High Added-Value by-Products from Biomass: A Case Study Unveiling Opportunities for Strengthening the Agroindustry Value Chain
Abstract
1. Introduction
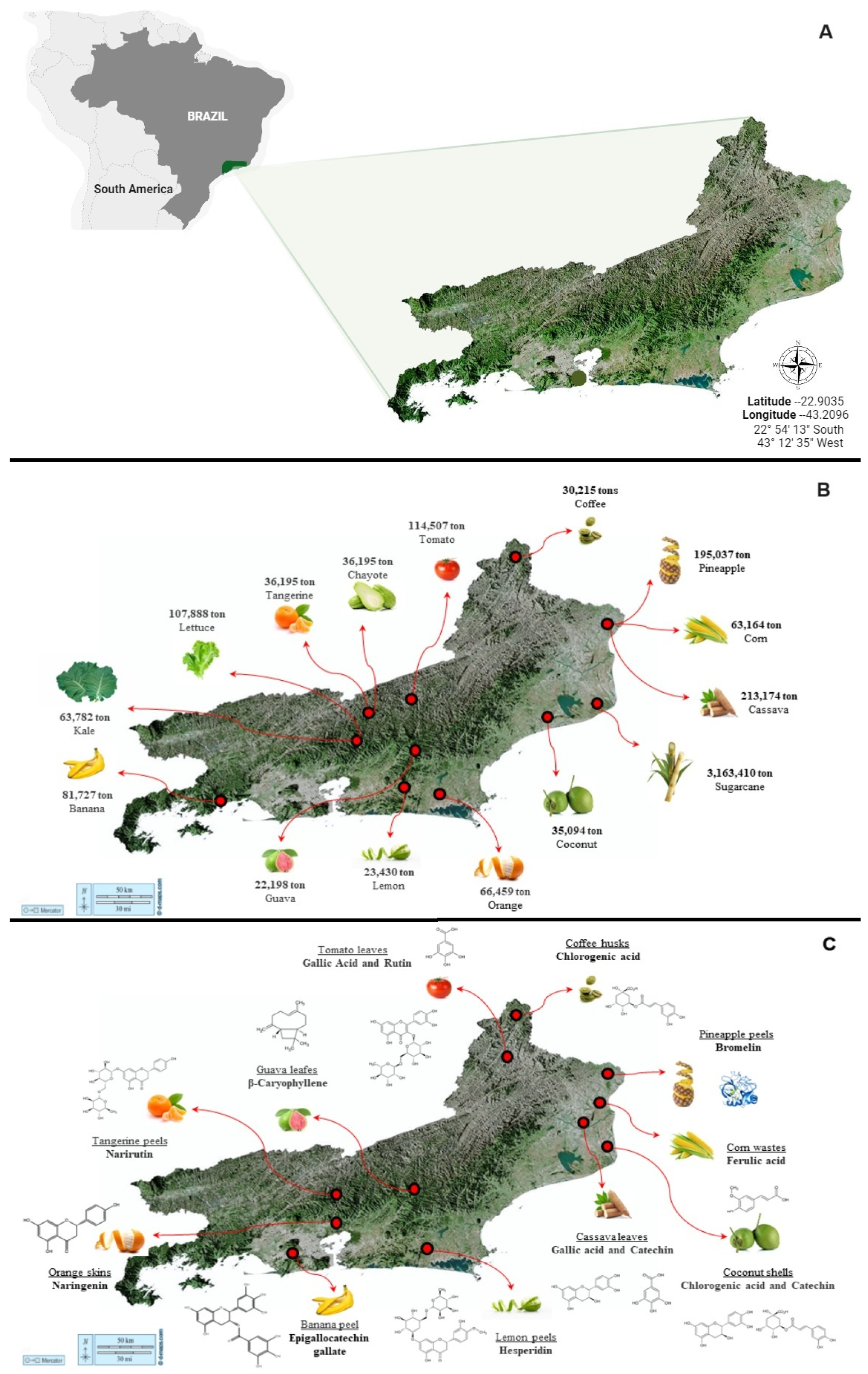
2. Rio de Janeiro State: A Case Study
2.1. Biodiversity and Climatic Patterns in Varied Vegetation Landscapes
2.2. Fruit and Vegetable Cultivation Belts
2.3. Biomass Residues from Agroindustry and Plant Processing
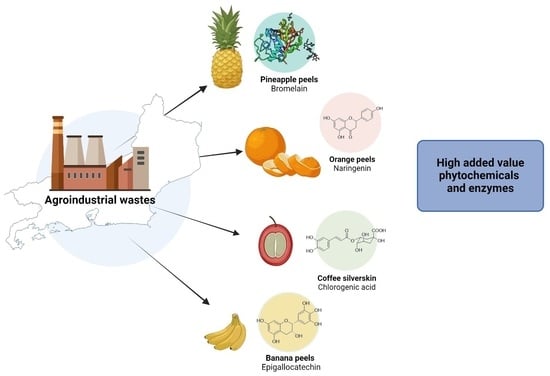
3. Waste Reuse
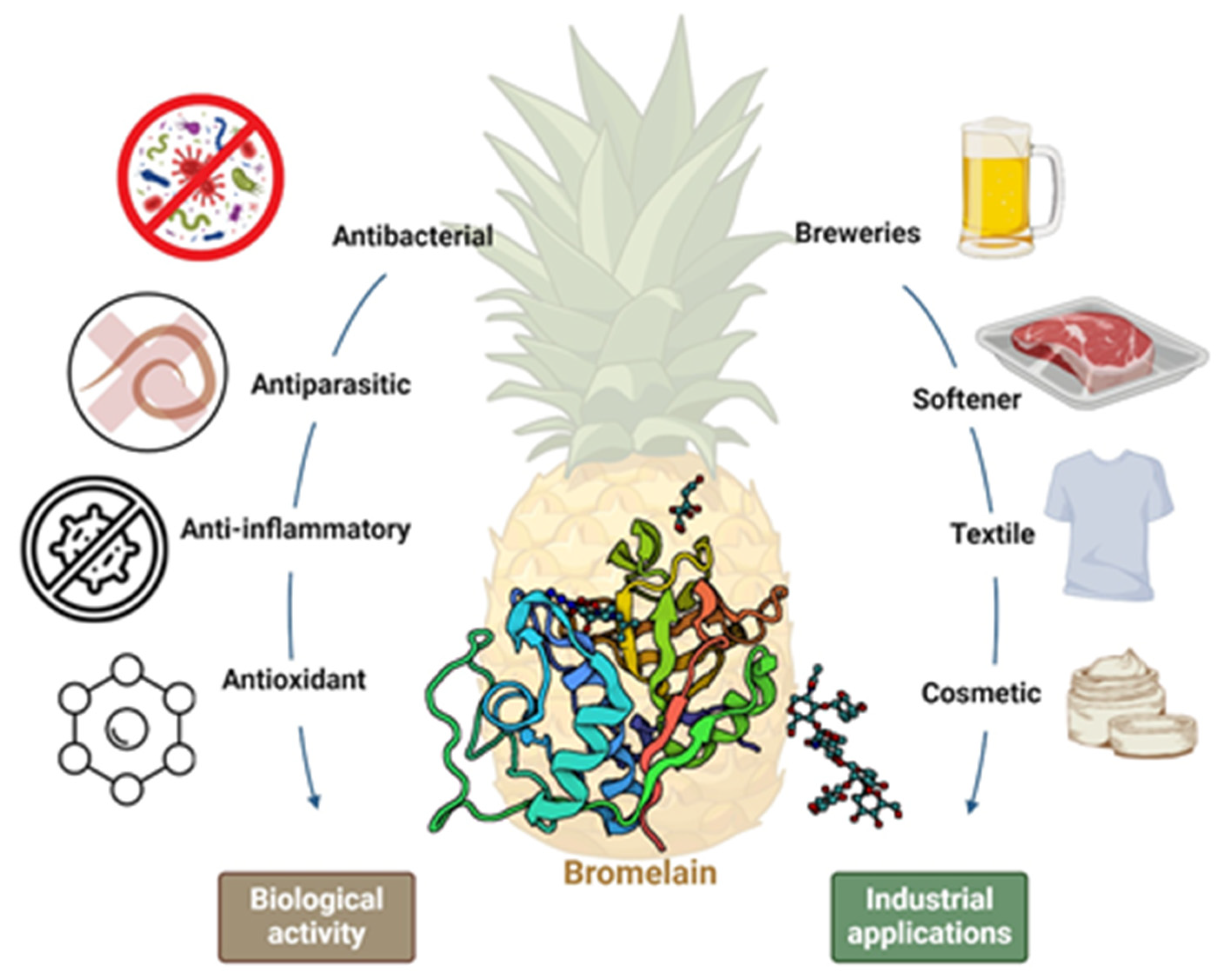
3.1. Pineapple (Ananas comosus)
3.2. Banana (Musa spp.)
3.3. Coffee (Coffea spp.)
3.4. Citrus (Citrus spp.)
4. Challenges in the Production Chain
4.1. Logistics
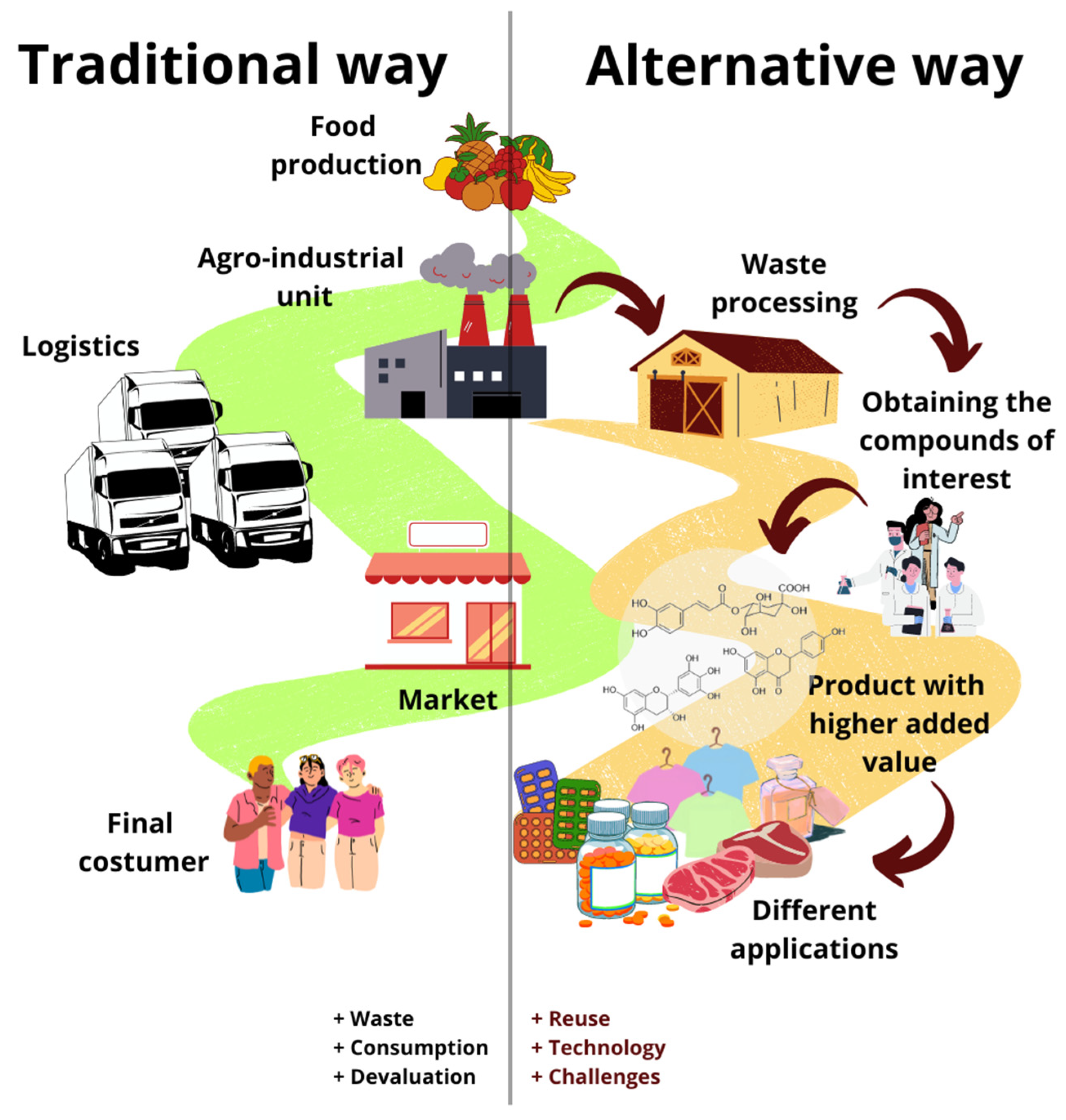
4.2. Market
4.3. Technology
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coleman-Jensen, A.; Rabbitt, M.P.; Gregory, C.A.; Singh, A. Household food security in the United States. Econ. Res. Rep. 2022, 155. [Google Scholar] [CrossRef]
- UNEP—United Nations Environment Programme. Food Waste Index Report. Available online: https://www.unep.org/resources/report/unep-food-waste-index-report-2021 (accessed on 18 January 2024).
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Bio-refinery approach for spent coffee grounds valorization. Bioresour. Technol. 2018, 247, 1077–1084. [Google Scholar] [CrossRef]
- De Azevedo, A.R.; Amin, M.; Hadzima-Nyarko, M.; Agwa, I.S.; Zeyad, A.M.; Tayeh, B.A.; Adesina, A. Possibilities for the application of agro-industrial wastes in cementitious materials: A brief review of the Brazilian perspective. Clean. Mater. 2022, 3, 100040. [Google Scholar] [CrossRef]
- Puglia, D.; Pezzolla, D.; Gigliotti, G.; Torre, L.; Bartucca, M.L.; Del Buono, D. The opportunity of valorizing agricultural waste, through its conversion into biostimulants, biofertilizers, and biopolymers. Sustainability 2021, 13, 2710. [Google Scholar] [CrossRef]
- Rashid, M.I.; Shahzad, K. Food waste recycling for compost production and its economic and environmental assessment as circular economy indicators of solid waste management. J. Clean. Prod. 2021, 317, 128467. [Google Scholar] [CrossRef]
- Sadhukhan, J.; Dugmore, T.I.; Matharu, A.; Martinez-Hernandez, E.; Aburto, J.; Rahman, P.K.; Lynch, J. Perspectives on “game changer” global challenges for sustainable 21st century: Plant-based diet, unavoidable food waste biorefining, and circular economy. Sustainability 2020, 12, 1976. [Google Scholar] [CrossRef]
- Kapoor, R.; Ghosh, P.; Tyagi, B.; Vijay, V.K.; Vijay, V.; Thakur, I.S.; Kamyab, H.; Nguyen, D.D.; Kumar, A. Advances in biogas valorization and utilization systems: A comprehensive review. J. Clean. Prod. 2020, 273, 123052. [Google Scholar] [CrossRef]
- Velasco-Muñoz, J.F.; Mendoza, J.M.F.; Aznar-Sánchez, J.A.; Gallego-Schmid, A. Circular economy implementation in the agricultural sector: Definition, strategies and indicators. Resour. Conserv. Recycl. 2021, 170, 105618. [Google Scholar] [CrossRef]
- Sabra, A.; Netticadan, T.; Wijekoon, C. Grape bioactive molecules, and the potential health benefits in reducing the risk of heart diseases. Food Chem. X 2021, 12, 100149. [Google Scholar] [CrossRef] [PubMed]
- Wegenast, C.A.; Meadows, I.D.; Anderson, R.E.; Southard, T.; González Barrientos, C.R.; Wismer, T.A. Acute kidney injury in dogs following ingestion of cream of tartar and tamarinds and the connection to tartaric acid as the proposed toxic principle in grapes and raisins. J. Vet. Emerg. Crit. Care 2022, 32, 812–816. [Google Scholar] [CrossRef]
- Cavalcante, B.D.M.; Scapini, T.; Camargo, A.F.; Ulrich, A.; Bonatto, C.; Dalastra, C.; Mossi, A.J.; Fongaro, G.; Di Piero, R.M.; Treichel, H. Orange peels and shrimp shell used in a fermentation process to produce an aqueous extract with bioherbicide potential to weed control. Biocatal. Agric. Biotechnol. 2021, 32, 101947. [Google Scholar] [CrossRef]
- De Almeida Cunha, A.; Madureira, C.B.; da Fonseca, G.A.B. Legal Atlantic Forest (Mata Atlântica Legal): Integrating biogeography to public policies towards the conservation of the biodiversity hotspot. Sustain. Debate 2019, 10, 320–353. [Google Scholar] [CrossRef]
- ASPA/AGROGEO—Acompanhamento Sistemático da Produção Agrícola. Anuário do Agronegócio do Estado do Rio de Janeiro 2022; AQUI Noticias: Rio de Janeiro, Brazil, 2022; pp. 1–19. ISBN 978-65-997409-09. [Google Scholar]
- Da SilvaNeiva, H.; Da Silva, M.S.; Cardoso, C. Analysis of climate behavior and land use in the city of Rio de Janeiro, RJ, Brazil. Climate 2017, 5, 52. [Google Scholar]
- Pougy, N.; Martins, E.; Verdi, M.; Fernandez, E.; Loyola, R.; Silveira-Filho, T.B.; Martinelli, G. Plano de Ação Nacional para a Conservação da Flora Endêmica Ameaçada de Extinção do Estado do Rio de Janeiro; Secretaria de Estado do Ambiente—SEA, Andrea Jakobsson Estúdio: Rio de Janeiro, Brazil, 2018; p. 80. [Google Scholar]
- Sans, L.M.A.; Santana, D.P. Clima e solo. In Cultivo do Milho; Cruz, J.C., Versiani, R.P., Ferreira, M.T.R., Eds.; Embrapa Milho e Sorgo: Sete Lagoas, MG, Brazil, 2000; pp. 1–20. [Google Scholar]
- Grelle, C.E.; Rajão, H.; Marques, M.C. The Future of the Brazilian Atlantic Forest. In The Atlantic Forest: History, Biodiversity, Threats and Opportunities of the Mega-Diverse Forest; Marques, M.C.M., Grelle, C.E.V., Eds.; Springer: Cham, Switzerland, 2021; pp. 487–503. [Google Scholar]
- Sharma, L.D.; Sadhukhan, R.; Hota, D. Neutralising Climate Change Through Fruit Crops. Res. Rev. J. Crop Sci. Technol. 2021, 10, 28–36. [Google Scholar]
- Pereira, V.R.; Fiore, F.A. Fatores influenciadores da segregação de resíduos orgânicos na fonte geradora para a viabilização de sistemas de compostagem. Eng. Sanit. Ambient. 2022, 27, 643–652. [Google Scholar] [CrossRef]
- Nirmal, N.P.; Khanashyam, A.C.; Mundanat, A.S.; Shah, K.; Babu, K.S.; Thorakkattu, P.; Al-Asmari, F.; Pandiselvam, R. Valorization of Fruit Waste for Bioactive Compounds and Their Applications in the Food Industry. Foods 2023, 12, 556. [Google Scholar] [CrossRef]
- Pereira, B.S.; de Freitas, C.; Vieira, R.M.; Brienzo, M. Brazilian banana, guava, and orange fruit and waste production as a potential biorefinery feedstock. J. Mater. Cycles Waste Manag. 2022, 24, 2126–2140. [Google Scholar] [CrossRef]
- He, C.; Wei, J.; Zeng, L.; Deng, J. Triterpenoids and Flavonoids from Cassava Leaves. Chem. Nat. Compd. 2020, 56, 331–333. [Google Scholar] [CrossRef]
- Silva-Beltrán, N.P.; Ruiz-Cruz, S.; Chaidez, C.; de Jesus Ornelas-Paz, J.; López-Mata, M.A.; Márquez-Ríos, E.; Estrada, M.I. Chemical constitution and effect of extracts of tomato plants byproducts on the enteric viral surrogates. IJEHR 2014, 25, 299–311. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, H.; Feng, X.; Ma, L.; Zhang, Y.; Dai, H. Lignocellulose nanocrystals from pineapple peel: Preparation, characterization and application as efficient Pickering emulsion stabilizers. Int. Food Res. J. 2021, 150, 110738. [Google Scholar] [CrossRef]
- Liu, N.; Li, X.; Zhao, P.; Zhang, X.; Qiao, O.; Huang, L.; Guo, L.; Gao, W. A review of chemical constituents and health-promoting effects of citrus peels. Food Chem. 2021, 365, 130585. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic composition, antioxidant potential and health benefits of citrus peel. Int. Food Res. J. 2020, 132, 109114. [Google Scholar] [CrossRef]
- Lima, E.B.; de Sousa, C.N.; Vasconcelos, G.S.; Meneses, L.N.; E Silva Pereira, Y.F.; Ximenes, N.C.; Santos Júnior, M.A.; Matos, N.C.; Brito, R.; Miron, D.; et al. Antidepressant, antioxidant and neurotrophic properties of the standardized extract of Cocos nucifera husk fiber in mice. J. Nat. Med. 2016, 70, 510–521. [Google Scholar] [CrossRef]
- Lau, T.; Harbourne, N.; Oruna-Concha, M. Valorisation of sweet corn (Zea mays) cob by extraction of valuable compounds. Int. J. Food Sci. Technol. 2019, 54, 1240–1246. [Google Scholar] [CrossRef]
- Zema, D.A.; Calabrò, P.S.; Folino, A.; Tamburino, V.; Zappia, G.; Zimbone, S.M. Valorisation of citrus processing waste: A review. Waste Manag. 2018, 80, 252–273. [Google Scholar] [CrossRef]
- Madia, V.N.; Vita, D.; Ialongo, D.; Tudino, V.; Leo, A.; Scipione, L.; Santos, R.; Costi, R.; Messore, A. Recent Advances in Recovery of Lycopene from Tomato Waste: A Potent Antioxidant with Endless Benefits. Molecules 2021, 26, 4495. [Google Scholar] [CrossRef]
- Zahari, C.N.M.C.; Mohamad, N.V.; Akinsanya, M.A.; Gengatharan, A. The crimson gem: Unveiling the vibrant potential of lycopene as a functional food ingredient. Food Chem. Adv. 2023, 3, 100510. [Google Scholar] [CrossRef]
- Dahiya, A. (Ed.) Bioenergy: Biomass to Biofuels and Waste to Energy, 2nd ed.; Academic Press: London, UK, 2020; pp. 105–450. [Google Scholar]
- Singh, N.; Ogunseitan, O.A.; Wong, M.H.; Tang, Y. Sustainable materials alternative to petrochemical plastics pollution: A review analysis. Sustain. Horiz. 2022, 2, 100016. [Google Scholar] [CrossRef]
- Wang, H.; Pu, Y.; Ragauskas, A.; Yang, B. From lignin to valuable products–strategies, challenges, and prospects. Bioresour. Technol. 2019, 271, 449–461. [Google Scholar] [CrossRef]
- Shukla, A.; Kumar, D.; Girdhar, M.; Kumar, A.; Goyal, A.; Malik, T.; Mohan, A. Strategies of pretreatment of feedstocks for optimized bioethanol production: Distinct and integrated approaches. Biotechnol. Biofuels Bioprod. 2023, 16, 44. [Google Scholar] [CrossRef]
- Broda, M.; Yelle, D.J.; Serwańska, K. Bioethanol production from lignocellulosic biomass—Challenges and solutions. Molecules 2022, 27, 8717. [Google Scholar] [CrossRef] [PubMed]
- Shirahigue, L.D.; Ceccato-Antonini, S.R. Agro-industrial wastes as sources of bioactive compounds for food and fermentation industries. Ciênc. Rural 2020, 50, e20190857. [Google Scholar] [CrossRef]
- Roy, A.; Khan, A.; Ahmad, I.; Alghamdi, S.; Rajab, B.S.; Babalghith, A.O.; Alshahrani, M.Y.; Islam, M.R. Flavonoids a bioactive compound from medicinal plants and its therapeutic applications. BioMed Res. Int. 2022, 2022, 5445291. [Google Scholar] [CrossRef] [PubMed]
- Setyorini, D.; Antarlina, S.S. Secondary metabolites in sorghum and its characteristics. LWT Food Sci. 2022, 42, e49822. [Google Scholar] [CrossRef]
- Rico, X.; Gullón, B.; Alonso, J.L.; Yáñez, R. Recovery of high value-added compounds from pineapple, melon, watermelon and pumpkin processing by-products: An overview. Int. Food Res. J. 2020, 132, 109086. [Google Scholar] [CrossRef] [PubMed]
- Colletti, A.; Li, S.; Marengo, M.; Adinolfi, S.; Cravotto, G. Recent advances and insights into bromelain processing, pharmacokinetics and therapeutic uses. Appl. Sci. 2021, 11, 8428. [Google Scholar] [CrossRef]
- Varilla, C.; Marcone, M.; Paiva, L.; Baptista, J. Bromelain, a group of pineapple proteolytic complex enzymes (Ananas comosus) and their possible therapeutic and clinical effects. A summary. Foods 2021, 10, 2249. [Google Scholar] [CrossRef]
- Ali, M.M.; Hashim, N.; Abd Aziz, S.; Lasekan, O. Pineapple (Ananas comosus): A comprehensive review of nutritional values, volatile compounds, health benefits, and potential food products. Int. Food Res. J. 2020, 137, 109675. [Google Scholar]
- Chakraborty, A.J.; Mitra, S.; Tallei, T.E.; Tareq, A.M.; Nainu, F.; Cicia, D.; Dhama, K.; Emran, T.B.; Simal-Gandara, J.; Capasso, R. Bromelain a potential bioactive compound: A comprehensive overview from a pharmacological perspective. Life 2021, 11, 317. [Google Scholar] [CrossRef]
- Yow, A.G.; Bostan, H.; Young, R.; Valacchi, G.; Gillitt, N.; Perkins-Veazie, P.; Xiang, Q.; Iorizzo, M. Identification of bromelain subfamily proteases encoded in the pineapple genome. Sci. Rep. 2023, 13, 11605. [Google Scholar] [CrossRef]
- Banerjee, S.; Ranganathan, V.; Patti, A.; Arora, A. Valorisation of pineapple wastes for food and therapeutic applications. Trends Food Sci. 2018, 82, 60–70. [Google Scholar] [CrossRef]
- Yusree, F.I.F.M.; Peter, A.P.; Mohd Nor, M.Z.; Show, P.L.; Mokhtar, M.N. Latest Advances in Protein-Recovery Technologies from Agricultural Waste. Foods 2021, 10, 2748. [Google Scholar] [CrossRef] [PubMed]
- EMBRAPA—Empresa Brasileira de Pesquisa Agropecuária. Embrapa Mandioca e Fruticultura. Portal Embrapa. 2021. Available online: https://www.embrapa.br/mandioca-e-fruticultura/cultivos/abacaxi (accessed on 31 January 2024).
- Ortiz-Ulloa, J.A.; Abril-González, M.F.; Pelaez-Samaniego, M.R.; Zalamea-Piedra, T.S. Biomass yield and carbon abatement potential of banana crops (Musa spp.) in Ecuador. Environ. Sci. Pollut. Res. 2021, 28, 18741–18753. [Google Scholar] [CrossRef] [PubMed]
- Varma, V.; Bebber, D.P. Climate change impacts on banana yields around the world. Nat. Clim. Chang. 2019, 9, 752–757. [Google Scholar] [CrossRef] [PubMed]
- FAO—Food and Agriculture Organization. Food Wastage Foot Print Impacts on Natural Resources. 2021. Available online: https://www.fao.org/news/story/en/item/196402/icode/ (accessed on 20 January 2024).
- FAO—Food and Agriculture Organization. Perdas e Desperdícios de Alimentos na América Latina e no Caribe. 2021. Available online: https://www.fao.org/americas/noticias/ver/pt/c/239394/ (accessed on 21 January 2024).
- Vishala, J.; Singh, G. A review on product development through pulp and peel of banana. Plant Arch. 2021, 21, 693–698. [Google Scholar] [CrossRef]
- Maseko, K.H.; Regnier, T.; Meiring, B.; Wokadala, O.C.; Anyasi, T.A. Musa species variation, production, and the application of its processed flour: A review. Sci. Hortic. 2024, 325, 112688. [Google Scholar] [CrossRef]
- D’Amato, D.; Malkamäki, A.; Hogarth, N.J.; Baral, H. A descriptive plantation typology and coding system to aid the analysis of ecological and socio-economic outcomes. Curr. For. Rep. 2017, 3, 296–307. [Google Scholar] [CrossRef]
- Stragliotto, L.K.; Ferrari, G.T.; Campagnol, P.C.B.; Strasburg, V.J.; Zandonadi, R.P.; de Oliveira, V.R. Green banana by-products on the chemical, technological and sensory quality of meat products. Int. J. Gastron. Food Sci. 2022, 30, 100614. [Google Scholar] [CrossRef]
- Rebello, L.P.G.; Ramos, A.M.; Pertuzatti, P.B.; Barcia, M.T.; Castillo-Muñoz, N.; Hermosín-Gutiérrez, I. Flour of banana (Musa AAA) peel as a source of antioxidant phenolic compounds. Int. Food Res. J. 2014, 55, 397–403. [Google Scholar] [CrossRef]
- Justine, J.L.; Gastineau, R.; Gros, P.; Gey, D.; Ruzzier, E.; Charles, L.; Winsor, L. Hammerhead flatworms (Platyhelminthes, Geoplanidae, Bipaliinae): Mitochondrial genomes and description of two new species from France, Italy, and Mayotte. PeerJ 2022, 10, e12725. [Google Scholar] [CrossRef]
- Bernatova, I. Biological activities of (−)-epicatechin and (−)-epicatechin-containing foods: Focus on cardiovascular and neuropsychological health. Biotechnol. Adv. 2018, 36, 666–681. [Google Scholar] [CrossRef]
- Yusuf, I.; Flagiello, F.; Ward, N.I.; Arellano-García, H.; Avignone-Rossa, C.; Felipe-Sotelo, M. Valorisation of banana peels by hydrothermal carbonisation: Potential use of the hydrochar and liquid by-product for water purification and energy conversion. Bioresour. Technol. Rep. 2020, 12, 100582. [Google Scholar] [CrossRef]
- Borges, R.M.; Taujale, R.; de Souza, J.S.; de Andrade Bezerra, T.; E Silva, E.L.; Herzog, R.; Ponce, F.V.; Wolfender, J.-L.; Edison, A.S. Dereplication of plant phenolics using a mass-spectrometry database independent method. Phytochem. Anal. 2018, 29, 601–612. [Google Scholar] [CrossRef]
- Khalifa, M.K.; Abdel-Sattar, S.A.; Amin, O.M.; Kohaf, N.A.; Zaky, H.S.; Abd El-Fattah, M.A.; Mohammed, K.H.A.; Badawi, N.M.; Mansoor, I.; Eassa, H.A. Effectiveness of epigallocatechin gallate nanoparticles on the in-vivo treatment of Alzheimer’s disease in a rat/mouse model: A systematic review. DARU J. Pharm. Sci. 2023, 1–19. [Google Scholar] [CrossRef]
- Wang, J.; Gao, L.; Song, J.; Li, S. Study of EGCG composite hydrogel for the treatment of radiation-induced skin injuries. J. Appl. Biomater. Funct. Mater. 2023, 21, 1–12. [Google Scholar] [CrossRef]
- Legeay, S.; Rodier, M.; Fillon, L.; Faure, S.; Clere, N. Epigallocatechin gallate: A review of its beneficial properties to prevent metabolic syndrome. Nutrients 2015, 7, 5443–5468. [Google Scholar] [CrossRef]
- Wang, H.; Chen, N.; Feng, C.; Deng, Y.; Gao, Y. Research on efficient denitrification system based on banana peel waste in sequencing batch reactors: Performance, microbial behavior and dissolved organic matter evolution. Chemosphere 2020, 253, 126693. [Google Scholar] [CrossRef]
- Zaini, H.M.; Roslan, J.; Saallah, S.; Munsu, E.; Sulaiman, N.S.; Pindi, W. Banana peels as a bioactive ingredient and its potential application in the food industry. J. Funct. Foods 2022, 92, 105054. [Google Scholar] [CrossRef]
- Avram, I.; Gatea, F.; Vamanu, E. Functional Compounds from Banana Peel Used to Decrease Oxidative Stress Effects. Processes 2022, 10, 248. [Google Scholar] [CrossRef]
- Behiry, S.I.; Okla, M.K.; Alamri, S.A.; El-Hefny, M.; Salem, M.Z.; Alaraidh, I.A.; Ali, H.M.; Al-Ghtani, S.M.; Monroy, J.C.; Salem, A.Z. Antifungal and antibacterial activities of Musa paradisiaca L. peel extract: HPLC analysis of phenolic and flavonoid contents. Processes 2019, 7, 215. [Google Scholar] [CrossRef]
- Saeed, S.; Baig UU, R.; Tayyab, M.; Altaf, I.; Irfan, M.; Raza, S.Q.; Nadeem, F.; Mehmood, T. Valorization of banana peels waste into biovanillin and optimization of process parameters using submerged fermentation. Biocatal. Agric. Biotechnol. 2021, 36, 102154. [Google Scholar] [CrossRef]
- Tallapally, M.; Sadiq, A.S.; Mehtab, V.; Chilakala, S.; Vemula, M.; Chenna, S.; Upadhyayula, V. GC-MS based targeted metabolomics approach for studying the variations of phenolic metabolites in artificially ripened banana fruits. LWT 2020, 130, 109622. [Google Scholar] [CrossRef]
- González-Montelongo, R.; Lobo, M.G.; González, M. Antioxidant activity in banana peel extracts: Testing extraction conditions and related bioactive compounds. Food Chem. 2010, 119, 1030–1039. [Google Scholar] [CrossRef]
- Sigma-Aldrich Corporation. Available online: https://www.sigmaaldrich.com/BR/pt/search/rutin?focus=products&page=1&perpage=30&sort=relevance&term=rutin&type=product (accessed on 20 January 2024).
- Adooq Bioscience, L.L.C. Available online: https://www.adooq.com/rutin-rutoside.html (accessed on 22 January 2024).
- Biosynth: Life Science Products and Manufacturing. Available online: https://www.biosynth.com/advanced/search-results?searchtype=1&query=RUTIN (accessed on 21 January 2024).
- Ferreira, A.M.; Barros, G.S.B.; Wojeicchowski, J.P.; Coutinho, J.A. Valorizing banana peels by extracting rutin with hydrated organic acids. Food Chem. Adv. 2024, 4, 100612. [Google Scholar] [CrossRef]
- ICO—International Coffee Organization. Coffee Report and Outlook. 2023. Available online: https://icocoffee.org/pt/resources/public-market-information/ (accessed on 8 February 2024).
- Durán, C.A.; Tsukui, A.; Santos, F.K.; Martinez, S.T.; Bizzo, H.R.; Rezende, C. Coffee: General aspects and its use beyond drink. Rev. Virtual Quim. 2017, 9, 107–134. [Google Scholar] [CrossRef]
- Rosa, D.S.; Nunes, A.C.C.; Santos, L.J.M. A adequação ambiental na torrefação de café. Rev. GeTeC 2017, 6, 167–180. [Google Scholar]
- Nzekoue, F.K.; Borsetta, G.; Navarini, L.; Abouelenein, D.; Xiao, J.; Sagratini, G.; Vittori, S.; Caprioli, G.; Angeloni, S. Coffee silverskin: Characterization of B-vitamins, macronutrients, minerals and phytosterols. Food Chem. 2022, 372, 131188. [Google Scholar] [CrossRef] [PubMed]
- Machado, S.; Costa, A.S.; Pimentel, F.; Oliveira, M.B.P.P.; Alves, R.C. A study on the protein fraction of coffee silverskin: Protein/non-protein nitrogen and free and total amino acid profiles. Food Chem. 2020, 326, 126940. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Chemical, functional, and structural properties of spent coffee grounds and coffee silverskin. Food Bioprocess Technol. 2014, 7, 3493–3503. [Google Scholar] [CrossRef]
- Nzekoue, F.K.; Angeloni, S.; Navarini, L.; Angeloni, C.; Freschi, M.; Hrelia, S.; Vitali, L.A.; Sagratini, G.; Vittori, S.; Caprioli, G. Coffee silverskin extracts: Quantification of 30 bioactive compounds by a new HPLC-MS/MS method and evaluation of their antioxidant and antibacterial activities. Int. Food Res. J. 2020, 133, 109128. [Google Scholar] [CrossRef] [PubMed]
- IBGE—Instituto Brasileiro de Geografia e Estatística. Produção Agropecuária. Produção de Café. 2022. Available online: https://www.ibge.gov.br/explica/producao-agropecuaria/cafe/rj (accessed on 7 February 2024).
- Costa, A.S.; Alves, R.C.; Vinha, A.F.; Costa, E.; Costa, C.S.; Nunes, M.A.; Almeida, A.A.; Santos-Silva, A.; Oliveira, M.B.P.P. Nutritional, chemical and antioxidant/pro-oxidant profiles of silverskin, a coffee roasting by-product. Food Chem. 2018, 267, 28–35. [Google Scholar] [CrossRef]
- Dos Santos, F.K.F.; Silva, I.G.C.B.; Nunes Filho, A.L.; da Veiga Júnior, V.F. Coffee and Folk Medicine: Mechanisms and Activities. In Ethnobotany; CRC Press: Boca Raton, FL, USA, 2023; pp. 65–80. [Google Scholar]
- Dos Santos, F.K.F.; de Almeida Cavalcante, S.F.; Rezende, C.M.; Veiga-Junior, V.F. Extraction and enrichment of furanoid glycosylated diterpenes present in green coffee beans: New methodology in the QuEChERS concept. J. Food Compos. Anal. 2021, 104, 104142. [Google Scholar] [CrossRef]
- Lu, H.; Tian, Z.; Cui, Y.; Liu, Z.; Ma, X. Chlorogenic acid: A comprehensive review of the dietary sources, processing effects, bioavailability, beneficial properties, mechanisms of action, and future directions. CRFSFS 2020, 19, 3130–3158. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Machado, M.; Espírito Santo, L.; Machado, S.; Lobo, J.C.; Costa, A.S.; Oliveira, M.B.P.P.; Ferreira, H.; Alves, R.C. Bioactive Potential and Chemical Composition of Coffee By-Products: From Pulp to Silverskin. Foods 2023, 12, 2354. [Google Scholar] [CrossRef] [PubMed]
- Regazzoni, L.; Saligari, F.; Marinello, C.; Rossoni, G.; Aldini, G.; Carini, M.; Orioli, M. Coffee silver skin as a source of polyphenols: High resolution mass spectrometric profiling of components and antioxidant activity. J. Funct. Foods 2016, 20, 472–485. [Google Scholar] [CrossRef]
- Bresciani, L.; Calani, L.; Bruni, R.; Brighenti, F.; Del Rio, D. Phenolic composition, caffeine content and antioxidant capacity of coffee silverskin. Int. Food Res. J. 2014, 61, 196–201. [Google Scholar] [CrossRef]
- Spagnol, C.M.; Assis, R.P.; Brunetti, I.L.; Isaac, V.L.B.; Salgado, H.R.N.; Corrêa, M.A. In vitro methods to determine the antioxidant activity of caffeic acid. Spectrochim. Acta Part A SAA 2019, 219, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Saivish, M.V.; Pacca, C.C.; da Costa, V.G.; de Lima Menezes, G.; da Silva, R.A.; Nebo, L.; Dutra da Silva, G.C.; de Aguiar Milhim, B.H.G.; da Silva Teixeira, I.; Henrique, T.; et al. Caffeic Acid Has Antiviral Activity against Ilhéus Virus In Vitro. Viruses 2023, 15, 494. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Li, S.; Liu, X.; An, H.; Kang, X.; Guo, S. Caffeic acid, an active ingredient in coffee, combines with DOX for multitarget combination therapy of lung cancer. J. Agric. Food Chem. 2022, 70, 8326–8337. [Google Scholar] [CrossRef] [PubMed]
- Inácio, H.P.; Santetti, G.S.; Dacoreggio, M.V.; da Silva Haas, I.C.; Baranzelli, J.; Emanuelli, T.; Hoff, R.R.; Kempka, A.P.; Freire, C.B.F.; de Mello Castanho Amboni, R.D. Effects of different extraction methods on the phenolic profile, antioxidant and antimicrobial activity of the coffee grounds and coffee silverskin (Coffea arabica L.). JSFA Rep. 2023, 3, 354–363. [Google Scholar] [CrossRef]
- Rodrigues, R.; Oliveira, M.B.P.P.; Alves, R.C. Chlorogenic acids and caffeine from coffee by-products: A review on skincare applications. Cosmetics 2023, 10, 12. [Google Scholar] [CrossRef]
- Turan, M.; Mammadov, R. Overview of Characteristics of the Citrus Genus. In Overview on Horticulture; IKSAD: Ankara, Turkey, 2021; ISBN 978-625-7562-92-8. [Google Scholar]
- FAO—Food and Agriculture Organization. Citrus Fruit Statistical Compendium; FAO: Rome, Italy, 2020; pp. 1–40. [Google Scholar]
- Lucia, C.; Laudicina, V.A.; Badalucco, L.; Galati, A.; Palazzolo, E.; Torregrossa, M.; Viviani, G.; Corsino, S.F. Challenges and opportunities for citrus wastewater management and valorisation: A review. Environ. Manag. 2022, 321, 115924. [Google Scholar] [CrossRef] [PubMed]
- Russo, C.; Maugeri, A.; Lombardo, G.E.; Musumeci, L.; Barreca, D.; Rapisarda, A.; Cirmi, S.; Navarra, M. The Second Life of Citrus Fruit Waste: A Valuable Source of Bioactive Compounds. Molecules 2021, 26, 5991. [Google Scholar] [CrossRef] [PubMed]
- Kundu, D.; Banerjee, S.; Karmakar, S.; Banerjee, R. Valorization of citrus lemon wastes through biorefinery approach: An industrial symbiosis. Bioresour. Technol. Rep. 2021, 15, 100717. [Google Scholar] [CrossRef]
- Melchior, C.; dos Santos Franciscato, L.M.S.; Barbosa, V.A.; da Silva, M.R.; Bittencourt, P.R.S.; Barros, B.C.B.; Picolloto, A.M.; Moritz, C.M.F. Chemical composition and thermal properties of commercial essential oils and their antimicrobial and antioxidant activities. Res. Soc. Dev. 2023, 12, e15412139694. [Google Scholar] [CrossRef]
- Fakayode, O.A.; Abobi, K.E. Optimization of oil and pectin extraction from orange (Citrus sinensis) peels: A response surface approach. J. Anal. Sci. Technol. 2018, 9, 20. [Google Scholar] [CrossRef]
- Suárez, G.G.; Ramírez, J.A.; Castañón, J.F.; Galavíz, J.A.; Meléndez, P.C. Valorization of Albedo Orange Peel Waste to Develop Electrode Materials in Supercapacitors for the Electric Industry. J. Chem. 2021, 2021, 3022815. [Google Scholar] [CrossRef]
- Li, D.Q.; Li, J.; Dong, H.L.; Li, X.; Zhang, J.Q.; Ramaswamy, S.; Xu, F. Pectin in biomedical and drug delivery applications: A review. Int. J. Biol. Macromol. 2021, 185, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Singhal, S.; Swami Hulle, N.R. Citrus pectins: Structural properties, extraction methods, modifications and applications in food systems—A review. Appl. Food Res. 2022, 2, 100215. [Google Scholar] [CrossRef]
- Roman-Benn, A.; Contador, C.A.; Li, M.W.; Lam, H.M.; Ah-Hen, K.; Ulloa, P.E.; Ravanal, M.C. Pectin: An overview of sources, extraction and applications in food products and health. Food Chem. Adv. 2023, 2, 100192. [Google Scholar] [CrossRef]
- Suri, S.; Singh, A.; Nema, P.K. Current applications of citrus fruit processing waste: A scientific outlook. Appl. Food Res. 2022, 2, 100050. [Google Scholar] [CrossRef]
- IBGE—Instituto Brasileiro de Geografia e Estatística. Produção Agropecuária: Produção Vegetal. Available online: https://www.ibge.gov.br/explica/producao-agropecuaria/laranja/rj (accessed on 6 February 2024).
- Sigma-Aldrich Corporation. Available online: https://www.sigmaaldrich.com/BR/pt/product/sigma/p9135 (accessed on 5 February 2024).
- Gómez-Mejía, E.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. Citrus peels waste as a source of value-added compounds: Extraction and quantification of bioactive polyphenols. Food Chem. 2019, 295, 289–299. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, D.; Tan, C.; Hu, Y.; Sundararajan, B.; Zhou, Z. Profiling of flavonoid and antioxidant activity of fruit tissues from 27 Chinese local citrus cultivars. Plants 2020, 9, 196. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Rehman, M.T.; Ismael, M.A.; AlAjmi, M.F.; Alruwaished, G.I.; Alokail, M.S.; Khan, M.R. Bioflavonoid (hesperidin) restrains protein oxidation and advanced glycation end product formation by targeting AGEs and glycolytic enzymes. Cell Biochem. Biophys. 2021, 79, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Yao, X.; Zhou, Q.; Meng, X.; Zhou, T.; Gu, Q. Citrus peel flavonoid extracts: Health-beneficial bioactivities and regulation of intestinal microecology in vitro. Front. Nutr. 2022, 9, 888745. [Google Scholar] [CrossRef]
- Zaidun, N.H.; Thent, Z.C.; Abd Latiff, A. Combating oxidative stress disorders with citrus flavonoid: Naringenin. Life Sci. 2018, 208, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Stabrauskiene, J.; Kopustinskiene, D.M.; Lazauskas, R.; Bernatoniene, J. Naringin and naringenin: Their mechanisms of action and the potential anticancer activities. Biomedicines 2022, 10, 1686. [Google Scholar] [CrossRef]
- Sigma-Aldrich Corporation. Available online: https://www.sigmaaldrich.com/BR/pt/product/supelco/phl89707 (accessed on 5 February 2024).
- Tiwari, A.; Khawas, R. Food Waste and Agro By-Products: A Step towards Food Sustainability. In Innovation in the Food Sector Through the Valorization of Food and Agro-Food By-Products; IntechOpen: London, UK, 2021; pp. 1–15. [Google Scholar]
- Alves, R.C.; Rodrigues, F.; Nunes, M.A.; Vinha, A.F.; Oliveira, M.B.P. State of the art in coffee processing by-products. In Handbook of Coffee Processing by-Products; Academic Press: Cambridge, MA, USA, 2017; pp. 1–26. [Google Scholar]
- Ben-Othman, S.; Jõudu, I.; Bhat, R. Bioactives from Agri-Food Wastes: Present Insights and Future Challenges. Molecules 2020, 25, 510. [Google Scholar] [CrossRef] [PubMed]
- Do, Q.; Ramudhin, A.; Colicchia, C.; Creazza, A.; Li, D. A systematic review of research on food loss and waste prevention and management for the circular economy. Int. J. Prod. Econ. 2021, 239, 108209. [Google Scholar] [CrossRef]
- Suhartini, S.; Rohma, N.A.; Elviliana; Santoso, I.; Paul, R.; Listiningrum, P.; Melville, L. Food waste to bioenergy: Current status and role in future circular economies in Indonesia. Energy Ecol. Environ. 2022, 7, 297–339. [Google Scholar] [CrossRef]
- Nunes, L.J.R.; Causer, T.P.; Ciolkosz, D. Biomass for energy: A review on supply chain management models. Renew. Sustain. Energy Rev. 2020, 120, 109658. [Google Scholar] [CrossRef]
- Mishra, K.; Siwal, S.S.; Nayaka, S.C.; Guan, Z.; Thakur, V.K. Waste-to-chemicals: Green solutions for bioeconomy markets. Sci. Total Environ. 2023, 887, 164006. [Google Scholar] [CrossRef]
- Gontard, N.; Sonesson, U.; Birkved, M.; Majone, M.; Bolzonella, D.; Celli, A.; Angellier-Coussy, H.; Jang, G.-W.; Verniquet, A.; Broeze, J.; et al. A research challenge vision regarding management of agricultural waste in a circular bio-based economy. Crit. Rev. Environ. Sci. Technol. 2018, 48, 614–654. [Google Scholar] [CrossRef]
- Santos, B.L.P.; Vieira, I.M.M.; Ruzene, D.S.; Silva, D.P. Unlocking the potential of biosurfactants: Production, applications, market challenges, and opportunities for agro-industrial waste valorization. Environ. Res. 2024, 244, 117879. [Google Scholar] [CrossRef]
- Pradhan, S.; Sahoo, N.K.; Satapathy, S.; Mishra, S. A report on green extraction procedures for separation of flavonoids and its bio activities. J. Herb. Med. 2023, 41, 100716. [Google Scholar] [CrossRef]
- Mir-Cerdà, A.; Nuñez, O.; Granados, M.; Sentellas, S.; Saurina, J. An overview of the extraction and characterization of bioactive phenolic compounds from agri-food waste within the framework of circular bioeconomy. TrAC Trends Anal. Chem. 2023, 161, 116994. [Google Scholar] [CrossRef]
- Sarangi, P.K.; Vivekanand, V.; Mohanakrishna, G.; Pattnaik, B.; Muddapur, U.M.; Aminabhavi, T.M. Production of bioactive phenolic compounds from agricultural by-products towards bioeconomic perspectives. J. Clean. Prod. 2023, 414, 137460. [Google Scholar] [CrossRef]
- Zhu, K.; Ma, J.; Cong, J.; Zhang, T.; Lei, H.; Xu, H.; Luo, Z.; Li, M. The road to reuse of walnut by-products: A comprehensive review of bioactive compounds, extraction and identification methods, biomedical and industrial applications. Trends Food Sci. 2024, 143, 104264. [Google Scholar] [CrossRef]
- da Silva, L.C.; Viganó, J.; de Souza Mesquita, L.M.; Dias, A.L.B.; de Souza, M.C.; Sanches, V.L.; Chaves, J.O.; Pizani, R.S.; Contieri, L.S.; Rostagno, M.A. Recent advances and trends in extraction techniques to recover polyphenols compounds from apple by-products. Food Chem. X 2021, 12, 100133. [Google Scholar] [CrossRef] [PubMed]
- Ameer, K.; Shahbaz, H.M.; Kwon, J.H. Green extraction methods for polyphenols from plant matrices and their byproducts: A review. CRFSFS 2017, 16, 295–315. [Google Scholar] [CrossRef] [PubMed]
- Bajkacz, S.; Adamek, J. Evaluation of new natural deep eutectic solvents for the extraction of isoflavones from soy products. Talanta 2017, 168, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Paradiso, V.M.; Clemente, A.; Summo, C.; Pasqualone, A.; Caponio, F. Towards green analysis of virgin olive oil phenolic compounds: Extraction by a natural deep eutectic solvent and direct spectrophotometric detection. Food Chem. 2016, 212, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Galviz-Quezada, A.; Ochoa-Aristizábal, A.M.; Arias Zabala, M.E.; Ochoa, S.; Osorio-Tobón, J.F. Valorization of iraca (Carludovica palmata, Ruiz & Pav.) infructescence by ultrasound-assisted extraction: An economic evaluation. Food Bioprod. Process. 2019, 118, 91–102. [Google Scholar]
- Santana, A.P.R.; Andrade, D.F.; Mora-Vargas, J.A.; Amaral, C.D.B.; Oliveira, A.; Gonzalez, M.H. Natural deep eutectic solvents for sample preparation prior to elemental analysis by plasma-based techniques. Talanta 2019, 199, 361–369. [Google Scholar] [CrossRef]
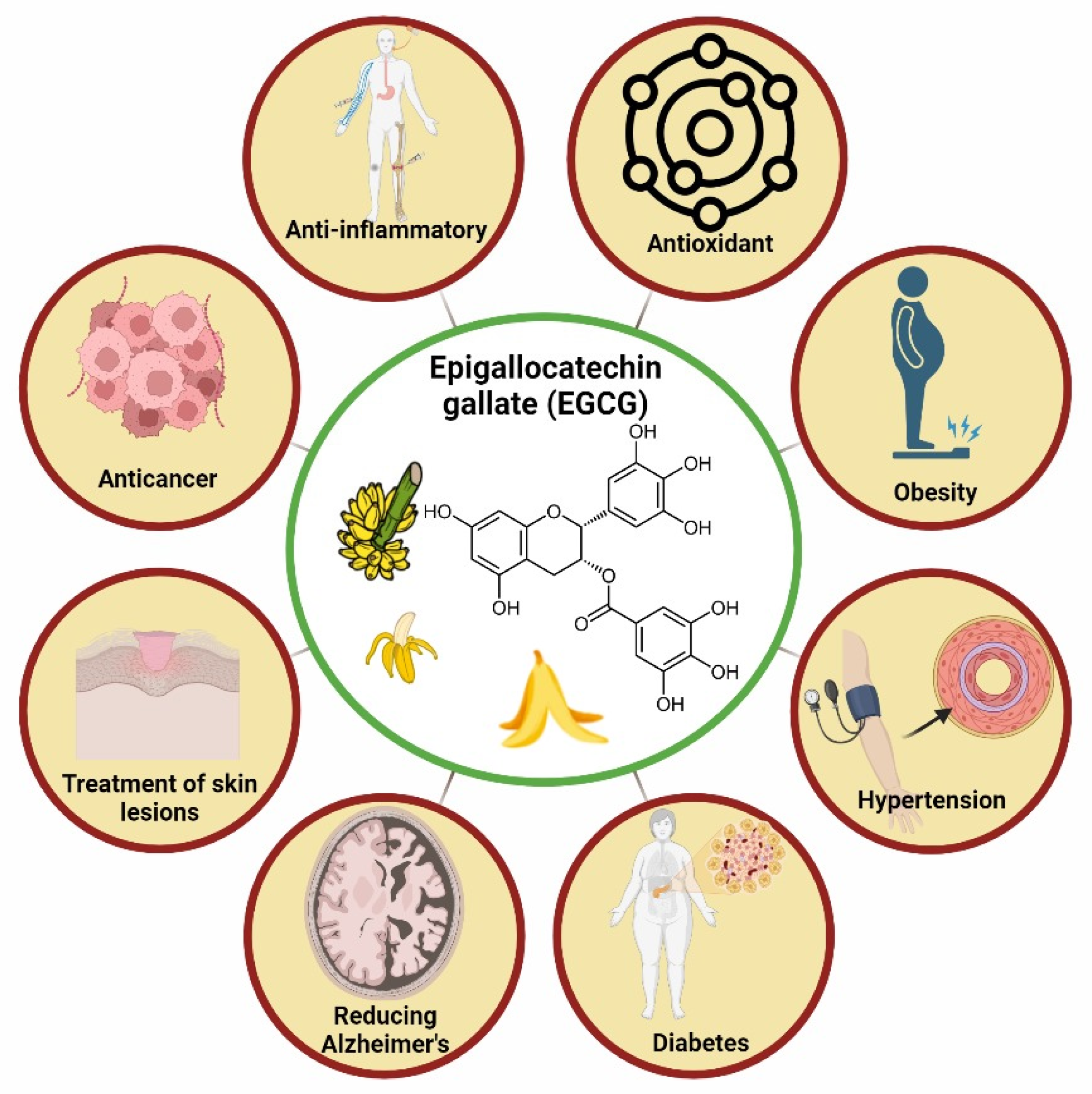
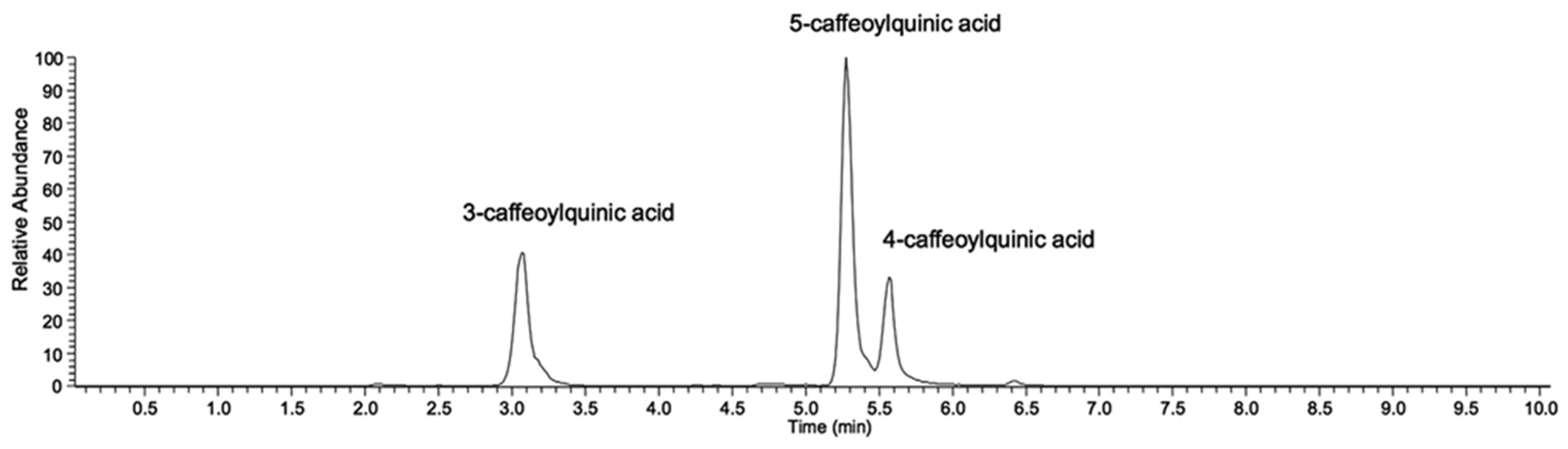

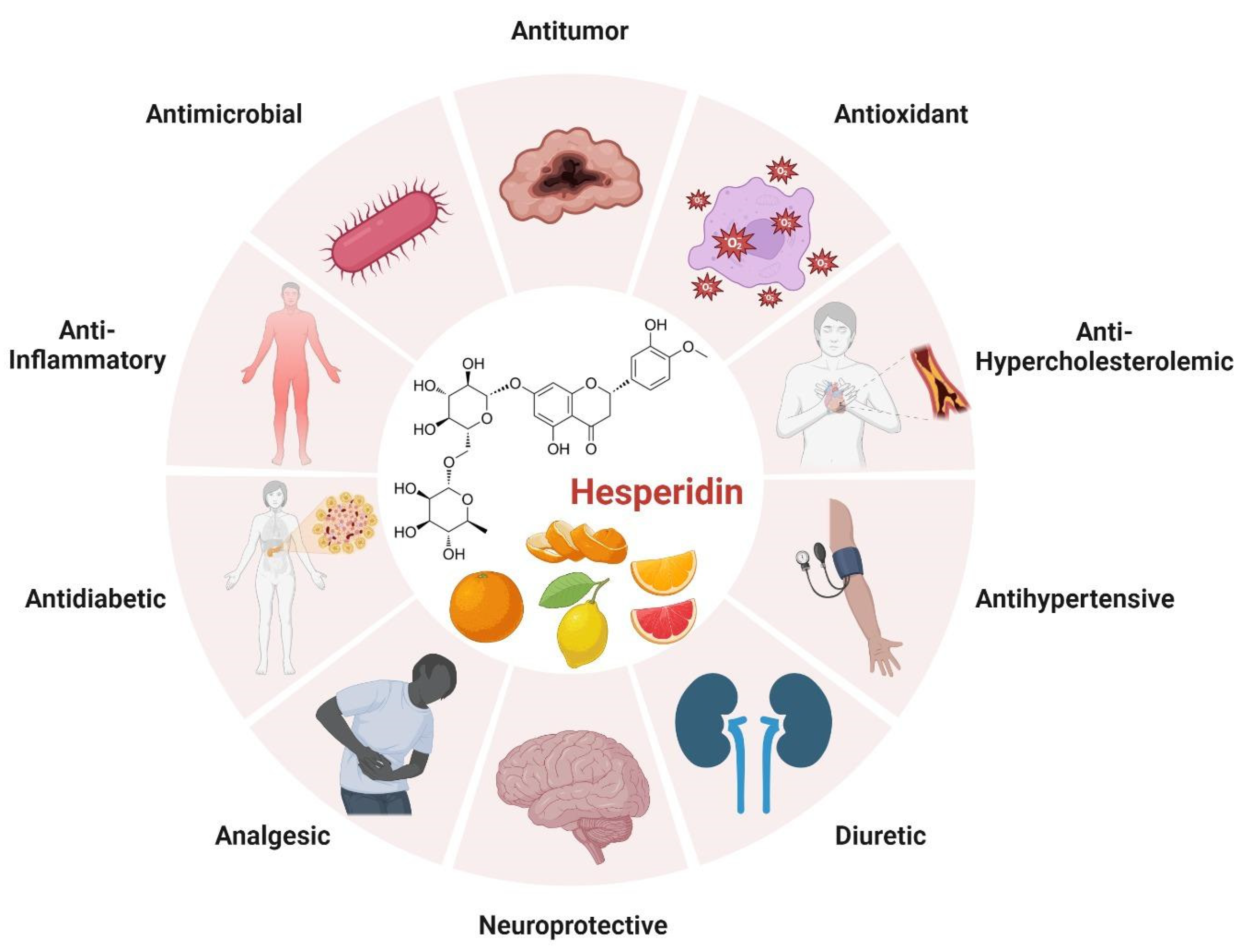
| Phenolic Compounds | Banana Peel | Quantity | References |
|---|---|---|---|
| Kaempferol | Red banana | 28.80 µg/mL | [68] |
| Yellow banana | 9.30 µg/mL | [68] | |
| Isoquercitrin | Red banana | 14.54 µg/mL | [68] |
| Yellow banana | 10.47 µg/mL | [68] | |
| Rutin | M. paradisiaca | 973.08 mg/100 g | [69] |
| Myricetin | 11.52 mg/100 g | ||
| Naringenin | 8.47 mg/100 g | ||
| Ferulic acid | 1.63 mg/100 g | ||
| Musa spp. | 60 mg/100 g | [70] | |
| Cinnamic acid | Karpooravalli | 1.93 ng/g | [71] |
| Alpha-hydroxycinnamic acid | 40.66 ng/g | ||
| Sinapic acid | 10.29 ng/g | ||
| p-Coumaric acid | 8.05 ng/g | ||
| Dopamine | Grande Naine | 1.72 mg/g | [72] |
| Gruesa | 1.17 mg/g | ||
| L-dopa | Grande Naine | 0.31 mg/g | |
| Gruesa | 0.56 mg/g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dos Santos, F.K.F.; Barcellos-Silva, I.G.C.; Leite-Barbosa, O.; Ribeiro, R.; Cunha-Silva, Y.; Veiga-Junior, V.F. High Added-Value by-Products from Biomass: A Case Study Unveiling Opportunities for Strengthening the Agroindustry Value Chain. Biomass 2024, 4, 217-242. https://doi.org/10.3390/biomass4020011
Dos Santos FKF, Barcellos-Silva IGC, Leite-Barbosa O, Ribeiro R, Cunha-Silva Y, Veiga-Junior VF. High Added-Value by-Products from Biomass: A Case Study Unveiling Opportunities for Strengthening the Agroindustry Value Chain. Biomass. 2024; 4(2):217-242. https://doi.org/10.3390/biomass4020011
Chicago/Turabian StyleDos Santos, Filipe Kayodè Felisberto, Ian Gardel Carvalho Barcellos-Silva, Odilon Leite-Barbosa, Rayssa Ribeiro, Yasmin Cunha-Silva, and Valdir Florencio Veiga-Junior. 2024. "High Added-Value by-Products from Biomass: A Case Study Unveiling Opportunities for Strengthening the Agroindustry Value Chain" Biomass 4, no. 2: 217-242. https://doi.org/10.3390/biomass4020011
APA StyleDos Santos, F. K. F., Barcellos-Silva, I. G. C., Leite-Barbosa, O., Ribeiro, R., Cunha-Silva, Y., & Veiga-Junior, V. F. (2024). High Added-Value by-Products from Biomass: A Case Study Unveiling Opportunities for Strengthening the Agroindustry Value Chain. Biomass, 4(2), 217-242. https://doi.org/10.3390/biomass4020011