Optimizing the Enzymatic Hydrolysis of Microchloropsis salina Biomass for Single-Cell Oil Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Algae Biomass
2.2. Enzyme Formulation—Hydrolases
2.3. Experimental Setup for Enzymatic Hydrolysis
2.4. Response Surface Methodology and Further Statistical Analysis—Design of Experiment
2.5. Fermentation
2.6. Sugar Analysis
2.7. Fatty Acid Analysis
2.8. Dry Cell Weight
2.9. Optical Density at 600 nm
2.10. Determination of Lipid Titer
3. Results and Discussion
3.1. Enzymatic Hydrolysis
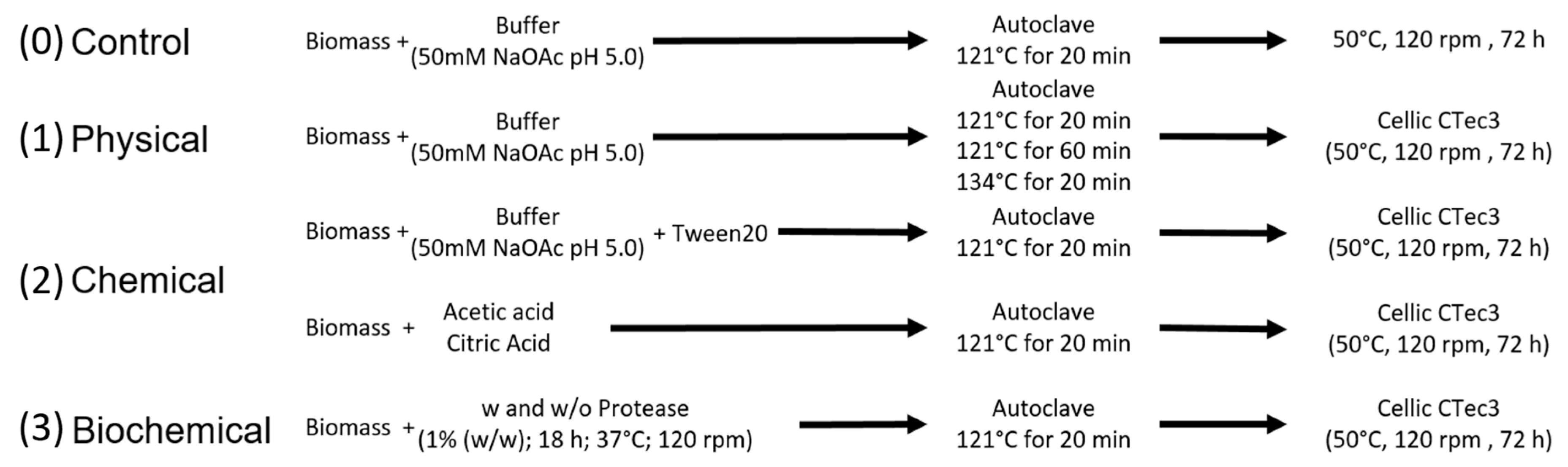
3.1.1. Pre-Treatment
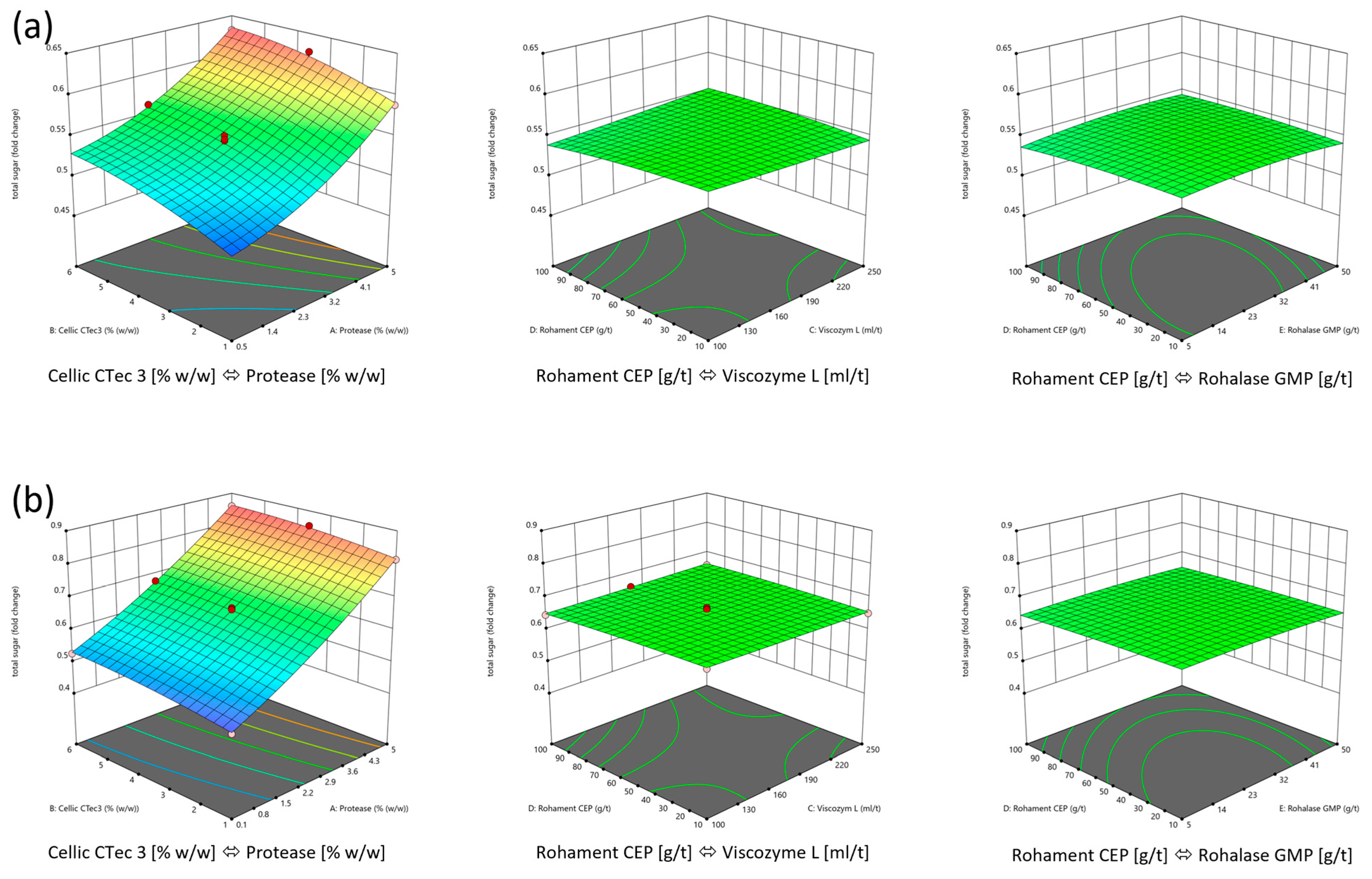
3.1.2. Optimization of the Enzyme Mix
3.1.3. Validation and Scale-Up
3.2. Algae Hydrolysate-Based Fed-Batch Fermentation with Consumption-Based Acetic Acid Feed
3.3. Overview of the Overall Process
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| SCO | Single-cell oil |
| DoE | Design of experiment |
| OD600 | Optical density at 600 nm |
| DCW | Dry cell weight |
| FA | Fatty acid |
| TAG | Triacyl glyceride |
Appendix A
Appendix A.1. Nutrient-Replete Biomass
| Run | Protease [wt%] | Cellic Ctec3 [wt%] | Viscozym L [mL/t] | Rohament CEP [g/t] | Rohalase GMP [g/t] | Pre-Treatment [% Chemical Hydrolysis] | Enzymatic Hydrolysis [% Chemical Hydrolysis] | Total Sugar [% Chemical Hydrolysis] |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.1 | 3.5 | 175 | 55 | 50 | 39 | 10 | 50 |
| 2 | 0.1 | 1 | 175 | 55 | 27.5 | 39 | 8 | 48 |
| 3 | 0.1 | 3.5 | 175 | 55 | 5 | 39 | 11 | 51 |
| 4 | 0.1 | 3.5 | 175 | 10 | 27.5 | 39 | 12 | 51 |
| 5 | 0.1 | 3.5 | 250 | 55 | 27.5 | 39 | 12 | 52 |
| 6 | 0.1 | 3.5 | 175 | 100 | 27.5 | 39 | 12 | 51 |
| 7 | 0.1 | 3.5 | 100 | 55 | 27.5 | 39 | 12 | 52 |
| 8 | 0.1 | 6 | 175 | 55 | 27.5 | 39 | 13 | 52 |
| 9 | 2.55 | 6 | 175 | 10 | 27.5 | 44 | 12 | 56 |
| 10 | 2.55 | 1 | 175 | 55 | 5 | 44 | 7 | 51 |
| 11 | 2.55 | 1 | 100 | 55 | 27.5 | 44 | 7 | 51 |
| 12 | 2.55 | 3.5 | 175 | 55 | 27.5 | 44 | 10 | 55 |
| 13 | 2.55 | 3.5 | 175 | 55 | 27.5 | 44 | 9 | 53 |
| 14 | 2.55 | 6 | 175 | 55 | 5 | 44 | 11 | 55 |
| 15 | 2.55 | 3.5 | 175 | 10 | 50 | 44 | 9 | 54 |
| 16 | 2.55 | 3.5 | 100 | 55 | 50 | 44 | 9 | 54 |
| 17 | 2.55 | 3.5 | 175 | 10 | 5 | 44 | 10 | 54 |
| 18 | 2.55 | 3.5 | 250 | 100 | 27.5 | 44 | 10 | 54 |
| 19 | 2.55 | 6 | 100 | 55 | 27.5 | 44 | 12 | 56 |
| 20 | 2.55 | 1 | 175 | 55 | 50 | 44 | 7 | 51 |
| 21 | 2.55 | 3.5 | 100 | 100 | 27.5 | 44 | 9 | 53 |
| 22 | 2.55 | 6 | 175 | 100 | 27.5 | 44 | 11 | 56 |
| 23 | 2.55 | 3.5 | 175 | 55 | 27.5 | 44 | 11 | 55 |
| 24 | 2.55 | 1 | 175 | 100 | 27.5 | 44 | 7 | 51 |
| 25 | 2.55 | 3.5 | 250 | 10 | 27.5 | 44 | 9 | 54 |
| 26 | 2.55 | 1 | 175 | 10 | 27.5 | 44 | 7 | 51 |
| 27 | 2.55 | 3.5 | 100 | 55 | 5 | 44 | 10 | 54 |
| 28 | 2.55 | 1 | 250 | 55 | 27.5 | 44 | 7 | 52 |
| 29 | 2.55 | 3.5 | 100 | 10 | 27.5 | 44 | 10 | 55 |
| 30 | 2.55 | 3.5 | 175 | 55 | 27.5 | 44 | 10 | 55 |
| 31 | 2.55 | 3.5 | 175 | 55 | 27.5 | 44 | 11 | 55 |
| 32 | 2.55 | 3.5 | 175 | 55 | 27.5 | 44 | 10 | 54 |
| 33 | 2.55 | 3.5 | 175 | 100 | 50 | 44 | 10 | 54 |
| 34 | 2.55 | 3.5 | 175 | 100 | 5 | 44 | 9 | 54 |
| 35 | 2.55 | 3.5 | 250 | 55 | 50 | 44 | 10 | 54 |
| 36 | 2.55 | 3.5 | 250 | 55 | 5 | 44 | 10 | 55 |
| 37 | 2.55 | 6 | 250 | 55 | 27.5 | 44 | 11 | 56 |
| 38 | 2.55 | 6 | 175 | 55 | 50 | 44 | 12 | 56 |
| 39 | 5 | 3.5 | 175 | 100 | 27.5 | 50 | 12 | 62 |
| 40 | 5 | 3.5 | 175 | 55 | 5 | 50 | 13 | 63 |
| 41 | 5 | 3.5 | 175 | 10 | 27.5 | 50 | 12 | 62 |
| 42 | 5 | 3.5 | 100 | 55 | 27.5 | 50 | 12 | 63 |
| 43 | 5 | 3.5 | 175 | 55 | 50 | 50 | 12 | 62 |
| 44 | 5 | 3.5 | 250 | 55 | 27.5 | 50 | 12 | 62 |
| 45 | 5 | 1 | 175 | 55 | 27.5 | 50 | 9 | 59 |
| 46 | 5 | 6 | 175 | 55 | 27.5 | 50 | 13 | 63 |

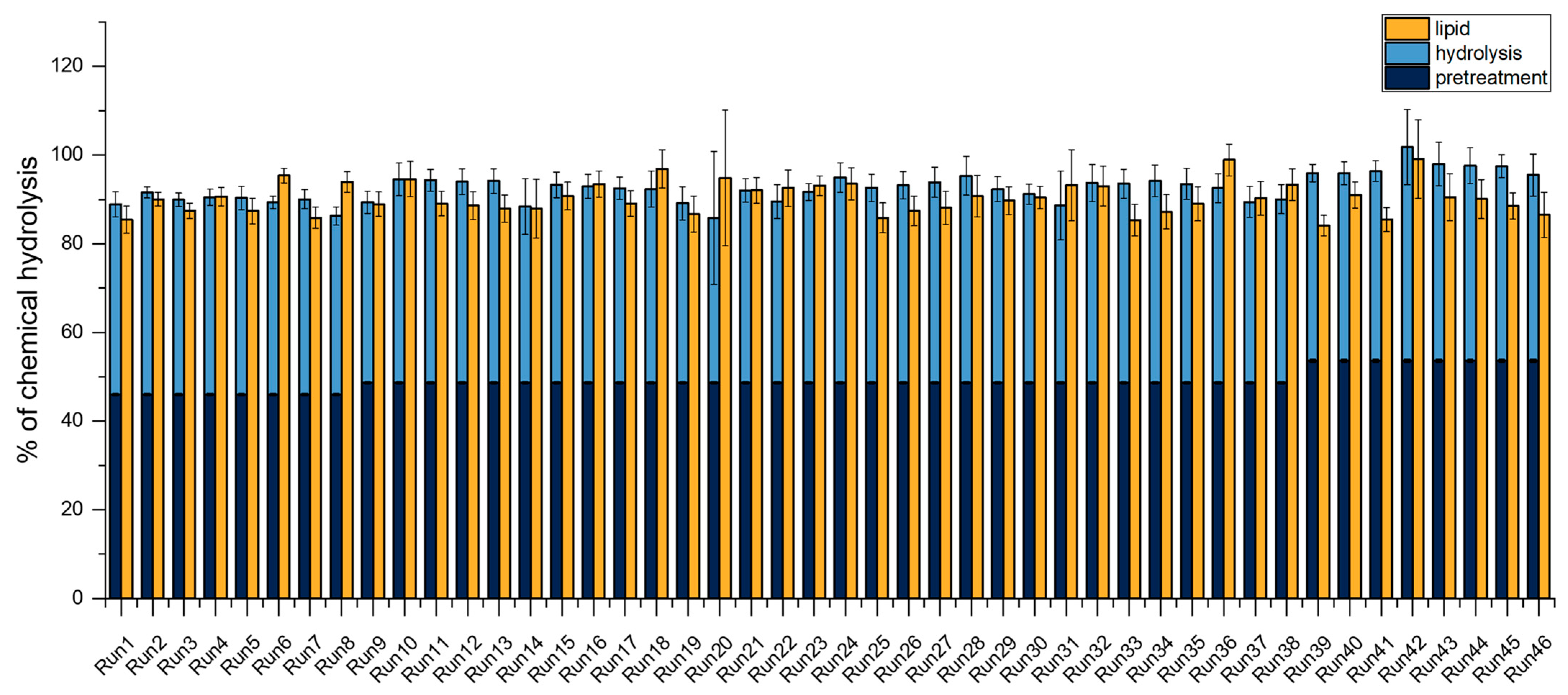
Appendix A.2. Lipid-Rich Biomass
| Run | Protease [wt%] | Cellic Ctec3 [wt%] | Viscozym L [mL/t] | Rohament CEP [g/t] | Rohalase GMP [g/t] | Pre-Treatment [% Chemical Hydrolysis] | Enzymatic Hydrolysis [% Chemical Hydrolysis] | Total Sugar [% Chemical Hydrolysis] | Lipid [% Chemical Hydrolysis] |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.1 | 3.5 | 175 | 55 | 50 | 46 | 43 | 89 | 85 |
| 2 | 0.1 | 1 | 175 | 55 | 27.5 | 46 | 46 | 92 | 90 |
| 3 | 0.1 | 3.5 | 175 | 55 | 5 | 46 | 44 | 90 | 87 |
| 4 | 0.1 | 3.5 | 175 | 10 | 27.5 | 46 | 44 | 91 | 91 |
| 5 | 0.1 | 3.5 | 250 | 55 | 27.5 | 46 | 44 | 90 | 87 |
| 6 | 0.1 | 3.5 | 175 | 100 | 27.5 | 46 | 43 | 89 | 95 |
| 7 | 0.1 | 3.5 | 100 | 55 | 27.5 | 46 | 44 | 90 | 86 |
| 8 | 0.1 | 6 | 175 | 55 | 27.5 | 46 | 40 | 86 | 94 |
| 9 | 2.55 | 6 | 175 | 10 | 27.5 | 49 | 41 | 89 | 89 |
| 10 | 2.55 | 1 | 175 | 55 | 5 | 49 | 46 | 95 | 95 |
| 11 | 2.55 | 1 | 100 | 55 | 27.5 | 49 | 46 | 94 | 89 |
| 12 | 2.55 | 3.5 | 175 | 55 | 27.5 | 49 | 45 | 94 | 89 |
| 13 | 2.55 | 3.5 | 175 | 55 | 27.5 | 49 | 45 | 94 | 88 |
| 14 | 2.55 | 6 | 175 | 55 | 5 | 49 | 40 | 88 | 88 |
| 15 | 2.55 | 3.5 | 175 | 10 | 50 | 49 | 45 | 93 | 91 |
| 16 | 2.55 | 3.5 | 100 | 55 | 50 | 49 | 44 | 93 | 93 |
| 17 | 2.55 | 3.5 | 175 | 10 | 5 | 49 | 44 | 93 | 89 |
| 18 | 2.55 | 3.5 | 250 | 100 | 27.5 | 49 | 44 | 92 | 97 |
| 19 | 2.55 | 6 | 100 | 55 | 27.5 | 49 | 40 | 89 | 87 |
| 20 | 2.55 | 1 | 175 | 55 | 50 | 49 | 37 | 86 | 95 |
| 21 | 2.55 | 3.5 | 100 | 100 | 27.5 | 49 | 43 | 92 | 92 |
| 22 | 2.55 | 6 | 175 | 100 | 27.5 | 49 | 41 | 90 | 93 |
| 23 | 2.55 | 3.5 | 175 | 55 | 27.5 | 49 | 43 | 92 | 93 |
| 24 | 2.55 | 1 | 175 | 100 | 27.5 | 49 | 46 | 95 | 94 |
| 25 | 2.55 | 3.5 | 250 | 10 | 27.5 | 49 | 44 | 93 | 86 |
| 26 | 2.55 | 1 | 175 | 10 | 27.5 | 49 | 45 | 93 | 87 |
| 27 | 2.55 | 3.5 | 100 | 55 | 5 | 49 | 45 | 94 | 88 |
| 28 | 2.55 | 1 | 250 | 55 | 27.5 | 49 | 47 | 95 | 91 |
| 29 | 2.55 | 3.5 | 100 | 10 | 27.5 | 49 | 44 | 92 | 90 |
| 30 | 2.55 | 3.5 | 175 | 55 | 27.5 | 49 | 43 | 91 | 90 |
| 31 | 2.55 | 3.5 | 175 | 55 | 27.5 | 49 | 40 | 89 | 93 |
| 32 | 2.55 | 3.5 | 175 | 55 | 27.5 | 49 | 45 | 94 | 93 |
| 33 | 2.55 | 3.5 | 175 | 100 | 50 | 49 | 45 | 94 | 85 |
| 34 | 2.55 | 3.5 | 175 | 100 | 5 | 49 | 46 | 94 | 87 |
| 35 | 2.55 | 3.5 | 250 | 55 | 50 | 49 | 45 | 94 | 89 |
| 36 | 2.55 | 3.5 | 250 | 55 | 5 | 49 | 44 | 93 | 99 |
| 37 | 2.55 | 6 | 250 | 55 | 27.5 | 49 | 41 | 89 | 90 |
| 38 | 2.55 | 6 | 175 | 55 | 50 | 49 | 41 | 90 | 93 |
| 39 | 5 | 3.5 | 175 | 100 | 27.5 | 54 | 42 | 96 | 84 |
| 40 | 5 | 3.5 | 175 | 55 | 5 | 54 | 42 | 96 | 91 |
| 41 | 5 | 3.5 | 175 | 10 | 27.5 | 54 | 43 | 96 | 85 |
| 42 | 5 | 3.5 | 100 | 55 | 27.5 | 54 | 48 | 102 | 99 |
| 43 | 5 | 3.5 | 175 | 55 | 50 | 54 | 44 | 98 | 91 |
| 44 | 5 | 3.5 | 250 | 55 | 27.5 | 54 | 44 | 98 | 90 |
| 45 | 5 | 1 | 175 | 55 | 27.5 | 54 | 44 | 97 | 89 |
| 46 | 5 | 6 | 175 | 55 | 27.5 | 54 | 42 | 95 | 87 |
References
- Mat Aron, N.S.; Khoo, K.S.; Chew, K.W.; Show, P.L.; Chen, W.H.; Nguyen, T.H.P. Sustainability of the four generations of biofuels—A review. Int. J. Energy Res. 2020, 44, 9266–9282. [Google Scholar] [CrossRef]
- Lü, J.; Sheahan, C.; Fu, P. Metabolic engineering of algae for fourth generation biofuels production. Energy Environ. Sci. 2011, 4, 2451–2466. [Google Scholar] [CrossRef]
- Li, Q.; Du, W.; Liu, D. Perspectives of microbial oils for biodiesel production. Appl. Microbiol. Biotechnol. 2008, 80, 749–756. [Google Scholar] [CrossRef]
- Peng, B.; Yao, Y.; Zhao, C.; Lercher, J.A. Towards quantitative conversion of microalgae oil to diesel-range alkanes with bifunctional catalysts. Angew. Chem.-Int. Ed. 2012, 51, 2072. [Google Scholar] [CrossRef]
- Yang, C.; Li, R.; Cui, C.; Liu, S.; Qiu, Q.; Ding, Y.; Wu, Y.; Zhang, B. Catalytic hydroprocessing of microalgae-derived biofuels: A review. Green Chem. 2016, 18, 3684–3699. [Google Scholar] [CrossRef]
- Jenck, J.F.; Agterberg, F.; Droescher, M.J. Products and processes for a sustainable chemical industry: A review of achievements and prospects. Green Chem. 2004, 6, 544–556. [Google Scholar] [CrossRef]
- Reinhardt, N.; Breitsameter, J.M.; Drechsler, K.; Rieger, B. Fully Bio-Based Epoxy Thermoset Based on Epoxidized Linseed Oil and Tannic Acid. Macromol. Mater. Eng. 2022, 307, 2200455. [Google Scholar] [CrossRef]
- Braun, M.K.; Lorenzen, J.; Masri, M.; Liu, Y.; Baráth, E.; Brück, T.; Lercher, J.A. Catalytic decomposition of the oleaginous yeast Cutaneotrichosporon oleaginosus and subsequent biocatalytic conversion of liberated free fatty acids. ACS Sustain. Chem. Eng. 2019, 7, 6531–6540. [Google Scholar] [CrossRef]
- Hayes, D.G.; Kleiman, R. Lipase-catalyzed synthesis and properties of estolides and their esters. J. Am. Oil Chem. Soc. 1995, 72, 1309–1316. [Google Scholar] [CrossRef]
- Lorenzen, J.; Driller, R.; Waldow, A.; Qoura, F.; Loll, B.; Brück, T. Rhodococcus erythropolis oleate hydratase: A new member in the oleate hydratase family tree—Biochemical and structural studies. ChemCatChem 2018, 10, 407–414. [Google Scholar] [CrossRef]
- Prem, S.; Helmer, C.P.; Dimos, N.; Himpich, S.; Brück, T.; Garbe, D.; Loll, B. Towards an understanding of oleate hydratases and their application in industrial processes. Microb. Cell Factories 2022, 21, 58. [Google Scholar] [CrossRef]
- Melcher, F.; Vogelgsang, F.; Haack, M.; Masri, M.; Ringel, M.; Roth, A.; Garbe, D.; Brück, T. Lipase-mediated plant oil hydrolysis—Toward a quantitative glycerol recovery for the synthesis of pure allyl alcohol and acrylonitrile. Eur. J. Lipid Sci. Technol. 2023, 125, 2200196. [Google Scholar] [CrossRef]
- Abeln, F.; Chuck, C.J. The history, state of the art and future prospects for oleaginous yeast research. Microb. Cell Factories 2021, 20, 221. [Google Scholar] [CrossRef]
- Flach, B.; Lieberz, S.; Bolla, S. EU-28 Biofuels Annual EU Biofuels Annual 2019; USDA Foreign Agricultural Service: Washington, DC, USA, 2019.
- Huang, C.; Chen, X.-F.; Xiong, L.; Chen, X.-D.; Ma, L.-L.; Chen, Y. Single cell oil production from low-cost substrates: The possibility and potential of its industrialization. Biotechnol. Adv. 2013, 31, 129–139. [Google Scholar] [CrossRef]
- Melcher, F.; Paper, M.; Brück, T.B. Chapter 9 Photosynthetic Conversion of CO2 into Bioenergy and Materials Using Microalgae. In Photosynthesis: Biotechnological Applications with Microalgae; Rögner, M., Ed.; De Gruyter: Berlin, Germany, 2021. [Google Scholar] [CrossRef]
- Boussiba, S.; Vonshak, A.; Cohen, Z.; Avissar, Y.; Richmond, A. Lipid and biomass production by the halotolerant microalga Nannochloropsis salina. Biomass 1987, 12, 37–47. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef]
- Zittelli, G.C.; Rodolfi, L.; Bassi, N.; Biondi, N.; Tredici, M.R. Photobioreactors for microalgal biofuel production. In Algae for Biofuels and Energy; Springer: New York, NY, USA, 2012; pp. 115–131. [Google Scholar]
- Schädler, T.; Cerbon, D.C.; de Oliveira, L.; Garbe, D.; Brück, T.; Weuster-Botz, D. Production of lipids with Microchloropsis salina in open thin-layer cascade photobioreactors. Bioresour. Technol. 2019, 289, 121682. [Google Scholar] [CrossRef]
- Shivakumar, S.; Serlini, N.; Esteves, S.M.; Miros, S.; Halim, R. Cell Walls of Lipid-Rich Microalgae: A Comprehensive Review on Characterisation, Ultrastructure, and Enzymatic Disruption. Fermentation 2024, 10, 608. [Google Scholar] [CrossRef]
- Mirsiaghi, M.; Reardon, K.F. Conversion of lipid-extracted Nannochloropsis salina biomass into fermentable sugars. Algal Res. 2015, 8, 145–152. [Google Scholar] [CrossRef]
- Awad, D.; Bohnen, F.; Mehlmer, N.; Brueck, T. Multi-factorial-guided media optimization for enhanced biomass and lipid formation by the oleaginous yeast Cutaneotrichosporon oleaginosus. Front. Bioeng. Biotechnol. 2019, 7, 54. [Google Scholar] [CrossRef]
- Fuchs, T.; Melcher, F.; Rerop, Z.S.; Lorenzen, J.; Shaigani, P.; Awad, D.; Haack, M.; Prem, S.A.; Masri, M.; Mehlmer, N. Identifying carbohydrate-active enzymes of Cutaneotrichosporon oleaginosus using systems biology. Microb. Cell Factories 2021, 20, 205. [Google Scholar] [CrossRef]
- Masri, M.A.; Garbe, D.; Mehlmer, N.; Brück, T.B. A sustainable, high-performance process for the economic production of waste-free microbial oils that can replace plant-based equivalents. Energy Environ. Sci. 2019, 12, 2717–2732. [Google Scholar] [CrossRef]
- Koruyucu, A.; Blums, K.; Peest, T.; Schmack-Rauscher, L.; Brück, T.; Weuster-Botz, D. High-cell-density yeast oil production with diluted substrates imitating microalgae hydrolysate using a membrane bioreactor. Energies 2023, 16, 1757. [Google Scholar] [CrossRef]
- Meo, A.; Priebe, X.L.; Weuster-Botz, D. Lipid production with Trichosporon oleaginosus in a membrane bioreactor using microalgae hydrolysate. J. Biotechnol. 2017, 241, 1–10. [Google Scholar] [CrossRef]
- Rerop, Z.S.; Stellner, N.I.; Graban, P.; Haack, M.; Mehlmer, N.; Masri, M.; Brück, T.B. Bioconversion of a lignocellulosic hydrolysate to single cell oil for biofuel production in a cost-efficient fermentation process. Fermentation 2023, 9, 189. [Google Scholar] [CrossRef]
- Younes, S.; Bracharz, F.; Awad, D.; Qoura, F.; Mehlmer, N.; Brueck, T. Microbial lipid production by oleaginous yeasts grown on Scenedesmus obtusiusculus microalgae biomass hydrolysate. Bioprocess Biosyst. Eng. 2020, 43, 1629–1638. [Google Scholar] [CrossRef]
- Koruyucu, A.; Schädler, T.; Gniffke, A.; Mundt, K.; Krippendorf, S.; Urban, P.; Blums, K.; Halim, B.; Brück, T.; Weuster-Botz, D. Energy-efficient production of Microchloropsis salina biomass with high CO2 fixation yield in open thin-layer cascade photobioreactors. Processes 2024, 12, 1303. [Google Scholar] [CrossRef]
- Jurkowski, W.; Paper, M.; Brück, T.B. Isolation and investigation of natural rare earth metal chelating agents from Calothrix brevissima-a step towards unraveling the mechanisms of metal biosorption. Front. Bioeng. Biotechnol. 2022, 10, 833122. [Google Scholar] [CrossRef]
- Engelhart-Straub, S.; Cavelius, P.; Hölzl, F.; Haack, M.; Awad, D.; Brueck, T.; Mehlmer, N. Effects of Light on Growth and Metabolism of Rhodococcus erythropolis. Microorganisms 2022, 10, 1680. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Stellner, N.; Rerop, Z.; Kyselka, J.; Alishevich, K.; Beneš, R.; Filip, V.; Celik, G.l.; Haack, M.; Ringel, M.; Masri, M. Value-Added Squalene in Single-Cell Oil Produced with Cutaneotrichosporon oleaginosus for Food Applications. J. Agric. Food Chem. 2023, 71, 8540–8550. [Google Scholar] [CrossRef]
- Scholz, M.J.; Weiss, T.L.; Jinkerson, R.E.; Jing, J.; Roth, R.; Goodenough, U.; Posewitz, M.C.; Gerken, H.G. Ultrastructure and composition of the Nannochloropsis gaditana cell wall. Eukaryot. Cell 2014, 13, 1450–1464. [Google Scholar] [CrossRef]
- Gelin, F.i.; Boogers, I.; Noordeloos, A.A.; Damsté, J.S.; Hatcher, P.; de Leeuw, J.W. Novel, resistant microalgal polyethers: An important sink of organic carbon in the marine environment? Geochim. et Cosmochim. Acta 1996, 60, 1275–1280. [Google Scholar] [CrossRef]
- Jeong, S.W.; Nam, S.W.; HwangBo, K.; Jeong, W.J.; Jeong, B.-R.; Chang, Y.K.; Park, Y.-I. Transcriptional Regulation of Cellulose Biosynthesis during the Early Phase of Nitrogen Deprivation in Nannochloropsis salina. Sci. Rep. 2017, 7, 5264. [Google Scholar] [CrossRef] [PubMed]
- Klassen, V.; Blifernez-Klassen, O.; Hoekzema, Y.; Mussgnug, J.H.; Kruse, O. A novel one-stage cultivation/fermentation strategy for improved biogas production with microalgal biomass. J. Biotechnol. 2015, 215, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Koruyucu, A.; Peest, T.; Korzin, E.; Gröninger, L.; Patricia; Brück, T.; Weuster-Botz, D. Cell Disruption and Hydrolysis of Microchloropsis salina Biomass as a Feedstock for Fermentation. Appl. Sci. 2024, 14, 9667. [Google Scholar] [CrossRef]
- Chen, L.; Li, R.; Ren, X.; Liu, T. Improved aqueous extraction of microalgal lipid by combined enzymatic and thermal lysis from wet biomass of Nannochloropsis oceanica. Bioresour. Technol. 2016, 214, 138–143. [Google Scholar] [CrossRef]
- Ometto, F.; Quiroga, G.; Pšenička, P.; Whitton, R.; Jefferson, B.; Villa, R. Impacts of microalgae pre-treatments for improved anaerobic digestion: Thermal treatment, thermal hydrolysis, ultrasound and enzymatic hydrolysis. Water Res. 2014, 65, 350–361. [Google Scholar] [CrossRef]
- Cássio, F.; Leao, C. Low-and high-affinity transport systems for citric acid in the yeast Candida utilis. Appl. Environ. Microbiol. 1991, 57, 3623–3628. [Google Scholar] [CrossRef]
- Kwon, O.-M.; Kim, D.-H.; Kim, S.-K.; Jeong, G.-T. Production of sugars from macro-algae Gracilaria verrucosa using combined process of citric acid-catalyzed pretreatment and enzymatic hydrolysis. Algal Res. 2016, 13, 293–297. [Google Scholar] [CrossRef]
- Abdel-Kader, H.A.; Abdel-Basset, R.; Danial, A.W. Yeast and enzymatic hydrolysis in converting Chlorella biomass into hydrogen gas by Rhodobacter sp. and Rhodopseudomonas palustris. Int. J. Hydrogen Energy 2022, 47, 1516–1528. [Google Scholar] [CrossRef]
- Kumar, S.; Gupta, R.; Kumar, G.; Sahoo, D.; Kuhad, R.C. Bioethanol production from Gracilaria verrucosa, a red alga, in a biorefinery approach. Bioresour. Technol. 2013, 135, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Kumar, M.; Chakraborty, S.; Gupta, R.; Kumar, S.; Sahoo, D.; Kuhad, R.C. Process development for the production of bioethanol from waste algal biomass of Gracilaria verrucosa. Bioresour. Technol. 2016, 220, 584–589. [Google Scholar] [CrossRef]
- Vandanjon, L.; Vallet, L.; Le Glatin, T.; Deleris, P.; Bourseau, P.; Dumay, J. Valorization of the macroalga Sargassum muticum by enzymatic hydrolysis. Interest of surfactants to improve the extraction of phlorotannins and polysaccharides. J. Mar. Biol. Aquac. 2016, 2, 7. [Google Scholar] [CrossRef]
- Seo, D.-J.; Fujita, H.; Sakoda, A. Structural changes of lignocelluloses by a nonionic surfactant, Tween 20, and their effects on cellulase adsorption and saccharification. Bioresour. Technol. 2011, 102, 9605–9612. [Google Scholar] [CrossRef]
- Seo, D.-J.; Fujita, H.; Sakoda, A. Effects of a non-ionic surfactant, Tween 20, on adsorption/desorption of saccharification enzymes onto/from lignocelluloses and saccharification rate. Adsorption 2011, 17, 813–822. [Google Scholar] [CrossRef]
- Saenge, C.; Cheirsilp, B.; Suksaroge, T.T.; Bourtoom, T. Potential use of oleaginous red yeast Rhodotorula glutinis for the bioconversion of crude glycerol from biodiesel plant to lipids and carotenoids. Process Biochem. 2011, 46, 210–218. [Google Scholar] [CrossRef]
- Troncoso, E.; Aguilera, J.M.; McClements, D.J. Fabrication, characterization and lipase digestibility of food-grade nanoemulsions. Food Hydrocoll. 2012, 27, 355–363. [Google Scholar] [CrossRef]
- Safi, C.; Rodriguez, L.C.; Mulder, W.; Engelen-Smit, N.; Spekking, W.; Van den Broek, L.; Olivieri, G.; Sijtsma, L. Energy consumption and water-soluble protein release by cell wall disruption of Nannochloropsis gaditana. Bioresour. Technol. 2017, 239, 204–210. [Google Scholar] [CrossRef]
- Nishinoaki, M.; Asakura, T.; Watanabe, T.; Kunizaki, E.; Matsumoto, M.; Eto, W.; Tamura, T.; Minami, M.; Obata, A.; Abe, K. Application of an Aspergillus saitoi protease preparation to soybean curd to modify its functional and rheological properties. Biosci. Biotechnol. Biochem. 2008, 72, 587–590. [Google Scholar] [CrossRef]
- Wu, C.; Xiao, Y.; Lin, W.; Li, J.; Zhang, S.; Zhu, J.; Rong, J. Aqueous enzymatic process for cell wall degradation and lipid extraction from Nannochloropsis sp. Bioresour. Technol. 2017, 223, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Zuorro, A.; Miglietta, S.; Familiari, G.; Lavecchia, R. Enhanced lipid recovery from Nannochloropsis microalgae by treatment with optimized cell wall degrading enzyme mixtures. Bioresour. Technol. 2016, 212, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Ramos, M.J.; Fernández, C.M.; Casas, A.; Rodríguez, L.; Pérez, Á. Influence of fatty acid composition of raw materials on biodiesel properties. Bioresour. Technol. 2009, 100, 261–268. [Google Scholar] [CrossRef]




| Name | Supplier | Activities | Activity Units | Recommended Dosing |
|---|---|---|---|---|
| Protease from Aspergillus saitoi | Sigma-Aldrich | Protease, glucanase | ≥600 U/g | Not available |
| Cellic CTec3 | Novozymes | Cellulase | ≥1000 BHU-2-HS/g | 1–6% (w/w) |
| Rohament CEP | AB Enzymes | Cellulase | ≥100,000 ECU/g | 10–100 g/t |
| Rohalase GMP | AB Enzymes | Mannanase, ß-glucanase | ≥600,000 MNU/g | 5–50 g/t |
| Viscozym L | Novozymes | Arabanase, cellulase, ß-glucanase, hemicellulase, xylanase, pectinase | ≥120 FBGU/mL | 100–250 mL/t |
| Suggestions | ||||||||
|---|---|---|---|---|---|---|---|---|
| Biomass | Enzyme Dosing | Protease [wt%] | Cellic CTec3 [wt%] | Viscozym L [ml/t] | Rohament CEP [g/t] | Rohalase GMP [g/t] | Sugar Yield | Desirability |
| lipid-rich | in range | 5 | 1 | 175 | 55 | 27.5 | 0.983 | 0.785 |
| min | 2.996 | 1.482 | 137.89 | 29.366 | 13.841 | 0.95 | 0.67 | |
| nutrient-replete | in range | 4.981 | 5.204 | 243.923 | 54.884 | 47.898 | 0.633 | 1 |
| min | 3.282 | 2.352 | 100 | 10 | 5 | 0.554 | 0.695 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melcher, F.; Schneider, M.; Paper, M.; Ringel, M.; Garbe, D.; Brück, T. Optimizing the Enzymatic Hydrolysis of Microchloropsis salina Biomass for Single-Cell Oil Production. Biomass 2025, 5, 56. https://doi.org/10.3390/biomass5030056
Melcher F, Schneider M, Paper M, Ringel M, Garbe D, Brück T. Optimizing the Enzymatic Hydrolysis of Microchloropsis salina Biomass for Single-Cell Oil Production. Biomass. 2025; 5(3):56. https://doi.org/10.3390/biomass5030056
Chicago/Turabian StyleMelcher, Felix, Max Schneider, Michael Paper, Marion Ringel, Daniel Garbe, and Thomas Brück. 2025. "Optimizing the Enzymatic Hydrolysis of Microchloropsis salina Biomass for Single-Cell Oil Production" Biomass 5, no. 3: 56. https://doi.org/10.3390/biomass5030056
APA StyleMelcher, F., Schneider, M., Paper, M., Ringel, M., Garbe, D., & Brück, T. (2025). Optimizing the Enzymatic Hydrolysis of Microchloropsis salina Biomass for Single-Cell Oil Production. Biomass, 5(3), 56. https://doi.org/10.3390/biomass5030056








