Definition
Matrix certified reference materials (mCRMs) are materials characterized by suitable homogeneity, stability, and traceability, with certified values, including uncertainties, and a specific matrix. mCRMs constitute a reference for instrumental analytical methods and ensure their metrological consistency. Matrix certified reference materials (mCRMs) are essential tools for ensuring the accuracy and traceability of analytical measurements, particularly for samples with complex matrices. These mCRMs are carefully manufactured materials that closely mimic the composition and properties of real samples, allowing laboratories to validate their analytical methods, calibrate analytical instruments, or check the classical methods. This article highlights the challenges associated with the production and characterization of these complex mCRMs, including obtaining homogeneous materials, establishing accurate target values, and ensuring stability for different types of materials, such as gases, liquids, and metal alloys. Additionally, the process of statistical evaluation through the use of advanced statistical methods is discussed, as is the systems approach associated with the implementation of the ISO 17034 standard, which specifies the requirements for manufacturers of reference materials. This paper also includes a summary of the current status in trends of normalization as well as mCRM production.
1. Introduction
Contral tasks for modern laboratories include ensuring the reliability of chemical and instrumental analysis results and meeting quality requirements. For this purpose, laboratories use certified reference materials (CRMs). According to the definition of the ISO 17034:2016 standard, CRMs are reference materials for which one or more properties have been characterized by a metrologically recognized procedure, and should be accompanied by a certificate specifying the value of the listed properties and the associated uncertainty, and confirming the maintenance of metrological traceability [1]. This means that CRMs are materials with an exceptional degree of homogeneity and a very well-selected and defined composition that serve as a reference point for laboratories worldwide [2].
Laboratories use CRMs in several key ways to ensure the accuracy and reliability of analytical results. They are used for (a) instrument calibration, ensuring measurement accuracy and traceability over time (including the development of new methods), (b) validation of new analytical methods, confirming that the methods produce reliable and accurate results, (c) routine quality control to monitor the performance of analytical methods and instruments, (d) proficiency testing and interlaboratory comparisons, where laboratories analyze the same CRM and compare results to ensure traceability, consistency, and accuracy across different laboratories, (e) training laboratory personnel, ensuring they can accurately perform analyses and interpret results, (f) procedure uncertainty estimation, (g) demonstration of regulatory compliance of results, (h) troubleshooting and detection of potential issues with instruments or reagents, and (i) stability studies for analytical instrumentation. Additionally, their usage in the above-mentioned areas supports the evaluation of laboratories and audits carried out by regulatory bodies.
The standardized system of units, known as the International System of Units (SI), was approved in 1960 at the General Conference on Weights and Measures. Since then, the standards or definitions associated with it have officially set reference points in many areas of life. Although it is straightforward to refer to a mass standard in classical gravimetric methods or a volume standard in volumetry, it is much more challenging to refer to SI units in instrumental techniques that rely, for example, on determining the interaction of electromagnetic radiation with matter. In these methods, the recorded signals from samples are compared with signals previously obtained for well-known reference materials. In this case, there arises a need for reference materials that, on the one hand, allow for the calibration of the apparatus, and on the other, prove it is operating correctly. This situation and the need for standardization have prompted various institutions to take actions to produce appropriate materials. Alongside the development of the market and producers, systemic development has progressed, requiring the establishment of universal standards. The International Organization for Standardization (ISO) has been involved for years in processes aimed at standardizing rules to ensure the metrological recognition of CRM systems. Further sections describe the standards system developed by the organization with the most important sets of guidelines—ISO Guide 30, ISO 33401:2024, ISO 33405:2024 [3,4,5], and ISO 17034:2016 [1], which replaced ISO Guide 34 [6]. According to ISO 17034 [1], reference material producers must meet a range of requirements regarding quality management, documentation, traceability, and certification. The process of producing reference standards must be strict and controlled to ensure that the resulting products comply with specified specifications and standards. Laboratories analyze very different samples, operating in various areas of industry and science, and therefore require different reference materials. Hence, producers offer CRMs in various forms with certified physical or chemical properties (biological, solution-based, solid, liquid, metal alloys, and many others). Whenever possible, laboratories choose CRMs with compositions corresponding to the real samples they analyze. In this context, it is often necessary to use matrix certified reference materials (mCRMs), i.e., those in which the matrix is defined and relevant from the point of view of the analytical methods used. Such materials are often used in the area of solid sample analysis and techniques where various matrix effects occur. Given the great diversity of analyzed materials, it cannot be expected that mCRMs will be commercially available for all types of matrices. Furthermore, the production process for matrix standards is complicated and often takes many years, resulting in limited offerings and a constant race in which standard producers strive to keep up with new materials produced in the industry. As a result of systemic changes and introduced standards (laboratories with a quality system implemented in accordance with ISO/IEC 17025 [7] are required to use certified reference materials produced by a manufacturer meeting the requirements of ISO 17034 [1]), interest in CRMs is steadily increasing. In the Scopus database, 75 documents were found under the keyword “Matrix certified reference materials” in the period of 1998–2025, with a significant increase in the number of scientific documents observed after 2018. By subject area, chemistry accounts for as much as 40% of all articles since the initiation of Scopus, followed by biochemistry (15.2%), engineering (9%), physics and astronomy (8.3%), chemical engineering (6.2%), and environmental sciences (5.5%). Other subject areas account for less than 5% [8]. Therefore, there is a need to ensure the availability of sufficient quantities of these materials. Among mCRM producers that compete on the global market, the following should be mentioned: NIST (National Institute of Standard and Technology, USA), LGC Standards (UK), BAM (Bundesanstalt für Materialforschung und-Prüfung, Germany), RIST (Research Institute of Industrial Science and Technology, Republic of Korea), KTR (Korea Testing and Research Institute), IRMM (Institute for Reference Materials and Measurements, European Union), and NMIJ (National Metrology Institute of Japan). Certified reference materials can be found by searching the Internet, but with the growth of the CRM market and the diversity of producers, finding the right choice has become difficult. This is why using a reference material database such as COMAR (platform of BAM) or CNRM (The National Sharing Platform for Reference Materials of China) can help users find materials more easily. For example, the COMAR database has 2030 CRMs with additional division by ISO 17034-accreditedCRMs (1218) [1] and CRMs from NMIs (national metrology institutes) (285). Moreover, it offers the function to search for CRMs by producer, material, properties, or fragments. In turn, the CNRM database can be divided by category or subcategory: ferrous metals (carbon steel, low-alloy steel, ferro-alloy, etc.), non-ferrous metals and gases (heavy metals and their alloys, light metals and their alloys, gases in metals, rare-earth element ores), geological materials (ore, rock), energy resources (coal, fuel, petroleum products), and many others. All of these are designed to help choose the right material.
2. The Process of Manufacturing Materials for Matrix CRMs
The production of matrix certified reference materials (mCRMs) is a meticulous, multi-stage process that is governed by strict international guidelines (mainly ISO 17034 [1] and ISO 17025 [6]). The aim of these guidelines is to achieve the highest possible levels of homogeneity, stability, and traceability, ensuring that CRMs can be reliably used for instrument calibration, method validation, and quality control. Depending on the type of CRM that is being developed, production is carried out in different ways, but the general scheme is as follows:
- Planning and material selection (defining the need);
- Material processing and preparation;
- Homogeneity testing;
- Stability testing;
- Characterization (value assignment);
- Certification and documentation;
- Post-certification monitoring.
Table 1 briefly describes, in a very general way, how the individual stages differ depending on the type of material (gas, liquid or solid state), with regard to metal alloys, polymers, organic substances, geological samples, etc.

Table 1.
The general differences between three states of mCRMs.
The development process also involves the preparation of a certificate that contains information about the CRM, including its certified values, uncertainties, and intended use. Once the value assignment process is complete, the CRM may be included in the accreditation scope of the accredited reference material producer.
3. Process of Statistical Assessment
A very important constituent of the mCRM production process is the determination of the measured value and its extended uncertainty. The approach to this depends on the type of CRM. There are two types of measurand determination approaches—independent of the particular procedure and operationally determined, where the completely described procedure is the one accepted. In the case of matrix CRMs, both of the approaches may be used for different values; however, as the matrix has an essential meaning in analysis of composition or physical properties, the independence of mass or mole or other SI-traceable values may be reached. This is always a favorable situation, where CRM may be used for different analytical methods and procedures, and thus the majority of certified values are treated in this way. The choice of approach has an impact on further laboratory steps and analysis, and thus for any particular procedure, only that procedure can be used in all analytical processes, while in the independent variant, different authorized methods may be used. However, the statistical approach will be the same in both cases. A new CRM is statistically processed according to its main properties: homogeneity, stability, and characteristic (certified) values. The final aim is the determination of the traceable certified values and their extended uncertainties. The general guidelines for all of these three stages are included in ISO 33405:2024 [5]. Finally, each of the properties delivers a constituent of the final extended uncertainty. Table 2 summarizes the main uncertainty constituents, their importance, and the general most-often-used approach to determination.

Table 2.
Main uncertainty constituents for matrix CRMs.
The determined certified value has to be noted with its expanded uncertainty U. The final budget of the uncertainty includes uncertainty of homogeneity, uncertainties of stabilities (long and short term/transportation), and uncertainty of characterization [1,5]. It has to be noted that the expanded uncertainty is calculated as standard uncertainty multiplied using the coverage factor k. Commonly used values for k are 2 and 3, which correspond to confidence levels of approximately 95% and 99% for a normal distribution. For mCRMs, a value of k = 2 is more often used, meaning that the measurement result with expanded uncertainty covers 95% probability that the true value lies within this interval.
It has to be emphasized that certified value uncertainty is not equal to measurement uncertainty. The result of the analysis of a certified reference material does not have to fall within the uncertainty of the certified value, because in the measurement uncertainty budget, in addition to the uncertainty of the certified value resulting from the characteristics of the CRM itself (homogeneity, stability, characterization process), the uncertainty arising from the measurement method used, and sometimes also from environmental factors (e.g., temperature or pressure), must be taken into account. It is worth noting that mCRMs are often used as a reference in the determination of expanded uncertainties of measurements, as is described in ISO/IEC Guide 98-3 (GUM) [37].
It should be clearly stated that there is no one universal statistical approach to the production of mCRMs, as they encompass a very wide scope of materials and the process of statistical treatment of the results has to be considered separately for each new product. The general methodology described is probably the one most commonly used by mCRM producers. However, general rules and examples are included in the ISO 33405 standard [5].
4. A Systematic Approach to the Production of Certified Reference Materials
A systematic approach to the production of certified reference materials (CRMs) is fundamental to ensuring the accuracy, traceability, and reliability of measurement results in laboratories worldwide [38]. International standards such as ISO 17034:2016—General requirements for the competence of reference material producers—and the ISO 33400 series establish uniform guidelines for terminology, production, characterization, and documentation [1,39]. Compliance with international guidelines ensures that CRMs are metrologically consistent, enabling a reliable comparison of results between different laboratories [40]. This directly translates to the accuracy of test results and customer trust. Additionally, in some cases covered by legal regulations, meeting legal requirements often depends on the use of CRMs that comply with international standards.
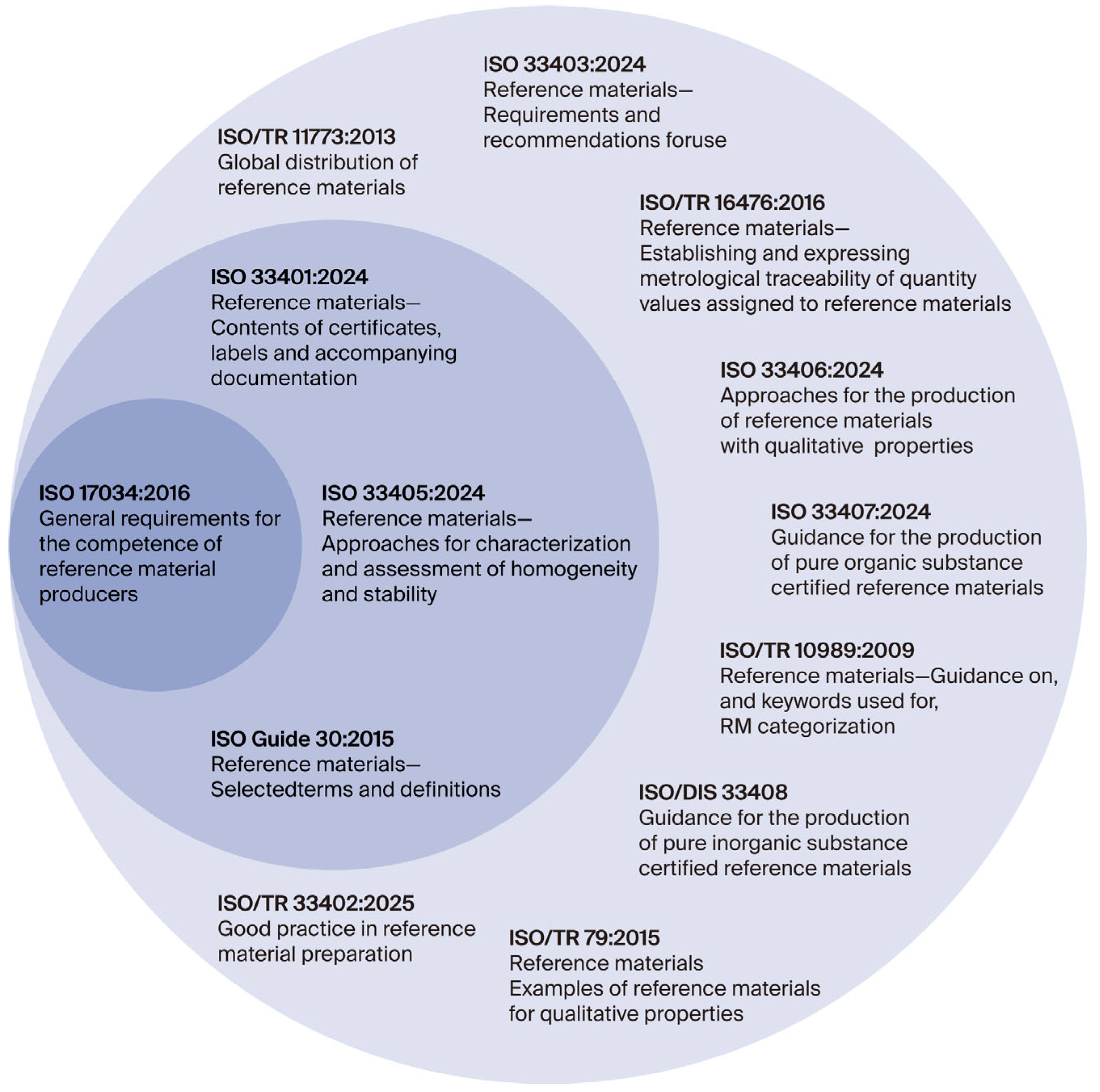
The most important standards that closely support the implementation of ISO 17034 requirements include ISO Guide 30:2015, Reference materials—Selected terms and definitions; ISO 33401:2024, Reference materials—Contents of certificates, labels and accompanying documentation; and ISO 33405:2024, Reference materials—Approaches for characterization and assessment of homogeneity and stability [3,4,5]. The terminology used in the field of reference materials is also aligned with the ISO/IEC Guide 99:2007 [41] International vocabulary of metrology—Basic and general concepts and associated terms (VIM), which provides standardized definitions essential for clear communication in metrology and quality assurance. A cross-referenced and comprehensive overview of the most relevant and up-to-date ISO standards and technical reports related to the production and application of reference materials is presented in Figure 1 and Table 3.
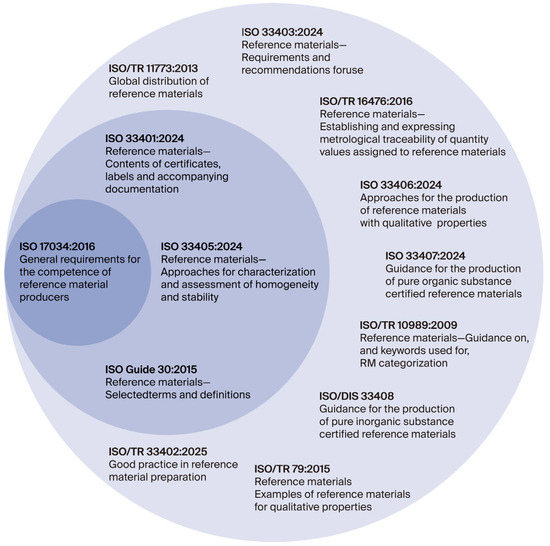
Figure 1.
Relationship between ISO 17034 [1], ISO 33400 [4,5] series, and technical reports in CRM production [42].

Table 3.
Overview of ISO Standards and Technical Reports relevant to the production and use of reference materials.
In summary, a systematic approach to CRM production, based on international standards such as ISO 17034 [1] and the ISO 33400 [5] series standards, as well as the indicated technical reports, is essential for ensuring high-quality and reliable measurement results. This approach promotes the continuous improvement of production processes and raises quality standards.
5. Conclusions and Prospects
Certified reference materials are crucial for ensuring measurement traceability and the accuracy of results in instrumental analytical chemistry. mCRM production is challenging because it usually requires a non-regular approach to the development of the material, many tests (e.g., homogeneity, long-term and short-term stability, characterization tests), statistical treatment of the analytical results suitable to the process, and working within quality systems, standards, and formal regulations. Because of the variety of mCRMs, the description or establishment of one universal procedure is impossible; different producers use different production processes, strategies, and different statistical treatment of the results to produce materials with the most suitable properties for use. The dynamic development of technology poses new challenges and opens new opportunities. Currently, the greatest challenges in the production of new CRMs are linked to advanced analytical techniques, such as next-generation mass spectrometry and high-resolution spectroscopy, which require CRMs of higher quality, stability, and homogeneity to meet their specific requirements. Moreover, the development of “small spot” techniques for direct analysis of solid samples, such as laser ablation inductively coupled plasma spectrometry, micro X-ray fluorescence spectrometry, or glow discharge optical emission spectrometry, has resulted in the requirement of mCRMs with confirmed homogeneity in very small sample volumes. The development of new materials, including nanomaterials and biomaterials, poses a challenge for mCRM manufacturers to develop appropriate matrix reference materials considering their unique properties. As a result, Łukasiewicz-IMN, an mCRM producer, has observed an increasing interest in the market for the development of individual and specific reference materials well suited for specific processes or customers. The other observed demand is for a short production time; mCRM production usually takes years, but the need for direct and immediate availability of CRMs is common. Increasing awareness of standardization requirements in the market will result in demand for mCRMs produced in reference to the most current standards. This is, now and in the future, challenging because of the dynamic characteristics of such regulations, especially the implementation of new ISO standards in place of preliminary ISO Guides and other formal and informal documents. The unexpected result of such dynamic regulations is the decreasing demand for CRMs produced in the past, when the specific regulations were not present. As the development of new mCRMs always results from the development of new materials, similar progress may be expected in the current trends in material engineering. The future challenges posed to reference material manufacturers include, among others, the use of artificial intelligence and machine learning to design and produce CRMs with optimal properties that are adapted to specific applications. The CRM market is shaping up as a dynamic area in which technological innovation and changing analytical needs will shape the development of new reference materials. Taking into account all the observed changes and trends, it can be assumed that technological and formal requirements for mCRMs are growing. Therefore, the past strategy used by producers for the production of large batches which will satisfy the market for many years is no longer relevant. All these factors also affect the cost of production and, as a result, the prices of mCRMs, but also stimulate producers to change and develop. Currently, no factors have been identified that could reduce the dynamics of changes in the certified reference material market in the future.
Author Contributions
Conceptualization and writing, T.G.; writing, J.K., M.W., and E.J.; review and editing, J.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- ISO 17034:2016; General Requirements for the Competence of Reference Material Producers. International Organization for Standardization: Geneva, Switzerland, 2016.
- Outaki, M.; Loukhmas, S.; Gmouh, S.; Kerak, E. Reference Materials: A review. J. Turk. Chem. Soc. Chem. A 2024, 11, 751–764. [Google Scholar] [CrossRef]
- ISO Guide 30:2015; Reference Materials—Selected Terms and Definitions. International Organization for Standardization: Geneva, Switzerland, 2015.
- ISO 33401:2024; Reference Materials—Contents of Certificates, Labels and Accompanying Documentation. International Organization for Standardization: Geneva, Switzerland, 2024.
- ISO 33405:2024; Reference Materials—Approaches for Characterization and Assessment of Homogeneity and Stability Standard. International Organization for Standardization: Geneva, Switzerland, 2024.
- ISO Guide 34:2009; General Requirements for the Competence of Reference Material Producers. International Organization for Standardization: Geneva, Switzerland, 2009.
- ISO/IEC 17025:2017; General Requirements for Competence of Testing and Calibration Laboratories. International Organization for Standardization: Geneva, Switzerland, 2017.
- Scopus—Analyze Search Results. Available online: https://www.scopus.com/results/results.uri?st1=%22matrix+certified+reference+materials%22&st2=&s=TITLE-ABS-KEY%28%22matrix+certified+reference+materials%22%29&limit=10&origin=searchbasic&sort=plf-f&src=s&sot=b&sdt=b&sessionSearchId=2e08ca783c109311a3004f4ee55cf2b9 (accessed on 20 February 2025).
- JCGM. Evaluation of Measurement Data—Guide to the Expression of Uncertainty in Measurement; JCGM: Paris, France, 2010. [Google Scholar]
- Durbiano, F.; Pavarelli, S.; Rolle, F.; Pennecchi, F.R.; Sega, M. Production of gaseous certified reference materials at inrim for amount of substance fraction of CO2. In Proceedings of the IMEKO TC8 TC11 TC24 Conference: New Perspectives in Chemical Measurements, Madeira, Portugal, 11–13 October 2023; pp. 38–41. Available online: https://www.imeko.org/index.php/proceedings/9379-production-of-gaseous-certified-reference-materials-at-inrim-for-amount-of-substance-fraction-of-co2 (accessed on 15 May 2025).
- ISO 6145-10:2002; Gas analysis—Preparation of Calibration Gas Mixtures Using Dynamic Volumetric Methods, Part 10: Permeation Method. International Organization for Standardization: Geneva, Switzerland, 2002.
- O’Keeffe, A.E.; Ortman, G.C. Primary standards for trace gas analysis. Anal. Chem. 1966, 38, 760. [Google Scholar] [CrossRef]
- Ricci, M.; Lava, R.; Koleva, B. Matrix Certified Reference Materials for environmental monitoring under the EU. Water Framework Directive: An update. Trends Anal. Chem. 2016, 76, 194–202. [Google Scholar] [CrossRef]
- Wise, S.A.; Hilpert, L.R.; Rebbert, R.E.; Sander, L.C.; Schantz, M.M.; Chesler, S.N.; May, W.E. Standard reference materials for the determination of polycyclic aromatic hydrocarbons. Fresenius’ J. Anal. Chem. 1988, 332, 589–596. [Google Scholar] [CrossRef]
- Tadić, Đ.; Pires de Lima, A.; Ricci, M. Quality assurance and quality control for human biomonitoring data—Focus on matrix reference materials. Anal. Bioanal. Chem. 2025, 107476. [Google Scholar] [CrossRef] [PubMed]
- Janko, P.; Malejczyk, E.; Nawotka, M. Development of certified reference materials of ethanol in aqueous solution resulting from the participation of GUM in EMPIR 16RPT02 ALCOREF project. Accredit. Qual. Assur. 2023, 28, 35–48. [Google Scholar] [CrossRef]
- Inagaki, S.; Numata, M.; Kitamaki, Y.; Hanari, N.; Iwasawa, R. Characterization of water content in biodiesel fuel certified reference material (NMIJ CRM 8302-a). Accredit. Qual. Assur. 2016, 21, 361–366. [Google Scholar] [CrossRef]
- Kostrzewa, J.; Anyszkiewicz, J.; Gorewoda, T.; Jamroz, E.; Blandhol, K.; Guldhav, A.Y.; Knapik, M.; Charasińska, J.; Jakóbik-Kolon, A. Development of New Series of Certified Reference Materials for Ferrosilicon Magnesium Alloys. Processes 2024, 125, 1017. [Google Scholar] [CrossRef]
- Karar, N.; Jain, V. Alloys as Certified Reference Materials (CRMs), Handbook of Metrology and Applications; Springer: Berlin/Heidelberg, Germany, 2023; pp. 697–730. [Google Scholar] [CrossRef]
- Dias, F.A.; Carlos, C.J. Evaluation of Homogeneity in Certified Reference Materials (CRM). In Proceedings of the 19th International Congress of Metrology, Caparica, Portugal, 21 October 2019; p. 20001. [Google Scholar] [CrossRef]
- Hioki, A.; Ohata, M.; Matsuyama, S.; Kinugasa, S. Development of plastic certified reference materials (CRMs) to cope with restrictions on hazardous substances. Synthesiology 2015, 8, 27–40. [Google Scholar] [CrossRef]
- Vasil’eva, I.E.; Shabanova, E. Certified reference materials of geological and environmental objects: Problems and solutions. J. Anal. Chem. 2017, 72, 129–146. [Google Scholar] [CrossRef]
- Linsinger, T.P.J. Evaluation of CRM homogeneity in cases of insufficient method repeatability: Comparison of Bayesian analysis with substitutes for ANOVA based estimates. Anal. Chim. Acta X 2020, 5, 100049. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.J.C.; Walker, R.J. Homogeneity and stability assessment of reference materials. Accredit. Qual. Assur. 2006, 11, 428–435. [Google Scholar]
- van der Veen, A.M.H.; Pauwels, J. Uncertainty calculations in the certification of reference materials. Principles of analysis of variance. Accredit. Qual. Assur. 2001, 6, 26–30. [Google Scholar] [CrossRef]
- Inagaki, K.; Jung, C.; Riedel, J.; Mauch, T.; Sauer, A.; Koch, M. Development of certified reference materials for polycyclic aromatic hydrocarbons in sediment: Homogeneity and stability assessment. Anal. Sci. 2015, 31, 655–660. [Google Scholar]
- Snell, J.; Emteborg, H.; Schimmel, H. Certification Report the Certification of the Mass Fractions of Elements in Fish Muscle Certified Reference Material ERM®-BB422; Institute for Reference Materials and Measurements (IRMM): Geel, Belgium, 2012. [Google Scholar]
- Toth, K.; Jakopic, R.; Bauwens, J.; Hennessy, C.; Kehoe, F.; Jakobsson, U.; Richter, S.; Aregbe, Y. Certification Report Preparation and Certification of Large-Sized Dried (LSD) Spike: IRMM-1027v; JRC Reference Materials Report; Institute for Reference Materials and Measurements (IRMM): Geel, Belgium, 2022. [Google Scholar]
- Garzón, D.A.; Ahumada, D.A.; Paredes, C.; Castillo, E. Development of a reference material for mercury in fish: Certified for total mercury and characterized for methylmercury. Anal. Bioanal. Chem. 2025, 417, 2717–2726. [Google Scholar] [CrossRef] [PubMed]
- Lamberty, A.; Schimmel, H.; Pauwels, J. The study of the stability of reference materials by isochronous measurements. Fresenius J. Anal. Chem. 1998, 360, 359–361. [Google Scholar] [CrossRef]
- Paredes, C.; Meija, J. Reference material stability challenge. Anal. Bioanal. Chem. 2024, 416, 5215–5216. [Google Scholar] [CrossRef] [PubMed]
- van der Veen, A.; Linsinger, T.; Lamberty, A.; Pauwels, J. Uncertainty calculations in the certification of reference materials 3. Stability study. Accredit. Qual. Assur. 2001, 6, 257–263. [Google Scholar] [CrossRef]
- Recknagel, S. Certification Report Certified Reference Material BAM-M321 Al-Alloy 2024 AlCu4Mg1; Bundesanstalt für Materialforschung und -Prüfung (BAM): Berlin, Germany, 2020. [Google Scholar]
- Cho, H.; Dasari, K.B.; Lim, M.C.; Hong, S.P.; Sun, G.-M.; Heo, S.W.; Lee, K.-S. Determination of certifed values for Co, Cr, and Zn in lake sediment CRM (KRISS 109-05-002) using the INAA method. J. Radioanal. Nucl. Chem. 2024, 333, 6609–6618. [Google Scholar] [CrossRef]
- Andrzejuk, W.; Bau’, A.; Charoud-Got, J.; de Vos, P.; Emteborg, H.; Lamberty, A.; Linsinger, T.; Oostra, A.; Quétel, C.; Roebben, G.; et al. Certification Report Certification of the Sulphur Mass Fraction in Three Commercial Petrol Materials Certified Reference Materials ERM®-EF211, ERM®-EF212, ERM®-EF213; Bundesanstalt für Materialforschung und -Prüfung (BAM): Berlin, Germany, 2007. [Google Scholar]
- Ricci, M.; de Boer, J.; Johansen, J.E.; Liu, H.; Dumas, P.; Warner, N.A.; Pērkons, I.; McGrath, T.J.; Borgen, A.R.; Bjørneby, S.M.; et al. Stepping-Up accurate quantification of chlorinated paraffins: Successful certification of the first matrix reference material. Anal. Chim. Acta 2024, 1315, 342757. [Google Scholar] [CrossRef] [PubMed]
- ISO Guide 98-3:2008; Uncertainty of Measurement Part 3: Guide to the Expression of Uncertainty in Measurement (GUM:1995). International Organization for Standardization: Geneva, Switzerland, 2008.
- Producenci materiałów odniesienia (Reference Materials Producers). Available online: https://www.pca.gov.pl/obszary-akredytacji/producenci-materialow-odniesienia (accessed on 14 February 2025).
- Introduction to ISO/TC 334 and the ISO 33400 Standard Series for Reference Materials. Available online: https://www.eurachem.org/images/stories/workshops/2024-09_QRM/pdf/0-03-Botha.pdf (accessed on 14 February 2025).
- European Parliament and Council. Directive 2004/22/EC of the European Parliament and of the Council of 31 March 2004 on Measuring Instruments; European Parliament and Council: Brussels, Belgium, 2004. [Google Scholar]
- ISO Guide 99:2007; International Vocabulary of Metrology—Basic and General Concepts and Associated Terms (VIM). International Organization for Standardization: Geneva, Switzerland, 2007.
- Hammond, J.P. Quality Matters. In Proceedings of the ISO Technical Committee on Reference Materials (ISO TC 334): Annual Meeting 2022 and ISO Future Developments 2022, Hainault, UK, 7–14 June 2022; Volume 34, pp. 36–38. [Google Scholar]
- ISO/TR 33402:2025; Good Practice in Reference Material Preparation. International Organization for Standardization: Geneva, Switzerland, 2025.
- ISO 33403:2024; Reference Materials—Requirements and Recommendations for Use. International Organization for Standardization: Geneva, Switzerland, 2024.
- ISO 33406:2024; Approaches for the Production of Reference Materials with Qualitative Properties. International Organization for Standardization: Geneva, Switzerland, 2024.
- ISO/TR 79:2015; Reference Materials—Examples of Reference Materials for Qualitative Properties. International Organization for Standardization: Geneva, Switzerland, 2015.
- ISO/TR 10989:2009; Reference materials—Guidance on, and Keywords Used for, RM Categorization. International Organization for Standardization: Geneva, Switzerland, 2009.
- ISO/TR 11773:2013; Global Distribution of Reference Materials. International Organization for Standardization: Geneva, Switzerland, 2013.
- ISO/TR 16476:2016; Reference Materials—Establishing and Expressing Metrological Traceability of Quantity Values Assigned to Reference Materials. International Organization for Standardization: Geneva, Switzerland, 2016.
- ISO 15194:2009; In Vitro Diagnostic Medical Devices—Measurement of Quantities in Samples of Biological Origin—Requirements for Certified Reference Materials and the Content of Supporting Documentation. International Organization for Standardization: Geneva, Switzerland, 2009.
- ISO 33407:2024; Guidance for the Production of Pure Organic Substance Certified Reference Materials. International Organization for Standardization: Geneva, Switzerland, 2024.
- ISO/DIS 33408; Guidance for the Production of Pure Inorganic Substance Certified Reference Materials. International Organization for Standardization: Geneva, Switzerland, 2024.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).