2. Case Presentation
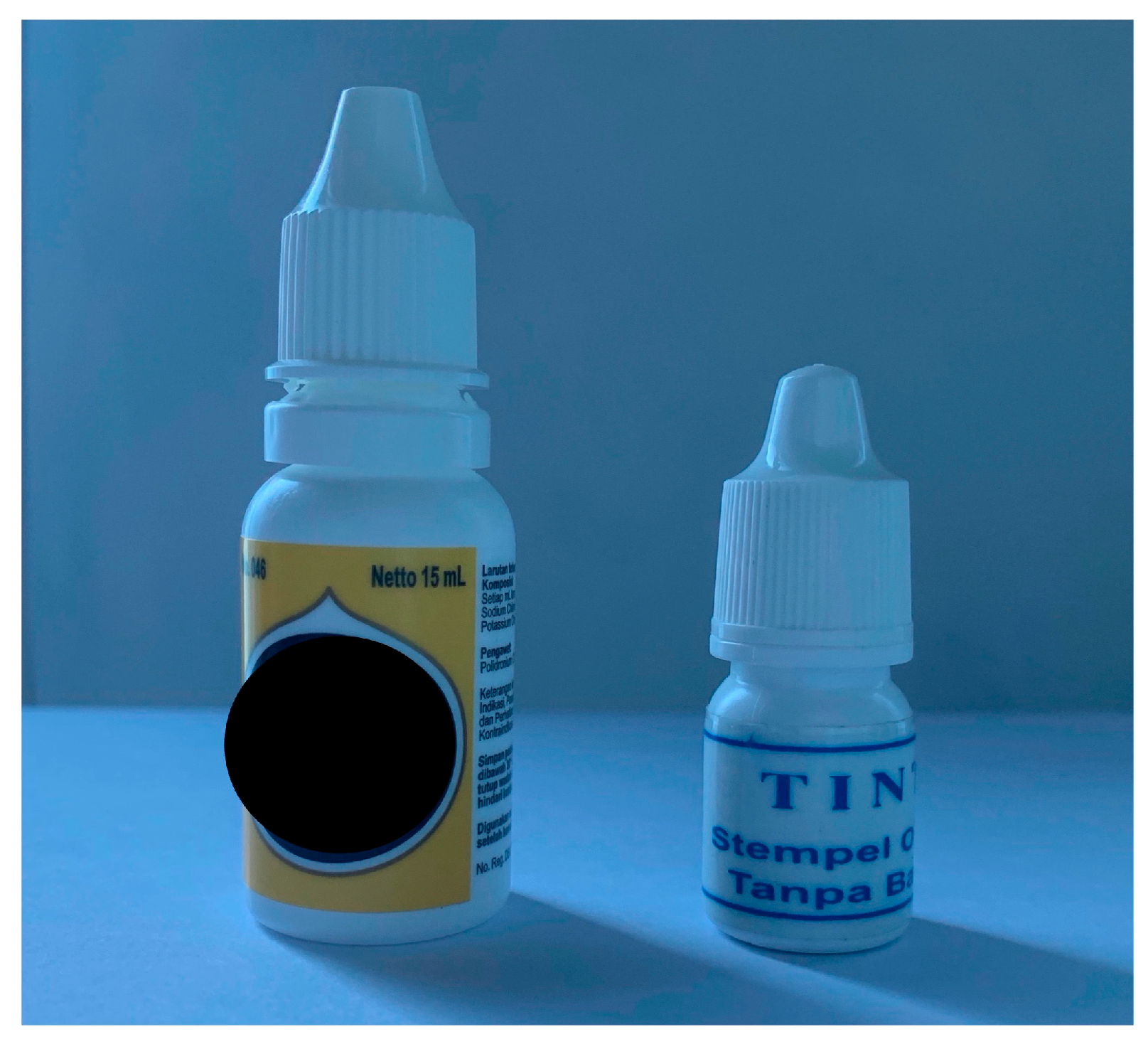
A 73-year-old man presented with blurred vision and mild pain in both eyes (BEs) after accidentally instilling blue stamper ink, mistaken for artificial tears. The ink was initially intended to refill the inkpad for the name stamp. The patient routinely instilled the artificial tears before sleeping while the lights were off. The patient also declared putting more drops in the right eye (RE) than in the left eye (LE), about six and two drops, respectively. The patient’s family admitted the patient to our Emergency Department (ED) 30 min after the injury. The patient had a history of dry eye syndrome in BEs and was treated with artificial tears containing sodium chloride and potassium chloride. The patient had diabetes mellitus, hypertension, and heart disease with good control of medication. The patient also had previous cataract surgeries in BEs 5 years ago in another hospital. The family showed both artificial tears and stamp ink bottles. They appeared identical, with the same white bottle packaging and cap structure, but different in height (
Figure 1).
Upon arrival at the ED, the blue color from the stamper ink was darker in the RE than in the LE, including the periocular. At first, topical anesthesia (Tetracaine HCl eyedrop) was administered in BEs, and then the eyes were immediately rinsed with copious amounts of water in our eye irrigation station. Cotton-bud applicators were used to sweep the fornices of BEs. We then performed a litmus test, using red and blue litmus paper, to find the pH. On the first testing attempt in the RE, it could not be interpreted because it was stained as dark as the stamper ink color. Then, we tested the LE, and it showed an alkaline pH. The irrigation was continued for another 30 min. A total of 45 min of irrigation was performed, with the subsequent changes in the pH of BEs recorded, and the ink showed a faded color in the LE but was still a darker blue color in the RE.
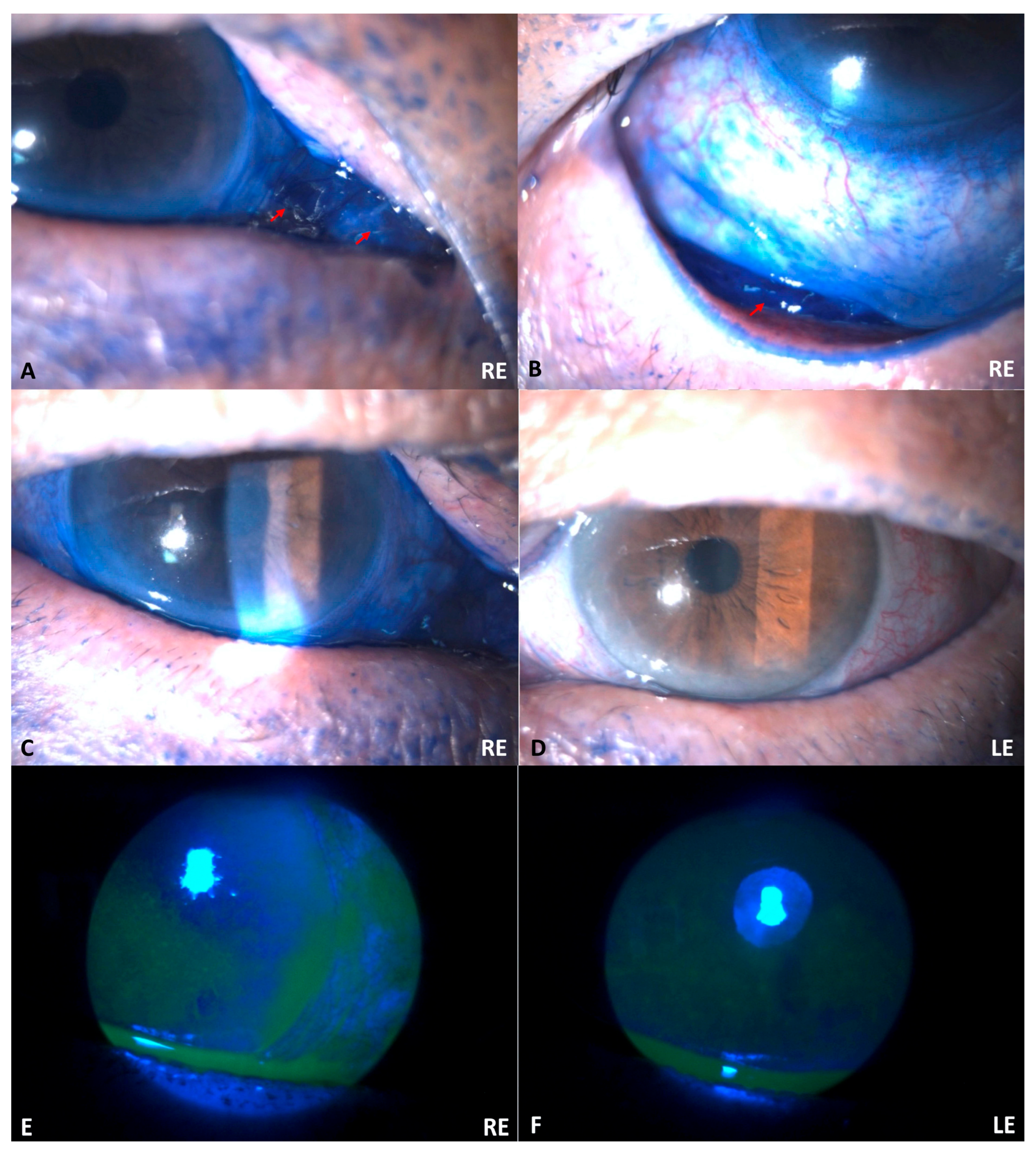
Upon examination, the initial uncorrected visual acuity (UCVA) was 0.4 in the RE and 0.8 in the LE. The intraocular pressure was not measured. Slit lamp biomicroscopy examination revealed bilateral edema palpebra with a blue ink-stained periocular. The conjunctiva of the RE was seen as dark blue and more chemotic than the LE (
Figure 2). No limbal ischemia was discovered in BEs. Both corneas showed epithelial defects with a positive fluorescein test. The intraocular lens (IOL) was in a good position for BEs. The anterior chamber, iris, and posterior segment of BEs were also normal. A diagnosis of bilateral corneal abrasion with chemotic conjunctiva due to alkaline chemical trauma, with bilateral pseudophakia, was established. The classification of severity was Grade I based on the Roper–Hall Classification (corneal epithelial damage and no limbal ischemia) [
9], and Grade III based on the Dua Classification (0/50: zero clock hours of limbus ischemia and >30–50% of conjunctival involvement) [
10]. Levofloxacin mini-dose eyedrops (every three hours) and sodium hyaluronate mini-dose eyedrops (every hour) were prescribed. Mefenamic acid and ascorbic acid tablets were also given twice daily. The patient was then scheduled to meet an ophthalmologist for a follow-up in the morning.
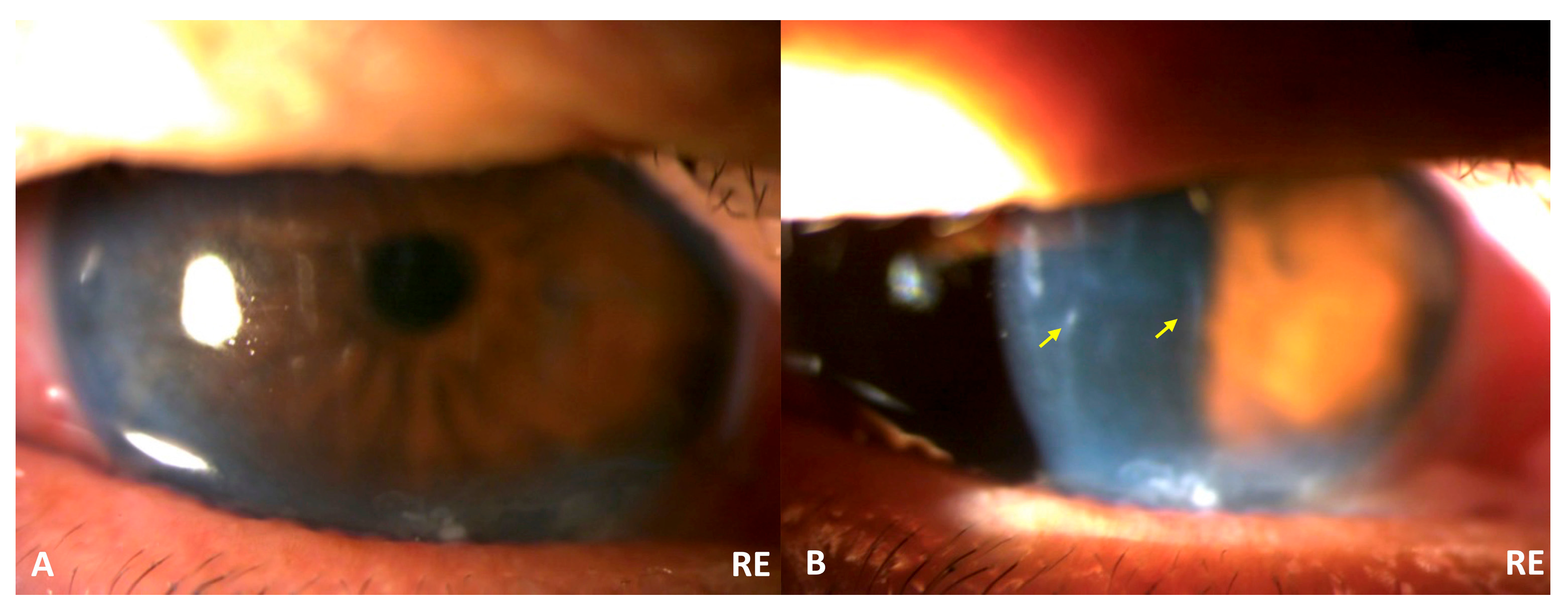
The patient saw an ophthalmologist in the morning for a follow-up appointment (ten hours after the ED visit). The UCVA in the RE and LE was 0.2 and 1.0, respectively. The RE UCVA decreased significantly, from 0.4 to 0.2. The slit lamp biomicroscopy examination showed Descemet’s fold in the RE with epithelial defects in BEs (
Figure 3). The additional diagnosis of RE corneal edema was established. The severity classification was then changed into Grade II based on the Roper–Hall Classification (corneal haze, iris details visible, and no limbal ischemia) [
9], and Grade III based on the Dua Classification (0/50: zero clock hours of limbus ischemia and >30–50% of conjunctival involvement) [
10]. The previous medications from the ED were continued, and the patient was given additional eyedrops, including sodium chloride eyedrops and combined polymyxin sulfate–neomycin sulfate and dexamethasone mini-dose eyedrops.
After one week, the patient visited the same ophthalmologist. The patient still complained about blurred vision and discomfort in BEs. The UCVA was 0.16 in the RE and 0.4 in the LE, and the BCVA was 0.4 in the RE and 0.5 in the LE. Intraocular pressure was 9.7 and 9.0 in the RE and LE, respectively. The slit lamp biomicroscopy examination revealed edema and Descemet’s fold in the RE cornea still occurred, with epithelial defects in BEs. The patient had an endothelial cell density test and showed results of 1952 cells/mm2 and 987 cells/mm2 in the RE and LE, respectively. A diagnosis of RE corneal edema, bilateral pseudophakia, and dry eye syndrome was established. The previous medication was continued for another two weeks. Two weeks after the last follow-up, corneal edema in the RE was resolved entirely, and visual acuity recovered to 0.4 and 0.8 for RE and LE, respectively.
3. Discussion
Ocular traumas resulting from chemical agents are a true ocular emergency that requires immediate evaluation and management [
1]. The incidence is still frequent [
11], and around 6% resulted from the inadvertent instillation of non-ophthalmic eyedrops mistaken for ophthalmic medication [
2]. Ophthalmologists around the world have been urged and proposed different packages for ophthalmic and non-ophthalmic products for almost 40 years [
3,
4,
5,
6,
7,
8,
12]. However, unfortunate events from non-ophthalmic products mistaken for eyedrops continue to occur. This case report adds to the continuing problem of chemical eye trauma.
Prompt irrigation is crucial in managing chemical trauma. Immediate evaluation and treatment contribute to a better prognosis. The severity of clinical presentation, which also contributes greatly to the prognosis, is determined by the causative agents, how long the substance contaminated the eye, and the time from injury to initial treatment [
1,
13]. To our knowledge, only one case report previously documented a similar accident to our study due to blue stamper ink mistaken for glaucoma medication [
12]. The patient had bilateral corneal abrasion, and the visual acuity returned to baseline after 24 h [
12]. Good prognosis also correlates with the rate of re-epithelialization and edema of the cornea, which, in our study, takes around three weeks to recover fully. After the injury, keratocytes migrate to repopulate the cornea, starting from the posterior stroma [
13]. In mild cases, type I collagen can be seen at the edge of the wound since the first day, while in moderate cases, it can take 7–14 days. Collagen synthesis can take 7–56 days, peaking at 21 days [
13]. In severe cases, keratocyte repopulation may not occur, and fibroblasts can lead to corneal scarring. Ascorbic acid is crucial in synthesizing collagen, and a lower level of ascorbic acid after the chemical injury can compromise collagen synthesis and result in corneal ulceration [
13]. Corneal re-epithelialization after chemical injuries, as reported by some reports, ranges from 24 h to 13 days [
6,
12].
Packaging non-ophthalmic consumer products in plastic “eyedropper” bottles poses a significant risk of accidental chemical ocular trauma, especially in elderly patients. Changes in the packaging, both the type of caps and the bottle height and labeling, of non-ophthalmic products in plastic eyedropper bottles are crucial to reducing these ocular injuries. Eyedropper bottles for various non-ophthalmic solutions must differ from the ophthalmic medication bottles. Some studies suggest that non-ophthalmic products in bottle packaging could incorporate the following features: (1) distinctly colored bottles or bottle caps (e.g., black-colored top or bright orange-colored bottle), (2) bottles with distinct shapes or ridges along the side, (3) child-safe caps with a locking mechanism, (4) and, for cyanoacrylate glue, the addition of a distinctive odor [
4,
8]. The US FDA has recommended that medications intended for topical use should be packaged in containers that differ from those for ophthalmic use. However, these recommendations were non-binding [
14]. Currently, no governmental or industry regulations in Indonesia mandate these essential safety features. One of the ophthalmic medication manufacturers in Indonesia is starting to produce products in mini-dose packages. The main purpose of their mini-dose packages is to minimize microorganism contamination. However, this can be considered a solution to reduce the ocular injuries resulting from mistaken instillation of eye medication. Education on distinguishing the non-ophthalmic and ophthalmic products is essential.
Some manufacturers of stamper ink have started to release material safety data sheets about the chemical ingredients and potential hazards that may affect health if any injuries occur [
15,
16,
17,
18]. Unfortunately, the ink producer that caused this injury is a home manufacturer and does not have any fact sheets. However, the main ingredients could be the same as others, which include dissolving dyes in a glycerol and water mixture, phosphates, colorants, and glycol [
15,
16,
17,
18]. It is important to note that even though some manufacturers have released toxicological information in their fact sheets, the information about the injury to the eye is still limited, with some writing “may cause eye irritation” or “none known” [
15,
17,
18], and only one manufacturer stated that bronopol (2-bromo-2-nitropropane-1,3-diol) can cause serious eye damage in the ingredients fact sheet [
16]. No information regarding the pH is available from these ink manufacturers. These facts add new insight into how potentially hazardous the ingredients’ stamper ink is for the eye and the importance of knowing the need to differentiate the package between stamper ink and ophthalmic medication.