Abstract
Each year, a substantial amount of food is discarded around the globe. A significant portion of this waste consists of by-products derived from Citrus fruits such as lemons. The purpose of this research is to examine the polyphenol extraction and the antioxidant ability of lemon peel using cloud point extraction (CPE), a sustainable approach. CPE was conducted using three steps with a 20% w/v concentration of Span 20 as the surfactant, which has a critical micellar concentration of 6.13 × 10−5 mol/L. The pH was set at 7 and a salt concentration of 20% was maintained at 45 °C for 20 min. The subsequent outcomes of the analysis were obtained: total polyphenol content (TPC): 526.32 mg gallic acid equivalents per liter; total flavonoid content (TFC): 90.22 mg rutin equivalents per liter; FRAP, DPPH, and hydrogen peroxide assays: 2.40, 2.68 and 1.03 mmol ascorbic acid equivalents per liter, respectively, and 168.63 mg/L ascorbic acid content. The quantification of the polyphenolic compounds through High-Performance Liquid Chromatography showed that the most abundant compounds in the lemon peels are eriocitrin (159.43 mg/L) and hesperidin (135.21 mg/L). The results indicate that the proposed CPE technique is successful in extracting antioxidant compounds from lemon peels. The generated extracts have the potential to be exploited as dietary additives to enhance human health and can also be utilized for nutraceuticals or pharmaceutical purposes.
1. Introduction
In the European Union, food waste surpassed 58 million tons in 2021 [1]. Households were responsible for 31 million tons of fresh mass, or 54% of the total. With over 12 million tons of fresh mass in food waste, processing and manufacturing ranked second, scoring a percentage of 21% [1]. Citruses are a highly abundant fruit worldwide, and their processing generates significant amounts of by-products [2,3]. The majority of these produced residues are either fed to animals or discarded into the environment, without appropriate processing [4]. The peels, pulps, and seeds of fruits usually contain beneficial substances that can be isolated and used as natural antioxidants [5]. These antioxidants can prevent the oxidation of certain foods or be incorporated into functional food products [6,7].
The lemon, a member of the Rutaceae family, is a quite significant Citrus fruit [8]. Lemon fruit peels account for 50–65% of the overall weight of the fruit [9]. Peels are commonly regarded as a primary source of environmental pollution [9,10]. Nevertheless, lemon peels contain a substantial amount of polyphenols [10]. Furthermore, they contain components that have health-promoting qualities, like vitamin C and flavonoids, which enhance their natural antioxidant abilities [11,12]. Lemon peels have been documented [13] to possess antifungal properties against plant infections both in vivo and in vitro [14,15], as well as anticancer properties in both in vivo and in vitro applications [16]. Lemon peel extract acts both as an avoiding and as a regulating agent for the development of urinary system calcifications by inhibiting the formation of calcium oxalate solid concretions. As such, it protects the urinary tract from damage caused by gall/kidney stones [17]. Narirutin and hesperidin, found in lemon peels, function as therapeutic agents that effectively enhance the angiogenic activities in disorders related to the arteries [18]. Furthermore, eriocitrin is a flavonoid that is abundant in lemon peels, and it is proven to possess anti-inflammatory effects both in vivo and in vitro [19]. More specifically, this flavonoid has been proved to have many health effects, such as anticancer, and antioxidant effects, and also serves as an antioxidative stress agent [20]. Moreover, hesperidin also holds health benefits, such as antimicrobial, anticancer, antihypertensive, and antiulcer effects [21]. The phenolic compounds found in lemon peels have the potential to be employed as natural beneficial components, antioxidants, and antibacterial agents in food products [22,23,24,25].
The investigation of bioactive compound extraction from lemon peels has served as a topic of extensive studies, with many extraction methods, such as extraction with reflux [11], microwave hydrodistillation [26], stirring and Soxhlet [27], and ultrasound extraction [13]. The Soxhlet extraction technique necessitates a significant amount of time and solvents. Furthermore, the process requires the utilization of a specialized apparatus, commonly referred to as a Soxhlet extractor [28]. However, the implementation of stirring necessitates more electrical energy consumption and is linked with a prolonged operation time, potentially leading to increased expenditures [29]. Nevertheless, the development or application of green techniques with reduced costs and environmental footprint is necessary. In that case, there is a lack of literature regarding the cloud point extraction (CPE) approach. With regard to the existing limitations on the widespread use of volatile organic compounds, which are frequently hazardous, CPE emerges as an eco-friendly and economically efficient alternative [30]. The CPE application for extracting bioactive compounds from plants serves as a sustainable and efficient approach [31].
Isolating bioactive compounds from liquid matrices can be performed rapidly and cost-effectively, as CPE utilizes surfactants [32]. In brief, the experimental methodology involves introducing salt and surfactant into a liquid sample, regulating the cloud point temperature, employing centrifugal force, and ultimately isolating the surfactant from the aqueous phase of the sample [33,34,35]. Surfactants that meet food-grade criteria can be used to directly include specific chemicals in food products, enabling the extraction of these molecules [36]. Various sectors, such as the pharmaceutical and the food ones, might benefit from implementing this extraction technique. Micelles are created in water-based solutions in which the molecular concentration approaches a specific threshold. Once formed, micelles maintain a state of equilibrium with the individual molecules present in the surrounding aqueous solution [37]. The association of hydrophobic and hydrophilic molecules with these structures is enhanced by dipole–dipole interactions and hydrogen bonds, leading to their separation [38]. Repeating CPE twice or more times could increase the efficiency of bioactive compounds recovery to a greater degree [39]. In light of the above, several surfactants, including Span 20, Genapol X-080, Tween 80, Triton X-100, and lecithin, have been investigated to aid in the effective extraction of bioactive molecules [39].
Currently, there is limited research on the process of extracting polyphenols from lemon peels through CPE. This type of extraction has the capacity to be employed in diverse industries, including the pharmaceuticals and food industries. Utilizing surfactants, the CPE method is a straightforward and cost-effective way to extract bioactive compounds from liquid matrices [32,37]. Although there is an increasing amount of research focused on extracting bioactive components from other kinds of food waste through CPE [39,40,41,42,43,44,45,46], there is a lack of studies specifically dedicated to extracting polyphenols from lemon peels. This is especially remarkable given the substantial amount of waste generated. This investigation aimed to assess the potential of CPE, utilizing a non-toxic food surfactant (namely Span 20) to extract polyphenols from lemon peels. The purpose of this investigation was to establish an integrated approach to effectively control and optimize the utilization of waste produced by lemons. The optimal surfactant, its concentration, the salting-out effect, and the influence of pH on the CPE procedure, along with the application of multiple steps of CPE, were investigated. This study also assessed the total polyphenol content derived from lemon peel extracts, focusing on their antioxidant and antiradical capacity.
2. Materials and Methods
2.1. Chemicals, Materials and Reagents
L-ascorbic acid, sodium hydroxide, hydrochloric acid, methanol, 2,4,6-tris(2-pyridyl)-s-triazine (TPTZ), Genapol X-080, Span 20, ethanol, DPPH• (1,1-diphenyl-2-picrylhydrazyl), iron chloride (hexahydrate), and any chemical standard (at least HPLC grade) used in HPLC-based analysis, such as neochlorogenic acid, eriocitrin, chlorogenic acid, catechin, rutin, syringic acid, caffeic acid, epicatechin, luteolin 7-glucoside, kaempferol 3-glucoside and hesperidin, were purchased from Sigma-Aldrich (Steinheim, Germany). Hydrogen peroxide, Folin-Ciocalteu reagent, anhydrous sodium carbonate, phosphate buffer, and gallic acid were obtained from Penta (Prague, Czech Republic). Tween 80 was from Panreac (Barcelona, Spain). Sodium chloride was bought from Carlo Erba (Milano, Italy). Triton X-100 was from Scharlau (Barcelona, Spain). Citric acid anhydrous was obtained from Merck (Darmstadt, Germany). Lecithin soya (>97%) was from ABS Food (Vignoza, PD, Italy). A deionizing column was utilized to generate the deionized water used throughout all experiments.
Farmers from the region of Corinth (Peloponnese, Greece) provided us with Interdonato lemons (Citrus limon). Tap water was used to wash the lemons, and then a paper towel was used to dry them. Next, the peels were separated from the fruit by hand, cut into pieces, and humidity was removed by freeze-drying with a Biobase BK-FD10P lyophilizer (Jinan, China). The dried peels were subsequently pulverized into a fine powder with Analysette 3 PRO (Fritsch GmbH, Oberstein, Germany), so particles ranged from 1.6 to 0.8 mm in diameter on average, and then put in the freezer (−40 °C) until further analysis.
2.2. The CPE Procedure
An amount of 1 g of dried grounded lemon peels was weighed (Kern PLS 3100-2F, Kern & Sohn GmbH, Balingen, Germany) and combined with 40 mL of water (1:40 solid-to-liquid ratio). The mixture was then subjected to ultrasonic treatment with an Elmasonic P instrument (manufactured by Elma Schmidbauer GmbH, Singen, Germany), operating at a frequency of 37 kHz and ambient temperature. The ultrasonic treatment was carried out for 20 min and was followed by centrifugation in a NEYA centrifuge (Remi Elektrotechnik Ltd., Palghar, India) to separate the solids from the liquids, and the supernatant was moved to a Duran™ bottle to conduct the CPE procedure.
For the CPE procedure, 20 mL of the lemon peel extract was mixed with a surfactant. The mixture was agitated (Heidolph MR Hei-Standard, Schwabach, Germany) at a speed of 800 rpm at 45 °C for 20 min. A temperature of 45 °C was chosen, aiming to minimize energy consumption and avoid increased temperatures that can result in the degradation of polyphenols. The aqueous phase was separated from the surfactant through centrifugation at 4500× g for 5 min before decantation, constituting the initial step of extraction. The amounts of both surfactant and water were measured following centrifugation. Additional steps of CPE extractions were carried out according to the following procedure: the micellar (surfactant) phase was separated from the aqueous phase and stored, while a new amount of surfactant was introduced in the aqueous phase, and the CPE procedure was conducted under the same conditions.
2.3. Recovery of Polyphenols
The assessment of polyphenol recovery was conducted via a polyphenol mass balance. The calculation of surfactant retrieval was performed utilizing a method that had been previously established [47] and the Equation (1):
where Cs denotes the concentration of polyphenols in the volume vs. of the surfactant phase, C0 corresponds to the concentration of polyphenols in the initial sample volume V0 (20 mL), and Cw represents the concentration of polyphenols in the volume of the water phase Vw.
The average concentration of each phase was obtained using the Folin-Ciocalteu (vide infra) method (see further explanation below). The result was then expressed in mg GAE/L.
2.4. Quantification of the Total Polyphenol Content (TPC)
Photometric determination of the total polyphenol content (TPC) was performed utilizing the Folin-Ciocalteu method [48]. Upon combining 200 μL of the diluted (1:20) extract with 200 μL of the Folin-Ciocalteu reagent for two minutes, 1600 μL of 5% w/v sodium carbonate solution was introduced. The solution was incubated for 20 min at 40 °C, lacking light exposure. Then, the absorbance at 740 nm was measured with a Shimadzu spectrophotometer (UV-1700, Shimadzu Europa GmbH, Duisburg, Germany). The results were expressed as mg gallic acid equivalents (GAE) per liter.
2.5. Determination of the Total Flavonoid Content (TFC)
In accordance with a methodology that had been previously documented [49], 200 μL of the extract (diluted in a ratio of 1:5) was combined with 80 μL of a reagent comprising 0.5 M sodium acetate and 5% w/v aluminum chloride and 1740 μL of aqueous ethanol (35% v/v). Following a 30 min incubation period at ambient temperature, the absorbance at 415 nm was obtained. The TFC was calculated using a calibration curve of rutin (quercetin 3-O-rutinoside) in methanol, ranging from 30 to 300 mg/L. The TFC was quantified as mg of rutin equivalents (RtE) per liter.
2.6. Ferric-Reducing Antioxidant Power (FRAP) Assay
A formerly established method [50] was implemented to perform the FRAP assay. A volume of 100 μL of the diluted (1:20) sample was combined with 100 μL of iron (III) chloride solution (4 mM in 0.05 M HCl) in an Eppendorf tube. Subsequently, the resultant mixture was subjected to incubation at 37 °C for 30 min. Next, 1800 μL of TPTZ solution (1 mM in 0.05 M hydrochloric acid) was introduced. Following a 5 min interval, the absorbance was measured at 620 nm. The ferric-reducing capacity (PR) was determined using a calibration curve, which was created using ascorbic acid diluted in 0.05 M hydrochloric acid. The concentrations of ascorbic acid varied between 0.05 and 0.5 mmol/L. The PR of the samples was quantified in mmol of ascorbic acid equivalents (AAE) per liter.
2.7. DPPH• Scavenging Andiradical Activity
The DPPH• scavenging activity was calculated using a procedure that had been previously specified [50]. A volume of 25 μL of diluted (1:5) extract sample was combined with 975 μL of DPPH• solution (100 μmol/L in methanol), and the absorbance at 515 nm was measured immediately (A515(i)) and 30 min later (A515(f)). The DPPH• radical scavenging capacity was expressed as described in Equation (2):
Antiradical activity (AAR) was expressed as mmol ascorbic acid equivalents (AAE) per liter, using an ascorbic acid calibration curve.
2.8. Hydrogen Peroxide (H2O2) Scavenging Assay
The H2O2 scavenging assay was conducted using a previously described approach [51]. In brief, 400 μL of the diluted (1:50) extract and 600 μL of a 40 mM H2O2 solution, prepared in phosphate buffer with pH 7.4, were combined in an Eppendorf tube. The absorbance at 230 nm was measured after 10 min. The ability to scavenge H2O2 was quantified as seen in Equation (3):
where A0 is the absorbance of the blank solution, Ac is the absorbance of the extract solution in the absence of hydrogen peroxide, and A the absorbance of the sample.
A calibration curve of ascorbic acid (CAA) ranging from 0.05 and 0.5 mmol/L in 0.05 M hydrochloric acid was utilized. The activity of antihydrogen peroxide (AAHP) in terms of mmol ascorbic acid equivalents (AAE) per liter was expressed.
2.9. Determination of Ascorbic Acid Content
The content of ascorbic acid was measured using a colorimetric technique developed before [52]. A volume of 900 μL of a 10% w/v trichloroacetic acid solution was combined with 100 μL of the extract. Subsequently, 500 μL of a solution containing 10% (v/v) Folin-Ciocalteu reagent was introduced into the solution. The measurement of absorbance at 760 nm was conducted after 10 min. An ascorbic acid calibration curve at concentrations ranging from 10 to 80 mg/L was used to quantify the results.
2.10. High-Performance Liquid Chromatography Coupled with Diode Array Detector (HPLC-DAD) Analysis
The liquid chromatograph (type CBM-20A) and diode array detector (model SPD-M20A) utilized in the present research were purchased by Shimadzu Europa GmbH, located in Duisburg, Germany. The compound separation process was executed utilizing a Phenomenex Luna C18(2) column (100 Å, 5 μm, 4.6 mm × 250 mm) obtained from Phenomenex Inc. in Torrance, CA, USA. The procedure for separation was conducted at a temperature of 40 °C. The mobile phase consisted of a 0.5% aqueous solution of formic acid (A) and a 0.5% solution of formic acid in acetonitrile (B). The gradient program employed a linear increase in solvent B concentration, starting at 0% and reaching 40% after 10 min. Subsequently, the concentration of B increased to 50% within the next 10 min, followed by a further increase to 70% within another 10 min. The concentration of B was then maintained at 70% for an additional 10 min. The mobile phase flow had a velocity of 1 mL/min. The concentration range (0 to 50 μg/mL) of the compounds of interest was determined using calibration curves. By comparing the absorbance and retention time spectra to those of purified chemical standards, this was accomplished.
2.11. Statistical Analysis
The analyses were conducted three times in total. The results were reported as the mean values of three repetitions, alongside the standard deviation. The Kolmogorov–Smirnov test was utilized to assess the normality of the data. To identify statistically significant differences, an IBM SPSS Statistics (Version 29.0) one-way analysis of variance (ANOVA) was conducted. A significance level of p < 0.05 was utilized in order to assess the statistical significance.
3. Results and Discussion
3.1. Optimization of the CPE Procedure
3.1.1. Selection of the Optimal Surfactant
This research aimed to establish the optimal conditions for polyphenol extraction from lemon peel extract. To achieve this goal, the initial stage was to identify an appropriate surfactant that would lead to higher TPC recoveries. Five surfactants were evaluated, specifically Tween 80, Triton X-100, Span 20, Genapol X-080, and lecithin. The efficacy of the surfactants was assessed by measuring the percentage of polyphenol recovery. The results are depicted in Figure 1.
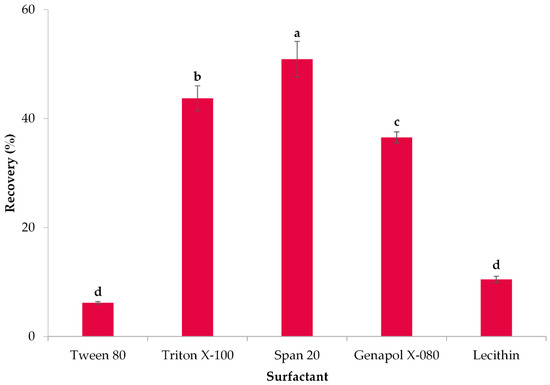
Figure 1.
Effect of various surfactants on the recovery of polyphenols from lemon peels. Error bars are used to denote standard deviations, whereas lowercase letters (e.g., (a–d)) are used to indicate means that have statistically significant differences (p < 0.05).
According to Figure 1, Span 20 resulted in the highest polyphenol recovery (50.90%), followed by Triton X-100 (p < 0.05) and Genapol X-080 (p < 0.05). On the other hand, Tween 80 and lecithin resulted in relatively poor recoveries. Hence, Span 20 was chosen as the optimal surfactant for the present study. Span 20 is a non-ionic, low-hazard, biodegradable surfactant consisting of a natural fatty acid, lauric acid, and sugar alcohol sorbitol [53]. The critical micellar concentration of Span 20 is 6.13 × 10−5 mol/L [54]. Span 20 was utilized as a surfactant in several studies. Al-Shamrani et al. [55] employed Span 20 to facilitate the separation of oil from water through the process of dissolved air flotation. Likewise, Papaioannou et al. [56] utilized Span 20 to recover lycopene from tomato peels.
3.1.2. Determination of the Optimal pH Value
The pH level of the sample significantly impacts the efficiency of the extraction process and should be carefully taken into account [57]. Therefore, a series of experiments was conducted to assess how various pH levels affected the recovery of polyphenols. The pH of the initial extract was 2.36 and it was adjusted to the reported values by adding hydrochloric acid or sodium hydroxide. The pH values of the lemon peel extracts were acquired with a pH meter (XS Instruments, PC 60 VioLab with XS 201T DHS digital electrode, Carpi, Modena, Italy). In Figure 2, the recovery of polyphenols from the extract and the impact of pH on them are presented. The impact of the pH level on the extraction efficiency of polyphenols is apparent. The highest level of recovery is achieved at a pH of 7, resulting in a yield of 58.85%. At pH 8, the recovery of polyphenols was 53.94%; this was not statistically different from pH 7 (p > 0.05), but the surfactant phase was more viscous at this point, making it hard to process, hence the value was rejected. This result is in line with our previous study [58], where pH 7 was chosen as optimal for the recovery of polyphenols from banana peels through CPE. Moreover, Zain et al. [59] established an environmentally friendly CPE technique, at a 7 pH value, to eliminate phenolic species from water samples.
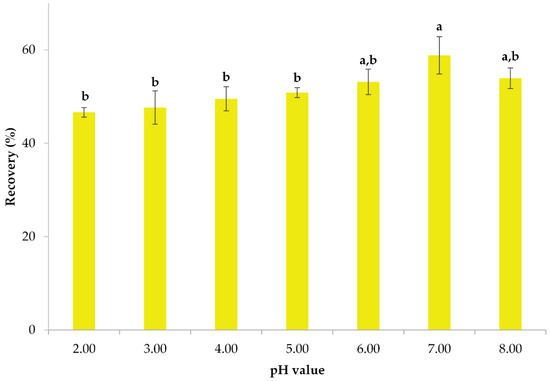
Figure 2.
The impact of pH on the extraction efficiency of polyphenols from lemon peels. Error bars are used to denote standard deviations, whereas lowercase letters (e.g., (a, b)) denote means that have statistically significant differences (p < 0.05).
3.1.3. Assessment of the Optimal Salt Concentration
Salt, namely sodium chloride, was added to the sample to increase the ionic strength of the aqueous phase and hasten the process of separating various phases. Because of its ionic strength, salt has been shown to improve the extraction process by lowering the cloud point temperature thus facilitating phase separation [33,60,61,62]. The solubility of organic molecules decreases when the ionic strength of a solution is increased, which is referred to as the salting-out effect. Therefore, this impact is beneficial in facilitating the extraction [63]. The effect of sodium chloride on polyphenol recovery is illustrated in Figure 3.
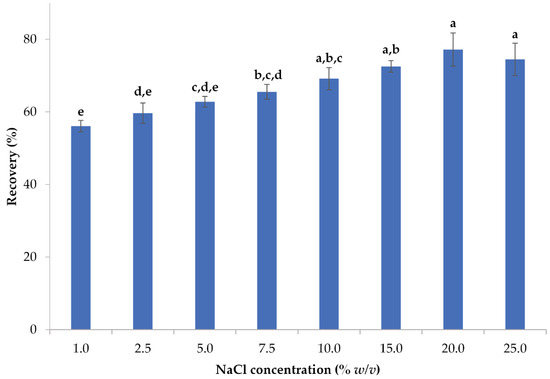
Figure 3.
The impact of salt concentration on the recovery of polyphenols from lemon peels. Error bars are used to denote standard deviations, whereas lowercase letters (e.g., (a–e)) denote statistically significant differences in means (p < 0.05).
When a 20% w/v of sodium chloride is introduced into the mixture, the highest polyphenol recovery (77.20%) is achieved. Although no significant differences (p > 0.05) between the concentrations of 10–25% w/v were observed, the 20% w/v salt concentration served as the optimum one, as it resulted in the highest yield. Karadag et al. [40] also reported the same salt concentration in their attempt to optimize the enrichment of lecithin with polyphenols from olive mill wastewater via CPE.
3.1.4. Assessment of the Optimal Surfactant Concentration
Span 20 or sorbitan monolaurate is a non-ionic surfactant that can form bigger micelles from the other surfactants [64]. This ability is attributed mainly to the fact that it is composed of lipids, which easily form micelles [65]. Span 20 was studied at various concentrations, ranging from 1 to 25% w/v. The recoveries of the Span 20 concentrations along with their polyphenol recoveries are displayed in Figure 4. It is apparent that concentrations 10, 15, 20, and 25% w/v were of no statistical significance (p > 0.05), 20% w/v was chosen as the optimal one as it yielded the highest recovery of polyphenols (81.70%). This percentage is relatively high and is expected to elevate if multiple CPE steps are implemented.
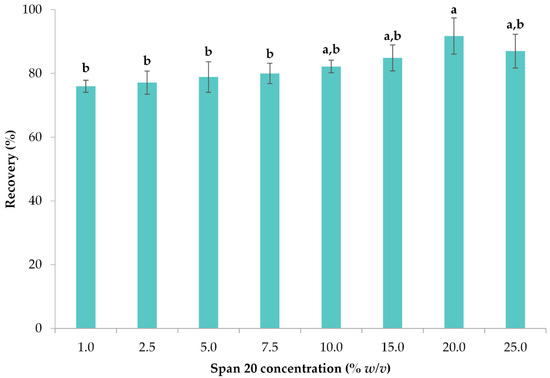
Figure 4.
The impact of varying concentrations of Span 20 on the extraction efficiency of polyphenols from lemon peels. Error bars are used to denote standard deviations, whereas lowercase letters (e.g., (a, b)) denote means that have differences of statistical significance (p < 0.05).
3.2. Analysis of the Optimal CPE Extract
After the optimization experiments, the optimal conditions of CPE were determined to be 20% w/v salt concentration and 20% w/v Span 20 concentration at pH 7. Three CPE steps were performed under optimal conditions to achieve the highest possible recovery of bioactive compounds from the lemon peel extract. For each step, a new surfactant was employed. TPC recoveries from both the surfactant (micellar) phase (SP) and the aqueous phase (WP) are displayed in Figure 5. In the first step of CPE, in SP, 512.04 mg GAE/L (or 91% recovery) of polyphenols was measured; the second step gave 15.36 mg GAE/L (or 3% recovery) and the third step gave 5.65 mg GAE/L (1% recovery) of polyphenols. In the initial lemon peel extract, 563.91 mg GAE/L was found. Thus, in total, 533.05 mg GAE/L of polyphenols was measured in SP, leading to an overall 95% recovery rate. Since the use of the two additional extraction steps resulted in a minor increase in the total recovery of polyphenols, their employment may be unnecessary.
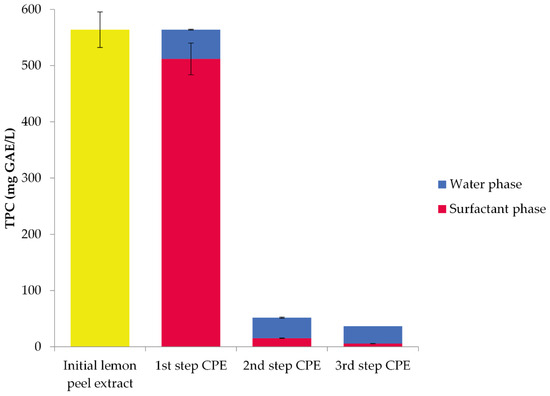
Figure 5.
Total polyphenol content (TPC) from lemon peel extract with 20% w/v Span 20. Standard deviations are presented with error bars.
The analyses were conducted on the final extract (optimal total SP), which comprised the combination of the SP from all three CPE steps. Measurements were conducted on the initial extract (the ultrasound-derived extract prior to any treatment) as well as the final extract. The results are displayed in Table 1. The difference between the TPC of the initial sample and the final sample was 6.67%. The TPC measured in the SP phase was 526.32 mg GAE/L, which is 60% higher than the one assessed by Danacioğlu et al. [66] on a combination of onion peel, lemon peel, and walnut shell tea. The TFC assessed in the optimal total SP was 50.55% lower than the one in the initial lemon peel extract. Nevertheless, a strong antioxidant capacity of the extracts was observed. The FRAP value on the final extract was ~52% lower than the one measured in the initial extract. Rodríguez-Solana [67] also reported a FRAP value close to ours, on a liqueur macerated with Ceratonia siliqua L. As for the DPPH• value, a ~20% reduction was observed in the final sample. The H2O2 values of the initial and the final extract were close to one another, as only ~28% reduction was observed in the optimal total SP. These results suggest that most of the antioxidants in the initial extract were encapsulated within the micelles of the surfactant and thus no statistically significant decrease in the antioxidant capacity of SP was observed. Comparable results were observed in our previous work [37], where CPE was applied to two different cultivars of clingstone cannery peaches, and small differences in the antioxidant capacity of peaches before and after CPE were noted. The ascorbic acid content of the extracts was also determined, and it was found to be ~10% higher in the initial extract.

Table 1.
Parameters and polyphenolic compounds on the initial lemon peel extract and the surfactant phase (SP) under optimal CPE conditions.
Table 1 also shows the polyphenolic compounds quantified by HPLC-DAD in both the initial and final extract, while Figure 6 illustrates a representative chromatogram of these compounds. The most abundant polyphenol identified through HPLC-DAD was eriocitrin, followed by hesperidin. The eriocitrin content determined in our study is ~21% higher than the one reported by Saeidi et al. [68], who determined 94.8 mg/L of eriocitrin in lime juice. The same research team reported a value of hesperidin on the same fruit juice which is ~44% lower than the one determined in our study. Mare et al. [69] also reported a hesperidin value close to ours. Figure 7 depicts a bivariate analysis of the initial extract by the final extract. There is a strong correlation between the two extracts, with a covariance of 19.5. The p-value of the model is <0.0001, which indicates that there is no significant lack of fit between the variables. The RSquare value is 0.95, which ensures that there is no high possibility of random errors in the model. Eriocitrin was identified as the polyphenolic compound in the higher amount in lemon peels in this study. Eriocitrin was also measured by Hajimahmoodi et al. [70] in various lemon juices, and the value obtained ranged from 3.24 to 10.68 mg/L, ~971–3430% lower than the value reported in this study. The same research team also determined hesperidin on the juices, and the values reported were 3.24–104.84 mg/L, ~29–1946% lower than our study.
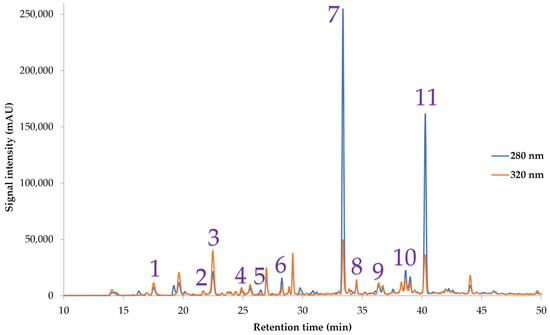
Figure 6.
Representative HPLC chromatogram at 280 and 320 nm of lemon peel extract, demonstrating polyphenolic compounds that were identified. 1: neochlorogenic acid; 2: catechin; 3: chlorogenic acid; 4: caffeic acid; 5: syringic acid; 6: epicatechin; 7: eriocitrin; 8: rutin; 9: luteolin 7-glucoside; 10: kaempferol 3-glucoside; 11: hesperidin.
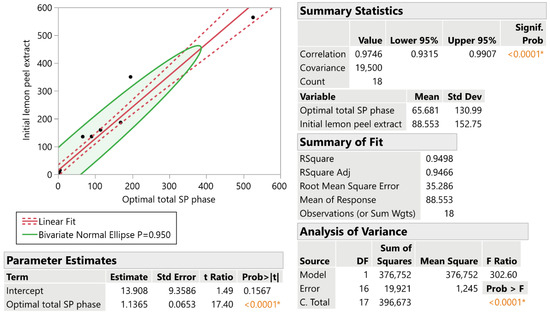
Figure 7.
Bivariate analysis of initial lemon peel extract by optimal total SP phase sample. Line of fit and confidence limits (curves) for the expected values are also presented. Asterisks and colored values denote statistically significant values, while inset tables include statistics relevant to the evaluation of the resulting bivariate platform model.
4. Conclusions
In this study, CPE was employed in lemon peel extract in order to assess the recovery of bioactive compounds. The optimal CPE parameters were established as a three-stepped CPE with 20% w/v Span 20 and 20% w/v sodium chloride at pH 7 and 45 °C for 20 min. As such, the optimal procedure can easily be used to obtain extracts rich in polyphenols, at the same time utilizing industrial waste and bestowing added value to them. Although it is known that lemon peels exhibit great potential for various applications within the food industry, such as being utilized as food ingredients that possess nutritional, antioxidant, and antibacterial properties, obtaining extracts from lemon peels was rendered more environmentally friendly with the proposed procedure compared to procedures that use organic solvents. Moreover, CPE ensures the encapsulation of bioactive substances within the surfactant, protecting them from oxidative or destructive agents, thus making this technique suitable for the production of more stable food additives. Overall, CPE is a highly promising technique that should be further exploited for other by-products. Further investigations should be undertaken regarding combining lemon peels with other by-products derived from fruits, vegetables, cereals, and legumes in order to develop innovative nutraceutical and pharmaceutical applications.
Author Contributions
Conceptualization, V.A., T.C. and S.I.L.; methodology, V.A. and T.C.; software, V.A.; validation, V.A. and T.C.; formal analysis, V.A. and T.C.; investigation, M.M., V.A., T.C. and E.B.; resources, S.I.L.; data curation, M.M. and V.A.; writing—original draft preparation, M.M.; writing—review and editing, V.A., T.C., M.M., E.B. and S.I.L.; visualization, V.A.; supervision, S.I.L.; project administration, V.A. and T.C.; funding acquisition, S.I.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Food Waste and Food Waste Prevention—Estimates. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Food_waste_and_food_waste_prevention_-_estimates (accessed on 11 December 2023).
- Imeneo, V.; Romeo, R.; De Bruno, A.; Piscopo, A. Green-Sustainable Extraction Techniques for the Recovery of Antioxidant Compounds from “Citrus Limon” by-Products. J. Environ. Sci. Health Part B 2022, 57, 220–232. [Google Scholar] [CrossRef]
- Russo, C.; Maugeri, A.; Lombardo, G.E.; Musumeci, L.; Barreca, D.; Rapisarda, A.; Cirmi, S.; Navarra, M. The Second Life of Citrus Fruit Waste: A Valuable Source of Bioactive Compounds. Molecules 2021, 26, 5991. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Mejía, E.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. Citrus Peels Waste as a Source of Value-Added Compounds: Extraction and Quantification of Bioactive Polyphenols. Food Chem. 2019, 295, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Putnik, P.; Bursać Kovačević, D.; Režek Jambrak, A.; Barba, F.J.; Cravotto, G.; Binello, A.; Lorenzo, J.M.; Shpigelman, A. Innovative “Green” and Novel Strategies for the Extraction of Bioactive Added Value Compounds from Citrus Wastes—A Review. Molecules 2017, 22, 680. [Google Scholar] [CrossRef]
- El-ghfar, M.H.A.A.; Ibrahim, H.M.; Hassan, I.M.; Fattah, A.A.A.; Mahmoud, M.H. Peels of Lemon and Orange as Value-Added Ingredients: Chemical and Antioxidant Properties. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 777–794. [Google Scholar] [CrossRef]
- Garcia-Castello, E.M.; Rodriguez-Lopez, A.D.; Mayor, L.; Ballesteros, R.; Conidi, C.; Cassano, A. Optimization of Conventional and Ultrasound Assisted Extraction of Flavonoids from Grapefruit (Citrus paradisi L.) Solid Wastes. LWT—Food Sci. Technol. 2015, 64, 1114–1122. [Google Scholar] [CrossRef]
- Khaledian, S.; Basiri, S.; Shekarforoush, S.S. Shelf-Life Extension of Pacific White Shrimp Using Tragacanth Gum-Based Coatings Containing Persian Lime Peel (Citrus latifolia) Extract. LWT 2021, 141, 110937. [Google Scholar] [CrossRef]
- Nawaz, R.; Safdar, N.; Ainee, A.; Jabbar, S. Development and Storage Stability Studies of Functional Fruit Drink Supplemented with Polyphenols Extracted from Lemon Peels. J. Food Process. Preserv. 2021, 45, e15268. [Google Scholar] [CrossRef]
- Martínez-Abad, A.; Ramos, M.; Hamzaoui, M.; Kohnen, S.; Jiménez, A.; Garrigós, M.C. Optimisation of Sequential Microwave-Assisted Extraction of Essential Oil and Pigment from Lemon Peels Waste. Foods 2020, 9, 1493. [Google Scholar] [CrossRef]
- Rizaldy, D.; Insanu, M.; Sabila, N.; Haniffadli, A.; Zahra, A.A.; Pratiwi, S.N.E.; Mudrika, S.N.; Hartati, R.; Fidrianny, I. Lemon (Citrus Limon L.): Antioxidative Activity and Its Marker Compound. Biointerface Res. Appl. Chem. 2022, 13, 21. [Google Scholar] [CrossRef]
- Haida, Z.; Ab Ghani, S.; Juju Nakasha, J.; Hakiman, M. Determination of Experimental Domain Factors of Polyphenols, Phenolic Acids and Flavonoids of Lemon (Citrus Limon) Peel Using Two-Level Factorial Design. Saudi J. Biol. Sci. 2022, 29, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Papoutsis, K.; Pristijono, P.; Golding, J.B.; Stathopoulos, C.E.; Bowyer, M.C.; Scarlett, C.J.; Vuong, Q.V. Optimizing a Sustainable Ultrasound-Assisted Extraction Method for the Recovery of Polyphenols from Lemon by-Products: Comparison with Hot Water and Organic Solvent Extractions. Eur. Food Res. Technol. 2018, 244, 1353–1365. [Google Scholar] [CrossRef]
- Arcas, M.C.; Botıa, J.M.; Ortuno, A.M.; Rıo, J.A.D. UV Irradiation Alters the Levels of Flavonoids Involved in the Defence Mechanism of Citrus Aurantium Fruits against Penicillium Digitatum. Eur. J. Plant Pathol. 2000, 196, 617–622. [Google Scholar] [CrossRef]
- Ortuño, A.; Báidez, A.; Gómez, P.; Arcas, M.C.; Porras, I.; García-Lidón, A.; Río, J.A.D. Citrus paradisi and Citrus sinensis Flavonoids: Their Influence in the Defence Mechanism against Penicillium Digitatum. Food Chem. 2006, 2, 351–358. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Fang, L.; Zheng, Z.; Zhi, D.; Wang, S.; Li, S.; Ho, C.-T.; Zhao, H. Anticancer Activities of Citrus Peel Polymethoxyflavones Related to Angiogenesis and Others. BioMed Res. Int. 2014, 2014, e453972. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, B.; Mehra, Y.; Ganesh, R.N.; Viswanathan, P. Regulation of Urinary Crystal Inhibiting Proteins and Inflammatory Genes by Lemon Peel Extract and Formulated Citrus Bioflavonoids on Ethylene Glycol Induced Urolithic Rats. Food Chem. Toxicol. 2016, 94, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic Composition, Antioxidant Potential and Health Benefits of Citrus Peel. Food Res. Int. 2020, 132, 109114. [Google Scholar] [CrossRef]
- Rustam, M.; Ifora, I.; Fauziah, F. Potential Anti-Inflammatory Effects of Eriocitrin: A Review. J. Drug Deliv. Ther. 2022, 12, 187–192. [Google Scholar] [CrossRef]
- Yao, L.; Liu, W.; Bashir, M.; Nisar, M.F.; Wan, C.C. Eriocitrin: A Review of Pharmacological Effects. Biomed. Pharmacother. 2022, 154, 113563. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Saluja, A. The Pharmacological Potential of Hesperidin; CSIR-National Institute of Science Communication and Policy Research: New Delhi, India, 2019; Volume 564. [Google Scholar]
- Jiang, H.; Zhang, W.; Xu, Y.; Chen, L.; Cao, J.; Jiang, W. An Advance on Nutritional Profile, Phytochemical Profile, Nutraceutical Properties, and Potential Industrial Applications of Lemon Peels: A Comprehensive Review. Trends Food Sci. Technol. 2022, 124, 219–236. [Google Scholar] [CrossRef]
- Fu, J.-T.; Chang, Y.-H.; Shiau, S.-Y. Rheological, Antioxidative and Sensory Properties of Dough and Mantou (Steamed Bread) Enriched with Lemon Fiber. LWT—Food Sci. Technol. 2015, 61, 56–62. [Google Scholar] [CrossRef]
- Huang, R.; Zhang, Y.; Shen, S.; Zhi, Z.; Cheng, H.; Chen, S.; Ye, X. Antioxidant and Pancreatic Lipase Inhibitory Effects of Flavonoids from Different Citrus Peel Extracts: An in Vitro Study. Food Chem. 2020, 326, 126785. [Google Scholar] [CrossRef] [PubMed]
- Pandiyan, P.; Soni, A.; Elumalai, P. Effects of Lemon and Pomelo Peel Extracts on Quality and Melanosis of Indian White Prawn during Chilled Storage. J. Food Process. Preserv. 2022, 46, e15952. [Google Scholar] [CrossRef]
- Fidalgo, A.; Ciriminna, R.; Carnaroglio, D.; Tamburino, A.; Cravotto, G.; Grillo, G.; Ilharco, L.M.; Pagliaro, M. Eco-Friendly Extraction of Pectin and Essential Oils from Orange and Lemon Peels. ACS Sustain. Chem. Eng. 2016, 4, 2243–2251. [Google Scholar] [CrossRef]
- Lopresto, C.G.; Petrillo, F.; Casazza, A.A.; Aliakbarian, B.; Perego, P.; Calabrò, V. A Non-Conventional Method to Extract D-Limonene from Waste Lemon Peels and Comparison with Traditional Soxhlet Extraction. Sep. Purif. Technol. 2014, 137, 13–20. [Google Scholar] [CrossRef]
- Sankeshwari, R.M.; Ankola, A.V.; Bhat, K.; Hullatti, K. Soxhlet versus Cold Maceration: Which Method Gives Better Antimicrobial Activity to Licorice Extract Against: Streptococcus Mutans:? J. Sci. Soc. 2018, 45, 67. [Google Scholar] [CrossRef]
- Sun, T.; Beiyuan, J.; Gielen, G.; Mao, X.; Song, Z.; Xu, S.; Ok, Y.S.; Rinklebe, J.; Liu, D.; Hou, D.; et al. Optimizing Extraction Procedures for Better Removal of Potentially Toxic Elements during EDTA-Assisted Soil Washing. J. Soils Sediments 2020, 20, 3417–3426. [Google Scholar] [CrossRef]
- Haddou, B.; Canselier, J.P.; Gourdon, C. Use of Cloud Point Extraction with Ethoxylated Surfactants for Organic Pollution Removal. In The Role of Colloidal Systems in Environmental Protection; Fanun, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 97–142. ISBN 978-0-444-63283-8. [Google Scholar]
- Chen, Y.; Du, K.; Li, J.; Bai, Y.; An, M.; Tan, Z.; Chang, Y. A Green and Efficient Method for the Preconcentration and Determination of Gallic Acid, Bergenin, Quercitrin, and Embelin from Ardisia Japonica Using Nononic Surfactant Genapol X-080 as the Extraction Solvent. Int. J. Anal. Chem. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Al_Saadi, M.R.; Al-Garawi, Z.S.; Thani, M.Z. Promising Technique, Cloud Point Extraction: Technology & Applications. J. Phys. Conf. Ser. 2021, 1853, 012064. [Google Scholar] [CrossRef]
- Mortada, W.I.; Hassanien, M.M.; El-Asmy, A.A. Cloud Point Extraction of Some Precious Metals Using Triton X-114 and a Thioamide Derivative with a Salting-out Effect. Egypt. J. Basic Appl. Sci. 2014, 1, 184–191. [Google Scholar] [CrossRef]
- Carabias-Martίnez, R.; Rodrίguez-Gonzalo, E.; Moreno-Cordero, B.; Pérez-Pavón, J.L.; Garcίa-Pinto, C.; Fernández Laespada, E. Surfactant Cloud Point Extraction and Preconcentration of Organic Compounds Prior to Chromatography and Capillary Electrophoresis. J. Chromatogr. A 2000, 902, 251–265. [Google Scholar] [CrossRef]
- Pytlakowska, K.; Kozik, V.; Dabioch, M. Complex-Forming Organic Ligands in Cloud-Point Extraction of Metal Ions: A Review. Talanta 2013, 110, 202–228. [Google Scholar] [CrossRef]
- Jie, Y.; Chen, F. Progress in the Application of Food-Grade Emulsions. Foods 2022, 11, 2883. [Google Scholar] [CrossRef] [PubMed]
- Giovanoudis, I.; Athanasiadis, V.; Chatzimitakos, T.; Kalompatsios, D.; Bozinou, E.; Gortzi, O.; Nanos, G.D.; Lalas, S.I. Isolation of Polyphenols from Two Waste Streams of Clingstone Peach Canneries Utilizing the Cloud Point Extraction Method. Biomass 2023, 3, 291–305. [Google Scholar] [CrossRef]
- Mortada, W.I. Recent Developments and Applications of Cloud Point Extraction: A Critical Review. Microchem. J. 2020, 157, 105055. [Google Scholar] [CrossRef]
- Arya, S.S.; Kaimal, A.M.; Chib, M.; Sonawane, S.K.; Show, P.L. Novel, Energy Efficient and Green Cloud Point Extraction: Technology and Applications in Food Processing. J. Food Sci. Technol. 2019, 56, 524–534. [Google Scholar] [CrossRef]
- Karadag, A.; Kayacan Cakmakoglu, S.; Metin Yildirim, R.; Karasu, S.; Avci, E.; Ozer, H.; Sagdic, O. Enrichment of Lecithin with Phenolics from Olive Mill Wastewater by Cloud Point Extraction and Its Application in Vegan Salad Dressing. J. Food Process. Preserv. 2022, 46, e16645. [Google Scholar] [CrossRef]
- Giovanoudis, I.; Athanasiadis, V.; Chatzimitakos, T.; Kalompatsios, D.; Bozinou, E.; Gortzi, O.; Nanos, G.D.; Lalas, S.I. Implementation of Cloud Point Extraction Using Surfactants in the Recovery of Polyphenols from Apricot Cannery Waste. Eng 2023, 4, 1225–1235. [Google Scholar] [CrossRef]
- Khani, R.; Sheykhi, R.; Bagherzade, G. An Environmentally Friendly Method Based on Micro-Cloud Point Extraction for Determination of Trace Amount of Quercetin in Food and Fruit Juice Samples. Food Chem. 2019, 293, 220–225. [Google Scholar] [CrossRef]
- Guo, N.; Jiang, Y.-W.; Kou, P.; Liu, Z.-M.; Efferth, T.; Li, Y.-Y.; Fu, Y.-J. Application of Integrative Cloud Point Extraction and Concentration for the Analysis of Polyphenols and Alkaloids in Mulberry Leaves. J. Pharm. Biomed. Anal. 2019, 167, 132–139. [Google Scholar] [CrossRef]
- Mohammadzadeh, M.; Honarvar, M.; Zarei, A.R.; Mashhadi Akbar Boojar, M.; Bakhoda, H. A New Approach for Separation and Recovery of Betaine from Beet Molasses Based on Cloud Point Extraction Technique. J. Food Sci. Technol. 2018, 55, 1215–1223. [Google Scholar] [CrossRef]
- Leite, A.C.; Ferreira, A.M.; Morais, E.S.; Khan, I.; Freire, M.G.; Coutinho, J.A.P. Cloud Point Extraction of Chlorophylls from Spinach Leaves Using Aqueous Solutions of Nonionic Surfactants. ACS Sustain. Chem. Eng. 2018, 6, 590–599. [Google Scholar] [CrossRef]
- De Araújo Padilha, C.E.; De Azevedo, J.C.S.; De Sousa, F.C.; De Oliveira, S.D.; Souza, D.F.D.S.; De Oliveira, J.A.; De Macedo, G.R.; Dos Santos, E.S. Recovery of Polyphenols from Camu-Camu (Myrciaria dubia H.B.K. McVaugh) Depulping Residue by Cloud Point Extraction. Chin. J. Chem. Eng. 2018, 26, 2471–2476. [Google Scholar] [CrossRef]
- Kiai, H.; Raiti, J.; El-Abbassi, A.; Hafidi, A. Recovery of Phenolic Compounds from Table Olive Processing Wastewaters Using Cloud Point Extraction Method. J. Environ. Chem. Eng. 2018, 6, 1569–1575. [Google Scholar] [CrossRef]
- Cicco, N.; Lanorte, M.T.; Paraggio, M.; Viggiano, M.; Lattanzio, V. A Reproducible, Rapid and Inexpensive Folin–Ciocalteu Micro-Method in Determining Phenolics of Plant Methanol Extracts. Microchem. J. 2009, 91, 107–110. [Google Scholar] [CrossRef]
- Manousaki, A.; Jancheva, M.; Grigorakis, S.; Makris, D.P. Extraction of Antioxidant Phenolics from Agri-Food Waste Biomass Using a Newly Designed Glycerol-Based Natural Low-Transition Temperature Mixture: A Comparison with Conventional Eco-Friendly Solvents. Recycling 2016, 1, 194–204. [Google Scholar] [CrossRef]
- Shehata, E.; Grigorakis, S.; Loupassaki, S.; Makris, D.P. Extraction Optimisation Using Water/Glycerol for the Efficient Recovery of Polyphenolic Antioxidants from Two Artemisia Species. Sep. Purif. Technol. 2015, 149, 462–469. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Athanasiadis, V.; Kotsou, K.; Bozinou, E.; Lalas, S.I. Response Surface Optimization for the Enhancement of the Extraction of Bioactive Compounds from Citrus Limon Peel. Antioxidants 2023, 12, 1605. [Google Scholar] [CrossRef]
- Jagota, S.K.; Dani, H.M. A New Colorimetric Technique for the Estimation of Vitamin C Using Folin Phenol Reagent. Anal. Biochem. 1982, 127, 178–182. [Google Scholar] [CrossRef]
- Katsoyannos, E.; Gortzi, O.; Chatzilazarou, A.; Athanasiadis, V.; Tsaknis, J.; Lalas, S. Evaluation of the Suitability of Low Hazard Surfactants for the Separation of Phenols and Carotenoids from Red-Flesh Orange Juice and Olive Mill Wastewater Using Cloud Point Extraction. J. Sep. Sci. 2012, 35, 2665–2670. [Google Scholar] [CrossRef]
- Kim, C.; Hsieh, Y.-L. Wetting and Absorbency of Nonionic Surfactant Solutions on Cotton Fabrics. Colloids Surf. Physicochem. Eng. Asp. 2001, 187–188, 385–397. [Google Scholar] [CrossRef]
- Al-Shamrani, A.A.; James, A.; Xiao, H. Separation of Oil from Water by Dissolved Air Flotation. Colloids Surf. Physicochem. Eng. Asp. 2002, 209, 15–26. [Google Scholar] [CrossRef]
- Papaioannou, E.H.; Karabelas, A.J. Lycopene Recovery from Tomato Peel under Mild Conditions Assisted by Enzymatic Pre-Treatment and Non-Ionic Surfactants. Acta Biochim. Pol. 2012, 59, 71–74. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Athanasiadis, V.; Mantiniotou, M.; Kalompatsios, D.; Bozinou, E.; Giovanoudis, I.; Lalas, S.I. Exploring the Feasibility of Cloud-Point Extraction for Bioactive Compound Recovery from Food Byproducts: A Review. Biomass 2023, 3, 306–322. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Chatzimitakos, T.; Mantiniotou, M.; Kalompatsios, D.; Bozinou, E.; Lalas, S.I. Investigation of the Polyphenol Recovery of Overripe Banana Peel Extract Utilizing Cloud Point Extraction. Engineering 2023, 4, 3026–3038. [Google Scholar] [CrossRef]
- Zain, N.N.M.; Abu Bakar, N.K.; Mohamad, S.; Saleh, N.M. Optimization of a Greener Method for Removal Phenol Species by Cloud Point Extraction and Spectrophotometry. Spectrochim. Acta A. Mol. Biomol. Spectrosc. 2014, 118, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Santalad, A.; Burakham, R.; Srijaranai, S.; Srijaranai, S.; Deming, R.L. Role of Different Salts on Cloud-Point Extraction of Isoprocarb and Promecarb Insecticides Followed by High-Performance Liquid Chromatography. J. Chromatogr. Sci. 2012, 50, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Mori, M.; Itabashi, H. Cloud Point Extraction of Cu(II) Using a Mixture of Triton X-100 and Dithizone with a Salting-out Effect and Its Application to Visual Determination. Talanta 2013, 117, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Sosa Ferrera, Z.; Padrón Sanz, C.; Mahugo Santana, C.; Santana Rodrίguez, J.J. The Use of Micellar Systems in the Extraction and Pre-Concentration of Organic Pollutants in Environmental Samples. TrAC Trends Anal. Chem. 2004, 23, 469–479. [Google Scholar] [CrossRef]
- Chatzimitakos, T.G.; Stalikas, C.D. Melamine Sponge Decorated with Copper Sheets as a Material with Outstanding Properties for Microextraction of Sulfonamides Prior to Their Determination by High-Performance Liquid Chromatography. J. Chromatogr. A 2018, 1554, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Peltonen, L.; Hirvonen, J.; Yliruusi, J. The Behavior of Sorbitan Surfactants at the Water–Oil Interface: Straight-Chained Hydrocarbons from Pentane to Dodecane as an Oil Phase. J. Colloid Interface Sci. 2001, 240, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Shimanouchi, T.; Hayashi, T.; Toramoto, K.; Fukuma, S.; Hayashi, K.; Yasuhara, K.; Kimura, Y. Microfluidic and Hydrothermal Preparation of Vesicles Using Sorbitan Monolaurate/Polyoxyethylene (20) Sorbitan Monolaurate (Span 20/Tween 20). Colloids Surf. B Biointerfaces 2021, 205, 111836. [Google Scholar] [CrossRef] [PubMed]
- Danacioğlu, D.; Pekel, M. Utilization of Some Plant Based Wastes for a Possible Formulation of Tea Infusion. Afyon Kocatepe Üniversitesi Fen Ve Mühendis. Bilim. Derg. 2021, 21, 122–129. [Google Scholar] [CrossRef]
- Rodríguez-Solana, R.; Dantas, M.; Romano, A. Influence of Carob Pod (Ceratonia Siliqua L.) Variety and Processing on the Antioxidant Capacity and Total Phenolic Content of Carob Liquors. In Proceedings of the INCREaSE; Mortal, A., Aníbal, J., Monteiro, J., Sequeira, C., Semião, J., Moreira da Silva, M., Oliveira, M., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 216–226. [Google Scholar]
- Saeidi, I.; Hadjmohammadi, M.R.; Peyrovi, M.; Iranshahi, M.; Barfi, B.; Babaei, A.B.; Dust, A.M. HPLC Determination of Hesperidin, Diosmin and Eriocitrin in Iranian Lime Juice Using Polyamide as an Adsorbent for Solid Phase Extraction. J. Pharm. Biomed. Anal. 2011, 56, 419–422. [Google Scholar] [CrossRef]
- Mare, R.; Pujia, R.; Maurotti, S.; Greco, S.; Cardamone, A.; Coppoletta, A.R.; Bonacci, S.; Procopio, A.; Pujia, A. Assessment of Mediterranean Citrus Peel Flavonoids and Their Antioxidant Capacity Using an Innovative UV-Vis Spectrophotometric Approach. Plants 2023, 12, 4046. [Google Scholar] [CrossRef]
- Hajimahmoodi, M.; Moghaddam, G.; Mousavi, S.M.; Sadeghi, N.; Oveisi, M.R.; Jannat, B. Total Antioxidant Activity, and Hesperidin, Diosmin, Eriocitrin and Quercetin Contents of Various Lemon Juices. Trop. J. Pharm. Res. 2014, 13, 951–956. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).