and Euterpe precatoria): Traditional uses, phytochemical composition, market trends, and emerging applications. Food Res. Int. 2023, 173, e113304. [Google Scholar] [CrossRef] [PubMed]
- Lima, R.C.; Carvalho, A.P.A.; Almeida, A.E.C.C.; Conte-Junior, C.A. Bioactive compounds and benefits of by-products of Amazon babassu oil production: Potential for dietary supplement, biomedical and food applications. Food Funct. 2024, 15, 6232–6253. [Google Scholar] [CrossRef] [PubMed]
- Dias, K.K.B.; Cardoso, A.L.; Costa, A.A.F.; Passos, M.F.; Costa, C.E.F.; Rocha Filho, G.N.; Andrade, E.H.A.; Luque, R.; Nascimento, L.A.S.; Noronha, R.C.R. Biological activities from andiroba (Carapa guianensis Aublet.) and its biotechnological applications: A systematic review. Arab. J. Chem. 2023, 16, e104629. [Google Scholar] [CrossRef]
- Morais, R.A.; Teixeira, G.L.; Ferreira, S.R.S.; Cifuentes, A.; Block, J.M. Nutritional composition and bioactive compounds of native brazilian fruits of the Arecaceae family and its potential applications for health promotion. Nutrients 2022, 14, 4009. [Google Scholar] [CrossRef]
- Steur, G.; ter Steege, H.; Verburg, R.W.; Sabatier, D.; Molino, J.-F.; Bánki, O.S.; Castellanos, H.; Stropp, J.; Fonty, É.; Ruysschaert, S.; et al. Relationships between species richness and ecosystem services in Amazonian forests strongly influenced by biogeographical strata and forest types. Sci. Rep. 2022, 12, e5960. [Google Scholar] [CrossRef]
- Medina, G.S.; Cruz, J.E. Estudos em Agronegócio: Participação Brasileira nas Cadeias Produtivas; Kelps: Goiânia, Brazil, 2021. [Google Scholar]
- Zuñiga-Martínez, B.S.; Domínguez-Avila, J.A.; Robles-Sánchez, R.M.; Ayala-Zavala, J.F.; Villegas-Ochoa, M.A.; González-Aguilar, G.A. Agro-industrial fruit byproducts as health-promoting ingredients used to supplement baked food products. Foods 2022, 11, 3181. [Google Scholar] [CrossRef]
- Yafetto, L.; Odamtten, G.T.; Wiafe-Kwagyan, M. Valorization of agro-industrial wastes into animal feed through microbial fermentation: A review of the global and Ghanaian case. Heliyon 2023, 9, e14814. [Google Scholar] [CrossRef]
- Yadav, N.; Yadav, G.; Bakthavachalam, V.; Potturaja, L.; Roy, J.K.; Elumalai, S. Agro-industrial residue torrefaction to bio-coal: Its physico-chemical characterization and potential applications in energy and environmental protection. Bioresour. Technol. 2025, 418, e131948. [Google Scholar] [CrossRef]
- Ryšavý, J.; Čespiva, J.; Kuboňová, L.; Dej, M.; Szramowiat-Sala, K.; Molchanov, O.; Niedzwiecki, L.; Yan, W.-M.; Thangavel, S. Co-gasification of pistachio shells with wood pellets in a semi-industrial hybrid cross/updraft reactor for producer gas and biochar production. Fire 2024, 7, 87. [Google Scholar] [CrossRef]
- Vurukonda, S.S.K.P.; Fotopoulos, V.; Saeid, A. Production of a rich fertilizer base for plants from waste organic residues by microbial formulation technology. Microorganisms 2024, 12, 541. [Google Scholar] [CrossRef]
- Astudillo, Á.; Rubilar, O.; Briceño, G.; Diez, M.C.; Schalchli, H. Advances in agroindustrial waste as a substrate for obtaining eco-friendly microbial products. Sustainability 2023, 15, 3467. [Google Scholar] [CrossRef]
- Sundaram, T.; Govindarajan, R.K.; Vinayagam, S.; Krishnan, V.; Nagarajan, S.; Gnanasekaran, G.R.; Baek, K.-H.; Rajamani Sekar, S.K. Advancements in biosurfactant production using agro-industrial waste for industrial and environmental applications. Front. Microbiol. 2024, 15, e1357302. [Google Scholar] [CrossRef]
- Vučurović, D.; Bajić, B.; Trivunović, Z.; Dodić, J.; Zeljko, M.; Jevtić-Mučibabić, R.; Dodić, S. Biotechnological utilization of agro-industrial residues and by-products—Sustainable production of biosurfactants. Foods 2024, 13, 711. [Google Scholar] [CrossRef] [PubMed]
- Sá, G.C.S.; Bezerra, P.V.V.; Ramos, E.O.; Orsato, A.; Leite, K.; Feio, A.M.; Pimentel, L.M.S.; Alves, J.A.; Gomes, G.S.; Rodrigues, P.D.; et al. Pseudomonas aeruginosa rhamnolipids produced by andiroba (Carapa guianensis Aubl.) (Sapindales: Meliaceae) biomass waste from Amazon: A potential weapon against Aedes aegypti L. (Diptera: Culicidae). Molecules 2025, 30, 618. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.C.; Torquato, C.A.; Santos, D.A.; Orsato, A.; Leite, K.; Serpeloni, J.M.; Losi-Guembarovski, R.; Pereira, E.R.; Dyna, A.L.; Barboza, M.G.L.; et al. Production and characterization of rhamnolipids by Pseudomonas aeruginosa isolated in the Amazon region, and potential antiviral, antitumor, and antimicrobial activity. Sci. Rep. 2024, 14, e4629. [Google Scholar] [CrossRef] [PubMed]
- Albert, J.S.; Carnaval, A.C.; Flantua, S.G.A.; Lohmann, L.G.; Ribas, C.C.; Riff, D.; Carrillo, J.D.; Fan, Y.; Figueiredo, J.J.P.; Guayasamin, J.M.; et al. Human impacts outpace natural processes in the Amazon. Science 2023, 379, eabo5003. [Google Scholar] [CrossRef]
- AOAC. Official Method 950.46, Moisture in Meat; Association of Official Analytical Collaboration (AOAC) International: Washington, DC, USA, 2007. [Google Scholar]
- AOAC. Official Method 923.03, Ash of Flour. Direct Method; Association of Official Analytical Collaboration (AOAC) International: Washington, DC, USA, 2007. [Google Scholar]
- AOAC. Official Method 981.10, Crude Protein in Meat. Block Digestion Method; Association of Official Analytical Collaboration (AOAC) International: Washington, DC, USA, 2007. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Sá, G.C.S.; Silva, M.A.T.; Silva, D.F.; Santi-Gadelha, T.; Fragoso, S.P.; Madruga, M.S.; Pacheco, M.T.B.; Lima, E.O.; Uchôa, A.F.; Gadelha, C.A.A. Nutritional composition and biological activities (antioxidant and antifungal) of Sesbania virgata (Cav.) Pers. seeds. Rev. Bras. Tecnol. Agroind. 2021, 15, 3648–3672. [Google Scholar] [CrossRef]
- Bodour, A.A.; Miller-Maier, R.M. Application of a modified drop-collapse technique for surfactant quantitation and screening of biosurfactant-producing microorganisms. J. Microbiol. Methods 1998, 32, 273–280. [Google Scholar] [CrossRef]
- Sievers, F.; Higgins, D.G. Clustal Omega for making accurate alignments of many protein sequences. Protein Sci. 2018, 27, 135–145. [Google Scholar] [CrossRef]
- Capella-Gutiérrez, S.; Silla-Martínez, J.M.; Gabaldón, T. trimAl: A tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 2009, 25, 1972–1973. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT: Iterative refinement and additional methods. Methods Mol. Biol. 2014, 1079, 131–146. [Google Scholar]
- Souza, G.S.; Alves, J.A.; Lima, E.P.; Pimentel, L.M.S.; Sá, G.C.S.; Gomes, C.G.; Silva, E.C.; Santos, S.C. Application of rhamnolipid-cell free broth in remediation of soil contaminated with potentially toxic metals: A study of metal contaminant adsorption. Water Air Soil Pollut. 2025, 236, e409. [Google Scholar] [CrossRef]
- Lotfabad, T.B.; Shourian, M.; Roostaazad, R.; Najafabadi, A.R.; Adelzadeh, M.R.; Noghabi, K.A. An efficient biosurfactant-producing bacterium Pseudomonas aeruginosa MR01, isolated from oil excavation areas in south of Iran. Colloids Surf. B Biointerfaces 2009, 69, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Springer, J. Determination of interfacial tension from the profile of a pendant drop using computer-aided image processing. J. Colloid. Interface Sci. 1996, 184, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Orhan, I.E.; Banach, M.; Rollinger, J.M.; Barreca, D.; Weckwerth, W.; Bauer, R.; Bayer, E.A.; et al. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Zheng, X.; Gao, S.; Wu, J.; Hu, X. Recent advances in aptamer-based biosensors for detection of Pseudomonas aeruginosa. Front. Microbiol. 2020, 11, e605229. [Google Scholar] [CrossRef]
- Crone, S.; Vives-Flórez, M.; Kvich, L.; Saunders, A.M.; Malone, M.; Nicolaisen, M.H.; Martínez-García, E.; Rojas-Acosta, C.; Catalina Gomez-Puerto, M.; Calum, H.; et al. The environmental occurrence of Pseudomonas aeruginosa. APMIS 2020, 128, 220–231. [Google Scholar] [CrossRef]
- Soberón-Chávez, G.; González-Valdez, A.; Soto-Aceves, M.P.; Cocotl-Yañez, M. Rhamnolipids produced by Pseudomonas: From molecular genetics to the market. Microb. Biotechnol. 2021, 14, 136–146. [Google Scholar] [CrossRef]
- George, S.; Jayachandran, K. Analysis of rhamnolipid biosurfactants produced through submerged fermentation using orange fruit peelings as sole carbon source. Appl. Biochem. Biotechnol. 2009, 158, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Elshafie, A.E.; Joshi, S.J.; Al-Wahaibi, Y.M.; Al-Bemani, A.S.; Al-Bahry, S.N.; Al-Maqbali, D.; Banat, I.M. Sophorolipids production by Candida bombicola ATCC 22214 and its potential application in microbial enhanced oil recovery. Front. Microbiol. 2015, 6, e1324. [Google Scholar] [CrossRef] [PubMed]
- Pele, M.A.; Ribeaux, D.R.; Vieira, E.R.; Souza, A.F.; Luna, M.A.C.; Rodríguez, D.M.; Andrade, R.F.S.; Alviano, D.S.; Alviano, C.S.; Barreto-Bergter, E.; et al. Conversion of renewable substrates for biosurfactant production by Rhizopus arrhizus UCP 1607 and enhancing the removal of diesel oil from marine soil. Electron. J. Biotechnol. 2019, 38, 40–48. [Google Scholar] [CrossRef]
- Kaskatepe, B.; Yildiz, S. Rhamnolipid biosurfactants produced by Pseudomonas species. Braz. Arch. Biol. Technol. 2016, 59, e16160786. [Google Scholar] [CrossRef]
- Zhu, Z.; Wen, Y.; Yi, J.; Cao, Y.; Liu, F.; McClements, D.J. Comparison of natural and synthetic surfactants at forming and stabilizing nanoemulsions: Tea saponin, Quillaja saponin, and Tween 80. J. Colloid Interface Sci. 2019, 536, 80–87. [Google Scholar] [CrossRef]
- GMI. Global Market Insights. Biosurfactants Market Size. 2025. Available online: https://www.gminsights.com/industry-analysis/biosurfactants-market-report (accessed on 1 July 2025).
- Zhao, K.; Li, J.; Yuan, Y.; Lin, J.; Wang, X.; Guo, Y.; Chu, Y. Nutrient factor-dependent performance of bacterial quorum sensing system during population evolution. Arch. Microbiol. 2020, 202, 2181–2188. [Google Scholar] [CrossRef]
- Carolin, F.C.; Kumar, P.S.; Mohanakrishna, G.; Hemavathy, R.V.; Rangasamy, G.; Aminabhavi, T.M. Sustainable production of biosurfactants via valorisation of industrial wastes as alternate feedstocks. Chemosphere 2023, 312, e137326. [Google Scholar] [CrossRef]
- Al-Marri, S.; Eldos, H.I.; Ashfaq, M.Y.; Saeed, S.; Skariah, S.; Varghese, L.; Mohamoud, Y.A.; Sultan, A.A.; Raja, M.M. Isolation, identification, and screening of biosurfactant-producing and hydrocarbon-degrading bacteria from oil and gas industrial waste. Biotechnol. Rep. 2023, 39, e00804. [Google Scholar] [CrossRef]
- Raaijmakers, J.M.; Bruijn, I.; Nybroe, O.; Ongena, M. Natural functions of lipopeptides from Bacillus and Pseudomonas: More than surfactants and antibiotics. FEMS Microbiol. Rev. 2010, 34, 1037–1062. [Google Scholar] [CrossRef]
- Brazil. Resolution RDC No. 12, 1978—Special Technical Norms Relating to Food and Beverages for Use Throughout Brazilian Territory. Brazilian Ministry of Health. National Commission for Norms and Standards for Food. 1978. Available online: https://bvsms.saude.gov.br/bvs/saudelegis/cnnpa/1978/res0012_30_03_1978.html (accessed on 1 July 2025).
- Liu, S.; Roopesh, M.S.; Tang, J.; Wu, Q.; Qin, W. Recent development in low-moisture foods: Microbial safety and thermal process. Food Res. Int. 2022, 155, e111072. [Google Scholar] [CrossRef]
- Cruz-Paredes, C.; Tájmel, D.; Rousk, J. Can moisture affect temperature dependences of microbial growth and respiration? Soil Biol. Biochem. 2021, 156, e108223. [Google Scholar] [CrossRef]
- Wason, S.; Verma, T.; Subbiah, J. Validation of process technologies for enhancing the safety of low-moisture foods: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4950–4992. [Google Scholar] [CrossRef]
- Nkhata, S.G.; Ayua, E.; Kamau, E.H.; Shingiro, J. Fermentation and germination improve nutritional value of cereals and legumes through activation of endogenous enzymes. Food Sci. Nutr. 2018, 6, 2446–2458. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Paredes, C.; Bang-Andreasen, T.; Christensen, S.; Ekelund, F.; Frøslev, T.G.; Jacobsen, C.S.; Johansen, J.L.; Mortensen, L.H.; Rønn, R.; Vestergård, M.; et al. Bacteria respond stronger than fungi across a steep wood ash-driven pH gradient. Front. For. Glob. Chang. 2021, 4, e781844. [Google Scholar] [CrossRef]
- Turp, G.A.; Turp, S.M.; Ozdemir, S.; Yetilmezsoy, K. Vermicomposting of biomass ash with bio-waste for solubilizing nutrients and its effect on nitrogen fixation in common beans. Environ. Technol. Innov. 2021, 23, e101691. [Google Scholar] [CrossRef]
- Hammer, M.L.A.; Johns, E.A. Tapping an Amazonian plethora: Four medicinal plants of Marajó island, Pará (Brazil). J. Ethnopharmacol. 1993, 40, 53–75. [Google Scholar] [CrossRef]
- Mendonça, F.A.C.; Silva, K.F.S.; Santos, K.K.; Ribeiro Júnior, K.A.L.; Sant’Ana, A.E.G. Activities of some Brazilian plants against larvae of the mosquito Aedes aegypti. Fitoterapia 2005, 76, 629–636. [Google Scholar] [CrossRef]
- Chia, C.Y.; Medeiros, A.D.; Corraes, A.M.S.; Manso, J.E.F.; Silva, C.S.C.; Takiya, C.M.; Vanz, R.L. Healing effect of andiroba-based emulsion in cutaneous wound healing via modulation of inflammation and transforming growth factor beta 3. Acta Cir. Bras. 2018, 33, 1000–1015. [Google Scholar] [CrossRef]
- Noordman, W.H.; Janssen, D.B. Rhamnolipid stimulates uptake of hydrophobic compounds by Pseudomonas aeruginosa. Appl. Environ. Microbiol. 2002, 68, 4502–4508. [Google Scholar] [CrossRef]
- Price, C.L.; Warrilow, A.G.; Rolley, N.J.; Parker, J.E.; Thoss, V.; Kelly, D.E.; Corcionivoschi, N.; Kelly, S.L. Cytochrome P450 168A1 from Pseudomonas aeruginosa is involved in the hydroxylation of biologically relevant fatty acids. PLoS ONE 2022, 17, e0265227. [Google Scholar] [CrossRef]
- Jurado-Martín, I.; Sainz-Mejías, M.; McClean, S. Pseudomonas aeruginosa: An audacious pathogen with an adaptable arsenal of virulence factors. Int. J. Mol. Sci. 2021, 22, 3128. [Google Scholar] [CrossRef]
- Bibi, F.; Ilyas, N.; Saeed, M.; Shabir, S.; Shati, A.A.; Alfaifi, M.Y.; Amesho, K.T.T.; Chowdhury, S.; Sayyed, R.Z. Innovative production of value-added products using agro-industrial wastes via solid-state fermentation. Environ. Sci. Pollut. 2023, 30, 125197–125213. [Google Scholar] [CrossRef] [PubMed]
- Apprich, S.; Tirpanalan, Ö.; Hell, J.; Reisinger, M.; Böhmdorfer, S.; Siebenhandl-Ehn, S.; Novalin, S.; Kneifel, W. Wheat bran-based biorefinery 2: Valorization of products. LWT Food Sci. Technol. 2014, 56, 222–231. [Google Scholar] [CrossRef]
- Ramachandran, S.; Singh, S.K.; Larroche, C.; Soccol, C.R.; Pandey, A. Oil cakes and their biotechnological applications—A review. Bioresour. Technol. 2007, 98, 2000–2009. [Google Scholar] [CrossRef] [PubMed]
- Brazil. Resolution-RDC No. 269, 2005—Technical Regulation on Recommended Daily Intake of Protein, Vitamins and Minerals. Brazilian Ministry of Health. National Health Surveillance Agency. 2005. Available online: https://bvsms.saude.gov.br/bvs/saudelegis/anvisa/2005/rdc0263_22_09_2005.html (accessed on 1 July 2025).
- Peydayesh, M.; Bagnani, M.; Soon, W.L.; Mezzenga, R. Turning food protein waste into sustainable technologies. Chem. Rev. 2023, 123, 2112–2154. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.; Lv, M.; Shao, Z.; Hungwe, M.; Wang, J.; Bai, X.; Xie, J.; Wang, Y.; Geng, W. Metabolism characteristics of lactic acid bacteria and the expanding applications in food industry. Front. Bioeng. Biotechnol. 2021, 9, e612285. [Google Scholar] [CrossRef]
- Begum, W.; Saha, B.; Mandal, U. A comprehensive review on production of bio-surfactants by bio-degradation of waste carbohydrate feedstocks: An approach towards sustainable development. RSC Adv. 2023, 13, 25599–25615. [Google Scholar] [CrossRef]
- Schulz, R.; Slavin, J. Perspective: Defining carbohydrate quality for human health and environmental sustainability. Adv. Nutr. 2021, 12, 1108–1121. [Google Scholar] [CrossRef]
- Panja, A.; Paul, S.; Jha, P.; Ghosh, S.; Prasad, R. Waste and their polysaccharides: Are they worth bioprocessing? Bioresour. Technol. Rep. 2023, 24, e101594. [Google Scholar] [CrossRef]
- Pathak, N.; Singh, S.; Singh, P.; Singh, P.K.; Singh, R.; Bala, S.; Thirumalesh, B.V.; Gaur, R.; Tripathi, M. Valorization of jackfruit waste into value added products and their potential applications. Front. Nutr. 2022, 9, e1061098. [Google Scholar] [CrossRef]
- Salazar-López, N.J.; Domínguez-Avila, J.A.; Yahia, E.M.; Belmonte-Herrera, B.H.; Wall-Medrano, A.; Montalvo-González, E.; González-Aguilar, G.A. Avocado fruit and by-products as potential sources of bioactive compounds. Food Res. Int. 2020, 138, e109774. [Google Scholar] [CrossRef]
- Mata-Sandoval, J.C.; Karns, J.; Torrents, A. Effect of nutritional and environmental conditions on the production and composition of rhamnolipids by P. aeruginosa UG2. Microbiol. Res. 2001, 155, 249–256. [Google Scholar] [CrossRef]
- Cortés-Sánchez, A.J.; Hernández-Sánchez, H.; Jaramillo-Flores, M.E. Biological activity of glycolipids produced by microorganisms: New trends and possible therapeutic alternatives. Microbiol. Res. 2013, 168, 22–32. [Google Scholar] [CrossRef]
- Araujo, J.S.; Rocha, J.C.; Filho, M.A.O.; Ribeiro, V.T.; Vasconcelos, L.T.C.P.; Araujo, N.K.; Neto, E.L.B.; Santos, E.S. Production of rhamnolipids by Pseudomonas aeruginosa AP029-GLVIIA and application on bioremediation and as a fungicide. Biosci. Biotechnol. Res. Asia 2020, 17, 467–477. [Google Scholar] [CrossRef]
- Oliva, A.; García-Carrillo, S.; Ortiz, A.; Aranda, F.J.; Teruel, J.A. Interaction of a dirhamnolipid biosurfactant with sarcoplasmic reticulum calcium ATPase (SERCA1a). Arch. Biochem. Biophys. 2021, 699, e108764. [Google Scholar] [CrossRef]
- Oliva, A.; Teruel, J.A.; Aranda, F.J.; Ortiz, A. Effect of a dirhamnolipid biosurfactant on the structure and phase behaviour of dimyristoylphosphatidylserine model membranes. Colloids Surf. B Biointerfaces 2020, 185, e110576. [Google Scholar] [CrossRef] [PubMed]
- Haesendonck, I.P.H.V.; Vanzeveren, E.C.A. Rhamnolipids in Bakery Products. U.S. Patent 2006/0233935 A1, 19 October 2006. [Google Scholar]
- DeSanto, K. Rhamnolipids-Based Formulations. Patent US 7,985,722 B2, 21 July 2011. [Google Scholar]
- Yang, Y.; Zhang, M.; Li, J.; Su, Y.; Gu, L.; Yang, Y.; Chang, C. Construction of egg white protein particle and rhamnolipid based emulsion gels with β-sitosterol as gelation factor: The application in cookie. Food Hydrocoll. 2022, 127, e107479. [Google Scholar] [CrossRef]
- Azevedo, M.A.; Cerqueira, M.A.; Gonçalves, C.; Amado, I.R.; Teixeira, J.A.; Pastrana, L. Encapsulation of vitamin D3 using rhamnolipids-based nanostructured lipid carriers. Food Chem. 2023, 427, e136654. [Google Scholar] [CrossRef]
- Cheng, C.; Wu, Z.; McClements, D.J.; Zou, L.; Peng, S.; Zhou, W.; Liu, W. Improvement on stability, loading capacity and sustained release of rhamnolipids modified curcumin liposomes. Colloids Surf. B Biointerfaces 2019, 183, e110460. [Google Scholar] [CrossRef]
- Twigg, M.S.; Adu, S.A.; Sugiyama, S.; Marchant, R.; Banat, I.M. Mono-rhamnolipid biosurfactants synthesized by Pseudomonas aeruginosa detrimentally affect colorectal cancer cells. Pharmaceutics 2022, 14, 2799. [Google Scholar] [CrossRef]
- McClure, C.D.; Schiller, N.L. Inhibition of macrophage phagocytosis by Pseudomonas aeruginosa rhamnolipids in vitro and in vivo. Curr. Microbiol. 1996, 33, 109–117. [Google Scholar] [CrossRef]
- Onlamool, T.; Saimmai, A.; Maneerat, S. Antifungal activity of rhamnolipid biosurfactant produced by Pseudomonas aeruginosa A4 against plant pathogenic fungi. Trends Sci. 2022, 20, e6524. [Google Scholar] [CrossRef]
- Li, D.; Tao, W.; Yu, D.; Li, S. Emulsifying properties of rhamnolipids and their in vitro antifungal activity against plant pathogenic fungi. Molecules 2022, 27, 7746. [Google Scholar] [CrossRef] [PubMed]
- Malakar, C.; Kashyap, B.; Bhattacharjee, S.; Chandra Kalita, M.; Mukherjee, A.K.; Deka, S. Antibiofilm and wound healing efficacy of rhamnolipid biosurfactant against pathogenic bacterium Staphylococcus aureus. Microb. Pathog. 2024, 195, e106855. [Google Scholar] [CrossRef] [PubMed]
- Firdose, A.; Chong, N.H.H.; Ramli, R.; Aqma, W.S. Antimicrobial, antiadhesive, and antibiofilm actions of rhamnolipids on ESKAPE pathogens. Lett. Appl. Microbiol. 2023, 76, ovad013. [Google Scholar] [CrossRef]
- Worakitsiri, P.; Pornsunthorntawee, O.; Thanpitcha, T.; Chavadej, S.; Weder, C.; Rujiravanit, R. Synthesis of polyaniline nanofibers and nanotubes via rhamnolipid biosurfactant templating. Synth. Met. 2011, 161, 298–306. [Google Scholar] [CrossRef]
- Hazra, C.; Kundu, D.; Chaudhari, A.; Jana, T. Biogenic synthesis, characterization, toxicity and photocatalysis of zinc sulfide nanoparticles using rhamnolipids from Pseudomonas aeruginosa BS01 as capping and stabilizing agent. J. Chem. Technol. Biotechnol. 2013, 88, 1039–1048. [Google Scholar] [CrossRef]
- Sana, S.; Datta, S.; Biswas, D.; Auddy, B.; Gupta, M.; Chattopadhyay, H. Excision wound healing activity of a common biosurfactant produced by Pseudomonas sp. Wound Med. 2018, 23, 47–52. [Google Scholar] [CrossRef]
- Panbehchouleh, F.A.; Amani, H.; Saeedi, M. Menadione sodium bisulfite loaded rhamnolipid based solid lipid nanoparticle as skin lightener formulation: A green production beside in vitro/in vivo safety index evaluation. Adv. Pharm. Bull. 2024, 14, 623–633. [Google Scholar] [CrossRef]
- Liu, X.-M. Mechanical response of composite materials prepared with polyurethane elastomers and polyvinyl chloride films. J. Mech. Behav. Biomed. Mater. 2023, 146, e106006. [Google Scholar] [CrossRef]
- Rocha, V.A.L.; Castilho, L.V.A.; Castro, R.P.V.; Teixeira, D.B.; Magalhães, A.V.; Abreu, F.d.A.; Cypriano, J.B.S.; Gomez, J.G.C.; Freire, D.M.G. Antibiofilm effect of mono-rhamnolipids and di-rhamnolipids on carbon steel submitted to oil produced water. Biotechnol. Prog. 2021, 37, e3131. [Google Scholar] [CrossRef] [PubMed]
- Alves, J.A.; Silva, E.C.; Sá, G.C.S.; Feio, A.M.; Ramos, E.O.; Gomes, G.S.; Pimentel, L.M.S.; Santos, S.C. Biosurfactant production by Pseudomonas aeruginosa using cocoa (Theobroma cacao L.) residue and its potential in oil dispersion. BioBrasil 2025, 15, 1–12. [Google Scholar] [CrossRef]
- Lopes, P.R.M.; Montagnolli, R.N.; Dilarri, G.; Mendes, C.R.; Cruz, J.M.; Bergamini-Lopes, M.P.; Moreira, B.R.A.; Contiero, J.; Bidoia, E.D. Combination of Pseudomonas aeruginosa and rhamnolipid for bioremediation of soil contaminated with waste lubricant oil. Appl. Biochem. Microbiol. 2024, 60, 627–639. [Google Scholar] [CrossRef]
- Samadi, S.; Amani, H.; Najafpour, G.D.; Kariminezhad, H.; Banaei, A. Investigating the potential of rhamnolipid as an eco-friendly surfactant for environmental protection in oil spill clean-up. Int. J. Environ. Sci. Technol. 2023, 20, 7277–7292. [Google Scholar] [CrossRef]
- Haag, A.P.; Maier, R.M.; Combie, J.; Geesey, G.G. Bacterially derived biopolymers as wood adhesives. Int. J. Adhes. Adhes. 2004, 24, 495–502. [Google Scholar] [CrossRef]
- Sousa, N.S.O.; Souza, E.S.; Canto, E.S.M.; Silva, J.P.A.; Carneiro, L.M.; Franco-de-Sá, J.F.O.; Souza, J.V.B. Amazonian fermentations: An analysis of industrial and social technology as tools for the development of bioeconomy in the region. Braz. J. Biol. 2023, 83, e276493. [Google Scholar] [CrossRef]
- Nathalia, T.C.; Hapsara, V.; Pramono, R. Food waste management on restaurants in Jakarta. Rev. Gest. Soc. Ambient. 2024, 18, e05169. [Google Scholar] [CrossRef]
- Martins, M.R.S.F.; Viana, L.F.; Cappato, L.P. Food waste profile in Brazilian Food and Nutrition Units and the implemented corrective actions. Food Sci. Technol. 2022, 42, e100421. [Google Scholar] [CrossRef]
- Impact Economist. Fixing Food 2021: An Opportunity for G20 Countries to Lead the Way. 2021. Available online: https://impact.economist.com/projects/foodsustainability/g20/fixing-food-2021-paper/food-loss-and-waste/ (accessed on 1 July 2025).

| NCBI Code | Bacterium | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | NR_041702.1 | Pseudomonas knackmussii B13 | 100 | 95.92 | 95.04 | 97.95 | 96.04 | 96.11 | 96.44 | 96.47 | 95.96 |
| 2 | NR_179771.1 | Pseudomonas lalkuanensis PE08 | 95.92 | 100 | 98.60 | 95.98 | 97.59 | 97.93 | 98.52 | 98.47 | 98.39 |
| 3 | NR_181770.1 | Pseudomonas boanensis DB1 | 95.04 | 98.60 | 100 | 96.18 | 97.90 | 97.51 | 97.98 | 97.92 | 98.03 |
| 4 | NR_180597.1 | Pseudomonas nicosulfuronedens LAM 1902 | 97.95 | 95.98 | 96.18 | 100 | 97.90 | 97.11 | 96.71 | 96.61 | 96.78 |
| 5 | NR_179382.1 | Pseudomonas tohonis TUM 18999 | 96.04 | 97.59 | 97.90 | 97.90 | 100 | 98.50 | 98.05 | 97.99 | 98.10 |
| 6 | NR_043289.1 | Pseudomonas otitidis MCC 10330 | 96.11 | 97.93 | 97.51 | 97.11 | 98.50 | 100 | 98.52 | 98.62 | 98.62 |
| 7 | NR_114471.1 | Pseudomonas aeruginosa ATCC 10145 | 96.44 | 98.52 | 97.98 | 96.71 | 98.05 | 98.52 | 100 | 99.93 | 99.87 |
| 8 | - | P23G-02 | 96.47 | 98.47 | 97.92 | 96.61 | 97.99 | 98.62 | 99.93 | 100 | 100 |
| 9 | NR_117678.1 | Pseudomonas aeruginosa DSM 50071 | 95.96 | 98.39 | 98.03 | 96.78 | 98.10 | 98.62 | 99.87 | 100 | 100 |
| Biosurfactant/Control | EI (%) | ST (mN/m) | YD (mg/mL) |
|---|---|---|---|
| Açai biosurfactant | 56.0 ± 2.00 | 35.7 ± 0.31 | 0.8 ± 0.22 |
| Babassu biosurfactant | 54.1 ± 1.00 | 31.6 ± 0.28 | 1.6 ± 0.36 |
| Andiroba biosurfactant | 60.8 ± 2.01 | 29.0 ± 0.08 | 5.6 ± 0.43 |
| Non-inoculated mineral saline medium | 0.0 ± 0.00 | 69.9 ± 0.74 | 0.0 ± 0.00 |
| 1% sodium dodecyl sulfate | 69.9 ± 1.10 | Nt | Nt |
| Parameters | Açai | Babassu | Andiroba |
|---|---|---|---|
| Moisture (%) | 8.81 ± 0.05 | 10.05 ± 0.03 | 2.75 ± 0.03 |
| Ash (%) | 1.31 ± 0.22 | 1.25 ± 0.18 | 4.42 ± 0.08 |
| Total lipids (%) | 3.72 ± 0.48 | 0.98 ± 0.18 | 57.02 ± 0.58 |
| Total proteins (%) | 3.73 ± 0.07 | 2.51 ± 0.09 | 10.76 ± 0.23 |
| Total carbohydrates (%) | 82.43 ± 0.46 | 85.21 ± 0.25 | 25.06 ± 0.46 |
| pH | 5.00 ± 0.07 | 5.91 ± 0.11 | 6.74 ± 0.14 |
| Energetic value (Kcal/100 g) | 378.12 | 359.70 | 656.46 |
| Industry/Activity | P. aeruginosa Strain | Reference |
|---|---|---|
| Pharmaceutical Industry | ||
| Antitumor | PAO1 | [79] |
| BM02 | [17] | |
| Immunomodulation | Mc210 | [80] |
| Antifungal | A4 | [81] |
| ZJU211 | [82] | |
| Antibiofilm | JS29 | [83] |
| UKMP14T | [84] | |
| Nanoparticles for drug delivery | SP4 | [85] |
| BS01 | [86] | |
| Wound healing | C2 | [87] |
| JS29 | [83] | |
| Skin treatment | ATCC 27853 | [88] |
| Automotive Industry | ||
| Mechanical response on automotive, railway and aeronautical materials | Unidentified strain | [89] |
| Steel Industry | ||
| Steel corrosion inhibition | ATCC 9027, LFM634, and ATCC 9027 | [90] |
| Petrochemical Industry | ||
| Bioremediation of soil contaminated | BM02 | [91] |
| LBI | [92] | |
| PTCC 1340 | [93] | |
| Timber Industry | ||
| Wood adhesives | ATCC 9027 | [94] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feio, A.M.; Sá, G.C.d.S.; Orsato, A.; Leite, K.; Pimentel, L.M.S.; Alves, J.d.A.; Gomes, G.S.; Ramos, E.O.; Quintella, C.M.; Fragoso, S.P.; et al. Valorization of Amazonian Fruit Biomass for Biosurfactant Production and Nutritional Applications. Biomass 2025, 5, 60. https://doi.org/10.3390/biomass5040060
Feio AM, Sá GCdS, Orsato A, Leite K, Pimentel LMS, Alves JdA, Gomes GS, Ramos EO, Quintella CM, Fragoso SP, et al. Valorization of Amazonian Fruit Biomass for Biosurfactant Production and Nutritional Applications. Biomass. 2025; 5(4):60. https://doi.org/10.3390/biomass5040060
Chicago/Turabian StyleFeio, Alan Moura, Giulian César da Silva Sá, Alexandre Orsato, Karoline Leite, Lucas Mariano Siqueira Pimentel, Joane de Almeida Alves, Glenda Soares Gomes, Evelly Oliveira Ramos, Cristina M. Quintella, Sinara Pereira Fragoso, and et al. 2025. "Valorization of Amazonian Fruit Biomass for Biosurfactant Production and Nutritional Applications" Biomass 5, no. 4: 60. https://doi.org/10.3390/biomass5040060
APA StyleFeio, A. M., Sá, G. C. d. S., Orsato, A., Leite, K., Pimentel, L. M. S., Alves, J. d. A., Gomes, G. S., Ramos, E. O., Quintella, C. M., Fragoso, S. P., Bitencourt, J. A. P., da Silva, E. C., & Santos, S. C. d. (2025). Valorization of Amazonian Fruit Biomass for Biosurfactant Production and Nutritional Applications. Biomass, 5(4), 60. https://doi.org/10.3390/biomass5040060







