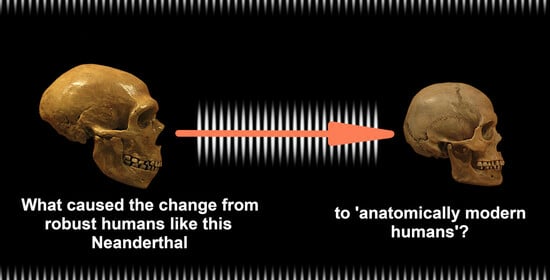
The Domestication of Humans
Definition
1. Introduction
2. Auto-Domestication of Hominins
3. Testing the Domestication Hypothesis
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Green, R.E.; Krause, J.; Briggs, A.W.; Maricic, T.; Stenzel, U.; Kircher, M.; Patterson, N.; Li, H.; Zhai, W.; Fritz, M.H.; et al. A draft sequence of the Neandertal genome. Science 2010, 328, 710–722. [Google Scholar] [CrossRef]
- Reich, D.; Green, R.E.; Kircher, M.; Krause, J.; Patterson, N.; Durand, E.Y.; Viola, B.; Briggs, A.W.; Stenzel, U.; Johnson, P.L.; et al. Genetic history of an archaic hominin group from Denisova Cave in Siberia. Nature 2010, 468, 1053–1060. [Google Scholar] [CrossRef]
- Sankararaman, S.; Patterson, N.; Li, H.; Pääbo, S.; Reich, D. The date of interbreeding between Neandertals and modern humans. PloS Genet. 2012, 8, e1002947. [Google Scholar] [CrossRef]
- Prüfer, K.; Racimo, F.; Patterson, N.; Jay, F.; Sankararaman, S.; Sawyer, S.; Heinze, A.; Renaud, G.; Sudmant, P.H.; De Filippo, C.; et al. The complete genome sequence of a Neanderthal from the Altai Mountains. Nature 2014, 505, 43–49. [Google Scholar] [CrossRef]
- Sankararaman, S.; Mallick, S.; Dannemann, M.; Prüfer, K.; Kelso, J.; Pääbo, S.; Patterson, N.; Reich, D. The genomic landscape of Neanderthal ancestry in present day humans. Nature 2014, 507, 354–357. [Google Scholar] [CrossRef]
- Viegas, J. Ancient Human with 10% Neandertal Genes Found. 2015. Available online: https://www.seeker.com/ancient-human-with-10-percent-neanderthal-genes-found-1769961373.html (accessed on 15 June 2023).
- Kuhlwilm, M.; Gronau, I.; Hubisz, M.J.; De Filippo, C.; Prado-Martinez, J.; Kircher, M.; Fu, Q.; Burbano, H.A.; Lalueza-Fox, C.; de La Rasilla, M.; et al. Ancient gene flow from early modern humans into eastern Neanderthals. Nature 2016, 530, 429–433. [Google Scholar] [CrossRef]
- Vernot, B.; Tucci, S.; Kelso, J.; Schraiber, J.G.; Wolf, A.B.; Gittelman, R.M.; Dannemann, M.; Grote, S.; McCoy, R.C.; Norton, H.; et al. Excavating Neandertal and Denisovan DNA from the genomes of Melanesian individuals. Science 2016, 352, 235–239. [Google Scholar] [CrossRef]
- Bednarik, R.G. Doing with less: Hominin brain atrophy. Homo—J. Comp. Human Biol. 2014, 65, 433–449. [Google Scholar] [CrossRef]
- Bednarik, R.G. The domestication of humans. Anthropologie 2008, 46, 1–17. [Google Scholar]
- Bednarik, R.G. The Human Condition; Springer: New York, NY, USA, 2011. [Google Scholar]
- Hammer, K. Das Domestikationssyndrom. Kulturpflanze 1984, 32, 11–34. [Google Scholar] [CrossRef]
- Wilkins, A.S.; Wrangham, R.W.; Fitch, W.T. The ‘domestication syndrome’ in mammals: A unified explanation based on neural crest cell behavior and genetics. Genetics 2014, 197, 795–808. [Google Scholar] [CrossRef]
- Wilson, P. The Domestication of the Human Species; Yale University Press: New Haven, CT, USA, 1988. [Google Scholar]
- Hodder, I. The Domestication of Europe: Structure and Contingency in Neolithic Societies; Basil Blackwell: Oxford, UK, 1990. [Google Scholar]
- Leach, H.M. Human domestication reconsidered. Curr. Anthr. 2003, 44, 349–368. [Google Scholar] [CrossRef]
- Darwin, C. The Variation of Animals and Plants under Domestication; John Murray: London, UK, 1868; Volume 2. [Google Scholar]
- Rindos, D. The Origins of Agriculture: An Evolutionary Perspective; Academic Press: Orlando, FL, USA, 1984. [Google Scholar]
- Hölldobler, B.; Wilson, E.O. The Ants; Belknap: Cambridge, MA, USA, 1990. [Google Scholar]
- Munkacsi, A.B.; Pan, J.J.; Villesen, P.; Mueller, U.G.; Blackwell, M.; McLaughlin, D.J. Convergent coevolution in the domestication of coral mushrooms by fungus-growing ants. Proc. Roy. Soc. B Biol. Sci. 2004, 271, 1777–1782. [Google Scholar] [CrossRef]
- Mueller, U.G.; Gerardo, N.M.; Aanen, D.K.; Six, D.L.; Schultz, T.R. The evolution of agriculture in insects. Ann. Rev. Ecol. Evol. Syst. 2005, 36, 563–595. [Google Scholar] [CrossRef]
- Boyd, R.; Richerson, P.J. The Origin and Evolution of Cultures; Oxford University Press: New York, NY, USA, 2005. [Google Scholar]
- Richerson, P.J.; Boyd, R. Not by Genes Alone: How Culture Transformed Human Evolution; University of Chicago Press: Chicago, IL, USA, 2005. [Google Scholar]
- Price, E.O. Animal Domestication and Behavior; CABI Publishing: New York, NY, USA, 2002. [Google Scholar]
- Lord, K.A.; Larson, G.; Coppinger, R.P.; Karlsson, E.K. The history of farm foxes undermines the animal domestication syndrome. Trends Ecol. Evol. 2020, 35, 125–136. [Google Scholar] [CrossRef]
- Shilton, D.; Breski, M.; Dor, D.; Jablonka, E. Human social evolution: Self-domestication or self-control? Front. Psychol. 2020, 11, 1–22. [Google Scholar] [CrossRef]
- Sánchez-Villagra, M.R.; van Schaik, C. Evaluating the selfdomestication hypothesis of human evolution. Evol. Anthropol. 2019, 28, 133–143. [Google Scholar] [CrossRef]
- Gleeson, B.T.; Wilson, L.A.B. Shared reproductive disruption, not neural crest or tameness, explains the domestication syndrome. Proc. Roy. Soc. B 2023, 290, 20222464. [Google Scholar] [CrossRef]
- Trut, L.; Oskina, I.; Kharlamova, A. Animal evolution during domestication: The domesticated fox as a model. BioEssays 2009, 31, 349–360. [Google Scholar] [CrossRef]
- Bednarik, R.G. The Domestication of Humans; Routledge: London, UK, 2020. [Google Scholar] [CrossRef]
- Lacruz, R.S.; Stringer, C.B.; Kimbel, W.H.; Wood, B.; Harvati, K.; O’Higgins, P.; Bromage, T.G.; Arsuaga, J.-L. The evolutionary history of the human face. Nat. Ecol. Evol. 2019, 3, 726–736. [Google Scholar] [CrossRef]
- Ashley Montagu, M.F. An Introduction to Physical Anthropology; Thomas: Springfield, IL, USA, 1960. [Google Scholar]
- De Beer, G.R. Embryos and Ancestors; Oxford University Press: Oxford, UK, 1940. [Google Scholar]
- Bednarik, R.G.; Sreenathan, M. Traces of the ancients: Ethnographic vestiges of Pleistocene ‘art’. Rock Art Res. 2012, 29, 191–217. [Google Scholar]
- Bednarik, R.G. Children as Pleistocene artists. Rock Art Res. 2008, 25, 173–182. [Google Scholar]
- Bednarik, R.G. Exograms. Rock Art Res. 2014, 31, 47–62. [Google Scholar]
- Buss, D.M.; Barnes, M.L. Preferences in human mate selection. J. Pers. Soc. Psychol. 1986, 50, 559–570. [Google Scholar] [CrossRef]
- Laland, K.N. Sexual selection with a culturally transmitted mating preference. Theor. Popul. Biol. 1994, 45, 1–15. [Google Scholar] [CrossRef]
- Ford, C.S.; Beach, F. Patterns of Sexual Behavior; Harper: New York, NY, USA, 1951. [Google Scholar]
- Gregersen, G. Sexual Practices: The Story of Human Sexuality; F. Watts: New York, NY, USA, 1983. [Google Scholar]
- Buss, D.M.; Abbott, M.; Angleitner, A.; Asherian, A.; Biaggio, A.; Blanco-Villasenor, A.; Bruchon-Schweitzer, M.; Ch’u, H.Y.; Czapinski, J.; Deraad, B.; et al. International preferences in selecting mates: A study of 37 societies. J. Cross Cult. Psychol. 1990, 21, 5–47. [Google Scholar] [CrossRef]
- Grammer, K.; Thornhill, R. Human facial attractiveness and sexual selection: The role of symmetry and averageness. J. Comp. Psychol. 1994, 108, 233–242. [Google Scholar] [CrossRef]
- Jones, D.M. Sexual selection, physical attractiveness and facial neoteny: Cross-cultural evidence and implications. Curr. Anthropol. 1995, 36, 723–748. [Google Scholar] [CrossRef]
- Jones, D.M. An evolutionary perspective on physical attractiveness. Evol. Anthropol. 1996, 5, 97–109. [Google Scholar] [CrossRef]
- Shackelford, T.K.; Larsen, R.J. Facial asymmetry as an indicator of psychological, emotional, and physiological distress. J. Personal. Soc. Psychol. 1997, 72, 456–466. [Google Scholar] [CrossRef]
- Barkow, J.H. Universals and evolutionary psychology. In Universals and Constructivism; Hejl, P.M., Ed.; Suhrkamp Verlag: Frankfurt, Germany, 2001; pp. 126–138. [Google Scholar]
- Buss, D.M.; Schmitt, D.P. Mate preferences and their behavioral manifestations. Ann. Rev. Psychol. 2019, 70, 23.1–23.34. [Google Scholar] [CrossRef]
- Walker, M.L.; Herndon, J.G. Menopause in nonhuman primates? Biol. Reprod. 2008, 79, 398–406. [Google Scholar] [CrossRef]
- Lovejoy, C.O. The origin of man. Science 1981, 211, 341–350. [Google Scholar] [CrossRef]
- Leonard, W.R.; Robertson, M.L. Nutritional requirements and human evolution: A bioenergetics model. Amer. J. Human Biol. 1992, 4, 179–195. [Google Scholar] [CrossRef]
- Leonard, W.R.; Robertson, M.L. Comparative primate energetics and hominid evolution. Amer. J. Phys. Anthropol. 1997, 102, 265–281. [Google Scholar] [CrossRef]
- Biesele, M. Women like Meat. In The Folklore and Foraging Ideology of the Kalahari Ju/’Hoan; Witwatersrand University Press: Johannesburg, South Africa, 1993. [Google Scholar]
- Aiello, L.C.; Wheeler, P. The expensive-tissue hypothesis: The brain and the digestive system in human and primate evolution. Curr. Anthropol. 1995, 36, 199–221. [Google Scholar] [CrossRef]
- Leonard, W.R. Food for thought: Dietary change was a driving force in human evolution. Sci. Am. 2002, 287, 106–115. [Google Scholar] [CrossRef]
- Williams, G.C. Pleiotropy, natural selection, and the evolution of senescence. Evolution 1957, 11, 398–411. [Google Scholar] [CrossRef]
- Hawkes, K. The centrality of ancestral grandmothering in human evolution. Integr. Comp. Biol. 2020, 60, 765–781. [Google Scholar] [CrossRef]
- Paquin, D.; Kato, D.; Kim, P. A mathematical model for the effects of grandmothering on human longevity. Math. Biosci. Eng. 2020, 17, 3175–3189. [Google Scholar] [CrossRef]
- Thomas, J.G. Self-Domestication and Language Evolution. Ph.D. thesis, Linguistics and English Language School of Philosophy, Psychology and Language Sciences, University of Edinburgh, Edinburgh, UK, 2013. [Google Scholar]
- Eckhardt, R.B.; Henneberg, M. Foreword. In The Domestication of Humans; Bednarik, R.G., Ed.; Routledge: Abingdon, VI, USA; New York, NY, USA, 2020; pp. xi–xvi. [Google Scholar]
- Benítez-Burraco, A.; Lattanzi, W.; Murphy, E. Language impairments in ASD resulting from a failed domestication of the human brain. Front. Neurosci. 2016, 10, 373. [Google Scholar] [CrossRef]
- Benítez-Burraco, A.; Theofanopoulou, C.; Boeckx, C. Globularization and domestication. Topoi 2016, 37, 265–278. [Google Scholar] [CrossRef]
- Theofanopoulou, C.; Gastaldon, S.; O’Rourke, T.; Samuels, B.D.; Messner, A.; Martins, P.T.; Delogu, F.; Alamri, S.; Boeckx, C. Self-domestication in Homo sapiens: Insights from comparative genomics. PLoS ONE 2017, 12, e0185306. [Google Scholar] [CrossRef] [PubMed]
- Terberger, T.; Street, M. Jungpaläolithische Menschenreste im westlichen Mitteleuropa und ihr Kontext. In Erkenntnisjäger: Kultur und Umwelt des frühen Menschen; Burdukiewicz, J.M., Fiedler, L., Heinrich, W.-D., Justus, A., Brühl, E., Eds.; Veröffentlichungen des Landesamtes für Archäologie Sachsen-Anhalt, Vol. 57/2; Landesmuseum für Vorgeschichte: Halle, Germany, 2003; pp. 579–591. [Google Scholar]
- Schulz, M. Die Regeln mache ich. Spiegel 2004, 34, 128–131. [Google Scholar]
- Bednarik, R.G. The mythical Moderns. J. World Prehist. 2008, 21, 85–102. [Google Scholar] [CrossRef]
- Racimo, F. Testing for ancient selection using cross-population allele frequency differentiation. Genetics 2016, 202, 733–750. [Google Scholar] [CrossRef] [PubMed]
- Peyrégne, S.; Boyle, M.J.; Dannemann, M.; Prüfer, K. Detecting ancient positive selection in humans using extended lineage sorting. Genome Res. 2017, 27, 1563–1572. [Google Scholar] [CrossRef]
- Castellano, S.; Parra, G.; Sánchez-Quinto, F.A.; Racimo, F.; Kuhlwilm, M.; Kircher, M.; Sawyer, S.; Fu, Q.; Heinze, A.; Nickel, B.; et al. Patterns of coding variation in the complete exomes of three Neandertals. Proc. Nat. Acad. Sci. USA 2014, 111, 6666–6671. [Google Scholar] [CrossRef]
- Björnerfeldt, S.; Webster, M.T.; Vilà, C. Relaxation of selective constraint on dog mitochondrial DNA following domestication. Genome Res. 2006, 16, 990–994. [Google Scholar] [CrossRef]
- Cruz, F.; Vilà, C.; Webster, M.T. The legacy of domestication: Accumulation of deleterious mutations in the dog genome. Mol. Biol. Evol. 2008, 25, 2331–2336. [Google Scholar] [CrossRef]
- Marsden, C.D.; Ortega-Del Vecchyo, D.; O’Brien, D.P.; Taylor, J.F.; Ramirez, O.; Vilà, C.; Marques-Bonet, T.; Schnabel, R.D.; Wayne, R.K.; Lohmueller, K.E. Bottlenecks and selective sweeps during domestication have increased deleterious genetic variation in dogs. Proc. Nat. Acad. Sci. USA 2016, 113, 152–157. [Google Scholar] [CrossRef]
- Makino, T.; Rubin, C.J.; Carneiro, M.; Axelsson, E.; Andersson, L.; Webster, M.T. Elevated proportions of deleterious genetic variation in domestic animals and plants. Genome Biol. Evol. 2018, 10, 276–290. [Google Scholar] [CrossRef]
- Voight, B.F.; Kudaravalli, S.; Wen, X.; Pritchard, J.K. A map of recent positive selection in the human genome. PLoS Biol. 2006, 4, e72. [Google Scholar]
- Evans, P.D.; Gilbert, S.L.; Mekel-Bobrov, N.; Vallender, E.J.; Anderson, J.R.; Vaez-Azizi, L.M.; Tishkoff, S.A.; Hudson, R.R.; Lahn, B.T. Microcephalin, a gene regulating brain size, continues to evolve adaptively in humans. Science 2005, 309, 1717–1720. [Google Scholar] [CrossRef] [PubMed]
- Mekel-Bobrov, N.; Gilbert, S.L.; Evans, P.D.; Vallender, E.J.; Anderson, J.R.; Tishkoff, S.A.; Lahn, B.T. Ongoing adaptive evolution of ASPM, a brain size determinant in Homo sapiens. Science 2005, 309, 1720–1722. [Google Scholar] [CrossRef]
- Zanella, M.; Vitriolo, A.; Andirko, A.; Martins, P.T.; Sturm, S.; O’Rourke, T.; Laugsch, M.; Malerba, N.; Skaros, A.; Trattaro, S.; et al. 7q11. 23 syndromes reveal BAZ1B as a master regulator of the modern human face and validate the self-domestication hypothesis. bioRxiv 2019, 570036. [Google Scholar] [CrossRef]
- Clark, G.; Henneberg, M. Ardipithecus ramidus and the evolution of language and singing: An early origin for hominin vocal capability. Homo—J. Comp. Human Biol. 2017, 68, 101–121. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bednarik, R.G. The Domestication of Humans. Encyclopedia 2023, 3, 947-955. https://doi.org/10.3390/encyclopedia3030067
Bednarik RG. The Domestication of Humans. Encyclopedia. 2023; 3(3):947-955. https://doi.org/10.3390/encyclopedia3030067
Chicago/Turabian StyleBednarik, Robert G. 2023. "The Domestication of Humans" Encyclopedia 3, no. 3: 947-955. https://doi.org/10.3390/encyclopedia3030067
APA StyleBednarik, R. G. (2023). The Domestication of Humans. Encyclopedia, 3(3), 947-955. https://doi.org/10.3390/encyclopedia3030067