Abstract
In situations where animal models (AMs) are necessary, as in the field of neuroscience, a strong culture of care must be supported and established. The pivotal question remains: how can we uphold a robust “culture of care”? In the multifaceted domain of neuroscience research, AMs traverse a spectrum shaped by conflicting viewpoints, anthropocentrism and pathocentrism, where established scientific norms intersect with ethical deliberations. Anthropocentrism, representative of conventional scientific approaches, may prioritize scientific goals potentially to the detriment of animal welfare. Conversely, pathocentrism places significant importance on the ethical treatment and well-being of AMs. This divergence of approach prompts the imperative development of a robust culture of care framework within research institutions, advocating for animal welfare, ethical responsibility, and adherence to regulatory standards. In this review, we refer to a European view of animal care, discussing internationally valid concepts that find rebuttal in the current European legislation. This review meticulously analyzes the many facets of the culture of care, particularly for neuroscience studies involving AMs, illustrating the principles, practices, and collaborations critical to overcoming ethical expectations. This commitment increases credibility and builds trust in the public and research spheres, underscoring the critical importance of a culture of care in the ethics of neuroscience research.
1. Introduction
Exploring the human brain and nervous system (NS) represents one of scientific inquiry’s most intricate and promising frontiers [1,2,3]. In this area, neuroscience research often relies on animal models (AMs) to unravel the complexities of neural function, behavior, and disease mechanisms [3,4,5]. However, ethical considerations surrounding using AMs in research have attracted increasing public and research community attention [6,7]. To navigate this moral landscape effectively, institutions engaged in research using AMs must adhere to a comprehensive culture of care framework [8]. The concept of a “culture of care” within the context of utilizing animals in scientific research pertains to the organizational environment that fosters continuous improvement in various aspects: animal care and welfare, the well-being of staff engaged in the animal care and use program, scientific quality, openness, and transparency [9]. While European Directive 2010/63/EU on protecting animals for scientific purposes does not explicitly mention “culture of care,” it underscores the importance of fostering a “climate of care” within animal welfare bodies (AWBs), as indicated in Recital 31 [9]. Although not expressly stated in the directive, guidance documents from the European Commission, member states, and stakeholders refer to the significance of a culture of care. For instance, the Education and Training Framework promotes a “Culture of Care” among staff at all levels. Additionally, guidance on inspections and enforcement incorporates the concept of a culture of care, offering insights into factors influencing its determination and leveraging inspectors or inspections to promote it. A culture of care transcends mere compliance with legal requirements [9]. It encompasses an organizational culture that values and supports compassionate and respectful behavior towards animals and colleagues. Everyone involved in animal studies, from those directly conducting research to animal facility management, planners, engineers, biologists, chemists, statisticians, project leaders, and senior leaders, is responsible for cultivating a culture that emphasizes ethical practices and continuous improvement [9,10,11,12]. Within this context, addressing the expectations of the public, who rightly advocate for ethical animal treatment, and meeting the stringent ethical standards set by the research community become imperative for these institutions [13]. This review explores the multifaceted dimensions within a culture of care framework [14,15]. Specifically tailored to neuroscience research involving animal subjects, this review delineates the principles, practices, and collaborations essential to meeting and exceeding the ethical expectations of the public and the research community [6,7].
From transparent communication to continuous improvement, welfare-centric practices, and collaborative education, this review delves into the intricacies of fostering ethical excellence within neuroscience research [16].
1.1. Ethical Considerations: Prioritizing Animal Welfare and Scientific Progress
The interface between animal welfare and research necessitates a delicate balance between scientific progress and ethical responsibility [16,17]. Animal welfare refers to the appropriate condition of a species based on science and ethics. It encompasses multifaceted considerations, from the initial stages of experimental design to the implementation of procedures and the overall well-being of animal subjects [18,19]. Ensuring animal welfare involves providing optimal living conditions that mimic natural environments tailored to the species’ behavioral and physiological needs [10,19]. Enrichment strategies, such as cognitive stimulation and social interactions, are integrated into housing environments to promote mental well-being and prevent boredom or distress [11,16,20]. The PREPARE Guidelines (https://norecopa.no/prepare, accessed on 15 December 2023) are pivotal to guiding researchers toward a meticulous and ethically sound approach [21]. They emphasize not only the scientific rigor but also the ethical responsibilities towards animal subjects [18,22,23]. These guidelines advocate for reducing animals used in experiments, refining procedures to minimize pain or distress, and replacing new alternative models (NAMs) wherever feasible [24,25]. Researchers navigate ethical considerations by continually refining methodologies and embracing innovative technologies to reduce the reliance on AMs [13,22,26]. Techniques such as non-invasive imaging or in vitro models offer alternatives that minimize the need for animal subjects, promoting ethical practices while advancing scientific knowledge [19,20]. Moreover, fostering a culture of care within research centers should be an institutional commitment to the ethical treatment of animals [10,14,27,28,29]. This involves comprehensive training for researchers, veterinarians, and support staff, ensuring they understand and adhere to ethical animal handling and experimentation guidelines [30,31]. The ethical landscape within research involving animals encapsulates a collective commitment to upholding rigorous scientific standards while prioritizing the welfare of sentient beings [10,19].
1.2. Ethical Paradigms: Anthropocentrism and Pathocentrism Examined
In the dynamic landscape of scientific research, AM utilization navigates a spectrum influenced by opposing perspectives, anthropocentrism and pathocentrism, where traditional scientific paradigms clash with ethical considerations [32,33]. Anthropocentrism, often reflective of traditional scientific practices, might prioritize achieving scientific objectives at the potential expense of animal welfare [34,35,36]. It ensures that the animals involved are treated ethically and compassionately throughout the research process, acknowledging their capacity to experience emotions and sensations [34,35,36]. Pathocentrism in scientific research ensures ethical standards and yields more reliable and translatable results. Within a pathocentric framework, researchers embracing animal experimentation must initially assess its indispensability [37,38,39,40]. Can valuable insights be derived from robust meta-analyses, probabilistic computational tools, or NAMs [41]? However, it is crucial to acknowledge that scientific inquiry frequently demands exploration beyond cellular or molecular levels, necessitating more intricate investigations [38,39,40,41,42]. When the trajectory of research mandates animal experimentation, the paramount concern for the researcher becomes prioritizing the welfare of these subjects [38,39,40,41,42]. By emphasizing the well-being of AMs, researchers reduce confounding factors such as stress-induced responses that might skew experimental outcomes [38,39,40,41,42,43]. Moreover, ethical research practices often contribute to better animal health, reducing variables that might interfere with neurological studies [37,42]. Adopting pathocentrism does not necessarily hinder scientific progress; instead, it encourages researchers to refine methodologies, explore alternative approaches, and create enriched environments for AMs [37,42]. Pathocentrism and the culture of care shape the ethical landscape of using AMs in scientific research [44,45]. Pathocentrism, by recognizing the intrinsic value and conscious nature of AMs, advocates for their ethical treatment, emphasizing the reduction in distress and promoting their overall well-being throughout the research process [44,45]. This ethical standpoint aligns with the culture of care, fostering an environment where researchers prioritize animal welfare by implementing refined methodologies, providing enriched environments, and continuously evaluating protocols to minimize discomfort and stress for the AMs involved [44,45]. The culture of care embodies a collective commitment within research institutions to uphold stringent ethical standards, training researchers in handling and fostering an ethos that values the ethical treatment of animal subjects [39,42]. Pathocentrism and the culture of care establish an ethical framework that advances scientific knowledge in the research community, acknowledging their intrinsic value and promoting ethical responsibility [42,46,47].
1.3. Synergizing Ethical Compasses: Comparing the Five Freedoms and the Five Domains
The five freedoms framework embodies essential principles guiding ethical treatment of animals involved in scientific studies [48,49]. Freedom from hunger and thirst, discomfort, pain, and distress, and the ability to express natural behaviors hold profound implications in experiments [48,49]. AMs must have access to fresh water and a diet that maintains health and vigor, meeting their nutritional needs [50]. AMs should have a suitable environment that offers shelter and living conditions that avoid discomfort [50,51,52]. This includes appropriate resting areas, protection from adverse weather, and clean living spaces [50,51,52]. Measures should be in place to prevent or rapidly diagnose and treat injuries or diseases to minimize suffering [50,51,52]. AMs should have sufficient space and suitable conditions to exhibit natural behaviors characteristic of their species [50,51,52]. This includes providing opportunities for social interactions, exploration, and activities that allow them to express their innate behaviors [50,51,52]. The environment and handling should not induce mental suffering or distress in AMs [50,51,52]. Integrating these principles into a broader culture of care within research institutions elevates their significance [27,28]. The culture of care fosters an environment where these freedoms become embedded in the institutional ethos, influencing the practices and behaviors of researchers and staff [13,28]. It emphasizes creating enriched environments that cater to the animals’ needs, refining methodologies to minimize distress and prioritizing their welfare throughout the research process [14,29]. This approach ensures that research upholds ethical standards, minimizing potential suffering for AMs while fostering a commitment to responsible and compassionate scientific inquiry [14,29].
In 1994, Professor David Mellor and Dr. Cam Reid introduced a novel model, transforming the established five freedoms into “five domains” to systematically assess and grade the severity of welfare compromise in various aspects: nutrition, environment, health, behavior, and mental state/experiences [53,54,55]. This approach distinguishes between the physical and functional factors affecting an animal’s welfare and the overall mental state arising from these factors [53,55]. Widely embraced over the past two decades, this paradigm has become a valuable tool for evaluating the welfare impacts of research procedures, pest control methods, and other animal life interventions [53,54,55].
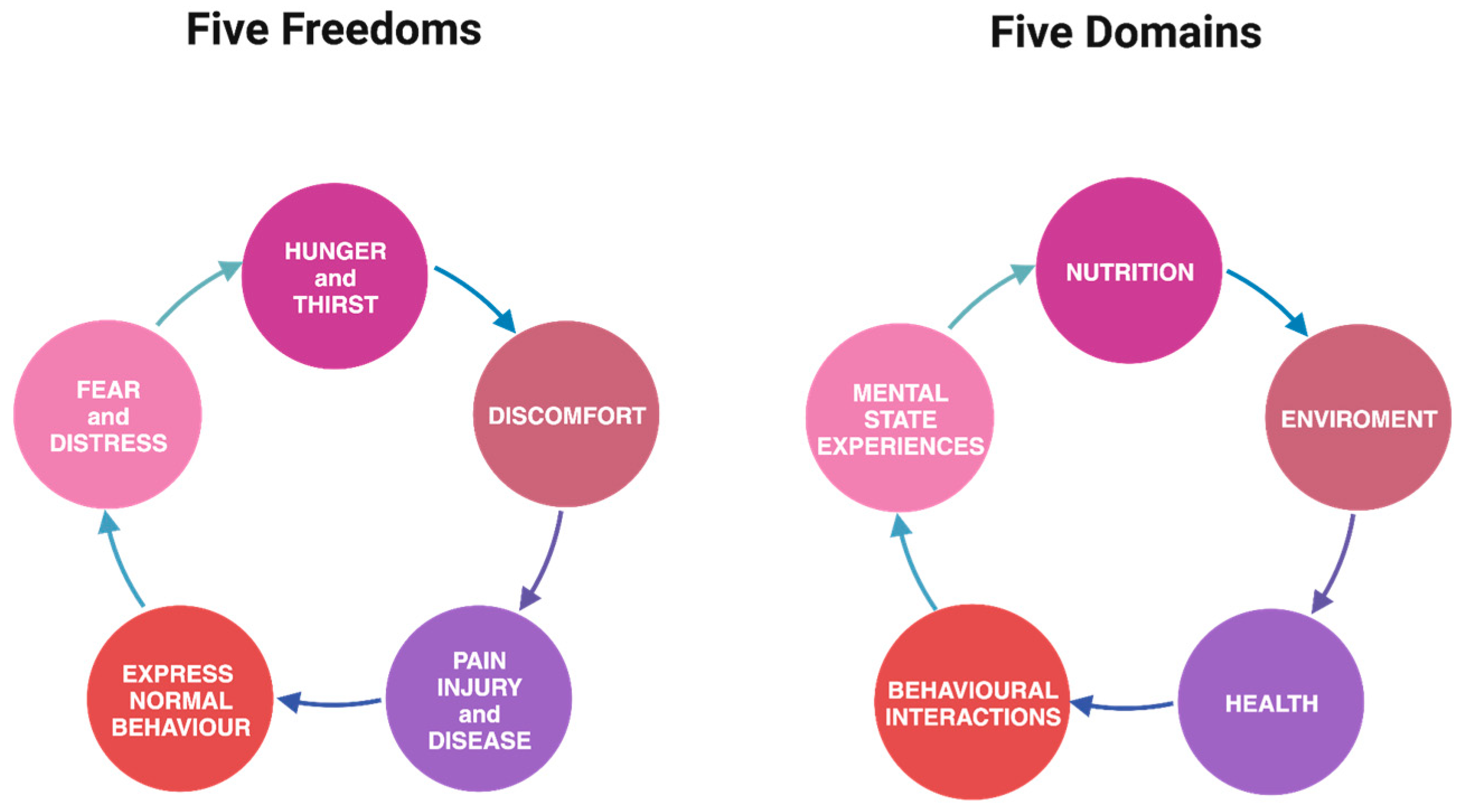
While both the five freedoms and five domains frameworks share the same core elements, the latter delves more deeply into the mental state of animals, recognizing that an emotion or subjective experience may accompany every physical aspect influencing welfare [53,54,55]. This nuanced exploration reinforces that emotional needs are as significant as physical needs for animals (Figure 1)
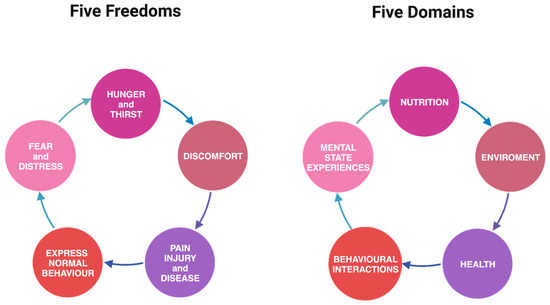
Figure 1.
Comparison of five freedoms and five domains versus one another.
A notable strength of the five domains framework lies in its clarity, emphasizing that merely alleviating negative physical or mental states does not necessarily guarantee positive welfare; it may, at best, achieve a neutral state. True good welfare for animals goes beyond the mere resolution of negatives [53,55].
Ensuring animals lead a “good life” involves providing opportunities for positive experiences, including anticipation, satisfaction, and satiation. Caretakers must create environments that encourage animals to engage in rewarding behaviors [53,55]. This shift in perspective forms the foundation of the five domains model, which integrates positive welfare states into its assessment, extending beyond the traditional focus on minimizing negatives [53,55].
Therefore, the five domains model serves as a comprehensive evaluation tool for assessing the welfare of individuals or groups of animals, prioritizing mental well-being and emphasizing the importance of facilitating positive experiences, thus expanding our considerations beyond the original five freedoms [53,55].
2. The Culture of Care
In the early 2000s, researchers introduced the concept of a “culture of care” regarding AMs [27,29]. This concept, borrowed from the health and patient care field, has gained significant importance since European Directive 2010/63/EU and the subsequent publication of the working document on Animal Welfare Bodies and National Committees to fulfill the requirements under the Directive in 2021 [56]. The concept of culture of care encapsulates not only the conduct and behavior of individuals but also the underlying principles and guiding beliefs upheld by institutions [14,28,29]. These elements collectively influence the successful implementation of replacement, reduction, and refinement (3Rs) and the ethical treatment of animals in research settings [42,57]. this framework emphasizes comprehensive protocols to ensure optimal living conditions, tailored care, and enrichment strategies that cater to the species’ behavioral and physiological needs [51,58,59,60]. The culture of care does not merely advocate for ethical standards [51,58,59,60]. However, it fosters a collective responsibility within research institutions, promoting continuous refinement of methodologies and exploring innovative technologies to minimize reliance on AMs [51,58,59,60]. The concept of culture of care includes not only the conduct and behavior of individuals but also the underlying principles and guiding beliefs upheld by institutions [27,28]. These elements collectively influence the successful implementation of the 3Rs and the ethical treatment of AMs in research settings [42,57]. Individual behaviors and institutional values are crucial to shaping practices that prioritize reducing, refining, and replacing animal use, promoting a humane approach to scientific research [51,58,59,60].
2.1. Embracing Ethical Excellence: Cultivating a “Culture of Care” in Animal Research
Research facilities should go beyond legal obligations to ensure kindness and respect for animals and employees [28].
Essential practices include constant animal welfare monitoring, administering analgesics and anesthetics, and minimizing the use of animals whenever possible [10]. Furthermore, transparent data sharing and results foster a culture of scientific collaboration and accountability [58,61].
The culture of care not only addresses ethical considerations but also reflects positively on the quality of the scientific results obtained, ensuring that the gathered information is reliable and pertinent [58,61].
The culture of care includes several aspects essential to ensuring research and ethical animal welfare, namely, animal welfare, ethical framework, education and training, collaboration and transparency, health monitoring and environmental control, enrichment programs, and communication [27,28,29], which are further detailed below:
- Animal welfare: Animal welfare precedes research objectives or convenience. It involves the provision of adequate housing, veterinary care, and attention to the physical and psychological needs of animals [19,45,62]. Knowledge about AMs to be acquired includes information about the species and the different strains, focusing on transgenic ones [19,45,62]. Adopting score sheets can help identify these signs of distress early, facilitating prompt intervention [63,64,65]. Several models are available in the bibliography (e.g., [25,66,67,68]). It is essential to customize models to fit specific needs based on the type of research and animal model being used.
- Ethical framework: The culture of care should be actively implemented daily, not just as an abstract concept [16,35,45]. Establishing a robust ethical framework involves defining clear guidelines and policies that promote respect, kindness, and ethical treatment of animals [7,39,42]. This includes exceeding minimal legal requirements and ensuring compliance with ethical standards in all research activities [7,39,42].
- Education and training: To ensure proper animal care, all personnel must receive adequate training as described in the referred normative (European Directive 2010/63/EU) [69]. This helps maintain high morale, skill development, and compliance with best practices in animal welfare [27,49,69]. All personnel working with laboratory animals must acquire the information and updates essential to embracing the culture of care carefully, including those who perform procedures on animals (Function A), those who design the experimental study (Function B), those who take care of the animals (Function C), and those who euthanize the animals (Function D) [27,49,69]. Everyone needs to be well versed in current regulations; the specific animal model they are working with; and all strategies to ensure animal welfare, starting with the 3Rs principle [16,17]. A comprehensive knowledge of the animal model, encompassing its characteristics and limitations, and careful analysis of the relevant literature can guide efforts towards replacement and reduction [17]. This knowledge empowers people to consider an NAM that is more suitable and informative for all or part of the study [27,49,69].
- Collaboration and transparency: In AM studies, transparency concerns the accurate sharing of the results and the chosen experimental methods, including the selection, care, and use of laboratory animals [70,71,72]. Detailed information on experiment design, ethical procedures adopted, and animal welfare monitoring should be provided [70,71,72]. Transparency and effective communication also include disclosing any limitations or challenges encountered during the research study [70,71,72]. This honest approach fosters a deeper comprehension of the studies conducted and facilitates mutual learning among researchers [70,71,72]. Collaboration and transparency play pivotal roles in establishing a robust knowledge base in neuroscience, ensuring that research is ethically grounded and that results are beneficial for the progress of science and medicine. Clarity in communication is equally important, not only among researchers [70,71,72].
- Health monitoring and environmental control: Monitoring the health of animal colonies is crucial to obtaining reliable scientific data [31]. It helps prevent variables in experimental designs and safeguards personnel health [31,69,73]. All animal facilities must have a periodic health monitoring program for pathogens [69,73]. Health controls can be either direct or indirect: tests on the animals themselves, their products, the environment in which they live, and the personnel involved in their management [69]. Effective research requires proper planning with established timelines and a clear list of pathogens. The Federation of European Laboratory Animal Science Associations (FELASA) guidelines and standardized health reports aid information exchange among cooperating labs [56,69,73]. Daily observations made by staff are crucial, in addition to routine health surveillance, assays, sampling, and testing [69,74,75]. Environmental control is also essential to ensuring animal health. Monitoring environmental parameters such as temperature, humidity, and ventilation carefully and regularly helps prevent the spread of diseases [75].
Equally crucial in this field is the training and ongoing education of colony care and management staff. A deep understanding of the health status enables the staff to be vigilant in recognizing any signs of disease in animals. Moreover, it heightens attention to procedures to prevent contact with zoonotic agents or the development of laboratory animal allergies [22].
A proactive approach is paramount in an animal facility. Health monitoring protocols must continually be improved based on the latest research and advancements in veterinary medicine. Feeding and watering are crucial for the well-being of laboratory animals. It is necessary to provide them with a well-balanced diet and clean water [31,69,73]. High-quality feeds are now available, optimizing most species’ growth, maintenance, and reproduction [31,69,73]. Feed types include natural, purified ingredients and chemically formulated options. It is essential to use certified diets that meet contaminant concentrations below predetermined levels [31,69,73]. For example, the nutritional requirements of pregnant and lactating females differ from those of adult males [76]. Moreover, it is essential to consider whether the animals are conventional, specific pathogen-free (SPF), or germ-free (GF), as supplementation of vitamins, especially K and B, may be necessary. Therefore, feed formulations must be tailored to meet the biological needs of the animals [31,69,73].
Water must be clean and easily accessible, and different watering methods must be carefully and regularly maintained to prevent contamination [31,69,73]. Changes in drinking habits can indicate potential health or stress problems in animals [35,77,78]. Regularly monitoring animal body weight and water intake ensures their health and well-being [35,77,78,79,80]. Finally, it should not be forgotten that the feeding and watering of laboratory animals are regulated by ethical and regulatory guidelines designed to ensure animal welfare (European Recommendations 526/2007) [77].
- Enrichment programs: Environmental and social enrichment are crucial to ensuring the welfare of laboratory animals [81,82,83]. Programs should be customized based on the specific needs and behaviors of the species involved while complying with applicable regulations and ethical principles. Providing adequate space and complexity is essential to allowing animals to express normal species-specific behaviors [81,82,83,84].
Enrichment programs should grant some control over the surroundings to minimize stress-related behaviors, like stereotypies. Social enrichment is crucial; isolation should only be for health and experimental reasons [81,82,83,84,85]. Varying the diet and mode of feeding, hiding food, and involving animals in training activities are ways to offer environmental enrichment (EE) [81,82,83,84,85]. Humans can also be part of ecological enrichment, particularly for rats, by stimulating them cognitively and creating a bond with handlers [81,82,83,84,85]. Positive interactions with animals can contribute to their emotional well-being, benefiting the overall outcome of experimental procedures [81,82,83,84,85].
- Communication: To have a successful team, it is essential to have individuals inclined towards communication and collaboration while having clearly defined roles [59,86,87]. This is essential to ensuring animal welfare and generate reliable scientific results that can be easily reproduced and translated to humans [27,87].
Personnel include animal facility technicians, researchers, a designed veterinarian, an animal welfare responsible, and other members of the animal welfare body [30].
European Directive 2010/63/EU also highlights the importance of communication, especially transparency, as these principles are essential to fostering sustainable scientific progress and establishing an open dialogue between science and society [69].
Harmonizing procedures and cultivating a culture of care that serves as a common thread throughout the animals’ lifespan and the entire study period is also beneficial to establishing international scientific collaborations [59,86,87].
Moreover, effective communication channels are imperative to disseminate and reinforce good laboratory animal practices (GLAPs) across research teams. Open dialogue, training sessions, and regular forums enable the sharing of experiences, challenges, and successful strategies [59,86,87]. This cultivates a shared understanding of ethical principles, ensuring that everyone comprehensively embraces and implements GLAPs within their respective roles [59,86,87]. Furthermore, dissemination is pivotal to extending these practices beyond institutional boundaries [59,86,87]. Encouraging publication and participation in conferences and contributing to training programs ensure that the broader scientific community remains updated on evolving GLAPs [59,86,87]. This enriches collective knowledge and fosters a global commitment to ethical animal research [59,86,87].
A concerted effort to foster collaboration, effective communication, and widespread dissemination of GLAPs solidifies a culture of care. It promotes a unified commitment among all stakeholders towards ethical standards, advancing research while prioritizing the animals’ well-being [59,86,87].
2.2. Challenges and Strategies in Implementing a Culture of Care in Animal Facilities
Implementing a robust culture of care encounters multifaceted challenges [27,28,29]. Limited funding often hampers the acquisition of crucial resources, such as state-of-the-art equipment, adequate staffing, and essential facility upgrades imperative for optimal animal care [27,28,29]. This financial shortfall further impedes the execution of enrichment programs and advanced veterinary care initiatives [27,28,29]. Moreover, while comprehensive training for staff, researchers, and technicians remains vital to upholding high animal welfare standards, high turnover rates or limited access to specialized training resources undermines the consistent delivery of such programs [86,88,89]. Balancing scientific pursuits with ethical considerations poses another significant challenge in research settings, requiring meticulous planning and monitoring, particularly in long-term studies or invasive procedures [86,88,89]. Striking compliance with stringent regulatory requirements and ethical standards demands rigorous oversight, meticulous record keeping, and frequent audits, which becomes labor-intensive when managing various research programs [86,88,89]. Ethical dilemmas often arise in experimental designs that demand alignment with animal welfare principles and necessitate ongoing ethical reviews and consultations [86,88,89]. Technological limitations still tether specific research fields’ reliance on AMs, delaying the transition to NAMs despite available advancements [90,91,92]. Instituting a culture of care also hinges on overcoming a cultural shift and obtaining stakeholder buy-in, demanding a paradigm shift in mindset and practices across all levels [86,88,89]. Addressing these challenges requires a concerted effort to allocate adequate resources, foster continuous education, promote interdisciplinary collaboration, and establish transparent communication channels to navigate and mitigate these impediments effectively [86,88,89].
3. Bridging Animal Models with a Culture of Care in Neuroscience
The culture of care is integral to using AMs in neuroscience research. It revolves around ethical considerations, ensuring the welfare of animals utilized in scientific studies [27,28,29]. In neuroscience, where AMs are crucial to understanding complex brain functions, the culture of care emphasizes humane treatment, minimizing discomfort and promoting animal well-being throughout the research process [27,28]. By prioritizing ethical animal handling, providing enriched environments, and considering the animals’ physiological and behavioral needs, the culture of care supports using AMs in neuroscience while upholding ethical standards [27,28,29]. This approach aligns with regulatory compliance and enhances the credibility and reliability of neuroscience research outcomes, emphasizing researchers’ ethical responsibility toward their animal subjects [27,28,29]. Hence, fostering a culture of care in neuroscience research ensures that scientific knowledge is pursued responsibly and compassionately while leveraging the indispensable role of AMs in advancing our understanding of the brain and neurological disorders [27,28,29]. However, we cannot but be honest with ourselves and point out how, within scientific institutions, resistance to change is deeply rooted. The prospect of doing things differently, particularly in terms of embracing a culture of care, poses a formidable challenge. Researchers are rewarded with publications and funding, and shifting paradigms requires a willingness to endure downtime for labs and a potential decrease in productivity as new approaches are integrated. The path toward a “culture of care” is fraught with disruption and storms of resistance and demands extensive planning, consensus building, and adept change management. Recidivism, or the tendency to revert to familiar practices, is a common hurdle that necessitates delicate handling. Surprisingly, the difficulties inherent in this transformative process often go unmentioned in academic papers, where the status quo is implicitly favored. Embracing a “culture of care” signifies a departure from the conventional, urging for a paradigm shift involving extensive animal habituation to handling, procedures, and positive reinforcement training. It compels scientists to delve into the lived experiences of the animals, prioritizing their well-being over the convenience of established laboratory norms.
3.1. Comprehensive Perspectives in Neuroscience Research: Animal Models, Advances, and Ethical Considerations
Throughout history, using AMs has been pivotal to advancing neuroscience. From Galen’s pioneering anatomical studies to Pasteur’s and Bernard’s pivotal experiments, AMs have elucidated neurological functions and disease mechanisms [93,94,95]. The 20th century witnessed a paradigm shift with genetic modifications and refined studies on genes and diseases, while technological strides like MRI transformed non-invasive brain research [1,91,96,97]. Despite ethical considerations prompting the exploration of NAMs, the historical contributions of AMs remain indispensable to shaping our understanding of the brain [1,3,4,91,98].
Continued progress in genetic engineering, molecular biology, and neuroscience has expanded the scope and precision of AMs [99]. Researchers now use genetically modified animals to study specific genes’ roles in brain function and disease [100,101,102]. Neuroscience researchers rely on sophisticated measurement tools and AMs as they grapple with the inherent complexity of organisms [100,101,102]. While cell cultures shed light on cellular mechanisms, organoids enable the study of cellular communication, synaptosomes aid in assessing synaptic function, and organs-on-chips simulate motion–neural interaction, none of the above can wholly emulate the intricacies of a peripheral nervous circuit, nuanced motor skills, or the complexities inherent in behaviors and cognitive functions [1,3,4,91,98]. AMs are indispensable tools for measuring and comprehensively studying these intricate aspects of the NS [103,104,105]. Integrating findings from these diverse models enhances our understanding of the brain and neurological disorders, paving the way for potential treatments and interventions [106]. Furthermore, there is an increasing emphasis on developing NAMs, such as organoids (miniature organ-like structures grown in the lab) and computer simulations, to complement or replace animal studies where feasible [107,108,109].
Nevertheless, AMs remain fundamental in neuroscience research due to several advantages that current NAMs might not fully replicate [110]. Animal brains, especially in mammals and primates, share structural and functional similarities with human brains, enabling researchers to study complex behaviors and cognitive processes that simpler models might not exhibit [104,110,111].
Many neurological diseases manifest differently in humans than in simpler models or cell cultures. AMs allow for a closer representation of disease pathology, aiding in understanding disease mechanisms and testing potential treatments [111]. Mammalian AMs, like mice and rats, share a significant portion of the genetic background of humans [112]. This genetic similarity makes them valuable for studying genetic factors underlying neurological disorders [106]. AMs allow researchers to study behaviors related to memory, learning, cognition, and social interactions in ways that might be challenging to replicate in NAMs [106,112,113].
AMs offer the advantage of studying the brain in the context of a whole organism, considering the brain’s interactions with other organ systems and the body [114]. Long-term studies and interventions can be conducted in animals to observe developmental changes, disease progression, and responses to therapeutic interventions over time, which might be more challenging in AMs [114,115,116]. Regulatory agencies often require data from animal studies before approving human clinical trials. Animal studies provide critical data on treatments’ safety, efficacy, and potential side effects before human trials [117,118,119].
However, despite these advantages, there are ethical considerations, limitations, and challenges associated with AMs, leading to the development and exploration of NAMs in neuroscience. While NAMs [102], like cell cultures, organoids, computer simulations, non-invasive diagnostic imaging, and human-based models, are advancing, they might not fully encompass the complexity of the whole brain or exhibit certain behaviors or disease phenotypes observed in AMs [102]. Hence, a combination of animal and NAMs is often used to address questions in neuroscience [2] comprehensively.
Xenopus laevis, commonly known as the African clawed frog, represents a significant model for studying developmental disorders due to its unique characteristics; their large and externally developing embryos allow for the easy manipulation and observation of neural development, offering insights into early neurodevelopmental processes [119].
Caenorhabditis elegans (C. elegans), a microscopic roundworm, is invaluable in neuroscience due to its well-characterized NS comprising just 302 neurons, allowing for the comprehensive mapping and understanding of neural circuits [120,121]. Its genetic manipulability and short lifecycle enable precise studies on neural development, synaptic plasticity, and aging, offering insights into fundamental principles of neural function and behavior [122].
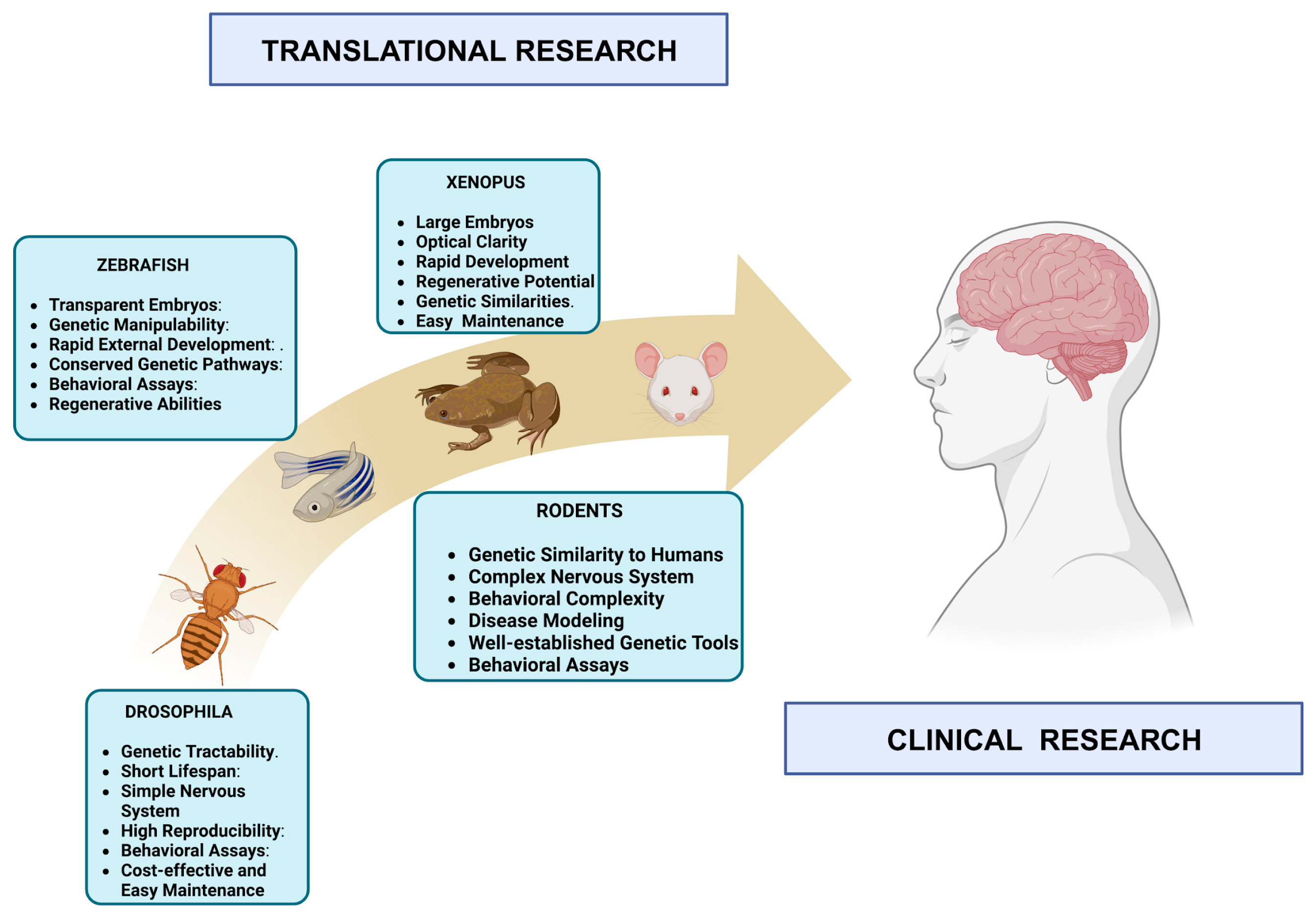
Due to their unique advantages, Drosophila melanogaster (the fruit fly) and zebrafish (Danio rerio) are essential models in neuroscience research [123,124]. Drosophila melanogaster offers rapid generation times, genetic tractability, and a relatively simple NS, facilitating studies on fundamental neurobiological processes like synaptic transmission and neural circuitry [125]. With their transparent embryos and rapid external development, zebrafish allow for the real-time observation of neural development, making them invaluable for studying early neurodevelopmental events [126]. While these models provide crucial insights into basic neural mechanisms, rats and mice remain the preferred species for many neuroscience studies due to their closer genetic and physiological resemblance to humans [1,57]. Their more complex NS, cognitive abilities, and similarity in pathology make rats and mice superior models for studying complex behaviors, cognitive functions, neurodegenerative diseases, and cellular bases of learning and memory, offering greater translational relevance to human conditions [52,127]. Their established genetic tools, ease of genetic manipulation, and extensive behavioral assays further enhance their importance in neuroscience research [52,127] (Figure 2).
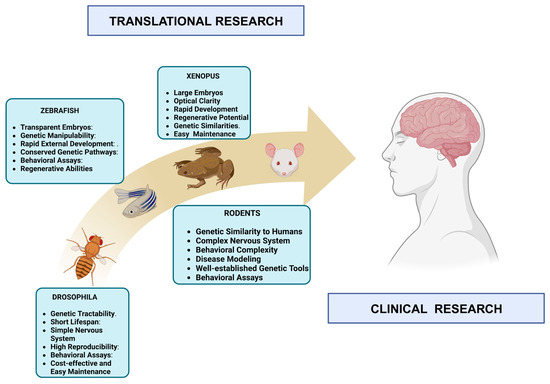
Figure 2.
From lab to clinic: bridging neuroscience with diverse animal models. The image illustrates several AMs, i.e., Drosophila melanogaster, zebrafish, Xenopus, and rodent, which are integral to neuroscience research. These models bridge preclinical studies and clinical applications, enabling the understanding of neural development, disease mechanisms, and therapeutic approaches. Their collective contributions translate essential research findings from the laboratory into clinical advances, offering promise for understanding neurological disorders and developing potential treatments. (Created with BioRender.com. and adapted from [128].)
However, we must never forget that AMs require a mandatory focus on adherence to ethical principles regarding reducing their numbers and exploring NAMs [129]. Indeed, the usefulness of these models for scientific research is closely linked to their welfare [57,101,127,129]. Ensuring the welfare of animals involved in research is an ethical responsibility and integral to the quality and reliability of scientific results [100,101,129]. By providing optimal care, minimizing stress, and meeting ethical standards, researchers ensure that the data collected from these models are more reliable, consistent, and translatable to human health [100,101,129]. Ethical treatment of animals is in line with moral responsibility. It enhances the credibility and validity of neuroscience research, promoting progress and respecting the dignity of the creatures that contribute to our scientific exploration [130].
3.2. Advancing Neuroscience Responsibly: Exploring Alternative Methods within a Culture of Care
NAMs in neuroscience represents a pivotal shift in research paradigms, aiming to minimize or eliminate the use of animals while retaining scientific rigor [27,30,33,91]. These methods encompass diverse approaches, such as computer simulations, organoids, neural networks, and advanced imaging techniques like fMRI and PET scans [131,132]. They offer unique advantages, including cost effectiveness, reproducibility, and the ability to accurately replicate specific neural functions or diseases [27,29,32,87]. Moreover, these methods permit detailed and controlled investigations into cellular and molecular mechanisms without ethical concerns about animal use [27,30,33,91]. Embracing such alternatives is rooted in the culture of care, aligning with ethical principles by prioritizing the welfare of animals and promoting a more humane approach to neuroscience research [27,30,33,91]. However, while these methods offer significant promise, they face challenges, such as accurately replicating complex neural systems and further validation and refinement to ensure their reliability and relevance in neuroscientific studies [27,30,33,91].
Various NAMs have been suggested to avoid AM use in neuroscience (Table 1):
- -
- Computer models: Computer models in neuroscience serve as a transformative tool for researchers and practitioners, providing a comprehensive platform for simulating intricate neural processes [132,133]. This simulation enables a deep exploration of the behaviors of neurons, synaptic connections, and neural networks, shedding light on how the brain processes information and generates complex behaviors. Beyond fundamental neuroscience, these computational models play a pivotal role in drug discovery and development, with applications like computer-aided drug design (CADD) predicting drug–receptor interactions and expediting the identification of therapeutic compounds [134]. Moreover, these models contribute significantly to understanding the underlying mechanisms of various neurological and psychiatric disorders, offering insights into conditions such as epilepsy, Alzheimer’s disease, and schizophrenia [135,136]. In diagnostics, advanced computational techniques analyze neuroimaging data, employing machine learning algorithms to identify patterns in brain scans and enhance diagnostic and prognostic capabilities [135,136]. Additionally, computer models are integral to developing brain–machine interfaces, fostering communication between the brain and external devices, with potential applications in assisting individuals with paralysis [135,136]. These models also contribute to cognitive modeling, helping unravel the intricacies of how the brain processes information, learns, and makes decisions. In personalized medicine, computational models analyze individual genetic, neuroimaging, and clinical data to predict responses to specific treatments, paving the way for more tailored and effective therapeutic interventions [135,136]. Furthermore, these models serve educational initiatives by providing interactive and visual tools for learning, allowing students to explore complex concepts and enhance their understanding of neural processes. In summary, computer models in neuroscience represent a versatile and powerful toolbox, contributing to advancements in drug development, the understanding of brain function and disorders, and the improvement in diagnostic and therapeutic strategies [135,136].
- -
- Cells and tissue cultures are vital components in neuroscience research, offering versatile platforms for delving into the intricacies of the NS [137,138,139]. These in vitro models serve many purposes, from studying fundamental aspects of neuronal function and communication to modeling neurological disorders. In the context of drug screening and development, these cultures provide a controlled environment to assess the effects of potential therapeutic compounds on neuronal cells [137,138,139]. Additionally, they play a pivotal role in toxicology studies, allowing researchers to evaluate the impact of various substances on neuronal health without resorting to animal experimentation [137,138,139]. The application of these cultures extends to electrophysiological studies, offering insights into the electrical activity of neurons and their networks [137,138,139]. Furthermore, neural stem cell-derived cultures contribute to exploring neuroregeneration and repair mechanisms, providing valuable information for developing strategies to promote neural recovery [137,138,139]. These in vitro models also play a crucial role in investigating neurodevelopment, gene expression, and other facets that collectively enhance our understanding of the complex workings of the NS [137,138,139].
- -
- Organoids, miniature three-dimensional tissue structures cultivated in vitro, represent a revolutionary tool in neuroscience research [140,141,142]. These self-organizing structures, resembling simplified organs, offer a unique opportunity to study complex aspects of brain development and function in a controlled environment [140,141,142]. In neuroscience, organoids are employed to model various aspects of the brain, allowing researchers to explore neuronal connectivity, synapse formation, and the development of specific brain regions [140,141,142]. Furthermore, organoids derived from patient cells enable the modeling of neurological disorders, providing insights into disease mechanisms and potential therapeutic interventions [140,141,142]. Their application extends to drug testing, where organoids serve as a valuable platform for screening and evaluating the efficacy of pharmaceutical compounds [140,141,142]. The ability to reproduce critical features of the brain’s architecture and functionality makes organoids a powerful tool for advancing our understanding of neurobiology and addressing intricate questions related to brain development, diseases, and potential treatment strategies [140,141,142].

Table 1.
Summary of some types of NAMs and their contribution to research in the field of neuroscience.
Table 1.
Summary of some types of NAMs and their contribution to research in the field of neuroscience.
| NAMs | Implication in Neuroscience | Ref. |
|---|---|---|
| fMRI and PET | Detailed and controlled investigations into cellular and molecular mechanisms without ethical concerns about animal use. | [27,30,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,131] |
| Computer models | Transformative tool for researchers and practitioners providing a comprehensive platform for simulating intricate neuronal processes allowing for the following:
| [133,134,135,136] |
| Cells and tissue cultures | Used for studying fundamental aspects of neuronal function and communication to model neurological disorders, allowing for the following:
| [137,138,139] |
| Organoids | Resembling simplified organs, they offer a unique opportunity to study complex aspects of brain development and function in a controlled environment, allowing for the following:
| [140,141,142] |
The culture of care within neuroscience embodies a foundational principle of ethical alignment, advocating for a conscientious approach in research practices to diminish reliance on AMs [28,97]. It encourages scientists to contemplate the ethical ramifications of their work and prioritize humane methodologies within neuroscience research [28,97]. Within this ethos, there is a robust emphasis on exploring and validating NAMs that minimize or supplant AMs [28,97]. This culture actively nurtures an environment that fosters innovation and supports the development of progressive techniques, like in vitro studies, computer simulations, stem cell-derived models, and advanced imaging technologies [28,97]. Crucially, these alternatives demand rigorous validation and reliability, ensuring they provide credible and accurate data equivalent to data obtained from traditional AMs [28,97]. Collaboration among researchers, institutions, and regulatory bodies is encouraged, advocating for a collective effort to advance and integrate alternative methods [28,97]. Education and awareness initiatives within the scientific community are vital, enlightening researchers about the availability and utility of these alternatives [28,97]. Furthermore, a core facet is a stringent adherence to regulatory guidelines, emphasizing that ethical considerations and animal welfare are paramount, even when employing innovative methodologies [28,97]. Ultimately, the culture of care strives for a lasting impact by fostering a shift towards reduced animal use in neuroscience research, aligning with the ethical responsibility to curtail animal suffering while advancing scientific knowledge [28,97].
4. Conclusions
While laboratory animals have significantly contributed to scientific neuroscience research, numerous alternative methods and computational analyses are available. However, when an organic study of an animal is necessary to understand the complexity of an organism fully, it is crucial to hunt the animal in the most humane way possible and to provide proper care and treatment. In neuroscience, the culture of care is an essential paradigm that intertwines ethical considerations, animal welfare, and scientific progress. It emphasizes a conscientious approach toward research methodologies, advocating for humane practices while exploring alternatives to traditional AMs [27,28,29]. Communication is the linchpin for fostering this culture, enabling collaboration among researchers, institutions, and regulatory bodies to promote innovative techniques that minimize animal use [27,28,29]. Moving forward, integrating a robust communication framework is crucial for the future of neuroscience research [27,28,29]. Enhanced communication channels will bridge gaps among stakeholders, facilitating the exchange of ideas, knowledge, and best practices [97]. This concerted effort will lead to greater awareness and education within the scientific community about the availability and utility of NAMs [97]. Moreover, transparent communication aids in aligning research goals with ethical standards, ensuring that the pursuit of scientific excellence remains deeply intertwined with animal welfare. The prospects of the culture of care and AMs in neuroscience hinge upon a collective commitment to open dialogue and shared responsibility [27,28,29,97]. Embracing communication as a cornerstone will propel scientific advancements while upholding ethical standards and ensuring the welfare of AMs [143,144].
Author Contributions
Conceptualization, M.M., A.W., P.B. and G.M.; resources, M.M., P.B. and G.M.; data curation, M.M., A.W. and G.M.; writing—original draft preparation, M.M. and A.W.; writing—review and editing, M.M. and G.M.; visualization, M.M., A.W., P.B. and G.M.; supervision, M.M. and G.M.; project administration, G.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We would like to thank Massimiliano Di Virgilio, Alessio Fieramosca, Gianni Catini, and Marzia Scarfò for their excellent help in applying culture of the cure protocols.
Conflicts of Interest
The authors declare no conflicts of interest. No sponsors participated in the choice of the items; in the design of the paper; in the collection of the literature or the interpretation of analyzed papers; in the writing of the manuscript; or in the decision to publish in the Encyclopedia journal.
Abbreviations
| AMs | animal models |
| NS | nervous system |
| NAMs | new alternative models |
| AWBs | animal welfare bodies |
| 3Rs | replacement, reduction, and refinement |
| SPF | specific pathogen-free |
| LAA | laboratory animal allergies |
| GF | germ-free |
| FELASA | Federation of European Laboratory Animal Science Associations |
| GLAPs | good laboratory animal practices |
| EE | environmental enrichment |
References
- Lambert, K. Wild brains: The value of neuroethological approaches in preclinical behavioral neuroscience animal models. Neurosci. Biobehav. Rev. 2023, 146, 105044. [Google Scholar] [CrossRef]
- Romanova, E.V.; Sweedler, J.V. Animal model systems in neuroscience. ACS Chem. Neurosci. 2018, 9, 1869–1870. [Google Scholar] [CrossRef]
- Bovenkerk, B.; Kaldewaij, F. The use of animal models in behavioural neuroscience research. Curr. Top. Behav. Neurosci. 2015, 19, 17–46. [Google Scholar] [CrossRef]
- Crystal, J.D. Elements of episodic-like memory in animal models. Behav. Process. 2009, 80, 269–277. [Google Scholar] [CrossRef]
- Yanshree; Yu, W.S.; Fung, M.L.; Lee, C.W.; Lim, L.W.; Wong, K.H. The monkey head mushroom and memory enhancement in Alzheimer’s disease. Cells 2022, 11, 2284. [Google Scholar] [CrossRef]
- Fine, A.H.; Beck, A.M.; Ng, Z. The State of Animal-Assisted Interventions: Addressing the Contemporary Issues that will Shape the Future. Int. J. Environ. Res. Public Health 2019, 16, 3997. [Google Scholar] [CrossRef]
- DeGrazia, D.; Beauchamp, T.L. Beyond the 3Rs to a more comprehensive framework of principles for animal research ethics. ILAR J. 2021, 60, 308–317. [Google Scholar] [CrossRef]
- Gruen, L. Ethics and Animals: An Introduction; Cambridge University Press: Cambridge, UK, 2021; ISBN 9781108988544. [Google Scholar]
- Robinson, S.; Sparrow, S.; Williams, B.; Decelle, T.; Bertelsen, T.; Reid, K.; Chlebus, M. The European Federation of the Pharmaceutical Industry and Associations’ Research and Animal Welfare Group: Assessing and benchmarking “Culture of Care” in the context of using animals for scientific purpose. Lab. Anim. 2019, 54, 23677219887998. [Google Scholar] [CrossRef] [PubMed]
- Arndt, S.S.; Goerlich, V.C.; van der Staay, F.J. A dynamic concept of animal welfare: The role of appetitive and adverse internal and external factors and the animal’s ability to adapt to them. Front. Anim. Sci. 2022, 3. [Google Scholar] [CrossRef]
- Davies, G.; Gorman, R.; Greenhough, B.; Hobson-West, P.; Kirk, R.G.W.; Message, R.; Myelnikov, D.; Palmer, A.; Roe, E.; Ashall, V.; et al. Animal research nexus: A new approach to the connections between science, health and animal welfare. Med. Humanit. 2020, 46, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Regan, T. Animal Rights, Human Wrongs: An Introduction to Moral Philosophy; Rowman & Littlefield Publishers: Lanham, MD, USA, 2003; ISBN 9780742599383. [Google Scholar]
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Collection: Reports funded by National Institutes of Health; National Academies Press (US): Washington, DC, USA, 2011; ISBN 0309154006. [Google Scholar]
- Ferrara, F.; Hiebl, B.; Kunzmann, P.; Hutter, F.; Afkham, F.; LaFollette, M.; Gruber, C. Culture of care in animal research—Expanding the 3Rs to include people. Lab. Anim. 2022, 56, 511–518. [Google Scholar] [CrossRef]
- Soulsbury, C.; Gray, H.; Smith, L.; Braithwaite, V.; Cotter, S.; Elwood, R.W.; Wilkinson, A.; Collins, L.M. The welfare and ethics of research involving wild animals: A primer. Methods Ecol. Evol. 2020, 11, 1164–1181. [Google Scholar] [CrossRef]
- Wahyuwardani, S.; Noor, S.M.; Bakrie, B. Animal welfare ethics in research and testing: Implementation and its barrier. WARTAZOA 2020, 30, 211. [Google Scholar] [CrossRef]
- Lee, K.H.; Lee, D.W.; Kang, B.C. The “R” principles in laboratory animal experiments. Lab. Anim. Res. 2020, 36, 45. [Google Scholar] [CrossRef]
- Blumer, K. Ethical aspects of animal experiments and the principle of solidarity. In Deutsche Forschungsgemeinschaft (DFG). Animal Experiments in Research; Exner, C., Bode, H.-J., Blumer, C., Giese, C., Eds.; Lemmens Medien: Bonn, Germany, 2007. [Google Scholar]
- Martinez, J.; von Nolting, C. Review: “Animal welfare”—A European concept. Animal 2023, 17 (Suppl. 4), 100839. [Google Scholar] [CrossRef]
- Maple, T.L.; Bloomsmith, M.A. Introduction: The science and practice of optimal animal welfare. Behav. Process. 2018, 156, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.J.; Clutton, R.E.; Lilley, E.; Hansen, K.E.A.; Brattelid, T. PREPARE: Guidelines for planning animal research and testing. Lab. Anim. 2018, 52, 135–141. [Google Scholar] [CrossRef] [PubMed]
- National Research Council, Division on Earth and Life Studies, Institute for Laboratory Animal Research. Guidance for the Description of Animal Research in Scientific Publications; National Academies Press: Washington, DC, USA, 2011; ISBN 9780309219518. [Google Scholar]
- Garner, J.P. The significance of meaning: Why do over 90% of behavioral neuroscience results fail to translate to humans, and what can we do to fix it? ILAR J. 2014, 55, 438–456. [Google Scholar] [CrossRef] [PubMed]
- Coscas, R.; Senemaud, J. Experimenters or Amateurs? Eur. J. Vasc. Endovasc. Surg. 2020, 60, 253. [Google Scholar] [CrossRef] [PubMed]
- van der Velden, J.; Asselbergs, F.W.; Bakkers, J.; Batkai, S.; Bertrand, L.; Bezzina, C.R.; Bot, I.; Brundel, B.J.J.M.; Carrier, L.; Chamuleau, S.; et al. Animal models and animal-free innovations for cardiovascular research: Current status and routes to be explored. Consensus document of the ESC Working Group on Myocardial Function and the ESC Working Group on Cellular Biology of the Heart. Cardiovasc. Res. 2022, 118, 3016–3051. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, D.M.; Feeney, W.P. The influence of feed and drinking water on terrestrial animal research and study replicability. ILAR J. 2020, 60, 175–196. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.J.; Symonowicz, C.; Medina, L.V.; Bratcher, N.A.; Buckmaster, C.A.; Klein, H.; Anderson, L.C. Culture of care: Organizational responsibilities. In Management of Animal Care and Use Programs in Research, Education, and Testing; Weichbrod, R.H., Thompson, G.A., Norton, J.N., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2018; ISBN 9781315152189. [Google Scholar]
- Bertelsen, T.; Øvlisen, K. Assessment of the Culture of Care working with laboratory animals by using a comprehensive survey tool. Lab. Anim. 2021, 55, 453–462. [Google Scholar] [CrossRef]
- Williams, A. Caring for those who care: Towards a more expansive understanding of ‘cultures of care’ in laboratory animal facilities. Soc. Cult. Geogr. 2023, 24, 31–48. [Google Scholar] [CrossRef]
- Hubrecht, R.C.; Carter, E. The 3Rs and humane experimental technique: Implementing change. Animals 2019, 9, 754. [Google Scholar] [CrossRef] [PubMed]
- Buchheister, S.; Bleich, A. Health Monitoring of Laboratory Rodent Colonies-Talking about (R)evolution. Animals 2021, 11, 1410. [Google Scholar] [CrossRef] [PubMed]
- Wu, J. Landscape sustainability science: Ecosystem services and human well-being in changing landscapes. Landsc. Ecol. 2013, 28, 999–1023. [Google Scholar] [CrossRef]
- Liz Paola, N.Z.; Torgerson, P.R.; Hartnack, S. Alternative paradigms in animal health decisions: A framework for treating animals not only as commodities. Animals 2022, 12, 1845. [Google Scholar] [CrossRef]
- Kopnina, H.; Washington, H.; Taylor, B.; Piccolo, J.J. Anthropocentrism: More than Just a Misunderstood Problem. J. Agric. Environ. Ethics 2018, 31, 109–127. [Google Scholar] [CrossRef]
- Croney, C.C.; Anthony, R. Engaging science in a climate of values: Tools for animal scientists tasked with addressing ethical problems. J. Anim. Sci. 2010, 88, E75–E81. [Google Scholar] [CrossRef]
- Beausoleil, N.J. I am a compassionate conservation welfare scientist: Considering the theoretical and practical differences between compassionate conservation and conservation welfare. Animals 2020, 10, 257. [Google Scholar] [CrossRef]
- Eggel, M.; Camenzind, S. Authorization of animal research proposals—A comparison of harm concepts in different European regulations. Berl. Münchener Tierärztliche Wochenschr. 2020. online first. [Google Scholar] [CrossRef]
- Baertschi, B.; Gyger, M. Ethical considerations in mouse experiments. Curr. Protoc. Mouse Biol. 2011, 1, 155–167. [Google Scholar] [CrossRef]
- Gross, D.; Tolba, R.H. Ethics in Animal-Based Research. Eur. Surg. Res. 2015, 55, 43–57. [Google Scholar] [CrossRef]
- Grimm, H. Ethics in laboratory animal science. In Comparative Medicine; Jensen-Jarolim, E., Ed.; Springer: Vienna, Austria, 2014; pp. 281–300. ISBN 978-3-7091-1558-9. [Google Scholar]
- Millar, K.M. Translational stem cell research and animal use: Examining ethical issues and opportunities. In Translational Stem Cell Research; Hug, K., Hermerén, G., Eds.; Stem Cell Biology and Regenerative Medicine; Humana Press: Totowa, NJ, USA, 2011; pp. 113–124. ISBN 978-1-60761-958-1. [Google Scholar]
- Vorstenbosch, J.M.G. The ethics of the Three Rs principle: A reconsideration. Anim. Welf. 2005, 14, 339–345. [Google Scholar] [CrossRef]
- Grunwald, A. Living Technology: Philosophy and Ethics at the Crossroads between Life and Technology; CRC Press: Boca Raton, FL, USA, 2021; ISBN 9781000346428. [Google Scholar]
- Schindler, S. The animal’s dignity in Swiss Animal Welfare Legislation—Challenges and opportunities. Eur. J. Pharm. Biopharm. 2013, 84, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K. Concepts of animal welfare in relation to positions in animal ethics. Acta Biotheor. 2011, 59, 153–171. [Google Scholar] [CrossRef] [PubMed]
- Mannhold, R.; Kubinyi, H.; Folkers, G. Animal Models for Human Cancer: Discovery and Development of Novel Therapeutics; John Wiley & Sons: Hoboken, NJ, USA, 2016; ISBN 9783527339976. [Google Scholar]
- Arlinghaus, R.; Cooke, S.J.; Lyman, J.; Policansky, D.; Schwab, A.; Suski, C.; Sutton, S.G.; Thorstad, E.B. Understanding the Complexity of Catch-and-Release in Recreational Fishing: An Integrative Synthesis of Global Knowledge from Historical, Ethical, Social, and Biological Perspectives. Rev. Fish. Sci. 2007, 15, 75–167. [Google Scholar] [CrossRef]
- McCausland, C. The five freedoms of animal welfare are rights. J. Agric. Environ. Ethics 2014, 27, 649–662. [Google Scholar] [CrossRef]
- Mellor, D.J. Moving beyond the “Five Freedoms” by Updating the “Five Provisions” and Introducing Aligned “Animal Welfare Aims”. Animals 2016, 6, 59. [Google Scholar] [CrossRef] [PubMed]
- Serpell, J.A.; Coppinger, R.; Fine, A.H.; Peralta, J.M. Welfare considerations in therapy and assistance animals. In Handbook on Animal-Assisted Therapy; Elsevier: Amsterdam, The Netherlands, 2010; pp. 481–503. ISBN 9780123814531. [Google Scholar]
- Jaasma, L. A Review of the Housing Conditions for Laboratory Animals. Master’s Thesis, Utrecht University, Utrecht, The Netherlands, July 2014. [Google Scholar]
- Gregory, N.G. Physiology and Behaviour of Animal Suffering; John Wiley & Sons: Hoboken, NJ, USA, 2008; ISBN 9781405173025. [Google Scholar]
- Mellor, D.J. Operational details of the five domains model and its key applications to the assessment and management of animal welfare. Animals 2017, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Mellor, D.J.; Beausoleil, N.J.; Littlewood, K.E.; McLean, A.N.; McGreevy, P.D.; Jones, B.; Wilkins, C. The 2020 Five Domains Model: Including Human-Animal Interactions in Assessments of Animal Welfare. Animals 2020, 10, 1870. [Google Scholar] [CrossRef]
- Mellor, D.J.; Beausoleil, N.J. Extending the ‘Five Domains’ model for animal welfare assessment to incorporate positive welfare states. Anim. Welf. 2015, 24, 241–253. [Google Scholar] [CrossRef]
- Gyger, M.; Berdoy, M.; Dontas, I.; Kolf-Clauw, M.; Santos, A.I.; Sjöquist, M. FELASA accreditation of education and training courses in laboratory animal science according to the Directive 2010/63/EU. Lab. Anim. 2019, 53, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Azkona, G.; Sanchez-Pernaute, R. Mice in translational neuroscience: What R we doing? Prog. Neurobiol. 2022, 217, 102330. [Google Scholar] [CrossRef] [PubMed]
- McLeod, C.; Hartley, S. Responsibility and laboratory animal research governance. Sci. Technol. Hum. Values 2018, 43, 723–741. [Google Scholar] [CrossRef] [PubMed]
- Davies, G.F.; Greenhough, B.J.; Hobson-West, P.; Kirk, R.G.W.; Applebee, K.; Bellingan, L.C.; Berdoy, M.; Buller, H.; Cassaday, H.J.; Davies, K.; et al. Developing a collaborative agenda for humanities and social scientific research on laboratory animal science and welfare. PLoS ONE 2016, 11, e0158791. [Google Scholar] [CrossRef] [PubMed]
- Davies, G.; Greenhough, B.; Hobson-West, P.; Kirk, R.G.W. Science, culture, and care in laboratory animal research. Sci. Technol. Hum. Values 2018, 43, 016224391875703. [Google Scholar] [CrossRef]
- Rolland, B.; Burnside, E.S.; Voils, C.I.; Shah, M.N.; Brasier, A.R. Enhancing reproducibility using interprofessional team best practices. J. Clin. Transl. Sci. 2020, 5, e20. [Google Scholar] [CrossRef]
- Mellor, D.J. Updating Animal Welfare Thinking: Moving beyond the “Five Freedoms” towards “A Life Worth Living”. Animals 2016, 6, 21. [Google Scholar] [CrossRef]
- Planchez, B.; Surget, A.; Belzung, C. Animal models of major depression: Drawbacks and challenges. J. Neural Transm. 2019, 126, 1383–1408. [Google Scholar] [CrossRef]
- Leenaars, M.; Hooijmans, C.R.; van Veggel, N.; ter Riet, G.; Leeflang, M.; Hooft, L.; van der Wilt, G.J.; Tillema, A.; Ritskes-Hoitinga, M. A step-by-step guide to systematically identify all relevant animal studies. Lab. Anim. 2012, 46, 24–31. [Google Scholar] [CrossRef]
- Hawkins, P. Recognizing and assessing pain, suffering and distress in laboratory animals: A survey of current practice in the UK with recommendations. Lab. Anim. 2002, 36, 378–395. [Google Scholar] [CrossRef] [PubMed]
- Ludolph, A.C.; Bendotti, C.; Blaugrund, E.; Chio, A.; Greensmith, L.; Loeffler, J.-P.; Mead, R.; Niessen, H.G.; Petri, S.; Pradat, P.-F.; et al. Guidelines for preclinical animal research in ALS/MND: A consensus meeting. Amyotroph. Lateral Scler. 2010, 11, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Workman, P.; Aboagye, E.O.; Balkwill, F.; Balmain, A.; Bruder, G.; Chaplin, D.J.; Double, J.A.; Everitt, J.; Farningham, D.A.H.; Glennie, M.J.; et al. Guidelines for the welfare and use of animals in cancer research. Br. J. Cancer 2010, 102, 1555–1577. [Google Scholar] [CrossRef] [PubMed]
- Zemanova, M.A. Crucial but neglected: Limited availability of animal welfare courses in education of wildlife researchers. Animals 2023, 13, 2907. [Google Scholar] [CrossRef] [PubMed]
- Scavizzi, F.; Galligioni, V.; Vasina, V.; Raspa, M. Animal health management and hygiene. In Practical Handbook on the 3Rs in the Context of the Directive 2010/63/EU.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 151–179. ISBN 9780128211809. [Google Scholar]
- Hooijmans, C.R.; de Vries, R.B.M.; Ritskes-Hoitinga, M.; Rovers, M.M.; Leeflang, M.M.; IntHout, J.; Wever, K.E.; Hooft, L.; de Beer, H.; Kuijpers, T.; et al. Facilitating healthcare decisions by assessing the certainty in the evidence from preclinical animal studies. PLoS ONE 2018, 13, e0187271. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; Emerson, M.; et al. Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 2020, 18, e3000411. [Google Scholar] [CrossRef]
- Carbone, L.; Austin, J. Pain and laboratory animals: Publication practices for better data reproducibility and better animal welfare. PLoS ONE 2016, 11, e0155001. [Google Scholar] [CrossRef]
- Nicklas, W. International harmonization of health monitoring. ILAR J. 2008, 49, 338–346. [Google Scholar] [CrossRef][Green Version]
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals Environment, Housing, and Management. 2011. Available online: https://grants.nih.gov/grants/olaw/guide-for-the-care-and-use-of-laboratory-animals.pdf (accessed on 20 November 2023).
- National Research Council, Division on Earth and Life Studies, Institute for Laboratory Animal Research, Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2010; ISBN 9780309186636. [Google Scholar]
- Temple, D.; Manteca, X. Animal welfare in extensive production systems is still an area of concern. Front. Sustain. Food Syst. 2020, 4, 545902. [Google Scholar] [CrossRef]
- Martin, A.L.; Franklin, A.N.; Perlman, J.E.; Bloomsmith, M.A. Systematic assessment of food item preference and reinforcer effectiveness: Enhancements in training laboratory-housed rhesus macaques. Behav. Process. 2018, 157, 445–452. [Google Scholar] [CrossRef]
- Germ-Free Animals: A Key Tool in Unraveling How the Microbiota Affects the Brain and Behavior. Available online: https://www.sciencedirect.com/science/article/abs/pii/B9780323999717000126 (accessed on 29 December 2023).
- Luczynski, P.; McVey Neufeld, K.-A.; Oriach, C.S.; Clarke, G.; Dinan, T.G.; Cryan, J.F. Growing up in a Bubble: Using Germ-Free Animals to Assess the Influence of the Gut Microbiota on Brain and Behavior. Int. J. Neuropsychopharmacol. 2016, 19, pyw020. [Google Scholar] [CrossRef]
- Weiskirchen, S.; Weiper, K.; Tolba, R.H.; Weiskirchen, R. All You Can Feed: Some Comments on Production of Mouse Diets Used in Biomedical Research with Special Emphasis on Non-Alcoholic Fatty Liver Disease Research. Nutrients 2020, 12, 163. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Gao, L.; Zhang, X. Environmental enrichment increases aquatic animal welfare: A systematic review and meta-analysis. Rev. Aquacult. 2022, 14, 1120–1135. [Google Scholar] [CrossRef]
- Lee, G.-H.; Kim, K.; Jo, W. Stress evaluation of mouse husbandry environments for improving laboratory animal welfare. Animals 2023, 13, 249. [Google Scholar] [CrossRef] [PubMed]
- Baumans, V.; Van Loo, P.L.P. How to improve housing conditions of laboratory animals: The possibilities of environmental refinement. Vet. J. 2013, 195, 24–32. [Google Scholar] [CrossRef] [PubMed]
- de Azevedo, C.S.; Cipreste, C.F.; Pizzutto, C.S.; Young, R.J. Review of the effects of enclosure complexity and design on the behaviour and physiology of zoo animals. Animals 2023, 13, 1277. [Google Scholar] [CrossRef] [PubMed]
- Baumans, V. Environmental enrichment for laboratory rodents and rabbits: Requirements of rodents, rabbits, and research. ILAR J. 2005, 46, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.; White, W.; Wilkes, J.; Wilkinson, C. Improving culture of care through maximising learning from observations and events: Addressing what is at fault. Lab. Anim. 2022, 56, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Klein, H.J.; Bayne, K.A. Establishing a culture of care, conscience, and responsibility: Addressing the improvement of scientific discovery and animal welfare through science-based performance standards. ILAR J. 2007, 48, 3–11. [Google Scholar] [CrossRef]
- Bekoff, M.; Meaney, C.A. (Eds.) Encyclopedia of Animal Rights and Animal Welfare; Routledge: Oxfordshire, UK, 2013; ISBN 9781135930028. [Google Scholar]
- Chow, P.K.H.; Ng, T.H.R.; Ogden, B.E. Using Animal Models in Biomedical Research: A Primer for the Investigator; World Scientific: Singapore, 2008; ISBN 9789812812025. [Google Scholar]
- Van Norman, G.A. Limitations of animal studies for predicting toxicity in clinical trials: Part 2: Potential alternatives to the use of animals in preclinical trials. JACC Basic Transl. Sci. 2020, 5, 387–397. [Google Scholar] [CrossRef]
- Mushtaq, S.; Daş, Y.K.; Aksoy, A. Alternative methods to animal experiments. Turkiye Klinikleri J. Lab. Anim. 2018, 170, 68–76. [Google Scholar] [CrossRef][Green Version]
- Scott, J.T.; Bourne, J.A. Modelling behaviors relevant to brain disorders in the nonhuman primate: Are we there yet? Prog. Neurobiol. 2022, 208, 102183. [Google Scholar] [CrossRef]
- Hajar, R. Animal testing and medicine. Heart Views 2011, 12, 42. [Google Scholar] [CrossRef]
- Distelzweig, P. “Mechanics” and mechanism in william harvey’s anatomy: Varieties and limits. In Early Modern Medicine and Natural Philosophy; Distelzweig, P., Goldberg, B., Ragland, E.R., Eds.; History, philosophy and theory of the life sciences; Springer: Dordrecht, The Netherlands, 2016; Volume 14, pp. 117–140. ISBN 978-94-017-7352-2. [Google Scholar]
- van Helvoort, T. Bacteriological and physiological research styles in the early controversy on the nature of the bacteriophage phenomenon. Med. Hist. 1992, 36, 243–270. [Google Scholar] [CrossRef]
- Wang, N.; Anderson, R.J.; Ashbrook, D.G.; Gopalakrishnan, V.; Park, Y.; Priebe, C.E.; Qi, Y.; Laoprasert, R.; Vogelstein, J.T.; Williams, R.W.; et al. Variability and heritability of mouse brain structure: Microscopic MRI atlases and connectomes for diverse strains. Neuroimage 2020, 222, 117274. [Google Scholar] [CrossRef]
- Mitchell, A.S.; Hartig, R.; Basso, M.A.; Jarrett, W.; Kastner, S.; Poirier, C. International primate neuroscience research regulation, public engagement and transparency opportunities. Neuroimage 2021, 229, 117700. [Google Scholar] [CrossRef]
- Muley, M.M.; Krustev, E.; McDougall, J.J. Preclinical assessment of inflammatory pain. CNS Neurosci. Ther. 2016, 22, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Sporns, O. The human connectome: A complex network. Ann. N. Y. Acad. Sci. 2011, 1224, 109–125. [Google Scholar] [CrossRef] [PubMed]
- Ohl, F.; Meijboom, F. Ethical issues associated with the use of animal experimentation in behavioral neuroscience research. Curr. Top. Behav. Neurosci. 2015, 19, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Vieira de Castro, A.C.; Olsson, I.A.S. Does the goal justify the methods? Harm and benefit in neuroscience research using animals. Curr. Top. Behav. Neurosci. 2015, 19, 47–78. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Yunta, E. Ethical issues concerning genetically modified animals for the study of human diseases. In Handbook of Bioethical Decisions. Volume I: Decisions at the Bench; Valdés, E., Lecaros, J.A., Eds.; Collaborative Bioethics; Springer International Publishing: Cham, Switzerland, 2023; Volume 2, pp. 513–525. ISBN 978-3-031-29450-1. [Google Scholar]
- Tello, J.A.; Williams, H.E.; Eppler, R.M.; Steinhilb, M.L.; Khanna, M. Animal models of neurodegenerative disease: Recent advances in fly highlight innovative approaches to drug discovery. Front. Mol. Neurosci. 2022, 15, 883358. [Google Scholar] [CrossRef] [PubMed]
- Greene, A.S.; Horien, C.; Barson, D.; Scheinost, D.; Constable, R.T. Why is everyone talking about brain state? Trends Neurosci. 2023, 46, 508–524. [Google Scholar] [CrossRef] [PubMed]
- Silverman, J.L.; Thurm, A.; Ethridge, S.B.; Soller, M.M.; Petkova, S.P.; Abel, T.; Bauman, M.D.; Brodkin, E.S.; Harony-Nicolas, H.; Wöhr, M.; et al. Reconsidering animal models used to study autism spectrum disorder: Current state and optimizing future. Genes Brain Behav. 2022, 21, e12803. [Google Scholar] [CrossRef] [PubMed]
- Moon, C. New Insights into and Emerging Roles of Animal Models for Neurological Disorders. Int. J. Mol. Sci. 2022, 23, 4957. [Google Scholar] [CrossRef] [PubMed]
- Eichmüller, O.L.; Knoblich, J.A. Human cerebral organoids—A new tool for clinical neurology research. Nat. Rev. Neurol. 2022, 18, 661–680. [Google Scholar] [CrossRef] [PubMed]
- Panoutsopoulos, A.A. Organoids, Assembloids, and Novel Biotechnology: Steps Forward in Developmental and Disease-Related Neuroscience. Neuroscientist 2021, 27, 463–472. [Google Scholar] [CrossRef]
- Wilcox, R.R.; Rousselet, G.A. A guide to robust statistical methods in neuroscience. Curr. Protoc. Neurosci. 2018, 82, 8.42.1–8.42.30. [Google Scholar] [CrossRef]
- Jucker, M. The benefits and limitations of animal models for translational research in neurodegenerative diseases. Nat. Med. 2010, 16, 1210–1214. [Google Scholar] [CrossRef]
- Feng, G.; Jensen, F.E.; Greely, H.T.; Okano, H.; Treue, S.; Roberts, A.C.; Fox, J.G.; Caddick, S.; Poo, M.-M.; Newsome, W.T.; et al. Opportunities and limitations of genetically modified nonhuman primate models for neuroscience research. Proc. Natl. Acad. Sci. USA 2020, 117, 24022–24031. [Google Scholar] [CrossRef]
- Kafkafi, N.; Agassi, J.; Chesler, E.J.; Crabbe, J.C.; Crusio, W.E.; Eilam, D.; Gerlai, R.; Golani, I.; Gomez-Marin, A.; Heller, R.; et al. Reproducibility and replicability of rodent phenotyping in preclinical studies. Neurosci. Biobehav. Rev. 2018, 87, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Pereira, T.D.; Shaevitz, J.W.; Murthy, M. Quantifying behavior to understand the brain. Nat. Neurosci. 2020, 23, 1537–1549. [Google Scholar] [CrossRef] [PubMed]
- Brenowitz, E.A.; Zakon, H.H. Emerging from the bottleneck: Benefits of the comparative approach to modern neuroscience. Trends Neurosci. 2015, 38, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Forstmann, B.U.; Ratcliff, R.; Wagenmakers, E.J. Sequential sampling models in cognitive neuroscience: Advantages, applications, and extensions. Annu. Rev. Psychol. 2016, 67, 641–666. [Google Scholar] [CrossRef] [PubMed]
- Strickland, J.C.; Smith, M.A. Animal models of resistance exercise and their application to neuroscience research. J. Neurosci. Methods 2016, 273, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Sécher, T.; Bodier-Montagutelli, E.; Guillon, A.; Heuzé-Vourc’h, N. Correlation and clinical relevance of animal models for inhaled pharmaceuticals and biopharmaceuticals. Adv. Drug Deliv. Rev. 2020, 167, 148–169. [Google Scholar] [CrossRef] [PubMed]
- Dixit, R.; Boelsterli, U.A. Healthy animals and animal models of human disease(s) in safety assessment of human pharmaceuticals, including therapeutic antibodies. Drug Discov. Today 2007, 12, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Lee-Liu, D.; Méndez-Olivos, E.E.; Muñoz, R.; Larraín, J. The African clawed frog Xenopus laevis: A model organism to study regeneration of the central nervous system. Neurosci. Lett. 2017, 652, 82–93. [Google Scholar] [CrossRef]
- Chalfie, M.; Jorgensen, E.M. C. elegans neuroscience: Genetics to genome. Trends Genet. 1998, 14, 506–512. [Google Scholar] [CrossRef]
- Sengupta, P.; Samuel, A.D.T. Caenorhabditis elegans: A model system for systems neuroscience. Curr. Opin. Neurobiol. 2009, 19, 637–643. [Google Scholar] [CrossRef]
- Husson, S.J.; Gottschalk, A.; Leifer, A.M. Optogenetic manipulation of neural activity in C. elegans: From synapse to circuits and behaviour. Biol. Cell 2013, 105, 235–250. [Google Scholar] [CrossRef]
- Gerlai, R. Zebrafish (Danio rerio): A newcomer with great promise in behavioral neuroscience. Neurosci. Biobehav. Rev. 2023, 144, 104978. [Google Scholar] [CrossRef]
- Davis, R.L. Learning and memory using Drosophila melanogaster: A focus on advances made in the fifth decade of research. Genetics 2023, 224, iyad085. [Google Scholar] [CrossRef] [PubMed]
- Liguori, F.; Pandey, U.B.; Digilio, F.A. Editorial: Drosophila as a model to study neurodegenerative diseases. Front. Neurosci. 2023, 17, 1275253. [Google Scholar] [CrossRef] [PubMed]
- Rinkwitz, S.; Mourrain, P.; Becker, T.S. Zebrafish: An integrative system for neurogenomics and neurosciences. Prog. Neurobiol. 2011, 93, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Ellenbroek, B.; Youn, J. Rodent models in neuroscience research: Is it a rat race? Dis. Model. Mech. 2016, 9, 1079–1087. [Google Scholar] [CrossRef]
- Bédard, P.; Gauvin, S.; Ferland, K.; Caneparo, C.; Pellerin, È.; Chabaud, S.; Bolduc, S. Innovative Human Three-Dimensional Tissue-Engineered Models as an Alternative to Animal Testing. Bioengineering 2020, 7, 115. [Google Scholar] [CrossRef] [PubMed]
- Nelson, N. Model homes for model organisms: Intersections of animal welfare and behavioral neuroscience around the environment of the laboratory mouse. Biosocieties 2016, 11, 46–66. [Google Scholar] [CrossRef]
- Prescott, M.J.; Lidster, K. Improving quality of science through better animal welfare: The NC3Rs strategy. Lab. Anim. 2017, 46, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.T.; Far, Y.K.; Ghaemi, Z.; Kamran, Z.; Andalibian, M.; Farhanian, A.; Taleifard, A.; Sanaei, A.; Chaboki, F.; Heidary, S. Neuroscience and Technology: Innovations in Brain Research and Therapy; Nobel Sciences: Dighi, India, 2023. [Google Scholar]
- Doke, S.K.; Dhawale, S.C. Alternatives to animal testing: A review. Saudi Pharm. J. 2015, 23, 223–229. [Google Scholar] [CrossRef]
- Dewhurst, D.G.; Hardcastle, J.; Hardcastle, P.T.; Stuart, E. Comparison of a computer simulation program and a traditional laboratory practical class for teaching the principles of intestinal absorption. Am. J. Physiol. 1994, 267, S95–S104. [Google Scholar] [CrossRef]
- Knight, A.; Bailey, J.; Balcombe, J. Animal carcinogenicity studies: 3. Alternatives to the bioassay. Altern. Lab. Anim. 2006, 34, 39–48. [Google Scholar] [CrossRef]
- Levenstein, D.; Alvarez, V.A.; Amarasingham, A.; Azab, H.; Chen, Z.S.; Gerkin, R.C.; Hasenstaub, A.; Iyer, R.; Jolivet, R.B.; Marzen, S.; et al. On the role of theory and modeling in neuroscience. J. Neurosci. 2023, 43, 1074–1088. [Google Scholar] [CrossRef]
- Santamaría-Vázquez, E.; Martínez-Cagigal, V.; Marcos-Martínez, D.; Rodríguez-González, V.; Pérez-Velasco, S.; Moreno-Calderón, S.; Hornero, R. MEDUSA©: A novel Python-based software ecosystem to accelerate brain-computer interface and cognitive neuroscience research. Comput. Methods Programs Biomed. 2023, 230, 107357. [Google Scholar] [CrossRef]
- Laerum, O.D.; Steinsvåg, S.; Bjerkvig, R. Cell and tissue culture of the central nervous system: Recent developments and current applications. Acta Neurol. Scand. 1985, 72, 529–549. [Google Scholar] [CrossRef]
- Wu, V.W.; Schwartz, J.P. Cell culture models for reactive gliosis: New perspectives. J. Neurosci. Res. 1998, 51, 675–681. [Google Scholar] [CrossRef]
- Gibbons, H.M.; Dragunow, M. Adult human brain cell culture for neuroscience research. Int. J. Biochem. Cell Biol. 2010, 42, 844–856. [Google Scholar] [CrossRef] [PubMed]
- Marx, V. Reality check for organoids in neuroscience. Nat. Methods 2020, 17, 961–964. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, M.; Zhou, J. Brain organoids: A promising living biobank resource for neuroscience research. Biopreserv. Biobank. 2020, 18, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Sun, W. Neural organoids, a versatile model for neuroscience. Mol. Cells 2022, 45, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Bayne, K.; Turner, P.V. Animal welfare standards and international collaborations. ILAR J. 2019, 60, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Medina, L.V. building a culture of animal welfare: Past, present and future. Annu. Rev. Biomed. Sci. 2008, 10, 104–111. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).