Cassia grandis L.f. Pods as a Source of High-Value-Added Biomolecules: Optimization of Extraction Conditions and Extract Rheology
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction Protocol
2.3. Analytical Determination
2.4. Physical Properties
2.5. Statistical Analyses
3. Results
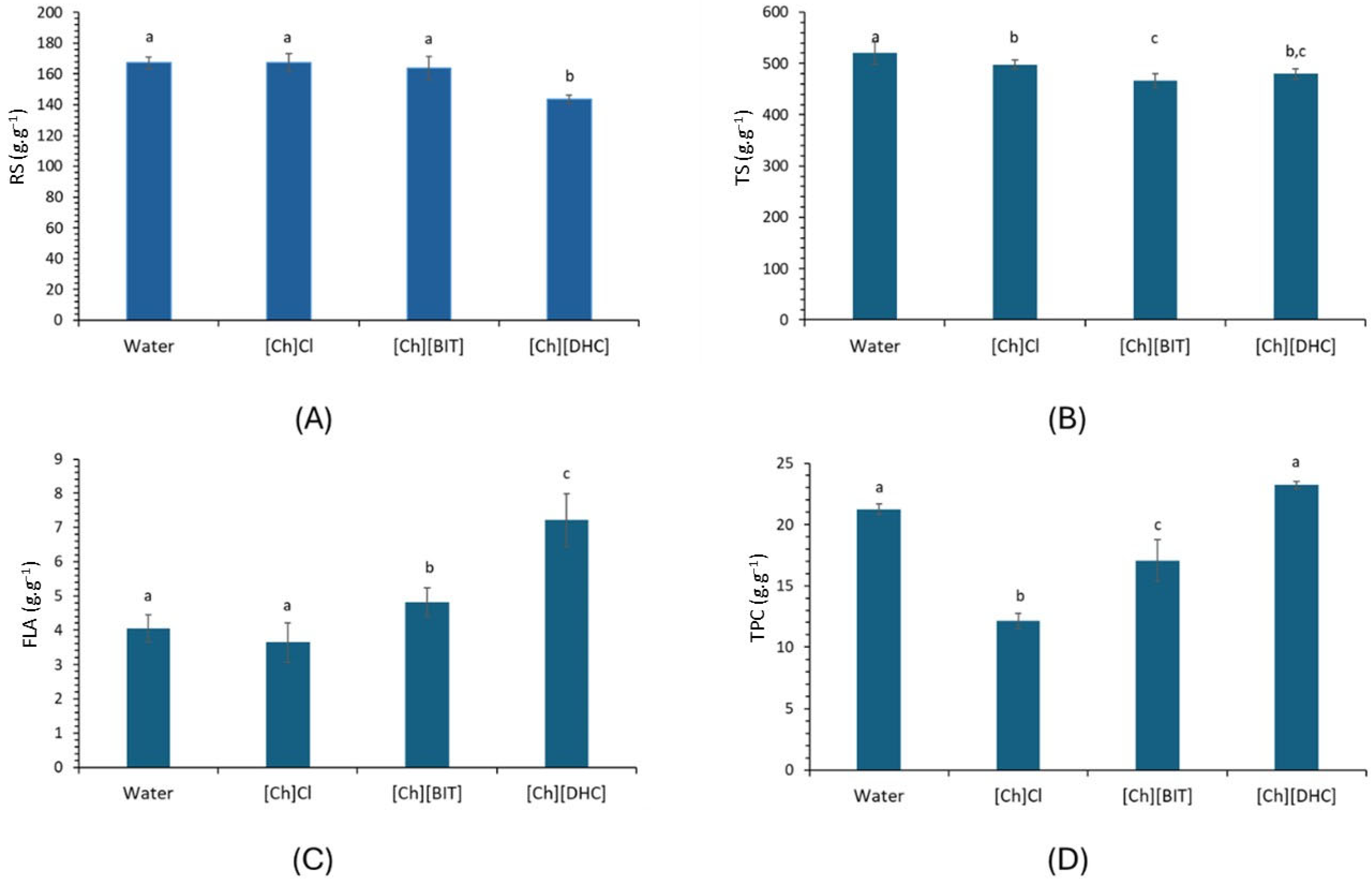
3.1. Extraction of Biocompounds from C. grandis L.f.
3.2. Physical Properties of C. grandis L.f. Extract and Its Concentrates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Lodha, S.R.; Joshi, S.V.; Vyas, B.A.; Upadhye, M.C.; Kirve, M.S.; Salunke, S.S.; Kadu, S.K.; Rogye, M.V. Assessment of the Antidiabetic Potential of Cassia grandis Using an in Vivo Model. J. Adv. Pharm. Technol. Res. 2010, 1, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, J.A.M.; Fernández, I.M.; Fernández, H.Z.; Sánchez, J.L.; Alemán, R.S.; Navarro-Alarcon, M.; Borrás-Linares, I.; Maldonado, S.A.S. Quantification of Bioactive Molecules, Minerals and Bromatological Analysis in Carao (Cassia grandis). J. Agric. Sci. 2020, 12, 88. [Google Scholar] [CrossRef]
- Luciméri, P.M.M.; Bárbara, A.R.; Márcia, V.d.S.; Maria, T.d.S.C.; Kêsia, X.F.R.d.S.; Túlio, D.d.S.; André, d.L.A.; Mônica, C.P.d.A.A.; Gardênia, C.G.M.; Teresinha, G.d.S.; et al. Antibacterial, Cytotoxic, and Schistosomicidal Activities of the Methanolic Extract from Cassia grandis L.f. (Fabaceae) Stem Bark and Its Fractions. J. Med. Plants Res. 2020, 14, 265–282. [Google Scholar] [CrossRef]
- Souza, A.A.; Ribeiro, K.A.; Seixas, J.R.P.C.; Silva Neto, J.C.; Santiago, M.G.P.F.; Aragão-Neto, A.C.; Lima-Ribeiro, M.H.M.; Borba, E.F.O.; Silva, T.G.; Kennedy, J.F.; et al. Effects Including Photobiomodulation of Galactomannan Gel from Cassia grandis Seeds in the Healing Process of Second-Degree Burns. Int. J. Biol. Macromol. 2023, 251, 126213. [Google Scholar] [CrossRef]
- Adimurti Kusumaningtyas Jenderal Achmad, V.; dewi Juliawaty, L.; Syah, Y.M.; Adimurti Kusumaningtyas, V.; Maolana Syah, Y.; Dewi Juliawaty, L. Two Stilbenes from Indonesian Cassia grandis and their antibacterial activities. Res. J. Chem. Environ. 2020, 24, 61–63. [Google Scholar]
- Harika, K.; Rao, B.G.; Ramadevi, D. Qualitative physicochemical, phytochemical analysis and quantitative estimation of total phenols, flavonoids and alkaloids of Cassia grandis. J. Glob. Trends Pharm. Sci. 2017, 8, 3860–3867. [Google Scholar]
- Gabsi, K.; Trigui, M.; Barrington, S.; Helal, A.N.; Taherian, A.R. Evaluation of Rheological Properties of Date Syrup. J. Food Eng. 2013, 117, 165–172. [Google Scholar] [CrossRef]
- Ferreira, K.; Cardoso, K.; Brandão-Costa, R.; Martins, J.T.; Botelho, C.; Neves, A.; Nascimento, T.; Batista, J.; Ferreira, É.; Damasceno, F.; et al. Physicochemical Properties of a Bioactive Polysaccharide Film from Cassia grandis with Immobilized Collagenase from Streptomyces Parvulus (DPUA/1573). Cosmetics 2024, 11, 86. [Google Scholar] [CrossRef]
- Medina, L.; Aleman, R.S.; Cedillos, R.; Aryana, K.; Olson, D.W.; Marcia, J.; Boeneke, C. Effects of Carao (Cassia grandis L.) on Physico-Chemical, Microbiological and Rheological Characteristics of Yogurt. LWT 2023, 183, 114891. [Google Scholar] [CrossRef]
- Osorio-Tobón, J.F. Recent Advances and Comparisons of Conventional and Alternative Extraction Techniques of Phenolic Compounds. J. Food Sci. Technol. 2020, 57, 4299–4315. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of Phenolic Compounds: A Review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef]
- Ventura, S.P.M.; E Silva, F.A.; Quental, M.V.; Mondal, D.; Freire, M.G.; Coutinho, J.A.P. Ionic-Liquid-Mediated Extraction and Separation Processes for Bioactive Compounds: Past, Present, and Future Trends. Chem. Rev. 2017, 117, 6984–7052. [Google Scholar] [CrossRef]
- Kaur, G.; Kumar, H.; Singla, M. Diverse Applications of Ionic Liquids: A Comprehensive Review. J. Mol. Liq. 2022, 351, 118556. [Google Scholar] [CrossRef]
- Greer, A.J.; Jacquemin, J.; Hardacre, C. Industrial Applications of Ionic Liquids. Molecules 2020, 25, 5207. [Google Scholar] [CrossRef]
- Singh, S.K.; Savoy, A.W. Ionic Liquids Synthesis and Applications: An Overview. J. Mol. Liq. 2020, 297, 112038. [Google Scholar] [CrossRef]
- Gadilohar, B.L.; Shankarling, G.S. Choline Based Ionic Liquids and Their Applications in Organic Transformation. J. Mol. Liq. 2017, 227, 234–261. [Google Scholar] [CrossRef]
- Buarque, F.S.; Monteiro e Silva, S.A.; Ribeiro, B.D. Choline Chloride-Based Deep Eutectic Solvent as an Inhibitor of Metalloproteases (Collagenase and Elastase) in Cosmetic Formulation. 3 Biotech. 2023, 13, 219. [Google Scholar] [CrossRef]
- Ventura, S.P.M.; e Silva, F.A.; Gonçalves, A.M.M.; Pereira, J.L.; Gonçalves, F.; Coutinho, J.A.P. Ecotoxicity Analysis of Cholinium-Based Ionic Liquids to Vibrio Fischeri Marine Bacteria. Ecotoxicol. Environ. Saf. 2014, 102, 48–54. [Google Scholar] [CrossRef]
- Lafourcade Prada, A.; Achod, L.D.R.; Keita, H.; Carvalho, J.C.T.; de Souza, T.P.; Rodríguez Amado, J.R. Development, Pharmacological and Toxicological Evaluation of a New Tablet Formulation Based on Cassia grandis Fruit Extract. Sustain. Chem. Pharm. 2020, 16, 100244. [Google Scholar] [CrossRef]
- Inocêncio, E.S.; Buarque, F.S.; Ferreira, L.F.R.; Soares, C.M.F.; Lima, Á.S.; Souza, R.L.d. Exploring the Potential of Licuri (Syagrus Coronata) Using Sustainable Techniques and Solvents for Extracting Bioactive Compounds. Sustainability 2025, 17, 1507. [Google Scholar] [CrossRef]
- Lima, T.S.P.; Borges, M.M.; Buarque, F.S.; de Souza, R.L.; Soares, C.M.F.; Lima, Á.S. Purification of Vitamins from Tomatoes (Solanum Lycopersicum) Using Ethanolic Two-Phases Systems Based on Ionic Liquids and Polypropylene Glycol. Fluid Phase Equilibria 2022, 557, 113434. [Google Scholar] [CrossRef]
- Hillis, W.E.; Swain, T. The Phenolic Constituents of Prunus Domestica. II.—The Analysis of Tissues of the Victoria Plum Tree. J. Sci. Food Agric. 1959, 10, 135–144. [Google Scholar] [CrossRef]
- Sheng, Z.L.; Wan, P.F.; Dong, C.L.; Li, Y.H. Optimization of Total Flavonoids Content Extracted from Flos Populi Using Response Surface Methodology. Ind. Crops Prod. 2013, 43, 778–786. [Google Scholar] [CrossRef]
- Buarque, F.S.; Lima, T.S.P.; Carniel, A.; Ribeiro, B.D.; Coelho, M.A.Z.; Souza, R.L.; Soares, C.M.F.; Pereira, M.M.; Lima, Á.S. Hormones Concentration in an Aqueous Two-Phase System: Experimental and Computational Analysis. Chem. Eng. Technol. 2024, 47, 716–721. [Google Scholar] [CrossRef]
- Docoslis, A.; Giese, R.F.; Van Oss, C.J. Influence of the water–air interface on the apparent surface tension of aqueous solutions of hydrophilic solutes. Colloids Surf. B Biointerfaces 2000, 19, 147–162. [Google Scholar] [CrossRef]
- Ismail, B.B.; Yusuf, H.L.; Pu, Y.; Zhao, H.; Guo, M.; Liu, D. Ultrasound-Assisted Adsorption/Desorption for the Enrichment and Purification of Flavonoids from Baobab (Adansonia digitata) Fruit Pulp. Sonochemistry 2020, 65, 104980. [Google Scholar] [CrossRef]
- Mahindrakar, K.V.; Rathod, V.K. Ultrasonic Assisted Aqueous Extraction of Catechin and Gallic Acid from Syzygium Cumini Seed Kernel and Evaluation of Total Phenolic, Flavonoid Contents and Antioxidant Activity. Chem. Eng. Process.-Process Intensif. 2020, 149, 107841. [Google Scholar] [CrossRef]
- Mané, C.; Souquet, J.M.; Ollé, D.; Verriés, C.; Véran, F.; Mazerolles, G.; Cheynier, V.; Fulcrand, H. Optimization of Simultaneous Flavanol, Phenolic Acid, and Anthocyanin Extraction from Grapes Using an Experimental Design: Application to the Characterization of Champagne Grape Varieties. J. Agric. Food Chem. 2007, 55, 7224–7233. [Google Scholar] [CrossRef]
- Sousa, K.M.; Maciel, G.E.L.O.; Buarque, F.S.; Santos, A.J.; Marques, M.N.; Cavalcanti, E.B.; Soares, C.M.F.; Lima, Á.S. Novel Phase Diagrams of Aqueous Two-Phase Systems Based on Tetrahydrofuran + Carbohydrates + Water: Equilibrium Data and Partitioning Experiments. Fluid Phase Equilib. 2017, 433, 1–9. [Google Scholar] [CrossRef]
- Patel, S.B.; Attar, U.A.; Sakate, D.M.; Ghane, S.G. Efficient Extraction of Cucurbitacins from Diplocyclos palmatus (L.) C. Jeffrey: Optimization Using Response Surface Methodology, Extraction Methods and Study of Some Important Bioactivities. Sci. Rep. 2020, 10, 2109. [Google Scholar] [CrossRef]
- Xu, B.J.; Chang, S.K.C. A Comparative Study on Phenolic Profiles and Antioxidant Activities of Legumes as Affected by Extraction Solvents. J. Food Sci. 2007, 72, S159–S166. [Google Scholar] [CrossRef]
- Velho, P.; Sousa, E.; Macedo, E.A. Binary Aqueous Solutions of Choline Salts: Determination and Modelling of Liquid Density (298.15 or 313.15 K) and Vapour Pressure Osmometry (313.15 K). Fluid Phase Equilibria 2024, 587, 114197. [Google Scholar] [CrossRef]
- Rathnasamy, S.K.; sri Rajendran, D.; Balaraman, H.B.; Viswanathan, G. Functional Deep Eutectic Solvent-Based Chaotic Extraction of Phycobiliprotein Using Microwave-Assisted Liquid-Liquid Micro-Extraction from Spirulina (Arthrospira platensis) and Its Biological Activity Determination. Algal Res. 2019, 44, 101709. [Google Scholar] [CrossRef]
- Buarque, F.S.; Camêlo, L.C.A.; Soares, C.M.F.; Mattedi, S.; Ferreira, A.F.B.; Feitosa, F.X.; Souza, R.L.; de Sant’Ana, H.B.; Lima, Á.S. Binary Mixture of Double Protic Ionic Liquid: Density, Viscosity, Refractive Index, Surface Tension, and Derivative Properties. J. Chem. Eng. Data 2021, 66, 4309–4325. [Google Scholar] [CrossRef]
- Tan, C.Y.; Huang, Y.X. Dependence of Refractive Index on Concentration and Temperature in Electrolyte Solution, Polar Solution, Nonpolar Solution, and Protein Solution. J. Chem. Eng. Data 2015, 60, 2827–2833. [Google Scholar] [CrossRef]




| Run | X1 (CIL, wt%) | X2 (RS/L, g mL−1) | X3 (T, °C) | RS (mg g−1) | TS (mg g−1) | FLA (mg g−1) | TPC (mg g−1) |
|---|---|---|---|---|---|---|---|
| 1 | −1 (5) | −1 (1:5) | −1 (25) | 149.55 | 401.44 | 6.96 | 12.95 |
| 2 | +1 (15) | −1 (1:5) | −1 (25) | 127.46 | 427.73 | 11.67 | 22.57 |
| 3 | −1 (5) | +1 (1:15) | −1 (25) | 151.94 | 447.44 | 7.18 | 15.40 |
| 4 | +1(15) | +1 (1:15) | −1 (25) | 136.69 | 459.44 | 14.04 | 30.93 |
| 5 | −1 (5) | −1 (1:5) | +1 (45) | 157.06 | 504.40 | 8.74 | 16.48 |
| 6 | +1 (15) | −1 (1:5) | +1 (45) | 146.25 | 499.84 | 13.54 | 28.22 |
| 7 | −1 (5) | +1 (1:15) | +1 (45) | 153.34 | 457.49 | 10.89 | 24.05 |
| 8 | +1 (15) | +1 (1:15) | +1 (45) | 153.71 | 483.51 | 15.79 | 38.10 |
| 9 | 0 (10) | 0 (1:10) | 0 (35) | 142.57 | 481.26 | 6.67 | 22.80 |
| 10 | 0 (10) | 0 (1:10) | 0 (35) | 139.78 | 490.16 | 8.00 | 23.42 |
| 11 | 0 (10) | 0 (1:10) | 0 (35) | 143.35 | 479.04 | 6.34 | 22.99 |
| 12 | 0 (10) | 0 (1:10) | 0 (35) | 147.73 | 491.07 | 8.31 | 23.57 |
| 13 | 0 (10) | 0 (1:10) | 0 (35) | 143.29 | 464.44 | 6.82 | 23.22 |
| 14 | 0 (10) | 0 (1:10) | 0 (35) | 145.51 | 473.31 | 7.15 | 23.34 |
| Variable | Equation | R2 |
|---|---|---|
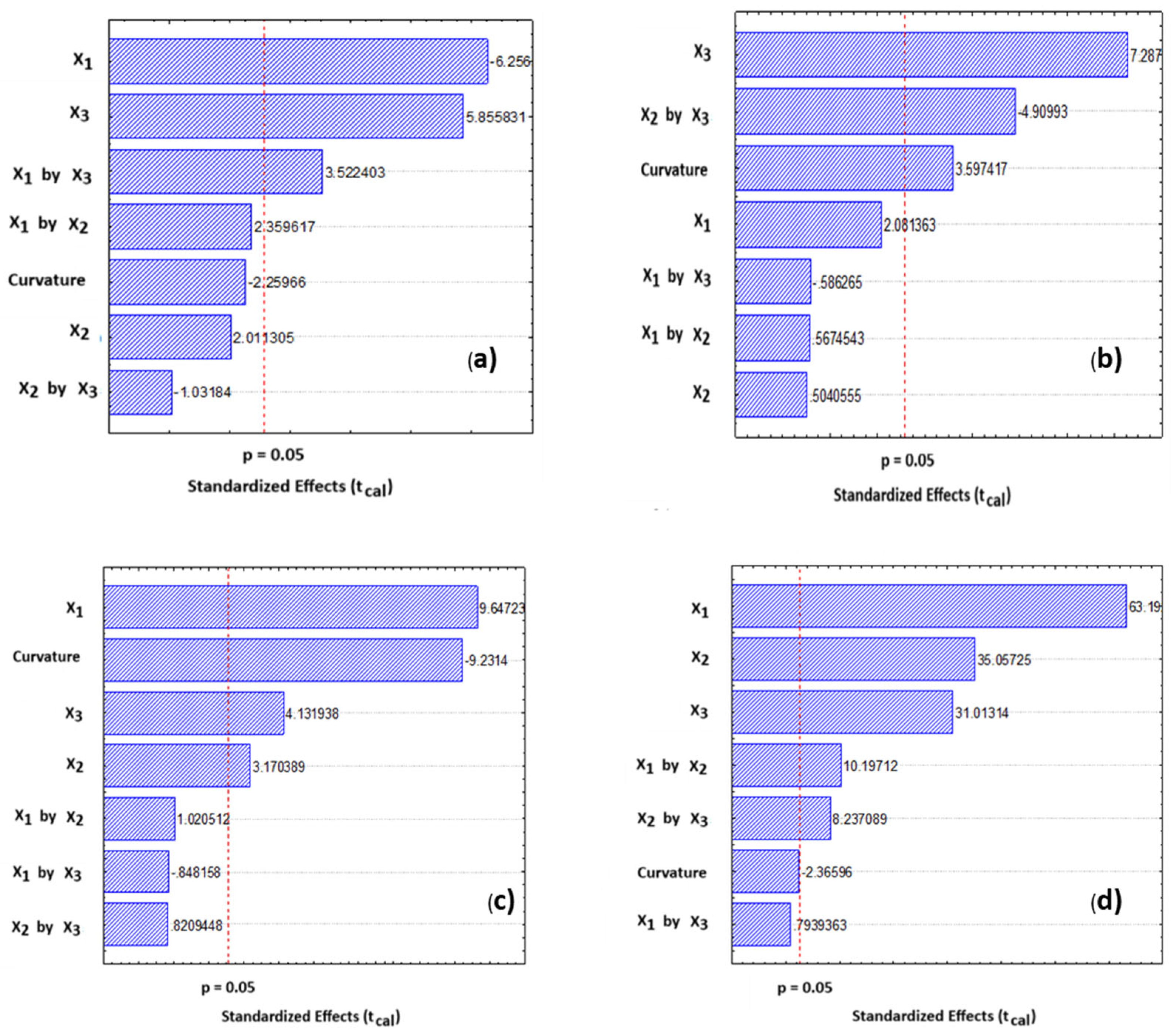
| Reducing Sugar—RS | RS = 147.00 − 5.97X1 + 1.92X2 + 5.59X3 + 2.25X1X2 + 3.36X1X3 − 0.99X2X3 | 0.9502 |
| Total Sugar—TS | TS = 460.16 + 7.47X1 + 1.81X2 + 26.15X3 + 2.04X1X2 − 2.10X1X3 − 17.62X2X3 | 0.9276 |
| Flavonoids—FLA | FLA = 11.10 + 2.66X1 + 0.87X2 + 1.14X3 + 0.28X1X2 − 0.23X1X3 + 0.23X2X3 | 0.9726 |
| Total Phenolic Compounds—TPCs | TPC = 23.59 + 6.37X1 + 3.53X2 + 3.13X3 + 1.03X1X2 + 0.08X1X3 + 0.83X2X3 | 0.9915 |
| Sample | a | b | R2 |
| Crude | 1.230 ± 0.008 | 6.0 × 10−4 ± 2.0 × 10−5 | 0.9863 |
| Concentrated 2× | 1.283 ± 0.005 | 6.0 × 10−4 ± 1.5 × 10−5 | 0.9957 |
| Concentrated 4× | 1.386 ± 0.003 | 7.0 × 10−4 ± 1.0 × 10−5 | 0.9983 |
| Concentrated 8× | 1.510 ± 0.003 | 8.0 × 10−4 ± 1.1 × 10−5 | 0.9987 |
| Viscosity—η | |||
| Sample | η0 | Eη | R2 |
| Crude | 1.1 × 10−4 ± 8.8 × 10−5 | 1.8 × 105 ± 2.1 × 102 | 0.9991 |
| Concentrated 2× | 9.0 × 10−4 ± 1.0 × 10−4 | 2.0 × 105 ± 3.9 × 102 | 0.9977 |
| Concentrated 4× | 1.0 × 10−4 ± 2.5 × 10−5 | 3.1 × 105 ± 4.8 × 102 | 0.9987 |
| Concentrated 8× | 7.6 × 10−7 ± 8.8 × 10−8 | 5.6 × 105 ± 2.8 × 102 | 0.9999 |
| Refraction Index—nD | |||
| Sample | c | d | R2 |
| Crude | 1.394 ± 0.002 | 1.0 × 10−4 ± 5.4 × 10−6 | 0.9861 |
| Concentrated 2× | 1.451 ± 0.001 | 2.0 × 10−4 ± 3.9 × 10−6 | 0.9980 |
| Concentrated 4× | 1.498 ± 0.002 | 3.0 × 10−4 ± 8.0 × 10−6 | 0.9933 |
| Concentrated 8× | 1.548 ± 0.001 | 3.0 × 10−4 ± 1.5 × 10−6 | 0.9998 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cordeiro, F.M.M.; Bedoya, S.G.; Santos, D.A.P.; Santos, R.S.; Bacelar, T.V.M.; Buarque, F.S.; Simonelli, G.; Silva, A.C.M.; Lima, Á.S. Cassia grandis L.f. Pods as a Source of High-Value-Added Biomolecules: Optimization of Extraction Conditions and Extract Rheology. Biomass 2025, 5, 24. https://doi.org/10.3390/biomass5020024
Cordeiro FMM, Bedoya SG, Santos DAP, Santos RS, Bacelar TVM, Buarque FS, Simonelli G, Silva ACM, Lima ÁS. Cassia grandis L.f. Pods as a Source of High-Value-Added Biomolecules: Optimization of Extraction Conditions and Extract Rheology. Biomass. 2025; 5(2):24. https://doi.org/10.3390/biomass5020024
Chicago/Turabian StyleCordeiro, Filipe M. M., Salomé G. Bedoya, Daniel A. P. Santos, Rebeca S. Santos, Thomas V. M. Bacelar, Filipe S. Buarque, George Simonelli, Ana C. M. Silva, and Álvaro S. Lima. 2025. "Cassia grandis L.f. Pods as a Source of High-Value-Added Biomolecules: Optimization of Extraction Conditions and Extract Rheology" Biomass 5, no. 2: 24. https://doi.org/10.3390/biomass5020024
APA StyleCordeiro, F. M. M., Bedoya, S. G., Santos, D. A. P., Santos, R. S., Bacelar, T. V. M., Buarque, F. S., Simonelli, G., Silva, A. C. M., & Lima, Á. S. (2025). Cassia grandis L.f. Pods as a Source of High-Value-Added Biomolecules: Optimization of Extraction Conditions and Extract Rheology. Biomass, 5(2), 24. https://doi.org/10.3390/biomass5020024









