Tautomerism of β-Diketones and β-Thioxoketones
Abstract
1. Introduction
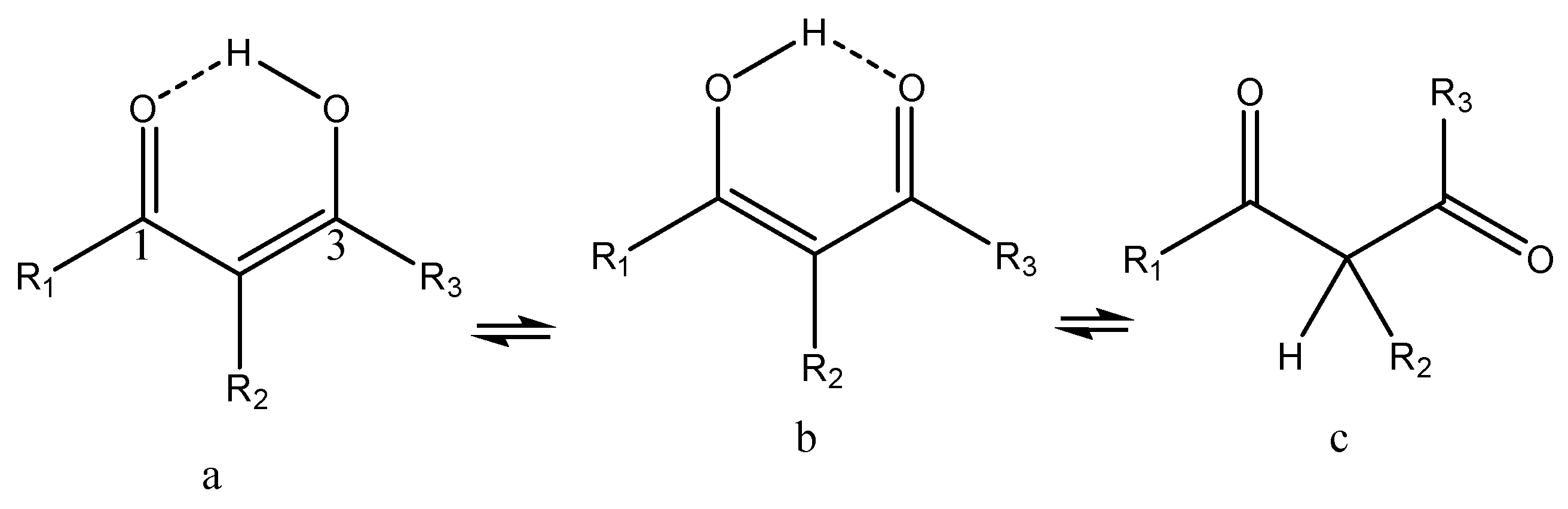
1.1. β-Diketones
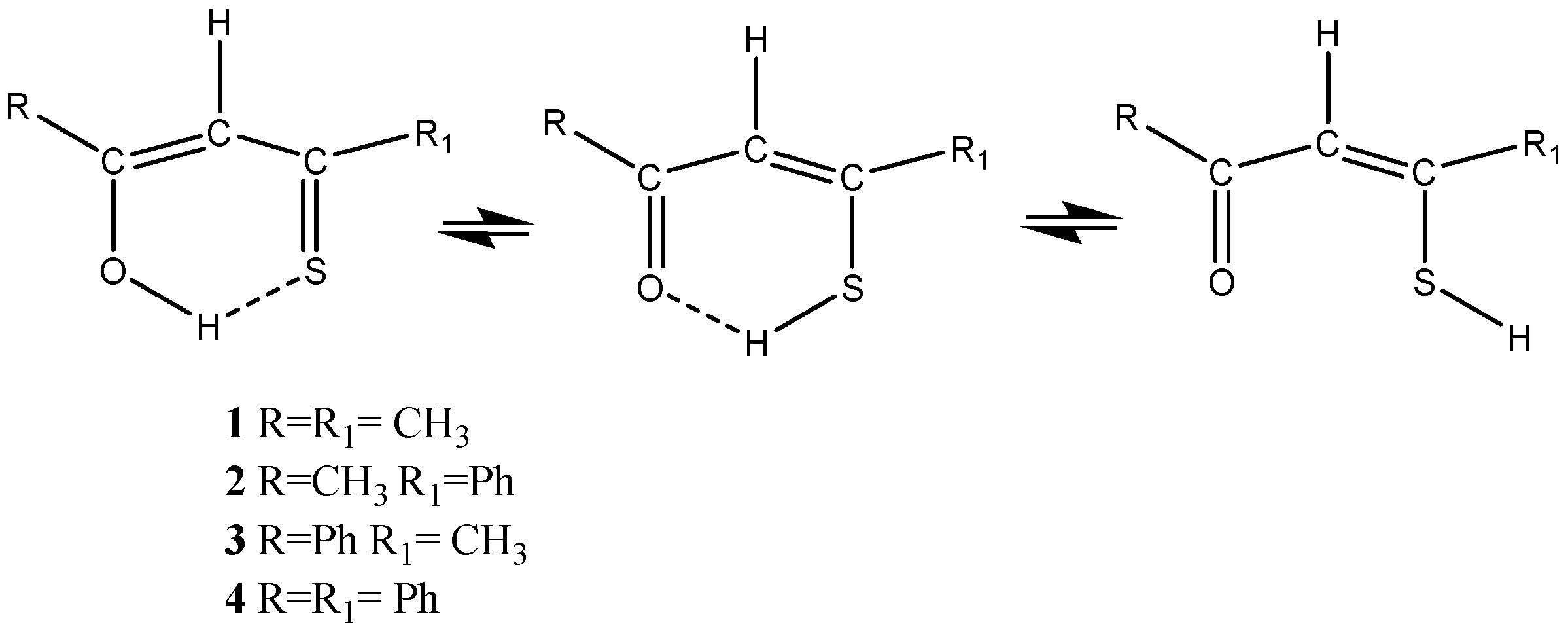
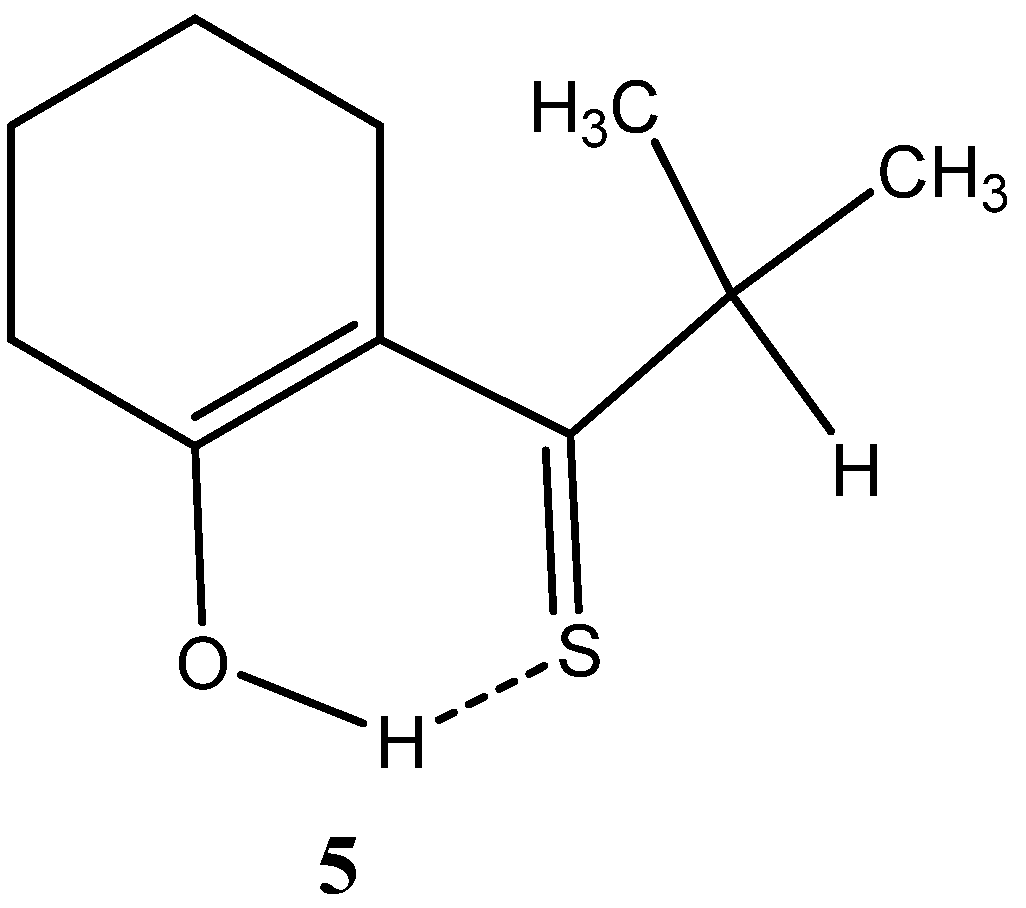
1.2. β-Thioxoketones
2. Tautomerism
2.1. Gas Phase
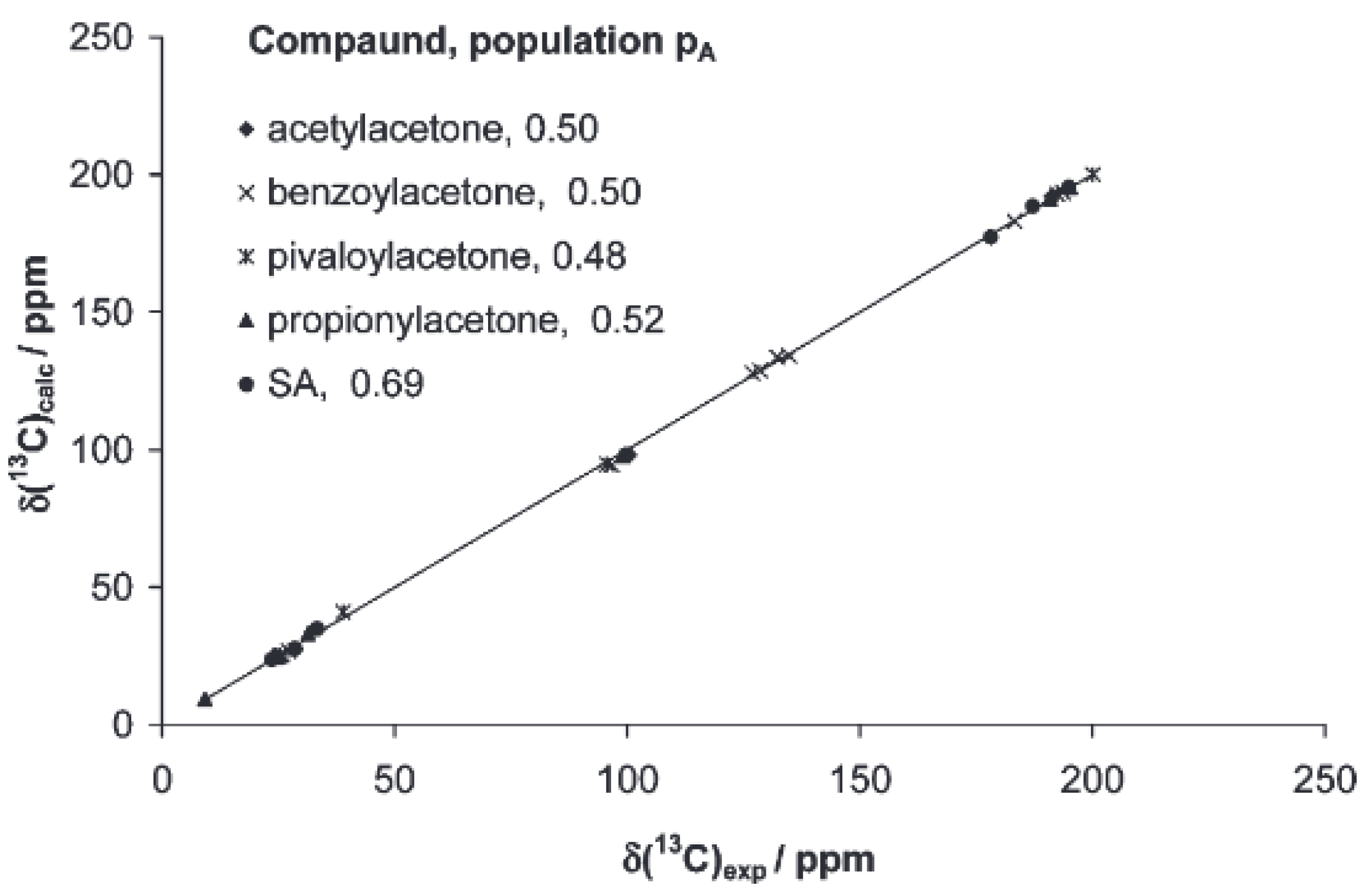
2.2. Liquid State
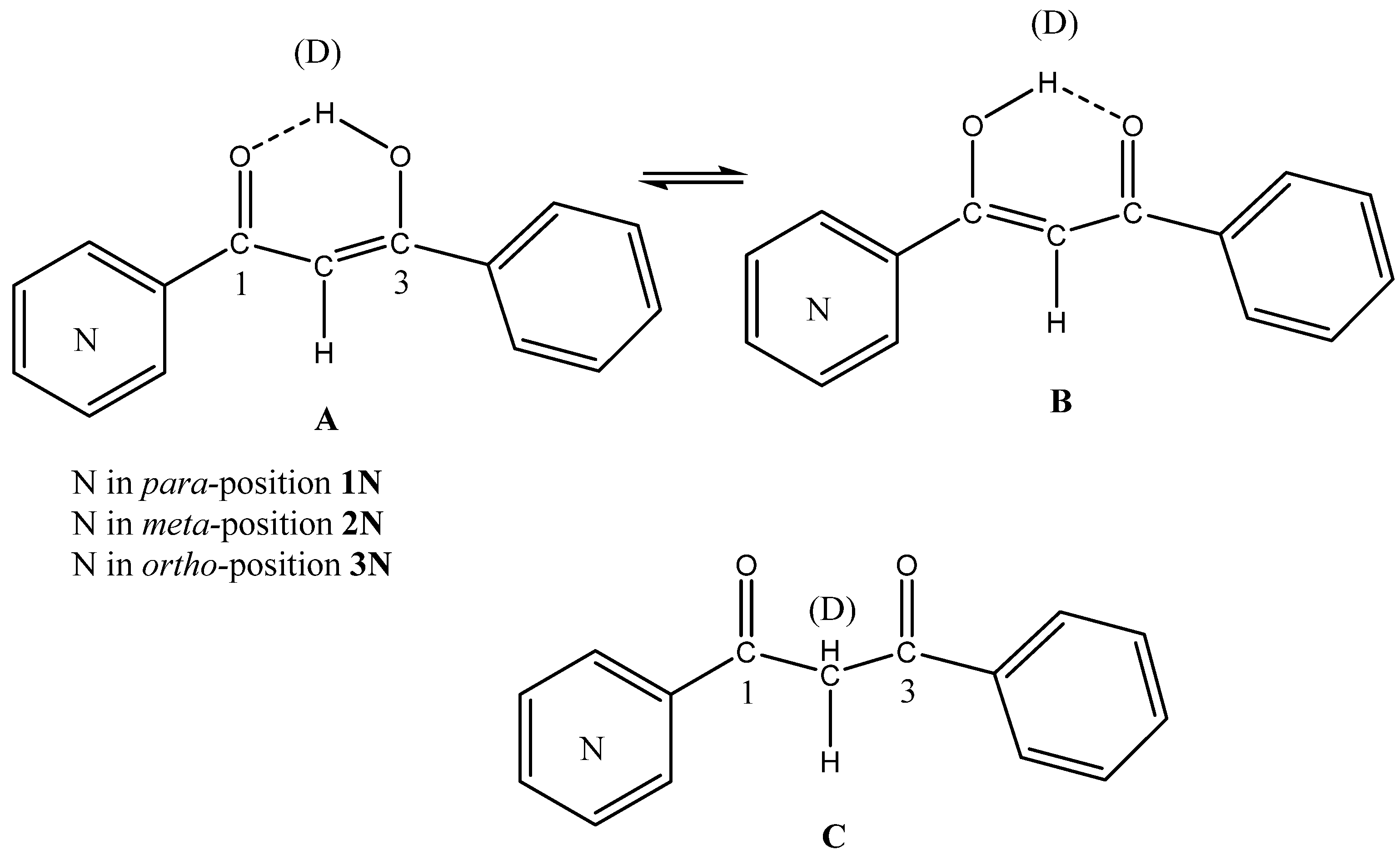
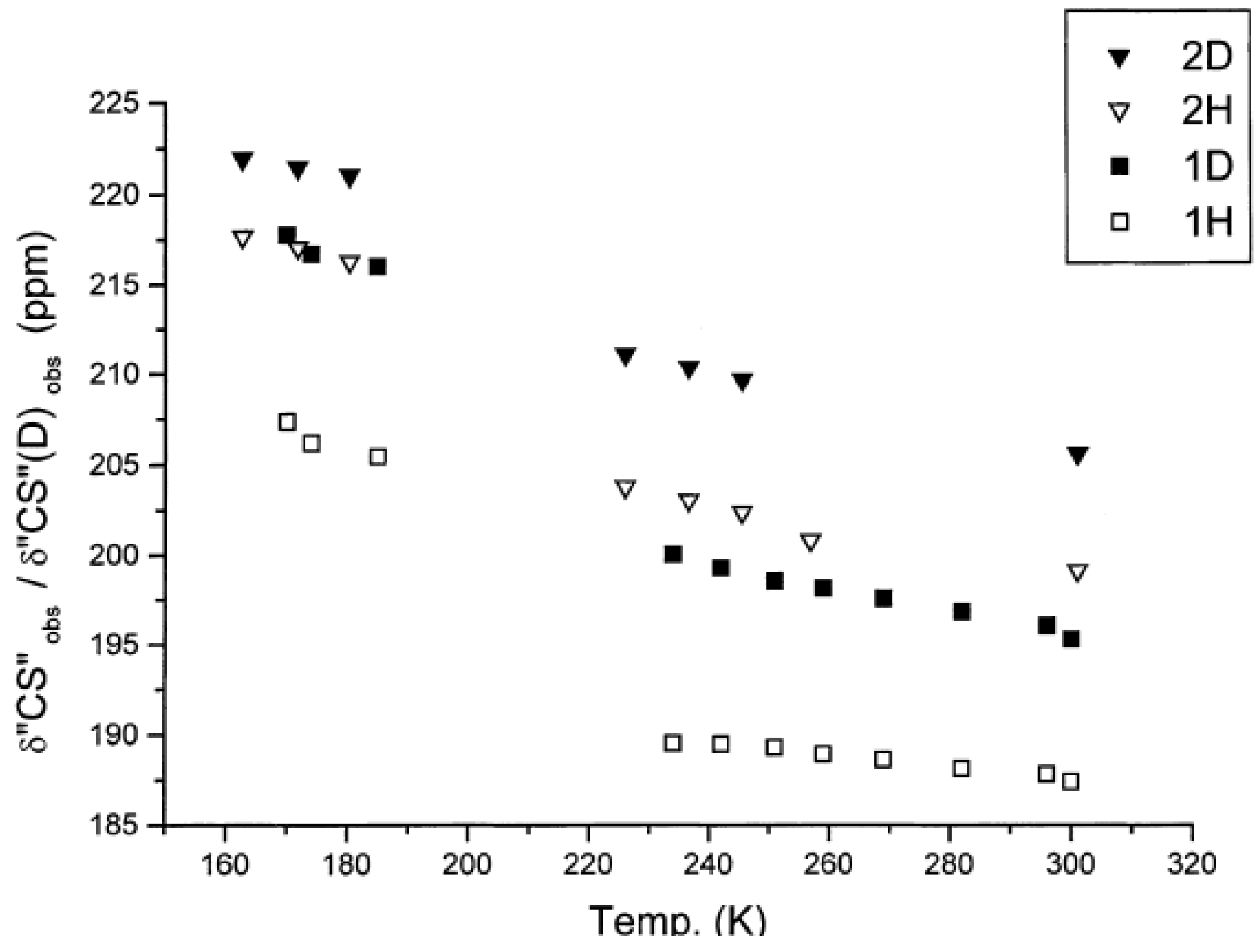
2.3. Solid State
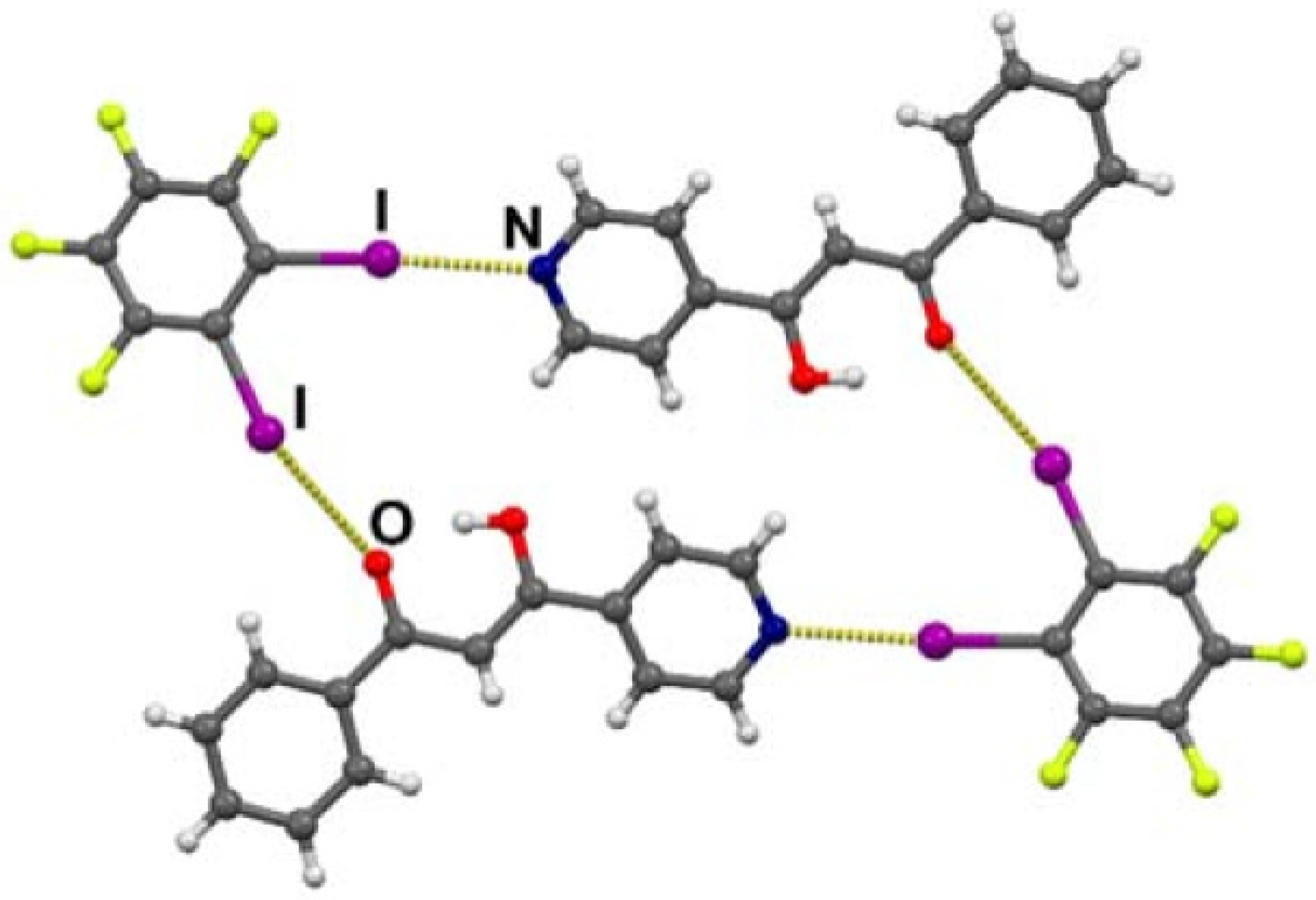
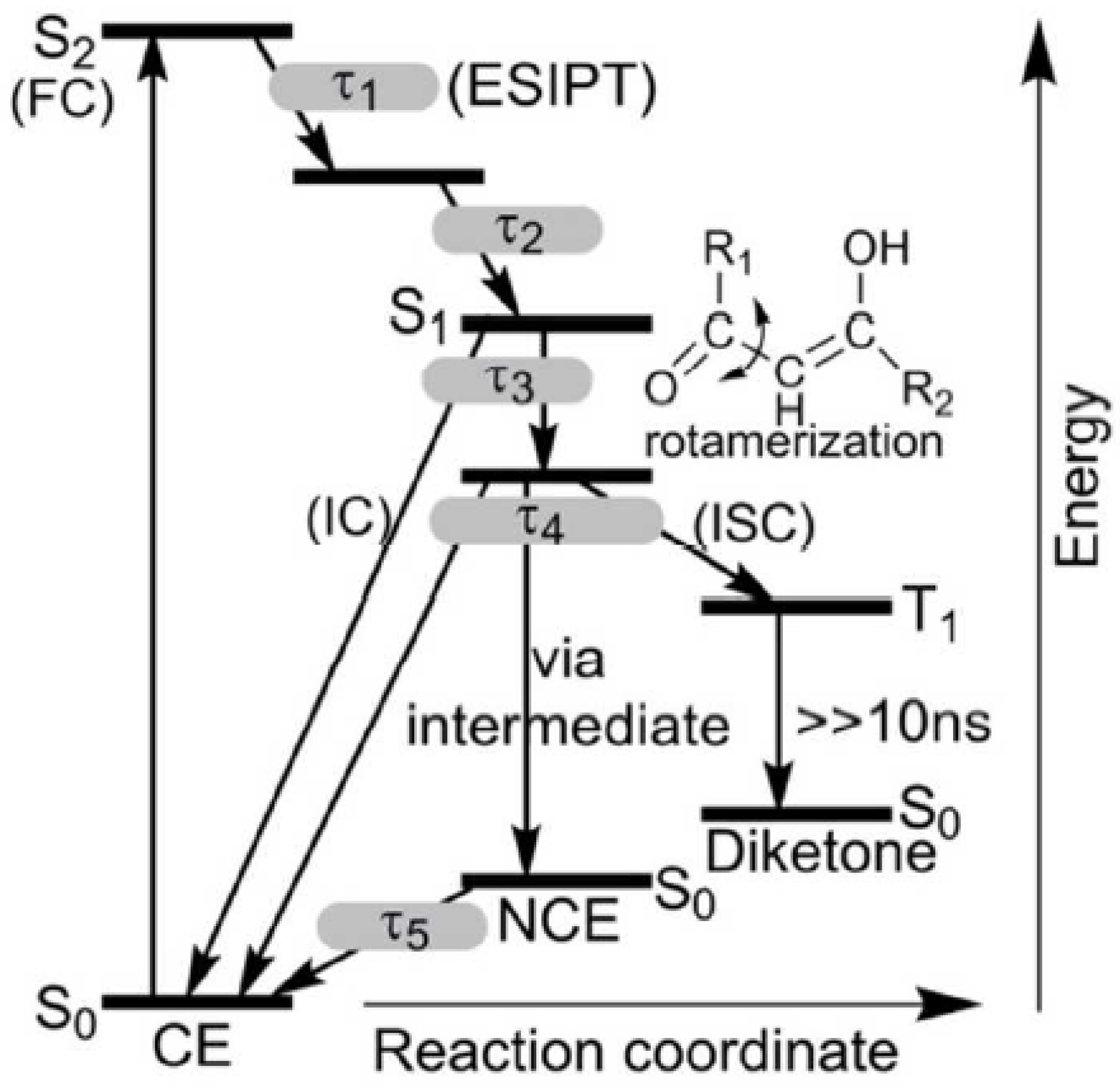
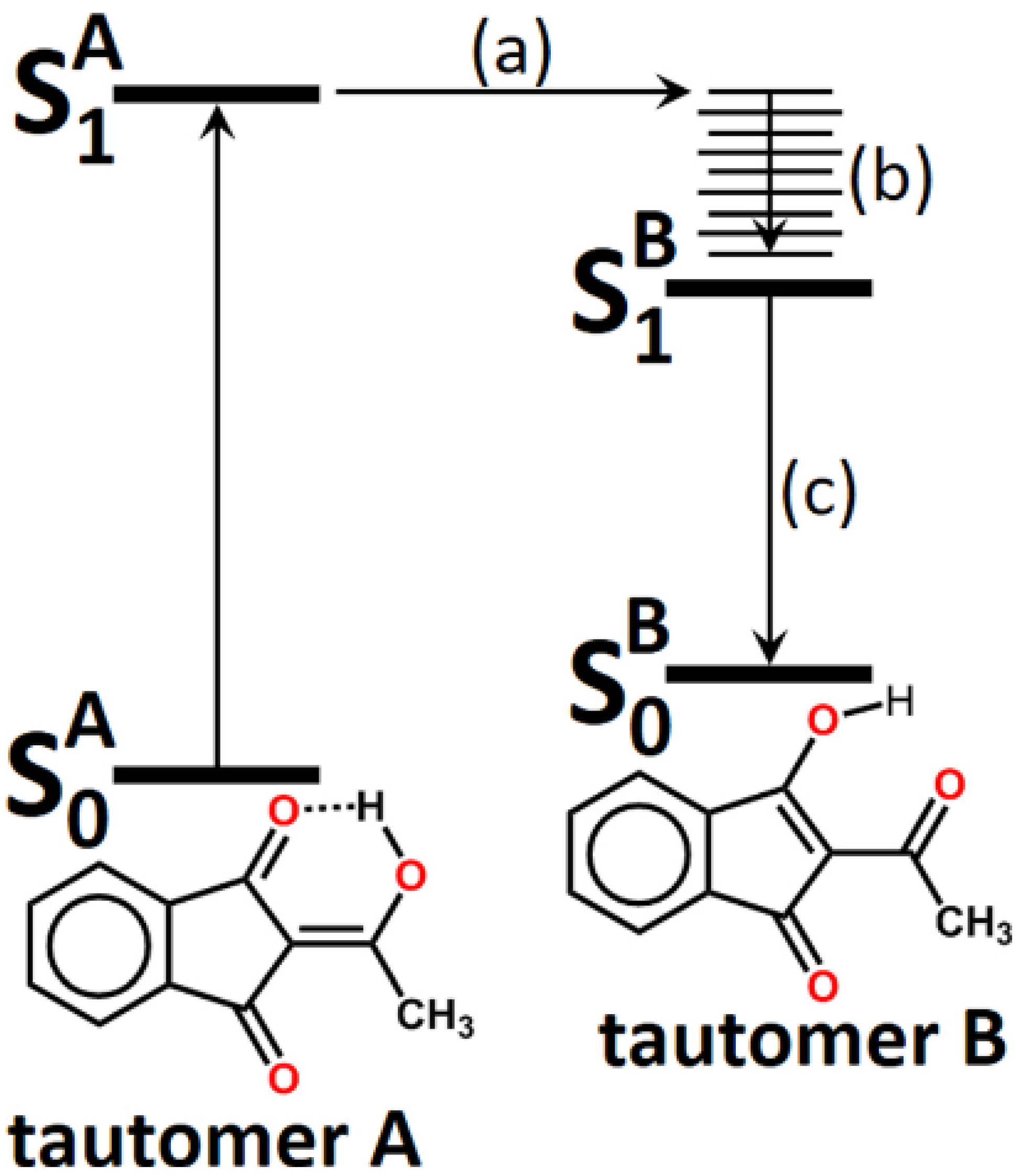
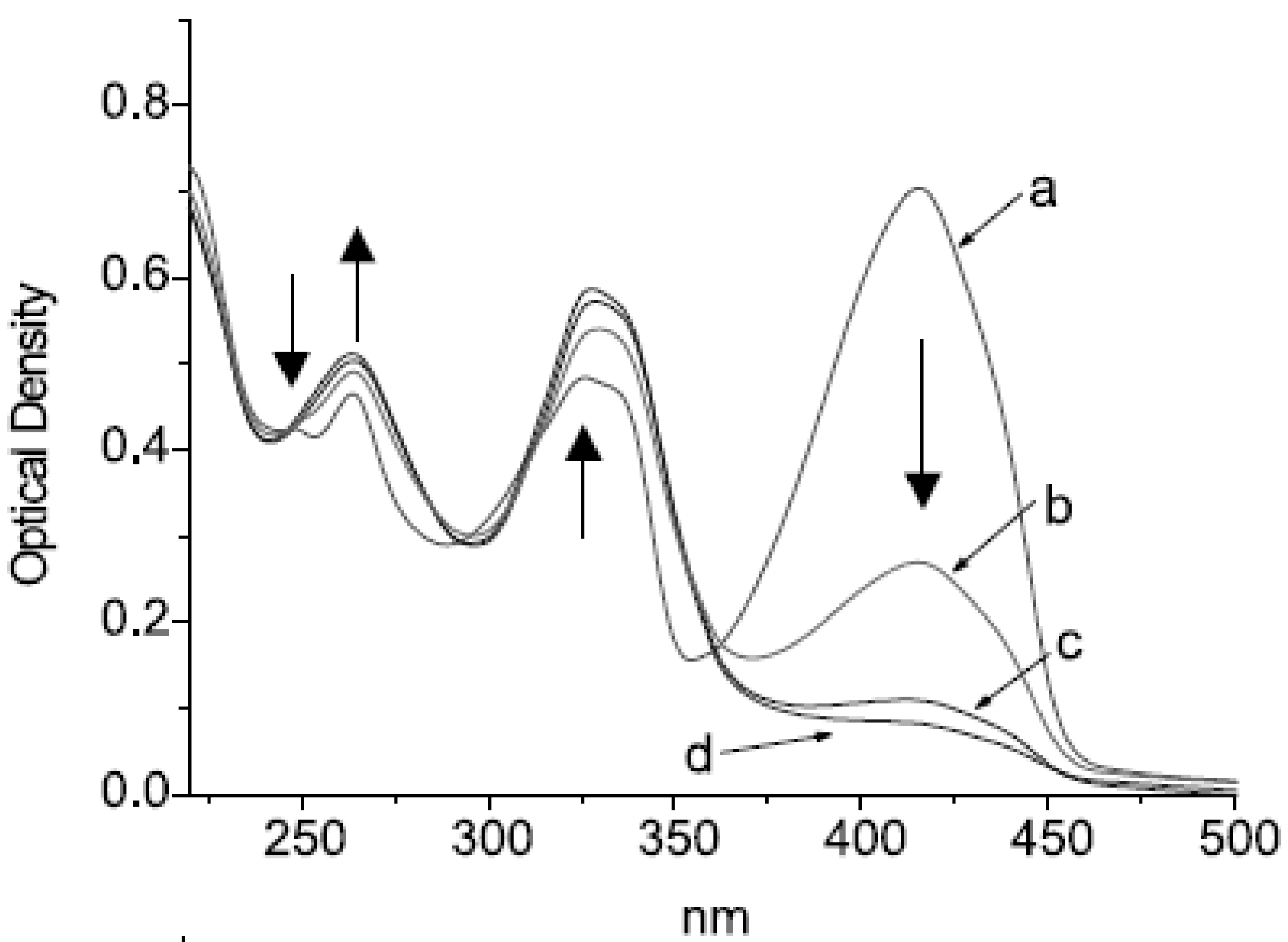
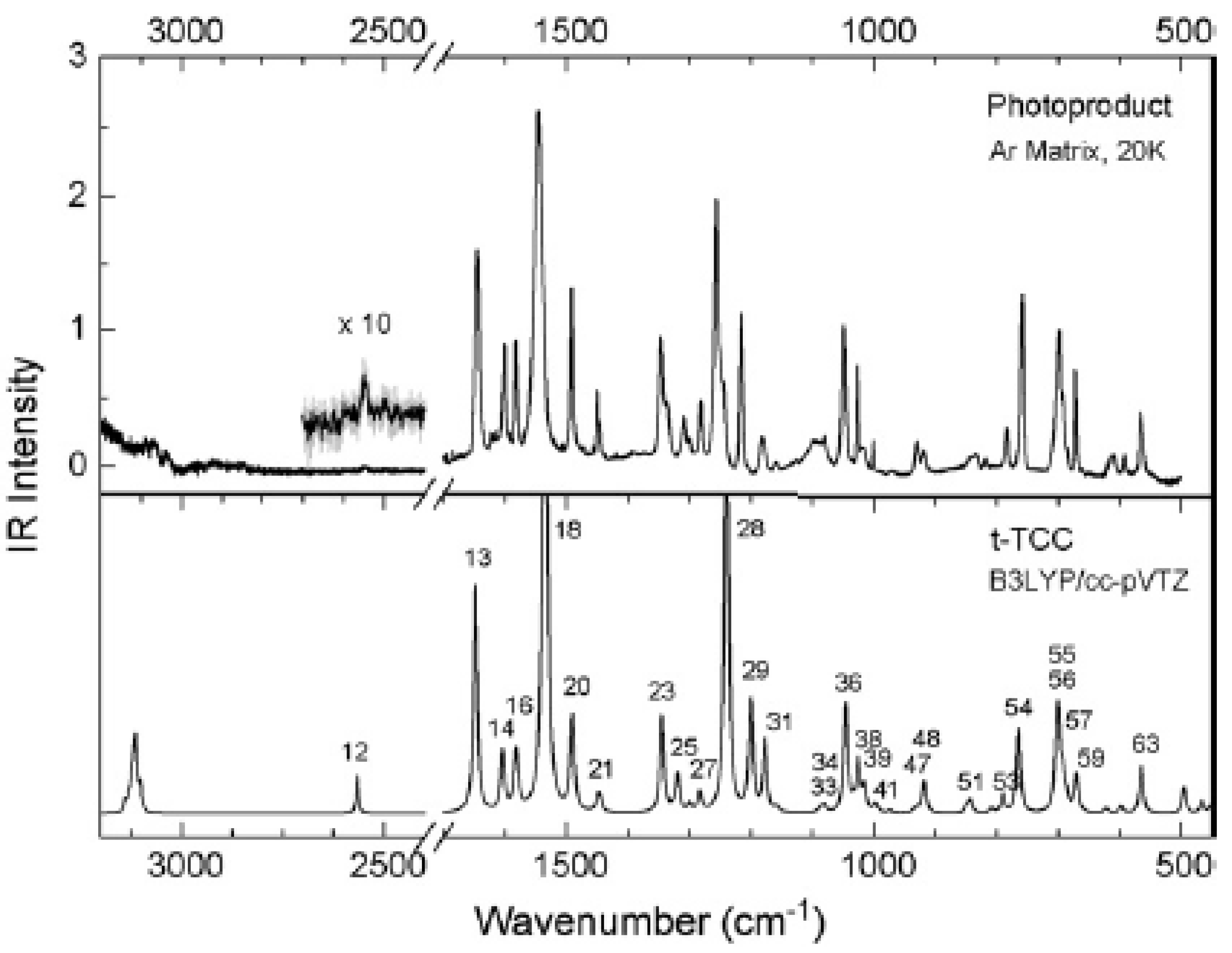
3. Photoconversion
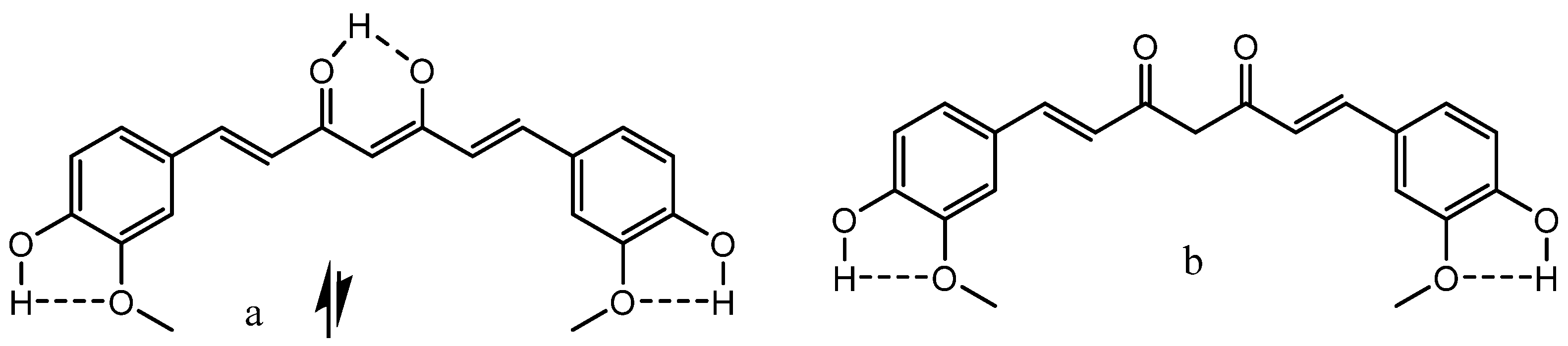
3.1. β-Diketones
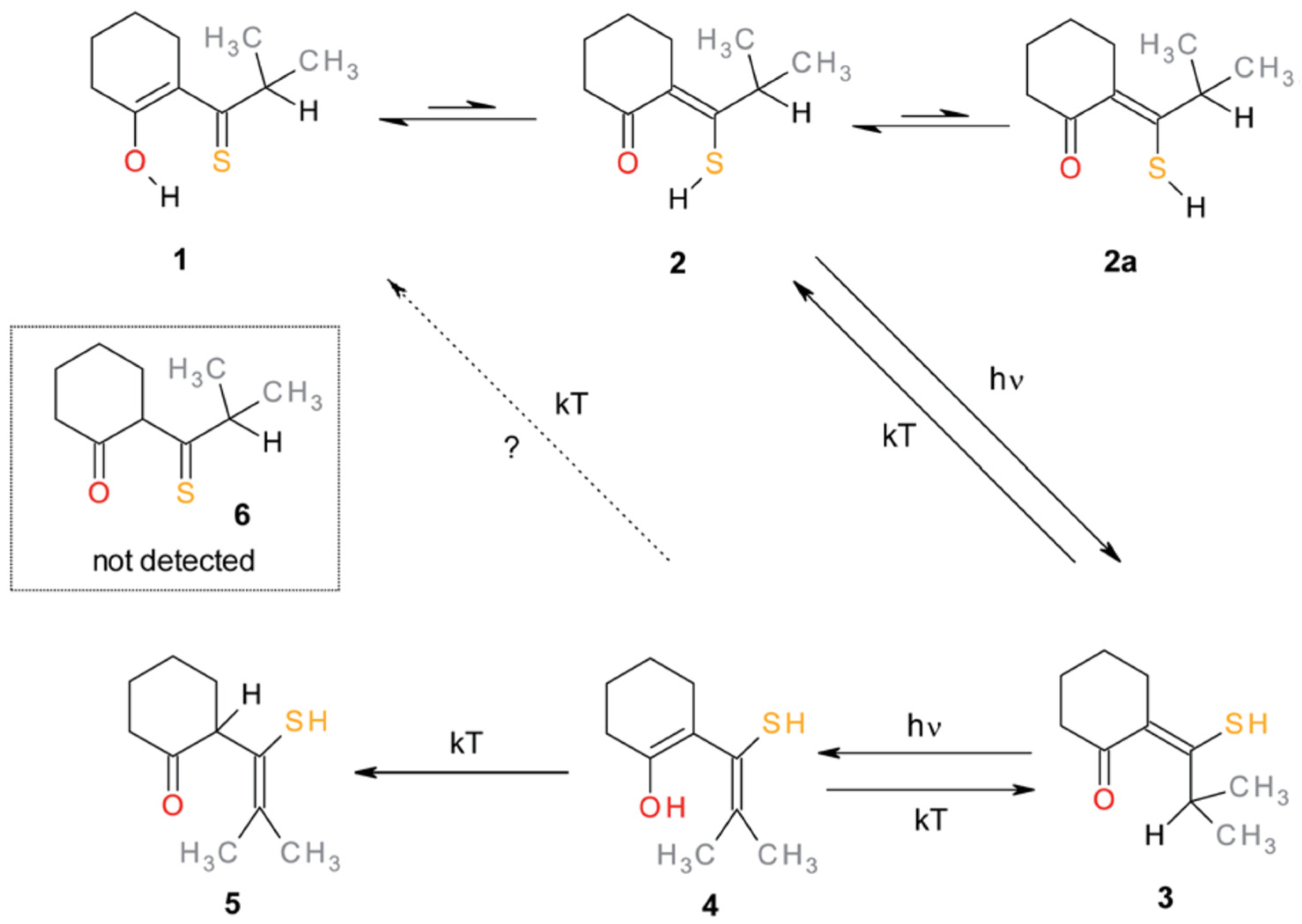
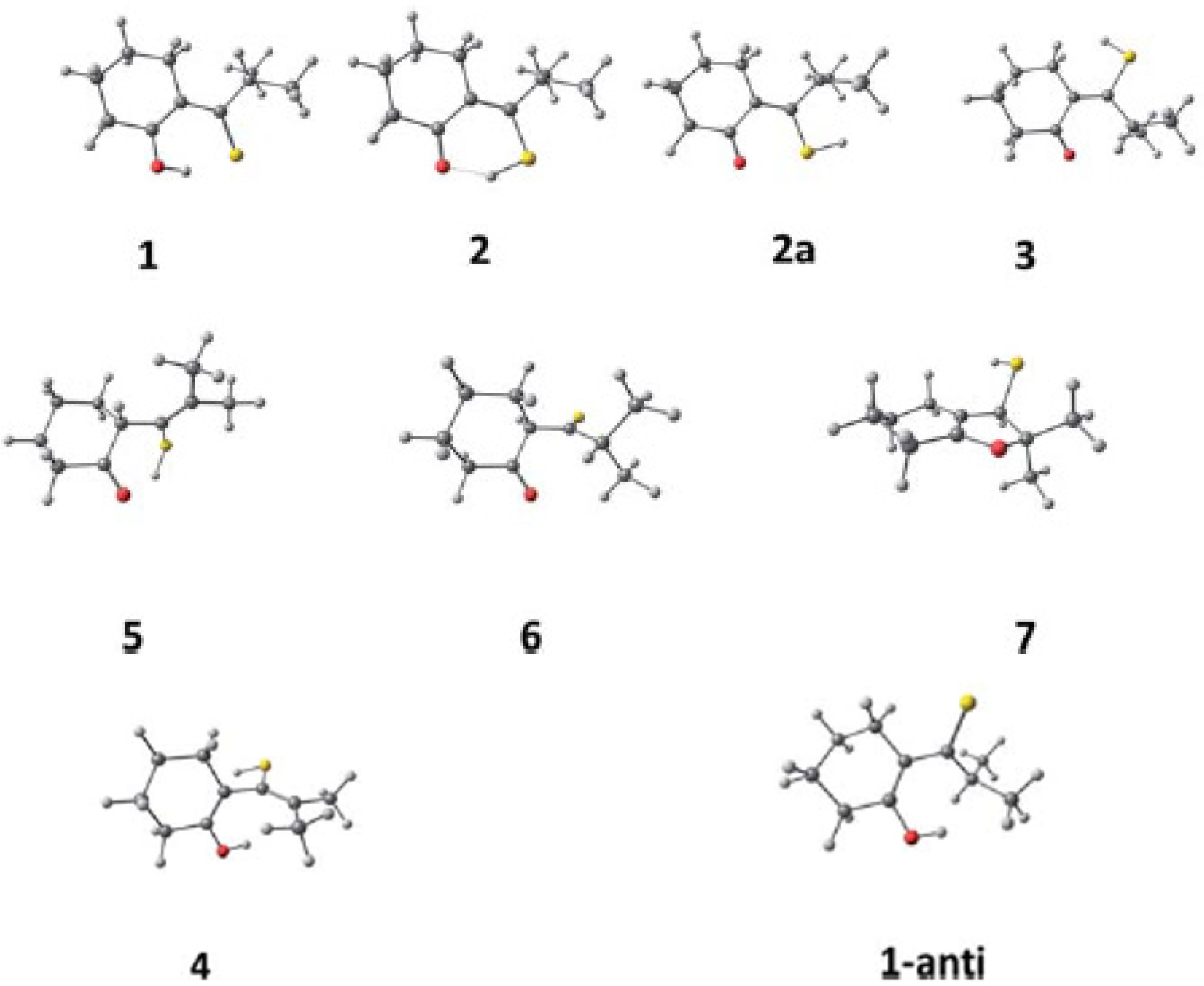
3.2. β-Thioxoketones
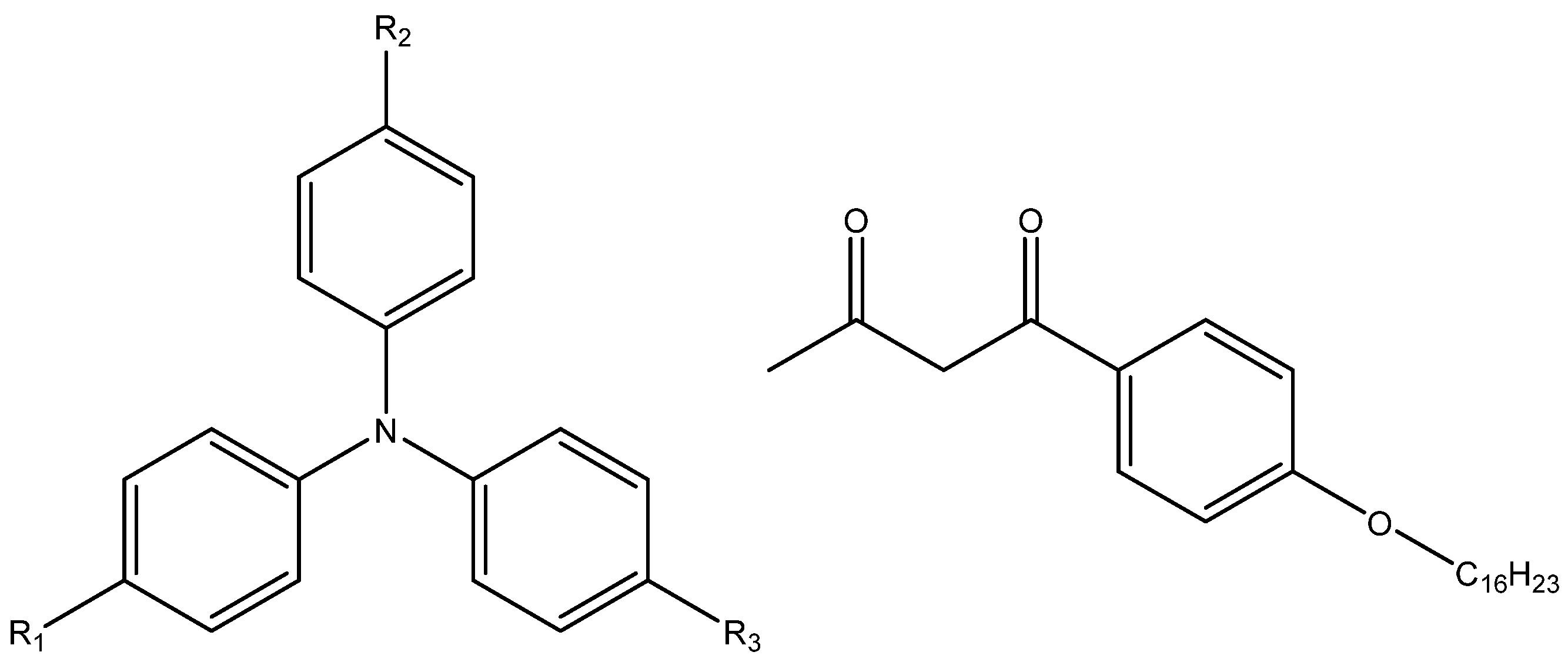
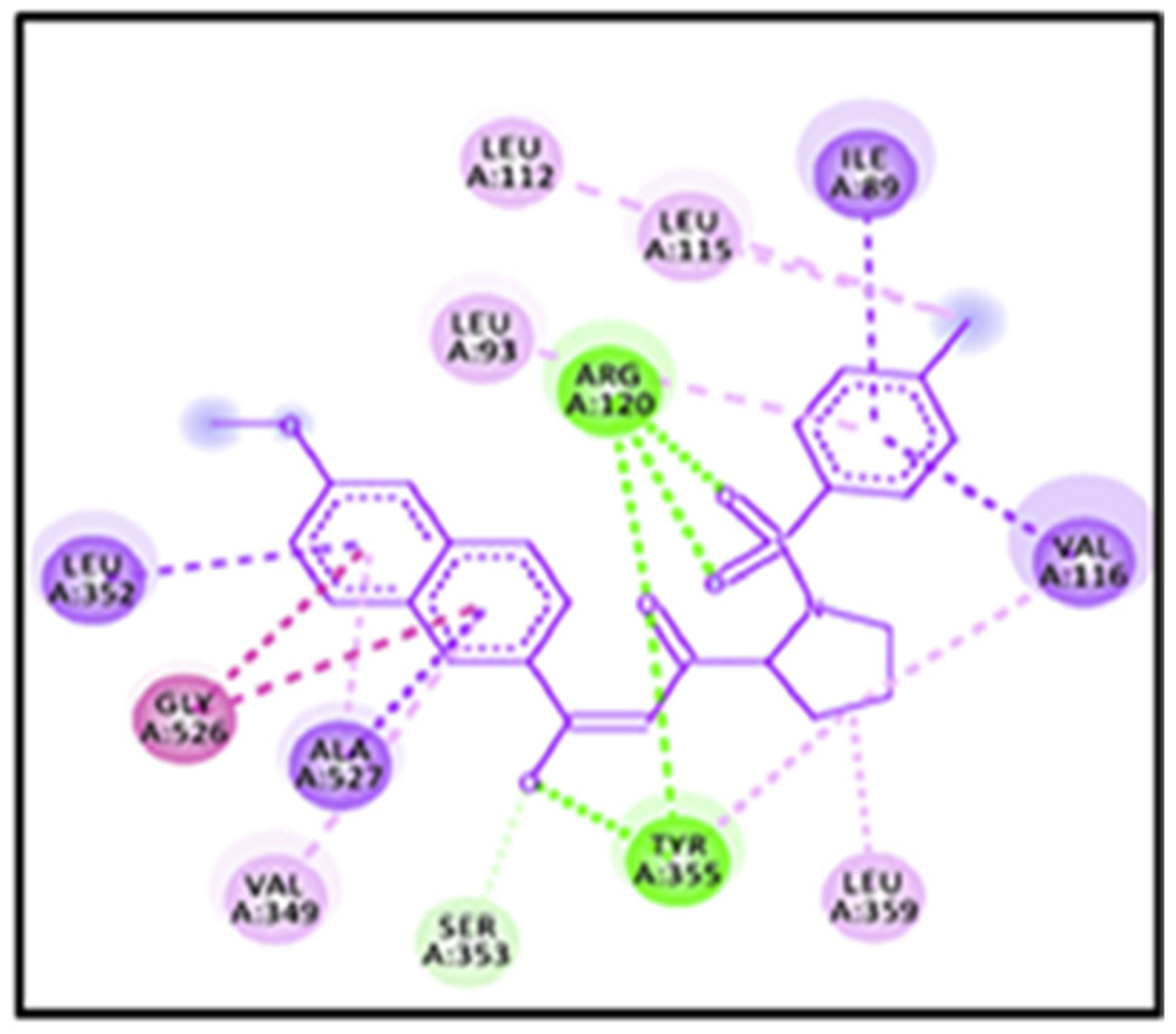
4. Docking
5. Experimental
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Gonzalo, G.; Alcántara, A.R. Recent Development in the Synthesis of β-diketones. Pharmaceuticals 2021, 14, 1043. [Google Scholar]
- Hansen, P.E. Structural Studies of β-diketones and its implications on Biological Effects. Pharmaceuticals 2021, 14, 1189. [Google Scholar] [CrossRef]
- Kljun, J.; Ture, I. β-Diketones as Scaffolds for Anticancer Drug Design–From Organic Building Blocks to Natural Products and Metallodrug Components. Eur. J. Inorg. Chem. 2017, 12, 1655–1666. [Google Scholar] [CrossRef]
- Anand, P.; Thomas, S.G.; Kunnumakkara, A.B.; Sundaram, C.; Harikumar, K.B.; Sung, B.; Tharakan, S.T.; Misra, K.; Priyadarsini, I.K.; Rajasekharan, K.N. Biological activities of curcumin and its analogues (Congeners) made by man and Mother. Nat. Biochem. Pharmacol. 2008, 76, 1590–1611. [Google Scholar] [CrossRef]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The Essential Medicinal Chemistry of Curcumin. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef]
- Priyadarsini, K.I. Chemical and Structural Features Influencing the Biological Activity of Curcumin. Curr. Pharm. Des. 2013, 19, 2093–2100. [Google Scholar]
- Slika, L.; Patra, D. A short review on chemical properties, stability and nano-technological advances for curcumin delivery. Expert Opin. Drug Deliv. 2020, 17, 61–75. [Google Scholar] [CrossRef]
- Malekshah, R.E.; Salehi, M.; Kubicki, M.; Khaleghian, A.J. Biological studies and computational modeling of two new copper complexes derived from β-diketones and their nano-complexes. Coord. Chem. 2019, 72, 1697–1714. [Google Scholar] [CrossRef]
- Hansen, P.E.; Mortensen, J.; Kamounah, F.S. The importance of correct tautomeric structures for biological molecules. JSM Chem. 2015, 3, 1014–1019. [Google Scholar]
- Jezierska, A.; Panek, J.J. Investigation of an O-H….S hydrogen bond via Carr-Parrinello and Path Integral molecular dynamics. J. Comput. Chem. 2009, 30, 1241–1250. [Google Scholar] [CrossRef]
- Mayoral, M.J.; Cornago, P.; Claramunt, R.; Cano, M. Pyridyl and pyridiniumyl β-diketonates as building blocks for palladium(III) and allyl-platinium(II) isomers. Multinuclear NMR structural elucidation and liquid crystal behavior. New. J. Chem. 2011, 35, 1020–1030. [Google Scholar] [CrossRef]
- Andrews, P.C.; Blair, V.L.; Ferrero, R.L.; Junk, P.C.; Kedzierski, L.; Peiris, R.M. Bismuth(III) beta-thioxoketonates as antibiotics against Helicobacter pylori and as anti-leishmanial agents. Dalton Trans. 2014, 43, 1279–1291. [Google Scholar] [CrossRef]
- Duus, F.; Antonsen, J.W. Thioxoketones. I. Preparation and Structure of Thioacetylacetone. Acta Chem. Scand. 1977, B 31, 40–46. [Google Scholar] [CrossRef]
- Semenova, I.S.; Yarovenko, V.N.; Levchenko, K.S.; Krayushkin, M.M. Synthesis of 1,3-thioxoketones from salicylaldehyde. Russ. Chem. Bull. 2013, 62, 1022–1025. [Google Scholar] [CrossRef]
- Becke, A.D. Density functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Belova, N.V.; Oberhammer, H.; Trang, N.H.; Girichev, G.V. Tautomeric Properties and Gas-Phase Structure of Acetylacetone. J. Org. Chem. 2014, 79, 5412–5419. [Google Scholar] [CrossRef]
- Belova, N.V.; Oberhammer, H.; Girichev, G.V. Tautomeric and conformational properties of dibenzoylmethane, C6H5-C(O)-CH2-C(O)-C6H5: Gas-phase electron diffraction and quantum chemical study. Struct. Chem. 2011, 22, 269–277. [Google Scholar] [CrossRef]
- Belova, N.V.; Oberhammer, H.; Girichev, G.V.; Shlykov, S.A. Automeric properties and gas-phase structure of 3-chloro-2,4-pentanedione. J. Phys. Chem. A 2008, 112, 3209–3214. [Google Scholar] [CrossRef]
- Belova, N.V.; Trang, N.H.; Trang, N.H.; Girichev, G.V. Tautomeric and conformational properties of dipivaloylmethane. J. Mol. Struct. 2017, 1132, 63–69. [Google Scholar] [CrossRef]
- Gilli, G.; Gilli, P. Towards an unified hydrogen-bond theory. J. Mol. Struct. 2000, 552, 1–15. [Google Scholar] [CrossRef]
- Anjomshoa, S.; Namazian, M.; Noorbala, M.R. The Effect of Solvent on Tautomerism, Acidity and Radical Stability of Curcuminand Its Derivatives Based on Thermodynamic Quantities. J. Sol. Chem. 2016, 45, 1021–1030. [Google Scholar] [CrossRef]
- Sandler, I.; Harper, J.B.; Ho, J. Explanation of Substituent Effects on the Enolization of β-Diketones and β-Ketoesters. J. Chem. Educ. 2021, 98, 1043–1048. [Google Scholar] [CrossRef]
- Cortney, C.H.; Krishnan, V.V. Keto–Enol Tautomerization of Acetylacetone in Mixed Solvents byNMR Spectroscopy. A Physical Chemistry Experiment on the Application of the Onsager-Kirkwood Model for Solvation Thermodynamics. J. Chem. Educ. 2020, 97, 825–830. [Google Scholar] [CrossRef]
- Schweitzer, G.K.; Benson, W. Enol Content of Some Beta-Diketones. J. Chem. Eng. Data 1968, 3, 454–455. [Google Scholar]
- Sloop, J.C.; Boyle, P.D.; Fountain, A.W.; Pearman, W.F.; Swann, J.A. Electron-Deficient Aryl beta-Diketones: Synthesis and Novel Tautomeric Preferences. Eur. J. Org. Chem. 2011, 936–941. [Google Scholar] [CrossRef]
- Belova, N.V.; Sliznev, V.V.; Oberhammer, H.; Girichev, G.V. Tautomeric and conformational properties of beta-diketones. J. Mol. Struct. 2010, 978, 282–293. [Google Scholar] [CrossRef]
- Zawadiak, J.; Mrzyczek, M. UV absorption and keto-enol tautomerism equilibrium of methoxy and dimethoxy 1,3-diphenylpropane-1,3-diones. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2010, 75, 925–929. [Google Scholar] [CrossRef]
- Manolova, Y.; Deneva, V.; Antonov, L.; Drakalska, E.; Momekova, D.; Lambov, N. The effect of the water on the curcumin tautomerism: A quantitative approach. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 132, 815–820. [Google Scholar] [CrossRef]
- Adeniyi, A.A.; Conradie, J. The stability, kinetics and inter-fragment electron communication of the tautomers of twelve selected beta-diketone molecules: A computational study. J. Mol. Graph. Model. 2018, 85, 25–39. [Google Scholar] [CrossRef]
- Henry, M.C.; Yonker, C.R. FT-IR studies of acetylacetonates in supercritical CO2 using a capillary cell at pressures up to 3.1 kbar. Anal. Chem. 2004, 76, 4684–4689. [Google Scholar] [CrossRef]
- Levina, E.O.; Khrenova, M.G.; Astakhov, A.A.; Tsirelson, V.G. Keto-enol tautomerism from the electron delocalization perspective. J. Comput. Chem. 2022, 43, 1000–1010. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Biswas, S.; Pramanik, A.; Sarkar, P. Computational Studies on the Keto-Enol Tautomerism of Acetylacetone. Int. J. Res. Soc. Nat. Sci. 2017, 2, 2455–5916. [Google Scholar]
- Tsukahara, T.; Nagaoka, K.; Morikawa, K.; Mawatari, K.; Kitamori, T. Keto–Enol Tautomeric Equilibrium of Acetylacetone SolutionConfined in Extended Nanospaces. J. Phys. Chem. B 2015, 119, 14750–14755. [Google Scholar] [CrossRef] [PubMed]
- Tayyari, S.F.; Najafi, A.; Lorestani, F.; Sammelson, R.E. Hydrogen bond strength and vibrational assignment of the enol form of 3-(methylthio)pentane-2,4-dione. J. Mol. Struct.-Theochem. 2008, 854, 54–62. [Google Scholar] [CrossRef]
- Sammelson, R.E.; Najafi, A.; Lorestani, F.; Azizkhani, M.; Tayyari, S.F. Hydrogen bond strength and vibrational assignment of the enol form of 3-(phenylthio)pentane-2,4-dione. J. Mol. Struct. 2008, 889, 165–176. [Google Scholar] [CrossRef]
- Dolati, F.; Tayyari, S.F.; Vakili, M. Tautomerism, conformational analysis, and spectroscopy studies of 3-bromo-pentane-2,4-dione. J. Mol. Struct. 2015, 1094, 264–273. [Google Scholar] [CrossRef]
- Zahedi-Tabrizi, M.; Tayyari, F.; Moosavi-Tekyeh, Z.; Jalali, A.; Tayyari, S.F. Structure and vibrational assignment of the enol form of 1,1,1-trifluoro-2,4-pentanedione. Spectrochim. Acta A-Mol. Biomol. Spectros. 2006, 65, 387–396. [Google Scholar] [CrossRef]
- Cornago, P.; Claramunt, R.M.; Bouissane, L.; Alkorta, I.; Elguero, J. A study of the tautomerism of beta-dicarbonyl compounds with special emphasis on curcuminoids. Tetrahedron 2008, 64, 8089–8094. [Google Scholar] [CrossRef]
- Carlsen, L.; Hansen, P.E.; Saeed, B.A.; Elias, R.S. Curcumin analogues for possible cancer treatment. A QSAR study. World J. Biol. Pharm. Res. 2021, 1, 1–16. [Google Scholar] [CrossRef]
- Mennucci, B.; Tomasi, J.; Cammi, R.; Cheeseman, J.R.; Frisch, M.J.; Devlin, F.J.; Gabriel, S.; Stephens, P.J. Polarizable Continuum Model (PCM) Calculations of Solvent Effects on Optical Rotations of Chiral Molecules. J. Phys. Chem. A 2002, 106, 6102–6113. [Google Scholar] [CrossRef]
- Takano, Y.; Houk, K.N. Benchmarking the Conductor-like Polarizable Continuum Model (CPCM) for Aqueous Solvation Free Energies of Neutral and Ionic Organic Molecules. J. Chem. Theory Comput. 2005, 1, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]
- Mehrani, S.; Tayyari, S.F.; Herav, M.M.; Morsali, A. Theoretical investigation of solvent effect on the keto-enol tautomerization of pentane-2,4-dione and a comparison between experimental data and theoretical calculations. Can. J. Chem. 2021, 99, 411–424. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules: A Quantum Theory; Oxford University Press: Oxford, UK, 1990. [Google Scholar]
- Casier, B.; Sisourat, N.; Carniato, S.; Capron, N. Keto-enol tautomerism in micro-hydrated acetylacetone: An atoms-in-molecules study. Theor. Chem. Acc. 2018, 137, 1–10. [Google Scholar] [CrossRef]
- Serdiuk, I.E.; Wera, M.; Roshal, A.S.D.; Sowinsky, P.; Zadykowicz, B. Tautomerism, structure and properties of 1,1′,1″-(2,4,6-trihydroxybenzene-1,3,5-triyl)triethanone. Tetrahedron Lett. 2011, 52, 2737–2740. [Google Scholar] [CrossRef]
- Hansen, P.E.; Kamounah, F.S.; Zhiryakova, D.; Manolova, Y.; Antonov, L. 1,1′,1″-(2,4,6-hydroxybenzene-1,3,5-triyl)triethanone non-tautomerism. Tetrahedron Lett. 2014, 55, 354–357. [Google Scholar] [CrossRef]
- Waluk, J.; Pietrzak, M.; Dobkowski, J.; Gorski, A.; Gawinkowski, S.; Kijak, M.; Luboradzki, R.; Hansen, P.E. Arresting consecutive steps of a photochromic reaction: Studies of β-thioxoketones combining laser photolysis with NMR detection. Chem. Phys. Phys. Chem. 2014, 16, 9128–9137. [Google Scholar]
- Kong, X.; Brinkmann, A.; Terskikh, V.; Wasylishen, R.E.; Bernard, G.M.; Duan, Z.; Wu, Q.; Wu, G. Proton Probability Distribution in the O…H…H Low-Barrier Hydrogen Bond: A Combined Solid-State NMR and Quantum Chemical Computational study of Dibenzoylmethane and Curcumin. J. Phys. Chem. B 2016, 120, 11692–11704. [Google Scholar] [CrossRef]
- Herbstein, F.H.; Iversen, B.B.; Kapon, M.; Krebs Larsen, F.; Madsen, G.K.H.; Reisner, G.M. X-ray and neutron diffraction study of benzoylacetone in the temperature range 8-300 K: Comparison with other cis-enol molecules. Acta Cryst. 1999, B55, 767–787. [Google Scholar] [CrossRef]
- Thomas, L.H.; Florence, A.J.; Wilson, C.C. Hydrogen atom behavior imaged in a short intramolecular hydrogen bond using the combined approach of X-ray and neutron diffraction. New J. Chem. 2009, 33, 2486–2490. [Google Scholar] [CrossRef]
- Srinivasan, R.; Feenstra, J.S.; Park, S.T.; Xu, S.; Zewail, A.H. Direct determination of hydrogen-bonded structures in resonant and tautomeric reactions using ultrafast electron diffraction. J. Am. Chem. Soc. 2004, 126, 2266–2267. [Google Scholar] [CrossRef]
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.I.J.; Refson, K.; Payne, M.C. First principles methods using CASTEP. Z. Kristallogr. 2005, 220, 567–570. [Google Scholar] [CrossRef]
- Conradie, M.M.; Muller, A.J.; Conradie, J. Thienyl-containing β-Diketones: Synthesis, Characterization, Crystal structure and Keto-enol Kinetics. S. Afr. J. Chem. 2008, 61, 13–21. [Google Scholar]
- Nieto, C.I.; Cabildo, P.; Claramunt, R.M.; Carnago, P.; Sanz, D.; Torralba, M.C.; Torres, M.R.; Ferraro, M.B.; Alkorta, I.; Marín-Luna, M.; et al. The structure of β-diketones related to curcumin determined by X-ray crystallography, NMR (solution and solid state) and theoretical calculations. Strukt. Chem. 2016, 27, 705–730. [Google Scholar] [CrossRef]
- Jiménez-Cruz, F.; Mar, L.F.; García-Gutierrez, J.L. Molecular structure and OAHO hydrogen bond in 1-aryl-1,3-diketone malonates. J. Mol. Struct. 2013, 1034, 43–50. [Google Scholar] [CrossRef]
- Babjakova, E.; Dastychova, L.; Hanulíková, B.; Kuřitka, I.; Nečas, M.; Vašková, H.; Vicha, R. Synthesis, molecular structure and vibrational spectra of 1,3-bis(1-adamantyl)-2-phenylpropan-1,3-diones. J. Mol. Struct. 2015, 1085, 207–214. [Google Scholar] [CrossRef]
- Chatterjee, C.; Incarvito, C.D.; Burns, L.A.; Vaccaro, P.H. Electronic Structure and Proton Transfer in Ground-State Hexafluoroacetylacetone. J. Phys. Chem. A 2010, 114, 6630–6640. [Google Scholar] [CrossRef]
- Oyarce, J.; Hernandez, L.; Ahumada, G.; Soto, J.P.; Del Valle, M.A.; Dorcet, V.; Carrillo, D.; Hamon, J.-R.; Manzur, C. Thiophene-containing beta-diketonate complex of copper(II): X-ray crystal structure and electropolymerization. Polyhedron 2017, 123, 277–284. [Google Scholar] [CrossRef]
- Car, R.; Parrinello, M. Unified Approach for Molecular Dynamics and Density-Functional Theory. Phys. Rev. Lett. 1985, 55, 2471–2474. [Google Scholar] [CrossRef]
- Marx, D.; Parrinello, M. Ab initio path integral molecular dynamics: Basic ideas. J. Chem. Phys. 1996, 104, 4077–4082. [Google Scholar] [CrossRef]
- Durlak, P.; Latajka, Z. Car-Parrinello and path integral molecular dynamics study of the intramolecular hydrogen bonds in the crystals of benzoylacetone and dideuterobenzoylacetone. Phys. Chem. Chem. Phys. 2014, 16, 23026–23037. [Google Scholar] [CrossRef]
- Hansen, P.E.; Borisov, E.V.; Lindon, J.C. Determination of the Tautomeric Equilibria of Pyridoyl Benzoyl β-Diketones in the Liquid and Solid State through the use of Deuterium Isotope Effects on 1H and 13C NMR Chemical Shifts and Spin Coupling Constants. Spectrochim. Acta 2015, 136, 107–112. [Google Scholar] [CrossRef]
- Dudek, M.; Clegg, J.K.; Glasson, C.R.K.; Kelly, N.; Gloe, K.; Gloe, K.; Kelling, A.; Buschmann, H.-J.; Jolliffe, K.A.; Lindoy, L.F.; et al. Interaction of Copper(II) with Ditopic Pyridyl-β-diketone Ligands: Dimeric, Framework, and Metallogel Structures. Cryst. Growth DES 2011, 11, 1697–1704. [Google Scholar] [CrossRef]
- Martinez, V.; Bedekovíc, N.; Stilinovíc, V.; Cincic, D. Tautomeric Equilibrium of an Asymmetric β-diketone in Halogen-Bonded Cocrystals with perfluorinated iodo benzenes. Crystal 2021, 11, 699. [Google Scholar] [CrossRef]
- Wasylishen, R.E.; Matlinska, M.A.; Bernard, G.M.; Terskikh, V.V.; Brinkmann, A. Hydrogen-Bonding in the Enol Tautomer of 1,3-Diketones: Insights from 2/1H Isotope Effects on NMR Parameters in the Solid State as well as Computational Chemistry. Acta Cryst. 2019, A75, a292. [Google Scholar] [CrossRef]
- Bolvig, S.; Hansen, P.E.; Wemmer, D.; Williams, P. Deuterium Isotope Effects on 17O Chemical Shifts of Intramolecularly Hydrogen Bonded Systems. J. Mol. Struct. 1999, 509, 171–181. [Google Scholar] [CrossRef]
- Noerskov-Lauritsen, L.; Carlsen, L.; Duus, F. Definitive Evidence for the Existence of the Hydrogen-bonding Enol Form of Non-aromatic β–Thioxoketones. X-Ray Crystal Structure of I-(I-Methylcyclopropyl)-3-thioxobutan-l–one. J. Chem. Soc. Chem. Commun. 1983, 9, 496–498. [Google Scholar] [CrossRef]
- Rozatian, N.; Beeby, A.; Ashworth, I.W.; Sandford, G.; Hodgson, D.R.W. Enolization rates control mono-versus di-fluorination of 1,3-dicarbonyl derivatives. Chem. Sci. 2019, 10, 10318–10330. [Google Scholar] [CrossRef]
- Kojic, M.; Petkovic, M.; Etinski, M. A new insight into the photochemistry of avobenzone in gas phase and acetonitrile from ab initio calculations. Phys. Chem. Chem. Phys. 2016, 18, 22168–22178. [Google Scholar] [CrossRef]
- Suwa, Y.; Yamaji, M. Steady state and laser photolysis studies of keto-enol tautomerizations in 2-alkyl-1,3-diketones having five-membered rings in acetonitrile: Temporal UV-A sunscreen. J. Photochem. Photobiol. A Chem. 2016, 316, 69–74. [Google Scholar] [CrossRef]
- Chi, T.X.-C.; Wang, Y.-H.; Gao, Y.; Sui, N.; Zhang, L.-Q.; Wang, W.-Y.; Lu, R.; Ji, W.-Y.; Yang, Y.-Q.; Zhang, H.-Z. Acceptor number-dependent ultrafast photo-physical properties of push-pull chromophores using time-resolved methods. Chem. Phys. Lett. 2018, 698, 127–131. [Google Scholar] [CrossRef]
- Verma, P.K.; Steinbacher, A.; Koch, F.; Nuernberger, P.; Brixner, T. Monitoring Ultrafast Intramolecular proton Transfer Processes in an Unsymmetrical β-Diketone. Phys. Chem. Chem. Phys. 2015, 17, 8459–8466. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.K.; Steinbacher, A.; Koch, F.; Nuernberger, P.; Brixner, T. Excited–state Intramolecular proton transfer of 2-acetylindan-1,3-dione studied by ultrafast absorption and fluorescence spectroscopy. Struct. Dyn. 2016, 3, 023606. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ren, W.; He, Z.; Zhu, Y. The Investigation of Excited-State Intramolecular proton transfer Mechanism of 2-acetyl-1,3-Dion: The solvation effect. J. Clust. Sci. 2017, 28, 2111–2122. [Google Scholar] [CrossRef]
- Wu, K.; Zhang, T.; Wang, Z.; Wang, L.; Zhan, L.; Gong, S.; Zhong, C.; Lu, Z.-H.; Zhang, S.; Yang, C. Do Novo Design of Excited-State Intramolecular Proton Transfer Emitters via a Thermally Activated Delayed fluorescence Channel. J. Am. Chem. Soc. 2018, 140, 887–8886. [Google Scholar]
- Leen, V.; Laine, M.; Ngongo, J.M.; Lipkowski, P.; Verbelen, B.; Kochel, A.; Dehaen, W.; Van der Auweraer, M.; Nadtochenko, V.; Filarowski, A. Impact of the Keto–Enol Tautomeric Equilibrium on the BODIPY Chromophore. J. Phys. Chem. A 2018, 122, 5955–5961. [Google Scholar] [CrossRef]
- Tang, B.; Zhang, H.; Cheng, X.; Ye, K.; Zhang, H. 1,3-Diaryl-β-diketone Organic Crystals with Red Amplified Spontaneous Emission. ChemPlusChem 2016, 81, 1320–1325. [Google Scholar] [CrossRef]
- Cheg, X.; Li, F.; Han, S.; Zhang, Y.; Jiao, C.; Wei, J.; Ye, K.; Wang, Y.; Zhang, H. Emission behaviors of unsymmetrical 1,3-diaryl-β-diketones: A model perfectly disclosing the effect of molecular conformation on luminescence of organic solids. Sci. Rep. 2015, 5, 9140. [Google Scholar]
- Wu, D.; Fang, X.; Song, J.; Qu, L.; Zhou, X.; Xiang, H.; Wang, J.; Liu, J.J. Multi-stimuli-responsive fluorescence of axially chiral 4-en-β–diketones. Dye. Pigment. 2021, 184, 108851. [Google Scholar] [CrossRef]
- Andresen, B.; Duus, F.; Bolvig, S.; Hansen, P.E. Variable Temperature 1H and 13C NMR Spectroscopic Investigation of the Enol-Enethiol tautomerism of β-Thioxoketones. Isotope Effects due to Deuteron Chelation. J. Mol. Struct. 2000, 552, 45–63. [Google Scholar] [CrossRef]
- Pietrzak, M.; Buczyńska, J.; Duus, F.; Waluk, J.; Hansen, P. Photoinduced and Ground State Conversions in a Cyclic β-Thioxoketone. RSC Adv. 2022, 12, 681–689. [Google Scholar] [CrossRef]
- Posokhov, A.; Gorsky, A.; Spanget-Larsen, J.; Duus, F.; Hansen, P.E.; Waluk, J. The Structure of the Phototransformation product in monothiodibenzoylmethane. Chem. Phys. Lett. 2001, 350, 502–508. [Google Scholar] [CrossRef]
- Hansen, B.K.V.; Gorski, A.; Posokhov, Y.; Duus, F.; Hansen, P.E.; Waluk, J.; Spanget-Larsen, J. Monothiobenzoylmethane: Structural and vibrational assignments. Vib. Spectros. 2007, 43, 53–63. [Google Scholar] [CrossRef]
- Nitschke, P.; Lokesh, N. Combination of illumination and high resolution NMR spectroscopy: Key features and practical aspects, photochemical applications, and new concepts. Progr. NMR 2019, 114–115, 86–134. [Google Scholar] [CrossRef]
- Dosli´c, N.; Abdel-Latif, M.K.; Kühn, O. Laser control of single and double proton transfer reactions. Acta Chim. Slov. 2011, 58, 411–424. [Google Scholar]
- Iglesias, E. Substituent effects on enol nitrosation of 1,3-diketones. Int. J. Chem. Kin. 2012, 44, 668–679. [Google Scholar] [CrossRef]
- Parameswari, A.R.; Rajalakshmi, G.; Kumaradhas, P. A combined molecular docking and charge density analysis is a new approach for medicinal research to understand drug-receptor interaction: Curcumin-AChE model. Chem. Biol. Interact. 2015, 225, 21–31. [Google Scholar] [CrossRef]
- Porchezhiyan, V.; Kalaivani, D.; Sobana, J.; Noorjahan, S.E. Synthesis, docking and in vitro evaluation of L-proline derived 1,3-diketones possessing anti-cancer and anti-inflammatory activities. J. Mol. Struct. 2020, 1206, 127754. [Google Scholar] [CrossRef]
- Manbeck, K.A.; Boaz, N.C.; Bair, N.C.; Sanders, A.M.S.; Marsh, A.L. Substituent Effects on Keto-Enol Equilibria Using NMR Spectroscopy. J. Chem. Educ. 2011, 88, 1444–1445. [Google Scholar] [CrossRef]
- Claramunt, R.M.; López, C.; Maria, M.D.S.; Sanz, D.; Elguero, J. The use of NMR spectroscopy to study tautomerism. Prog. Nucl. Magn. Reson. Spectrosc. 2006, 49, 169–206. [Google Scholar] [CrossRef]
- Bal, D.; Kraska-Dziadecka, A.; Gryff-Keller, A. Solution Structure of Succinylacetone, An Unsymmetrical beta-Diketone, As Studied by C-13 NMR and GIAO-DFT Calculations. J. Org. Chem. 2009, 74, 8604–8609. [Google Scholar] [CrossRef] [PubMed]
- Buceta, N.N.; Della Ve´dova, C.O.; Romanelli, G.P.; Autino, J.C.; Jios, J.L. Deuterium isotopic effect on 13C NMR chemical shifts of 1-(2-hydroxyphenyl)-3-aryl-1,3-propanediones: Hydrogen bond and substituent effects. J. Mol. Struct. 2008, 878, 50–59. [Google Scholar] [CrossRef]
- Perrin, C.L.; Kim, Y.J. Symmetry of the hydrogen bond in malonaldehyde enol in solution. J. Am. Chem. Soc. 1998, 120, 12641–12645. [Google Scholar] [CrossRef]
- Hansen, P.E. Methods to distinguish tautomeric cases from static ones. In Tautomerism: Ideas, Compounds, Applications; Antonov, L., Ed.; Wiley-VCH: Weinheim, Germany, 2016. [Google Scholar]
- Altman, L.J.; Laungani, D.; Gunnarsson, G.; Wennerström, H.; Forsén, S. Proton, deuterium and tritium nuclear magnetic–resonance of intra-molecular hydrogen bonds–isotope effects and shape of potential-energy function. J. Am. Chem. Soc. 1978, 100, 8264–8266. [Google Scholar] [CrossRef]
- Bolvig, S.; Hansen, P.E.; Morimoto, H.; Wemmer, D.; Williams, P. Primary Tritium and Deuterium Isotope Effects on Chemical Shifts of Compounds having an Intramolecular Hydrogen bond. Magn. Reson. Chem. 2000, 38, 525–535. [Google Scholar] [CrossRef]
- Bolvig, S.; Hansen, P.E. Deuterium Isotope Effects on 13C Chemical Shifts as a Probe for Tautomerism in Enolic β-Diketones. Magn. Reson. Chem. 1996, 34, 467–478. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hansen, P.E. Tautomerism of β-Diketones and β-Thioxoketones. Encyclopedia 2023, 3, 182-201. https://doi.org/10.3390/encyclopedia3010013
Hansen PE. Tautomerism of β-Diketones and β-Thioxoketones. Encyclopedia. 2023; 3(1):182-201. https://doi.org/10.3390/encyclopedia3010013
Chicago/Turabian StyleHansen, Poul Erik. 2023. "Tautomerism of β-Diketones and β-Thioxoketones" Encyclopedia 3, no. 1: 182-201. https://doi.org/10.3390/encyclopedia3010013
APA StyleHansen, P. E. (2023). Tautomerism of β-Diketones and β-Thioxoketones. Encyclopedia, 3(1), 182-201. https://doi.org/10.3390/encyclopedia3010013








