Abstract
Biomass pyrolysis is a thermochemical process that breaks down organic matter in the absence of oxygen, offering a sustainable route for converting biomass into bio-oil, biochar, and syngas. This review provides a comprehensive overview of pyrolysis, focusing on its fundamental principles, modes, and its applications across different industries. It covers major pyrolysis types and explores the reactors used in these processes and how key parameters, such as temperature, heating rate, and residence time, impact the distribution and quality of pyrolysis products. Special attention is given to bio-oil upgrading methods, including catalytic and non-catalytic processes, and how they affect fuel quality. The study also presents techno-economic assessments of various pathways, identifying cost-effective configurations like pyrolysis combined with hydrotreatment and heat integration. Despite encouraging advancements, scaling up bio-oil technologies continues to face significant challenges, primarily due to cost competitiveness and variability in feedstock supply. This review emphasizes the critical need for continued innovation in reactor design, catalyst efficiency, and integrated process optimization, alongside supportive policy frameworks and strategic investments to accelerate commercial deployment. Finally, this review aims to help researchers, engineers, and policymakers work together to advance pyrolysis technology as a practical solution for producing low-carbon fuels and chemicals.
1. Introduction
Rapid global population growth and accelerating energy demand are placing unprecedented pressure on existing energy systems, highlighting the need to consider renewable and sustainable alternatives. Renewable energy sources can supplant energy supply, address national security, and environmental issues [1]. In particular, the use of biomass materials to produce liquid transportation fuels has garnered significant interest [2]. Based on projections, as much as 40% of global energy demand in the upcoming decades could be satisfied by biomass-based energy [3].
To understand the potential of biomass, it is important to first define biomass and its conversion options. Microbes and vegetation create biomass [4]. It comprises all organic and biological substances obtained from living organisms through direct or indirect processing methods [5]. It can be categorized into agricultural biomass, forestry biomass, crops, wood-based biomass, municipal and industrial wastes, food waste, and animal- and human-generated wastes. It ranks as the fourth largest main energy source, currently supplying 14% of the global primary energy supply [6].
Biomass can be converted into biofuels using either biological or thermal approaches. At the commercial level, the biological conversion approach is limited as it relies heavily on food-based raw materials [7]. However, the thermal conversion approach, including widely applied pyrolysis, gasification, and combustion methods, can process a diverse range of raw materials in short residence time and handle various complex biofuels [8,9,10]. Biofuels that are derived from biomass address specific practical and economic challenges by exhibiting diverse physicochemical properties [11].
One of the most effective and versatile thermal conversion methods is biomass pyrolysis, which transforms low-value, easily accessible biomass materials like grasses, shells, bagasse, husks, waste woods, stalks, and sawdust into high-value products [2]. Unlike traditional thermochemical routes that emphasize combustion for heat or electricity, pyrolysis offers a flexible platform to produce liquid bio-oils and chemicals suitable for refining into transportation fuels and value-added chemicals. Its ability to handle heterogeneous feedstocks and operate at moderate scales makes it particularly attractive for decentralized energy systems and rural deployment [8].
Despite its potential, biomass pyrolysis is not yet a mainstream commercial technology. This is primarily due to several technical and economic hurdles including variability in bio-oil quality, limited catalyst lifetimes, scale-up complexities, and the need for cost-effective reactor designs [12]. Furthermore, the high oxygen content, acidity, and instability of raw bio-oil necessitate extensive upgrading processes before it can substitute for conventional fuels [13].
This review aims to address these gaps by offering a detailed, interdisciplinary examination of biomass pyrolysis. The paper covers (1) basic principles and mechanisms of pyrolysis; (2) various types of pyrolysis and associated reactors for processing; (3) bio-oil upgrading techniques; (4) techno-economic assessments of different processing pathways; and (5) an overview of leading global biomass companies contributing to the development and commercialization of pyrolysis technologies.
2. Biomass to Bio-Oil Production
2.1. Pyrolysis Mechanism
Biomass pyrolysis typically involves three key stages [14]: (1) initial evaporation of free moisture: evaporating free moisture in the biomass is the first stage of biomass pyrolysis; (2) primary decomposition: this stage involves the primary decomposition of the biomass, forming solid char between 200 and 400 °C, which leads to the degradation of biomass; and (3) secondary reactions: these include oil cracking and repolymerization, taking place within the solid matrix as the temperature rises further. The main stages of this process and the resulting products are summarized in Figure 1.
2.2. Decomposition of Biomass Components
Lignocellulosic biomass is mainly made up of three components—cellulose, hemicellulose, and lignin—along with smaller amounts of extractives and ash. Hemicellulose breaks down between 250 and 350 °C, with xylan serving as a representative component in this temperature range. Cellulose decomposes between 325 and 400 °C, producing levoglucosan as the main product. Lignin is the most stable and breaks down over a wider range (between 300 to 550 °C). Of these, cellulose decomposition is the most studied and well understood, following the Waterloo mechanism, which involves dehydrogenation, depolymerization, and fragmentation happening at different temperatures [14].
In addition to the main components, ash, which consists mostly of inorganic minerals, can affect the biomass breakdown during pyrolysis. The amount and type of ash can change the reaction speed, the amounts of products formed, and their qualities. Higher ash content tends to lower the yields of levoglucosan and bio-oil and increase the amount of non-condensable gases. Potassium and other minerals in ash can promote different breakdown reactions by acting as catalysts [15].
2.3. Definition of Pyrolysis and Overview of Its Chemistry
Pyrolysis occurs when organic material is subjected to thermochemical decomposition in an atmosphere that is devoid of oxygen. The process is irreversible and changes both the chemical composition and physical structure of the organic material. The term “pyrolysis” is derived from Greek, with “pyro” meaning fire and “lysis” meaning separating. Unlike complete combustion, which occurs with oxygen and produces carbon dioxide and water, pyrolysis transforms organic materials differently [16,17].
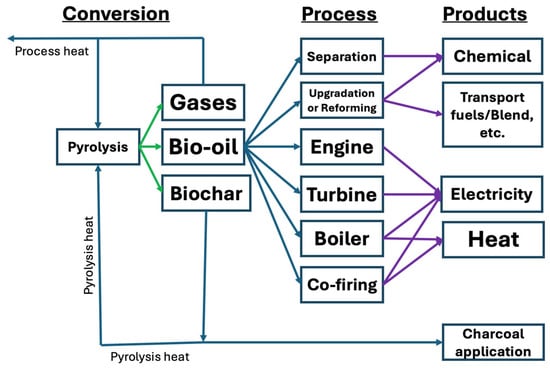
Figure 1.
Schematic representation of biomass processing pathways and the major products obtained via pyrolysis. Adapted from [18].
Pyrolysis of organic materials typically yields three distinct phases. The gaseous phase consists of carbon monoxide (CO) and hydrogen (H2) (referred to as syngas in industrial settings), methane (CH4), short-chain hydrocarbons, and carbon dioxide (CO2). The liquid phase, known commercially as bio-oil and tars, contains a variety of compounds including aliphatic and aromatic substances, phenols, aldehydes, levoglucosan, hydroxy acetaldehyde, hydrocarbon chains, and water. Tar, a key component of this liquid phase, is a viscous, dark-colored fluid made up of hydrocarbons and free carbon. While bio-oil and tar are closely related, bio-oil is distinguished by its composition of lower molecular weight organic compounds compared to tar [16,17]. Therefore, bio-oil exhibits lower fluidity compared to tar, and it is of higher purity than tar. To make it suitable for commercial use, raw bio-oil needs to go through an upgrading process. It is worth noting that there is no strict scientific definition of tar. Generally, any pyrolysis product heavier than benzene falls in this category [19]. Furthermore, the process yields a carbon-rich solid by-product, commonly referred to as char or biochar. This solid fraction may also contain trace contaminants, such as aromatic compounds. When the process is conducted at high temperatures with the primary goal of producing solid carbon residue, it is termed carbonization [13,14]. An overview of biomass processing pathways and the main products generated through pyrolysis is illustrated in Figure 1.
3. Types of Pyrolysis
Pyrolysis can be categorized based on the operational parameters, including slow, intermediate, fast, flash, and hydro-pyrolysis. There are strategies that can be employed to boost the production of specific products like using special catalysts to enhance the production of pyrolytic gas and specific pretreatment processes to increase the generation of bio-oil [1]. The following subsections provide a detailed overview of each pyrolysis type, highlighting their operating conditions and product distributions.
3.1. Slow Pyrolysis
Slow pyrolysis is associated with a relatively long residence time that can last several days. It is typically carried out at relatively low temperatures not exceeding 500 °C and involves slow heating rates, ranging from 0.1 to 2 °C per second. Char and tar are the dominant products in slow pyrolysis, as the extended residence time of gases and other pyrolysis products within the pyrolytic converter allows for repolymerization and recombination reactions to occur. Additionally, the type of biomass used is a crucial factor in determining the ratios of char, tar, and gas produced [17].
Traditional slow pyrolysis is primarily utilized when char is the desired product. This method, known as carbonization, is traditionally used to produce char (charcoal) and involves heating biomass to around 400 °C for several days. The vapors generated are typically left uncondensed and redirected to provide thermal energy for the process [1].
Torrefaction is another form of slow pyrolysis, operating at moderate temperatures of 225–300 °C to improve the heating properties and energy density of biomass [20]. Research by Gaitan-Alvarez et al. [21] has shown that 200 °C and 225 °C are optimal temperatures for light and medium torrefaction, respectively, while a temperature of 250 °C can lead to severe degradation of the material.
Conventional pyrolysis, which yields balanced quantities of biochar, bio-oil, and syngas, is generally conducted at approximately 600 °C with a moderate heating rate. Studies by Balagurumurthy et al. [22] on slow pyrolysis of rice straw at 300–450 °C found that 400 °C was the best temperature for slow pyrolysis, as the bio-oil yield increased up to this point but decreased beyond it. Slow pyrolysis of sugarcane bagasse at heating rates of 45–50 °C/min and temperatures of 663–1253 K showed more syngas production at higher temperatures. Moreover, methane production was favored at lower temperatures, while hydrogen production increased at higher temperatures [23].
3.2. Intermediate Pyrolysis
Intermediate pyrolysis produces a combination of products characteristic of both slow and fast pyrolysis. Slow pyrolysis tends to generate a larger proportion of solid residues and fewer liquids, whereas fast pyrolysis favors higher liquid yields with reduced solid formation (as detailed below). Typically, intermediate pyrolysis is conducted at temperatures ranging from 300 °C to 600 °C and heating rates between 0.1 °C/min and 10 °C/min. In the low-temperature range of intermediate pyrolysis, favorable chemical transformations take place, allowing for more flexibility in optimizing the process. Intermediate pyrolysis typically yields 15–25% biochar, 40–60% bio-oil, and 20–30% gas [24]. The bio-oil produced has lower tar content and viscosity compared to bio-oil from fast pyrolysis. Intermediate pyrolysis can also use particles of varying sizes, unlike fast pyrolysis, which requires finely ground particles [1].
3.3. Fast Pyrolysis
The fast pyrolysis technique resembles flash pyrolysis, but it entails slower heating rates. The primary products of rapid pyrolysis are liquid and gaseous fractions, specifically bio-oil and biogas. Rapid pyrolysis is distinguished by elevated heating rates (>10–200 °C/s) and short residence times (0.5–10 s, typically <2 s), yielding bio-oil at 50–70 weight percent of the total products. Conversely, the flash pyrolysis approach is characterized by even higher heating rates and shorter residence times (<0.5 s), which can result in remarkably high bio-oil yields of up to 75–80 weight percent [14].
During fast pyrolysis, biomass rapidly breaks down into mostly vapors and aerosols, with minimal char and gas produced. These vapors and aerosols condense into a dark brown liquid, bio-oil, which has a higher heating value (HHV) of approximately 15–25 MJ/kg [25], compared to fossil fuels such as gasoline and diesel oils, which typically feature HHVs of around 42–46 MJ/kg [26]. Thus, bio-oil possesses roughly half the calorific value of fossil oils. Biomass with low ash content produces higher yields of bio-oil. For high liquid yields, the biomass feedstock should be finely ground (less than 3 mm) to ensure efficient heat transfer due to low thermal conductivity of biomass [8]. To minimize secondary cracking reactions, a short reaction time (typically less than 2 s) and rapid cooling of hot vapors are necessary. A temperature of around 500 °C is generally suitable for producing high yields of bio-oil from most biomass feedstocks. Interest in developing advanced fast pyrolysis processes has increased due to its potential for bio-oil production from biomass. Fast pyrolysis is an efficient way to convert low-value biomass into bio-oil and other valuable products, typically using fluidized bed or circulating fluidized bed reactors [1].
Arni [23] investigated the differences between fast and slow pyrolysis of sugarcane bagasse. The researcher used heating rates of 120–127 K/min, a residence time of 20 min, and temperatures ranging from 653 K to 1053 K for the fast pyrolysis process. The researchers found that higher temperatures led to an increase in both product losses and solid yields. However, the maximum loss observed was less than 15%, which was still higher than the losses recorded for conventional pyrolysis. This greater loss was linked to the extended residence time used in the process.
3.4. Flash Pyrolysis
Fast pyrolysis utilizes rapid heating rates to convert biomass into valuable products, primarily bio-oil. In comparison, flash pyrolysis employs even higher heating rates, resulting in the production of gases and bio-oil. Flash pyrolysis has gained popularity for producing liquid fuels from biomass due to its extremely high temperatures and short reaction times, which help prevent the repolymerization of decomposed products [27]. This process can achieve bio-oil yields exceeding 75% [28] by rapidly devolatilizing biomass in an oxygen-deficient environment at around 1000 °C and high heating rates within a very short time. Madhu et al. [29] stated a bio-oil yield of approximately 48.2% at 500 °C using a 1 mm particle size and a 2 m3/h sweep gas flow rate.
Nevertheless, flash pyrolysis encounters several technological hurdles, including low thermal stability of bio-oil, high acidity and viscosity, the generation of pyrolytic water, the presence of char within the bio-oil, and dissolution of alkali substances in the char [30].
3.5. Hydro-Pyrolysis
Hydro-pyrolysis is a developing method for generating bio-oil with improved quality from biomass sources. In this process, pyrolysis is carried out under an inert atmosphere where hydrogen serves as the inert gas. Both biomass and hydrogen are fed into the reactor under elevated pressures, typically between 5 and 20 MPa. The presence of hydrogen plays a key role in minimizing the generation of free radicals during the reaction, which leads to fewer unsaturated hydrocarbons and higher-quality bio-oil [18]. Hydro-pyrolysis is similar to fast pyrolysis but occurs under conditions where hydrogen is used as the atmosphere, and the process is performed at elevated pressure and share comparable parameters including operating temperature, the rate of heating, and the duration of the reaction [24].
Balagurumurthy et al. [22] carried out hydro-pyrolysis experiments with rice straw, identifying 400 °C and 30 bar as the optimal temperature and pressure. The resulting bio-oil contained a high concentration of phenolic substances, but its yield was less than when nitrogen was used instead of hydrogen under identical operational settings. A comparative summary of the main pyrolysis types and their operational characteristics is presented in Table 1. Microwave pyrolysis is also emerging as a pathway [31] but is still in infancy, so it is not covered here.

Table 1.
Summary of comparison of pyrolysis processes and their operating parameters.
4. Biomass to Bio-Oil Process: Types of Reactors
The reactors used for biomass pyrolysis are a key to the pyrolysis process because the type of reactor mainly determines quality, quantity, properties, and characteristics of the subsequent pyrolysis products [32]. Various types of reactors have been developed to optimize bio-oil yield and composition. In the following sections, the most commonly used reactor types are discussed.
4.1. Fixed-Bed Reactors
Fixed-bed reactors (Figure 2) are widely used to convert biomass into biofuels and other valuable products due to their simple design and operational efficiency. The following are key features and findings related to bio-oil production using fixed-bed reactors [1]: Fixed-bed reactors are typically constructed from firebricks or steel and comprise a feeding unit, cooling system, ash cleaning unit, and gas exit. These reactors are characterized by long biomass residence times, high carbon conservation, reduced ash entrainment, and low gas velocity. They are commonly used in small-scale energy generation systems. One of the significant challenges related to fixed-bed reactors is tar removal, which affects the quality of the product gas.
As for results, Chen et al. [33] used a fixed-bed reactor for pyrolyzing poplar wood. In their study, the optimal conditions for biochar production resulted in a Brunauer, Emmett, and Teller (This method calculates the pore volume and surface area of the char produced.) (BET) surface area of 411.06 m2/g at 600 °C and a heating rate of 30 K/min. Moreover, the HHV of non-condensable gas reached 14.56 MJ/m3 at 600 °C and 50 K/min, while the HHV of bio-oil was 14.39 MJ/kg at 550 °C and 50 K/min.
Messina et al. [34] pyrolyzed acid-treated peanut shells at various temperatures, finding the optimal temperature at 500 °C. Treated biomass yielded 42% bio-oil compared to 33% from untreated biomass and a char BET surface area of 300 m2/g, indicating potential use as an adsorbent. Açıkalın and Karaca [35] examined various parameters affecting product yields. They utilized different pyrolysis conditions: heating rate (40 K/min), pyrolysis temperature (350–650 °C), nitrogen gas flow rate (50–450 mL/min), and residence time (10–50 min). Maximum liquid yield was 48.2% at 500 °C at 30 min residence time and 150 mL/min nitrogen flow. Ma et al. [36] used a fixed-bed reactor for pyrolyzing rice husks. The researchers observed that higher pyrolysis temperatures favored phenol production, while increased condensation temperatures resulted in phenol compounds and dehydrated carbohydrates production.
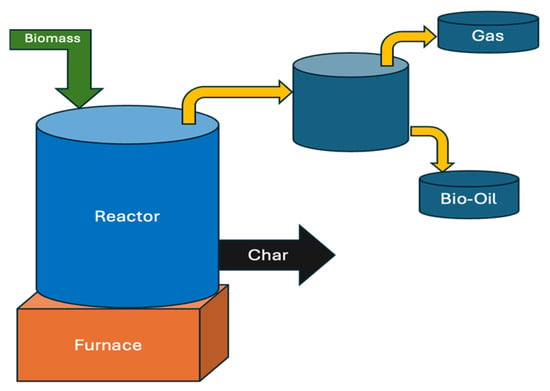
Figure 2.
Schematic diagram of a fixed-bed reactor for biomass pyrolysis. Adapted with permission from [37]; redrawn by the authors.
4.2. Fluidized Bed Reactors
Fluidized bed reactors are ideal for fast pyrolysis because they offer efficient heat transfer, enhanced velocity and surface area interaction, and allow for precise control over the vapor residence time [1].High-rate anaerobic digesters highlight the importance of reactor configurations in optimizing energy recovery [38]. Key aspects and findings related to bio-oil production using fluidized bed reactors [1] are as follows: Biomass is mixed with heated sand particles in a fluidized bed reactor to enhance heat and mass transfer. The reactor’s bed is externally heated, with thermal energy transferred through direct or indirect sources. Fluidized bed reactors can operate in three main configurations: bubbling fluidized bed, entrained fluidized bed, and circulating fluidized bed. Figure 3 illustrates schematic representations of bubbling fluidized bed and circulating fluidized bed reactors.
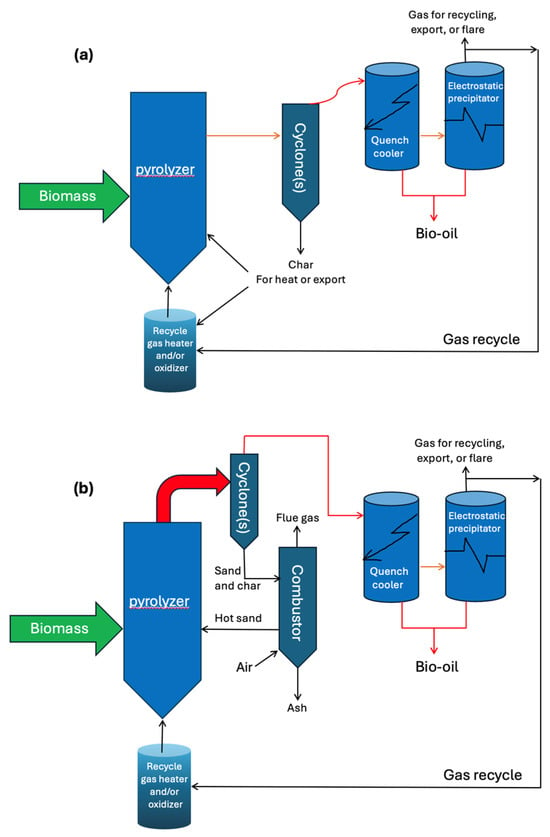
Figure 3.
Schematic diagrams of bubbling (a) and circulating (b) fluidized bed reactor configurations used in biomass pyrolysis. Adapted with permission from [1]; redrawn by the authors. © 2021 Springer Nature.
Among the studies and results, Carvalho et al. [39] investigated the fast pyrolysis of sweet sorghum bagasse in a fluidized bed reactor, using particle sizes ranging from 500 to 1000 μm. They observed variations in the outcomes attributed to the type of solvent employed, secondary reaction pathways, the efficiency of condensation, and polymerization occurring after condensation. Lisa et al. [40] carried out pyrolysis of pine using catalysts (in situ: added directly to the biomass before or during pyrolysis; ex situ: used in a separate stage after initial pyrolysis) in a bench-scale fluidized bed reactor. The study determined that the in situ system exhibited marginally superior performance compared to the ex situ system. Madhu et al. [29] conducted flash pyrolysis of palmyra palm fruit bunches, examining the influence of variables such as temperature, particle size, and sweep gas flow rate. The findings indicated that the highest bio-oil yield achieved was about 48%, occurring at a temperature of 500 °C, with a particle size of 1 mm and a sweep gas flow rate of 2 m3/h.
4.3. Microwave Reactors
Microwave reactors represent a recent advancement in biomass pyrolysis, offering several advantages over traditional slow pyrolysis reactors such as fixed-bed reactors. Key benefits and findings related to bio-oil production using microwave reactors [1] are as follows: The drying process in microwave reactor occurs in an oven chamber connected to a power source, while an inert gas is used to maintain an oxygen-free environment. Energy transfer occurs through interactions among molecules and atoms, which results in efficient heat transfer and controlled heating. Microwave reactors have some advantages like enhanced control over the heating process and improved chemical reactions that reduce the formation of unwanted products.
Among studies and results, Yu et al. [41] investigated bio-oil production from Chinese tallow kernel oil with a silicon carbide catalyst. The maximum achieved a yield of approached 90% at 300 °C, with a 1:2 catalyst/feed ratio and 1 mL/min feed rate, and the product mix was mostly aromatic components. Zhao et al. [42] analyzed the impact of microwave heating on biomass pyrolysis, finding that higher temperatures positively influenced the process, increasing the proportion of combustible gases and enhancing biosolid characteristics. Tarves et al. [43] studied the effect of different gas atmospheres on bio-oil properties from lignocellulosic biomass pyrolysis. They found that CO atmosphere had little impact, whereas H2, CH4, and model pyrolysis gas atmospheres produced more deoxygenated products with a lower oxygen content.
4.4. Ablative Reactors
Ablative reactors are complex systems that are used for biomass pyrolysis and characterized by intense, mechanically driven processes. Key features and findings related to bio-oil production using ablative reactors [1] include the observation that heat is transferred from the ablative reactor walls to the biomass particles under pressure, which removes volatiles quickly. It operates at temperatures around 600 °C, achieving high particle velocity. The advantages of this reactor are high heating rates, efficient heat transfer, energy and cost efficiency, compact design, and minimal size reduction requirements.
Two common types of ablative reactors are vortex reactors (Figure 4) and rotating cone reactors (Figure 5). In vortex reactors, particles contact the heated reactor walls, pushed by steam at a velocity of 1200 m/s. This velocity forces small particles of the biomass to rotate within the walls of the reactor at approximately 625 °C. Solid and liquid products are separated and removed, with unconverted solids fed back into the reactor. Vapors are quickly removed using carrier gas within 100 ms. High bio-oil yields of about 65% have been attained by using a vortex reactor. Biomass pyrolysis via vortex reactors have been used in both experimental and simulation studies with notable success (e.g., [44,45,46]).
Rotating cone reactors do not require inert gas. Biomass is mixed with hot sand and moved from the cone base to the tip by centrifugal force. Then, vapors are condensed at the top, and char is returned as feedstock. Despite their complex design, rotating cone reactors produce high bio-oil yield. Biomass pyrolysis using the rotating cone reactor design has been successfully used in various studies [47,48,49].
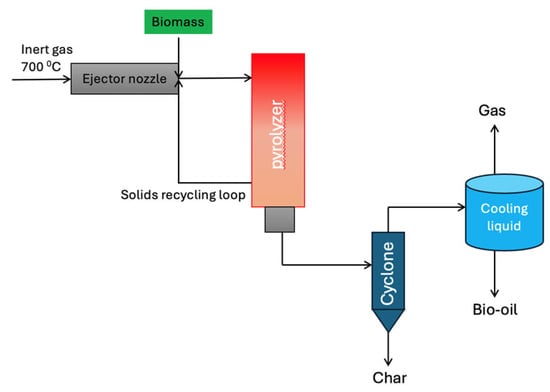
Figure 4.
Schematic of a vortex reactor. Image by National Renewable Energy Laboratory (NREL), Adapted with permission from [50]; redrawn by the authors. © 2021 Springer Nature.
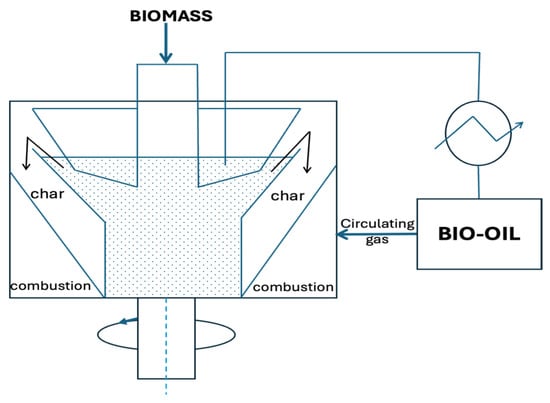
Figure 5.
Schematic of a rotating cone reactor. Redrawn by the authors based on [18].
4.5. Auger Reactor (Screw Reactor)
The auger reactor, or screw reactor, shown in Figure 6, is a pyrolysis technology suitable for mobile applications and areas with limited infrastructure due to its ease of operation and maintenance. Key features and findings related to bio-oil production using screw reactors [1] are as follows: Auger reactor works by mixing feedstock with hot sand using a screw conveyor to control reaction times. The advantages of this kind of reactor are low operating temperatures and the ability to use small reactor sizes. On the other hand, the drawbacks are long vapor residence times, resulting in lower pyrolysis oil yields, and mechanical unreliability due to exposure of moving parts to high temperatures.
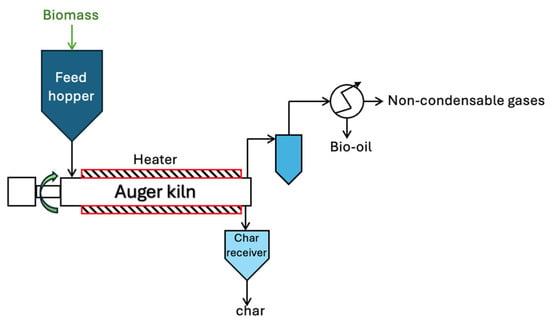
Figure 6.
An auger/screw pyrolysis reactor system. Redrawn by the authors based on [18].
As for results, Brassard et al. [51] investigated how pyrolysis conditions, including temperatures ranging from 450 °C to 650 °C, solid residence times between 60 and 120 s, and nitrogen flow rates from 1 to 5 L per minute, affect product yields and biochar properties in a vertical auger reactor. Papari et al. [52] conducted a parametric study using a pilot auger reactor on forest residues for bio-oil production, achieving a 53% oil yield at 450–475 °C with a 4 kg/h feed rate.
4.6. Free-Fall Reactor
Free-fall or drop-tube reactors, as illustrated in Figure 7, represent a simple yet effective technology for biomass pyrolysis. During bio-oil production using free-fall reactors, the particles descend through the length of the free-fall reactor. Since the biomass is introduced from the uppermost region of the reactor, the flow of sweep gas is diminished relative to other reactor types. Char is trapped in a collector, while the volatile gas transverses a cyclone to remove solid particles prior to entering a condenser; moreover, bio-oil is recovered by quenching volatile gases. These reactors operate at elevated heating rates, and the retention time may range from milliseconds to several seconds [53].
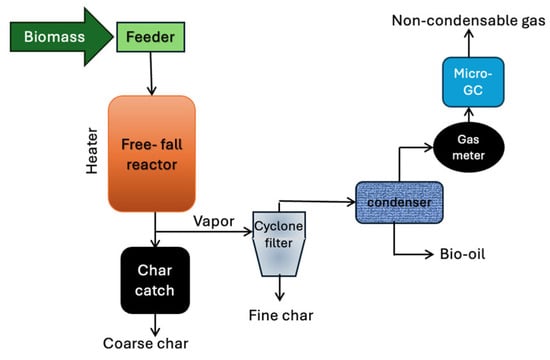
Figure 7.
Process flow diagram of a free-fall reactor for biomass pyrolysis. Redrawn by the authors based on [25].
Other pyrolysis reactors include vacuum reactors, which operate under vacuum conditions to increase bio-oil yield by reducing vapor residence times and secondary reactions; plasma reactors, which use high temperatures in an oxygen-deficient environment to convert waste into syngas; and solar reactors, which use solar energy for pyrolysis, potentially producing higher-calorific products with lower carbon emissions. Research on these reactors has focused on improving bio-oil properties and yields, optimizing reaction conditions, and enhancing energy efficiency [1]. A detailed comparison of the physical and chemical properties of bio-oil produced by various pyrolysis reactors is presented in Table 2. In this table, parameters above the “Product Yields” row refer to feedstock/process characteristics (input material), while those below refer to properties of products. Properties like pH, total acid number, viscosity, density, and HHV refer to bio-oil.

Table 2.
Comparison of physical and chemical properties of bio-oil obtained from various pyrolysis reactors. Reprinted from [25], with permission from Elsevier.
5. Bio-Oil Upgrading
The undesirable properties of crude bio-oil have restricted its direct use as transportation fuel. Bio-oil needs upgrading to enhance its stability, increase its heating value, reduce char and ash contents, lower viscosity and acidity, and improve its suitability for blending with fossil fuels [1]. This section offers a concise overview of bio-oil upgrading techniques. Table 3 provides a comprehensive overview of various techniques, detailing their specific operating parameters, beneficial aspects, and potential drawbacks [59].

Table 3.
Current bio-oil upgrading techniques with technical challenges and potential benefits. Adapted from [59], with permission from Elsevier.
5.1. Solvent Addition
The utilization of polar solvents like ethyl acetate, acetone, methanol, and ethanol has been a long-standing practice to improve homogenization and reduce the viscosity of biomass-derived oils [60,61,62]. Adding these solvents can increase the heating value because they have a higher heating value than most bio-oils. Furthermore, the introduction of solvents to bio-oil serves a dual purpose in viscosity reduction. Firstly, it physically dilutes the bio-oil, directly decreasing its viscosity. Secondly, it initiates chemical interactions between the solvent molecules and bio-oil constituents. These reactions inhibit the progression of chain growth and aging processes, effectively maintaining the bio-oil’s lower viscosity over time [63].
5.2. Emulsification/Emulsions
Emulsification with other suitable fuels is one of the upgrading bio-oil methods. Although pyrolysis-derived bio-oil is not naturally mixable with petroleum-based fuels, it can be emulsified with biodiesel and diesel oil through the addition of surfactants [59].
Although emulsification offers a straightforward approach to upgrading bio-oil, it comes with a significant cost since the process requires surfactants and high energy for emulsification. When bio-oil is emulsified, the resulting mixture demonstrates favorable ignition characteristics. However, there is a trade-off in engine applications. The emulsions tend to be more corrosive than either pure bio-oil or diesel fuel used independently, potentially leading to increased wear and maintenance issues in engine components [64,65]. This method does not involve chemical reactions and appears to be a short-term solution for bio-oil upgrading. While it improves some characteristics, such as ignition properties, it does not effectively enhance heating value, corrosiveness, and cetane number [66].
5.3. Esterification/Alcoholysis
In biodiesel production, esterification, also known as alcoholysis, converts free fatty acids into alkyl esters. This involves the reaction of fatty acids with alcohols in the presence of an acid catalyst at atmospheric pressure. Methanol is frequently employed as the alcohol because it is cost effective [67]. Esterification is usually conducted at 60 °C when using methanol, the temperature that is typically below the boiling point of the alcohol used. Esterification can also be conducted under supercritical conditions for enhanced efficiency [68,69].
Peng et al. [69] found that catalytic upgrading of bio-oil using supercritical ethanol is more effective than sub-critical methods. Water is a by-product of esterification, and its removal can increase reaction yield, often conducted by reactive distillation [70,71]. Although both homogeneous and heterogeneous catalysts can be used, heterogeneous ones are preferred due to their easier separation from products. HZSM-5 [69] and aluminum silicate [72] are examples of catalysts used in bio-oil upgrading.
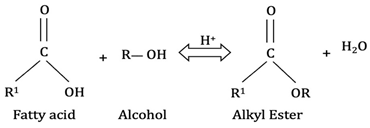
Bio-oil is rich in fatty acids and makes esterification a promising technique for its upgrading. Researchers [59,73] studied the tribological effects of bio-oil upgraded from Spirulina algae through esterification with methanol and ethanol. The results showed that catalytic esterification significantly enhanced the friction resistance and anti-wear properties of the bio-oil. The alcoholysis reaction for bio-oil upgrading can generally be represented by Equation (1) [70]:
Equation (2) shows the alcoholysis reaction of bio-oil [70]. Esterification enhances bio-oil quality by reducing viscosity, density, aging rate, acidity, oxygen content, water content, and increasing heating value. However, significant nitrogen removal effects have not been observed. Due to its simplicity, low temperature and pressure requirements, and the cost effectiveness of alcohols like methanol, esterification appears to be a promising technique for upgrading bio-oil [59].

5.4. Supercritical Fluids (SCFs)
A liquid becomes a supercritical fluid (SCF) at a temperature and pressure above its critical point, where distinct liquid and gas phases do not exist. SCFs can dissolve materials like a liquid and diffuse through solids like a gas, making them suitable substitutes for organic solvents. They possess unique properties, such as liquid-like density, gas-like diffusivity, and low viscosity, which allow for faster rates of mass and heat transfer [74]. SCFs have recently been used in hydrothermal liquefaction of biomass to increase bio-oil yield or quality and for bio-oil upgrading [75]. Various solvents, including ethanol [74,76], methanol [77], and water [78], have been investigated for this purpose.
Xu et al. [79] have shown that using SCFs, such as supercritical 1-butanol or supercritical water with catalysts, results in bio-oil with better properties compared to non-supercritical or subcritical conditions, making them comparable to petroleum-based fuels.
5.5. Hydrotreating/Hydro-Processing/Hydro-Refining/Hydrodeoxygenation
Hydrotreating is an established refinery process applied to decrease the amount of oxygen (O), nitrogen (N), and sulfur (S) atoms from oil cuts or petrochemical feedstocks. This is achieved through catalytic reactions with high-pressure hydrogen that removes oxygen as water, nitrogen as ammonia (NH3), and sulfur as hydrogen sulfide (H2S) [80]. The process typically operates at high pressures (up to 20 MPa) and moderate temperatures (300–450 °C) and needs hydrogen. The widely used hydrotreating catalysts are cobalt–molybdenum (CoMo)- and nickel–molybdenum (NiMo)-based catalysts [65].
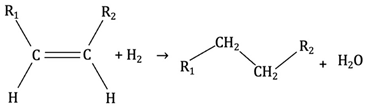
Hydrogenation can be carried out during hydrotreating to improve fuel product quality by increasing its hydrogen content. This process can enhance bio-oil quality during hydrotreating. While the primary goal of hydrotreating is not to crack heavy molecules, partial cracking may occur. The main reaction in hydrotreating is hydrodeoxygenation [81]. Hydrotreating has been widely used for bio-oil upgrading because bio-oil contains oxygen-rich compounds (e.g., acids, aldehydes, esters, ketones, and phenols) [82]. The simplest equation form of hydrotreating reaction of bio-oil can be written as Equation (3) [59]:
Many catalysts like Ru/C [83], Pd/C [84], Ni–Cu/Al2O3 [85], Ni–Cu/SiO2 [86], Ru/TiO2 [87], Ru/Al2O3 [87], CoMo/γ-Al2O3 [88], and NiMo/Al2O3 [89] have been evaluated for hydrotreating of bio-oil. Recently, algal bio-oil hydrotreatment has gained attention [90,91]. For example, Li and Savage [92] studied algal bio-oil treatment. The study used the HZSM-5 catalyst under a high pressure of hydrogen that significantly reduced heteroatoms (N, O, and S) in the resulting bio-oil. Also, it was noted that the reaction temperature considerably influenced the composition of upgraded bio-oil.
While hydrotreating is well established in oil refineries, it needs high-pressure hydrogen, which results in high equipment costs. Catalyst deactivation and coking are of significant concern as the reported results show an average catalyst lifetime around 200 h [12]. Despite these challenges, hydrotreating is a promising approach for bio-oil upgrading; however, more research is needed to increase catalyst lifetime and develop a sustainable hydrogen source [59]. Equations (4) and (5) exemplify the key chemical transformations that occur during hydrotreating (the main reaction is hydrodeoxygenation) [79].
Hydrodeoxygenation:
Hydrogenation:

5.6. Hydrocracking
Hydrocracking is a catalytic process that occurs at temperatures above 350 °C and high pressures (up to 14 MPa) in the presence of hydrogen gas and a suitable catalyst. This process comprises hydrogenation and the breaking of complex organic molecules into simpler ones. The products include coke, an aqueous phase, an organic liquid phase, and gases [81]. In hydrocracking, hydrogen breaks C–C bonds, whereas in hydrotreatment, hydrogen breaks C–N, C–O, and C–S bonds. A mixture of hydrotreating and hydrocracking has been studied for bio-oil upgrading. This integrated approach initially upgrades bio-oil through hydrotreating, followed by separation of heavy constituents, which are subsequently subjected to hydrocracking to produce lighter fractions [93].
Although hydrocracking is an effective way to break heavy molecules into lighter products, it needs high temperatures and hydrogen pressures for operation, which increases the process cost. Equation (6) shows an example of a hydrocracking reaction [59].
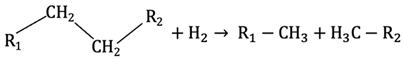
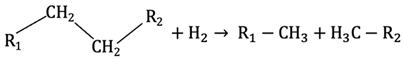
5.7. Catalytic Cracking
Zeolites, which are microporous aluminosilicate materials, are commonly employed as catalysts and adsorbents due to their high surface areas and adsorption capacities. They have acid active sites, the strength and concentration of which can help for specific applications. Zeolite cracking, a bio-oil upgrading technique performed at 350–500 °C, involves deoxygenation, and its products are gases (CO2, CO, and light alkanes), water-soluble organics, water, oil-soluble organics, and coke [65]. Unlike other methods, zeolite cracking does not use hydrogen, so the process is conducted at atmospheric pressure. But it leads to a low H/C ratio in upgraded bio-oil due to the lack of additional hydrogen and is associated with extensive coking and rapid catalyst deactivation [12].
Mortensen et al. [12] conducted experiments to compare zeolite cracking and hydrodeoxygenation methods for bio-oil upgrading. The study concluded that zeolite cracking is not a promising approach due to the low quality of produced bio-oil. Despite this known limitation, researchers have explored various zeolites as catalysts. Srinivas et al. [94] constructed a two-reactor system wherein thermal reactions occur in the first reactor, followed by catalytic reactions in the second reactor. The benefit of this two-reactor system was a reduction in coke deposition on the catalyst surface, thus improving the catalyst life.
5.8. Steam Reforming
Steam reforming is a technique for generating syngas, a blend of hydrogen and carbon monoxide, by processing hydrocarbon-based fuels like natural gas. In this process, fossil fuels react with steam at elevated temperatures (700–1000 °C). Nickel is the most widely utilized catalyst in industrial-scale applications. The well-developed steam reforming technology, originally applied to fossil fuels, has now been adapted for use with bio-oil as a feedstock.
The aim of steam reforming bio-oil is to produce synthesis gas, which can subsequently be transformed into various fuels. This process has been studied using both fixed-bed [95,96,97] and fluidized bed reactors [98]. Key parameters influencing steam reforming of bio-oils include the temperature, steam-to-carbon ratio, and catalyst-to-feed ratio [99]. Similar to the steam reforming of fossil fuels, nickel-based catalysts are predominantly used for bio-oils catalysts [100]. However, a significant challenge in this process, akin to hydrotreating and cracking techniques, is catalyst deactivation due to coking [59].
5.9. Extraction of Chemicals from Bio-Oil
Bio-oil is known to contain numerous compounds, with several having significant industrial value, including phenol for resin production, as well as various organic acids and alkanes [101]. Therefore, researchers have explored bio-oil upgrading methods, one of which is extracting valuable chemicals from bio-oil. The methods used are similar to separation technologies long employed in oil refineries and petrochemical plants. Various studies have implemented techniques such as absorption with acetone as a solvent [102], distillation [103], phase separation fractionation [104], and aqueous extraction fractionation [105]. Eboibi et al. [106] utilized vacuum distillation to upgrade bio-oil produced from the hydrothermal liquefaction of Spirulina sp. and Tetraselmis sp. The researchers demonstrated an increase in the higher heating value (HHV) and a notable reduction in the oxygen content and boiling point ranges in the distilled bio-crude oil.
6. Process Economics
Process economics plays an essential role in assessing the feasibility and sustainability of bio-oil production technologies. It helps quantify the capital and operating costs, evaluate profitability, and assess long-term viability under different configurations, scales, and feedstocks. Economic analysis also serves as a critical tool for guiding investment decisions and public policy aimed at accelerating the transition to low-carbon fuels. According to Wright et al. [107], techno-economic analysis (TEA) is essential not only for understanding cost structures but also for identifying process improvements and supporting commercialization strategies for thermochemical biofuel technologies.
The economic feasibility of bio-oil production is especially important given the continued dominance of low-cost fossil fuels and the challenges of scaling renewable alternatives. For bio-oil to be adopted in the energy market, its production must be cost competitive and financially sustainable. As Humbird et al. [108] emphasize, new technologies need to prove both technical performance and economic value to move from pilot to commercial scale. Key factors that improve economic feasibility include using flexible feedstocks, recovering value from co-products, and applying efficient upgrading methods that reduce overall costs and improve fuel quality.
6.1. Bio-Oil Production
6.1.1. Cost Components and Optimization Strategies
Economic analysis entails evaluating the economic feasibility of a process or product across progressive stages, which enables tracking of future research, expansion, and investment. Financial analysis focuses on determining the costs of manufacturing, selling, investing, and marketing. Furthermore, the calculated values can assist in forecasting future cash flow and return on investment [109].
Economic analysis of bio-oil production is based on the methods, plant size (laboratory, pilot, or commercial), and the availability and consistency of feedstock supply. Key challenges include analyzing feed supply and product costs to ensure market compatibility. Fixed capital investment (FCI) involves funds for purchasing manufacturing and plant infrastructure, while working capital covers operational costs. Total capital investment combines both FCI and working capital. FCI can be divided into manufacturing costs (like site preparation, piping, and equipment) and non-manufacturing costs (such as construction overheads) [110]. The overall direct production expense is calculated using feedstock and utility costs, while the total product cost is determined by combining both fixed capital expenses and production costs.
Utilizing a combined feedstock, which is a mix of two or more distinct types of biomass, can reduce risks and costs due to the variety of biomass options available [111]. Biofuel upgrading, especially through catalytic processes, enhances commercial viability. Catalysts such as Ni, zeolite, and Al2O3 reduce the acidity and oxygen compounds in bio-oil, making catalytic pyrolysis more economically favorable due to lower equipment needs [12,112,113]. Recycling gases produced during pyrolysis is another method to boost economic potential. Studies on materials like rape straw, corn stalks, and camphor wood have shown the benefits of this approach [114].
6.1.2. TEA Results and Economic Insights
Table 4 summarizes the TEA of bio-oil production through biomass fast pyrolysis from various reported studies. The final yield percentage of bio-oil significantly impacts the economic viability of the process [115]. Meyer et al. [116] assessed the economics of six types of lignocellulosic biomass to report that pine achieved the highest bio-oil yield, while switchgrass had the lowest. Wang et al. [117] studied the TEA aspects of products from cotton stalks. With an estimated production capacity of around 18,000 tons per year, the production cost was estimated to be USD 3/kg. Researchers also explored the economic feasibility of using rice straw in thermochemical conversion techniques, concluding that bio-oil production through pyrolysis of rice straw is economically feasible, with typical biomass conversion rates ranging from 46 to 65% [118].

Table 4.
Techno-economic analysis of bio-oil produced from fast pyrolysis. Adapted from [115]; licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/, accessed on 7 September 2025).
A previous study summarized bio-oil production cost versus refinery size for bio-oil produced from various feedstocks and via different methods [130] 9 April 2025 10:15:00 AM. The study showed that, depending on the method used, costs ranged from USD 1.73 per gallon at a refinery size of 2.4 metric tons per day to USD 0.41 per gallon at 1000 metric tons per day.
The data presented in Table 5 illustrates the production expenses associated with bio-oil derived from various biomass sources using the fast pyrolysis process. Patel et al. [131] examined the cost of producing bio-oil from the fast pyrolysis of 2000 tons of woodchips per day, reporting a cost of USD 1.09 per liter. They also explored the optimization of plant size, ranging from 500 to 5000 tons per day, and concluded that a capacity of 3000 tons per day is optimal based on economic analysis.
Xin et al. [132] analyzed the cost of producing bio-oil and co-products using an innovative method involving the cultivation, harvesting, dewatering, fast pyrolysis, and utilization of water-based waste algae. They estimated the price of bio-oil at USD 2.23 per gallon, which is considered acceptable. The return rate could increase to 18.7% if significant improvements are made in cultivation, harvesting, and conversion processes. Meanwhile, Li et al. [133] performed a cost analysis of biomass using both in situ and ex situ catalytic pyrolysis. They found that the minimum selling price for bio-oil was USD 1.11 per liter for the in situ process and USD 1.13 per liter for the ex situ process.
Implementing heat integration strategies in pyrolysis enhances the process’s sustainability through effective energy recovery, leading to a reduction in overall utility expenses. The pyrolysis process is endothermic and requires heat to complete key reactions. Combustible gases produced during fast pyrolysis can be used to supply this heat, reducing overall utility and operation costs, which positively affect the expense of producing bio-oil [134].

Table 5.
Cost of bio-oil produced from fast pyrolysis. Adapted from [115]; licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/, accessed on 7 September 2025).
Table 5.
Cost of bio-oil produced from fast pyrolysis. Adapted from [115]; licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/, accessed on 7 September 2025).
| Source of Bio-Oil | Process | Capacity | Cost of Bio-Oil | Reference |
|---|---|---|---|---|
| Municipal sewage sludge | Pyrolysis, gas chromatography, mass spectroscopy, and Aspen Plus | 50 kg/h | 3.66 (USD/kg) | [135] |
| Napier grass bagasse | Pyrolysis and in situ hydrodeoxygenation | 49 kg/h | USD 5.81/gallon (USD 1.45/L) gasoline equivalent | [136] |
| Sludge scum | Integrated system with pyrolysis | 3.5 wet tons of scum, 265 dry tons of sludge daily | USD 1.85/gallon | [137] |
| Horse manure | Tail gas reactive pyrolysis (TGRP) | 200 metric dry ton per day | (USD 1.35–USD 1.80 L−1) of jet fuel by upgraded bio-oil | [138] |
| Pine | Pretreatment, fast pyrolysis, catalytic upgrading, and heat integration | 1000 dry metric ton per day | 4.01–4.78 USD/gal for heat-integrated process 4.70–6.84 USD/gal without heat integration | [139] |
| Sorghum bagasse, corn stove, palm kernel, and switchgrass | Regression-based chemical process model | 2000 metric tons per day (MT/d) | USD 2.5 to USD 5 per gallon | [140] |
| Beechwood | Pre-treatment and catalytic circulating fluidized bed reactor | 500 MT/day | 2.32–3.08 USD/gallons | [141] |
| Pinewood | Fast pyrolysis, hydro-processing, and economic analyzer | 72 MT/day | USD 8.47/ gallon-gasoline equivalent | [11] |
| Red oak | Fast pyrolysis and five-stage fractionation system | 2000 dry metric tons per day | USD 3.09/gallon | [142] |
| Microalgae | Pretreatment, catalytic pyrolysis, and chemical process modeling | 2000 MT per day | USD 1.49 and USD 1.80 per liter | [143] |
6.2. Bio-Oil Upgrading to Fuels and Chemicals
6.2.1. Cost Overview of Biofuel Pathways
Figure 8 summarizes the average, range (high–low), and number of estimates (n) for fuel production costs from the reviewed literature. For cellulosic ethanol (EtOH), production costs range from USD 1.15 to USD 7.85 per gallon-gasoline equivalent (gge), with an average of USD 4 [144,145,146,147,148]. On average, feedstock costs made up 39% of the total costs, and operating costs were 33%. However, there was substantial variability across studies, with feedstock costs averaging 33% and operating costs at 42%. Two estimates are from a 2016 Lux Research study, showing the cost variation of USD 3.25 to USD 6.80 per gge for six operational cellulosic ethanol facilities worldwide. In this study, feedstock costs contributed 23–31%, with the highest share (40%) observed at POET’s Project Liberty Facility [147].
Among drop-in fuels, pyrolysis followed by hydrotreatment showed the lowest average production cost of approximately USD 3.25/gge (range: USD 2.15–USD 4.75/gge), with the highest share of operating costs (47%) and the lowest feedstock cost share (30%). Hydroprocessed esters and fatty acids (HEFAs) averaged USD 3.70/gge (range: USD 3.10–USD 4.40/gge), with feedstock contributing over half (52%) of total costs. Biomass-to-liquid (BTL) fuels averaged USD 3.80 per gge (range: USD 1.60–USD 6.30/gge), with feedstock costs contributing 46% and capital expenditures 30%, the highest of any fuels [133,149,150]. Notably, the lowest BTL estimate came from a high-capacity 2006 model plant, where the large scale significantly reduced unit costs [151].
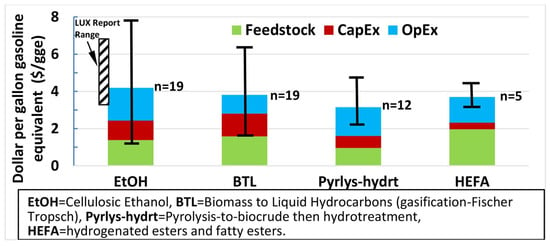
Figure 8.
Summary of biofuel production cost estimates [144,145,146,147,148,149,150]. Average costs, range (maximum to minimum), and number of scenarios examined (n) for facilities at the early commercial deployment stage but beyond “pioneer plants”. The Lux Report Range presents the highest and lowest projected values for six functioning cellulosic ethanol plants, based on their designed capacities rather than actual production figures. Reprinted from [152], with permission from Elsevier.
6.2.2. Barriers to Market Viability
Cost competitiveness is critical for market uptake. At a plant gate cost of USD 3.25–USD 4.00/gge, biofuels can only compete with retail gasoline or diesel without subsidies if oil prices rise above USD 100–130 per barrel, considering a USD 0.50/gge markup from plant to retail. However, oil prices have remained under this threshold since 2014 [153]. The advanced biofuel industry has yet to establish successful pioneer commercial plants, despite current policy incentives, except for the HEFA diesel facilities and potentially the POET cellulosic ethanol plant, which is now focused on R&D. The uncertainty of policy motivations makes them unreliable for project financing [154]. Pioneer plants typically cost significantly more than subsequent plants in a mature industry, which are the basis for the cost estimates reviewed.
The impact of technical and commercial success in sparking further industry activity is unpredictable and varies widely across industries [154]. The assumed USD 0.50 per gge markup is illustrative only. Actual markup from plant gate cost to retail price depends on factors such as fuel properties (e.g., drop-in or blend), delivery infrastructure, fuel taxes, market structure, and profit margins [152].
6.2.3. Case Studies and Economic Insights
Makepa et al. [155] conducted a study to evaluate the economic viability and environmental impact of microwave-assisted pyrolysis (MAP) of pine sawdust, followed by bio-oil esterification to produce biodiesel. They applied Aspen Plus® to simulate a facility processing 2000 metric tons of pine sawdust per day. The results showed a biodiesel yield of 631.7 tons per day with a minimum fuel selling price (MFSP) of USD 2.31 per liter. The overall capital investment required was USD 286.1 million, with yearly operating expenses of USD 164.9 million. The yields of pyrolysis products were estimated at 65.8 wt% bio-oil, 8.9 wt% biochar, and 25.3 wt% non-condensable gases. The study found that methanol cost and operational expenses were significant factors, and the global warming potential (GWP) of the production process was 70.97 kg CO2 equivalent. The researchers determined the process to be economically feasible, as the projected MFSP compares favorably with conventional diesel fuel. The findings emphasize the significance of optimizing critical process parameters to improve the economic viability of the pyrolysis method [155].
In another study [156], comparative TEA and environmental sustainability analysis of four biofuel production pathways for the marine sector were conducted. The four pathways included (1) hydrothermal liquefaction (HTL) of wet wastes like sewage sludge and manure (pathway 1); (2) fast pyrolysis (FP) of woody biomass (pathway 2); (3) landfill gas Fischer−Tropsch synthesis (LGFT) (pathway 3); and (4) the lignin−ethanol oil (LEO) pathway from a lignocellulosic ethanol biorefinery using reductive catalytic fractionation (RCF) (pathway 4). The biofuels examined in this research are viewed as potential drop-in fuels or blendstocks that are well matched with marine engines. This analysis relied on small-scale experimental data and precise process simulations to extrapolate the chosen pathways to larger scales.
Figure 9 shows the TEA of different pathways mentioned above. Pathway 1 is the wet waste HTL with two wet wastes (sewage sludge and manure). The plant scale is a key economic driver for this pathway. There are three different options for sludge hydrothermal liquefaction (SHTL) and three options for manure hydrothermal liquefaction (MHTL). To evaluate these three options, three hydrotreatment scenarios were assessed for each feedstock: no hydrotreatment, mild hydrotreatment, and full hydrotreatment [156]. Pathway 2 involves processes that convert a blend of forest residues and clean pine into bio-oil through three methods: FP without catalytic upgrading, FP with upgrading using a ZSM-5 catalyst, and FP with a Pt/TiO2 catalyst. These processes are designed for a plant with a daily feedstock rate of 2000 dry metric tons [156]. Pathway 3 is a gas-to-liquid process that uses landfill gas (LFG) as feedstock instead of common natural gas. This process involves steam methane reforming (SMR), syngas conditioning (compression and acid gas removal), and FT synthesis. LFG contains about 40% CO2. It must be compressed from 1.6 psig to the SMR operating pressure of 30 psi. H2S is removed using an iron bed, and siloxanes are removed with an activated carbon bed. SMR converts methane in the LFG to syngas (CO and H2) by the injection of steam [156]. Pathway 4, known as the LEO pathway, integrates a biorefinery design and uses reductive catalytic fractionation (RCF) instead of dilute acid pretreatment. This process uses hybrid poplar as the feedstock, which has a higher lignin content than herbaceous feedstocks, resulting in higher LEO yields [156].
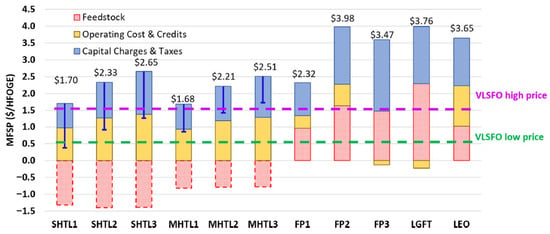
Figure 9.
Summary of MFSPs across four biofuel pathways. In the HTL cases, the feedstock costs marked with a dash symbolize sensitivity analyses that factor in the avoided disposal fees for potential wet waste. Additionally, the blue error bars represent potential cost reductions for HTL pathways based on avoided disposal fees. For reference, the high and low prices of very-low-sulfur fuel oil (VLSFO) reflect the historical price range observed at major North American ports [157]. Adapted from [156] and licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/, accessed on 7 September 2025). The $ on the y-axis represents USD.
The various fuel products are aggregated and presented as a unified fuel product using a heavy fuel oil gallon equivalent (HFOGE) basis for ease of comparison and understanding. All MFSPs were calculated and stated on this combined basis. The cost components of the MFSP include (i) capital charges and taxes, (ii) operating costs and co-product credits, and (iii) feedstock costs. MFSPs across different pathways range from USD 1.68 to USD 3.98 per HFOGE for scenarios without feedstock credits (Figure 9).
Recent interest in alternative methods for producing marine fuel has prompted a study that compared the cost of fuel by different biomass processing methods and sources. The data in Table 6 shows that among the pathways listed, the Fischer–Tropsch (FT) process and fast pyrolysis can yield fuel at relatively low costs: below USD 3 per gallon in some cases.

Table 6.
Potential marine fuel price in the open literature. Adapted from [156]; licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/, accessed on 7 September 2025).
7. Discussion and Policy Outlook
The techno-economic insights presented in this review highlight both the promise and the challenges of advancing biomass pyrolysis for renewable fuel and chemical production. Although a wide range of feedstocks and upgrading technologies have been developed and evaluated, cost competitiveness remains the most critical barrier to widespread commercialization, particularly in an energy market still dominated by inexpensive fossil fuels.
The comparative techno-economic analyses in Table 4 and Table 5 reveal key trends in bio-oil production through fast pyrolysis. First, bio-oil production costs vary widely depending on plant scale, feedstock type, and process configuration. Reported costs range from as low as USD 1.85 per gallon (~USD 0.5 per L), particularly for optimized systems utilizing heat integration with feedstocks such as sludge scum and rice husk, up to USD 5–6 per gallon in less efficient or smaller-scale operations. Systems using heat integration, catalytic upgrading, or co-product valorization consistently showed improved economic performance. The use of mobile pyrolysis systems and feedstock blending also appears to reduce production costs and increase resilience against biomass supply fluctuations.
The techno-economic analysis and comparisons in bio-oil upgrading section suggest that while many bio-oil upgrading methods are getting closer to being cost competitive, their success depends on factors like the type of feedstock used, the size of the facility, and how efficiently the process runs. Among the reviewed pathways, pyrolysis combined with hydrotreatment and hydrothermal liquefaction of wet waste stands out as the most promising from both a cost and sustainability standpoint. These not only show strong potential in terms of cost and environmental benefits but also perform even better when combined with strategies like recovering co-products and reusing heat.
These findings highlight the importance of creating policies that support more modular, scalable, and heat-integrated pyrolysis systems, particularly in areas with a wide variety of available raw materials. Investing in research to improve catalyst reuse, streamline biomass transport, and recover process heat could make these systems much more cost-effective. To encourage growth in this area, governments may consider including fast pyrolysis technologies in renewable fuel standards or bio-refinery initiatives. Doing so could reduce investment risks and help bring these innovations to market more quickly.
To unlock the full potential of bio-oil technologies, especially during their early deployment stages, governments play a crucial role. Supportive policies such as production tax credits, capital grants for pioneer plants, and inclusion of pyrolysis-derived fuels in renewable fuel mandates can provide the financial confidence needed for commercial scale-up.
Equally important is the need for consistent and harmonized evaluation methods. Standardizing life-cycle emissions data and TEA frameworks across countries and studies would make it easier to compare results and identify the most viable and sustainable biofuel pathways. Finally, stronger collaboration between governments, academic institutions, and industry can accelerate innovation, reduce investment risks, and facilitate knowledge-sharing platforms that accelerate the transition to commercial viability.
8. Leading Biomass Companies in the World
The global biomass market is experiencing significant growth, driven by the active participation of leading companies within the industry. These organizations are channeling investments into innovative biomass technologies and projects, contributing to the increased affordability and accessibility of biomass as a sustainable energy source. Table 7 highlights top biomass companies worldwide, showcasing the industry pioneers at the forefront of advancements in the biomass sector.

Table 7.
Top biomass companies in the world [161].
9. Conclusions
Biomass as a feedstock replacement represents an opportunity to produce fuels and chemicals from non-fossil-based feedstocks. The U.S. DOE billion-ton study gives a baseline to assess the quantitative target as a percentage of the total output of the end products. This review focused on four topics: (1) biomass to bio-oil chemistry, (2) processes to produce products through bio-oil upgrading, both catalytic and non-catalytic pathways, (3) reactors used to process biomass, and (4) process economics. The combination of these factors can serve as a guide to assess a targeted product for scale-up. The review could serve as a guide to a roadmap for transitioning to renewable biomass feedstock-based transportation fuels and chemicals. However, the key to this transition is process economics, advances in reactor design that, coupled with efficient catalysts, as listed in this review, could pave the way to global deployment. Other approaches under development, such as co-processing of biomass with plastic and sludge waste [171] and microwave heating for processing [31], could also make biomass processing attractive. When supported by enabling policies and strategic investments, these advances could help biomass emerge as competitive domestic feedstock in building a low-carbon energy future.
Author Contributions
Conceptualization D.M.; methodology, D.M.; validation, D.M. and E.P.; formal analysis, E.P. and D.M.; writing—original draft preparation, E.P.; writing—review and editing, D.M.; visualization, E.P. and D.M.; supervision, D.M.; project administration, D.M.; funding acquisition, D.M.; E.L.H., policy section editing. All authors have read and agreed to the published version of the manuscript.
Funding
The research was funded by the Institute of Gas Innovation and Technology (I-GIT) at Stony Brook University.
Data Availability Statement
Data supporting the reported results can be found, including links to publicly archived datasets analyzed or generated during the study, in the references cited throughout the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Amenaghawon, A.N.; Anyalewechi, C.L.; Okieimen, C.O.; Kusuma, H.S. Biomass pyrolysis technologies for value-added products: A state-of-the-art review. Environ. Dev. Sustain. 2021, 23, 14324–14378. [Google Scholar] [CrossRef]
- Bridgewater, A. Biomass fast pyrolysis. Therm. Sci. 2004, 8, 21–50. [Google Scholar] [CrossRef]
- Czernik, S.; Bridgwater, A.V. Overview of Applications of Biomass Fast Pyrolysis Oil. Energy Fuels 2004, 18, 590–598. [Google Scholar] [CrossRef]
- Boran, F.E. A new approach for evaluation of renewable energy resources: A case of Turkey. Energy Sources Part B Econ. Plan. Policy 2018, 13, 196–204. [Google Scholar] [CrossRef]
- Nachenius, R.W.; Ronsse, F.; Venderbosch, R.H.; Prins, W. Biomass Pyrolysis. In Advances in Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2013; Volume 42, pp. 75–139. [Google Scholar] [CrossRef]
- Tabakaev, R.; Kanipa, I.; Astafev, A.; Dubinin, Y.; Yazykov, N.; Zavorin, A.; Yakovlev, V. Thermal enrichment of different types of biomass by low-temperature pyrolysis. Fuel 2019, 245, 29–38. [Google Scholar] [CrossRef]
- Naik, S.N.; Goud, V.V.; Rout, P.K.; Dalai, A.K. Production of first and second generation biofuels: A comprehensive review. Renew. Sustain. Energy Rev. 2010, 14, 578–597. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Ghenai, C.; Inayat, A.; Shanableh, A.; Al-Sarairah, E.; Janajreh, I. Combustion and emissions analysis of Spent Pot lining (SPL) as alternative fuel in cement industry. Sci. Total Environ. 2019, 684, 519–526. [Google Scholar] [CrossRef]
- Shahbaz, M.; Yusup, S.; Pratama, A.; Inayat, A.; Patrick, D.O.; Ammar, M. Parametric Study and Optimization of Methane Production in Biomass Gasification in the Presence of Coal Bottom Ash. Procedia Eng. 2016, 148, 409–416. [Google Scholar] [CrossRef]
- Shemfe, M.B.; Gu, S.; Ranganathan, P. Techno-economic performance analysis of biofuel production and miniature electric power generation from biomass fast pyrolysis and bio-oil upgrading. Fuel 2015, 143, 361–372. [Google Scholar] [CrossRef]
- Mortensen, P.M.; Grunwaldt, J.-D.; Jensen, P.A.; Knudsen, K.G.; Jensen, A.D. A review of catalytic upgrading of bio-oil to engine fuels. Appl. Catal. Gen. 2011, 407, 1–19. [Google Scholar] [CrossRef]
- Oasmaa, A.; Czernik, S. Fuel Oil Quality of Biomass Pyrolysis OilsState of the Art for the End Users. Energy Fuels 1999, 13, 914–921. [Google Scholar] [CrossRef]
- Kan, T.; Strezov, V.; Evans, T.J. Lignocellulosic biomass pyrolysis: A review of product properties and effects of pyrolysis parameters. Renew. Sustain. Energy Rev. 2016, 57, 1126–1140. [Google Scholar] [CrossRef]
- Puri, L.; Hu, Y.; Naterer, G. Critical review of the role of ash content and composition in biomass pyrolysis. Front. Fuels 2024, 2, 1378361. [Google Scholar] [CrossRef]
- Östman, B.; Rydholm, D. National Fire Regulations in Relation to the Use of Wood in European and Some Other Countries; Trätek Publication No. 0212044; Trätek: Frankfurt am Main, Germany, 2002; 57p. [Google Scholar]
- Fahmy, T.Y.A.; Fahmy, Y.; Mobarak, F.; El-Sakhawy, M.; Abou-Zeid, R.E. Biomass pyrolysis: Past, present, and future. Environ. Dev. Sustain. 2020, 22, 17–32. [Google Scholar] [CrossRef]
- Dhyani, V.; Bhaskar, T. A comprehensive review on the pyrolysis of lignocellulosic biomass. Renew. Energy 2018, 129, 695–716. [Google Scholar] [CrossRef]
- Kiel, J.H.A.; Van Paasen, S.V.B.; Neeft, J.P.A.; Devi, L.; Ptasinski, K.J.; Janssen, F.J.J.G.; Meijer, R.; Berends, R.H.; Temmink, H.M.G.; Brem, G.; et al. Primary Measures to Reduce Tar Formation in Fluidised-Bed Biomass Gasifiers. In Final Report SDE Project P1999-012; Eindhoven University of Technology: Eindhoven, The Netherlands, 2004. [Google Scholar]
- Prins, M.J.; Ptasinski, K.J.; Janssen, F.J.J.G. Torrefaction of wood. J. Anal. Appl. Pyrolysis 2006, 77, 28–34. [Google Scholar] [CrossRef]
- Gaitán-Álvarez, J.; Moya, R.; Puente-Urbina, A.; Rodriguez-Zúñiga, A. Thermogravimetric, Devolatilization Rate, and Differential Scanning Calorimetry Analyses of Biomass of Tropical Plantation Species of Costa Rica Torrefied at Different Temperatures and Times. Energies 2018, 11, 696. [Google Scholar] [CrossRef]
- Balagurumurthy, B.; Srivastava, V.; Vinit; Kumar, J.; Biswas, B.; Singh, R.; Gupta, P.; Kumar, K.L.N.S.; Singh, R.; Bhaskar, T. Value addition to rice straw through pyrolysis in hydrogen and nitrogen environments. Bioresour. Technol. 2015, 188, 273–279. [Google Scholar] [CrossRef]
- Al Arni, S. Comparison of slow and fast pyrolysis for converting biomass into fuel. Renew. Energy 2018, 124, 197–201. [Google Scholar] [CrossRef]
- Tripathi, M.; Sahu, J.N.; Ganesan, P. Effect of process parameters on production of biochar from biomass waste through pyrolysis: A review. Renew. Sustain. Energy Rev. 2016, 55, 467–481. [Google Scholar] [CrossRef]
- Papari, S.; Hawboldt, K. A review on the pyrolysis of woody biomass to bio-oil: Focus on kinetic models. Renew. Sustain. Energy Rev. 2015, 52, 1580–1595. [Google Scholar] [CrossRef]
- Heat Values of Various Fuels—World Nuclear Association. Available online: https://world-nuclear.org/information-library/facts-and-figures/heat-values-of-various-fuels (accessed on 17 August 2025).
- Akhtar, J.; Saidina Amin, N. A review on operating parameters for optimum liquid oil yield in biomass pyrolysis. Renew. Sustain. Energy Rev. 2012, 16, 5101–5109. [Google Scholar] [CrossRef]
- Demirbas, A.; Arin, G. An Overview of Biomass Pyrolysis. Energy Sources 2002, 24, 471–482. [Google Scholar] [CrossRef]
- Madhu, P.; Kanagasabapathy, H.; Manickam, I.N. Flash pyrolysis of palmyra palm (Borassus flabellifer) using an electrically heated fluidized bed reactor. Energy Sources Part A Recovery Util. Environ. Eff. 2016, 38, 1699–1705. [Google Scholar] [CrossRef]
- Cornelissen, T.; Yperman, J.; Reggers, G.; Schreurs, S.; Carleer, R. Flash co-pyrolysis of biomass with polylactic acid. Part 1: Influence on bio-oil yield and heating value. Fuel 2008, 87, 1031–1041. [Google Scholar] [CrossRef]
- Robinson, J.; Dodds, C.; Stavrinides, A.; Kingman, S.; Katrib, J.; Wu, Z.; Medrano, J.; Overend, R. Microwave Pyrolysis of Biomass: Control of Process Parameters for High Pyrolysis Oil Yields and Enhanced Oil Quality. Energy Fuels 2015, 29, 1701–1709. [Google Scholar] [CrossRef]
- He, M.; Xiao, B.; Liu, S.; Hu, Z.; Guo, X.; Luo, S.; Yang, F. Syngas production from pyrolysis of municipal solid waste (MSW) with dolomite as downstream catalysts. J. Anal. Appl. Pyrolysis 2010, 87, 181–187. [Google Scholar] [CrossRef]
- Chen, D.; Li, Y.; Cen, K.; Luo, M.; Li, H.; Lu, B. Pyrolysis polygeneration of poplar wood: Effect of heating rate and pyrolysis temperature. Bioresour. Technol. 2016, 218, 780–788. [Google Scholar] [CrossRef]
- Gurevich Messina, L.I.; Bonelli, P.R.; Cukierman, A.L. Effect of acid pretreatment and process temperature on characteristics and yields of pyrolysis products of peanut shells. Renew. Energy 2017, 114, 697–707. [Google Scholar] [CrossRef]
- Açıkalın, K.; Karaca, F. Fixed-bed pyrolysis of walnut shell: Parameter effects on yields and characterization of products. J. Anal. Appl. Pyrolysis 2017, 125, 234–242. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, J.; Zhang, Y. Analysis of pyrolysis characteristics and kinetics of Euphausia superba shell waste using TG-FTIR and distributed activation energy model. Biomass Convers. Biorefinery 2018, 8, 329–337. [Google Scholar] [CrossRef]
- Sakthivel, R.; Harshini, G.V.; Vardhan, M.S.; Vinod, A.; Gomathi, K. Biomass energy conversion through pyrolysis: A ray of hope for the current energy crisis. In Green Energy Systems; Elsevier: Amsterdam, The Netherlands, 2023; pp. 37–68. [Google Scholar] [CrossRef]
- Tauseef, S.M.; Abbasi, T.; Abbasi, S.A. Energy recovery from wastewaters with high-rate anaerobic digesters. Renew. Sustain. Energy Rev. 2013, 19, 704–741. [Google Scholar] [CrossRef]
- Carvalho, W.S.; Santana Júnior, J.A.; De Oliveira, T.J.P.; Ataíde, C.H. Fast pyrolysis of sweet sorghum bagasse in a fluidized bed reactor: Product characterization and comparison with vapors generated in analytical pyrolysis. Energy 2017, 131, 186–197. [Google Scholar] [CrossRef]
- Iisa, M.; French, R.; Orton, K.; Yung, M.; Johnson, D.; Nimlos, M.; Dam, J.; Watson, M. In Situ and ex Situ Catalytic Pyrolysis of Pine in a Bench-Scale Fluidized Bed Reactor System. Energy Fuels 2016, 30, 2144–2157. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, Y.; Jiang, L.; Dai, L.; Liu, Y.; Ruan, R.; Duan, D.; Zhou, Y.; Fan, L.; Zhao, Y.; et al. Microwave-assisted catalytic pyrolysis of Chinese tallow kernel oil for aromatic production in a downdraft reactor. J. Anal. Appl. Pyrolysis 2018, 133, 16–21. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, M.; Liu, H.; Li, L.; Ma, C.; Song, Z. A microwave reactor for characterization of pyrolyzed biomass. Bioresour. Technol. 2012, 104, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Tarves, P.C.; Mullen, C.A.; Boateng, A.A. Effects of various reactive gas atmospheres on the properties of bio-oils produced using microwave pyrolysis. ACS Sustain. Chem. Eng. 2016, 4, 930–936. [Google Scholar] [CrossRef]
- Kulkarni, S.; Gonzalez-Quiroga, A.; Perreault, P.; Sewani, H.; Heynderickx, G.; Van Geem, K.; Marin, G. Cfd-based biomass fast pyrolysis simulations in a gas-solid vortex reactor demonstrating process intensification. Chem. Eng. Trans. 2018, 65, 19–24. [Google Scholar] [CrossRef]
- Gonzalez-Quiroga, A.; Reyniers, P.A.; Kulkarni, S.R.; Torregrosa, M.M.; Perreault, P.; Heynderickx, G.J.; Van Geem, K.M.; Marin, G.B. Design and cold flow testing of a Gas-Solid Vortex Reactor demonstration unit for biomass fast pyrolysis. Chem. Eng. J. 2017, 329, 198–210. [Google Scholar] [CrossRef]
- Miller, R.S.; Bellan, J. Numerical Simulation of Vortex Pyrolysis Reactors for Condensable Tar Production from Biomass. Energy Fuels 1998, 12, 25–40. [Google Scholar] [CrossRef]
- Wagenaar, B.M.; Prins, W.; Van Swaaij, W.P.M. Pyrolysis of biomass in the rotating cone reactor: Modelling and experimental justification. Chem. Eng. Sci. 1994, 49, 5109–5126. [Google Scholar] [CrossRef]
- Westerhout, R.W.J.; Waanders, J.; Kuipers, J.A.M.; Van Swaaij, W.P.M. Recycling of Polyethene and Polypropene in a Novel Bench-Scale Rotating Cone Reactor by High-Temperature Pyrolysis. Ind. Eng. Chem. Res. 1998, 37, 2293–2300. [Google Scholar] [CrossRef]
- Guoxin, H.; Xiwu, G.; Hao, H.; Haojie, F.; Zheng, W. Experimental studies on flow and pyrolysis of coal with solid heat carrier in a modified rotating cone reactor. Chem. Eng. Process. Process Intensif. 2008, 47, 1777–1785. [Google Scholar] [CrossRef]
- ResearchGate. Schematic of a Vortex Reactor Designed at the NREL. Available online: https://www.researchgate.net/figure/Schematic-of-a-vortex-reactor-designed-at-the-NREL-International-Energy-Agency-IEA_fig11_349365567 (accessed on 17 November 2024).
- Brassard, P.; Godbout, S.; Raghavan, V.; Palacios, J.; Grenier, M.; Zegan, D. The Production of Engineered Biochars in a Vertical Auger Pyrolysis Reactor for Carbon Sequestration. Energies 2017, 10, 288. [Google Scholar] [CrossRef]
- Papari, S.; Hawboldt, K.; Helleur, R. Production and Characterization of Pyrolysis Oil from Sawmill Residues in an Auger Reactor. Ind. Eng. Chem. Res. 2017, 56, 1920–1925. [Google Scholar] [CrossRef]
- Bahng, M.-K.; Mukarakate, C.; Robichaud, D.J.; Nimlos, M.R. Current technologies for analysis of biomass thermochemical processing: A review. Anal. Chim. Acta 2009, 651, 117–138. [Google Scholar] [CrossRef]
- Ingram, L.; Mohan, D.; Bricka, M.; Steele, P.; Strobel, D.; Crocker, D.; Mitchell, B.; Mohammad, J.; Cantrell, K.; Pittman, C.U. Pyrolysis of Wood and Bark in an Auger Reactor: Physical Properties and Chemical Analysis of the Produced Bio-oils. Energy Fuels 2008, 22, 614–625. [Google Scholar] [CrossRef]
- Meier, D.; Faix, O. State of the art of applied fast pyrolysis of lignocellulosic materials—A review. Bioresour. Technol. 1999, 68, 71–77. [Google Scholar] [CrossRef]
- Abnisa, F.; Arami-Niya, A.; Daud, W.M.A.W.; Sahu, J.N. Characterization of Bio-oil and Bio-char from Pyrolysis of Palm Oil Wastes. BioEnergy Res. 2013, 6, 830–840. [Google Scholar] [CrossRef]
- Junsheng, L. The Optimal of Pyrolysis Process in the Rotating Cone Reactor and Pyrolysis Product Analysis. In Proceedings of the 2010 International Conference on Challenges in Environmental Science and Computer Engineering, Wuhan, China, 6–7 March 2010; IEEE: New York, NY, USA, 2010; pp. 530–533. [Google Scholar] [CrossRef]
- Punsuwan, N.; Tangsathitkulchai, C. Product Characterization and Kinetics of Biomass Pyrolysis in a Three-Zone Free-Fall Reactor. Int. J. Chem. Eng. 2014, 2014, 986719. [Google Scholar] [CrossRef]
- Saber, M.; Nakhshiniev, B.; Yoshikawa, K. A review of production and upgrading of algal bio-oil. Renew. Sustain. Energy Rev. 2016, 58, 918–930. [Google Scholar] [CrossRef]
- Diebold, J.P.; Czernik, S. Additives To Lower and Stabilize the Viscosity of Pyrolysis Oils during Storage. Energy Fuels 1997, 11, 1081–1091. [Google Scholar] [CrossRef]
- Boucher, M.E.; Chaala, A.; Roy, C. Bio-oils obtained by vacuum pyrolysis of softwood bark as a liquid fuel for gas turbines. Part I: Properties of bio-oil and its blends with methanol and a pyrolytic aqueous phase. Biomass Bioenergy 2000, 19, 337–350. [Google Scholar] [CrossRef]
- Pidtasang, B.; Udomsap, P.; Sukkasi, S.; Chollacoop, N.; Pattiya, A. Influence of alcohol addition on properties of bio-oil produced from fast pyrolysis of eucalyptus bark in a free-fall reactor. J. Ind. Eng. Chem. 2013, 19, 1851–1857. [Google Scholar] [CrossRef]
- Liu, R.; Fei, W.; Shen, C. Influence of acetone addition on the physicochemical properties of bio-oils. J. Energy Inst. 2014, 87, 127–133. [Google Scholar] [CrossRef]
- Chiaramonti, D.; Bonini, M.; Fratini, E.; Tondi, G.; Gartner, K.; Bridgwater, A.V.; Grimm, H.P.; Soldaini, I.; Webster, A.; Baglioni, P. Development of emulsions from biomass pyrolysis liquid and diesel and their use in engines—Part 1: Emulsion production. Biomass Bioenergy 2003, 25, 85–99. [Google Scholar] [CrossRef]
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of Transportation Fuels from Biomass: Chemistry, Catalysts, and Engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, R.; Yin, R.; Mei, Y. Upgrading of bio-oil from biomass fast pyrolysis in China: A review. Renew. Sustain. Energy Rev. 2013, 24, 66–72. [Google Scholar] [CrossRef]
- Demirbaş, A. Production of Biodiesel from Algae Oils. Energy Sources Part Recovery Util. Environ. Eff. 2008, 31, 163–168. [Google Scholar] [CrossRef]
- Peng, J.; Chen, P.; Lou, H.; Zheng, X. Upgrading of Bio-oil over Aluminum Silicate in Supercritical Ethanol. Energy Fuels 2008, 22, 3489–3492. [Google Scholar] [CrossRef]
- Peng, J.; Chen, P.; Lou, H.; Zheng, X. Catalytic upgrading of bio-oil by HZSM-5 in sub- and super-critical ethanol. Bioresour. Technol. 2009, 100, 3415–3418. [Google Scholar] [CrossRef]
- Mahfud, F.H.; Melián-Cabrera, I.; Manurung, R.; Heeres, H.J. Biomass to Fuels. Process Saf. Environ. Prot. 2007, 85, 466–472. [Google Scholar] [CrossRef]
- Junming, X.; Jianchun, J.; Yunjuan, S.; Yanju, L. Bio-oil upgrading by means of ethyl ester production in reactive distillation to remove water and to improve storage and fuel characteristics. Biomass Bioenergy 2008, 32, 1056–1061. [Google Scholar] [CrossRef]
- Zhang, Q.; Chang, J.; Wang; Xu, Y. Upgrading Bio-oil over Different Solid Catalysts. Energy Fuels 2006, 20, 2717–2720. [Google Scholar] [CrossRef]
- Xu, Y.; Zheng, X.; Hu, X.; Dearn, K.D.; Xu, H. Effect of catalytic esterification on the friction and wear performance of bio-oil. Wear 2014, 311, 93–100. [Google Scholar] [CrossRef]
- Xu, C.; Etcheverry, T. Hydro-liquefaction of woody biomass in sub- and super-critical ethanol with iron-based catalysts. Fuel 2008, 87, 335–345. [Google Scholar] [CrossRef]
- Yin, W.; Venderbosch, R.H.; Bottari, G.; Krawzcyk, K.K.; Barta, K.; Heeres, H.J. Catalytic upgrading of sugar fractions from pyrolysis oils in supercritical mono-alcohols over Cu doped porous metal oxide. Appl. Catal. B Environ. 2015, 166, 56–65. [Google Scholar] [CrossRef]
- Chen, W.; Luo, Z.; Yu, C.; Li, G.; Yang, Y.; Zhang, H. Upgrading of bio-oil in supercritical ethanol: Catalysts screening, solvent recovery and catalyst stability study. J. Supercrit. Fluids 2014, 95, 387–393. [Google Scholar] [CrossRef]
- Li, W.; Pan, C.; Zhang, Q.; Liu, Z.; Peng, J.; Chen, P.; Lou, H.; Zheng, X. Upgrading of low-boiling fraction of bio-oil in supercritical methanol and reaction network. Bioresour. Technol. 2011, 102, 4884–4889. [Google Scholar] [CrossRef] [PubMed]
- Duan, P.; Xu, Y.; Bai, X. Upgrading of Crude Duckweed Bio-Oil in Subcritical Water. Energy Fuels 2013, 27, 4729–4738. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, C.; Zhai, Y.; Liu, Y.; Zhang, R.; Tang, X. Upgrading of Bio-Oil Using Supercritical 1-Butanol over a Ru/C Heterogeneous Catalyst: Role of the Solvent. Energy Fuels 2014, 28, 4611–4621. [Google Scholar] [CrossRef]
- Xiu, S.; Shahbazi, A. Bio-oil production and upgrading research: A review. Renew. Sustain. Energy Rev. 2012, 16, 4406–4414. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T. Microalgae biofuels: A critical review of issues, problems and the way forward. Biotechnol. Adv. 2012, 30, 673–690. [Google Scholar] [CrossRef]
- Zacher, A.H.; Olarte, M.V.; Santosa, D.M.; Elliott, D.C.; Jones, S.B. A review and perspective of recent bio-oil hydrotreating research. Green Chem. 2014, 16, 491–515. [Google Scholar] [CrossRef]
- Mullen, C.A.; Boateng, A.A.; Reichenbach, S.E. Hydrotreating of fast pyrolysis oils from protein-rich pennycress seed presscake. Fuel 2013, 111, 797–804. [Google Scholar] [CrossRef]
- Li, X.; Gunawan, R.; Wang, Y.; Chaiwat, W.; Hu, X.; Gholizadeh, M.; Mourant, D.; Bromly, J.; Li, C.-Z. Upgrading of bio-oil into advanced biofuels and chemicals. Part III. Changes in aromatic structure and coke forming propensity during the catalytic hydrotreatment of a fast pyrolysis bio-oil with Pd/C catalyst. Fuel 2014, 116, 642–649. [Google Scholar] [CrossRef]
- Ardiyanti, A.R.; Khromova, S.A.; Venderbosch, R.H.; Yakovlev, V.A.; Heeres, H.J. Catalytic hydrotreatment of fast-pyrolysis oil using non-sulfided bimetallic Ni-Cu catalysts on a δ-Al2O3 support. Appl. Catal. B Environ. 2012, 117, 105–117. [Google Scholar] [CrossRef]
- Ardiyanti, A.R.; Khromova, S.A.; Venderbosch, R.H.; Yakovlev, V.A.; Melián-Cabrera, I.V.; Heeres, H.J. Catalytic hydrotreatment of fast pyrolysis oil using bimetallic Ni–Cu catalysts on various supports. Appl. Catal. Gen. 2012, 449, 121–130. [Google Scholar] [CrossRef]
- Wildschut, J.; Mahfud, F.H.; Venderbosch, R.H.; Heeres, H.J. Hydrotreatment of Fast Pyrolysis Oil Using Heterogeneous Noble-Metal Catalysts. Ind. Eng. Chem. Res. 2009, 48, 10324–10334. [Google Scholar] [CrossRef]
- Parapati, D.R.; Guda, V.K.; Penmetsa, V.K.; Steele, P.H.; Tanneru, S.K. Single stage hydroprocessing of pyrolysis oil in a continuous packed-bed reactor. Environ. Prog. Sustain. Energy 2014, 33, 726–731. [Google Scholar] [CrossRef]
- Pstrowska, K.; Walendziewski, J.; Łużny, R.; Stolarski, M. Hydroprocessing of rapeseed pyrolysis bio-oil over NiMo/Al2O3 catalyst. Catal. Today 2014, 223, 54–65. [Google Scholar] [CrossRef]
- Guo, Q.; Wu, M.; Wang, K.; Zhang, L.; Xu, X. Catalytic Hydrodeoxygenation of Algae Bio-oil over Bimetallic Ni–Cu/ZrO2 Catalysts. Ind. Eng. Chem. Res. 2015, 54, 890–899. [Google Scholar] [CrossRef]
- Le, T.A.; Ly, H.V.; Kim, J.; Kim, S.-S.; Choi, J.H.; Woo, H.-C.; Othman, M.R. Hydrodeoxygenation of 2-furyl methyl ketone as a model compound in bio-oil from pyrolysis of Saccharina Japonica Alga in fixed-bed reactor. Chem. Eng. J. 2014, 250, 157–163. [Google Scholar] [CrossRef]
- Li, Z.; Savage, P.E. Feedstocks for fuels and chemicals from algae: Treatment of crude bio-oil over HZSM-5. Algal Res. 2013, 2, 154–163. [Google Scholar] [CrossRef]
- Zhu, Y.; Albrecht, K.O.; Elliott, D.C.; Hallen, R.T.; Jones, S.B. Development of hydrothermal liquefaction and upgrading technologies for lipid-extracted algae conversion to liquid fuels. Algal Res. 2013, 2, 455–464. [Google Scholar] [CrossRef]
- Srinivas, S.T.; Dalai, A.K.; Bakhshi, N.N. Thermal and catalytic upgrading of a biomass-derived oil in a dual reaction system. Can. J. Chem. Eng. 2000, 78, 343–354. [Google Scholar] [CrossRef]
- Wang, D.; Czernik, S.; Chornet, E. Production of Hydrogen from Biomass by Catalytic Steam Reforming of Fast Pyrolysis Oils. Energy Fuels 1998, 12, 19–24. [Google Scholar] [CrossRef]
- Garcia, L.; French, R.; Czernik, S.; Chornet, E. Catalytic steam reforming of bio-oils for the production of hydrogen: Effects of catalyst composition. Appl. Catal. Gen. 2000, 201, 225–239. [Google Scholar] [CrossRef]
- Wu, C.; Huang, Q.; Sui, M.; Yan, Y.; Wang, F. Hydrogen production via catalytic steam reforming of fast pyrolysis bio-oil in a two-stage fixed bed reactor system. Fuel Process. Technol. 2008, 89, 1306–1316. [Google Scholar] [CrossRef]
- Xu, Q.; Lan, P.; Zhang, B.; Ren, Z.; Yan, Y. Hydrogen Production via Catalytic Steam Reforming of Fast Pyrolysis Bio-oil in a Fluidized-Bed Reactor. Energy Fuels 2010, 24, 6456–6462. [Google Scholar] [CrossRef]
- Czernik, S.; French, R.J.; Magrini-Bair, K.A.; Chornet, E. The Production of Hydrogen by Steam Reforming of Trap GreaseProgress in Catalyst Performance. Energy Fuels 2004, 18, 1738–1743. [Google Scholar] [CrossRef]
- Trane-Restrup, R.; Jensen, A.D. Steam reforming of cyclic model compounds of bio-oil over Ni-based catalysts: Product distribution and carbon formation. Appl. Catal. B Environ. 2015, 165, 117–127. [Google Scholar] [CrossRef]
- Zhang, Q.; Chang, J.; Wang, T.; Xu, Y. Review of biomass pyrolysis oil properties and upgrading research. Energy Convers. Manag. 2007, 48, 87–92. [Google Scholar] [CrossRef]
- Cao, J.-P.; Zhao, X.-Y.; Morishita, K.; Li, L.-Y.; Xiao, X.-B.; Obara, R.; Wei, X.-Y.; Takarada, T. Triacetonamine formation in a bio-oil from fast pyrolysis of sewage sludge using acetone as the absorption solvent. Bioresour. Technol. 2010, 101, 4242–4245. [Google Scholar] [CrossRef]
- Capunitan, J.A.; Capareda, S.C. Characterization and separation of corn stover bio-oil by fractional distillation. Fuel 2013, 112, 60–73. [Google Scholar] [CrossRef]
- Lindfors, C.; Kuoppala, E.; Oasmaa, A.; Solantausta, Y.; Arpiainen, V. Fractionation of Bio-Oil. Energy Fuels 2014, 28, 5785–5791. [Google Scholar] [CrossRef]
- Vasilkovova, B.; Augustinova, J.; Buzetzki, E.; Cvengros, J.; Mikulec, J.; Kubinec, R.; Blasko, J. Fractionation of bio-oil from flash pyrolysis. Chem. Eng. Trans. 2014, 39, 1573–1578. [Google Scholar] [CrossRef]
- Eboibi, B.E.-O.; Lewis, D.M.; Ashman, P.J.; Chinnasamy, S. Hydrothermal liquefaction of microalgae for biocrude production: Improving the biocrude properties with vacuum distillation. Bioresour. Technol. 2014, 174, 212–221. [Google Scholar] [CrossRef]
- Wright, M.M.; Daugaard, D.E.; Satrio, J.A.; Brown, R.C. Techno-economic analysis of biomass fast pyrolysis to transportation fuels. Fuel 2010, 89, S2–S10. [Google Scholar] [CrossRef]
- Humbird, D.; Davis, R.; Tao, L.; Kinchin, C.; Hsu, D.; Aden, A.; Schoen, P.; Lukas, J.; Olthof, B.; Worley, M.; et al. Process Design and Economics for Biochemical Conversion of Lignocellulosic Biomass to Ethanol: Dilute-Acid Pretreatment and Enzymatic Hydrolysis of Corn Stover; UNT Digital Library: Denton, TX, USA, 2011. [Google Scholar] [CrossRef]
- Sharma, A.; Jakhete, A.; Sharma, A.; Joshi, J.B.; Pareek, V. Lowering greenhouse gas (GHG) emissions: Techno-economic analysis of biomass conversion to biofuels and value-added chemicals. Greenh. Gases Sci. Technol. 2019, 9, 454–473. [Google Scholar] [CrossRef]
- Inayat, A.; Ahmad, M.M.; Mutalib, M.I.A.; Yusup, S.; Khan, Z. Economic analysis and optimization for bio-hydrogen production from oil palm waste via steam gasification. Energy Sources Part B Econ. Plan. Policy 2017, 12, 158–165. [Google Scholar] [CrossRef]
- Oasmaa, A.; Solantausta, Y.; Arpiainen, V.; Kuoppala, E.; Sipilä, K. Fast Pyrolysis Bio-Oils from Wood and Agricultural Residues. Energy Fuels 2010, 24, 1380–1388. [Google Scholar] [CrossRef]
- Miandad, R.; Rehan, M.; Barakat, M.A.; Aburiazaiza, A.S.; Khan, H.; Ismail, I.M.I.; Dhavamani, J.; Gardy, J.; Hassanpour, A.; Nizami, A.-S. Catalytic Pyrolysis of Plastic Waste: Moving Toward Pyrolysis Based Biorefineries. Front. Energy Res. 2019, 7, 27. [Google Scholar] [CrossRef]
- Farooq, W.; Ali, I.; Raza Naqvi, S.; Sajid, M.; Abbas Khan, H.; Adamu, S. Evolved Gas Analysis and Kinetics of Catalytic and Non-Catalytic Pyrolysis of Microalgae Chlorella sp. Biomass With Ni/θ-Al2O3 Catalyst via Thermogravimetric Analysis. Front. Energy Res. 2021, 9, 775037. [Google Scholar] [CrossRef]
- Yang, M.; Luo, B.; Shao, J.; Zeng, K.; Zhang, X.; Yang, H.; Chen, H. The influence of CO2 on biomass fast pyrolysis at medium temperatures. J. Renew. Sustain. Energy 2018, 10, 013108. [Google Scholar] [CrossRef]
- Inayat, A.; Ahmed, A.; Tariq, R.; Waris, A.; Jamil, F.; Ahmed, S.F.; Ghenai, C.; Park, Y.-K. Techno-Economical Evaluation of Bio-Oil Production via Biomass Fast Pyrolysis Process: A Review. Front. Energy Res. 2022, 9, 770355. [Google Scholar] [CrossRef]
- Meyer, P.A.; Snowden-Swan, L.J.; Jones, S.B.; Rappé, K.G.; Hartley, D.S. The effect of feedstock composition on fast pyrolysis and upgrading to transportation fuels: Techno-economic analysis and greenhouse gas life cycle analysis. Fuel 2020, 259, 116218. [Google Scholar] [CrossRef]
- Wang, J.; Lu, Z.; Shah, A. Techno-economic analysis of levoglucosan production via fast pyrolysis of cotton straw in China. Biofuels Bioprod. Biorefining 2019, 13, 1085–1097. [Google Scholar] [CrossRef]
- Diehlmann, F.; Zimmer, T.; Glöser-Chahoud, S.; Wiens, M.; Schultmann, F. Techno-economic assessment of utilization pathways for rice straw: A simulation-optimization approach. J. Clean. Prod. 2019, 230, 1329–1343. [Google Scholar] [CrossRef]
- Van Schalkwyk, D.L.; Mandegari, M.; Farzad, S.; Görgens, J.F. Techno-economic and environmental analysis of bio-oil production from forest residues via non-catalytic and catalytic pyrolysis processes. Energy Convers. Manag. 2020, 213, 112815. [Google Scholar] [CrossRef]
- Brigagão, G.V.; De Queiroz Fernandes Araújo, O.; De Medeiros, J.L.; Mikulcic, H.; Duic, N. A techno-economic analysis of thermochemical pathways for corncob-to-energy: Fast pyrolysis to bio-oil, gasification to methanol and combustion to electricity. Fuel Process. Technol. 2019, 193, 102–113. [Google Scholar] [CrossRef]
- Ramirez, J.A.; Rainey, T.J. Comparative techno-economic analysis of biofuel production through gasification, thermal liquefaction and pyrolysis of sugarcane bagasse. J. Clean. Prod. 2019, 229, 513–527. [Google Scholar] [CrossRef]
- Pighinelli, A.L.M.T.; Schaffer, M.A.; Boateng, A.A. Utilization of eucalyptus for electricity production in Brazil via fast pyrolysis: A techno-economic analysis. Renew. Energy 2018, 119, 590–597. [Google Scholar] [CrossRef]
- Wang, W.-C.; Jan, J.-J. From laboratory to pilot: Design concept and techno-economic analyses of the fluidized bed fast pyrolysis of biomass. Energy 2018, 155, 139–151. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, H.; Xiao, R. Mobile Autothermal Pyrolysis System for Local Biomass Conversion: Process Simulation and Techno-Economic Analysis. Energy Fuels 2018, 32, 4178–4188. [Google Scholar] [CrossRef]
- Shemfe, M.; Gu, S.; Fidalgo, B. Techno-economic analysis of biofuel production via bio-oil zeolite upgrading: An evaluation of two catalyst regeneration systems. Biomass Bioenergy 2017, 98, 182–193. [Google Scholar] [CrossRef]
- Ji, L.-Q.; Zhang, C.; Fang, J.-Q. Economic analysis of converting of waste agricultural biomass into liquid fuel: A case study on a biofuel plant in China. Renew. Sustain. Energy Rev. 2017, 70, 224–229. [Google Scholar] [CrossRef]
- Michailos, S.; Parker, D.; Webb, C. A techno-economic comparison of Fischer–Tropsch and fast pyrolysis as ways of utilizing sugar cane bagasse in transportation fuels production. Chem. Eng. Res. Des. 2017, 118, 206–214. [Google Scholar] [CrossRef]
- Carrasco, J.L.; Gunukula, S.; Boateng, A.A.; Mullen, C.A.; DeSisto, W.J.; Wheeler, M.C. Pyrolysis of forest residues: An approach to techno-economics for bio-fuel production. Fuel 2017, 193, 477–484. [Google Scholar] [CrossRef]
- Do, T.X.; Lim, Y. Techno-economic comparison of three energy conversion pathways from empty fruit bunches. Renew. Energy 2016, 90, 307–318. [Google Scholar] [CrossRef]
- Mirkouei, A.; Haapala, K.R.; Sessions, J.; Murthy, G.S. A review and future directions in techno-economic modeling and optimization of upstream forest biomass to bio-oil supply chains. Renew. Sustain. Energy Rev. 2017, 67, 15–35. [Google Scholar] [CrossRef]
- Patel, M.; Oyedun, A.O.; Kumar, A.; Gupta, R. A Techno-Economic Assessment of Renewable Diesel and Gasoline Production from Aspen Hardwood. Waste Biomass Valorization 2019, 10, 2745–2760. [Google Scholar] [CrossRef]
- Xin, C.; Addy, M.M.; Zhao, J.; Cheng, Y.; Cheng, S.; Mu, D.; Liu, Y.; Ding, R.; Chen, P.; Ruan, R. Comprehensive techno-economic analysis of wastewater-based algal biofuel production: A case study. Bioresour. Technol. 2016, 211, 584–593. [Google Scholar] [CrossRef]
- Li, B.; Ou, L.; Dang, Q.; Meyer, P.; Jones, S.; Brown, R.; Wright, M. Techno-economic and uncertainty analysis of in situ and ex situ fast pyrolysis for biofuel production. Bioresour. Technol. 2015, 196, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Strezov, V. Thermochemical production of bio-oil: A review of downstream processing technologies for bio-oil upgrading, production of hydrogen and high value-added products. Renew. Sustain. Energy Rev. 2021, 135, 110152. [Google Scholar] [CrossRef]
- Shahbeig, H.; Nosrati, M. Pyrolysis of municipal sewage sludge for bioenergy production: Thermo-kinetic studies, evolved gas analysis, and techno-socio-economic assessment. Renew. Sustain. Energy Rev. 2020, 119, 109567. [Google Scholar] [CrossRef]
- Mohammed, I.Y.; Abakr, Y.A.; Mokaya, R. Integrated biomass thermochemical conversion for clean energy production: Process design and economic analysis. J. Environ. Chem. Eng. 2019, 7, 103093. [Google Scholar] [CrossRef]
- Xin, C.; Addy, M.M.; Zhao, J.; Cheng, Y.; Ma, Y.; Liu, S.; Mu, D.; Liu, Y.; Chen, P.; Ruan, R. Waste-to-biofuel integrated system and its comprehensive techno-economic assessment in wastewater treatment plants. Bioresour. Technol. 2018, 250, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Sorunmu, Y.E.; Billen, P.; Elkasabi, Y.; Mullen, C.A.; Macken, N.A.; Boateng, A.A.; Spatari, S. Fuels and Chemicals from Equine-Waste-Derived Tail Gas Reactive Pyrolysis Oil: Technoeconomic Analysis, Environmental and Exergetic Life Cycle Assessment. ACS Sustain. Chem. Eng. 2017, 5, 8804–8814. [Google Scholar] [CrossRef]
- Winjobi, O.; Zhou, W.; Kulas, D.; Nowicki, J.; Shonnard, D.R. Production of Hydrocarbon Fuel Using Two-Step Torrefaction and Fast Pyrolysis of Pine. Part 2: Life-Cycle Carbon Footprint. ACS Sustain. Chem. Eng. 2017, 5, 4541–4551. [Google Scholar] [CrossRef]
- Li, W.; Dang, Q.; Brown, R.C.; Laird, D.; Wright, M.M. The impacts of biomass properties on pyrolysis yields, economic and environmental performance of the pyrolysis-bioenergy-biochar platform to carbon negative energy. Bioresour. Technol. 2017, 241, 959–968. [Google Scholar] [CrossRef]
- Vasalos, I.A.; Lappas, A.A.; Kopalidou, E.P.; Kalogiannis, K.G. Biomass catalytic pyrolysis: Process design and economic analysis. WIREs Energy Environ. 2016, 5, 370–383. [Google Scholar] [CrossRef]
- Hu, W.; Dang, Q.; Rover, M.; Brown, R.C.; Wright, M.M. Comparative techno-economic analysis of advanced biofuels, biochemicals, and hydrocarbon chemicals via the fast pyrolysis platform. Biofuels 2016, 7, 57–67. [Google Scholar] [CrossRef]
- Thilakaratne, R.; Wright, M.M.; Brown, R.C. A techno-economic analysis of microalgae remnant catalytic pyrolysis and upgrading to fuels. Fuel 2014, 128, 104–112. [Google Scholar] [CrossRef]
- Anex, R.P.; Aden, A.; Kazi, F.K.; Fortman, J.; Swanson, R.M.; Wright, M.M.; Satrio, J.A.; Brown, R.C.; Daugaard, D.E.; Platon, A.; et al. Techno-economic comparison of biomass-to-transportation fuels via pyrolysis, gasification, and biochemical pathways. Fuel 2010, 89, S29–S35. [Google Scholar] [CrossRef]
- Ou, L.; Brown, T.R.; Thilakaratne, R.; Hu, G.; Brown, R.C. Techno-economic analysis of co-located corn grain and corn stover ethanol plants. Biofuels Bioprod. Biorefining 2014, 8, 412–422. [Google Scholar] [CrossRef]
- Zhao, X.; Brown, T.R.; Tyner, W.E. Stochastic techno-economic evaluation of cellulosic biofuel pathways. Bioresour. Technol. 2015, 198, 755–763. [Google Scholar] [CrossRef]
- LUX. Uncovering the Cost of Cellulosic Ethanol Production—State of the Market Report; LUX Research: Boston, MA, USA, 2016. [Google Scholar]
- OpenEI. Transparent Cost Database|Open Energy Information 2015. Available online: https://openei.org/wiki/Transparent_Cost_Database (accessed on 7 September 2025).
- De Jong, S.; Hoefnagels, R.; Wetterlund, E.; Pettersson, K.; Faaij, A.; Junginger, M. Cost optimization of biofuel production—The impact of scale, integration, transport and supply chain configurations. Appl. Energy 2017, 195, 1055–1070. [Google Scholar] [CrossRef]
- Brown, T.R.; Thilakaratne, R.; Brown, R.C.; Hu, G. Techno-economic analysis of biomass to transportation fuels and electricity via fast pyrolysis and hydroprocessing. Fuel 2013, 106, 463–469. [Google Scholar] [CrossRef]
- Hamelinck, C.N.; Faaij, A.P.C. Outlook for advanced biofuels. Energy Policy 2006, 34, 3268–3283. [Google Scholar] [CrossRef]
- Witcover, J.; Williams, R.B. Comparison of “Advanced” biofuel cost estimates: Trends during rollout of low carbon fuel policies. Transp. Res. Part Transp. Environ. 2020, 79, 102211. [Google Scholar] [CrossRef]
- US EIA. Cushing, OK WTI Spot Price; U.S. Energy Information Administration: Washington, DC, USA, 2019.
- Morrison, G.M.; Witcover, J.; Parker, N.C.; Fulton, L. Three routes forward for biofuels: Incremental, leapfrog, and transitional. Energy Policy 2016, 88, 64–73. [Google Scholar] [CrossRef]
- Makepa, D.C.; Chihobo, C.H.; Musademba, D. Techno-economic analysis and environmental impact assessment of biodiesel production from bio-oil derived from microwave-assisted pyrolysis of pine sawdust. Heliyon 2023, 9, e22261. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tan, E.C.D.; Dutta, A.; Snowden-Swan, L.J.; Thorson, M.R.; Ramasamy, K.K.; Bartling, A.W.; Brasington, R.; Kass, M.D.; Zaimes, G.G.; et al. Techno-economic Analysis of Sustainable Biofuels for Marine Transportation. Environ. Sci. Technol. 2022, 56, 17206–17214. [Google Scholar] [CrossRef] [PubMed]
- LA/Long Beach Bunker Prices. Ship Bunker. Available online: https://shipandbunker.com/prices/am/nampac/us-lax-la-long-beach (accessed on 5 November 2024).
- Tan, E.C.D.; Hawkins, T.R.; Lee, U.; Tao, L.; Meyer, P.A.; Wang, M.; Thompson, T. Biofuel Options for Marine Applications: Technoeconomic and Life-Cycle Analyses. Environ. Sci. Technol. 2021, 55, 7561–7570. [Google Scholar] [CrossRef]
- Carvalho, F.; Portugal-Pereira, J.; Junginger, M.; Szklo, A. Biofuels for Maritime Transportation: A Spatial, Techno-Economic, and Logistic Analysis in Brazil, Europe, South Africa, and the USA. Energies 2021, 14, 4980. [Google Scholar] [CrossRef]
- Tanzer, S.E.; Posada, J.; Geraedts, S.; Ramírez, A. Lignocellulosic marine biofuel: Technoeconomic and environmental assessment for production in Brazil and Sweden. J. Clean. Prod. 2019, 239, 117845. [Google Scholar] [CrossRef]
- The Top 10 Biomass Companies in the World|NES Fircroft. Available online: https://www.nesfircroft.com/resources/blog/the-top-10-biomass-companies-in-the-world/ (accessed on 29 December 2024).
- Largest, U.S. Biodiesel Producers 2024|Statista. Available online: https://www.statista.com/statistics/829958/largest-us-biodiesel-producer/ (accessed on 29 December 2024).
- POET-DSM Makes Major Technology, Process Purchase for Commercial Cellulosic Bio-Ethanol—POET. Available online: https://poet.com/pr/poet-dsm-makes-major-technology-process-purchase-for-cellulosic-bio-ethanol (accessed on 29 December 2024).
- Green Plains Inc. Green Plains Unveils New Specialty Feed Ingredient Brand. Available online: https://investor.gpreinc.com/news/news-details/2024/Green-Plains-Unveils-New-Specialty-Feed-Ingredient-Brand-c5dfcfa63/default.aspx (accessed on 29 December 2024).
- Shell and Raízen Sign Large Cellulosic Ethanol Deal|Shell Global. Available online: https://www.shell.com/business-customers/trading-and-supply/trading/news-and-media-releases/shell-and-raizen-sign-large-cellulosic-ethanol-deal.html (accessed on 29 December 2024).
- Enviva Kicks off Construction on Wood Pellet Plant in Sumter County—Made in Alabama. Available online: https://www.madeinalabama.com/2023/06/enviva-kicks-off-construction-on-wood-pellet-plant-in-sumter-county/ (accessed on 29 December 2024).
- Technology—VERBIO North America. Available online: https://www.verbio-north-america.com/technology (accessed on 29 December 2024).
- Company|Learn More About Our Story|Enerkem. Available online: https://enerkem.com/company/story (accessed on 29 December 2024).
- Biomethane & Gas Upgrading Plants|EnviTec Biogas. Available online: https://www.envitec-biogas.com/plant-construction/biomethane# (accessed on 29 December 2024).
- Syclus Choses Axens to Provide Atol® Technology for Europe’s First Ethanol-Based Renewable Ethylene Production Plant—Renewable Carbon News. Available online: https://renewable-carbon.eu/news/syclus-choses-axens-to-provide-atol-technology-for-europes-first-ethanol-based-renewable-ethylene-production-plant-2/ (accessed on 29 December 2024).
- Seah, C.C.; Tan, C.H.; Arifin, N.A.; Hafriz, R.S.R.M.; Salmiaton, A.; Nomanbhay, S.; Shamsuddin, A.H. Co-pyrolysis of biomass and plastic: Circularity of wastes and comprehensive review of synergistic mechanism. Results Eng. 2023, 17, 100989. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).