Vesicular Transport and Amyloids: The Growing Relationship
Abstract
1. Introduction
2. Protein Aggregates, Amyloids and Cellular Homeostasis
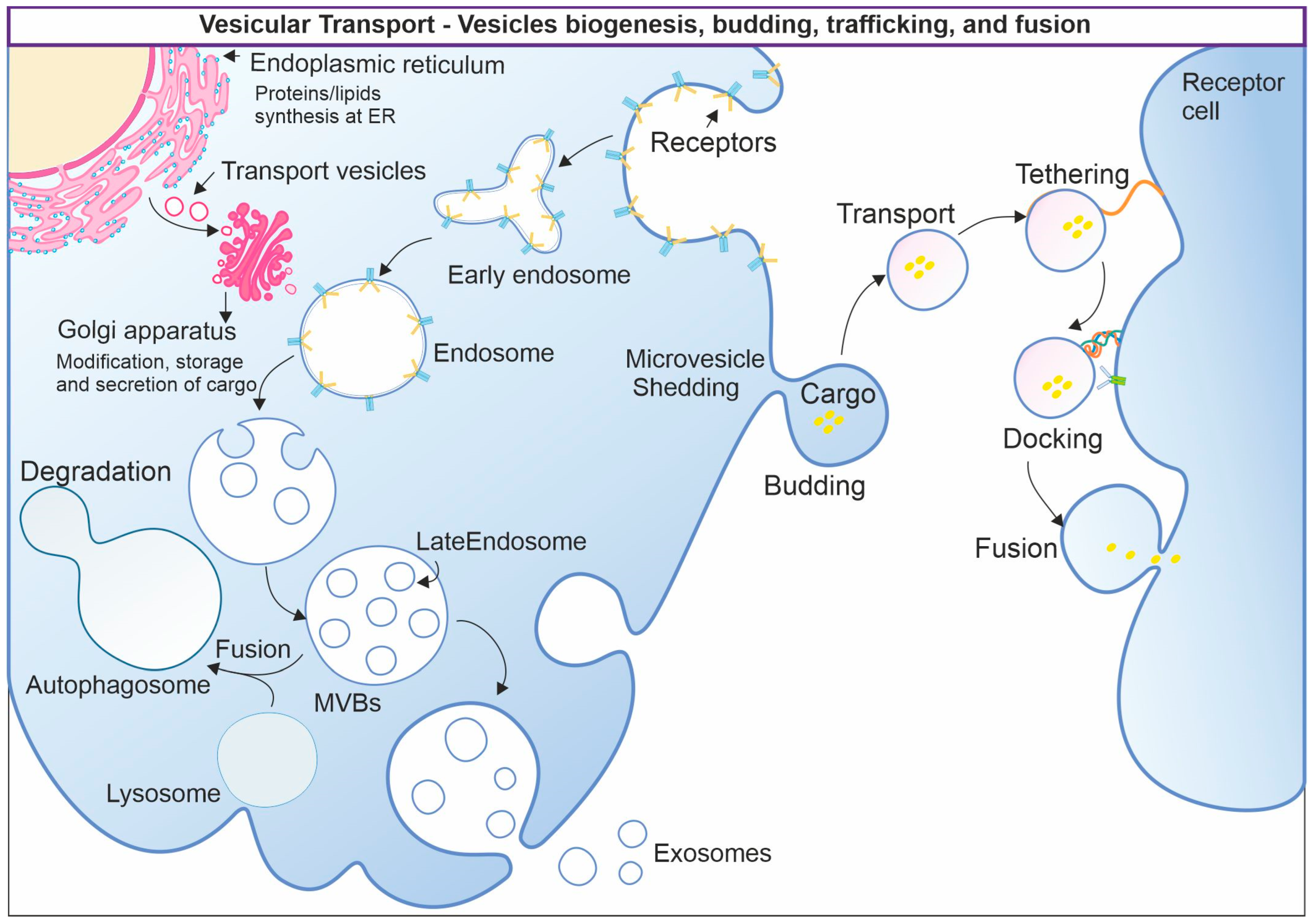
3. Vesicular Transport
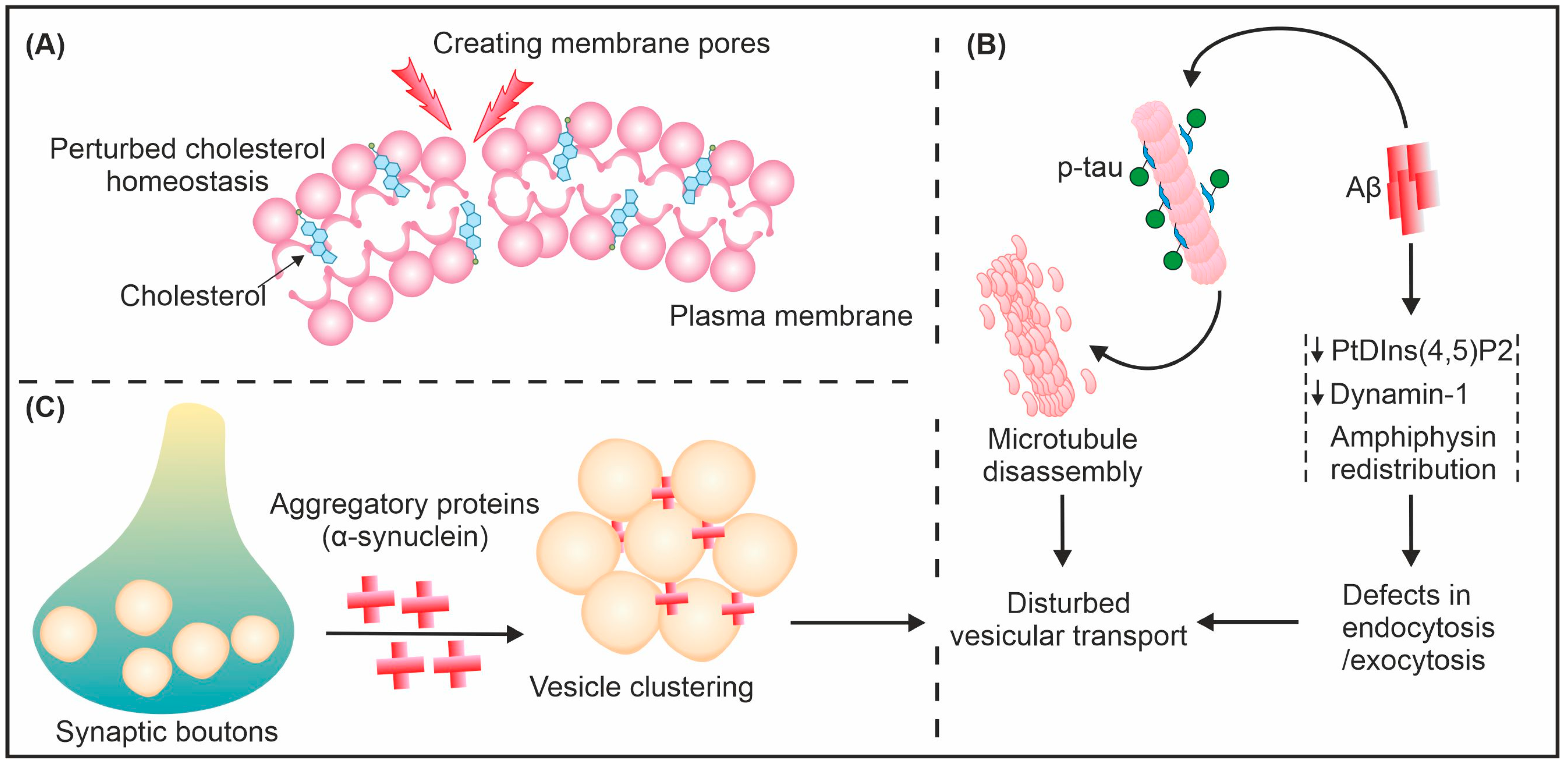
4. How Does Amyloid Interact and Interfere with Vesicular Transport?
5. Consequences of Amyloid-Induced Vesicular Transport Disruption
6. Vesicular Transport Facilitates Amyloid Propagation
7. Vesicular Transport System Plays Vital Role in Tau Propagation
8. Amyloids, Vesicles, and Transport—Avenues for Future Therapeutics
9. Discussion
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pearson, H.A.; Peers, C. Physiological roles for amyloid β peptides. J. Physiol. 2006, 575, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; LeVine, H., III. Alzheimer’s disease and the amyloid-beta peptide. J. Alzheimer’s Dis. 2010, 19, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Li, L.; Sang, S.; Pan, X.; Zhong, C. Physiological Roles of β-amyloid in Regulating Synaptic Function: Implications for AD Pathophysiology. Neurosci. Bull. 2023, 39, 1289–1308. [Google Scholar] [CrossRef] [PubMed]
- Koo, E.H.; Sisodia, S.S.; Archer, D.R.; Martin, L.J.; Weidemann, A.; Beyreuther, K.; Fischer, P.; Masters, C.L.; Price, D.L. Precursor of amyloid protein in Alzheimer disease undergoes fast anterograde axonal transport. Proc. Natl. Acad. Sci. USA 1990, 87, 1561–1565. [Google Scholar] [CrossRef]
- Marsh, J.; Alifragis, P. Synaptic dysfunction in Alzheimer’s disease: The effects of amyloid beta on synaptic vesicle dynamics as a novel target for therapeutic intervention. Neural Regen. Res. 2018, 13, 616–623. [Google Scholar] [CrossRef]
- Decker, H.; Lo, K.Y.; Unger, S.M.; Ferreira, S.T.; Silverman, M.A. Amyloid-beta peptide oligomers disrupt axonal transport through an NMDA receptor-dependent mechanism that is mediated by glycogen synthase kinase 3beta in primary cultured hippocampal neurons. J. Neurosci. 2010, 30, 9166–9171. [Google Scholar] [CrossRef]
- Gan, K.J.; Akram, A.; Blasius, T.L.; Ramser, E.M.; Budaitis, B.G.; Gabrych, D.R.; Verhey, K.J.; Silverman, M.A. GSK3β Impairs KIF1A Transport in a Cellular Model of Alzheimer’s Disease but Does Not Regulate Motor Motility at S402. eNeuro 2020, 7, 0176-20. [Google Scholar] [CrossRef]
- Canevari, L.; Abramov, A.Y.; Duchen, M.R. Toxicity of Amyloid β Peptide: Tales of Calcium, Mitochondria, and Oxidative Stress. Neurochem. Res. 2004, 29, 637–650. [Google Scholar] [CrossRef]
- Zhang, L.; Trushin, S.; Christensen, T.A.; Tripathi, U.; Hong, C.; Geroux, R.E.; Howell, K.G.; Poduslo, J.F.; Trushina, E. Differential effect of amyloid beta peptides on mitochondrial axonal trafficking depends on their state of aggregation and binding to the plasma membrane. Neurobiol. Dis. 2018, 114, 1–16. [Google Scholar] [CrossRef]
- Liu, Y.; Peterson, D.A.; Schubert, D. Amyloid β peptide alters intracellular vesicle trafficking and cholesterol homeostasis. Proc. Natl. Acad. Sci. USA 1998, 95, 13266–13271. [Google Scholar] [CrossRef]
- Gan, K.J.; Silverman, M.A. Dendritic and axonal mechanisms of Ca2+ elevation impair BDNF transport in Aβ oligomer-treated hippocampal neurons. Mol. Biol. Cell 2015, 26, 1058–1071. [Google Scholar] [CrossRef] [PubMed]
- Seifert, B.; Eckenstaler, R.; Rönicke, R.; Leschik, J.; Lutz, B.; Reymann, K.; Lessmann, V.; Brigadski, T. Amyloid-Beta Induced Changes in Vesicular Transport of BDNF in Hippocampal Neurons. Neural Plast. 2016, 2016, 4145708. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.Z.A.; Gleeson, P.A. The role of membrane trafficking in the processing of amyloid precursor protein and production of amyloid peptides in Alzheimer’s disease. Biochim. Biophys. Acta Biomembr. 2019, 1861, 697–712. [Google Scholar] [CrossRef] [PubMed]
- Muresan, V.; Ladescu Muresan, Z. Amyloid-β precursor protein: Multiple fragments, numerous transport routes and mechanisms. Exp. Cell Res. 2015, 334, 45–53. [Google Scholar] [CrossRef]
- Coleman, B.M.; Hill, A.F. Extracellular vesicles—Their role in the packaging and spread of misfolded proteins associated with neurodegenerative diseases. Semin. Cell Dev. Biol. 2015, 40, 89–96. [Google Scholar] [CrossRef]
- Zheng, T.; Pu, J.; Chen, Y.; Mao, Y.; Guo, Z.; Pan, H.; Zhang, L.; Zhang, H.; Sun, B.; Zhang, B. Plasma exosomes spread and cluster around β-amyloid plaques in an animal model of Alzheimer’s disease. Front. Aging Neurosci. 2017, 9, 12. [Google Scholar] [CrossRef]
- Burwinkel, M.; Lutzenberger, M.; Heppner, F.L.; Schulz-Schaeffer, W.; Baier, M. Intravenous injection of beta-amyloid seeds promotes cerebral amyloid angiopathy (CAA). Acta Neuropathol. Commun. 2018, 6, 23. [Google Scholar] [CrossRef]
- Cintron, A.F.; Dalal, N.V.; Dooyema, J.; Betarbet, R.; Walker, L.C. Transport of cargo from periphery to brain by circulating monocytes. Brain Res. 2015, 1622, 328–338. [Google Scholar] [CrossRef]
- Olsson, T.T.; Klementieva, O.; Gouras, G.K. Prion-like seeding and nucleation of intracellular amyloid-β. Neurobiol. Dis. 2018, 113, 1–10. [Google Scholar] [CrossRef]
- Walker, L.C.; Schelle, J.; Jucker, M. The Prion-Like Properties of Amyloid-β Assemblies: Implications for Alzheimer’s Disease. Cold Spring Harb. Perspect. Med. 2016, 6, a024398. [Google Scholar] [CrossRef]
- Lewis, P.A. Vesicular dysfunction and pathways to neurodegeneration. Essays Biochem. 2021, 65, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, A. Structure of proteins: Evolution with unsolved mysteries. Prog. Biophys. Mol. Biol. 2019, 149, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Labbadia, J.; Morimoto, R.I. The biology of proteostasis in aging and disease. Annu. Rev. Biochem. 2015, 84, 435–464. [Google Scholar] [CrossRef] [PubMed]
- Shastry, B.S. Neurodegenerative disorders of protein aggregation. Neurochem. Int. 2003, 43, 1–7. [Google Scholar] [CrossRef]
- Sundaria, N.; Upadhyay, A.; Prasad, A.; Prajapati, V.K.; Poluri, K.M.; Mishra, A. Neurodegeneration & imperfect ageing: Technological limitations and challenges? Mech. Ageing Dev. 2021, 200, 111574. [Google Scholar] [CrossRef]
- Upadhyay, A.; Sundaria, N.; Dhiman, R.; Prajapati, V.K.; Prasad, A.; Mishra, A. Complex Inclusion Bodies and Defective Proteome Hubs in Neurodegenerative Disease: New Clues, New Challenges. Neuroscientist 2022, 28, 271–282. [Google Scholar] [CrossRef]
- Upadhyay, A.; Mishra, A. Amyloids of multiple species: Are they helpful in survival? Biol. Rev. 2018, 93, 1363–1386. [Google Scholar] [CrossRef]
- Scheres, S.H.W.; Ryskeldi-Falcon, B.; Goedert, M. Molecular pathology of neurodegenerative diseases by cryo-EM of amyloids. Nature 2023, 621, 701–710. [Google Scholar] [CrossRef]
- Höhn, A.; Weber, D.; Jung, T.; Ott, C.; Hugo, M.; Kochlik, B.; Kehm, R.; König, J.; Grune, T.; Castro, J.P. Happily (n)ever after: Aging in the context of oxidative stress, proteostasis loss and cellular senescence. Redox Biol. 2017, 11, 482–501. [Google Scholar] [CrossRef]
- Munishkina, L.A.; Ahmad, A.; Fink, A.L.; Uversky, V.N. Guiding protein aggregation with macromolecular crowding. Biochemistry 2008, 47, 8993–9006. [Google Scholar] [CrossRef]
- Minton, A.P. Implications of macromolecular crowding for protein assembly. Curr. Opin. Struct. Biol. 2000, 10, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Sahu, M.; Srivastava, D.; Tiwari, S.; Ambasta, R.K.; Kumar, P. Post-translational modifications: Regulators of neurodegenerative proteinopathies. Ageing Res. Rev. 2021, 68, 101336. [Google Scholar] [CrossRef] [PubMed]
- Buxbaum, E. Vesicular Transport in Eukaryotic Cells. In Fundamentals of Protein Structure and Function; Buxbaum, E., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 393–420. [Google Scholar]
- Barr, F. Vesicular transport. Essays Biochem. 2000, 36, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Nickel, W.; Brügger, B.; Wieland, F.T. Vesicular transport: The core machinery of COPI recruitment and budding. J. Cell Sci. 2002, 115, 3235–3240. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. The molecular mechanisms of membrane transport and the maintenance of compartmental diversity. In Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Schmid, S.L. Clathrin-Coated Vesicle Formation and Protein Sorting: An Integrated Process. Annu. Rev. Biochem. 1997, 66, 511–548. [Google Scholar] [CrossRef]
- Paraan, M.; Mendez, J.; Sharum, S.; Kurtin, D.; He, H.; Stagg, S.M. The structures of natively assembled clathrin-coated vesicles. Sci. Adv. 2020, 6, eaba8397. [Google Scholar] [CrossRef]
- Pigino, G.; Morfini, G.A.; Brady, S.T. Chapter 7—Intracellular Trafficking. In Basic Neurochemistry, 8th ed.; Brady, S.T., Siegel, G.J., Albers, R.W., Price, D.L., Eds.; Academic Press: New York, NY, USA, 2012; pp. 119–145. [Google Scholar]
- Bonifacino, J.S.; Glick, B.S. The Mechanisms of Vesicle Budding and Fusion. Cell 2004, 116, 153–166. [Google Scholar] [CrossRef]
- Jahn, R.; Cafiso, D.C.; Tamm, L.K. Mechanisms of SNARE proteins in membrane fusion. Nat. Rev. Mol. Cell Biol. 2024, 25, 101–118. [Google Scholar] [CrossRef]
- Palfreyman, M.T.; West, S.E.; Jorgensen, E.M. SNARE Proteins in Synaptic Vesicle Fusion. Adv. Neurobiol. 2023, 33, 63–118. [Google Scholar] [CrossRef]
- Pérez-González, R.; Kim, Y.; Miller, C.; Pacheco-Quinto, J.; Eckman, E.A.; Levy, E. Extracellular vesicles: Where the amyloid precursor protein carboxyl-terminal fragments accumulate and amyloid-β oligomerizes. FASEB J. 2020, 34, 12922–12931. [Google Scholar] [CrossRef]
- Osborne, O.M.; Kowalczyk, J.M.; Louis, K.D.P.; Daftari, M.T.; Colbert, B.M.; Naranjo, O.; Torices, S.; András, I.E.; Dykxhoorn, D.M.; Toborek, M. Brain endothelium-derived extracellular vesicles containing amyloid-beta induce mitochondrial alterations in neural progenitor cells. Extracell. Vesicles Circ. Nucleic Acids 2022, 3, 340–362. [Google Scholar] [CrossRef]
- Das, A.K.; Pandit, R.; Maiti, S. Effect of amyloids on the vesicular machinery: Implications for somatic neurotransmission. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140187. [Google Scholar] [CrossRef] [PubMed]
- McLaurin, J.; Chakrabartty, A. Membrane Disruption by Alzheimer β-Amyloid Peptides Mediated through Specific Binding to Either Phospholipids or Gangliosides: Implications for Neurotoxicity. J. Biol. Chem. 1996, 271, 26482–26489. [Google Scholar] [CrossRef]
- Dietschy, J.M.; Turley, S.D. Thematic review series: Brain Lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J. Lipid Res. 2004, 45, 1375–1397. [Google Scholar] [CrossRef]
- Rudajev, V.; Novotny, J. Cholesterol as a key player in amyloid β-mediated toxicity in Alzheimer’s disease. Front. Mol. Neurosci. 2022, 15, 937056. [Google Scholar] [CrossRef] [PubMed]
- Berman, D.E.; Dall’Armi, C.; Voronov, S.V.; McIntire, L.B.J.; Zhang, H.; Moore, A.Z.; Staniszewski, A.; Arancio, O.; Kim, T.-W.; Di Paolo, G. Oligomeric amyloid-β peptide disrupts phosphatidylinositol-4,5-bisphosphate metabolism. Nat. Neurosci. 2008, 11, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Kelly, B.L.; Ferreira, A. Beta-amyloid disrupted synaptic vesicle endocytosis in cultured hippocampal neurons. Neuroscience 2007, 147, 60–70. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bathina, S.; Das, U.N. Brain-derived neurotrophic factor and its clinical implications. Arch. Med. Sci 2015, 11, 1164–1178. [Google Scholar] [CrossRef]
- Poon, W.W.; Carlos, A.J.; Aguilar, B.L.; Berchtold, N.C.; Kawano, C.K.; Zograbyan, V.; Yaopruke, T.; Shelanski, M.; Cotman, C.W. β-Amyloid (Aβ) oligomers impair brain-derived neurotrophic factor retrograde trafficking by down-regulating ubiquitin C-terminal hydrolase, UCH-L1. J. Biol. Chem. 2013, 288, 16937–16948. [Google Scholar] [CrossRef]
- Poon, W.W.; Blurton-Jones, M.; Tu, C.H.; Feinberg, L.M.; Chabrier, M.A.; Harris, J.W.; Jeon, N.L.; Cotman, C.W. β-Amyloid impairs axonal BDNF retrograde trafficking. Neurobiol. Aging 2011, 32, 821–833. [Google Scholar] [CrossRef]
- Eggert, S.; Kins, S.; Endres, K.; Brigadski, T. Brothers in arms: proBDNF/BDNF and sAPPα/Aβ-signaling and their common interplay with ADAM10, TrkB, p75NTR, sortilin, and sorLA in the progression of Alzheimer’s disease. Biol. Chem. 2022, 403, 43–71. [Google Scholar] [CrossRef] [PubMed]
- Icking, A.; Amaddii, M.; Ruonala, M.; Höning, S.; Tikkanen, R. Polarized transport of Alzheimer amyloid precursor protein is mediated by adaptor protein complex AP1-1B. Traffic 2007, 8, 285–296. [Google Scholar] [CrossRef] [PubMed]
- de Wilde, M.C.; Overk, C.R.; Sijben, J.W.; Masliah, E. Meta-analysis of synaptic pathology in Alzheimer’s disease reveals selective molecular vesicular machinery vulnerability. Alzheimer’s Dement. 2016, 12, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Greten-Harrison, B.; Polydoro, M.; Morimoto-Tomita, M.; Diao, L.; Williams, A.M.; Nie, E.H.; Makani, S.; Tian, N.; Castillo, P.E.; Buchman, V.L.; et al. αβγ-Synuclein triple knockout mice reveal age-dependent neuronal dysfunction. Proc. Natl. Acad. Sci. USA 2010, 107, 19573–19578. [Google Scholar] [CrossRef]
- Smith, R.; Bacos, K.; Fedele, V.; Soulet, D.; Walz, H.A.; Obermüller, S.; Lindqvist, A.; Björkqvist, M.; Klein, P.; Önnerfjord, P.; et al. Mutant huntingtin interacts with β-tubulin and disrupts vesicular transport and insulin secretion. Hum. Mol. Genet. 2009, 18, 3942–3954. [Google Scholar] [CrossRef]
- Zeineddine, R.; Pundavela, J.F.; Corcoran, L.; Stewart, E.M.; Do-Ha, D.; Bax, M.; Guillemin, G.; Vine, K.L.; Hatters, D.M.; Ecroyd, H.; et al. SOD1 protein aggregates stimulate macropinocytosis in neurons to facilitate their propagation. Mol. Neurodegener. 2015, 10, 57. [Google Scholar] [CrossRef]
- Wang, L.; Das, U.; Scott, D.A.; Tang, Y.; McLean, P.J.; Roy, S. α-Synuclein Multimers Cluster Synaptic Vesicles and Attenuate Recycling. Curr. Biol. 2014, 24, 2319–2326. [Google Scholar] [CrossRef]
- Jani, R.A.; Setty, S.R.G. Membrane trafficking and vesicle fusion: Post-Palade era researchers win the Nobel Prize. Resonance 2014, 19, 421–445. [Google Scholar] [CrossRef]
- Bodart-Santos, V.; Pinheiro, L.S.; da Silva-Junior, A.J.; Froza, R.L.; Ahrens, R.; Gonçalves, R.A.; Andrade, M.M.; Chen, Y.; Alcantara, C.d.L.; Grinberg, L.T.; et al. Alzheimer’s disease brain-derived extracellular vesicles reveal altered synapse-related proteome and induce cognitive impairment in mice. Alzheimer’s Dement. 2023, 19, 5418–5436. [Google Scholar] [CrossRef]
- Rui, Y.; Zheng, J.Q. Amyloid β oligomers elicit mitochondrial transport defects and fragmentation in a time-dependent and pathway-specific manner. Mol. Brain 2016, 9, 79. [Google Scholar] [CrossRef]
- Schindowski, K.; Belarbi, K.; Buée, L. Neurotrophic factors in Alzheimer’s disease: Role of axonal transport. Genes Brain Behav. 2008, 7 (Suppl. S1), 43–56. [Google Scholar] [CrossRef] [PubMed]
- Haass, C.; Kaether, C.; Thinakaran, G.; Sisodia, S. Trafficking and proteolytic processing of APP. Cold Spring Harb. Perspect. Med. 2012, 2, a006270. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, A.; Chhangani, D.; Rao, N.R.; Kofler, J.; Vassar, R.; Rincon-Limas, D.E.; Savas, J.N. Amyloid fibril proteomics of AD brains reveals modifiers of aggregation and toxicity. Mol. Neurodegener. 2023, 18, 61. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, U.; Nilson, A.N.; Kayed, R. The Role of Amyloid-β Oligomers in Toxicity, Propagation, and Immunotherapy. eBioMedicine 2016, 6, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Shankar, G.M.; Li, S.; Mehta, T.H.; Garcia-Munoz, A.; Shepardson, N.E.; Smith, I.; Brett, F.M.; Farrell, M.A.; Rowan, M.J.; Lemere, C.A.; et al. Amyloid-β protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat. Med. 2008, 14, 837–842. [Google Scholar] [CrossRef]
- Lambert, M.P.; Barlow, A.K.; Chromy, B.A.; Edwards, C.; Freed, R.; Liosatos, M.; Morgan, T.E.; Rozovsky, I.; Trommer, B.; Viola, K.L.; et al. Diffusible, nonfibrillar ligands derived from Aβ 1–42 are potent central nervous system neurotoxins. Proc. Natl. Acad. Sci. USA 1998, 95, 6448–6453. [Google Scholar] [CrossRef]
- Koffie, R.M.; Meyer-Luehmann, M.; Hashimoto, T.; Adams, K.W.; Mielke, M.L.; Garcia-Alloza, M.; Micheva, K.D.; Smith, S.J.; Kim, M.L.; Lee, V.M.; et al. Oligomeric amyloid β associates with postsynaptic densities and correlates with excitatory synapse loss near senile plaques. Proc. Natl. Acad. Sci. USA 2009, 106, 4012–4017. [Google Scholar] [CrossRef]
- Meyer-Luehmann, M.; Spires-Jones, T.L.; Prada, C.; Garcia-Alloza, M.; de Calignon, A.; Rozkalne, A.; Koenigsknecht-Talboo, J.; Holtzman, D.M.; Bacskai, B.J.; Hyman, B.T. Rapid appearance and local toxicity of amyloid-β plaques in a mouse model of Alzheimer’s disease. Nature 2008, 451, 720–724. [Google Scholar] [CrossRef]
- Shah, P.; Lal, N.; Leung, E.; Traul, D.E.; Gonzalo-Ruiz, A.; Geula, C. Neuronal and axonal loss are selectively linked to fibrillar amyloid-{beta} within plaques of the aged primate cerebral cortex. Am. J. Pathol. 2010, 177, 325–333. [Google Scholar] [CrossRef]
- Ricciarelli, R.; Fedele, E. The Amyloid Cascade Hypothesis in Alzheimer’s Disease: It’s Time to Change Our Mind. Curr. Neuropharmacol. 2017, 15, 926–935. [Google Scholar] [CrossRef]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Grothe, M.J.; Barthel, H.; Sepulcre, J.; Dyrba, M.; Sabri, O.; Teipel, S.J. In vivo staging of regional amyloid deposition. Neurology 2017, 89, 2031–2038. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Pozo, A.; Frosch, M.P.; Masliah, E.; Hyman, B.T. Neuropathological alterations in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2011, 1, a006189. [Google Scholar] [CrossRef] [PubMed]
- Thal, D.R.; Rüb, U.; Orantes, M.; Braak, H. Phases of Aβ-deposition in the human brain and its relevance for the development of AD. Neurology 2002, 58, 1791–1800. [Google Scholar] [CrossRef]
- Watts, J.C.; Prusiner, S.B. β-Amyloid Prions and the Pathobiology of Alzheimer’s Disease. Cold Spring Harb. Perspect. Med. 2018, 8, a023507. [Google Scholar] [CrossRef]
- Sinha, A.; Principe, S.; Alfaro, J.; Ignatchenko, A.; Ignatchenko, V.; Kislinger, T. Proteomic Profiling of Secreted Proteins, Exosomes, and Microvesicles in Cell Culture Conditioned Media. Methods Mol. Biol. 2018, 1722, 91–102. [Google Scholar] [CrossRef]
- Meyer-Luehmann, M.; Stalder, M.; Herzig, M.C.; Kaeser, S.A.; Kohler, E.; Pfeifer, M.; Boncristiano, S.; Mathews, P.M.; Mercken, M.; Abramowski, D.; et al. Extracellular amyloid formation and associated pathology in neural grafts. Nat. Neurosci. 2003, 6, 370–377. [Google Scholar] [CrossRef]
- Eisele, Y.S.; Obermüller, U.; Heilbronner, G.; Baumann, F.; Kaeser, S.A.; Wolburg, H.; Walker, L.C.; Staufenbiel, M.; Heikenwalder, M.; Jucker, M. Peripherally applied Abeta-containing inoculates induce cerebral beta-amyloidosis. Science 2010, 330, 980–982. [Google Scholar] [CrossRef]
- Sharples, R.A.; Vella, L.J.; Nisbet, R.M.; Naylor, R.; Perez, K.; Barnham, K.J.; Masters, C.L.; Hill, A.F. Inhibition of gamma-secretase causes increased secretion of amyloid precursor protein C-terminal fragments in association with exosomes. FASEB J. 2008, 22, 1469–1478. [Google Scholar] [CrossRef]
- Rajendran, L.; Honsho, M.; Zahn, T.R.; Keller, P.; Geiger, K.D.; Verkade, P.; Simons, K. Alzheimer’s disease beta-amyloid peptides are released in association with exosomes. Proc. Natl. Acad. Sci. USA 2006, 103, 11172–11177. [Google Scholar] [CrossRef]
- Falcicchia, C.; Tozzi, F.; Gabrielli, M.; Amoretti, S.; Masini, G.; Nardi, G.; Guglielmo, S.; Ratto, G.M.; Arancio, O.; Verderio, C.; et al. Microglial extracellular vesicles induce Alzheimer’s disease-related cortico-hippocampal network dysfunction. Brain Commun. 2023, 5, fcad170. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Wu, X.; Wei, X.; Wang, M.; Zhang, B. The release and transmission of amyloid precursor protein via exosomes. Neurochem. Int. 2018, 114, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Kawahara, H.; Kodera, N.; Kumaki, A.; Tada, Y.; Tang, Z.; Sakai, K.; Ono, K.; Yamada, M.; Hanayama, R. Extracellular vesicles contribute to the metabolism of transthyretin amyloid in hereditary transthyretin amyloidosis. Front. Mol. Biosci. 2022, 9, 839917. [Google Scholar] [CrossRef] [PubMed]
- Halipi, V.; Sasanian, N.; Feng, J.; Hu, J.; Lubart, Q.; Bernson, D.; van Leeuwen, D.; Ahmadpour, D.; Sparr, E.; Esbjörner, E.K. Extracellular Vesicles Slow Down Aβ(1–42) Aggregation by Interfering with the Amyloid Fibril Elongation Step. ACS Chem. Neurosci. 2024, 15, 944–954. [Google Scholar] [CrossRef] [PubMed]
- Gabrielli, M.; Tozzi, F.; Verderio, C.; Origlia, N. Emerging Roles of Extracellular Vesicles in Alzheimer’s Disease: Focus on Synaptic Dysfunction and Vesicle–Neuron Interaction. Cells 2023, 12, 63. [Google Scholar] [CrossRef]
- Perez-Gonzalez, R.; Gauthier, S.A.; Kumar, A.; Levy, E. The exosome secretory pathway transports amyloid precursor protein carboxyl-terminal fragments from the cell into the brain extracellular space. J. Biol. Chem. 2012, 287, 43108–43115. [Google Scholar] [CrossRef]
- Zhao, Y.; Gu, Y.; Zhang, Q.; Liu, H.; Liu, Y. The Potential Roles of Exosomes Carrying APP and Tau Cleavage Products in Alzheimer’s Disease. J. Clin. Med. 2023, 12, 1883. [Google Scholar] [CrossRef]
- Takeuchi, S.; Ueda, N.; Suzuki, K.; Shimozawa, N.; Yasutomi, Y.; Kimura, N. Elevated Membrane Cholesterol Disrupts Lysosomal Degradation to Induce β-Amyloid Accumulation: The Potential Mechanism Underlying Augmentation of β-Amyloid Pathology by Type 2 Diabetes Mellitus. Am. J. Pathol. 2019, 189, 391–404. [Google Scholar] [CrossRef]
- Burrinha, T.; Martinsson, I.; Gomes, R.; Terrasso, A.P.; Gouras, G.K.; Almeida, C.G. Upregulation of APP endocytosis by neuronal aging drives amyloid-dependent synapse loss. J. Cell Sci. 2021, 134, jcs255752. [Google Scholar] [CrossRef]
- Liu, L.; Drouet, V.; Wu, J.W.; Witter, M.P.; Small, S.A.; Clelland, C.; Duff, K. Trans-Synaptic Spread of Tau Pathology In Vivo. PLoS ONE 2012, 7, e31302. [Google Scholar] [CrossRef]
- Jucker, M.; Walker, L.C. Pathogenic protein seeding in Alzheimer disease and other neurodegenerative disorders. Ann. Neurol. 2011, 70, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Clavaguera, F.; Bolmont, T.; Crowther, R.A.; Abramowski, D.; Frank, S.; Probst, A.; Fraser, G.; Stalder, A.K.; Beibel, M.; Staufenbiel, M. Transmission and spreading of tauopathy in transgenic mouse brain. Nat. Cell Biol. 2009, 11, 909–913. [Google Scholar] [CrossRef] [PubMed]
- Clavaguera, F.; Akatsu, H.; Fraser, G.; Crowther, R.A.; Frank, S.; Hench, J.; Probst, A.; Winkler, D.T.; Reichwald, J.; Staufenbiel, M. Brain homogenates from human tauopathies induce tau inclusions in mouse brain. Proc. Natl. Acad. Sci. USA 2013, 110, 9535–9540. [Google Scholar] [CrossRef] [PubMed]
- Lasagna-Reeves, C.A.; Castillo-Carranza, D.L.; Sengupta, U.; Guerrero-Munoz, M.J.; Kiritoshi, T.; Neugebauer, V.; Jackson, G.R.; Kayed, R. Alzheimer brain-derived tau oligomers propagate pathology from endogenous tau. Sci. Rep. 2012, 2, 700. [Google Scholar] [CrossRef]
- Smolek, T.; Jadhav, S.; Brezovakova, V.; Cubinkova, V.; Valachova, B.; Novak, P.; Zilka, N. First-in-rat study of human Alzheimer’s disease tau propagation. Mol. Neurobiol. 2019, 56, 621–631. [Google Scholar] [CrossRef]
- Iba, M.; Guo, J.L.; McBride, J.D.; Zhang, B.; Trojanowski, J.Q.; Lee, V.M.-Y. Synthetic tau fibrils mediate transmission of neurofibrillary tangles in a transgenic mouse model of Alzheimer’s-like tauopathy. J. Neurosci. 2013, 33, 1024–1037. [Google Scholar] [CrossRef]
- Guo, J.L.; Lee, V.M. Neurofibrillary tangle-like tau pathology induced by synthetic tau fibrils in primary neurons over-expressing mutant tau. FEBS Lett. 2013, 587, 717–723. [Google Scholar] [CrossRef]
- Frost, B.; Jacks, R.L.; Diamond, M.I. Propagation of tau misfolding from the outside to the inside of a cell. J. Biol. Chem. 2009, 284, 12845–12852. [Google Scholar] [CrossRef]
- Calafate, S.; Buist, A.; Miskiewicz, K.; Vijayan, V.; Daneels, G.; de Strooper, B.; de Wit, J.; Verstreken, P.; Moechars, D. Synaptic contacts enhance cell-to-cell tau pathology propagation. Cell Rep. 2015, 11, 1176–1183. [Google Scholar] [CrossRef]
- Baker, S.; Polanco, J.C.; Götz, J. Extracellular vesicles containing P301L mutant tau accelerate pathological tau phosphorylation and oligomer formation but do not seed mature neurofibrillary tangles in ALZ17 mice. J. Alzheimer’s Dis. 2016, 54, 1207–1217. [Google Scholar] [CrossRef]
- Polanco, J.C.; Scicluna, B.J.; Hill, A.F.; Götz, J. Extracellular vesicles isolated from the brains of rTg4510 mice seed tau protein aggregation in a threshold-dependent manner. J. Biol. Chem. 2016, 291, 12445–12466. [Google Scholar] [CrossRef] [PubMed]
- Fiandaca, M.S.; Kapogiannis, D.; Mapstone, M.; Boxer, A.; Eitan, E.; Schwartz, J.B.; Abner, E.L.; Petersen, R.C.; Federoff, H.J.; Miller, B.L. Identification of preclinical Alzheimer’s disease by a profile of pathogenic proteins in neurally derived blood exosomes: A case-control study. Alzheimer’s Dement. 2015, 11, 600–607.e601. [Google Scholar] [CrossRef] [PubMed]
- Saman, S.; Kim, W.; Raya, M.; Visnick, Y.; Miro, S.; Saman, S.; Jackson, B.; McKee, A.C.; Alvarez, V.E.; Lee, N.C. Exosome-associated tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid in early Alzheimer disease. J. Biol. Chem. 2012, 287, 3842–3849. [Google Scholar] [CrossRef] [PubMed]
- Dujardin, S.; Bégard, S.; Caillierez, R.; Lachaud, C.; Delattre, L.; Carrier, S.; Loyens, A.; Galas, M.-C.; Bousset, L.; Melki, R. Ectosomes: A new mechanism for non-exosomal secretion of tau protein. PLoS ONE 2014, 9, e100760. [Google Scholar] [CrossRef] [PubMed]
- Lasagna-Reeves, C.A.; Sengupta, U.; Castillo-Carranza, D.; Gerson, J.E.; Guerrero-Munoz, M.; Troncoso, J.C.; Jackson, G.R.; Kayed, R. The formation of tau pore-like structures is prevalent and cell specific: Possible implications for the disease phenotypes. Acta Neuropathol. Commun. 2014, 2, 56. [Google Scholar] [CrossRef]
- Merezhko, M.; Brunello, C.A.; Yan, X.; Vihinen, H.; Jokitalo, E.; Uronen, R.-L.; Huttunen, H.J. Secretion of Tau via an Unconventional Non-vesicular Mechanism. Cell Rep. 2018, 25, 2027–2035.e24. [Google Scholar] [CrossRef]
- Patel, N.; Ramachandran, S.; Azimov, R.; Kagan, B.L.; Lal, R. Ion Channel Formation by Tau Protein: Implications for Alzheimer’s Disease and Tauopathies. Biochemistry 2015, 54, 7320–7325. [Google Scholar] [CrossRef]
- Kumar, M.A.; Baba, S.K.; Sadida, H.Q.; Marzooqi, S.A.; Jerobin, J.; Altemani, F.H.; Algehainy, N.; Alanazi, M.A.; Abou-Samra, A.-B.; Kumar, R.; et al. Extracellular vesicles as tools and targets in therapy for diseases. Signal Transduct. Target. Ther. 2024, 9, 27. [Google Scholar] [CrossRef]
- Soliman, H.M.; Ghonaim, G.A.; Gharib, S.M.; Chopra, H.; Farag, A.K.; Hassanin, M.H.; Nagah, A.; Emad-Eldin, M.; Hashem, N.E.; Yahya, G.; et al. Exosomes in Alzheimer’s Disease: From Being Pathological Players to Potential Diagnostics and Therapeutics. Int. J. Mol. Sci. 2021, 22, 10794. [Google Scholar] [CrossRef]
- Cui, L.; Li, H.; Xi, Y.; Hu, Q.; Liu, H.; Fan, J.; Xiang, Y.; Zhang, X.; Shui, W.; Lai, Y. Vesicle trafficking and vesicle fusion: Mechanisms, biological functions, and their implications for potential disease therapy. Mol. Biomed. 2022, 3, 29. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Upadhyay, A. Vesicular Transport and Amyloids: The Growing Relationship. Biologics 2024, 4, 376-389. https://doi.org/10.3390/biologics4040023
Upadhyay A. Vesicular Transport and Amyloids: The Growing Relationship. Biologics. 2024; 4(4):376-389. https://doi.org/10.3390/biologics4040023
Chicago/Turabian StyleUpadhyay, Arun. 2024. "Vesicular Transport and Amyloids: The Growing Relationship" Biologics 4, no. 4: 376-389. https://doi.org/10.3390/biologics4040023
APA StyleUpadhyay, A. (2024). Vesicular Transport and Amyloids: The Growing Relationship. Biologics, 4(4), 376-389. https://doi.org/10.3390/biologics4040023





