Emerging and Current Biologics for the Treatment of Intracranial Aneurysms
Abstract
1. Introduction
2. Clinical Relevance
3. Pathogenesis
4. Management History
5. Clinical Presentation
6. Diagnostic Techniques
7. Current Management
7.1. Medical Management
7.2. Surgical Treatment
7.3. Endovascular Treatment
8. Emerging Biologics for the Treatment of Intracranial Aneurysms
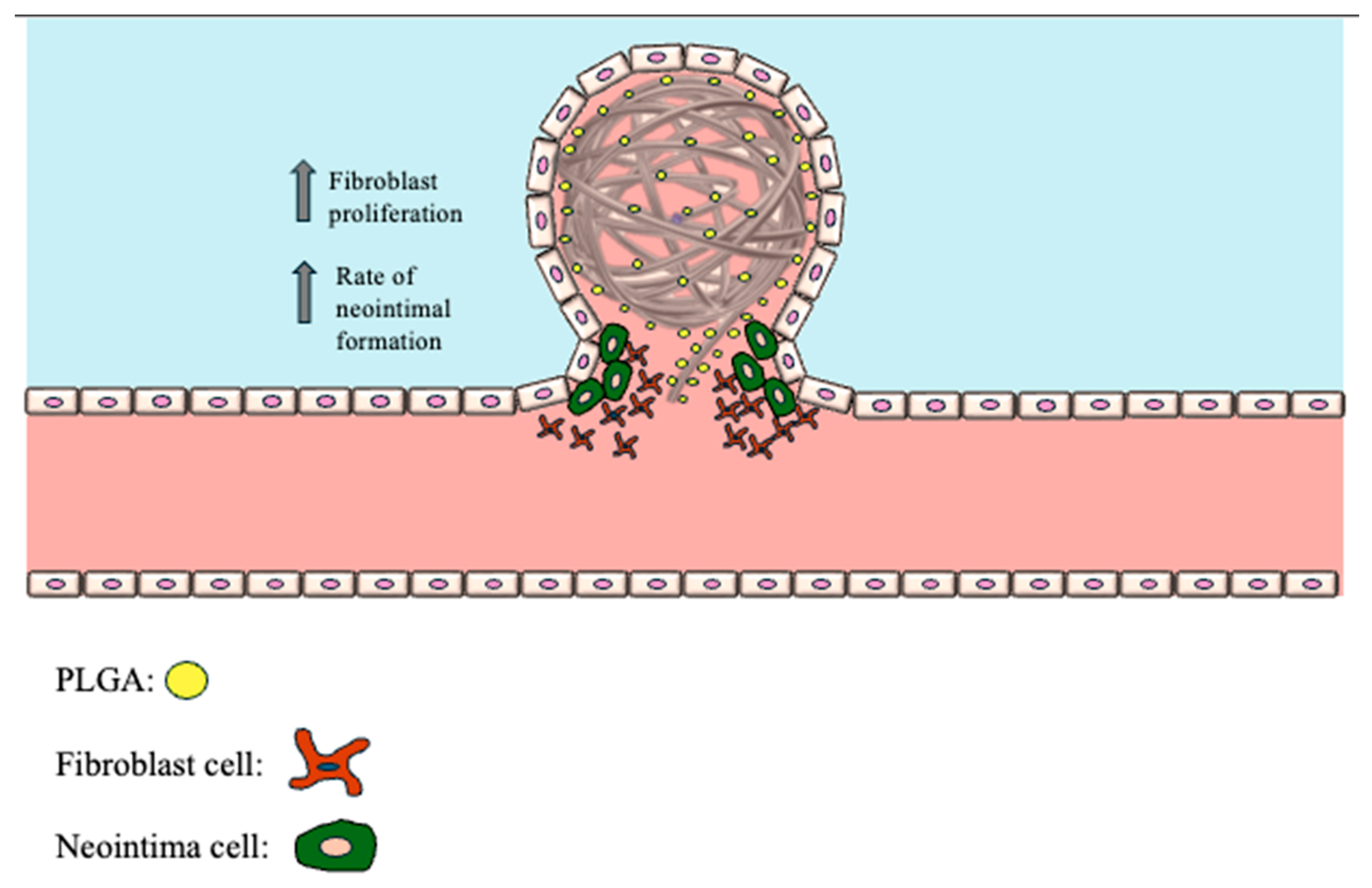
8.1. PLGA-Coated Coils
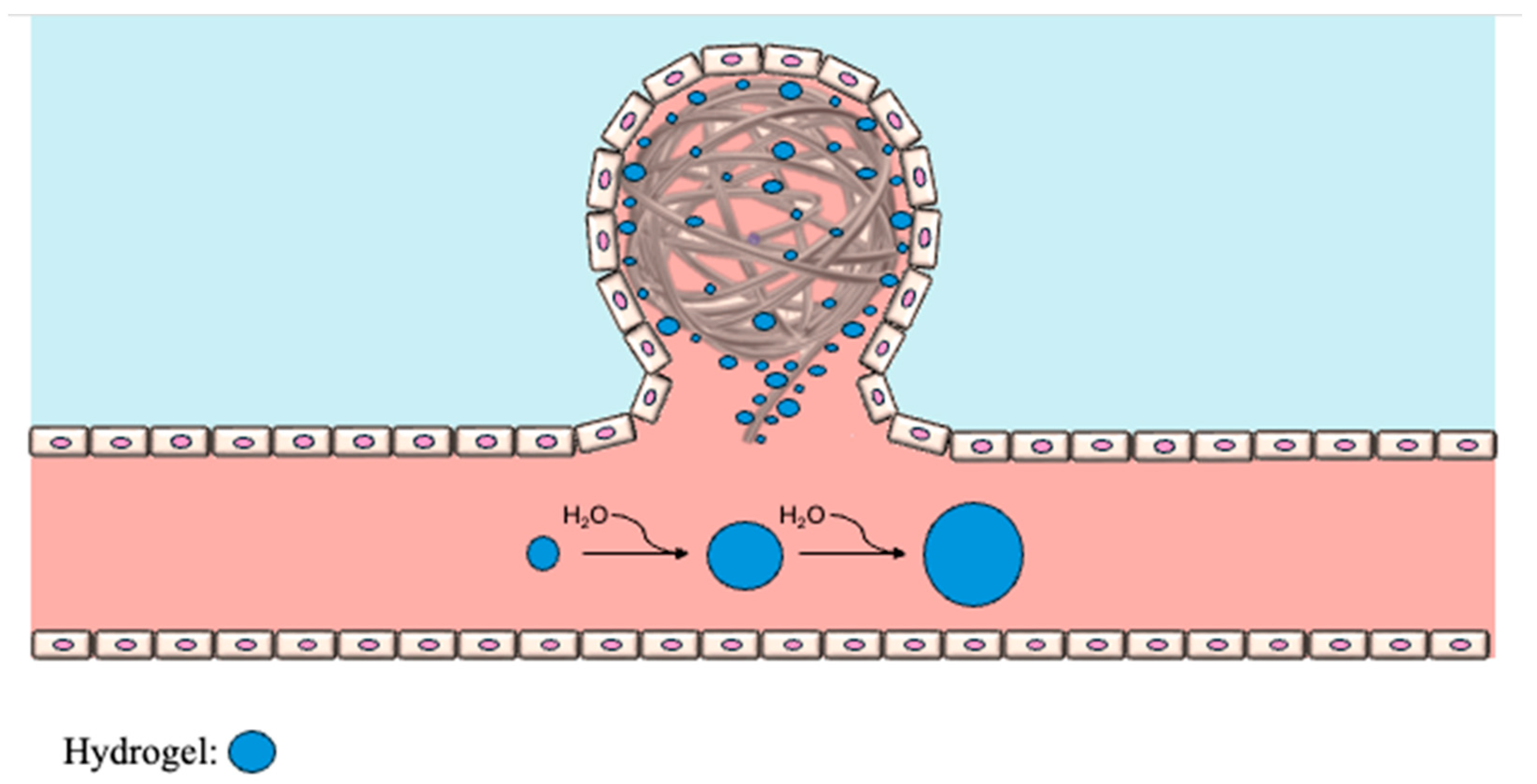
8.2. Hydrogel-Coated Coils
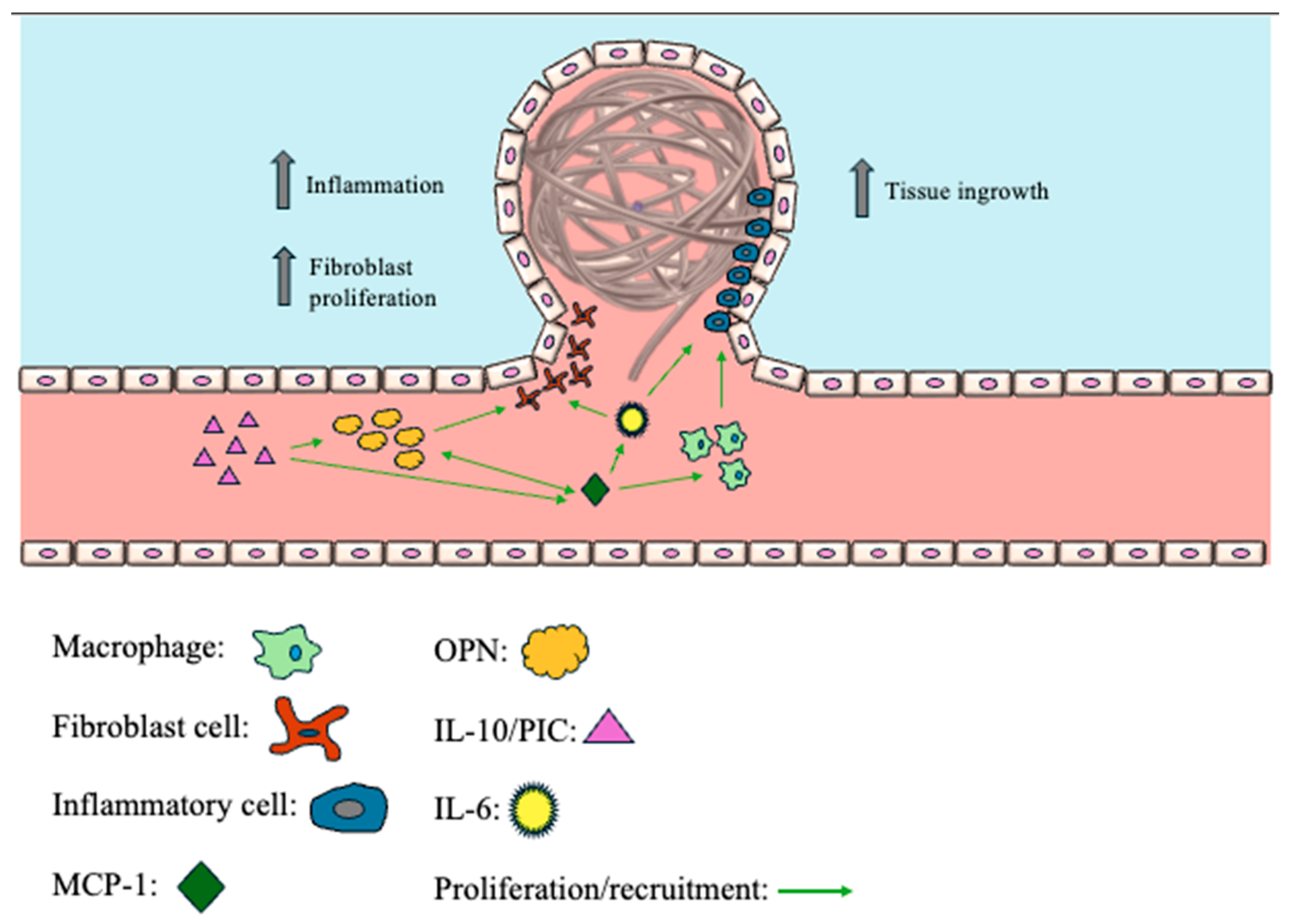
8.3. Monocyte Chemoattractant Protein-1 (MCP-1)
8.4. Osteopontin (OPN)
8.5. Interleukin-6/Interleukin-10 (IL-6/IL-10)
8.6. Priming the System
9. Future Directions
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Keedy, A. An overview of intracranial aneurysms. Mcgill J. Med. 2006, 9, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Mercadante, A.A.; Raja, A. Anatomy, Arteries. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2024. [Google Scholar]
- Merritt, W.C.; Berns, H.F.; Ducruet, A.F.; Becker, T.A. Definitions of intracranial aneurysm size and morphology: A call for standardization. Surg. Neurol. Int. 2021, 12, 506. [Google Scholar] [CrossRef] [PubMed]
- Barletta, E.; Ricci, R.; Silva, R.G.; Gaspar, R.M.L.; Araújo, J.M.; Neves, M.F.; de Aquino, J.B.; Belsuzarri, T.B. Fusiform aneurysms: A review from its pathogenesis to treatment options. Surg. Neurol. Int. 2018, 9, 189. [Google Scholar] [CrossRef] [PubMed]
- Fatterpekar, G.; Naidich, T.P.; Som, P.M. The Teaching Files: Brain and Spine; Elsevier/Saunders: Philadelphia, PA, USA, 2011. [Google Scholar]
- Majeed, H.; Ahmad, F. Mycotic Aneurysm. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2024. [Google Scholar]
- Vlak, M.H.; Algra, A.; Brandenburg, R.; Rinkel, G.J. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: A systematic review and meta-analysis. Lancet Neurol. 2011, 10, 626–636. [Google Scholar] [CrossRef]
- Lawton, M.T.; Vates, G.E. Subarachnoid Hemorrhage. N. Engl. J. Med. 2017, 377, 257–266. [Google Scholar] [CrossRef] [PubMed]
- van der Kamp, L.T.; Rinkel, G.J.E.; Verbaan, D.; Berg, R.v.D.; Vandertop, W.P.; Murayama, Y.; Ishibashi, T.; Lindgren, A.; Koivisto, T.; Teo, M.; et al. Risk of Rupture after Intracranial Aneurysm Growth. JAMA Neurol. 2021, 78, 1228–1235. [Google Scholar] [CrossRef]
- Greving, J.P.; Wermer, M.J.H.; Brown, R.D., Jr.; Morita, A.; Juvela, S.; Yonekura, M.; Ishibashi, T.; Torner, J.C.; Nakayama, T.; Rinkel, G.J.E.; et al. Development of the PHASES score for prediction of risk of rupture of intracranial aneurysms: A pooled analysis of six prospective cohort studies. Lancet Neurol. 2014, 13, 59–66. [Google Scholar] [CrossRef]
- Sforza, D.M.; Putman, C.M.; Cebral, J.R. Computational fluid dynamics in brain aneurysms. Int. J. Numer. Method Biomed. Eng. 2012, 28, 801–808. [Google Scholar] [CrossRef]
- Guo, H.; Yang, S.-T.; Wang, J.-W.; Li, H.; Gao, B.-L.; Li, C.-H. High hemodynamic stresses induce aneurysms at internal carotid artery bends. Medicine 2023, 102, e34587. [Google Scholar] [CrossRef]
- Katritsis, D.; Kaiktsis, L.; Chaniotis, A.; Pantos, J.; Efstathopoulos, E.P.; Marmarelis, V. Wall shear stress: Theoretical considerations and methods of measurement. Prog. Cardiovasc. Dis. 2007, 49, 307–329. [Google Scholar] [CrossRef]
- de Falco, F.A. Sentinel headache. Neurol. Sci. 2004, 25 (Suppl. 3), S215–S217. [Google Scholar] [CrossRef] [PubMed]
- Dott, N.M. Intracranial Aneurysms: Cerebral Arterio-Radiography: Surgical Treatment. Edinb. Med. J. 1933, 40, T219–T240. [Google Scholar] [PubMed]
- Yasargil, M.G.; Fox, J.L. The microsurgical approach to intracranial aneurysms. Surg. Neurol. 1975, 3, 7–14. [Google Scholar] [PubMed]
- Lee, K.S.; Zhang, J.J.Y.; Nguyen, V.; Han, J.; Johnson, J.N.; Kirollos, R.; Teo, M. The evolution of intracranial aneurysm treatment techniques and future directions. Neurosurg. Rev. 2022, 45, 1–25. [Google Scholar] [CrossRef]
- Loewenstein, J.E.; Gayle, S.C.; Duffis, E.J.; Prestigiacomo, C.J.; Gandhi, C.D. The natural history and treatment options for unruptured intracranial aneurysms. Int. J. Vasc. Med. 2012, 2012, 898052. [Google Scholar] [CrossRef]
- Tawk, R.G.; Hasan, T.F.; D’souza, C.E.; Peel, J.B.; Freeman, W.D. Diagnosis and Treatment of Unruptured Intracranial Aneurysms and Aneurysmal Subarachnoid Hemorrhage. Mayo Clin. Proc. 2021, 96, 1970–2000. [Google Scholar] [CrossRef]
- Chappell, E.T.; Moure, F.C.; Good, M.C. Comparison of computed tomographic angiography with digital subtraction angiography in the diagnosis of cerebral aneurysms: A meta-analysis. Neurosurgery 2003, 52, 624–631; discussion 630–631. [Google Scholar] [CrossRef]
- White, P.M.; Wardlaw, J.M.; Easton, V. Can noninvasive imaging accurately depict intracranial aneurysms? A systematic review. Radiology 2000, 217, 361–370. [Google Scholar] [CrossRef]
- Sailer, A.M.; Wagemans, B.A.; Nelemans, P.J.; de Graaf, R.; van Zwam, W.H. Diagnosing intracranial aneurysms with MR angiography: Systematic review and meta-analysis. Stroke 2014, 45, 119–126. [Google Scholar] [CrossRef]
- Yoon, D.Y.; Lim, K.J.; Choi, C.S.; Cho, B.M.; Oh, S.M.; Chang, S.K. Detection and characterization of intracranial aneurysms with 16-channel multidetector row CT angiography: A prospective comparison of volume-rendered images and digital subtraction angiography. AJNR Am. J. Neuroradiol. 2007, 28, 60–67. [Google Scholar]
- Teksam, M.; McKinney, A.; Casey, S.; Asis, M.; Kieffer, S.; Truwit, C.L. Multi-section CT angiography for detection of cerebral aneurysms. AJNR Am. J. Neuroradiol. 2004, 25, 1485–1492. [Google Scholar] [PubMed]
- Kucukay, F.; Okten, R.S.; Tekiner, A.; Dagli, M.; Gocek, C.; Bayar, M.A.; Cumhur, T. Three-dimensional volume rendering digital subtraction angiography in comparison with two-dimensional digital subtraction angiography and rotational angiography for detecting aneurysms and their morphological properties in patients with subarachnoid hemorrhage. Eur. J. Radiol. 2012, 81, 2794–2800. [Google Scholar] [CrossRef] [PubMed]
- Rinkel, G.J. Intracranial aneurysm screening: Indications and advice for practice. Lancet Neurol. 2005, 4, 122–128. [Google Scholar] [CrossRef]
- De Simone, M.; Fontanella, M.M.; Choucha, A.; Schaller, K.; Machi, P.; Lanzino, G.; Bijlenga, P.; Kurz, F.T.; Lövblad, K.-O.; De Maria, L. Current and Future Applications of Arterial Spin Labeling MRI in Cerebral Arteriovenous Malformations. Biomedicines 2024, 12, 753. [Google Scholar] [CrossRef]
- Flemming, K.D.; Lanzino, G. Management of Unruptured Intracranial Aneurysms and Cerebrovascular Malformations. Continuum 2017, 23, 181–210. [Google Scholar] [CrossRef]
- Muirhead, W.R.; Grover, P.J.; Toma, A.K.; Stoyanov, D.; Marcus, H.J.; Murphy, M. Adverse intraoperative events during surgical repair of ruptured cerebral aneurysms: A systematic review. Neurosurg. Rev. 2021, 44, 1273–1285. [Google Scholar] [CrossRef]
- Wiebers, D.O.; International Study of Unruptured Intracranial Aneurysms Investigators. Unruptured intracranial aneurysms: Natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 2003, 362, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Lin, H.; Summers, R.; Yang, M.; Cousins, B.G.; Tsui, J. Current Treatment Strategies for Intracranial Aneurysms: An Overview. Angiology 2018, 69, 17–30. [Google Scholar] [CrossRef]
- Li, H.; Pan, R.; Wang, H.; Rong, X.; Yin, Z.; Milgrom, D.P.; Shi, X.; Tang, Y.; Peng, Y. Clipping versus coiling for ruptured intracranial aneurysms: A systematic review and meta-analysis. Stroke 2013, 44, 29–37. [Google Scholar] [CrossRef]
- Sanai, N.; Zador, Z.; Lawton, M.T. Bypass surgery for complex brain aneurysms: An assessment of intracranial-intracranial bypass. Neurosurgery 2009, 65, 670–683; discussion 683. [Google Scholar] [CrossRef]
- Koch, M.J.; Stapleton, C.J.; Charbel, F.T.; Russin, J.; Amin-Hanjani, S. Intracranial-Intracranial Bypass for Aneurysms: Quantitative Intraoperative Assessment of Flow Preservation. Oper. Neurosurg. 2022, 22, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Guglielmi, G.; Viñuela, F.; Dion, J.; Duckwiler, G. Electrothrombosis of saccular aneurysms via endovascular approach. Part 2: Preliminary clinical experience. J. Neurosurg. 1991, 75, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Pierot, L.; Wakhloo, A.K. Endovascular treatment of intracranial aneurysms: Current status. Stroke 2013, 44, 2046–2054. [Google Scholar] [CrossRef]
- Laurent, D.; Lucke-Wold, B.; Dodd, W.S.; Martinez, M.; Chowdhury, M.A.B.; Hosaka, K.; Motwani, K.; Hoh, B. Combination release of chemokines from coated coils to target aneurysm healing. J. NeuroInterventional Surg. 2023, 15, 689–694. [Google Scholar] [CrossRef]
- Hosaka, K.; Rojas, K.; Fazal, H.Z.; Schneider, M.B.; Shores, J.; Federico, V.; McCord, M.; Lin, L.; Hoh, B. Monocyte Chemotactic Protein-1-Interleukin-6-Osteopontin Pathway of Intra-Aneurysmal Tissue Healing. Stroke 2017, 48, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- Hudson, J.S.; Hoyne, D.S.; Hasan, D.M. Inflammation and human cerebral aneurysms: Current and future treatment prospects. Future Neurol. 2013, 8, 663–676. [Google Scholar] [CrossRef]
- Vance, A.; Welch, B.G. The utility of bioactive coils in the embolization of aneurysms. Neurol. Res. 2014, 36, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jiang, G.; Song, Z.; Cheng, W.; Wu, W.; Chen, Z.; Wang, Z.; You, W.; Chen, G. Efficacy and Safety of Different Bioactive Coils in Intracranial Aneurysm Interventional Treatment, a Systematic Review and Bayesian Network Meta-Analysis. Brain Sci. 2022, 12, 1062. [Google Scholar] [CrossRef]
- Chen, J.; Yang, L.; Chen, Y.; Zhang, G.; Fan, Z. Controlled release of osteopontin and interleukin-10 from modified endovascular coil promote cerebral aneurysm healing. J. Neurol. Sci. 2016, 360, 13–17. [Google Scholar] [CrossRef]
- Kim, S.; Nowicki, K.W.; Ye, S.; Jang, K.; Elsisy, M.; Ibrahim, M.; Chun, Y.; Gross, B.A.; Friedlander, R.M.; Wagner, W.R. Bioabsorbable, elastomer-coated magnesium alloy coils for treating saccular cerebrovascular aneurysms. Biomaterials 2022, 290, 121857. [Google Scholar] [CrossRef]
- Abi-Aad, K.R.; Rahme, R.J.; Patra, D.P.; Turcotte, E.L.; Richter, K.R.; Merrill, S.A.; Syal, A.; Neville, M.R.; Hudson, M.; Garcia, J.O.; et al. Clinical outcomes of first- and second-generation hydrogel coils compared with bare platinum coils: A systematic literature review. Neurosurg. Rev. 2022, 45, 1873–1882. [Google Scholar] [CrossRef] [PubMed]
- Nickele, C.; Oravec, C.S.; Morris, S.D.; Hoit, D.; Elijovich, L.; Arthur, A.S. Long-Term Follow-up of Aneurysms Treated with Hydrogel-Coated Coils Shows Progressive Thrombosis and Improvement in Raymond-Roy Classification. Oper. Neurosurg. 2022, 22, 239–243. [Google Scholar] [CrossRef]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte chemoattractant protein-1 (MCP-1): An overview. J. Interferon Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Hourani, S.; Motwani, K.; Wajima, D.; Fazal, H.; Jones, C.H.; Doré, S.; Hosaka, K.; Hoh, B.L. Local Delivery Is Critical for Monocyte Chemotactic Protein-1 Mediated Site-Specific Murine Aneurysm Healing. Front. Neurol. 2018, 9, 158. [Google Scholar] [CrossRef]
- Kadirvel, R.; Ding, Y.-H.; Dai, D.; Lewis, D.A.; Kallmes, D.F. Differential gene expression in well-healed and poorly healed experimental aneurysms after coil treatment. Radiology 2010, 257, 418–426. [Google Scholar] [CrossRef]
- Girn, H.; Orsi, N.; Homer-Vanniasinkam, S. An overview of cytokine interactions in atherosclerosis and implications for peripheral arterial disease. Vasc. Med. 2007, 12, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. The inflammatory response in myocardial injury, repair, and remodelling. Nat. Rev. Cardiol. 2014, 11, 255–265. [Google Scholar] [CrossRef]
- Hoh, B.L.; Hosaka, K.; Downes, D.P.; Nowicki, K.W.; Fernandez, C.E.; Batich, C.D.; Scott, E.W. Monocyte chemotactic protein-1 promotes inflammatory vascular repair of murine carotid aneurysms via a macrophage inflammatory protein-1α and macrophage inflammatory protein-2-dependent pathway. Circulation 2011, 124, 2243–2252. [Google Scholar] [CrossRef]
- Hoh, B.L.; Fazal, H.Z.; Hourani, S.; Li, M.; Lin, L.; Hosaka, K. Temporal cascade of inflammatory cytokines and cell-type populations in monocyte chemotactic protein-1 (MCP-1)-mediated aneurysm healing. J. NeuroInterventional Surg. 2018, 10, 301–305. [Google Scholar] [CrossRef]
- Ghozy, S.; Lashin, B.I.; Elfil, M.; Bilgin, C.; Kobeissi, H.; Shehata, M.; Kadirvel, R.; Kallmes, D.F. The safety and effectiveness of the Contour Neurovascular System for the treatment of wide-necked aneurysms: A systematic review and meta-analysis of early experience. Interv. Neuroradiol. 2022, 15910199221139546. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tenhoeve, S.A.; Owens, M.-R.; Rezk, R.; Hanna, A.G.; Lucke-Wold, B. Emerging and Current Biologics for the Treatment of Intracranial Aneurysms. Biologics 2024, 4, 364-375. https://doi.org/10.3390/biologics4040022
Tenhoeve SA, Owens M-R, Rezk R, Hanna AG, Lucke-Wold B. Emerging and Current Biologics for the Treatment of Intracranial Aneurysms. Biologics. 2024; 4(4):364-375. https://doi.org/10.3390/biologics4040022
Chicago/Turabian StyleTenhoeve, Samuel A., Monica-Rae Owens, Rogina Rezk, Abanob G. Hanna, and Brandon Lucke-Wold. 2024. "Emerging and Current Biologics for the Treatment of Intracranial Aneurysms" Biologics 4, no. 4: 364-375. https://doi.org/10.3390/biologics4040022
APA StyleTenhoeve, S. A., Owens, M.-R., Rezk, R., Hanna, A. G., & Lucke-Wold, B. (2024). Emerging and Current Biologics for the Treatment of Intracranial Aneurysms. Biologics, 4(4), 364-375. https://doi.org/10.3390/biologics4040022






