Extraction and Depolymerization of Lignin from Pine Sawdust and Pistachio Shells
Abstract
1. Introduction
2. Materials
3. Experimental procedure:
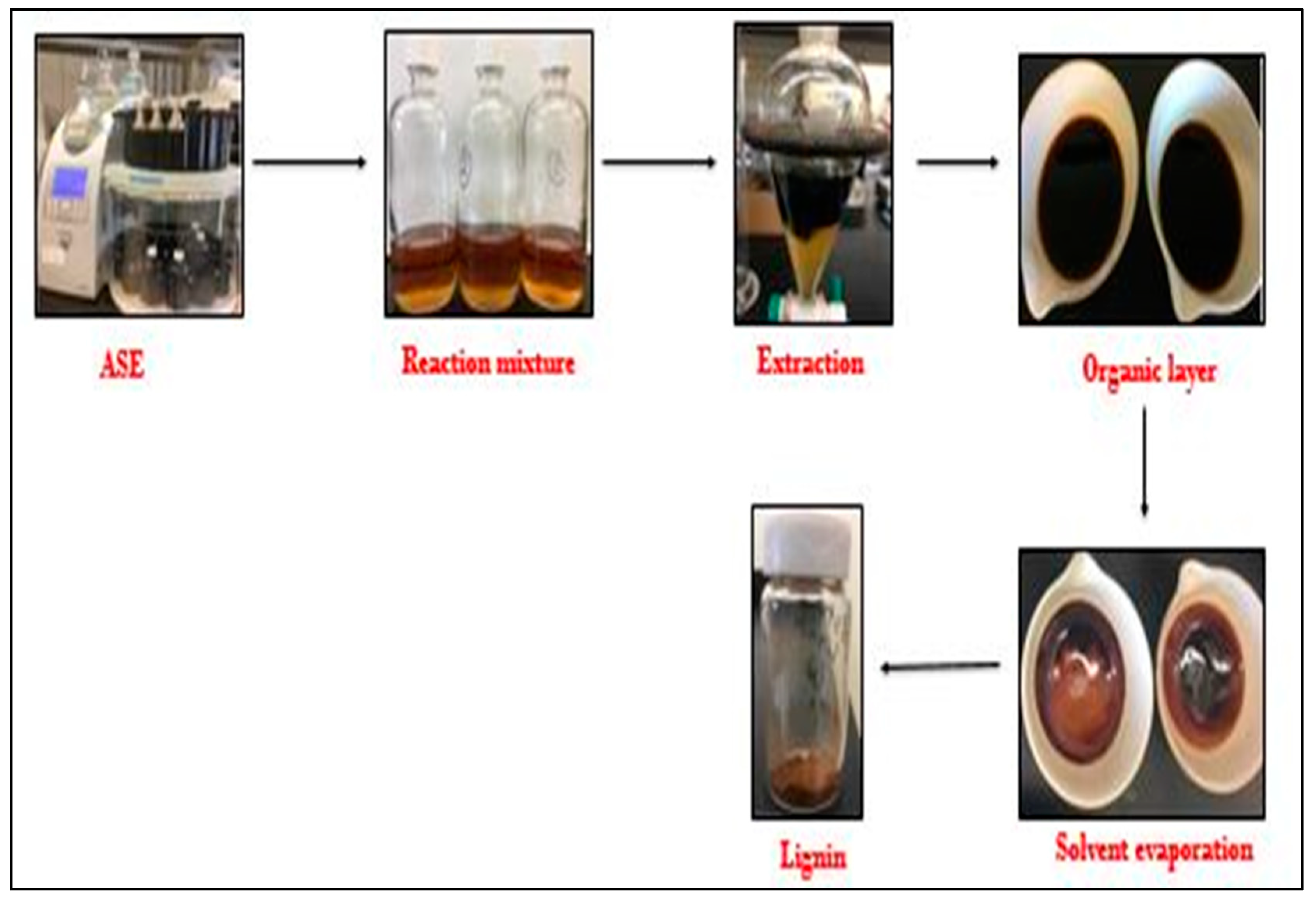
3.1. Extraction of Lignin from Pine Sawdust and Pistachio Shells
3.2. Depolymerization of Extracted Lignin from Pistachio Shells and Pine Sawdust
4. Results and Discussion
4.1. Extraction of Lignin from the Pine Sawdust and Pistachio Shell Biomass
4.2. Characterization of Extracted Lignin
4.2.1. FTIR Spectroscopy
4.2.2. NMR Spectroscopy
4.2.3. Thermogravimetric Analysis (TGA)
4.2.4. Identification of Phenolic Monomers Using GC-MS
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tursi, A. A review on biomass: Importance, chemistry, classification, and conversion. Biofuel Res. J. 2019, 6, 962. [Google Scholar] [CrossRef]
- Nanda, S.; Mohammad, J.; Reddy, S.N.; Kozinski, J.A.; Dalai, A.K. Pathways of lignocellulosic biomass conversion to renewable fuels. Biomass Convers. Biorefin. 2014, 4, 157–191. [Google Scholar] [CrossRef]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M.; Ladisch, M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, R.; von Wuehlisch, G. Mitigation of global warming through renewable biomass. Biomass Bioenergy 2013, 48, 75–89. [Google Scholar] [CrossRef]
- Lucas, M.; Macdonald, B.A.; Wagner, G.L.; Joyce, S.A.; Rector, K.D. Ionic Liquid Pretreatment of Poplar Wood at Room Temperature: Swelling and Incorporation of Nanoparticles. ACS Appl. Mater. Interfaces 2010, 2, 2198–2205. [Google Scholar] [CrossRef] [PubMed]
- Espinoza-Acosta, J.L.; Torres-Chávez, P.I.; Olmedo-Martínez, J.L.; Vega-Rios, A.; Flores-Gallardo, S.; Zaragoza-Contreras, E.A. Lignin in storage and renewable energy applications: A review. J. Energy Chem. 2018, 27, 1422–1438. [Google Scholar] [CrossRef]
- Lu, Y.; Lu, Y.C.; Hu, H.Q.; Xie, F.J.; Wei, X.Y.; Fan, X. Structural characterization of lignin and its degradation products with spectroscopic methods. J. Spectrosc. 2017, 2017, 8951658. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K.; Raghavan, P.; Kessler, M.R. Progress in green polymer composites from lignin for multifunctional applications: A review. ACS Sustain. Chem. Eng. 2014, 2, 1072–1092. [Google Scholar] [CrossRef]
- Pandey, M.P.; Kim, C.S. Lignin Depolymerization and Conversion: A Review of Thermochemical Methods. Chem. Eng. Technol. 2011, 34, 29–41. [Google Scholar] [CrossRef]
- Deuss, P.J.; Scott, M.; Tran, F.; Westwood, N.J.; de Vries, J.G.; Barta, K. Aromatic Monomers by in Situ Conversion of Reactive Intermediates in the Acid-Catalyzed Depolymerization of Lignin. J. Am. Chem. Soc. 2015, 137, 7456–7467. [Google Scholar] [CrossRef]
- Erdocia, X.; Prado, R.; Corcuera, M.A.; Labidi, J. Base catalyzed depolymerization of lignin: Influence of organosolv lignin nature. Biomass Bioenergy 2014, 66, 379–386. [Google Scholar] [CrossRef]
- Gall, D.L.; Kontur, W.S.; Lan, W.; Kim, H.; Li, Y.; Ralph, J.; Donohue, T.J.; Noguera, D.R. In vitro enzymatic depolymerization of lignin with release of syringyl, guaiacyl, and tricin units. Appl. Environ. Microbiol. 2018, 84, e02076-17. [Google Scholar] [CrossRef] [PubMed]
- Stärk, K.; Taccardi, N.; Bösmann, A.; Wasserscheid, P. Oxidative Depolymerization of Lignin in Ionic Liquids. ChemSusChem 2010, 3, 719–723. [Google Scholar] [CrossRef]
- Gosselink, R.J.; Teunissen, W.; van Dam, J.E.; de Jong, E.; Gellerstedt, G.; Scott, E.L.; Sanders, J.P. Lignin depolymerisation in supercritical carbon dioxide/acetone/water fluid for the production of aromatic chemicals. Bioresour. Technol. 2012, 106, 173–177. [Google Scholar] [CrossRef]
- Chio, C.; Sain, M.; Qin, W. Lignin utilization: A review of lignin depolymerization from various aspects. Renew. Sustain. Energy Rev. 2019, 107, 232–249. [Google Scholar] [CrossRef]
- Jadhav, B.; Roy, R.; Rahman, S.; Amit, T.A.; Subedi, S.; Hummel, M.; Gu, Z.; Raynie, D.E. Enhancing the Efficacy of the Subcritical Water-Based Alkali Lignin Depolymerization by Optimizing the Reaction Conditions and Using Heterogeneous Catalysts. Biomass 2022, 2, 178–187. [Google Scholar] [CrossRef]
- Muley, P.D.; Henkel, C.; Abdollahi, K.K.; Marculescu, C.; Boldor, D. A critical comparison of pyrolysis of cellulose, lignin, and pine sawdust using an induction heating reactor. Energy Convers. Manag. 2016, 117, 273–280. [Google Scholar] [CrossRef]
- Stoffel, R.B.; Neves, P.V.; Felissia, F.E.; Ramos, L.P.; Gassa, L.M.; Area, M.C. Hemicellulose extraction from slash pine sawdust by steam explosion with sulfuric acid. Biomass Bioenergy 2017, 107, 93–101. [Google Scholar] [CrossRef]
- Watkins, D.; Nuruddin, M.; Hosur, M.; Tcherbi-Narteh, A.; Jeelani, S. Extraction and characterization of lignin from different biomass resources. J. Mater. Res. Technol. 2015, 4, 26–32. [Google Scholar] [CrossRef]
- FAOSTAT. Food and Agriculture Data. 2018. Available online: http://www.fao.org/faostat/en/#home (accessed on 1 September 2022).
- Kasiri, N.; Fathi, M. Production of cellulose nanocrystals from pistachio shells and their application for stabilizing Pickering emulsions. Int. J. Biol. Macromol. 2018, 106, 1023–1031. [Google Scholar] [CrossRef]
- Ghasemynasabparizi, M.; Ahmadi, A.; Mazloomi, S. A review on pistachio: Its composition and benefits regarding the prevention or treatment of diseases. J. Occup. Health Epidemiol. 2015, 4, 57–69. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Hao, J.; Wang, W. Study of Almond Shell Characteristics. Materials 2018, 11, 1782. [Google Scholar] [CrossRef] [PubMed]
- Achinivu, E.C. Protic Ionic Liquids for Lignin Extraction—A Lignin Characterization Study. Int. J. Mol. Sci. 2018, 19, 428. [Google Scholar] [CrossRef] [PubMed]
- Obst, J.R.; Kirk, T.K. Isolation of lignin. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1988; pp. 3–12. [Google Scholar]
- Sun, R.; Tomkinson, J.; Jones, G.L. Fractional characterization of ash-AQ lignin by successive extraction with organic solvents from oil palm EFB fibre. Polym. Degrad. Stab. 2000, 68, 111–119. [Google Scholar] [CrossRef]
- Rahman, M.S.; Roy, R.; Jadhav, B.; Hossain, M.N.; Halim, M.A.; Raynie, D.E. Formulation, structure, and applications of therapeutic and amino acid-based deep eutectic solvents: An overview. J. Mol. Liq. 2021, 321, 114745. [Google Scholar] [CrossRef]
- Ma’Ruf, A.; Pramudono, B.; Aryanti, N. Lignin isolation process from rice husk by alkaline hydrogen peroxide: Lignin and silica extracted. AIP Conf. Proc. 2017, 1823, 020013. [Google Scholar]
- Riyadi, R.; Masyithah, Z.; Hutagalung, A.; Haryanto, B. The effect of temperature and reaction time on lignin content of Imperata Cylindrica. AIP Conf. Proc. 2021, 2342, 090001. [Google Scholar]
- Roy, R.; Jadhav, B.; Rahman, S.; Raynie, D.E. Characterization of residue from catalytic hydrothermal depolymerization of lignin. Curr. Res. Green Sustain. Chem. 2021, 4, 100052. [Google Scholar] [CrossRef]

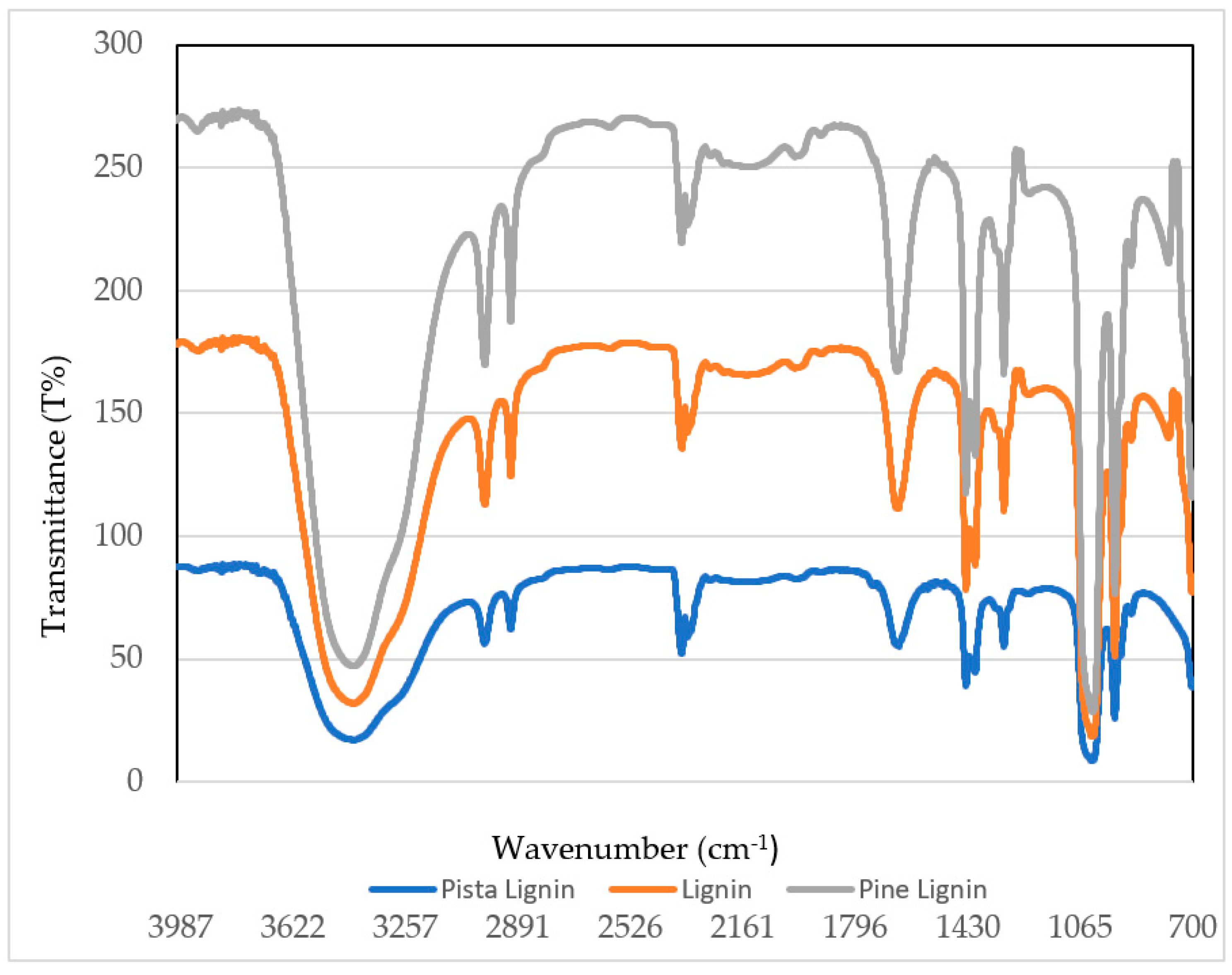




| Absorption Bands (cm−1) | Functional Groups |
|---|---|
| 3445 | O-H stretching vibration due to alcohols |
| 2358–2997 | C-H stretching in methyl and methylene groups |
| 1660.76 | C=O stretching in aromatic carbonyl |
| 1437.15 | Aliphatic CH2 vibrations |
| 1407.46 | Aromatic skeletal vibrations and C-H in-plane deformation |
| 1311.38 | Aliphatic C-H stretch in CH3 |
| 1056.16 | Aliphatic ether C-O and alcohol C-O stretching |
| 954.53 | Aromatic C-H out-of-plane deformation |
| Chemical Shift (ppm) | Functional Group |
|---|---|
| 6.5–7.0 | Aromatic protons |
| 5.75–6.25 | H-α with α-O-Ac in β-O-4 |
| 3.95–4.50 | H-γ in β-O-4, β-5, β-1, and β-β protons |
| 3.0–4.0 | -OCH3 protons |
| 2.50–3.55 | H-β in β-1 and β-β protons |
| 2.20–2.50 | H-in Ar-OAc |
| 1.50–2.20 | H-in aliphatic-OAc and Ar-OAc in 5-5 units |
| No | Retention Time (min) | Phenolic Monomer | Abundance (%) |
|---|---|---|---|
| 1 | 3.318 | Phenol | 0.63 |
| 2 | 4.429 | Guaiacol | 1.15 |
| 3 | 7.484 | Syringol | 2.02 |
| 4 | 7.674 | m-Hydroxy benzaldehyde | 2.23 |
| 5 | 7.948 | Vanillin | 5.52 |
| 6 | 8.548 | Propyl guaiacol | 1.95 |
| 7 | 10.584 | Syrinzaldehyde | 12.82 |
| 8 | 10.958 | Methoxy eugenol | 2.41 |
| 9 | 11.290 | Coniferaldehyde | 4.13 |
| 10 | 11.545 | Synapyl alcohol | 3.85 |
| 11 | 12.050 | Trimethoxy benzyl alcohol | 4.93 |
| 12 | 13.569 | Synapaldehyde | 3.51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jadhav, B.; Roy, R.; Rahman, M.S.; Raynie, D.E. Extraction and Depolymerization of Lignin from Pine Sawdust and Pistachio Shells. Biomass 2022, 2, 348-357. https://doi.org/10.3390/biomass2040023
Jadhav B, Roy R, Rahman MS, Raynie DE. Extraction and Depolymerization of Lignin from Pine Sawdust and Pistachio Shells. Biomass. 2022; 2(4):348-357. https://doi.org/10.3390/biomass2040023
Chicago/Turabian StyleJadhav, Balawanthrao, Ranen Roy, Md Sajjadur Rahman, and Douglas E. Raynie. 2022. "Extraction and Depolymerization of Lignin from Pine Sawdust and Pistachio Shells" Biomass 2, no. 4: 348-357. https://doi.org/10.3390/biomass2040023
APA StyleJadhav, B., Roy, R., Rahman, M. S., & Raynie, D. E. (2022). Extraction and Depolymerization of Lignin from Pine Sawdust and Pistachio Shells. Biomass, 2(4), 348-357. https://doi.org/10.3390/biomass2040023






